Abstract
The purpose of this review is to describe the functional anatomy of the precuneal cortex and outline some semiological features of precuneal seizures. The precuneal cortex is a structure that occupies the posterior medial portion of the parietal lobe, and it has broad cortical and subcortical connections. Neuroanatomical tracing, functional imaging, as well as electrical stimulation studies, of humans and other primates have elucidated many complex integrative functions of the precuneus including visuo-spatial imagery, sensorimotor functions, and consciousness (Cavanna and Trimble, 2006). Based on the understanding of its functions and connectivity, descriptions of potential seizure semiologies are hypothesized and compared to what is available in the literature. The latter is mostly in the form of case reports or case series. Seizures may involve simple or complex motor or sensory manifestations including abnormal eye movements, visual hallucinations, sensation of motion, or medial temporal-like seizures.
Keywords: Parietal lobe epilepsy, Focal epilepsies, Precuneus, Parietal lobe, Epilepsy surgery, SEEG
1. Introduction
The precuneus is a trapezoidal structure that defines the posterior medial portion of the parietal lobe. This region includes both unimodal and polymodal association regions and has been implicated in many complex integrative functions of the brain including visuo-spatial imagery, sensorimotor functions, and consciousness (Cavanna and Trimble, 2006). The precuneus is extensively involved in many different processes, as we review below, having multiple cortical and subcortical connections. The presentation of lesions in the precuneus can be diverse depending on the location of the lesion. Similarly, precuneal epilepsy represents the rich dynamic interaction between the precuneus and its interconnected structures. Consequently, the clinical manifestations can be difficult to recognize – a principal motivation for the present review.
Parietal lobe epilepsies make up 5% of focal epilepsies, and a proportion of these originate in the precuneus (Siegel, 2003; Salanova, 2012). However, this may be an underestimation as parietal lobe epilepsy is not frequently studied. Parietal lobe epilepsies can manifest with multiple auras in the same patient, frequently with secondary generalization (Francione et al., 2015). Only a handful of reports of precuneal epilepsy exist in the literature, likely because precuneal epilepsy is difficult to recognize, demonstrate and characterize given its heterogeneous presentations and complex circuitry with distinct connections in a relatively small region of the cerebral cortex.
The analysis of seizure semiology is an integral part of presurgical evaluation of epilepsy to better define the epileptogenic zone. Bancaud and Talairach defined the epileptogenic zone as the site of primary organization of the ictal discharges and its early spread (Talairach 1965). The resultant activation or inhibition of a network of interconnected brain regions gives rise to the symptom and signs of seizure, and the way in which these unfold in time – overall referred to as semiology. An implantation of intracranial electrodes requires the establishment of solid anatomo-electro-clinical (AEC) hypothesis. Therefore, of an equal importance is an in-depth understanding of functional anatomy in order to plan the implantations schema based on presurgical data. There is significant inter-rater variability in interpreting semiology as different centers and even different clinicians within the same center may define symptoms and signs of seizure differently (Tufenkjian and Lüders, 2012). This becomes even more problematic in the case of parietal lobe seizures, particularly those originating from the precuneus, given the wide range of possible semiologies.
For the systematic qualitative component of the present review, we comprehensively searched, evaluated and synthesized research evidence. Inclusion criteria were various types of studies including retrospective studies, review articles, systematic reviews, and case reports. Comprehensive search was performed in PubMed, using terms individually and using the Boolean ANDs and ORs. In the search strategy the following terms were included: Precuneus OR Precuneal epilepsy OR Parietal lobe epilepsy OR Medial parietal AND seizure AND semiology. Sixty-six articles were found in PubMed. A narrative approach was used to summarize and compare findings of semiologies in articles. Cited references were sometimes obtained, and a broader range of other literature was used in the review of anatomy, function, and organization of the precuneus.
2. Anatomy
2.1. Gross anatomy and histology
The precuneus, first illustrated by Soemmering and defined by Burdach (Swanson, 2015), refers to the posterior-medial aspect of the superior parietal lobule. The precuneus is defined anatomically as anterior to the cuneus of the occipital lobe, as defined by the parieto-occipital fissure. While typically straight, this fissure can be t-shaped or branched. Anteriorly the border of the precuneus is defined by the posterior paracentral gyrus and the posterior (or marginal) ramus of the cingulate sulcus, corresponding to the posterior margin of the primary sensorimotor cortices (Johns, 2014). The inferior border is formed by the subparietal sulcus, which can have a branching course or an H-shape (Cavanna and Trimble, 2006)
Given these anatomical landmarks, the medial portion of Brodmann’s area (BA) 7 and the dorsal part of BA31 together comprise the precuneus. Brodmann (1909) recognized a histological gradient from anterior to posterior in BA7, with reducing cell density more posteriorly. While BA31 is the dorsal posterior cingulate region, it does not have a clear border with BA7 and likely has more typical cingulate connections; this region extends above the subparietal sulcus and is considered part of the precuneus as defined on gross anatomical grounds (see Figure 2). More recent parcellations based on histology and neuroimaging are shown in Figure 1. Interestingly, the posterior and ventral portion of the region is architectonically similar to the ventroposterior lateral parietal lobe, which in the rhesus monkey is the parietal gyrus (area PG based on von Economo’s nomenclature ( ), see Figure 2), resulting in the terminology medial PG or PGm. These areas are extensively interconnected (Pandya and Seltzer, 1982). Rapidly evolving studies of regional cortical gene expression and regulatory genes for cortical development herald a new era of genetic-architectonics (Grasby et al., 2020) but are not refined enough on an areal basis at present to be used as a standard method of cortical parcellation for clinical use.
Figure 2. Functional regions of the precuneus and their connections.
