Abstract
We investigated the involvement of the recently described staphylococcal enterotoxins G and I in toxic shock syndrome. We reexamined Staphylococcus aureus strains isolated from patients with menstrual and nonmenstrual toxic shock syndrome (nine cases) or staphylococcal scarlet fever (three cases). These strains were selected because they produced none of the toxins known to be involved in these syndromes (toxic shock syndrome toxin 1 and enterotoxins A, B, C, and D), enterotoxin E or H, or exfoliative toxin A or B, despite the fact that superantigenic toxins were detected in a CD69-specific flow cytometry assay measuring T-cell activation. Sets of primers specific to the enterotoxin G and I genes (seg and sei, respectively) were designed and used for PCR amplification. All of the strains were positive for seg and sei. Sequence analysis confirmed that the PCR products, corresponded to the target genes. We suggest that staphylococcal enterotoxins G and I may be capable of causing human staphylococcal toxic shock syndrome and staphylococcal scarlet fever.
Toxic shock syndrome (TSS) is a life-threatening multisystem disorder caused by strains of Staphylococcus aureus. It is characterized by rapid onset of fever, arterial hypotension, scarlatiniform rash, and multiorgan failure (4). Originally described for children by Todd and Fishaut in 1978 (32), TSS has been extensively studied over the past 18 years, since the occurrence of major outbreaks associated with menstruation and the use of a newly introduced superabsorbant brand of tampon (3, 4). These tampons were withdrawn from the market, and most cases of TSS now occur in settings other than menstruation and among individuals of both sexes and all ages. Nonmenstrual TSS is usually secondary to S. aureus infection or to skin or mucosal trauma with S. aureus colonization (4).
S. aureus TSS toxin 1 (TSST-1) was the first toxin shown to be involved in TSS, in both menstrual and nonmenstrual cases (2, 30). Staphylococcal enterotoxins A to D and H (SEA to SED and SEH) also appear to have caused some cases of nonmenstrual TSS (8, 14, 16, 21, 28, 29). TSST-1 and SEA to SED have been linked to other staphylococcal syndromes such as staphylococcal scarlet fever (SSF) and recalcitrant erythematous desquamating disorder (REDD), both of which were suggested to be variants of TSS on the basis of toxin production and certain clinical similarities (6, 18). Another staphylococcal enterotoxin (SEE) was isolated from chicken and food specimens but has not been associated with TSS (7). All of these toxins exhibit superantigen activity, stimulating polyclonal T-cell proliferation through coligation between major histocompatibility complex class II molecules on antigen-presenting cells and the variable portion of the T-cell antigen receptor β chain (22). This superantigen activity can be detected with mitogenic assays (with mouse, rabbit, or human leukocytes) or in a CD69-specific flow cytometric assay of T-cell activation (15, 17).
In a French epidemiological survey of S. aureus isolates from patients with TSS, SSF, and REDD, several strains produced none of the known superantigenic toxins (TSST-1, SEA to SEE, and SEH), but superantigen activity was detected in culture supernatants in a CD69-specific flow cytometric assay, pointing to unknown superantigenic toxins (16). Recently, two new staphylococcal enterotoxins, SEG and SEI, have been isolated from S. aureus strains collected from human nares but have not been linked to TSS (24). In this study we used PCR to detect the SEG and SEI genes (seg and sei, respectively) in CD69-positive, SEA- to SEE-, SEH-, and TSST-1-negative strains isolated from patients with a diagnosis of TSS or SSF. seg and sei were detected in all of these strains, suggesting a clinical importance for these new toxins.
MATERIALS AND METHODS
Patients.
The 12 patients included in this study corresponded to nine cases of TSS (including two menstrual case) and three cases of SSF. They were all colonized or infected by S. aureus strains that did not produce the usual superantigenic toxins associated with TSS. They were selected from among 170 cases of TSS, 5 cases of REDD, and 105 cases of SSF reported to the Centre National de Référence des Toxémies à Staphylocoques (Lyon, France) between 1 January 1985 and 31 January 1999 from hospitals throughout France. Cases were first identified by chart review if the patient was from the Lyon area or otherwise from accompanying notes sent to the staphylococcal reference center. Cases met the definition of TSS, REDD, or SSF (4, 6, 16). The patients were epidemiologically unrelated.
