Abstract
Background
Tramadol is a widely used synthetic opioid for moderate to severe pain. Some studies have shown that tramadol can increase oxidative stress in different tissues of the body. Quercetin is also a substance with various biological effects, including antioxidant, anti-inflammatory, hepatoprotective, nephroprotective, and cardioprotective activities. The current investigation aimed at determining the effects of quercetin, with or without naloxone, on tramadol intoxication.
Methods
This study was performed on 30 male Wistar rats divided into five groups: Group I) control group: intraperitoneal injections of normal saline 0.9% for 14 days; Group II) tramadol: 25 mg/kg for 14 days, and then a 50 mg/kg acute dose injection on the last day; Group III) acute quercetin (single dose): tramadol injection as with the second group plus 100 mg/kg of quercetin on the last day; Group IV) chronic quercetin: tramadol injection similar to the second group plus quercetin 100 mg/kg for 14 days; Group V) quercetin plus naloxone: tramadol injection similar to the second group plus injection of quercetin 100 mg/kg + intravenous naloxone 2 mg/kg on the last day, followed by a 4 mg/kg/h injection of naloxone for six hours. The rats were monitored for six hours on the last day, relating to the number and severity of seizures. Finally, the samples were prepared for biochemical investigation of the serum level of oxidative stress markers (MDA, SOD, NOx), inflammatory factors (IL-6, TNF-α), biochemical parameters (ALT, AST, creatinine, glucose) and hematological assay. The liver, heart, kidney, cortex, cerebellum, and adrenal tissues were collected to investigate the redox state.
Results
None of the treatments had positive effects on the number and severity of seizures. Chronic administration of quercetin led to alteration of some blood parameters, including reduced hemoglobin level and elevated platelet counts. Acute on chronic tramadol administration resulted in a significant rise in AST, where different treatments failed to reduce their levels down to the control group.
Conclusion
chronic administration of quercetin showed decreased oxidative/nitrosative stress in the liver, kidney, adrenal, and heart tissues. Quercetin plus naloxone decreased oxidative stress in the heart and adrenal tissues, but adverse effects on the brain cortex and hepatic function. Single-dose quercetin reduced cardiac oxidative stress.
Keywords: Acute on chronic, Inflammation, Oxidative stress, Quercetin, Tramadol
Background
Tramadol (TRM) is a central analgesic that is mostly used in moderate to severe pain. Its analgesic effect results from its having an agonist function on opioid receptors and inhibiting the reuptake of serotonin and norepinephrine (1, 2). Today, Tramadol has become one of the most widely used drugs globally, resulting in increased toxicity, complications, and mortality (3, 4). A tramadol overdose can lead to impaired consciousness, seizure and possible damages, agitation, respiratory depression, and serotonin syndrome (4, 5). Further, long-term use of this synthetic opioid is associated with addiction as well as physical and psychological dependence, which, despite therapeutic effects, can affect different organs of the body (3). Tramadol may cause self-limiting tonic-clonic seizures within 4–6 h after administration. Some pathways like inhibitory effects on gamma-aminobutyric acid (GABA) receptors, the serotonin, nitric oxide, opioid, and glutaminergic pathways, have been proposed in tramadol-induced seizures (4, 6).
Studies have shown that oxidative stress is one of the critical causes of damages in tramadol toxicity (6–8). Tramadol can induce a high regulation of lipid peroxidation, inflammation, and apoptosis markers. Also, it can alter neurotransmission in the rat cerebrum (9).
Furthermore, chronic consumption of it has shown histological disorders, such as increased apoptosis in the brain cortex of rats through developing oxidative stress (3, 10). In cases of tramadol poisoning, choosing the right therapy can be challenging. Naloxone, as an opioid antagonist, is used to prevent respiratory depression caused by non-synthetic opioids. However, there are contradictory studies about its usage in tramadol toxicity and its effect on seizures (11). In addition to typical chemical drugs that may be used for tramadol toxicity, other compounds that have shown beneficial effects in similar situations may prove helpful during toxicity with this widely used drug. Therefore, it will be substantially useful to design strategies to prevent toxicity with this drug on different systems and limit its medical complications. Quercetin is a flavonoid present in fruits and vegetables to which various therapeutic and protective properties have been attributed (12, 13). Quercetin has multiple advantageous effects against oxidative stress-related diseases. This substance has multiple biological effects due to its antioxidant, anti-inflammatory, hepatoprotective, and anti-apoptotic properties as well as neuroprotective potential (12, 13). The protective effects of this substance have also been shown on the heart (14–16).
Quercetin has several phenolic hydroxyl groups that have strong antioxidant activity. It has been reported to be a potent ROS cleanser that can modulate oxidative parameters (17). For instance, it enhances superoxide dismutase activities, catalase, glutathione peroxidase, and glutathione levels. Also, it can reduce the malondialdehyde’s serum levels. Besides, quercetin increased nitric oxide synthase activity. It also can increase nitric oxide levels in serum (18). Also, neuroprotective effects of quercetin on various central nervous system disorders like memory impairment (19, 20) and seizure (12, 13, 21) have been documented. The previous study results showed that quercetin reduced kainic acid-induced seizures by reducing GABA gene expression (12). Also, some earlier studies showed that quercetin has anti-seizure activity in acute and chronic models of kindling induced using pentylenetetrazole (PTZ) (13, 20). Chronic administration of quercetin (20–50 mg/kg/orally) in the case of ethanol withdrawal showed a protective effect against PTZ seizures (22). However, in another study, it was found that quercetin could not protect against seizures induced by N-methyl D-aspartate (NMDA) in rats. It has been suggested that quercetin may have proconvulsant effect (23). The protective effects of quercetin in lead, methylmercury, and tungsten toxicity have been reported (24). However, its protective effects concerning tramadol administration have remained understudied. Due to the positive effects of quercetin on seizures, its effects on the GABA receptor and its well-known systemic antioxidant/anti-inflammatory properties, the present study was designed to confirm the hypothesis that this phenolic compound can have a protective mechanism against tramadol-induced seizures. Also, the possible oxidant/antioxidant effect on other tissues and hematological parameters was monitored. Furthermore, the present study examined the effects of quercetin alone and naloxone (a commonly used opioid receptor antagonist in the case of tramadol-induced seizures) in acute tramadol administration.
Methods
Animals and materials
This study was performed on 30 male Wistar rats (body weight: 200–250 g, 12 weeks) procured from the department for keeping laboratory animals at Birjand University of medical sciences. The animals were subject to standard laboratory conditions (constant room temperature: 22 ± 2 °C), the light-dark cycle of 12 h, and free access to food and water. All experiments and treatments of rats were conducted according to the international laws of handling laboratory animals. The study protocol was also confirmed by the ethics committee of Birjand University of medical sciences (code: IR.BUMS.REC.1397.194). Sigma-Aldrich supplied quercetin (Q4951-10G), Naloxone (PHR1802-300MG), and pentobarbital (P3761-5G) while tramadol was purchased from Temad Co. (Iran, Karaj). Quercetin and naloxone were dissolved in DMSO while tramadol was in normal saline. The sample size calculation is the basis of the mathematical formula for “Group Comparison” design for animal studies (25). Healthy animals with normal behavior and activity from the same species, genders, weighing 200 to 250 g, and 12 weeks were included. Previously used rats in other experiments were not included. Exclusion criteria were considered as death during experiments and animals with aberrant behavior.
Experimental design
After one week of adaptation to laboratory conditions, the animals were randomly assigned into five 6-member groups.
Group I (Control group): Normal saline 0.9%, intraperitoneally (IP) for 14 days.
Group II (Tramadol group): 25 mg/kg tramadol, IP for 14 days followed by acute administration of 50 mg/kg tramadol IP on the last day (the final dose of the last day: 75 mg/kg).
Group III (Acute quercetin group) (single dose): tramadol 25 mg/kg IP for 14 days. On the last day, acute IP injection of 50 mg/kg tramadol + quercetin 100 mg/kg IP following tramadol injection.