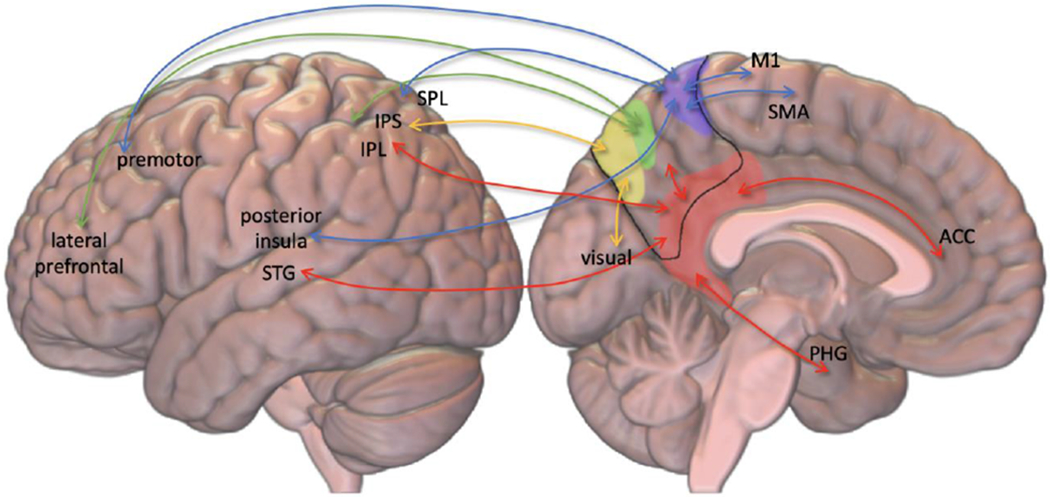
The four functional subdivisions of the precuneus are depicted: The sensorimotor (blue), cognitive (green), visual (yellow) and limbic (red). Basic connections are depicted. ACC: Anterior cingulate cortex/region. IPL: Inferior parietal lobule. IPS: Intraparietal sulcus. M1: Primary motor cortex. PHG: Parahippocampal gyrus. SMA: Supplementary motor area. SPL: Superior parietal lobule. STG: Superior temporal gyrus.
Figure 1. Cytoarchitectonic parcellation of the precuneus.
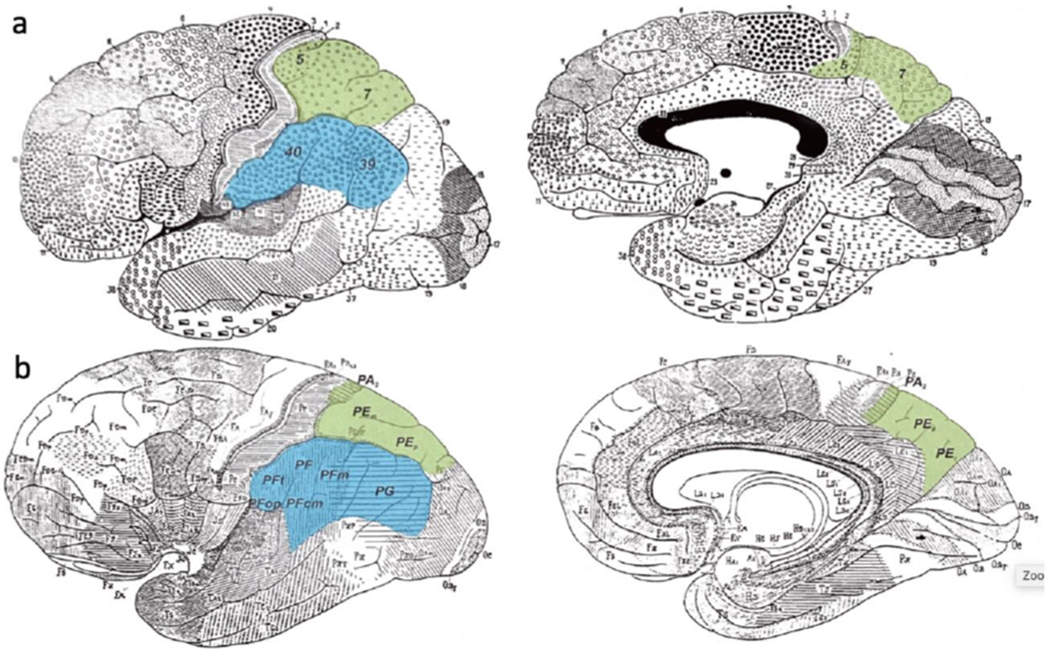
Parcellations that are commonly used in epilepsy literature of (a) Brodmann (1909) and (b) von Economo and Koskinas (1925). Note that the margins of the precuneus extend below the shaded area to include the dorsal part of Brodmann’s area (BA) 31. In the primate, the central precuneus is termed PGm given strong homology with von Economo and Koskinas’s region PG. From (Caspers et al. 2012) with permission
Developmentally, classical studies (e.g. Flechsig, 1901) show that myelinogenesis occurs later than primary sensorimotor regions, with the dorsal anterior precuneus myelinating around the same time as secondary somatosensory and motor cortices, with the remainder of the precuneus, in keeping with a higher order or polymodal role (see below), myelinating very late, shortly before the ultimate completion of myelination in the dorsolateral prefrontal area.
2.2. Chemical anatomy
Receptor expression patterns have helped establish the unique functional microarchitecture of the precuneus and have bolstered interspecies comparisons. Neurotransmitter receptors are distributed heterogeneously within the parietal lobe. Based on rhesus monkey autoradiographic data, expression of M3 receptors in the superficial layers of area PGm is high compared to other areas in the parietal lobe, highlighting the existence of this region as proposed on purely cytoarchitectonic grounds previously (Impieri et al., 2019). While the functional importance of receptor expression is yet to be elucidated, it is interesting that this region, being part of the default mode network (Utevsky, Smith and Huettel, 2014), expresses excitatory g-protein coupled receptors sensitive to cholinergic inputs provided by components of the basal forebrain arousal nuclei, particularly the nucleus basalis of Meynert (Mesulam et al., 1984).
