Abstract
Background
Emerging reports have shown the benefits of steroids in hospitalized COVID-19 patients as life-saving drugs. However, the use of steroids in COVID-19 patients is confusing among many physicians.
Aim
The aim of the current study was to find out the exact association of steroids in the deaths of COVID-19 patients.
Methods
The relevant studies were searched in PubMed, Google scholar, and Clinical trials registries till May 25, 2021 and sorted out based on inclusion and exclusion criteria. The quality of studies was assessed using a standard scale. The pooled odds ratio was calculated with a 95% confidence interval. The sensitivity and sub-group analyses were also done. The publication bias was assessed qualitatively. The Rev Man 5 was used for all analyses with a random-effect model.
Results
The quantitative analysis was done with 9922 patients (6265-male and 3657-females) from 21 relevant studies. The pooled estimate results i.e. 0.52 [0.34, 0.80] have shown a significant reduction in deaths of COVID-19 patients in the steroidal group as compared to the non-steroidal group. The sensitivity analyses did not alter our conclusions. In subgroup analysis, methylprednisolone has shown a significant reduction in deaths of COVID-19 patients as compared to the non-steroidal group, however, more clinical evidence is required for dexamethasone and hydrocortisone.
Conclusion
The use of steroids in hospitalized COVID-19 patients is useful to reduce deaths.
Keywords: COVID-19, Steroids, Methylprednisolone, Dexamethasone, Hydrocortisone, Meta-analysis
1. Introduction
The novel coronavirus (2019-nCoV) spreads from Wuhan City of China to the rest of the world (Singhal, 2020). The first case was reported on November 17, 2019 (Allam, 2020). The Severe acute respiratory syndrome- Coronavirus-2 (SARS-Cov-2) is a positive single-strand ribose nucleic acid (RNA) virus having spike proteins on its surface which attach with the angiotensin-converting enzyme (ACE2) of the host and helps in the entry of the virus (Jha et al., 2021). After entry, it uses the machinery of the host and results in the production of various viral proteins. The transmission of the infection occurs mainly through the inhalational route and symptoms appear between 2 and 14 days after the infection. The symptoms vary among the individuals and overlap with other viral infections. The reverse transcription polymaerase chain reaction (RT-PCR) is the most commonly used method for the detection of this infection. Other laboratory blood tests such as C-reactive protein (CRP) level, D-dimer, complete blood count (CBC) is also done to know about inflammatory and coagulation level of the individuals. It has been observed that patients with comorbid conditions like diabetes, obesity, hypertension, etc are more likely to get into serious conditions (Rahman et al., 2021; Yan et al., 2021). Many researchers across the globe are working on the development of new chemical entities (NCEs) against this infection, however, no specific drugs are available in the market for its treatment so far (Singhal, 2020). Various classes of drugs are repurposed for the management of COVID-19 cases including steroids, rapamycin, janus activated protein kinase (JAK) inhibitors (Patoulias et al., 2021) and antiandrogens (Mauvais-Jarvis, 2021; Fagone et al., 2020).
Steroids are well-known drugs that are available in the market for the treatment of various diseases like rheumatoid arthritis, multiple sclerosis, crohn's disease, etc. Various case reports, case series, as well as research articles, have shown the benefits of using steroids in hospitalized COVID-19 patients (Van Paassen et al., 2020; Kalfaoglu et al., 2020; Chaudhuri et al., 2021). Steroids are turned to be life-saving drugs for serious COVID-19 patients. The regulatory authorities also recommended the use of steroids in serious COVID-19 patients. On September 2, 2020, World Health Organization (WHO) has updated clinical care guidance and recommended the use of corticosteroids in severe and critical COVID-19 patients under medical supervision (WHO, 2020). As per National Institute of Health (NIH) guidelines (updated on November 3, 2020), corticosteroids can be used in severe COVID-19 patients to control systematic inflammation (Fagone et al., 2020). Recently, on April 22, 2021, Indian Council of Medical Research (ICMR) updated the guidelines and recommended the use of steroids like methylprednisolone in hospitalized COVID-19 patients if not contraindicated (ICMR, 2021). It has been observed that the life of serious COVID-19 patients was saved using steroids but the exact role of steroids in COVID-19 patients is still an open question to answer. Thus, we have conducted a meta-analysis of available clinical evidence on the use of steroids in COVID-19 patients.
2. Methodology
The study was conducted in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines. The study is registered with the International prospective register of systematic reviews Prospectively register of systematic reviews (PROSPERO), registration number CRD42021259891.
2.1. Search strategy
A search was done in PubMed, Google scholar, and Clinical trial registry for observational, randomized, and non-randomized controlled studies, cohort studies, and comparative cross-sectional studies with the following search strategies. Steroids OR corticosteroids OR corticoids OR dexamethasone OR prednisone OR methyl prednisone OR prednisolone OR dexamethasone sodium phosphate OR hydrocortisone OR betamethasone OR beclomethasone OR budesonide OR formoterol AND COVID-19 OR SARS-CoV-2 OR severe acute respiratory syndrome OR Novel coronavirus. The references of included studies were screened to boost the search.
2.2. Study selection
The inclusion criteria are as follows a) confirmed COVID-19 cases b) participants should be on steroidal therapy c) comparator group without steroidal therapy d) death should be one of the outcomes of study e) all age groups f) both male and female participants. Studies were excluded if - a.) study participants were not on steroidal therapy. b.) if there is no comparator group. c.) case reports, case series, narrative review, systematic review, meta-analysis d.) studies of poor quality as per standard scale. Two authors (MT and AK) separately screened all the titles and abstracts for eligibility criteria. Finally, the full-text articles were separately screened by two authors (MT and AK). In the case of conflicts over the inclusion, the third author (AKD) was consulted.
2.3. Quality assessment
The quality assessment was done using Newcastle-Ottawa Scale (NOS). The assessment was done by two reviewers (MT and AK) separately. The studies were categorized into three categories i.e., good, fair, and poor quality.
2.4. Data extraction
The data was extracted from studies by two authors (MT and AK) in an excel sheet. The information such as name of the first author with publication year, name of the country where the study was conducted, study design, the total number of subjects, subjects in the steroid group, number of deaths in steroidal group, subjects in the non-steroidal group, number of deaths in the non-steroidal group was extracted from full-text articles.
2.5. Sensitivity analysis
The sensitivity analysis was done to check the effect of high or low sample size on the outcome.
2.6. Statistical analysis
All the analyses were performed using RevMan 5. The overall estimate was calculated as an odds ratio with 95% confidence intervals using a random effect model. The heterogeneity among studies was calculated using Cochrane Q and I square statistics. The publication bias was analyzed qualitatively using a funnel plot.