Group IV (Chronic quercetin group): 25 mg/kg tramadol IP + 100 mg/kg quercetin IP for 14 days, followed by 50 mg/kg tramadol IP on the last day (12, 13).
Group V (Quercetin plus naloxone group): 25 mg/kg tramadol IP for 14 days and on the last day, 50 mg/kg tramadol IP + 100 mg/kg quercetin IP + 2 mg/kg intravenous naloxone + 4 mg/kg/h naloxone for 6 h.
The rats were monitored for six hours on the last day for the number and severity of seizures. Seizure severity was rated according to the Racine criteria. In stage 1, rats were immobile, with closed eyes and facial clonus, whereas the hair around the nose was shaking. In stage 2, rats showed head-shaking accompanied by more severe facial clonus. In stage 3, rats experienced forelimb clonus. In stage 4, rats rose on their hind legs and exhibited bilateral forelimb clonus. In stage 5, rats rose on their hind legs, had no balance, fell, and underwent generalized tonic-clonic seizures (4). Video camera recorded all behavioral tests. A trained observer afterward analyzed videos. The researcher was aware of the rats’ experimental group, but a researcher, blinded to the group allocation, carried out statistical analysis and outcome assessment.
Analyses of different parameters
Twenty-four hours after the last injection (for 12–14 h of fasting), the rats were sacrificed. 24 h after the end of the experiments, rats were profoundly anesthetized with 60 mg/kg sodium pentobarbital (Sigma-Aldrich, Germany, P 3761-5G) intraperitoneally before euthanization.
Then, blood specimens were taken from the heart. The serum was obtained through centrifugation and stored at − 20 °C until used. The EDTA-containing blood samples were used to determine complete blood count (CBC). The obtained serum was used to investigate biochemical (ALT, AST, creatinine, glucose), inflammatory (TNF-a, Interleukin-6), and oxidative stress (MDA, NOx, SOD) parameters. An IL-6 concentration was measured using a rat ELISA kit (ZellBio GmbH; Germany, Cat.No: ZB-10135C-R9648, the assay’s sensitivity: 2.5 ng/L). Also, Serum TNF-a was measured using a rat ELISA kit (Diaclone, France, Cat No:865.000.096); intra-and interassay coefficients of variations (CVs) were 8.2 and 6.5%, respectively. The liver, heart, kidney, cortex, cerebellum, and adrenal tissues were excised immediately and kept at − 80 °C until the time of analysis. The tissues were homogenized via a phosphate buffer cold solution (1:10, ph 7.7) by a homogenizer (MICCRA-D1, Germany) while being immersed in an ice bath. Next, the homogenated tissues were centrifuged at 15000 rpm for 20 min, where their supernatant was isolated. Homogenization and centrifugation were done carefully and in the same way in all samples. The serum and tissue concentration of NOx (nitrate and nitrite) was measured through the Griess reaction as previously described (26–28). As a byproduct of lipid peroxidation, the levels of thiobarbituric acid–reactive substances (TBARS) were determined using Uchiyama and Mihara’s method (29). MDA is the final product of the peroxidation of fatty acids, which creates a colored complex when combined with thiobarbituric acid (TBA). Based on the reaction with this acid at acidic pH at 90–100 °C, the absorption of the pink product resulting from the reaction was measured using spectrophotometry at the wavelength of 532 nm. A Nasdox™–Superoxide Dismutase Non-Enzymatic kit (Code: NS-15033, Navandsalamat, Iran) was employed to measure the level of superoxide dismutase (SOD). This test is based on inhibiting the pyrogallol autooxidation reaction, where the absorbance was read via an ELISA reader at the wavelength of 405 nm. It was then placed in the following formula as sample SOD activity (U/ml) × 200. All samples of a test variable were assessed together to minimize the interassay coefficient of variation measurement.
Statistical analysis
After collection, the data were introduced into SPSS 16. The assumption of data normality was investigated via the Shapiro Wilk test. In order to compare the means in the groups, the one-way ANOVA plus the Bonferroni post hoc test was used. In the case of abnormal data distribution, the Kruskal Wallis test and Dunn-Bonferroni test were employed. P < 0.05 was considered statistically significant. All data were included in the analysis.
Results
Biometrical data
In this study, one case of mortality was observed in the chronic quercetin (Group IV) and quercetin plus naloxone (Group V) groups. Before and after the intervention, the rats’ average weights were 218.08 ± 18.8 and 259.7 ± 34.6 g, respectively, whereas there was no difference between the groups before the study (p > 0.05). However, at the end of the study, the mean weight of the chronic quercetin group (Group IV) was significantly lower than that of the tramadol and control groups. Further, the chronic injection of quercetin for this group lowered the heart weight considerably compared to other groups (Table 1). Kidney and liver weight were similar in control, tramadol, and different treatment groups (Table 1). The ratio of tissue weight to body weight did not differ between groups. (p > 0.05).
Table 1.
Weight of rats and organ weight in different experimental groups
| Variables | Control | Tramadol | Tramadol + single Quercetin | Tramadol + chronic quercetin | Tramadol + single Quercetin + Naloxone |
|---|---|---|---|---|---|
| Weight of rats before intervention (g) | 219.1 ± 9.02 | 216.8 ± 15.5 | 217.2 ± 21.5 | 216.7 ± 27.8 | 220.6 ± 18.2 |
| Weight of rats after intervention (g) | 271.7 ± 15.7 | 271.6 ± 20.8 | 265.2 ± 23.19 | 229.33 ± 33.7*,** | 264.37 ± 4.52 |
| Weight of liver (g) | 9.1 ± 0.9 | 8.8 ± 1 | 8.05 ± 0.3 | 8.7 ± 0.3 | 9.4 ± 0.6 |
| Weight of kidney (g) | 1.7 ± 0.5 | 1.8 ± 0.1 | 1.8 ± 0.3 | 1.8 ± 0.1 | 1.8 ± 0.6 |
| Weight of heart (g) | 0.82 [0.79,0.88] † | 0.85 [0.83,0.91] † | 0.87 [0.83,0.88] † | 0.75 [0.71,0.8] | 0.86[0.86,0.86] † |
Statistical comparison between groups was made using one-way ANOVA followed by Bonferroni posthoc or Kruskal Wallis test and Dunn-Bonferroni post hoc
p < 0.05 significant as compared to the control group
** p < 0.05 significant as compared to tramadol group
† p < 0.05 significant as compared to Tramadol + chronic Quercetin group
Behavioral parameters
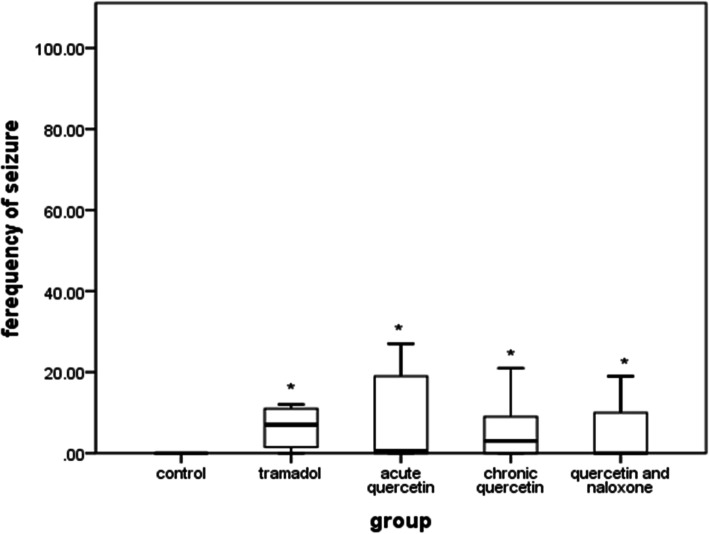
The results of this study showed that the number of seizures in all experimental groups was significantly higher than that of the control group (p < 0.05) (Fig. 1). However, none of the treatments significantly affected the number and severity of seizures compared to the tramadol group (p > 0.05).