Other notable findings seen on chemical studies of the precuneus in certain disease or aging states include decreased metabolism in the bilateral precuneus in early onset Alzheimer’s disease (Rabinovici and Miller, 2010; Shivamurthy et al., 2015), lowered amplitude of low frequency fluctuations on fMRI suggesting reduction in spontaneous neuronal activity in patients with anti-NMDA encephalitis (Cai et al., 2020), and a decline in aerobic glycolysis within the precuneus during normal aging that is greater than most other brain regions (Goyal et al., 2017).
3. Connections of the precuneus
3.1. Functional organization of the precuneus
While considered part of this task-negative network (Fox et al., 2005), neuroanatomical and functional imaging studies in non-human primates and humans highlight the central role of the precuneus in specific cognitive tasks related to somatosensory processing, medial temporal inputs from the forebrain, visuospatial processing (Costigan et al., 2019) and cognition. While the default mode network (DMN) hub is thought to reside in the precuneus (Utevsky, Smith and Huettel, 2014) since studies have shown decreased gamma power during cognitive tasks, present evidence suggests the DMN also, or perhaps only, involves the posterior cingulate cortex (PCC) (Jerbi et al., 2010; Li et al., 2019). Speculatively, the ventral ‘limbic’ medial temporal projecting regions of the precuneus (Margulies et al., 2009) may also be part of this DMN hub, given functional and anatomical similarities to the PCC and both areas are part of the cytoarchitectonic region BA 31.
3.2. Neuroanatomical tracing studies in the rhesus monkey
Neuroanatomical tracing, often anterograde tracing with autoradiography in non-human primates, along with other neuroanatomical tracing methods and histological analysis, is the gold-standard for determining anatomical connectivity. Given the limitations of diffusion tensor tractography, and the use of structural connectivity to denote this, we suggest using the term hodological connectivity to describe methods of neuroanatomical tracing. Studies of non-human primates using autoradiographic and ablation-degeneration techniques found two major rostral-to-caudal connectional sequences. The first sequence runs from dorsal postcentral gyrus, through divisions of the superior parietal lobule, and terminates in the medial surface of the parietal lobe. The second sequence begins in the ventral postcentral gyrus and passes through the rostral inferior parietal lobule to reach the caudal inferior parietal lobule (Pandya and Seltzer, 1982). More recent tracing studies have identified connections from the dorsal visual precuneus to the posterior dorsal prefrontal cortex (BA8), the posterior claustrum, the intraparietal sulcus, and, as widely appreciated, the inferior parietal lobule (Schmahmann and Pandya, 2006). Posterior tracer injections reveal anterograde labeling of efferent projections that are principally within the central precuneus, intraparietal sulcus, posterior parietal region, as well as visual areas V2 and V3, but not the calcarine fissure. When the injection is slightly more dorsal in the visual precuneus, fibers are also noted projecting rostrally into the prefrontal cortex Brodmann areas 6, 9 and 46 and into part of the dorsal V1 region (Yeterian and Pandya, 2010). These marked differences in projection patterns within one candidate domain of the precuneus highlight complex topographic projections that belie the four-sector segregation of the precuneus (Margulies et al., 2010), as described above on the basis of neuroimaging studies (see also Figure 1). A fine grained analysis of afferent connections with rhesus retrograde tracing reveal that the higher order visual area V6A provides input to both the central (PGm) and ventral (BA31) precuneus, but the limbic region has more medial frontal and denser superior/polymodal temporal input, while area PGm has densest input from the ventral precuneal region as well as the lateral parietal and dorsolateral frontal cortex (Passarelli et al., 2018). Together, these findings are consistent with a broadly polymodal function of both regions, albeit of a different flavor, with sensorimotor and visual input predominating for the central precuneus and affective and polymodal sensory inputs for the ventral precuneus. Further bolstering these findings and as a good general principle, connectivity of the prefrontal cortex and cingulate region are essentially reciprocal: for the central region, anterograde tracing from the frontal cortex highlights distinct networks of the dorsal posterior parietal cortex, presumed visual, medial and lateral, to the prefrontal cortex. For the ventral region, reciprocal connections, via the cingulate fasciculus, connect the ventral precuneus, presumably limbic, to the anterior cingulate and medial frontal cortex as well as to the prefrontal areas 8 and 46 (Yeterian et al., 2012). Subcortical projections are less well documented. The sensorimotor precuneus directly innervates the basis pontis in the rhesus monkey based on studies by Schmahmann and Pandya in 1989 but this is unknown in humans. In a study by Pandya et al (1971), callosal lesions in rhesus monkeys revealed callosal connections by the distribution of degenerating fibers. Based upon this work, the bulk of the precuneus is acollosal, with light contralateral innervation to the central precuneal area. While outside of the precuneus proper, collosal input was noted in the parietooccipital sulcus and surrounding the splenium in the posterior cingulate region. While these region are outside of the precuneus, both receives input from precuneus, and are thereby two of several likely oligosynaptic pathways to the contralateral hemisphere.
3.3. MR-based connectivity
Connectivity can be assessed in a number of ways, with typical terminology being structural, functional, and effective (Park and Friston, 2013), to which we add hodological connectivity, based in neuroanatomical tracing and described in detail below. In the setting of MR-based work, structural connectivity refers to diffusion-based methods such as diffusion tensor imaging, functional connectivity refers to correlated activity in functional imaging studies (e.g. blood oxygen level desaturation signal in MRI), and effective connectivity refers to techniques that perturb a network to see what changes occur. In the case of the precuneus, our ability to make claims about connectivity is based on having human and rhesus MRI-based tractography, which are similar, then making inferences about human connectivity based on neuroanatomical tracing studies in non-human primates, principally rhesus monkeys.