Strains.
S. aureus strains from patients with TSS or SSF were cultured from sites including the genital tract, blood, skin, throat, and soft tissues. Strains were identified as S. aureus by their ability to coagulate citrated rabbit plasma (bioMérieux, Marcy-l’Etoile, France) and to produce a clumping factor (Staphyslide test; bioMérieux). Isolates were typed by using phage and serotyping techniques (33).
Toxins.
Superantigen activity in culture supernatants was detected by measuring T-cell activation in a CD3- and CD69-specific flow cytometry assay (15). Since fewer than 1% of unstimulated CD3+ lymphocytes spontaneously expressed CD69, T cells were considered to be activated when more than 2% expressed CD69 (15).
Sequences specific for sea to see, seg to sei, eta, etb, and tst, encoding SEA to SEE, SEG to SEI, exfoliative toxin A (ETA), ETB, and TSST-1, respectively, were detected by PCR. Genomic DNA was extracted from staphylococcal cultures and used as a template for amplification with the primers described in Table 1 (Eurogentec, Seraing, Belgium). The thermal conditions were as follows: denaturation for 1 min at 94°C, annealing for 1 min at 55°C, and extension for 1 min at 72°C. Amplification of gyrA (11) was used as a control to confirm the quality of each DNA extract and the absence of PCR inhibitors. All PCR products were analyzed by electrophoresis through 1% agarose gels (Sigma, Saint Quentin Fallavier, France). The following S. aureus strains were used to control the specificity of PCR amplification: RN-450 (negative control), A970237 (negative control), A990204 (negative control), FDA-S6 (ATCC 13566) (sea+ seb+), FRI-137 (ATCC 19095) (sec+), FRI-1151m (sed+), FRI-326 (ATCC 27664) (see+), FRI-569 (ATCC 51811) (seh+), FRI-1169 (tst+), TC-7 (eta+), and TC-146 (etb+) (1, 7, 11, 13, 15, 20, 26, 27, 31). Since no control strains for SEG and SEI were available, the specificity of seg and sei amplification was assessed by DNA sequencing of selected PCR products (Genome Express, Grenoble, France). To rule out the possibility of false-negative PCRs due to minor variations in the DNA sequences, Southern blotting of selected strains was performed as follows: total DNA was digested with HindIII (Boehringer Mannheim, Meylan, France), separated on a 1% agarose gel, vacuum transferred to positively charged nylon membranes (Boehringer Mannheim), and cross-linked by exposure to UV light. The seg and sei PCR products were labelled with digoxigenin (DIG) by using a DIG DNA Labelling and Detection Kit (Boehringer Mannheim) for use as probes. Hybridization and washing steps were carried out at 68°C in standard buffer solutions (Boehringer Mannheim). Hybridizing bands were detected with anti-DIG–alkaline phosphatase conjugate and the chemiluminescent substrate CSPD, using the DIG Luminescent Detection kit in accordance with the instructions of the supplier (Boehringer Mannheim). Lumi-Film (Boehringer Mannheim) was subsequently exposed to the membranes for 1 h. The sizes of the hybridizing bands were estimated by using a 1-kb DNA ladder (Gibco BRL, Cergy Pontoise, France).
TABLE 1.