Fig. 1.
Comparison of the number of seizures in different groups. *p < 0.05 compared to the Control group. Values are median and IQR
Hematologic and biochemical parameters
Chronic administration of quercetin resulted in an alteration of some blood parameters, such as reduced hemoglobin level and elevated platelet counts. In this regard, the chronic quercetin group’s median hemoglobin was significantly lower than that of the tramadol, single-dose quercetin, and quercetin plus naloxone groups. On the other hand, the mean platelet count was significantly higher in the chronic quercetin group than in others. Furthermore, the mean white blood count was higher in the single-dose quercetin group than in control. The administration of naloxone and a single dose of quercetin (100 mg) resulted in a significantly increased ALT level compared to the chronic quercetin group (Group IV). Further, the AST/ALT ratio was prominently higher in all groups than in control. However, the treatment did not affect the serum level of glucose and creatinine (Table 2).
Table 2.
hematologic and biochemical parameters in different experimental groups
| Variables | Control | Tramadol | Tramadol + single Quercetin | Tramadol + chronic Quercetin | Tramadol + single Quercetin + Naloxone |
|---|---|---|---|---|---|
| WBC10*3/μL | 3.12 ± 0.9 | 4.02 ± 1.3 | 5.8 ± 1.4 * | 5.2 ± 1.4 | 4.8 ± 1.3 |
| RBC 10*6/μL | 8.5 ± 0.9 | 8.98 ± 0.4 | 9.2 ± 0.3 | 8.34 ± 0.5 | 9.23 ± 0.4 |
| HGB (g/dL) | 14.9 [13.1, 15.25] | 14.7 [14.25,15.75] † | 15.3[14.85, 16.2] † | 13.45 [13.05,14.05] | 15.5 [14.97, 15.95] † |
| HCT (%) | 45.5[40.85,46.9] | 46.1 [43.65, 48.15] | 47.1[45.1, 49.65] | 41.15 [40.55,44.17] | 47.15 [46.5, 48.7] |
| PLT (10*3/μL) | 516.7 ± 21.7 † | 478.2 ± 46.51 † | 516.8 ± 69.5 † | 727.16 ± 58.04 | 412.5 ± 38.31 † |
| MCV (μm*3) | 51.8[51.2,52.7] | 50.7 [50.6, 51.95] | 51.4[50, 52.85] | 50.05 [49.65, 51.9] | 50.7 [50.47, 50.7] |
| MCHC (g/dL) | 32.5[32,32.7] | 32.7 [32, 32.95] | 32.9[32.3, 33.05] | 32.35 [31.82, 32.7] | 32.7 [32, 33.17] |
| MCH (pg) | 16.84 ± 0.2 | 16.62 ± 0.3 | 16.82 ± 0.4 | 16.28 ± 0.4 | 16.77 ± 0.4 |
| LYM (%) | 64.2 ± 7.7 | 66.2 ± 9.4 | 52.1 ± 14.6 | 52 ± 9.1 | 61.5 ± 9.7 |
| MON (%) | 0.62 ± 0.4 | 0.66 ± 0.4 | 1.44 ± 0.9 | 1.76 ± 0.5 | 1.47 ± 0.8 |
| NEU (%) | 32 ± 7.9 | 31 ± 10.5 | 44.2 ± 15.4 | 43.7 ± 8.1 | 34.5 ± 10.6 |
| EOS (%) | 2.4 ± 1.3 | 1.96 ± 2.3 | 0.9 ± 0.1 | 0.8 ± 0.6 | 1.57 ± 0.2 |
| BAS (%) | 1.7 [0.07, 2.1] | 2.4 [0, 3.3] | 3.2 [0.1, 5.5] | 2.9 [0.4, 4.7] | 2.9 [0.3, 3.5] |
| NEU/LYM ratio | 0.51 ± 0.1 | 0.49 ± 0.2 | 0.94 ± 0.4 | 0.87 ± 0.2 | 0.59 ± 0.2 |
| Glucose (mg/dl) | 136.6 ± 18.08 | 154.3 ± 25.1 | 137.1 ± 18.3 | 123.3 ± 18.8 | 131.8 ± 12.4 |
| Cr (mg/dl) | 0.35 ± 0.04 | 0.36 ± 0.05 | 0.34 ± 0.08 | 0.32 ± 0.05 | 0.44 ± 0.21 |
| AST (IU/L) | 110.6 [91.6, 146.3] | 177 [135.6, 203.5] | 148.3 [138.2, 216.9] | 157.05 [125.3, 182.6] | 180.5 [145.6, 435.7] |
| ALT (IU/L) | 59.4 ± 11 | 52.3 ± 11.4 | 39.1 ± 5.7 | 34.5 ± 16.3 | 79.2 ± 50.8† |
| AST/ALT ratio | 1.9 [1.8, 2.1] | 3.3 [2.6, 4.2] * | 4.2 [3.1, 5.7] * | 4.1 [3.1, 13.5] * | 3.1 [2.7, 4.3] * |
WBC, White Blood count; RBC, Red Blood count; HGB, hemoglobin; HCT, hematocrit; PLT, platelet; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; LYM, Lymphocytes; MON, Monocytes; NEU, Neutrophils; EOS, Eosinophils; BAS, Basophils; Cr, creatinine; AST, aspartate aminotransferase; ALT, alanine aminotransferase
Statistical comparison between groups was made using one-way ANOVA followed by Bonferroniposthoc or Kruskal Wallis test and Dunn-Bonferroni post hoc
* p < 0.05 significant as compared to the control group
† p < 0.05 significant as compared to Tramadol + chronic Quercetin group
Inflammatory parameter and antioxidant enzyme activity of serum
The level of interleukin 6 (IL-6) did not significantly differ between groups. The serum concentration of TNF-a was significantly higher in chronic quercetin and quercetin-naloxone groups compared to the control group. The nitric oxide, TBARS, and SOD levels of the serum did not differ between the groups (p > 0.05) (Table 3).
Table 3.
Inflammatory and oxidative stress parameters of serum in different experimental groups
| Groups Variable | Control | Tramadol | Tramadol + single Quercetin | Tramadol + chronic quercetin | Tramadol + single Quercetin + Naloxone |
|---|---|---|---|---|---|
| NOx (μmol/l) | 24.9 [19, 54.8] | 51.4 [26.2, 73.9] | 27.9 [11.16, 81.9] | 22.1 [12.5, 27.6] | 33.39 [12.18, 108.6] |
| SOD (U/ml) | 439.5 ± 50.3 | 494.6 ± 99.1 | 527.9 ± 78.2 | 574.4 ± 76.5 | 441.5 ± 86.9 |
| MDA (μmol/l) | 2.15 ± 0.2 | 2.32 ± 0.3 | 2.39 ± 0.3 | 2.21 ± 0.4 | 2.5 ± 0.7 |
| IL-6 (ng/L) | 8.6 ± 3.4 | 11.4 ± 4.5 | 12.87 ± 4.4 | 13.01 ± 4 | 10.08 ± 1.7 |
| Tnf-a (pg/mL) | 10.32 ± 1.1 | 13.86 ± 4.3 | 13.7 ± 2.8 | 15.2 ± 1.3* | 14.6 ± 0.8* |
Statistical comparison between groups was made using one-way ANOVA followed by Bonferroniposthoc or Kruskal Wallis test and Dunn-Bonferroni post hoc
NOx: Nitric Oxide, SOD: Superoxide dismutase, MDA: Malondialdehyde, IL-6: interleukin-6, Tnf-a: Tumour Necrosis Factor-alpha
* p < 0.05 significant as compared to the control group
The concentration of nitric oxide metabolites (NOx) in different tissues
Tramadol significantly increased NOx in the kidneys; however, it had no effect on the liver, heart, brain, or adrenal tissues. A quercetin injection (100 mg) along with tramadol for 14 days significantly decreased the liver’s nitric oxide level and restored the high NOx level following a tramadol injection in the kidney. There was a significantly higher NOx level in the acute quercetin and chronic quercetin groups than in the quercetin/naloxone group in the heart tissue. On the other hand, in the cortex, the nitric oxide level of the quercetin/naloxone group was significantly higher than in control, the acute quercetin, and chronic quercetin groups.