Topographical connectivity of the precuneus is notable. Based on analysis of resting state functional connectivity in anesthetized rhesus monkeys and awake resting human subjects, similar patterns of connectivity were noted. Of course, this analysis does not establish anatomical connectivity with certainty, but shows that spatial patterns of correlated cerebral metabolism are similar between humans and rhesus monkeys. Based on these analyses, connectivity of the precuneus was divided into four regions (Margulies et al., 2009) (Figure 1): (A) The anterior segment contributes to sensorimotor function as it is connected to the primary and secondary sensory and motor cortices. (B) The central precuneus (Margulies et al., 2009), lumped with the ventral precuneus by Wang and coworkers based on tractography (Wang et al., 2019) is involved in cognition, but is noted by Marguiles to separate into the central region with principally frontal and parietal functional connectivity and the ventral region (C) that connects to ‘limbic’ structures and the medial temporal lobe. Finally, (D) the posterior portion is connected to the primary and secondary visual cortices and is thought to participate in higher order visual functions. Tractography in humans is broadly in agreement with these non-human primate findings (Wang et al., 2019). Tractography can suffer from length effects, inability to resolve the entering of exit of smaller bundles, direction, and can show fewer long-range connections that functional connectivity and neuroanatomical tracing approaches, highlighting the valuable contributions of the latter gold-standard.
3.4. Lesion studies
While simple discrete vascular lesion studies are few, there are numerous cases of other lesions of the precuneus or related brain regions that help understand the functional role of the precuneus. Narasimha reported a case of a 59-year-old with left precuneus granuloma presenting with seizures, kinetopsia and delusions of nihilism and guilt (Narasimha et al., 2019). One study by (Herbet et al., 2019) included 14 patients with low grade gliomas infiltrating either the right or left precuneus and the anterior-dorsal precuneus was found to be involved in body awareness and perception due to its connections to the primary and secondary somatosensory cortex (Herbet et al., 2019). Previous studies have identified body awareness disorders like macrosomatognosia, fading limb, and autotopagnosia to be associated with high order somatosensory cortices (Zhang and Li, 2012). This hypothesis is further supported by the lesion network mapping study by Darby el al that demonstrated 90% of patients with alien limb syndrome had lesions in areas with functional connectivity to the precuneus, specifically in the right hemisphere (Darby et al., 2018)
In addition to spatial awareness of the body and associated diseases, precuneal lesions have been implicated in inability to navigate through either familiar or new environments despite being able to describe its location and sometimes even being able to describe how to get to a certain location—a phenomenon called topographical disorientation. A case report by (Suzuki et al., 1998) described a woman who had suffered a hemorrhagic stroke in the right precuneus and subsequently was unable to find her way to places she knew despite being able to describe their locations and recognize buildings. Topographical disorientation and its association with predominantly right precuneal lesions highlight the importance of the precuneus for visuospatial functions. A similar study simulated lesions in the precuneus with transcranial magnetic stimulation to demonstrate the essential role of the precuneus in developing a working memory of object locations and then for using these locations to form a self-centered map with which one can navigate his environment (Müller et al., 2018). In a Stroke study by Yassi 2015, it was found that strokes involving precuneus in the left hemisphere is associated with a non-favorable outcome with higher Modified Rankin Scores (Yassi et al., 2015).
3.5. Direct electrical stimulation mapping
Stimulation studies have helped elucidate the precuneus’s role in vision, visual integration, and possibly vestibular function. Thierie and Andersen hypothesized ‘medial parietal eye field’ after eliciting saccades with electrical microsimulation of the posterior medial parietal cortex in primates (Thier and Andersen, 1998). A study analyzed clinical manifestations induced by bipolar electrical stimulation in 172 patients (Balestrini et al., 2015) with at least one electrode stereotactically implanted in the parietal cortex. Electrical stimulations in both hemispheres elicited visual illusions or hallucinations, consistent with a previous study by Richer et al (Richer et al., 1991). Precuneal stimulation was not helpful in lateralization, perhaps related to its associational nature and strong interhemispheric connections.
Another notable finding from this study by Balestrini, et al. included vertiginous symptoms in six subjects with non-dominant and one with dominant hemispheric stimulation (Balestrini et al., 2015). The study authors proposed the vestibular responses may be likely due to the role of the precuneus in the processing of spatial information relative to the position of the subject, allowing the control of body movements. Other studies suggest that the precuneus has a role in vestibular function. A study retrospectively analyzed electrical stimulation studies in 260 patients with focal epilepsy with vestibular symptoms as part of their epilepsy (Kahane et al., 2003). Four patients had vestibular symptoms with stimulation of the precuneus (one with left precuneal stimulation, three with right sided stimulation), all within BA7. The authors of this study suggest but report that they cannot definitively state that the precuneus, specifically an area within BA7, is involved in vestibular function. A case report described a 16-year-old boy with seizure semiology of lateral sensation of movement (Wiest et al., 2004), he had a known lesion in the right paramedian precuneus and electrical cortical stimulation of the area elicited the same vestibular sensation he described with his seizures, again highlighting a possible vestibular role for this region.
3.6. Functional correlations of precuneal connectivity
Anatomical connections described above imply and are known to be associated with particular cortical functions, as are reciprocal connections of the dorsal thalamus and precuneus. The main parietal lobe connections to the precuneus are through the caudal parietal operculum, inferior and superior parietal lobules, and internal parietal sulcus (Cavanna and Trimble, 2006). The latter is well studied and has been shown to function in visual-spatial processing, containing the lateral parietal eye fields. It is hypothesized that the precuneus contains a “medial parietal eye field” and is involved in both eye movement control and visual reaching based on monkey functional stimulation studies. This is likely by virtue of either medial to lateral parietal connections and association with the parietal eye field, or a direct projection to the frontal eye field.