Base sequences of the staphylococcal toxin-specific oligonucleotide primers and predicted sizes of amplified products
| Genea | GenBank accession no. | Primer | Oligonucleotide sequence (5′→3′) | Size of amplified productb (bp) | Reference |
|---|---|---|---|---|---|
| sea | M18970 | SEA-1 | TTGGAAACGGTTAAAACGAA | 120 | 12 |
| SEA-2 | GAACCTTCCCATCAAAAACA | ||||
| seb | M11118 | SEB-1 | TCGCATCAAACTGACAAACG | 478 | 12 |
| SEB-2 | GCAGGTACTCTATAAGTGCC | ||||
| sec | X05815 | SEC-1 | GCATAAAAGCTAGGAATTT | 257 | 12 |
| SEC-2 | AAATCGGATTAACATTATCC | ||||
| sed | M28521 | SED-1 | CTAGTTTGGTAATATCTCCT | 317 | 12 |
| SED-2 | TAATGCTATATCTTATAGGG | ||||
| see | M21319 | SEE-1 | CAAAGAAATGCTTTAAGCAATCTTAGGCCAC | 482 | 12 |
| SEE-2 | CTTACCGCCAAAGCTG | ||||
| seg | AF064773 | SEG-1 | AATTATGTGAATGCTCAACCCGATC | 642 | This study |
| SEG-2 | AAACTTATATGGAACAAAAGGTACTAGTTC | ||||
| seh | U11702 | SEH-1 | CAATCACATCATATGCGAAAGCAG | 375 | This study |
| SEH-2 | CATCTACCCAAACATTAGCACC | ||||
| sei | AF064774 | SEI-1 | CTCAAGGTGATATTGGTGTAGG | 576 | This study |
| SEI-2 | AAAAAACTTACAGGCAGTCCATCTC | ||||
| tst | J02615 | TSST-1 | ATGGCAGCATCAGCTTGATA | 350 | 12 |
| TSST-2 | TTTCCAATAACCACCCGTTT | ||||
| eta | M17347 | ETA-1 | CTAGTGCATTTGTTATTCAA | 119 | 12 |
| ETA-2 | TGCATTGACACCATAGTACT | ||||
| etb | M17348 | ETB-1 | ACGGCTATATACATTCAATT | 200 | 12 |
| ETB-2 | TCCATCGATAATATACCTAA |
Genes encoding SEA to SEE, SEG to SEI, TSST-1, ETA, and ETB.
Size of PCR product derived from published GenBank sequences.
RESULTS
The 12 S. aureus strains induced CD69 expression by over 2% of CD3+ lymphocytes (Table 2), in a manner similar to that observed with supernatants from control strains producing known superantigenic toxins (Table 3). In contrast, the toxin-negative control strain (RN450) induced CD69 expression in only 0.4% ± 0.2% of CD3+ lymphocytes, as observed with strains that do not produce superantigenic toxins (15).
TABLE 2.
Toxin production by S. aureus isolates from patients with TSS or SSF that do not produce TSST-1, SEA to SEE, SEH, ETA, or ETB
| Case | Bacterial characteristics
|
Patient characteristics
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| S. aureus strain | S. aureus-positive sample(s) | CD69 expression on CD3+ T cellsa (%) | Toxins detectedb | Serotype | Phage typing pattern | Age (yr)/sex | Clinical manifestation(s) | Outcome | |
| 1 | A850375 | Blood | 4.4 ± 1.6 | SEG, SEI | h2/m/1 | Untypeable | 77/M | Arteriovenous shunt abscess, TSS | Dead |
| 2 | A860376 | Abscess | 3.2 ± 0.6 | SEG, SEI | c1/o/1 | Group V: 94/96 | 17/M | Thigh abscess, TSS | Alive |
| 3 | A890326 | Vagina, placenta, and newborn blood | 6.1 ± 0.3 | SEG, SEI | c1/i1i2/o | Group III: 84 | 27/F | Puerperal SSF | Mother alive, newborn dead |
| 4 | A900322 | Tampon, vagina | 7.4 ± 2.9 | SEG, SEI | c1/o | Group I/III: 29/52/52A/79/80/47/53/54/83A/84/85 | 18/F | Menstrual TSS | Alive |
| 5 | A900422 | Pharynx | 5.2 ± 2.0 | SEG, SEI | e/h2/o/262-3) | Untypeable | 19/F | Pharyngitis, SSF | Alive |
| 6 | A910472 | Blood, bronchoalveolar lavage, cerebrospinal fluid | 5.9 ± 0.9 | SEG, SEI | c1/o | Group III: 53/54/83A/85 | 23/M | Bacteriemia, pneumonia, meningitis, and TSS in an intravenous-drug user | Alive |