3. Results
3.1. Search results and study characteristics
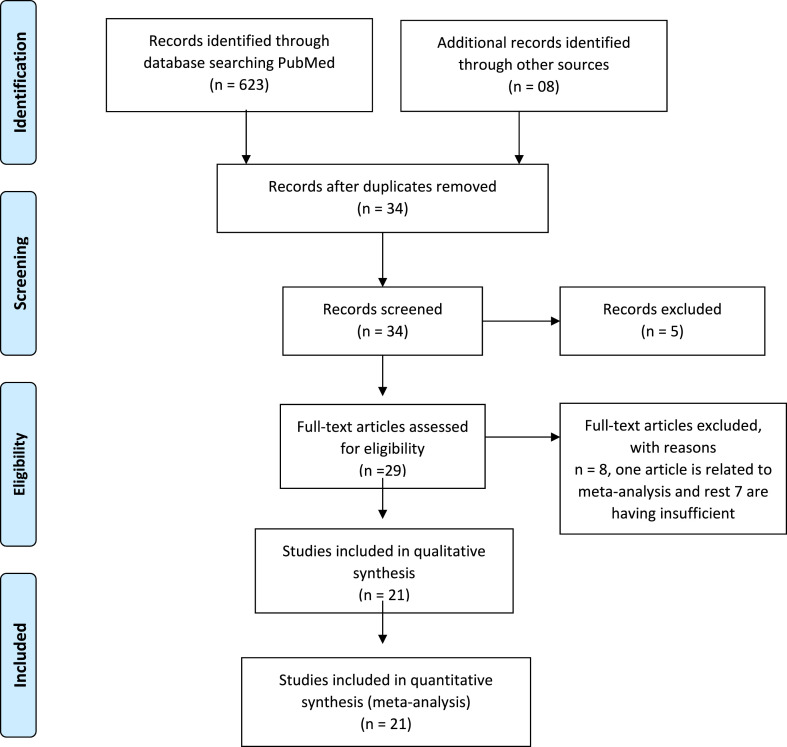
The 631 studies were found after the initial search. After removing duplicates and primarily screening of titles, 34 articles were retrieved, of which 21 articles (Villar et al., 2020; Salton et al., 2020; Petersen et al., 2020; Horby et al., 2021; Steroid SARI, 2020; Tomazini et al., 2020; Pontali et al., 2021; Rashad et al., 2021; Jamaati et al., 2021; Edalatifard et al., 2020; Ramiro et al., 2020; Tang et al., 2021; Corral-Gudino et al., 2021; Angus et al., 2020; Dequin et al., 2020; Liu et al., 2020; Ranjbar et al., 2021; Jeronimo et al., 2021; Fadel et al., 2020; Nelson et al., 2021; Ooi et al., 2020) were found to be appropriate for quantitative analysis as presented in Fig. 1 . The full-text or secondary screening with bibliography searching of the literature did not yield any additional article for inclusion. Out of the 21 studies, 13 studies were randomized control trials, 01 was retrospective, cohort, multi-centered quasi study each and the remaining 5 were clinical trials (CT). A total of 9922 patients (male: 6265, female: 3657) were found. The study characteristics are compiled in Table 1 .
Fig. 1.
Selection of studies as per the PRISMA Checklist.
Table 1.
Study characteristics of Included studies.
| References |
Country |
Study design |
Sample size |
Sex |
Steroid group |
Non steroid group |
|||
|---|---|---|---|---|---|---|---|---|---|
| Male | female | No. of Patients | Death | No. of Patients | Death | ||||
| Villar et al. (2020) | Spain, Canada, China, USA | Clinical trial | 19 | 13 | 6 | 7 | 2 | 12 | 2 |
| Salton et al. (2020) | Italy | Clinical trial | 173 | 120 | 53 | 83 | 6 | 90 | 21 |
| Steroid SARI (2020) | Wuhan | Clinical trial | 47 | 35 | 12 | 24 | 13 | 23 | 13 |
| Petersen et al. (2020) | Denmark | Clinical trial | 29 | 23 | 6 | 15 | 6 | 14 | 2 |
| Horby et al. (2021) | UK | Randomized controlled trial | 6425 | 4087 | 2338 | 2104 | 482 | 4321 | 1110 |
| Tomazini et al. (2020) | Brazil | Randomized clinical trial | 299 | 187 | 112 | 151 | 85 | 148 | 91 |
| Rashad et al. (2021) | Egypt | Randomized controlled trial | 173 | 81 | 92 | 127 | 33 | 46 | 32 |
| Jamaati et al. (2021) | Iran | Randomized clinical trial | 43 | 25 | 18 | 25 | 16 | 18 | 15 |
| Edalatifard et al. (2020) | Iran | Randomized controlled trial | 62 | 39 | 23 | 34 | 2 | 28 | 12 |
| Ramiro et al. (2020) | Netherlands | Randomized controlled trial | 172 | 136 | 36 | 86 | 14 | 86 | 41 |
| Tang et al. (2021) | China | Randomized controlled trial | 86 | 41 | 45 | 43 | 0 | 43 | 1 |
| Pontali et al. (2021) | Genoa | Randomized controlled trial | 128 | 87 | 41 | 63 | 9 | 65 | 28 |
| Corral-Gudino et al. (2021) | Spain | Randomized controlled trial | 64 | 39 | 25 | 35 | 14 | 29 | 14 |
| Angus et al. (2020) | Pittsburgh | Randomized clinical trial | 384 | 273 | 111 | 283 | 78 | 101 | 33 |
| Dequin et al. (2020) | France | Randomized clinical trial | 149 | 104 | 45 | 76 | 11 | 73 | 20 |
| Liu et al. (2020) | China | Randomized Clinical trial | 774 | 452 | 322 | 409 | 366 | 365 | 228 |
| Ranjbar et al. (2021) | Iran | Randomized controlled trial | 86 | 49 | 37 | 44 | 8 | 42 | 15 |
| Jeronimo et al. (2021) | Brazil | Clinical trial | 393 | 254 | 139 | 194 | 157 | 199 | 186 |
| Fadel et al. (2020) | USA | Multicenter quasi experimental | 213 | 109 | 104 | 132 | 18 | 81 | 21 |
| Nelson et al. (2021) | USA | Cohort study | 111 | 80 | 31 | 48 | 25 | 63 | 49 |
| Ooi et al. (2020) | Singapore | Retrospective study | 92 | 31 | 61 | 35 | 0 | 57 | 1 |
3.2. Quality assessment
All randomized controlled trials (RCTs), clinical trials (CTs), cohort studies with one protocol assessed for methodological quality using Newcastle-Ottawa Scale (NOS) were found to be of good and fair quality, based on the scores of the study in the selection, comparability, and outcome subscales. Out of 21 studies, 15 were of good quality, and remaining 6 were of fair quality (Table 2 ).
Table 2.
Quality assessment using Newcastle Ottawa scale.