The large group of cortical connections are those with the frontal lobe, but the precuneus innervates each lobe to some extent. The lateral prefrontal cortex, dorsal premotor cortex, supplementary motor area, anterior cingulate gyrus, and frontal eye fields all receive direct precuneal input (see Figure 1), and mostly project reciprocally back to the precuneus. The major projections from the precuneus to the lateral parietal area and premotor cortex play a major role in hand eye coordination (Cavanna and Trimble 2006). The precuneus is also connected to different high-order-associated cortices like the temporo-parietal-occipital polymodal cortex which is involved in major sensory processing including auditory, visual and somatosensory processes.
Functional subdivisions of the precuneus are consistent with its cortico-thalamic relations. Taking a broad view, the precuneus can be seen as composed of unimodal (and limbic) association cortices at its edges, with a central polymodal association area (e.g. the ‘cognitive’ precuneus). Relatedly, the precuneus does not receive direct input from somatosensory relay nuclei. Instead, and as general principal, the precuneus is connected to the lateral posterior and, somewhat topographically, to the anterior and lateral pulvinar nuclei (Jones, 2007). The ‘limbic’ portion of the precuneus, composed of BA31, in keeping with its functional role and connections, is connected with the anterior nuclei and lateral dorsal nucleus of the thalamus (Aggleton et al., 2014). It is worth noting, however, that it is the more ventral BA31 that projects into these regions, so the significance of these findings for the precuneal dorsal portion of BA31 is not resolved. Specifically, the function of this region is mnemonic, potentially internally-directed (e.g. default mode, although this may be the retrosplenial area) and likely visuospatial, and its connections are with the anterior cingulate gyrus and medial temporal lobe, all consistent with anterior thalamic connection. Retrograde tracing from the dorsal portion of area 31 shows the above inputs along with labeled neurons in the intralaminar mediodorsal and paracentral nuclei (Gamberini et al., 2020). The major thalamic connections of the precuneus, being the pulvinar and laterodorsal (Grodd et al., 2020) nuclei are likely similar in the human based on phylogenic considerations, diffusion imaging (Behrens et al., 2003), and functional imaging (Zhang et al., 2010). Functional imaging further shows relations with other network components of each precuneal region, along with expanded and expected thalamic relations (Cauda et al., 2010).
In addition to its involvement in auditory, visual and sensorimotor processes, the precuneus is thought to play a major role in consciousness. In overview, posterior cortices likely have a key role in consciousness (e.g. Koch et al., 2016), and we presume that is more specifically related to polymodal sensory perceptual consciousness. Clearly, the parietal cortices, including the precuneus, participate in the higher order processing of unimodal and polymodal information. Consistent with this idea, when there is loss of consciousness, such as in deep sleep or vegetative state, the precuneus has been found to be significantly less active and it is one of the first regions in the brain to resume activity once patients regain consciousness (Cavanna and Trimble 2006). Finally, reports of unusual seizure semiology with startle reflex seizures or sensation of imbalance with lesions identified in the precuneus suggest that it may also be involved in other processes including part of the startle induced network and the vestibular system.
4. Semiology of seizures
4.1. Semiology bases on the result of a systematic qualitative review
The majority of literature describing precuneal seizures attributes the wide range of semiologies to the extensive connectivity to multimodal association areas. As discussed earlier, there are multiple processes in which the precuneus is involved including sensorimotor functions, cognition/consciousness, visual integration, and vestibular sensations. Because of these wide connections throughout the cerebral cortex, seizures caused by precuneal involvement may propagate to different areas causing unusual seizure semiologies. Umeoka reported a patient with anterior precuneal cortical dysplasia with seizure semiology of bilateral symmetrical tonic posturing of the upper extremities and impaired consciousness, was thought to be a result of propagation to the ipsilateral supplementary motor and premotor cortex (Umeoka et al., 2007). In light of the connectivity described above, spread from or through the sensorimotor precuneus into the frontal motor regions seems likely.
Mailo et al. reported right posterior precuneus epilepsy and further discussed role of the precuneus in spatial awareness, visuo-spatial processing and consciousness, their patient reported seizures consisted of feeling an urge to move but without vertigo, syncope, or a sense of imbalance (Mailo and Tang-Wai, 2015). Scalp EEG localized seizures to the non-dominant parieto-occipital region, and imaging findings were consistent with a low-grade neoplasm in the medial parietal cortex. Semiology was explained by the hypothesized role of the precuneus in generating spatial information for imagined or planned body movement (Ogiso, Kobayashi and Sugishita, 2000).
Other case reports have reported unusual semiologies. For example, Saeki et al described startle-induced seizures had been associated with an epileptogenic zone in the precuneus (Saeki et al., 2009). A patient with seizures described as linear self-motion perception and occasional body tilts swaying sensations described as ‘being on a boat’. Intracranial electrophysiology showed seizure onset from circumscribed ependymoma in paramedian precuneus and hence the region was thought to be associated in processing of static and dynamic vestibular information (Wiest et al., 2004).
4.2. Possible semiological features of seizures arising in the precuneal cortex:
The Precuneus has strong cortical and subcortical connections which have been demonstrated in stimulation, lesional and functional studies. One can speculate, based on these findings, the theoretically varied manifestations of precuneal epilepsy originating from different sub-regions of the precuneus. These potential features are based on our current understanding of the functional connectivity and the aforementioned literature.
Sensorimotor Anterior Precuneal Region:
This can lead to simple or complex motor or sensory manifestations due to connections with paracentral lobule, BA2, BA6 and SMA. Tonic, clonic, versive, hypermotor, bilateral asymmetric tonic manifestations have been reported (Harroud et al., 2017). This area is also connected with the parietal operculum and the insula which may contribute to vestibular and body image disturbance.
Cognitive/Associative Central Precuneal Region:
This region connects with angular gyrus and the dorsolateral prefrontal cortex including area 8, and therefore, could be related to some of the eye movement-related semiology, such as eye deviation (Harroud et al., 2017).