Oxidative stress parameters of different tissues
The TBARS in adrenal tissue was significantly lower in a single dose and naloxone groups (III and V) than in control; it was also lower in the chronic quercetin group (IV) than in the control and tramadol groups. All of the three treatment methods in the adrenal tissue reduced the TBARS similarly. Further, the level of this factor was lower in the heart tissue of all groups than in the control, suggesting the antioxidant effects of all interventions. A significant rise of the TBARS in the liver tissue of the single-dose quercetin group (III) over tramadol and other treatment groups (II, IV, and V) indicates the pro-oxidant effects of acute quercetin. In kidney and brain tissue, the TBARS was similar among all groups. Tramadol administration with different treatments did not affect the SOD activity in different studied tissues (Table 4).
Table 4.
Oxidative stress parameters of organs in different experimental groups
| Groups Variable | Control | Tramadol | Tramadol + single Quercetin | Tramadol + chronic quercetin | Tramadol + single Quercetin + Naloxone | |
|---|---|---|---|---|---|---|
| Liver | SOD (U/ml) | 628.36 [604.3, 712.7] | 673.3 [557.5, 686.7] | 726.5[580.3, 898.5] | 617.8 [594.6, 644.3] | 759.4 [590.6, 855.48] |
| NOx (μmol/l) | 15.2 ± 1.8 | 14.5 ± 2.1 | 11.7 ± 2.4 | 10.6 ± 0.5* | 12.9 ± 2.5 | |
| MDA (μmol/l) | 11.9 ± 3.9 | 8.7 ± 2.2 | 14.4 ± 2.1**,†,†† | 9.5 ± 1.5 | 8.9 ± 3.2 | |
| Kidney | SOD(U/ml) | 832.2 ± 72.4 | 665.2 ± 78.7 | 758.7 ± 117.7 | 686.4 ± 83.7 | 741.9 ± 62.7 |
| NOx (μmol/l) | 16.1 ± 2.9 | 21.3 ± 8.3* | 16.8 ± 2.2 | 12.44 ± 1.6** | 16.34 ± 1.2 | |
| MDA (μmol/l) | 20 ± 1.7 | 19 ± 2.6 | 21.5 ± 1.8 | 18.6 ± 2.1 | 20.7 ± 2.9 | |
| Heart | SOD(U/ml) | 695.3 ± 183 | 792.3 ± 136.7 | 948 ± 95 | 725.3 ± 268 | 651 ± 120 |
| NOx (μmol/l) | 11.2 [10.6,12.25] | 11.06 [9.3, 13.1] | 12.6 [10.5,15.6] †† | 11.9 [11.9, 14.1] †† | 9.5 [7.08, 10.7] | |
| MDA (μmol/l) | 17.9 ± 4.2 | 10.2 ± 2.5* | 9.4 ± 0.7* | 8.6 ± 0.5* | 8.9 ± 0.9* | |
| Cerebellum | SOD(U/ml) | 292.3 ± 21.26 | 319.8 ± 35.5 | 298.6 ± 19.7 | 278.9 ± 20.7 | 295.5 ± 27.06 |
| NOx (μmol/l) | 8.08 ± 1.3 | 7.5 ± 0.6 | 7.64 ± 1.2 | 8.49 ± 1.4 | 8.01 ± 1.5 | |
| MDA (μmol/l) | 5.6 ± 1.3 | 4.5 ± 1.1 | 5.3 ± 0.4 | 5.8 ± 0.6 | 5.3 ± 1.5 | |
| Cortex | SOD(U/ml) | 258.9 ± 15.32 | 275.7 ± 23.1 | 272.2 ± 17.5 | 260.1 ± 14.58 | 273.08 ± 10.11 |
| NOx (μmol/l) | 3.46 ± 0.8 | 4.1 ± 2.2 | 2.65 ± 1.04†† | 1.53 ± 0.7†† | 6 ± 1.68* | |
| MDA (μmol/l) | 4.86 [4.03, 5.6] | 4.6 [4.2, 6.7] | 4.9 [4.4, 6.1] | 4.6 [4.2,6.1] | 4.5 [4.4, 5.2] | |
| Adrenal | SOD(U/ml) | 577.3 ± 77.2 | 572 ± 50.5 | 576.6 ± 85.9 | 460.9 ± 48.4 | 584 ± 135 |
| NOx (μmol/l) | 17.6 ± 3.5 | 17.1 ± 3.1 | 15.7 ± 4.2 | 15.3 ± 1.4 | 14.5 ± 2.8 | |
| MDA (μmol/l) | 4.5 [4.1, 4.8] | 3.02 [2.6, 3.3] | 2.4 [2.2,3.1] * | 2.06 [1.9, 2.2] *,** | 2.3 [2.1, 2.3] * | |
* p < 0.05 significant as compared to the control group
** p < 0.05 significant as compared to tramadol group
† p < 0.05 significant as compared to Tramadol + chronic Quercetin group
†† p < 0.05 significant as compared to Tramadol + single Quercetin + Naloxone
Statistical comparison between groups was made using one-way ANOVA followed by Bonferroni post hoc or Kruskal Wallis test and Dunn-Bonferroni post hoc
Values are mean ± SD or median [IQR]
Discussion
Biometrical data
In the current study, we investigated quercetin’s beneficial effects on tramadol overdose. Based on our information, this is the first study that assessed the effects of this flavonoid on tramadol intoxication. After the experimental period, the rats in the TRM + chronic quercetin group showed body weight loss and decreased heart weight. This result was reported in some previous studies (30). Differences in reports can be observed, however, depending on the experimental model and doses. One study investigated the effects of oral quercetin treatment on experimental renovascular hypertension in Wistar rats (10 mg/kg) within five weeks. According to the results, the treatment did not lead to kidney or heart weight variations compared to untreated controls (31). The examination did not reveal any alterations in food and water consumption, body weights, or organ weight of Swiss mice at any doses of flavonoids (30, 300, or 3000 mg/kg for 28 days) (32). One other study revealed that a 25 mg administration of quercetin/day within 28 days, as 0.5% of the diet, had an insignificant impact on Swiss mice’s body or organ weight gains compared to a control group (33). Organ weight can critically indicate an experimental compound effect, and a substantial difference in the weight of organs may happen in treated and control animals if no morphological variation occurs (34).
Behavioral parameters
None of the treatments had significant effects on the number and severity of seizures. To our knowledge, quercetin effectiveness in tramadol-induced seizures has not been studied till now. However, its protective effects in other drug-induced seizures have been documented. For example, quercetin decreased kainic acid-induced seizure severity in a dose-dependent manner (12). The anticonvulsant effects of quercetin have been demonstrated at doses of 10 to 200 mg/kg in 6 Hz-induced convulsive seizures, the highest quercetin-related anticonvulsant activity associated with high plasma and cerebral concentrations (21). The exact anticonvulsant effects of quercetin and other flavonoids are not well-known, possibly mediated by their interactions with GABA receptors, glycine (35), acetylcholine (21), serotonin receptors (36), and adenosine (37).
Hematologic and biochemical parameters
In our study, chronic administration of quercetin had effects on some hematological parameters, including decreased hemoglobin level and increased platelet count. It has been reported that the oral administration of quercetin decreased hemoglobin and hematocrit at 30, 300, or 3000 mg/kg within 28 days (32). Another study did not document any statistically significant changes in hematologic parameters along with chlorpyrifos in the quercetin treatment group compared to the control group (38). In contrast, some others reported beneficial effects of quercetin on RBC and hematocrit (39). The platelet rates’ values were significantly increased with simultaneous quercetin administration and polychlorinated biphenyls (40) or acute and sub-chronic manganese administration (41). Some studies have shown an inhibition of platelet aggregation following supplementation with flavonoids in vivo (42, 43). In an in vivo study, quercetin inhibited platelet activation by interacting with cell membranes by not scavenging free radicals (43). Other animal studies have shown unaltered blood platelet counts in treatment with quercetin (44, 45). Also, tramadol is reported to cause experimental animals to experience hepatotoxicological variation in acute and chronic administration (46, 47). The present study results indicated that TRM led to the increased AST/ALT ratio in the serum related to male rats. A muscle injury due to tramadol-induced seizures should be considered for an increase in these enzymes.