| References | Selection | Comparability | Exposure | Total Score | Quality of the Study |
|---|---|---|---|---|---|
| Villar et al. (2020) | ** | * | *** | 6 | Fair |
| Salton et al. (2020) | **** | ** | *** | 9 | Good |
| Steroid SARI (2020) | *** | * | ** | 6 | Good |
| Petersen et al. (2020) | *** | * | *** | 7 | Good |
| Horby et al. (2021) | *** | * | *** | 7 | Good |
| Tomazini et al. (2020) | *** | * | *** | 7 | Good |
| Rashad et al. (2021) | ** | ** | ** | 6 | Fair |
| Jamaati et al. (2021) | ** | * | ** | 5 | Fair |
| Edalatifard et al. (2020) | ** | * | *** | 6 | Fair |
| Ramiro et al. (2020) | *** | ** | ** | 7 | Good |
| Tang et al. (2021) | ** | * | ** | 5 | Fair |
| Pontali et al. (2021) | **** | ** | ** | 8 | Good |
| Corral-Gudino et al. (2021) | *** | ** | ** | 7 | Good |
| Angus et al. (2020) | *** | * | ** | 6 | Good |
| Dequin et al. (2020) | *** | * | *** | 7 | Good |
| Liu J et al. (2020) | *** | * | ** | 6 | Good |
|
Ranjbar et al. (2021) Jeronimo et al. (2021) |
*** | ** | ** | 7 | Good |
| Fadel et al. (2020) | **** | ** | ** | 8 | Good |
| Nelson et al. (2021) | *** | ** | ** | 7 | Good |
| Ranjbar et al. (2021) | *** | ** | ** | 7 | Good |
| Ooi et al. (2020) | ** | * | *** | 6 | Fair |
3.3. Use of steroids in COVID-19 patients
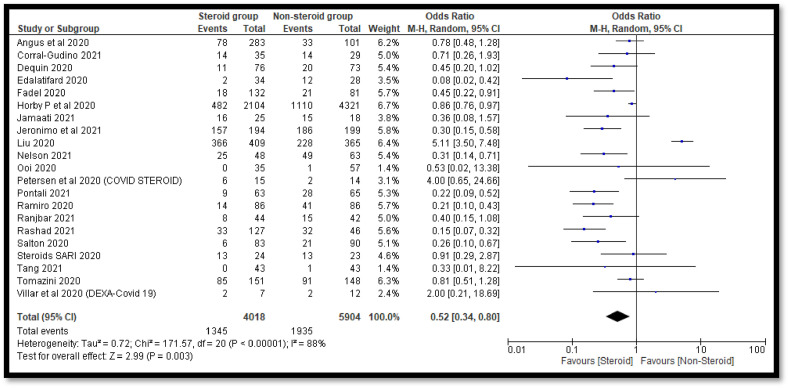
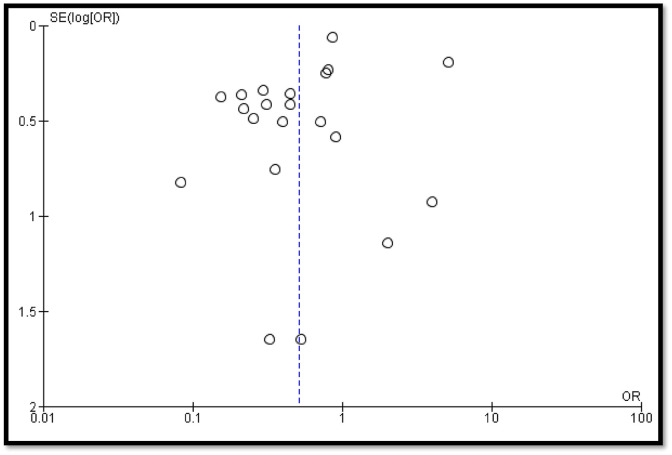
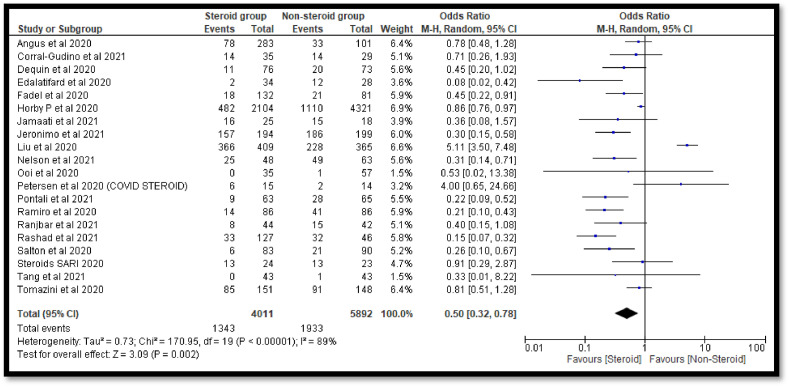
A total of 21 studies contains 9922 COVID-19 cases. Out of 9922 COVID-19 cases, 4018 were on steroids whereas 5904 were in the non-steroid group. The overall estimate was 0.52 [0.34, 0.80] which indicates a significant decrease in deaths of COVID-19 patients in the steroidal group as compared to the non-steroidal group (Fig. 2 ). The funnel plot was not found to be symmetrical in shape which indicates involvement of publication bias (Fig. 3 ).
Fig. 2.
Pooled analysis results using a random effect model (forest plot).
Fig. 3.
Funnel plot for qualitative analysis of publication bias.
3.4. Sensitivity analysis
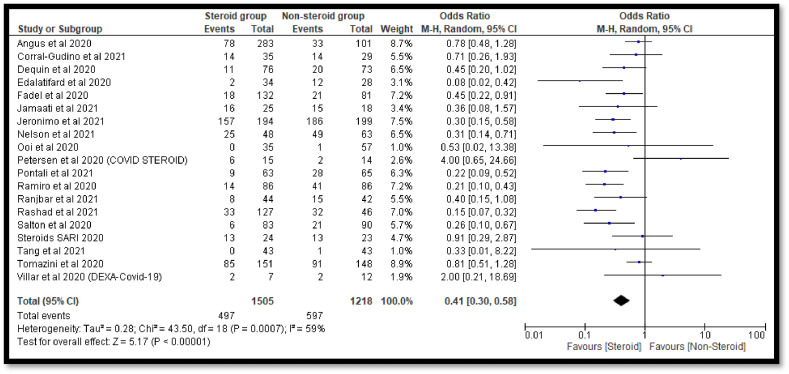
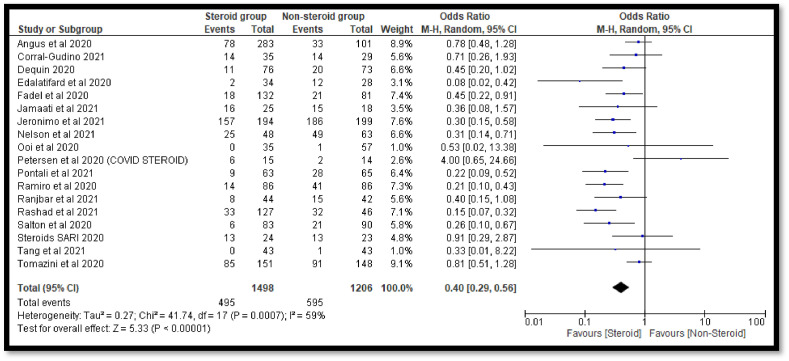
The sensitivity analysis was done to check the effect of outliers. In the current analysis, we have found two studies i.e. Horby et al. (2021) and Liu et al. (2020) with a high sample size whereas one study i.e. Villar et al. (2020) with low sample size. The pooled OR was found to be 0.41 [0.30, 0.58] after exclusion of both studies with high sample size which also indicates a significant decrease in the deaths of COVID-19 patients in the steroidal group as compared to the non-steroidal group (Fig. 4 ). The conclusion was also not affected after the removal of the study with a low sample size (Fig. 5 ). Finally, we have also excluded all the outliers with high and low sample sizes and found no effect on the conclusion of the study (Fig. 6 ).
Fig. 4.
Forest plot after removal of studies with high sample size.
Fig. 5.
Forest plot after removal of studies with low sample size.
Fig. 6.
Forest plot after removal of studies with low and high sample size.
3.5. Heterogeneity
The I2 (90%) and chi2 statics has shown high heterogeneity. However, after the removal of outliers, heterogeneity among studies was also reduced from 88% to 59%.
3.6. Sub-group analysis
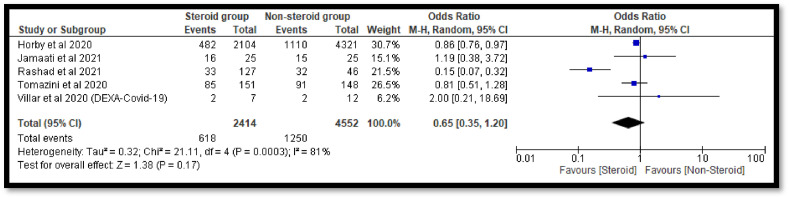
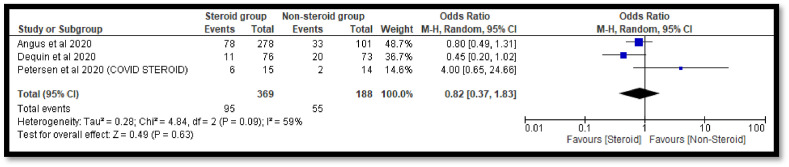
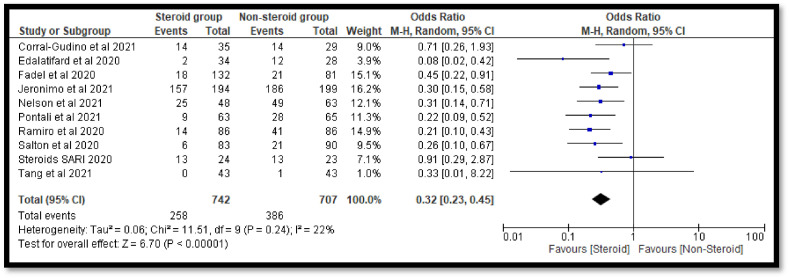
The sub-group analysis was done to check the effects of individual steroids in the deaths of COVID-19 patients. The pooled OR 0.65 [0.35, 1.20] with dexamethasone indicates a non-significant reduction in deaths of COVID-19 patients as compare to the non-steroidal group (Fig. 7 ). Similar non-significant results were also observed with hydrocortisone (Fig. 8 ). However, with methyl-prednisolone, a significant 0.32[0.23, 0.45] reduction in mortality was observed in the steroidal group as compared with the non-steroidal group (Fig. 9 ).
Fig. 7.
Pooled analysis results using a random effect model (forest plot) with dexamethasone.