| 7 | A930316 | Urine | 3.1 ± 0.8 | SEG, SEI | c1/l | Untypeable | 15/M | Postendoscopy urinary tract infection, TSS | Alive |
| 8 | A950260 | Pus | 2.9 ± 1.1 | SEG, SEI | c1 | Group III/D: 53/77/83A/84/85/87 | 9/F | Infection of finger pulp, TSS | Alive |
| 9 | A980044 | Sinus, blood | 8.6 ± 2.6 | SEG, SEI | NDc | ND | 39/F | Sinusitis, TSS | Alive |
| 10 | A980114 | Wound | 8.8 ± 0.5 | SEG, SEI | ND | ND | 35/M | Postoperative spondylitis, TSS | Alive |
| 11 | A980483 | Pharynx | 2.7 ± 0.7 | SEG, SEI | ND | ND | 2/F | Pharyngitidis, SSF | Alive |
| 12 | A990055 | Tampon, vagina, blood | 5.3 ± 1.2 | SEG, SEI | ND | ND | 44/F | Menstrual TSS | Dead |
The percentage of CD69-positive cells was determined after electronic gating of the CD3+ population. All experiments used separate blood samples from one donor. Results are shown as means ± standard deviations.
All toxins were detected by PCR assay of the corresponding genes.
ND, not done.
TABLE 3.
Toxin production by S. aureus control strains
| Control strain (reference) | Reference toxin production | CD69 expression on CD3+ T cells (%)a | Toxin production detected in this studyb
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SEA | SEB | SEC | SED | SEE | SEG | SEH | SEI | TST | ETA | ETB | |||
| RN-450 (26) | None | 0.5 ± 0.2 | − | − | − | − | − | − | − | − | − | − | − |
| FDA-S6 (20) | SEA, SEB | 14.7 ± 2.2 | + | + | − | − | − | − | − | − | − | − | − |
| FRI-137 (27) | SEC | 4.9 ± 1.5 | − | + | − | − | + | + | + | − | − | − | |
| FRI-1151m (13) | SED | 5.0 ± 1.8 | − | − | − | + | − | − | − | − | − | − | − |
| FRI-326 (7) | SEE | 9.8 ± 1.7 | − | − | − | − | + | − | − | − | − | − | − |
| FRI-569 (31) | SEH | 2.3 ± 0.2 | − | − | − | − | − | − | + | − | − | − | − |
| FRI-1169 (5) | TSST-1 | 7.3 ± 1.2 | − | − | − | − | − | − | − | − | + | − | − |
| TC-7 (1) | ETA | 10.5 ± 1.8 | − | − | − | − | − | + | − | + | − | + | − |
| TC-146 (1) | ETB | 5.7 ± 1.2 | − | − | − | − | − | + | − | + | − | − | + |
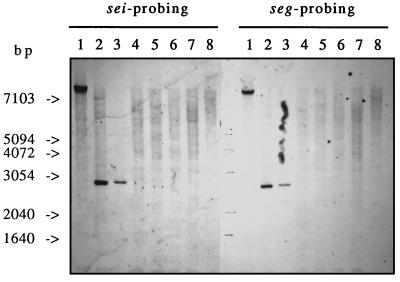
The 12 strains were examined for the presence of sea to see, seg to sei, eta, and etb by PCR amplification, and were all positive for seg and sei only (Table 2), while RN-450 was negative for all of these genes. Several of the control strains harboring sea to see, seh, tst, eta, or etb were also positive for both seg and sei (Table 3). seg and sei amplicons from 3 of the 12 clinical strains were sequenced, and the sequences were 100% identical to the published sequences (GenBank accession no. AF064773 and AF064774, respectively). Since a change in only a few bases could cause false-negative results in seg or sei PCRs, DNAs from three PCR-positive strains and five PCR-negative strains were analyzed by Southern blotting with seg and sei probes. Only strains which were positive for seg and sei by PCR hybridized to both DNA probes (Fig. 1), thus confirming the PCR results. The sizes of the hybridizing fragments were identical for both probes but differed between strains, from ≈2.9 kb (strains A900322 and TC7) to >7.1 kb (strain A980483) (Fig. 1).