Inflammatory and oxidative stress parameters
The levels of some oxidative stress markers in the tramadol group were no different than in the control group. Some studies have shown that chronic and acute administration of tramadol can lead to increased oxidative stress in the body (7, 10, 48–50). Some others have reported lower MDA levels in the tramadol receiving groups than in the control group. This confirms the hypothesis, which claims that tramadol reduces the effects of oxidative stress via peroxyl radicals scavenging. Also, in that study, the SOD data supported the possibility of a tramadol antioxidant effect when the drug is used during the pre-ischemic period (51). The differences in study design and experimental models may explain the discrepancies. In this study, the animals received acute on chronic administration of tramadol. The initial dose of tramadol may cause tolerance (52) and reduce adverse effects. Therefore, these results indicate the need for further studies.
The MDA content can be used to estimate the extent of lipid peroxidation (53). In the current study, all types of quercetin administration effectively reduced the MDA content of adrenal tissue. The increase of malondialdehyde in the liver in the acute quercetin group, which has not been seen in the chronic quercetin and naloxone-quercetin groups, presumably results from liver enzyme induction quercetin or positive effects of naloxone. However, we have not undertaken biochemical studies to verify this assumption. Our results also showed that chronic administration of quercetin in tramadol overdose decreased NOx formation in the liver, cortex, and kidneys, but not in the heart. As a member of the flavonoids family, Quercetin is demonstrated to function as a scavenger of both different reactive oxygen species and antioxidant activity (54). These support previous studies on different induced oxidative stressors (55). Quercetin administered orally and intraperitoneally can decrease the MDA level, which is induced by numerous toxins in rats (54). This ameliorative impact might be because of their greater diffusion into membranes, enabling them to scavenge free radicals at various sites (56) and regenerate exogenous and endogenous antioxidants, such as vitamins C and E as well as glutathione (45).
Cellular studies have revealed that quercetin can generate antioxidant and pro-oxidant impacts under certain conditions (57). It has been argued that the cellular oxidative balance and glutathione (GSH) content have an essential role in determining these effects (57). The quercetin pro-oxidant activity might have an auto-oxidizing capacity or might be able to convert to ortho-semiquinone and ortho-quinone/quinone methide intermediates through enzymatic oxidation, which may contribute to ROS generation and GSH depletion (58, 59). The activities of antioxidant enzymes have also been reported to be inhibited or increased by quercetin (44–46), and it depends on the experimental model (60–62) or the dose of the drug. Nevertheless, the significance of this mechanism has not been thoroughly resolved in vivo. It has been demonstrated that quercetin concentrations of up to 10 μM can protect fibroblasts from oxidative stress-induced damage and that following pre-treatment with quercetin concentrations of 30 μM, cytotoxicity occurred instead (63).
Additionally, the results revealed that the naloxone and quercetin co-treatment has toxic effects in cortex and liver tissues, compared to chronic quercetin administration, suggesting that naloxone has an adverse effect on some oxidative and liver function parameters. Most guidelines recommend naloxone as the first method of management in opioid poisoning, and there are some controversies between studies regarding using naloxone in tramadol overdose because of the possible risk for seizure (64–68).
In contrast to our results, in some reports (69), the quercetin anti-inflammatory effect was correlated with its free radical scavenging and anti-oxidative features; thus, eliminating reactive oxygen species can simultaneously prevent oxidation and inhibit inflammation. It has been noted that reactive oxygen species and the oxidation process contribute to the inflammatory responses via activating transfer variables, including nuclear factor-κ-gene binding (NF-κB) (70). Further, NF-κB has the capacity to induce TNF-α cytokine production (71). An in vivo study on mice reported a reduced inflammatory gene expression using the quercetin enriched diet (72). However, studies performed on healthy human volunteers with the help of quercetin demonstrated no effects on inflammatory agents existing in human blood (73).
Limitation
This study has some limitations that should be taken into consideration for interpreting the data. First, based on the animal study design, the findings can only be indicative rather than representative of humans’ complicated clinical situations. However, our model showed tramadol-induced seizures similar to that documented in humans (74–76). Second, the dose dependence effects of quercetin and its histopathological alteration were not investigated in this study. Third, we did not assess possible mechanisms by which quercetin alters hematologic, oxidative, and inflammatory parameters. Fourth, the seizures parameters were evaluated using visual assessment; other assessment methods are suggested for future studies. Moreover, further experimental research is required to reveal the effectiveness of quercetin in a different type of tramadol administration and the molecular pathways of quercetin’s effects on tramadol overdose with more powerful methods that provides more robust results.
Conclusion
None of the treatments had significant effects on the number and severity of seizures. Within the limitation of the animal model study, it can be concluded that acute on chronic administration of tramadol induced a significant increase in AST levels. Besides, chronic supplementation with quercetin during the experimental period decreased oxidative/nitrosative stress in the heart, adrenal, liver and kidney tissues. On the other hand, it imposed thrombocyte imbalance. Quercetin and naloxone decreased oxidative stress in the heart and adrenal tissue; however, they adversely affected cortex tissue. Contrary to the inconsistent effect of quercetin on liver tissue, a single dose of quercetin similar to its chronic administration reduced cardiac oxidative stress.
Acknowledgements
Not applicable.
Abbreviations
- TRM
Tramadol
- ROS
reactive oxygen species
- NF-κB
nuclear factor-kappaB
- IP
intraperitoneal
- TBA
thiobarbituric acid
- SOD
superoxide dismutase
- ANOVA
one-way analysis of variance
- GSH
glutathione
- NF-κB
Nuclear factor-kappa B
- CBC
complete blood count
- WBC
White Blood count
- RBC
Red Blood count
- HGB
hemoglobin
- HCT
hematocrit
- PLT
platelet
- MCV
mean corpuscular volume
- MCH
mean corpuscular hemoglobin
- MCHC
mean corpuscular hemoglobin concentration
- LYM
Lymphocytes
- MON
Monocytes
- NEU
Neutrophils
- EOS
Eosinophils
- BAS
Basophils
- Cr
creatinine
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- NOx
Nitric Oxide metabolites (nitrate+ nitrite)
- SOD
Superoxide dismutase
- MDA
Malondialdehyde
- IL-6
interleukin-6
- Tnf-a
Tumour Necrosis Factor-alpha
Authors’ contributions
SN, KF, EM, MF, OM contributed to the manuscript’s conception, design, and preparation. SN, KF, MA, OM contributed to conducting experiments, acquisition, analysis, and interpretation. SN, KF, EM, MF, MA, OM made substantial contributions in drafting the manuscript and revising it critically for important intellectual content. All authors have read and approved the final version of the manuscript.
Funding
This investigation was supported by the research council of the Birjand University of Medical Sciences (with the grant number: 455668), Birjand, Iran, and Iran National Science Foundation (INSF) (with the grant number: 97012231). The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets are available from the corresponding author on formal and logical requests.
Declarations
Ethics approval and consent to participate
All experiments and treatments of rats were conducted according to the international laws of handling laboratory animals. The study protocol was also confirmed by the ethics committee of Birjand University of medical sciences (code: IR.BUMS.REC.1397.194). The study is reported in accordance with ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
The authors take full responsibility for the writing and content of this article and confirm that there are no conflicts of interest associated with this academic publication.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Khadijeh Farrokhfall, Email: kfarrokhfall@yahoo.com.
Omid Mehrpour, Email: omid.mehrpour@yahoo.com.au.