Fig. 8.
Pooled analysis results using a random effect model (forest plot) with hydrocortisone.
Fig. 9.
Pooled analysis results using a random effect model (forest plot) with methylprednisolone.
4. Discussion
Steroids are found to be life saving drugs in the management of COVID-19. It enters the cytoplasm and acts on the nuclear receptors which results in the synthesis of specific mRNA causes protein synthesis which lead to responses. It downregulating the hyper activation of the components of both innate (neutrophils) and acquired (T and B lymphocytes) immune system and the cytokine storm that characterizes severe case of covid-19 (Vanderbeke et al., 2021; Kalfaoglu et al., 2020; Sunkara and Dewan, 2021).The current meta-analysis was performed to check the association of the use of steroids in the deaths of COVID-19 patients. The available meta-analysis results are contradictory and also contain a smaller number of patients. Thus, further analysis is required to help physicians to make better clinical decisions. The meta-analysis results of Sterne et al. (2020), have reported the role of corticosteroids in the decrease of deaths of COVID-19 serious patients. Sarma et al. (2020) have also conducted a meta-analysis to find out the role of steroidal therapy in mechanically ventilated COVID-19 patients and reported a reduction of mortality of mechanically ventilated patients as well as decreased the requirement of mechanical ventilation. Chaudhuri et al. (2021) have reported higher survival rates in acute respiratory distress syndrome (ARDS) patients treated with corticosteroids (longer duration) as compared to a shorter duration. Pulakurthi et al. (2021) meta-analysis results have reported a significant reduction in deaths of COVID-19 patients who were on steroidal therapy as compared to patients who were on non-steroidal therapy. Pasin et al. (2021) conducted a meta-analysis and concluded that steroids should be used only in the patients who are critically ill and require ventilation and should be avoided in the patients who do not require any oxygen. However, the meta-analysis results of Sarkar et al. (2021) have shown the use of systemic glucocorticoid did not result in a significant reduction of mortality as well as no significant reduction in duration of hospital stay of COVID-19 patients was observed. Wang et al., (2021) meta-analysis results have reported that the patients who were on steroid therapy have delayed the viral clearance time, thus, there was no significant difference in the use of steroids. Cano et al. (2021) meta-analysis results have also reported no effect (harmful or beneficial) between high and low-dose steroidal therapy. We have found a significant decrease in deaths of COVID-19 patients who are on steroids as compared to non-steroidal patients. The sensitivity analysis results also did not alter the findings of the study. The subgroup analysis results have shown a significant association of methyl-prednisolone in the reduction of deaths of COVID-19 patients, however, results with dexamethasone and hydrocortisone were found to be non-significant. Overall, to get a clear picture of individual steroids, more evidence is required.
Limitations
The study has the following limitations. The search for relevant articles has been performed on limited search engines. The articles which were published in the English language were only considered.
Conclusion
The steroids play a significant role in the decrease of demises of hospitalized COVID-19 patients.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Manisha Thakur: Data curation, extraction, Writing – original draft, preparation. Ashok Kumar Datusalia: Supervision, Writing – review & editing. Anoop Kumar: Conceptualization, Methodology, Software, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgments
The authors are thankful to Vice-Chancellor, Prof. R.K. Goyal, Delhi Pharmaceutical Sciences & Research University (DPSRU), New Delhi, and Director, National Institute of Pharmaceutical Education and Research (NIPER), Raebareli, India for their continuous support, motivation, and providing necessary facilities to carry out this work.
References
- Allam Z. The first 50 days of COVID-19: a detailed chronological timeline and extensive review of literature documenting the pandemic. Surv. Covid-19 Pandemic its Implic. 2020;1 doi: 10.1016/B978-0-12-824313-8.00001-2. [DOI] [Google Scholar]
- Angus D.C., Derde L., Al-Beidh F., Annane D., Arabi Y., Beane A., van Bentum-Puijk W., Berry L., Bhimani Z., Bonten M., Bradbury C., Brunkhorst F., Buxton M., Buzgau A., Cheng A.C., de Jong M., Detry M., Estcourt L., Fitzgerald M., Goossens H., Green C., Haniffa R., Higgins A.M., Horvat C., Hullegie S.J., Kruger P., Lamontagne F., Lawler P.R., Linstrum K., Litton E., Lorenzi E., Marshall J., McAuley D., McGlothin A., McGuinness S., McVerry B., Montgomery S., Mouncey P., Murthy S., Nichol A., Parke R., Parker J., Rowan K., Sanil A., Santos M., Saunders C., Seymour C., Turner A., van de Veerdonk F., Venkatesh B., Zarychanski R., Berry S., Lewis R.J., McArthur C., Webb S.A., Gordon A.C., Writing Committee for the REMAP-CAP Investigators. Al-Beidh F., Angus D., Annane D., Arabi Y., van Bentum-Puijk W., Berry S., Beane A., Bhimani Z., Bonten M., Bradbury C., Brunkhorst F., Buxton M., Cheng A., De Jong M., Derde L., Estcourt L., Goossens H., Gordon A., Green C., Haniffa R., Lamontagne F., Lawler P., Litton E., Marshall J., McArthur C., McAuley D., McGuinness S., McVerry B., Montgomery S., Mouncey P., Murthy S., Nichol A., Parke R., Rowan K., Seymour C., Turner A., van de Veerdonk F., Webb S., Zarychanski R., Campbell L., Forbes A., Gattas D., Heritier S., Higgins L., Kruger P., Peake S., Presneill J., Seppelt I., Trapani T., Young P., Bagshaw S., Daneman N., Ferguson N., Misak C., Santos M., Hullegie S., Pletz M., Rohde G., Rowan K., Alexander B., Basile K., Girard T., Horvat C., Huang D., Linstrum K., Vates J., Beasley R., Fowler R., McGloughlin S., Morpeth S., Paterson D., Venkatesh B., Uyeki T., Baillie K., Duffy E., Fowler R., Hills T., Orr K., Patanwala A., Tong S., Netea M., Bihari S., Carrier M., Fergusson D., Goligher E., Haidar G., Hunt B., Kumar A., Laffan M., Lawless P., Lother S., McCallum P., Middeldopr S., McQuilten Z., Neal M., Pasi J., Schutgens R., Stanworth S., Turgeon A., Weissman A., Adhikari N., Anstey M., Brant E., de Man A., Lamonagne F., Masse M.H., Udy A., Arnold D., Begin P., Charlewood R., Chasse M., Coyne M., Cooper J., Daly J., Gosbell I., Harvala-Simmonds H., Hills T., MacLennan S., Menon D., McDyer J., Pridee N., Roberts D., Shankar-Hari M., Thomas H., Tinmouth A., Triulzi D., Walsh T., Wood E., Calfee C., O'Kane C., Shyamsundar M., Sinha P., Thompson T., Young I., Bihari S., Hodgson C., Laffey J., McAuley D., Orford N., Neto A., Detry M., Fitzgerald M., Lewis R., McGlothlin A., Sanil A., Saunders C., Berry