Visual Posterior Precuneal Region:
Complex visual hallucinations and visual illusion can be predicted based on connectivity to posterior fusiform gyrus. The involvement of the primary visual cortex can lead to elementary visual hallucinations. Blurred vision has been reported which may also be related to this region.
Limbic Precuneal Region:
This area is closely related to the posterior Cingulate/retrosplenial region which connects to limbic medial temporal region, including the parahippocampal gyrus and hippocampus. This can lead to mesial temporal like- manifestations. Given its connectivity with BA32 and 25, a variety of autonomic and emotional responses could be seen.
5. Conclusion
The precuneus is a fascinating structure which has many known, and potentially still to be discovered, functions due to its extensive cortical and subcortical connections. In summary, the precuneus can be divided into four distinct regions based on each area’s known networks connecting it to other parts of the brain and its associated functions:
The anterior dorsal precuneus has strong connections to primary and secondary sensory-motor cortices, including frontal eye field which indicate sensory motor function. In addition, it has a role in specially guided movement (Zhang and Li, 2012).
The posterior dorsal precuneus is part of a network involving the primary and secondary visual cortices, likely making it essential for visuospatial coordination. It has also been implicated in developing episodic memory and reasoning (Wang et al., 2019).
The ventral precuneus is part of mnemonic networks and may be part of the default mode network (DMN), a network involved in memory, emotion and language processing during resting state (Wang et al. 2019). However, the most anterior part of the ventral precuneus is involved in attention control (Cauda et al., 2010).
Finally, the central precuneus connects to the prefrontal area through the multisensory posterior inferior parietal lobule and is thought to have function within the higher cognitive polymodal cortex (Passarelli et al., 2020 and Margulies et al. 2009).
Overall, the precuneus contributes to many different processes that include but are not limited to auditory, vestibular, visual, sensorimotor, coordination, and memory consolidation and retrieval functions.
Table 1:
Functional-anatomical divisions of the precuneus.
| Precuneus Subsections | Connections of the precuneus | Function |
|---|---|---|
| Anterior dorsal | Primary and secondary sensory-motor cortices | a. Sensory motor function b. Specially guided movement (Zhang and Li, 2012) |
| Posterior dorsal | Primary and secondary visual cortices | a. Episodic memory and reasoning (Wang et al., 2019) b. visual special coordination |
| Ventral precuneus | a. Posterior cingulate gyrus b. Limbic mesial temporal cortex |
a. Default mode network (DMN), a network involved in memory, emotion and language processing during resting state (Wang et al., 2019) b. Attention control (Cauda et al., 2010) |
| Central precuneus | Prefrontal area | Higher cognitive functions (Margulies et al., 2009; Passarelli et al., 2018) |
Table 2:
Summary of precuneal lesional study.
| Study Name | Anatomical Location | Lesion | Loss of Function |
|---|---|---|---|
| Suzuki et al., 1998 | Right precuneus | hemorrhagic stroke | Inability to navigate through either familiar or new environments |
| Müller et al., 2018 | “virtual lesion” over precuneus | Via transcranial magnetic stimulation | Loss of working memory of object locations |
| Narasimha et al., 2019 | Left precuneus | Granuloma | Kinetopsia; delusions of nihilism and guilt |
| Herbet et al., 2019 | Right or left precuneus and the anterior-dorsal precuneus | Gliomas | Changes in body awareness and perception |
Question 1: What are the functional subdivision of the Precuneus?
Sensorimotor anterior, cognitive central, visual posterior and limbic
Question 2: What are the principal connections to the subdivisions of the Precuneus?
Sensorimotor: Primary and secondary sensory-motor cortices
Cognitive: Prefrontal area
Visual : Primary and secondary visual cortices
Limbic: Posterior cingulate gyrus and mesial temporal cortex
Question 3: What are the possible semiological manifestations of the subdivisions of the precuneus?
Sensorimotor Anterior Precuneal Region: This can lead to simple or complex motor or sensory manifestations. Tonic, clonic, versive, hypermotor, bilateral asymmetric tonic seizures. It can also lead to vestibular and body image disturbance.
Cognitive/Associative Central Precuneal Region: eye movement-related semiology, such as eye deviation.
Visual Posterior Precuneal Region: Elementary and complex visual hallucinations and visual illusion. Blurred vision has been reported which may also be related to this region.
Limbic Precuneal Region: This can lead to mesial temporal like- manifestations and autonomic and emotional responses could be seen.
Funding and Acknowledgements
NPP is supported by National Institute of Neurological Disorders And Stroke of the National Institutes of Health under Award Number K08NS105929. Other authors have no disclosures.
Footnotes
Disclosure of Conflicts of Interest
NPP is a member of the Scientific Advisory Board for Dixi Medical USA.