References
- 1.Szkutnik-Fiedler D, Kus K, Ratajczak P, Antoniów M, Nowakowska E, Grześkowiak E. Coadministration of tramadol with aripiprazole and venlafaxine—the effect on spatial memory functions in male rats. Pharmacol Rep. 2016;68(2):451–456. doi: 10.1016/j.pharep.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Shadnia S, Brent J, Mousavi-Fatemi K, Hafezi P, Soltaninejad K. Recurrent seizures in tramadol intoxication: implications for therapy based on 100 patients. Basic & clinical pharmacology & toxicology. 2012;111(2):133–136. doi: 10.1111/j.1742-7843.2012.00874.x. [DOI] [PubMed] [Google Scholar]
- 3.Baghishani F, Mohammadipour A, Hosseinzadeh H, Hosseini M, Ebrahimzadeh-bideskan A. The effects of tramadol administration on hippocampal cell apoptosis, learning and memory in adult rats and neuroprotective effects of crocin. Metab Brain Dis. 2018;33(3):1–10. doi: 10.1007/s11011-018-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lagard C, Malissin I, Indja W, Risède P, Chevillard L, Mégarbane B. Is naloxone the best antidote to reverse tramadol-induced neuro-respiratory toxicity in overdose? An experimental investigation in the rat. Clin Toxicol. 2017;56(8):1–7. doi: 10.1080/15563650.2017.1401080. [DOI] [PubMed] [Google Scholar]
- 5.Lagard C, Chevillard L, Malissin I, Risède P, Callebert J, Labat L, Launay JM, Laplanche JL, Mégarbane B. Mechanisms of tramadol-related neurotoxicity in the rat: does diazepam/tramadol combination play a worsening role in overdose? Toxicol Appl Pharmacol. 2016;310:108–119. doi: 10.1016/j.taap.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Bameri B, Shaki F, Ahangar N, Ataee R, Samadi M, Mohammadi H. Evidence for the involvement of the dopaminergic system in seizure and oxidative damage induced by tramadol. Int J Toxicol. 2018;37(2):164–170. doi: 10.1177/1091581817753607. [DOI] [PubMed] [Google Scholar]
- 7.Abdel-Zaher AO, Abdel-Rahman MS, ELwasei FM. Protective effect of Nigella sativa oil against tramadol-induced tolerance and dependence in mice: role of nitric oxide and oxidative stress. Neurotoxicology. 2011;32(6):725–733. doi: 10.1016/j.neuro.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Mohamed TM, Ghaffar HMA, El Husseiny RM. Effects of tramadol, clonazepam, and their combination on brain mitochondrial complexes. Toxicol Ind Health. 2015;31(12):1325–1333. doi: 10.1177/0748233713491814. [DOI] [PubMed] [Google Scholar]
- 9.Mohamed HM, Mahmoud AM. Chronic exposure to the opioid tramadol induces oxidative damage, inflammation and apoptosis, and alters cerebral monoamine neurotransmitters in rats. Biomed Pharmacother. 2019;110:239–247. doi: 10.1016/j.biopha.2018.11.141. [DOI] [PubMed] [Google Scholar]
- 10.Ghoneim FM, Khalaf HA, Elsamanoudy AZ, Helaly AN. Effect of chronic usage of tramadol on motor cerebral cortex and testicular tissues of adult male albino rats and the effect of its withdrawal: histological, immunohistochemical and biochemical study. Int J Clin Exp Pathol. 2014;7(11):7323–7341. [PMC free article] [PubMed] [Google Scholar]
- 11.Eizadi-Mood N, Ozcan D, Sabzghabaee AM, Mirmoghtadaee P, Hedaiaty M. Does naloxone prevent seizure in tramadol intoxicated patients? Int J Prev Med. 2014;5(3):302–307. [PMC free article] [PubMed] [Google Scholar]
- 12.Moghbelinejad S, Alizadeh S, Mohammadi G, Khodabandehloo F, Rashvand Z, Najafipour R, Nassiri-Asl M. The effects of quercetin on the gene expression of the GABA a receptor α5 subunit gene in a mouse model of kainic acid-induced seizure. J Physiol Sci. 2017;67(2):339–343. doi: 10.1007/s12576-016-0497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nassiri-Asl M, Hajiali F, Taghiloo M, Abbasi E, Mohseni F, Yousefi F. Comparison between the effects of quercetin on seizure threshold in acute and chronic seizure models. Toxicol Ind Health. 2016;32(5):936–944. doi: 10.1177/0748233713518603. [DOI] [PubMed] [Google Scholar]
- 14.Kumar M, Kasala ER, Bodduluru LN, Kumar V, Lahkar M. Molecular and biochemical evidence on the protective effects of quercetin in isoproterenol-induced acute myocardial injury in rats. J Biochem Mol Toxicol. 2017;31(1):1–8. doi: 10.1002/jbt.21832. [DOI] [PubMed] [Google Scholar]
- 15.Roslan J, Giribabu N, Karim K, Salleh N. Quercetin ameliorates oxidative stress, inflammation and apoptosis in the heart of streptozotocin-nicotinamide-induced adult male diabetic rats. Biomed Pharmacother. 2017;86:570–582. doi: 10.1016/j.biopha.2016.12.044. [DOI] [PubMed] [Google Scholar]
- 16.Zakaria N, Khalil SR, Awad A, Khairy GM. Quercetin reverses altered energy metabolism in the heart of rats receiving Adriamycin chemotherapy. Cardiovasc Toxicol. 2018;18(2):109–119. doi: 10.1007/s12012-017-9420-4. [DOI] [PubMed] [Google Scholar]
- 17.Vieira EK, Bona S, Di Naso FC, Porawski M, Tieppo J, Marroni NP. Quercetin treatment ameliorates systemic oxidative stress in cirrhotic rats. ISRN gastroenterology. 2011;2011:1–6. doi: 10.5402/2011/604071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou J, Zhou S, Gao Y, Zeng S. Modulatory effects of quercetin on hypobaric hypoxic rats. Eur J Pharmacol. 2012;674(2–3):450–454. doi: 10.1016/j.ejphar.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 19.Abdalla FH, Schmatz R, Cardoso AM, Carvalho FB, Baldissarelli J, de Oliveira JS, Rosa MM, Gonçalves Nunes MA, Rubin MA, da Cruz IBM, Barbisan F, Dressler VL, Pereira LB, Schetinger MRC, Morsch VM, Gonçalves JF, Mazzanti CM. Quercetin protects the impairment of memory and anxiogenic-like behavior in rats exposed to cadmium: possible involvement of the acetylcholinesterase and Na+, K+-ATPase activities. Physiol Behav. 2014;135:152–167. doi: 10.1016/j.physbeh.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Nassiri-Asl M, Moghbelinejad S, Abbasi E, Yonesi F, Haghighi M-R, Lotfizadeh M, Bazahang P. Effects of quercetin on oxidative stress and memory retrieval in kindled rats. Epilepsy Behav. 2013;28(2):151–155. doi: 10.1016/j.yebeh.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 21.Nieoczym D, Socała K, Raszewski G, Wlaź P. Effect of quercetin and rutin in some acute seizure models in mice. Prog Neuro-Psychopharmacol Biol Psychiatry. 2014;54:50–58. doi: 10.1016/j.pnpbp.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Joshi D, Naidu P, Singh A, Kulkarni S. Protective effect of quercetin on alcohol abstinence-induced anxiety and convulsions. J Med Food. 2005;8(3):392–396. doi: 10.1089/jmf.2005.8.392. [DOI] [PubMed] [Google Scholar]
- 23.Nitsinskaya L, Ekimova I, Guzhova I, Feizulaev B, Pastukhov YF. Effects of quercetin on the severity of chemically induced convulsions and 70-kDal heat shock protein content in brain structures in rats. Neurosci Behav Physiol. 2011;41(7):680–686. doi: 10.1007/s11055-011-9472-z. [DOI] [Google Scholar]
- 24.Costa LG, Garrick JM, Roquè PJ, Pellacani C. Mechanisms of neuroprotection by quercetin: counteracting oxidative stress and more. Oxidative Med Cell Longev. 2016;2016:1–10. doi: 10.1155/2016/2986796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arifin WN, Zahiruddin WM. Sample size calculation in animal studies using resource equation approach. The Malaysian journal of medical sciences: MJMS. 2017;24(5):101–105. doi: 10.21315/mjms2017.24.5.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farrokhfall K, Khoshbaten A, Zahediasl S, Mehrani H, Karbalaei N. Improved islet function is associated with anti-inflammatory, antioxidant and hypoglycemic potential of cinnamaldehyde on metabolic syndrome induced by high tail fat in rats. J functional foods. 2014;10:397–406. doi: 10.1016/j.jff.2014.07.014. [DOI] [Google Scholar]