L., Lorenzi E., Miller E., Singh V., Zammit C., van Bentum Puijk W., Bouwman W., Mangindaan Y., Parker L., Peters S., Rietveld I., Raymakers K., Ganpat R., Brillinger N., Markgraf R., Ainscough K., Brickell K., Anjum A., Lane J.B., Richards-Belle A., Saull M., Wiley D., Bion J., Connor J., Gates S., Manax V., van der Poll T., Reynolds J., van Beurden M., Effelaar E., Schotsman J., Boyd C., Harland C., Shearer A., Wren J., Clermont G., Garrard W., Kalchthaler K., King A., Ricketts D., Malakoutis S., Marroquin O., Music E., Quinn K., Cate H., Pearson K., Collins J., Hanson J., Williams P., Jackson S., Asghar A., Dyas S., Sutu M., Murphy S., Williamson D., Mguni N., Potter A., Porter D., Goodwin J., Rook C., Harrison S., Williams H., Campbell H., Lomme K., Williamson J., Sheffield J., van’t Hoff W., McCracken P., Young M., Board J., Mart E., Knott C., Smith J., Boschert C., Affleck J., Ramanan M., D'Souza R., Pateman K., Shakih A., Cheung W., Kol M., Wong H., Shah A., Wagh A., Simpson J., Duke G., Chan P., Cartner B., Hunter S., Laver R., Shrestha T., Regli A., Pellicano A., McCullough J., Tallott M., Kumar N., Panwar R., Brinkerhoff G., Koppen C., Cazzola F., Brain M., Mineall S., Fischer R., Biradar V., Soar N., White H., Estensen K., Morrison L., Smith J., Cooper M., Health M., Shehabi Y., Al-Bassam W., Hulley A., Whitehead C., Lowrey J., Gresha R., Walsham J., Meyer J., Harward M., Venz E., Williams P., Kurenda C., Smith K., Smith M., Garcia R., Barge D., Byrne D., Byrne K., Driscoll A., Fortune L., Janin P., Yarad E., Hammond N., Bass F., Ashelford A., Waterson S., Wedd S., McNamara R., Buhr H., Coles J., Schweikert S., Wibrow B., Rauniyar R., Myers E., Fysh E., Dawda A., Mevavala B., Litton E., Ferrier J., Nair P., Buscher H., Reynolds C., Santamaria J., Barbazza L., Homes J., Smith R., Murray L., Brailsford J., Forbes L., Maguire T., Mariappa V., Smith J., Simpson S., Maiden M., Bone A., Horton M., Salerno T., Sterba M., Geng W., Depuydt P., De Waele J., De Bus L., Fierens J., Bracke S., Reeve B., Dechert W., Chassé M., Carrier F.M., Boumahni D., Benettaib F., Ghamraoui A., Bellemare D., Cloutier È., Francoeur C., Lamontagne F., D'Aragon F., Carbonneau E., Leblond J., Vazquez-Grande G., Marten N., Wilson M., Albert M., Serri K., Cavayas A., Duplaix M., Williams V., Rochwerg B., Karachi T., Oczkowski S., Centofanti J., Millen T., Duan E., Tsang J., Patterson L., English S., Watpool I., Porteous R., Miezitis S., McIntyre L., Brochard L., Burns K., Sandhu G., Khalid I., Binnie A., Powell E., McMillan A., Luk T., Aref N., Andric Z., Cviljevic S., Đimoti R., Zapalac M., Mirković G., Baršić B., Kutleša M., Kotarski V., Vujaklija Brajković A., Babel J., Sever H., Dragija L., Kušan I., Vaara S., Pettilä L., Heinonen J., Kuitunen A., Karlsson S., Vahtera A., Kiiski H., Ristimäki S., Azaiz A., Charron C., Godement M., Geri G., Vieillard-Baron A., Pourcine F., Monchi M., Luis D., Mercier R., Sagnier A., Verrier N., Caplin C., Siami S., Aparicio C., Vautier S., Jeblaoui A., Fartoukh M., Courtin L., Labbe V., Leparco C., Muller G., Nay M.A., Kamel T., Benzekri D., Jacquier S., Mercier E., Chartier D., Salmon C., Dequin P., Schneider F., Morel G., L'Hotellier S., Badie J., Berdaguer F.D., Malfroy S., Mezher C., Bourgoin C., Megarbane B., Voicu S., Deye N., Malissin I., Sutterlin L., Guitton C., Darreau C., Landais M., Chudeau N., Robert A., Moine P., Heming N., Maxime V., Bossard I., Nicholier T.B., Colin G., Zinzoni V., Maquigneau N., Finn A., Kreß G., Hoff U., Friedrich Hinrichs C., Nee J., Pletz M., Hagel S., Ankert J., Kolanos S., Bloos F., Petros S., Pasieka B., Kunz K., Appelt P., Schütze B., Kluge S., Nierhaus A., Jarczak D., Roedl K., Weismann D., Frey A., Klinikum Neukölln V., Reill L., Distler M., Maselli A., Bélteczki J., Magyar I., Á Fazekas, Kovács S., Szőke V., Szigligeti G., Leszkoven J., Collins D., Breen P., Frohlich S., Whelan R., McNicholas B., Scully M., Casey S., Kernan M., Doran P., O'Dywer M., Smyth M., Hayes L., Hoiting O., Peters M., Rengers E., Evers M., Prinssen A., Bosch Ziekenhuis J., Simons K., Rozendaal W., Polderman F., de Jager P., Moviat M., Paling A., Salet A., Rademaker E., Peters A.L., de Jonge E., Wigbers J., Guilder E., Butler M., Cowdrey K.A., Newby L., Chen Y., Simmonds C., McConnochie R., Ritzema Carter J., Henderson S., Van Der Heyden K., Mehrtens J., Williams T., Kazemi A., Song R., Lai V., Girijadevi D., Everitt R., Russell R., Hacking D., Buehner U., Williams E., Browne T., Grimwade K., Goodson J., Keet O., Callender O., Martynoga R., Trask K., Butler A., Schischka L., Young C., Lesona E., Olatunji S., Robertson Y., José N., Amaro dos Santos Catorze T., de Lima Pereira T.N.A., Neves Pessoa L.M., Castro Ferreira R.M., Pereira Sousa Bastos J.M., Aysel Florescu S., Stanciu D., Zaharia M.F., Kosa A.G., Codreanu D., Marabi Y., Al Qasim E., Moneer Hagazy M., Al Swaidan L., Arishi H., Muñoz-Bermúdez R., Marin-Corral J., Salazar Degracia A., Parrilla Gómez F., Mateo López M.I., Rodriguez Fernandez J., Cárcel Fernández S., Carmona Flores R., León López R., de la Fuente Martos C., Allan A., Polgarova P., Farahi N., McWilliam S., Hawcutt D., Rad L., O'Malley L., Whitbread J., Kelsall O., Wild L., Thrush J., Wood H., Austin K., Donnelly A., Kelly M., O'Kane S., McClintock D., Warnock M., Johnston P., Gallagher L.J., Mc Goldrick C., Mc Master M., Strzelecka A., Jha R., Kalogirou M., Ellis C., Krishnamurthy V., Deelchand V., Silversides J., McGuigan P., Ward K., O'Neill A., Finn S., Phillips B., Mullan D., Oritz-Ruiz de Gordoa L., Thomas M., Sweet K., Grimmer L., Johnson R., Pinnell J., Robinson M., Gledhill L., Wood T., Morgan M., Cole J., Hill H., Davies M., Antcliffe D., Templeton M., Rojo R., Coghlan P., Smee J., Mackay E., Cort J., Whileman A., Spencer T., Spittle N., Kasipandian V., Patel A., Allibone S., Genetu R.M., Ramali M., Ghosh A., Bamford P., London E., Cawley K., Faulkner M., Jeffrey H., Smith T., Brewer C., Gregory J., Limb J., Cowton A., O'Brien J., Nikitas N., Wells C., Lankester L., Pulletz M., Williams P., Birch J., Wiseman S., Horton S., Alegria A., Turki S., Elsefi T., Crisp N., Allen L., McCullagh I., Robinson P., Hays C., Babio-Galan M., Stevenson H., Khare D., Pinder M., Selvamoni S., Gopinath A., Pugh R., Menzies D., Mackay C., Allan E., Davies G., Puxty K., McCue C., Cathcart S., Hickey N., Ireland J., Yusuff H., Isgro G., Brightling C., Bourne M., Craner M., Watters M., Prout R., Davies L., Pegler S., Kyeremeh L., Arbane G., Wilson K., Gomm L., Francia F., Brett S., Sousa Arias S., Elin Hall R., Budd J., Small C., Birch J., Collins E., Henning J., Bonner S., Hugill K., Cirstea E., Wilkinson D., Karlikowski M., Sutherland H., Wilhelmsen E., Woods J., North J., Sundaran D., Hollos L., Coburn S., Walsh J., Turns M., Hopkins P., Smith J., Noble H., Depante M.T., Clarey E., Laha S., Verlander M., Williams A., Huckle A., Hall A., Cooke