Ethical Publication and Authorship Statements
| Name | Location | Role | Contribution |
|---|---|---|---|
| Ruba R. Al-Ramadhani | Emory University School of Medicine | Author | study concept and design, literature/ data analysis and interpretation, drafting manuscript |
| Veeresh Kumar N. Shivamurthy | Emory University School of Medicine | Author | study concept and design, literature/ data analysis and interpretation, drafting manuscript. |
| Kathryn Elkins | Emory University School of Medicine | Author | literature/ data analysis and interpretation, drafting manuscript. |
| Satyanarayana Gedela | Emory University; Children’s Healthcare of Atlanta | Author | revising manuscript, study supervision. |
| Nigel P. Pedersen | Emory University; Children’s Healthcare of Atlanta | Author | study concept and design, data analysis and interpretation, drafting and revising manuscript, study supervision. |
| Ammar Kheder | Emory University; Children’s Healthcare of Atlanta | Author | study concept and design, data analysis and interpretation, drafting and revising manuscript, study supervision, final approval. |
References
- Aggleton J, Saunders R, Wright N and Vann S (2014). The Origin Of Projections From The Posterior Cingulate And Retrosplenial Cortices To The Anterior, Medial Dorsal And Laterodorsal Thalamic Nuclei Of Macaque Monkeys. European Journal of Neuroscience; 39(1):107–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestrini S et al. (2015) ‘Multimodal responses induced by cortical stimulation of the parietal lobe: a stereo-electroencephalography study’, Brain: a journal of neurology, 138(Pt 9), pp. 2596–2607. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, et al. “Non-Invasive Mapping of Connections between Human Thalamus and Cortex Using Diffusion Imaging.” Nature Neuroscience, vol. 6, no. 7, 15 June 2003, pp. 750–757, 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Cai L et al. (2020) ‘Cerebral functional activity and connectivity changes in anti-N-methyl-D-aspartate receptor encephalitis: A resting-state fMRI study’, NeuroImage. Clinical, 25, p. 102189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers, Amunts K, and Zilles K, Posterior parietal cortex, The Human Nervous System, pp. 1036–1053, 2012. [Google Scholar]
- Cauda F et al. (2010) ‘Functional connectivity of the posteromedial cortex’, PloS one, 5(9). doi: 10.1371/journal.pone.0013107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE and Trimble MR (2006) ‘The precuneus: a review of its functional anatomy and behavioural correlates’, Brain: a journal of neurology. Oxford Academic, 129(3), pp. 564–583. [DOI] [PubMed] [Google Scholar]
- Costigan AG et al. (2019) ‘Neurochemical correlates of scene processing in the precuneus/posterior cingulate cortex: A multimodal fMRI and 1H-MRS study’, Human brain mapping. Wiley Online Library, 40(10), pp. 2884–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby RR et al. (2018) ‘Lesion network localization of free will’, Proceedings of the National Academy of Sciences, pp. 10792–10797. doi: 10.1073/pnas.1814117115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD et al. (2005) ‘The human brain is intrinsically organized into dynamic, anticorrelated functional networks’, Proceedings of the National Academy of Sciences of the United States of America, 102(27), pp. 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francione S et al. (2015) ‘Drug-resistant parietal epilepsy: polymorphic ictal semiology does not preclude good post-surgical outcome’, Epileptic disorders: international epilepsy journal with videotape. Wiley Online Library, 17(1), pp. 32–46. [DOI] [PubMed] [Google Scholar]
- Fransson P and Marrelec G (2008) ‘The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis’, NeuroImage, 42(3), pp. 1178–1184. [DOI] [PubMed] [Google Scholar]
- Gamberini Michela, et al. “Thalamic Afferents Emphasize the Different Functions of Macaque Precuneate Areas.” Brain Structure and Function, vol. 225, no. 2, 20 February. 2020, pp. 853–870 [DOI] [PubMed] [Google Scholar]
- Grasby KL, Jahanshad N, Painter JN, et al. The genetic architecture of the human cerebral cortex. Science. 2020;367(6484):eaay6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodd Wolfgang, et al. “The Anterior and Medial Thalamic Nuclei and the Human Limbic System: Tracing the Structural Connectivity Using Diffusion-Weighted Imaging.” Scientific Reports, vol. 10, no. 1, 2 July 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal MS et al. (2017) ‘Loss of Brain Aerobic Glycolysis in Normal Human Aging’, Cell metabolism, 26(2), pp. 353–360.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harroud A et al. (2017) ‘Precuneal epilepsy: Clinical features and surgical outcome’, Epilepsy & behavior: E&B, 73, pp. 77–82. [DOI] [PubMed] [Google Scholar]
- Herbet G et al. (2019) ‘The antero-dorsal precuneal cortex supports specific aspects of bodily awareness’, Brain: a journal of neurology, 142(8), pp. 2207–2214. [DOI] [PubMed] [Google Scholar]
- Impieri D et al. (2019) ‘Receptor density pattern confirms and enhances the anatomic-functional features of the macaque superior parietal lobule areas’, Brain structure & function, 224(8), pp. 2733–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerbi K et al. (2010) ‘Exploring the electrophysiological correlates of the default-mode network with intracerebral EEG’, Frontiers in systems neuroscience, 4, p. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones Edward G. (2007) The Thalamus, 2nd ed, Cambridge University Press, Cambridge UK [Google Scholar]
- Johns P (2014) ‘Chapter 3 - Functional neuroanatomy’, in Johns P (ed.) Clinical Neuroscience. Churchill Livingstone, pp. 27–47. [Google Scholar]
- Kahane P et al. (2003) ‘Reappraisal of the human vestibular cortex by cortical electrical stimulation study’, Annals of neurology, 54(5), pp. 615–624. [DOI] [PubMed] [Google Scholar]
- Li J et al. (2019) ‘Default mode and visual network activity in an attention task: Direct measurement with intracranial EEG’, NeuroImage, 201, p. 116003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailo J and Tang-Wai R (2015) ‘Insight into the precuneus: a novel seizure semiology in a child with epilepsy arising from the right posterior precuneus’, Epileptic disorders: international epilepsy journal with videotape, 17(3), pp. 321–327. [DOI] [PubMed] [Google Scholar]