- 27.Farrokhfall K, Hashtroudi MS, Ghasemi A, Mehrani H. Comparison of inducible nitric oxide synthase activity in pancreatic islets of young and aged rats. Iranian journal of basic medical sciences. 2015;18(2):115–121. [PMC free article] [PubMed] [Google Scholar]
- 28.Ataie Z, Mehrani H, Ghasemi A, Farrokhfall K. Cinnamaldehyde has beneficial effects against oxidative stress and nitric oxide metabolites in the brain of aged rats fed with long-term, high-fat diet. J Funct Foods. 2019;52:545–551. doi: 10.1016/j.jff.2018.11.038. [DOI] [Google Scholar]
- 29.Uchiyama M, Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86(1):271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 30.Abarikwu SO. Protective effect of quercetin on atrazine-induced oxidative stress in the liver, kidney, brain, and heart of adult Wistar rats. Toxicol Int. 2014;21(2):148–155. doi: 10.4103/0971-6580.139794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.García-Saura MF, Galisteo M, Villar IC, Bermejo A, Zarzuelo A, Vargas F, et al. Effects of chronic quercetin treatment in experimental renovascular hypertension. Mol Cell Biochem. 2005;270(1–2):147–155. doi: 10.1007/s11010-005-4503-0. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz MJ, Fernández M, Estela JM, Asensi MÁ, Mañes J, Picó Y. Short-term oral toxicity of quercetin and pterostibene in Swiss mice. Toxicol Lett. 2006;164:S275–S2S6. doi: 10.1016/j.toxlet.2006.07.232. [DOI] [Google Scholar]
- 33.Barrenetxe J, Aranguren P, Grijalba A, Martinez-Penuela J, Marzo F, Urdaneta E. Effect of dietary quercetin and sphingomyelin on intestinal nutrient absorption and animal growth. Br J Nutr. 2006;95(3):455–461. doi: 10.1079/BJN20051651. [DOI] [PubMed] [Google Scholar]
- 34.Bailey SA, Zidell RH, Perry RW. Relationships between organ weight and body/brain weight in the rat: what is the best analytical endpoint? Toxicol Pathol. 2004;32(4):448–466. doi: 10.1080/01926230490465874. [DOI] [PubMed] [Google Scholar]
- 35.Lee B-H, Lee J-H, Yoon I-S, Lee J-H, Choi S-H, Pyo MK, Jeong SM, Choi WS, Shin TJ, Lee SM, Rhim H, Park YS, Han YS, Paik HD, Cho SG, Kim CH, Lim YH, Nah SY Human glycine α1 receptor inhibition by quercetin is abolished or inversed by α267 mutations in transmembrane domain 2. Brain Res 2007;1161:1–10, 1, doi: 10.1016/j.brainres.2007.05.057. [DOI] [PubMed]
- 36.Lee B-H, Jung S-M, ngo Lee J-H, Kim J-H, Yoon I-S, Lee J-H, et al. Quercetin Inhibits the 5-Hydroxytryptamine Type 3 Receptormediated Ion Current by Interacting with Pre-Transmembrane Domain I. Molecules & Cells (Springer Science & Business Media BV). 2005;20(1). [PubMed]
- 37.Alexander SP. Flavonoids as antagonists at A1 adenosine receptors. Phytotherapy Research: An international journal devoted to pharmacological and toxicological evaluation of natural product derivatives. 2006;20(11):1009–1012. doi: 10.1002/ptr.1975. [DOI] [PubMed] [Google Scholar]
- 38.Uzun FG, Kalender Y. Chlorpyrifos induced hepatotoxic and hematologic changes in rats: the role of quercetin and catechin. Food Chem Toxicol. 2013;55:549–556. doi: 10.1016/j.fct.2013.01.056. [DOI] [PubMed] [Google Scholar]
- 39.Keskin E, DÖNMEZ NKG, Kandır S. Beneficial effect of quercetin on some Haematological parameters in Streptozotocin-induced diabetic rats. Bulletin of Environment, Pharmacology and Life Sciences. 2016;5(6):65–68. [Google Scholar]
- 40.Selvakumar K, Bavithra S, Suganya S, Ahmad Bhat F, Krishnamoorthy G, Arunakaran J. Effect of quercetin on haematobiochemical and histological changes in the liver of polychlorined biphenyls-induced adult male wistar rats. Journal of biomarkers. 2013;2013:1–12. doi: 10.1155/2013/960125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bahar E, Lee G-H, Bhattarai KR, Lee H-Y, Kim H-K, Handigund M, et al. Protective role of quercetin against manganese-induced injury in the liver, kidney, and lung; and hematological parameters in acute and subchronic rat models. Drug design, development and therapy. 2017;11:2605. doi: 10.2147/DDDT.S143875. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Mosawy S, Jackson D, Woodman O, Linden M. Treatment with quercetin and 3′, 4′-dihydroxyflavonol inhibits platelet function and reduces thrombus formation in vivo. J Thromb Thrombolysis. 2013;36(1):50–57. doi: 10.1007/s11239-012-0827-2. [DOI] [PubMed] [Google Scholar]
- 43.Chen Y, Deuster P. Comparison of quercetin and dihydroquercetin: antioxidant-independent actions on erythrocyte and platelet membrane. Chem Biol Interact. 2009;182(1):7–12. doi: 10.1016/j.cbi.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Chouhan S, Yadav A, Kushwah P, Kaul RK, Flora SJ. Silymarin and quercetin abrogates fluoride induced oxidative stress and toxic effects in rats. Molecular & Cellular Toxicology. 2011;7(1):25–32. doi: 10.1007/s13273-011-0004-2. [DOI] [Google Scholar]
- 45.Dwivedi N, Flora S. Dose dependent efficacy of quercetin in preventing arsenic induced oxidative stress in rat blood and liver. Journal of Cell and Tissue Research. 2011;11(1):2605. [Google Scholar]
- 46.Nna VU, Akpan UP, Okon VE, Atangwho IJ. Hepatotoxicity following separate administration of two phosphodiesterase-5 inhibitors (sildenafil & tadalafil) and opioid (tramadol); evaluation of possible reversal following their withdrawal. Journal of Applied Pharmaceutical Science. 2015;5(08):105–113. doi: 10.7324/JAPS.2015.50817. [DOI] [Google Scholar]
- 47.Youssef H, Azza Z. Histopathological and biochemical effects of acute & chronic tramadol drug toxicity on liver, kidney and testicular function in adult male albino rats. J Med Toxicol Clin Forens Med. 2016;2(4):00060–00068. doi: 10.15406/frcij.2016.02.00060. [DOI] [Google Scholar]
- 48.Awadalla EA, Salah-Eldin A-E. Histopathological and molecular studies on tramadol mediated hepato-renal toxicity in rats. J Pharm Biol Sci. 2015;10(6):90–102. [Google Scholar]
- 49.Baghishani F, Mohammadipour A, Hosseinzadeh H, Hosseini M, Ebrahimzadeh-bideskan A. The effects of tramadol administration on hippocampal cell apoptosis, learning and memory in adult rats and neuroprotective effects of crocin. Metab Brain Dis. 2018;33(3):907–916. doi: 10.1007/s11011-018-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Samaka R, Girgis N, Shams T. Acute toxicity and dependence of tramadol in albino rats: relationship of nestin and notch 1 as stem cell markers. J Am Sci. 2012;8(6):313–327. [Google Scholar]
- 51.Bilir A, Erkasap N, Koken T, Gulec S, Kaygisiz Z, Tanriverdi B, Kurt I. Effects of tramadol on myocardial ischemia-reperfusion injury. Scand Cardiovasc J. 2007;41(4):242–247. doi: 10.1080/14017430701227747. [DOI] [PubMed] [Google Scholar]
- 52.Leppert W. Tramadol as an analgesic for mild to moderate cancer pain. Pharmacol Rep. 2009;61(6):978–992. doi: 10.1016/S1734-1140(09)70159-8. [DOI] [PubMed] [Google Scholar]
- 53.Messarah M, Boumendjel A, Chouabia A, Klibet F, Abdennour C, Boulakoud MS, Feki AE. Influence of thyroid dysfunction on liver lipid peroxidation and antioxidant status in experimental rats. Exp Toxicol Pathol. 2010;62(3):301–310. doi: 10.1016/j.etp.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 54.Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585(2–3):325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 55.Abarikwu S, Adesiyan A, Oyeloja T, Farombi E. Quercetin potentiates oxidative stress in the liver of rats exposed to the herbicide, atrazine. Advances in Environmental Research New York: Nova Science Publishers. 2012:487–502.