J., Gardiner-Hill C., Maloney C., Qureshi H., Flint N., Nicholson S., Southin S., Nicholson A., Borgatta B., Turner-Bone I., Reddy A., Wilding L., Chamara Warnapura L., Agno Sathianathan R., Golden D., Hart C., Jones J., Bannard-Smith J., Henry J., Birchall K., Pomeroy F., Quayle R., Makowski A., Misztal B., Ahmed I., KyereDiabour T., Naiker K., Stewart R., Mwaura E., Mew L., Wren L., Willams F., Innes R., Doble P., Hutter J., Shovelton C., Plumb B., Szakmany T., Hamlyn V., Hawkins N., Lewis S., Dell A., Gopal S., Ganguly S., Smallwood A., Harris N., Metherell S., Lazaro J.M., Newman T., Fletcher S., Nortje J., Fottrell-Gould D., Randell G., Zaman M., Elmahi E., Jones A., Hall K., Mills G., Ryalls K., Bowler H., Sall J., Bourne R., Borrill Z., Duncan T., Lamb T., Shaw J., Fox C., Moreno Cuesta J., Xavier K., Purohit D., Elhassan M., Bakthavatsalam D., Rowland M., Hutton P., Bashyal A., Davidson N., Hird C., Chhablani M., Phalod G., Kirkby A., Archer S., Netherton K., Reschreiter H., Camsooksai J., Patch S., Jenkins S., Pogson D., Rose S., Daly Z., Brimfield L., Claridge H., Parekh D., Bergin C., Bates M., Dasgin J., McGhee C., Sim M., Hay S.K., Henderson S., Phull M.K., Zaidi A., Pogreban T., Rosaroso L.P., Harvey D., Lowe B., Meredith M., Ryan L., Hormis A., Walker R., Collier D., Kimpton S., Oakley S., Rooney K., Rodden N., Hughes E., Thomson N., McGlynn D., Walden A., Jacques N., Coles H., Tilney E., Vowell E., Schuster-Bruce M., Pitts S., Miln R., Purandare L., Vamplew L., Spivey M., Bean S., Burt K., Moore L., Day C., Gibson C., Gordon E., Zitter L., Keenan S., Baker E., Cherian S., Cutler S., Roynon-Reed A., Harrington K., Raithatha A., Bauchmuller K., Ahmad N., Grecu I., Trodd D., Martin J., Wrey Brown C., Arias A.M., Craven T., Hope D., Singleton J., Clark S., Rae N., Welters I., Hamilton D.O., Williams K., Waugh V., Shaw D., Puthucheary Z., Martin T., Santos F., Uddin R., Somerville A., Tatham K.C., Jhanji S., Black E., Dela Rosa A., Howle R., Tully R., Drummond A., Dearden J., Philbin J., Munt S., Vuylsteke A., Chan C., Victor S., Matsa R., Gellamucho M., Creagh-Brown B., Tooley J., Montague L., De Beaux F., Bullman L., Kersiake I., Demetriou C., Mitchard S., Ramos L., White K., Donnison P., Johns M., Casey R., Mattocks L., Salisbury S., Dark P., Claxton A., McLachlan D., Slevin K., Lee S., Hulme J., Joseph S., Kinney F., Senya H.J., Oborska A., Kayani A., Hadebe B., Orath Prabakaran R., Nichols L., Thomas M., Worner R., Faulkner B., Gendall E., Hayes K., Hamilton-Davies C., Chan C., Mfuko C., Abbass H., Mandadapu V., Leaver S., Forton D., Patel K., Paramasivam E., Powell M., Gould R., Wilby E., Howcroft C., Banach D., Fernández de Pinedo Artaraz Z., Cabreros L., White I., Croft M., Holland N., Pereira R., Zaki A., Johnson D., Jackson M., Garrard H., Juhaz V., Roy A., Rostron A., Woods L., Cornell S., Pillai S., Harford R., Rees T., Ivatt H., Sundara Raman A., Davey M., Lee K., Barber R., Chablani M., Brohi F., Jagannathan V., Clark M., Purvis S., Wetherill B., Dushianthan A., Cusack R., de Courcy-Golder K., Smith S., Jackson S., Attwood B., Parsons P., Page V., Zhao X.B., Oza D., Rhodes J., Anderson T., Morris S., Xia Le, Tai C., Thomas A., Keen A., Digby S., Cowley N., Wild L., Southern D., Reddy H., Campbell A., Watkins C., Smuts S., Touma O., Barnes N., Alexander P., Felton T., Ferguson S., Sellers K., Bradley-Potts J., Yates D., Birkinshaw I., Kell K., Marshall N., Carr-Knott L., Summers C. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. J. Am. Med. Assoc. 2020;324:1317–1329. doi: 10.1001/JAMA.2020.17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano E.J., Fonseca Fuentes X., Corsini Campioli C., O'Horo J.C., Abu Saleh O., Odeyemi Y., Yadav H., Temesgen Z. Impact of corticosteroids in coronavirus disease 2019 outcomes: systematic review and meta-analysis. Chest. 2021;159:1019–1040. doi: 10.1016/J.CHEST.2020.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri D., Sasaki K., Karkar A., Sharif S., Lewis K., Mammen M.J., Alexander P., Ye Z., Lozano L.E.C., Munch M.W., Perner A., Du B., Mbuagbaw L., Alhazzani W., Pastores S.M., Marshall J., Lamontagne F., Annane D., Gu Meduri, Rochwerg B. Corticosteroids in COVID-19 and non-COVID-19 ARDS: a systematic review and meta-analysis. Intensive Care Med. 2021;47:521–537. doi: 10.1007/S00134-021-06394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral-Gudino L., Bahamonde A., Arnaiz-Revillas F., Gómez-Barquero J., Abadía-Otero J., García-Ibarbia C., Mora V., Cerezo-Hernández A., Hernández J.L., López-Muñíz G., Hernández-Blanco F., Cifrián J.M., Olmos J.M., Carrascosa M., Nieto L., Fariñas M.C., Riancho J.A. Methylprednisolone in adults hospitalized with COVID-19 pneumonia : an open-label randomized trial (GLUCOCOVID) Wien Klin. Wochenschr. 2021;133:303–311. doi: 10.1007/S00508-020-01805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dequin P.F., Heming N., Meziani F., Plantefève G., Voiriot G., Badié J., François B., Aubron C., Ricard J.D., Ehrmann S., Jouan Y., Guillon A., Leclerc M., Coffre C., Bourgoin H., Lengellé C., Caille-Fénérol C., Tavernier E., Zohar S., Giraudeau B., Annane D., Le Gouge A. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. J. Am. Med. Assoc. 2020;324:1298–1306. doi: 10.1001/JAMA.2020.16761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edalatifard M., Akhtari M., Salehi M., Naderi Z., Jamshidi A., Mostafaei S., Najafizadeh S.R., Farhadi E., Jalili N., Esfahani M., Rahimi B., Kazemzadeh H., Mahmoodi Aliabadi M., Ghazanfari T., Sattarian M., Ebrahimi Louyeh H., Raeeskarami S.R., Jamalimoghadamsiahkali S., Khajavirad N., Mahmoudi M., Rostamian A. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. Eur. Respir. J. 2020;56:2002808. doi: 10.1183/13993003.02808-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel R., Morrison A.R., Vahia A., Smith Z.R., Chaudhry Z., Bhargava P., Miller J., Kenney R.M., Alangaden G., Ramesh M.S. Early short-course corticosteroids in hospitalized patients with COVID-19. Clin. Infect. Dis. 2020;71:2114–2120. doi: 10.1093/CID/CIAA601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagone P., Ciurleo R., Lombardo S.D., Iacobello C., Palermo C.I., Shoenfeld Y., Bendtzen K., Bramanti P., Nicoletti F. Transcriptional landscape of SARS-CoV-2 infection dismantles pathogenic pathways activated by the virus, proposes unique sex-specific differences and predicts tailored therapeutic strategies. Autoimmun. Rev. 2020;19:102571. doi: 10.1016/J.AUTREV.2020.102571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J. Dexamethasone in hospitalized patients with covid-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMOA2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indian Council of Medical Research (ICMR) Clinical guidance for management of COVID-19 patients. 2021. https://www.icmr.gov.in/pdf/covid/techdoc Available at: Accessed on 1st May 2021.