- Margulies DS et al. (2009) ‘Precuneus shares intrinsic functional architecture in humans and monkeys’, Proceedings of the National Academy of Sciences of the United States of America, 106(47), pp. 20069–20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM et al. (1984) ‘Atlas of cholinergic neurons in the forebrain and upper brainstem of the macaque based on monoclonal choline acetyltransferase immunohistochemistry and acetylcholinesterase histochemistry’, Neuroscience, 12(3), pp. 669–686. [DOI] [PubMed] [Google Scholar]
- Müller NG et al. (2018) ‘Repetitive transcranial magnetic stimulation reveals a causal role of the human precuneus in spatial updating’, Scientific reports, 8(1), p. 10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimha VL et al. (2019) ‘Precuneus and psychiatric manifestations: Novel neurobiological formulations through lesion based connectivity mapping of psychopathology’, Asian journal of psychiatry, 39, pp. 98–100. [DOI] [PubMed] [Google Scholar]
- Ogiso T, Kobayashi K and Sugishita M (2000) ‘The precuneus in motor imagery: a magnetoencephalographic study’, Neuroreport, 11(6), pp. 1345–1349. [DOI] [PubMed] [Google Scholar]
- Pandya DN and Seltzer B (1982) ‘Intrinsic connections and architectonics of posterior parietal cortex in the rhesus monkey’, The Journal of comparative neurology, 204(2), pp. 196–210. [DOI] [PubMed] [Google Scholar]
- Park H-J and Friston K (2013) ‘Structural and functional brain networks: from connections to cognition’, Science, 342(6158), p. 1238411. [DOI] [PubMed] [Google Scholar]
- Passarelli L et al. (2018) ‘Uniformity and Diversity of Cortical Projections to Precuneate Areas in the Macaque Monkey: What Defines Area PGm?’, Cerebral cortex , 28(5), pp. 1700–1717. [DOI] [PubMed] [Google Scholar]
- Rabinovici GD and Miller BL (2010) ‘Frontotemporal lobar degeneration: epidemiology, pathophysiology, diagnosis and management’, CNS drugs, 24(5), pp. 375–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richer F et al. (1991) ‘Visual motion perception from stimulation of the human medial parieto-occipital cortex’, Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale, 87(3), pp.649–652. [DOI] [PubMed] [Google Scholar]
- Saeki K et al. (2009) ‘Startle epilepsy associated with gait-induced seizures: Pathomechanism analysis using EEG, MEG, and PET studies’, Epilepsia, 50(5), pp. 1274–1279. [DOI] [PubMed] [Google Scholar]
- Salanova V (2012) ‘Parietal Lobe Epilepsy’, Journal of Clinical Neurophysiology pp. 392–396. doi: 10.1097/wnp.0b013e31826c9ebc. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD and Pandya DN (1989) ‘Anatomical investigation of projections to the basis pontis from posterior parietal association cortices in rhesus monkey’, The Journal of comparative neurology, 289(1), pp. 53–73. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD and Pandya DN (2006) ‘Superior longitudinal fasciculus and arcuate fasciculus’, Fiber Pathways of the brain. Oxford university press Oxford, pp. 393–408. [Google Scholar]
- Shivamurthy VKN et al. (2015) ‘Brain FDG PET and the diagnosis of dementia’, AJR. American journal of roentgenology, 204(1), pp. W76–85. [DOI] [PubMed] [Google Scholar]
- Siegel AM (2003) The parietal lobes. [Google Scholar]
- Suzuki K et al. (1998) ‘Pure topographical disorientation related to dysfunction of the viewpoint dependent visual system’, Cortex; a journal devoted to the study of the nervous system and behavior, 34(4), pp. 589–599. [DOI] [PubMed] [Google Scholar]
- Swanson LW (2015) ‘Response to Foley’s review of Swanson’s Neuroanatomical Terminology (2014)’, Journal of the history of the neurosciences, pp. 199–202. [DOI] [PubMed] [Google Scholar]
- Talairach J and Bancaud J (1966). Lesion, “Irritative” Zone and Epileptogenic Focus. Confin Neurol 27:91–94 [DOI] [PubMed] [Google Scholar]
- Thier P and Andersen RA (1998) ‘Electrical microstimulation distinguishes distinct saccade-related areas in the posterior parietal cortex’, Journal of neurophysiology, 80(4), pp. 1713–1735. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Wang G-J and Volkow ND (2013) ‘Energetic cost of brain functional connectivity’, Proceedings of the National Academy of Sciences of the United States of America, 110(33), pp. 13642–13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufenkjian K and Lüders HO (2012) ‘Seizure semiology: its value and limitations in localizing the epileptogenic zone’, Journal of clinical neurology , 8(4), pp. 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeoka S et al. (2007) ‘Bilateral symmetric tonic posturing suggesting propagation to the supplementary motor area in a patient with precuneate cortical dysplasia’, Epileptic disorders: international epilepsy journal with videotape, 9(4), pp. 443–448. [DOI] [PubMed] [Google Scholar]
- Utevsky AV, Smith DV and Huettel SA (2014) ‘Precuneus is a functional core of the default-mode network’, The Journal of neuroscience: the official journal of the Society for Neuroscience, 34(3), pp.932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J et al. (2019) ‘Corresponding anatomical and coactivation architecture of the human precuneus showing similar connectivity patterns with macaques’, NeuroImage, 200, pp. 562–574. [DOI] [PubMed] [Google Scholar]
- Wiest G et al. (2004) ‘Vestibular processing in human paramedian precuneus as shown by electrical cortical stimulation’, Neurology, 62(3), pp. 473–475. [DOI] [PubMed] [Google Scholar]
- Yassi N et al. (2015) ‘The association between lesion location and functional outcome after ischemic stroke’, International journal of stroke: official journal of the International Stroke Society, 10(8), pp. 1270–1276. [DOI] [PubMed] [Google Scholar]
- Yeterian EH et al. (2012) ‘The cortical connectivity of the prefrontal cortex in the monkey brain’, Cortex; a journal devoted to the study of the nervous system and behavior, 48(1), pp. 58–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeterian EH and Pandya DN (2010) ‘Fiber pathways and cortical connections of preoccipital areas in rhesus monkeys’, The Journal of comparative neurology, 518(18), pp. 3725–3751. [DOI] [PubMed] [Google Scholar]
- Zhang S and Li C-SR (2012) ‘Functional connectivity mapping of the human precuneus by resting state fMRI’, NeuroImage, pp. 3548–3562. doi: 10.1016/j.neuroimage.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Dongyang, et al. “Noninvasive Functional and Structural Connectivity Mapping of the Human Thalamocortical System.” Cerebral Cortex, vol. 20, no. 5, 3 Sept. 2009, pp. 1187–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]