- 56.Muthukumaran S, Sudheer AR, Menon VP, Nalini N. Protective effect of quercetin on nicotine-induced prooxidant and antioxidant imbalance and DNA damage in Wistar rats. Toxicology. 2008;243(1–2):207–215. doi: 10.1016/j.tox.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 57.Geetha T, Malhotra V, Chopra K, Kaur IP. Antimutagenic and antioxidant/prooxidant activity of quercetin. 2005. [PubMed] [Google Scholar]
- 58.Metodiewa D, Jaiswal AK, Cenas N, Dickancaité E, Segura-Aguilar J. Quercetin may act as a cytotoxic prooxidant after its metabolic activation to semiquinone and quinoidal product. Free Radic Biol Med. 1999;26(1–2):107–116. doi: 10.1016/S0891-5849(98)00167-1. [DOI] [PubMed] [Google Scholar]
- 59.Boots AW, Kubben N, Haenen GR, Bast A. Oxidized quercetin reacts with thiols rather than with ascorbate: implication for quercetin supplementation. Biochem Biophys Res Commun. 2003;308(3):560–565. doi: 10.1016/S0006-291X(03)01438-4. [DOI] [PubMed] [Google Scholar]
- 60.Choi EJ, Lee BH, Lee K, Chee K-M. Long-term combined administration of quercetin and daidzein inhibits quercetin-induced suppression of glutathione antioxidant defenses. Food Chem Toxicol. 2005;43(5):793–798. doi: 10.1016/j.fct.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 61.Choi EJ, Chee K-M, Lee BH. Anti-and prooxidant effects of chronic quercetin administration in rats. Eur J Pharmacol. 2003;482(1–3):281–285. doi: 10.1016/j.ejphar.2003.09.067. [DOI] [PubMed] [Google Scholar]
- 62.Guzy J, Chovanová Z, Mareková M, Chavková Z, Tomeckova V, Mojzisova G, et al. Effect of quercetin on paracetamol-induced rat liver mitochondria dysfunction. BIOLOGIA-BRATISLAVA-. 2004;59(3):399–404. [Google Scholar]
- 63.Spencer JP, Kuhnle GG, Williams RJ, Catherine R-E. Intracellular metabolism and bioactivity of quercetin and its in vivo metabolites. Biochem J. 2003;372(1):173–181. doi: 10.1042/bj20021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lagard C, Malissin I, Indja W, Risède P, Chevillard L, Mégarbane B. Is naloxone the best antidote to reverse tramadol-induced neuro-respiratory toxicity in overdose? An experimental investigation in the rat. Clin Toxicol. 2018;56(8):737–743. doi: 10.1080/15563650.2017.1401080. [DOI] [PubMed] [Google Scholar]
- 65.Yang L, Li F, Ge W, Mi C, Wang R, Sun R. Protective effects of naloxone in two-hit seizure model. Epilepsia. 2010;51(3):344–353. doi: 10.1111/j.1528-1167.2009.02250.x. [DOI] [PubMed] [Google Scholar]
- 66.Rehni AK, Singh I, Kumar M. Tramadol-induced seizurogenic effect: a possible role of opioid-dependent gamma-aminobutyric acid inhibitory pathway. Basic Clin Pharmacol Toxicol. 2008;103(3):262–6. Epub 2008/08/08. 18684224. 10.1111/j.1742-7843.2008.00276.x. [DOI] [PubMed]
- 67.Gilbert P, Martin W. Antagonism of the convulsant effects of heroin, d-propoxyphene, meperidine, normeperidine and thebaine by naloxone in mice. J Pharmacol Exp Ther. 1975;192(3):538–541. [PubMed] [Google Scholar]
- 68.Saidi H, Ghadiri M, Abbasi S, Ahmadi SF. Efficacy and safety of naloxone in the management of postseizure complaints of tramadol intoxicated patients: a self-controlled study. Emergency medicine journal: EMJ. 2010;27(12):928–30. Epub 2010/04/10. 20378740. 10.1136/emj.2009.083162. [DOI] [PubMed]
- 69.Wang W, Sun C, Mao L, Ma P, Liu F, Yang J, Gao Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: a review. Trends Food Sci Technol. 2016;56:21–38. doi: 10.1016/j.tifs.2016.07.004. [DOI] [Google Scholar]
- 70.MacNee W. Oxidative stress and lung inflammation in airways disease. Eur J Pharmacol. 2001;429(1–3):195–207. doi: 10.1016/S0014-2999(01)01320-6. [DOI] [PubMed] [Google Scholar]
- 71.Xu H, He Y, Yang X, Liang L, Zhan Z, Ye Y, et al. Anti-malarial agent artesunate inhibits TNF-α-induced production of proinflammatory cytokines via inhibition of NF-κB and PI3 kinase/Akt signal pathway in human rheumatoid arthritis fibroblast-like synoviocytes. 2007, Anti-malarial agent artesunate inhibits TNF- -induced production of proinflammatory cytokines via inhibition of NF- B and PI3 kinase/Akt signal pathway in human rheumatoid arthritis fibroblast-like synoviocytes. [DOI] [PubMed]
- 72.Boesch-Saadatmandi C, Loboda A, Wagner AE, Stachurska A, Jozkowicz A, Dulak J, Döring F, Wolffram S, Rimbach G. Effect of quercetin and its metabolites isorhamnetin and quercetin-3-glucuronide on inflammatory gene expression: role of miR-155. J Nutr Biochem. 2011;22(3):293–299. doi: 10.1016/j.jnutbio.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 73.Boots AW, Wilms LC, Swennen EL, Kleinjans JC, Bast A, Haenen GR. In vitro and ex vivo anti-inflammatory activity of quercetin in healthy volunteers. Nutrition. 2008;24(7–8):703–710. doi: 10.1016/j.nut.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 74.Marquardt KA, Alsop JA, Albertson TE. Tramadol exposures reported to statewide poison control system. Ann Pharmacother. 2005;39(6):1039–1044. doi: 10.1345/aph.1E577. [DOI] [PubMed] [Google Scholar]
- 75.Shadnia S, Soltaninejad K, Heydari K, Sasanian G, Abdollahi M. Tramadol intoxication: a review of 114 cases. Human & experimental toxicology. 2008;27(3):201–205. doi: 10.1177/0960327108090270. [DOI] [PubMed] [Google Scholar]
- 76.Hassanian-Moghaddam H, Farajidana H, Sarjami S, Owliaey H. Tramadol-induced apnea. Am J Emerg Med. 2013;31(1):26–31. doi: 10.1016/j.ajem.2012.05.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets are available from the corresponding author on formal and logical requests.