- Jamaati H., Hashemian S.M., Farzanegan B., Malekmohammad M., Tabarsi P., Marjani M., Moniri A., Abtahian Z., Haseli S., Mortaz E., Dastan A., Mohamadnia A., Vahedi A., Monjazebi F., Yassari F., Fadaeizadeh L., Saffaei A., Dastan F. No clinical benefit of high dose corticosteroid administration in patients with COVID-19: a preliminary report of a randomized clinical trial. Eur. J. Pharmacol. 2021;897:173947. doi: 10.1016/J.EJPHAR.2021.173947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeronimo C.M.P., Farias M.E.L., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Safe I.P., Borba M.G.S., Netto R.L.A., Maciel A.B.S., Neto J.R.S., Oliveira L.B., Figueiredo E.F.G., Oliveira Dinelly K.M., de Almeida Rodrigues M.G., Brito M., Mourão M.P.G., Pivoto João G.A., Hajjar L.A., Bassat Q., Romero G.A.S., Naveca F.G., Vasconcelos H.L., de Araújo Tavares M., Brito-Sousa J.D., Costa F.T.M., Nogueira M.L., Baía-da-Silva D.C., Xavier M.S., Monteiro W.M., Lacerda M.V.G. Methylprednisolone as adjunctive therapy for patients hospitalized with coronavirus disease 2019 (COVID-19; metcovid): a randomized, double-blind, phase IIb, placebo-controlled trial. Clin. Infect. Dis. 2021;72:e373–e381. doi: 10.1093/CID/CIAA1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha N.K., Jeyaraman M., Rachamalla M., Ojha S., Dua K., Chellappan D.K., Muthu S., Sharma A., Jha S.K., Jain R., Jeyaraman N., Gs P., Satyam R., Khan F., Pandey P., Verma N., Singh S.K., Roychoudhury S., Dholpuria S., Ruokolainen J., Kesari K.K. Current understanding of novel coronavirus: molecular pathogenesis, diagnosis, and treatment approaches. Immuno. 2021;1:30–66. doi: 10.3390/IMMUNO1010004. 2021. 30-66 1. [DOI] [Google Scholar]
- Kalfaoglu B., Almeida-Santos J., Tye C.A., Satou Y., Ono M. T-cell hyperactivation and paralysis in severe COVID-19 infection revealed by single-cell analysis. Front. Immunol. 2020;11 doi: 10.3389/FIMMU.2020.589380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Zhang S., Dong X., Li Z., Xu Q., Feng H., Cai J., Huang S., Guo J., Zhang L., Chen Y., Zhu W., Du H., Liu Y., Wang T., Chen L., Wen Z., Annane D., Qu J., Chen D. Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome. J. Clin. Invest. 2020;130:6417–6428. doi: 10.1172/JCI140617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauvais-Jarvis F. Do anti-androgens have potential as therapeutics for COVID-19? Endocrinology. 2021;162 doi: 10.1210/ENDOCR/BQAB114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B.C., Laracy J., Shoucri S., Dietz D., Zucker J., Patel N., Sobieszczyk M.E., Kubin C.J., Gomez-Simmonds A. Clinical outcomes associated with methylprednisolone in mechanically ventilated patients with COVID-19. Clin. Infect. Dis. 2021;72:e367–e372. doi: 10.1093/CID/CIAA1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi S.T., Parthasarathy P., Lin Y., Nallakaruppan V.D., Ng S., Tan T.C., Low S., Tang T. Antivirals with adjunctive corticosteroids prevent clinical progression of early coronavirus 2019 pneumonia: a retrospective cohort study. Open forum Infect. Dis. 2020;7 doi: 10.1093/OFID/OFAA486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasin L., Navalesi P., Zangrillo A., Kuzovlev A., Likhvantsev V., Hajjar L.A., Fresilli S., Lacerda M.V.G., Landoni G. Corticosteroids for patients with coronavirus disease 2019 (COVID-19) with different disease severity: a meta-analysis of randomized clinical trials. J. Cardiothorac. Vasc. Anesth. 2021;35:578–584. doi: 10.1053/J.JVCA.2020.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patoulias D., Doumas M., Papadopoulos C., Karagiannis A. Janus kinase inhibitors and major COVID-19 outcomes: time to forget the two faces of Janus! A meta-analysis of randomized controlled trials. Clin. Rheumatol. 2021;1 doi: 10.1007/S10067-021-05884-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M.W., Meyhoff T.S., Helleberg M., Kjaer M.N., Granholm A., Hjortsø C.J.S., Jensen T.S., Møller M.H., Hjortrup P.B., Wetterslev M., Vesterlund G.K., Russell L., Jørgensen V.L., Tjelle K., Benfield T., Ulrik C.S., Andreasen A.S., Mohr T., Bestle M.H., Poulsen L.M., Hitz M.F., Hildebrandt T., Knudsen L.S., Møller A., Sølling C.G., Brøchner A.C., Rasmussen B.S., Nielsen H., Christensen S., Strøm T., Cronhjort M., Wahlin R.R., Jakob S., Cioccari L., Venkatesh B., Hammond N., Jha V., Myatra S.N., Gluud C., Lange T., Perner A. Low-dose hydrocortisone in patients with COVID-19 and severe hypoxia (COVID STEROID) trial-Protocol and statistical analysis plan. Acta Anaesthesiol. Scand. 2020;64:1365–1375. doi: 10.1111/AAS.13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontali E., Volpi S., Signori A., Antonucci G., Castellaneta M., Buzzi D., Montale A., Bustaffa M., Angelelli A., Caorsi R., Giambruno E., Bobbio N., Feasi M., Gueli I., Tricerri F., Calautti F., Castagnola E., Moscatelli A., Rollandi G.A., Ravelli A., Cassola G., Sormani M.P., Gattorno M. Efficacy of early anti-inflammatory treatment with high doses of intravenous anakinra with or without glucocorticoids in patients with severe COVID-19 pneumonia. J. Allergy Clin. Immunol. 2021;147:1217–1225. doi: 10.1016/J.JACI.2021.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulakurthi Y.S., Pederson J.M., Saravu K., Gupta N., Balasubramanian P., Kamrowski S., Schmidt M., Vegivinti C.T.R., Dibas M., Reierson N.L., Pisipati S., Joseph B.A., Selvan P.T., Dmytriw A.A., Keesari P.R., Sriram V., Chittajallu S., Brinjikji W., Katamreddy R.R., Chibbar R., Davis A.R., Malpe M., Mishra H.K., Kallmes K.M., Hassan A.E., Evanson K.W. Corticosteroid therapy for COVID-19: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltim.) 2021;100 doi: 10.1097/MD.0000000000025719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M.R., Islam T., Shahjaman M., Islam M.R., Lombardo S.D., Bramanti P., Ciurleo R., Bramanti A., Tchorbanov A., Fisicaro F., Fagone P., Nicoletti F., Pennisi M. Discovering common pathogenetic processes between COVID-19 and diabetes mellitus by differential gene expression pattern analysis. Briefings Bioinf. 2021;2021:1–12. doi: 10.1093/BIB/BBAB262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramiro S., Mostard R.L.M., Magro-Checa C., van Dongen C.M.P., Dormans T., Buijs J., Gronenschild M., de Kruif M.D., van Haren E.H.J., van Kraaij T., Leers M.P.G., Peeters R., Wong D.R., Landewé R.B.M. Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study. Ann. Rheum. Dis. 2020;79:1143–1151. doi: 10.1136/ANNRHEUMDIS-2020-218479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjbar K., Moghadami M., Mirahmadizadeh A., Fallahi M.J., Khaloo V., Shahriarirad R., Erfani A., Khodamoradi Z., Gholampoor Saadi M.H. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial. BMC Infect. Dis. 2021;21 doi: 10.1186/S12879-021-06045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashad A., Mousa S., Nafady-Hego H., Nafady A., Elgendy H. Short term survival of critically ill COVID-19 Egyptian patients on assisted ventilation treated by either Dexamethasone or Tocilizumab. Sci. Rep. 2021;11 doi: 10.1038/S41598-021-88086-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salton F., Confalonieri P., Gu Meduri, Santus P., Harari S., Scala R., Lanini S., Vertui V., Oggionni T., Caminati A., Patruno V., Tamburrini M., Scartabellati A., Parati M., Villani M., Radovanovic D., Tomassetti S., Ravaglia C., Poletti V., Vianello A., Gaccione A.T., Guidelli L., Raccanelli R., Lucernoni P., Lacedonia D., Foschino Barbaro M.P., Centanni S., Mondoni M., Davì M., Fantin A., Cao X., Torelli L., Zucchetto A., Montico M., Casarin A., Romagnoli M., Gasparini S., Bonifazi M., D'Agaro P., Marcello A., Licastro D., Ruaro B., Volpe M.C., Umberger R., Confalonieri M. Prolonged low-dose methylprednisolone in patients with severe COVID-19 pneumonia. Open forum Infect. Dis. 2020;7 doi: 10.1093/OFID/OFAA421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Khanna P., Soni K.D. Are the steroids a blanket solution for COVID-19? A systematic review and meta-analysis. J. Med. Virol. 2021;93:1538–1547. doi: 10.1002/jmv.26483. [DOI] [PubMed] [Google Scholar]
- Sarma P., Bhattacharyya A., Kaur H., Prajapat M., Prakash A., Kumar S., Bansal S., Kirubakaran R., Reddy D.H., Muktesh G., Kaushal K., Sharma S., Shekhar N., Avti P., Thota P., Medhi B. Efficacy and safety of steroid therapy in COVID-19: a rapid systematic review and Meta-analysis. Indian J. Pharmacol. 2020;52:535. doi: 10.4103/IJP.IJP_1146_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J. Pediatr. 2020;87:281–286. doi: 10.1007/S12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne J.A.C., Murthy S., Diaz J.V., Slutsky A.S., Villar J., Angus D.C., Annane D., Azevedo L.C.P., Berwanger O., Cavalcanti A.B., Dequin P.F., Du B., Emberson J., Fisher D., Giraudeau B., Gordon A.C., Granholm A., Green C., Haynes R., Heming N., Higgins J.P.T., Horby P., Jüni P., Landray M.J., Le Gouge A., Leclerc M., Lim W.S., Machado F.R., McArthur C., Meziani F., Møller M.H., Perner A., Petersen M.W., Savovic J., Tomazini B., Veiga V.C., Webb S., Marshall J.C. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. J. Am. Med. Assoc. 2020;324:1330–1341. doi: 10.1001/JAMA.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steroid Sari Glucocorticoid therapy for COVID-19 critically ill patients with severe acute respiratory failure. 2020. https://clinicaltrials.gov/ Trial number: NCT04244591. Available at: Accessed on 22nd July 2021.
- Sunkara H., Dewan S.M.R. Coronavirus disease-2019: a review on the disease exacerbation via cytokine storm and concurrent management. Int. Immunopharm. 2021;99 doi: 10.1016/J.INTIMP.2021.108049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Feng Y.M., Ni J.X., Zhang J.Y., Liu L.M., Hu K., Wu X.Z., Zhang J.X., Chen J.W., Zhang J.C., Su J., Li Y.L., Zhao Y., Xie J., Ding Z., He X.L., Wang W., Jin R.H., Shi H.Z., Sun B. Early use of corticosteroid May prolong SARS-CoV-2 shedding in non-intensive care unit patients with COVID-19 pneumonia: a multicenter, single-blind, randomized control trial. Respiration. 2021;100:116–126. doi: 10.1159/000512063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomazini B.M., Maia I.S., Cavalcanti A.B., Berwanger O., Rosa R.G., Veiga V.C., Avezum A., Lopes R.D., Bueno F.R., Silva M.V.A.O., Baldassare F.P., Costa E.L.V., Moura R.A.B., Honorato M.O., Costa A.N., Damiani L.P., Lisboa T., Kawano-Dourado L., Zampieri F.G., Olivato G.B., Righy C., Amendola C.P., Roepke R.M.L., Freitas D.H.M., Forte D.N., Freitas F.G.R., Fernandes C.C.F., Melro L.M.G., Junior G.F.S., Morais D.C., Zung S., Machado F.R., Azevedo L.C.P. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA, J. Am. Med. Assoc. 2020;324:1307–1316. doi: 10.1001/JAMA.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Paassen J., Vos J.S., Hoekstra E.M., Neumann K.M.I., Boot P.C., Arbous S.M. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit. Care. 2020;24:696. doi: 10.1186/S13054-020-03400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderbeke L., Van Mol P., Van Herck Y., De Smet F., Humblet-Baron S., Martinod K., Antoranz A., Arijs I., Boeckx B., Bosisio F.M., Casaer M., Dauwe D., De Wever W., Dooms C., Dreesen E., Emmaneel A., Filtjens J., Gouwy M., Gunst J., Hermans G., Jansen S., Lagrou K., Liston A., Lorent N., Meersseman P., Mercier T., Neyts J., Odent J., Panovska D., Penttila P.A., Pollet E., Proost P., Qian J., Quintelier K., Raes J., Rex S., Saeys Y., Sprooten J., Tejpar S., Testelmans D., Thevissen K., Van Buyten T., Vandenhaute J., Van Gassen S., Velásquez Pereira L.C., Vos R., Weynand B., Wilmer A., Yserbyt J., Garg A.D., Matthys P., Wouters C., Lambrechts D., Wauters E., Wauters J. Monocyte-driven atypical cytokine storm and aberrant neutrophil activation as key mediators of COVID-19 disease severity. Nat. Commun. 2021;12:4117. doi: 10.1038/S41467-021-24360-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar J., Añón J.M., Ferrando C., Aguilar G., Muñoz T., Ferreres J., Ambrós A., Aldecoa C., Suárez-Sipmann F., Thorpe K.E., Jüni P., Slutsky A.S., Ferrando C., Mellado-Artigas R., Fernández J., Hernández M., Castellá M., Castro P., Badia J.R., Aguilar G., Carbonell J.A., Badenes R., Tornero C., Ferreres J., Blasco M.L., Carbonell N., Serrano A., Juan M., Gómez-Herreras J.I., López M.L., Ambrós A., Martín C., Del Campo R., Puig-Bernabeu J., Ferrer C., De Andrés J., Muñoz T., Serna-Grande P., Tamayo G., Martínez-Ruíz A., Bilbao-Villasante I., Villar J., Fernández R.L., Calvo C.P., Vidal Á., Añón J.M., Figueira J.C., Asensio M.J., Maseda E., Suárez-Sipmann F., Ramasco F., Varela-Durán M., Díaz-Parada P., Trenado-Álvarez J., Fernández M.M., Aldecoa C., Rico-Feijoo J., Fernández L., Sánchez-Ballesteros J., Blanco-Schweizer P., Martínez D., Soler J.A., Slutsky A.S., Jüni P., Thorpe K.E., Thomas R., Wysocki K., De Verno P., Lakhanpal G., Juando-Prats C. Efficacy of dexamethasone treatment for patients with the acute respiratory distress syndrome caused by COVID-19: study protocol for a randomized controlled superiority trial. Trials. 2020;21:717. doi: 10.1186/S13063-020-04643-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Yang W., Chen P., Guo J., Liu R., Wen P., Li K., Lu Y., Ma T., Li X., Qin S., Zhang Y., Wang Y. The proportion and effect of corticosteroid therapy in patients with COVID-19 infection: a systematic review and meta-analysis. PLoS One. 2021;16 doi: 10.1371/JOURNAL.PONE.0249481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) Clinical management of severe acute respiratory infection ( SARI) when COVID-19 disease is suspected: interim guidance. 2020. https://apps.who.int/iris/handle/10665/331446 13th March 2020. Avialble at. Accessed on 9th April 2021.
- Yan T., Xiao R., Wang N., Shang R., Lin G. Obesity and severe coronavirus disease 2019: molecular mechanisms, paths forward, and therapeutic opportunities. Theranostics. 2021;11:8234–8253. doi: 10.7150/THNO.59293. [DOI] [PMC free article] [PubMed] [Google Scholar]