FIG. 1.
Southern blot hybridization of DNAs of S. aureus strains with sei and seg probes. Total DNA was digested with HindIII, separated by agarose gel electrophoresis, transferred to positively charged nylon membranes, and probed with the indicated DIG-labelled probes. Lanes contain DNAs of three S. aureus strains found to be PCR positive for seg and sei (lanes 1, A980483; lanes 2, TC-7; and lanes 3, A900322) and five S. aureus strains found to be PCR negative for seg and sei (lanes 4, FRI-1169; lanes 5, FRI-569; lanes 6, RN-450; lanes 7, A970237; and lanes 8, A990204).
Since all 12 strains were positive for both genes by PCR and the sizes of the hybridizing fragments were identical for both probes, the possibility that the seg and sei loci were adjacent to each other was investigated by attempting to coamplify the two genes with all combinations of seg- and sei-specific primers. Only the combination of primer SEI-1 with primer SEG-2 produced an amplicon, of about 3.2 kb, while the other primer combinations gave negative results. Partial sequence analysis showed that the 3.2-kb amplicon contained portions of both seg and sei, in tandem orientation with a 1.9-kb intergenic segment.
Eight of the clinical strains harboring seg and sei were analyzed by phage typing and serotyping. They were not clonal, as they had distinct phage types. Four strains belonged to phage group III, one belonged to group V, and three were untypeable (Table 2). Serotyping confirmed the absence of clonality.
DISCUSSION
Among the staphylococcal superantigenic toxins, only TSST-1, SEA, SEB, SEC, and SED have been linked to TSS or SSF (8, 18, 21). This study shows that two additional staphylococcal enterotoxins, SEG and SEI, are likely associated with human TSS and SSF. We selected strains of S. aureus isolated from patients with TSS or SSF and which did not produce TSST-1, SEA to SEE, SEH, ETA, or ETB. Using PCR amplification with primer sets designed to be specific for seg or sei, we detected both genes in all 12 strains and also in several control strains (Tables 2 and 3). The specificity of seg and sei amplification was confirmed by sequence analysis of PCR products and Southern blotting (Fig. 1). SEG and SEI produced by these strains were probably responsible for the T-cell activation detected in a CD69-specific flow cytometry assay. It is also conceivable that the SEG and SEI produced by these strains caused the clinical manifestations of TSS or SSF.
As seg and sei were initially cloned from two different strains (FRI-572 and FRI-445, respectively) (24), we were surprised that both seg and sei were detected in all 12 clinical strains and also in several reference strains (Table 3). The positive PCR amplification obtained with the SEI-1 and SEG-2 primers in our study indicates that sei and seg are in tandem orientation and are separated by 1.9 kb of intergenic DNA. Sequencing of this intergenic region is under way to determine whether it contains additional open reading frames, as suggested by the reported observation that strain FRI-445 contains part of an enterotoxin-like gene upstream of sei (24). The link between two staphylococcal superantigenic toxins such as seg and sei is not uncommon; it has been described for plasmid pIB485, which contains both sed and sej (34), and for pathogenicity islands containing both tst and an enterotoxin-like gene (19). Phage typing and serotyping ruled out a clonal origin of our S. aureus clinical strains harboring seg and sei and responsible for TSS, contrasting with the clonal origin of strains isolated from patients with menstrual TSS that produced TSST-1 (25).
Three of the strains that produced SEG and SEI were associated with two cases of menstrual TSS and a case of puerperal SSF, respectively. Previous findings suggested that the vast majority of cases of menstrual TSS were due to TSST-1-producing strains (29). However, some vaginal isolates from women with menstrual TSS did not produce TSST-1, suggesting that other staphylococcal toxins might be responsible for the clinical manifestations (4, 9, 10, 21); indeed, cases of menstrual TSS related to strains producing only SEA to SED have been described (8, 23). Our data suggest that S. aureus strains producing SEG and SEI but not TSST-1 can also cause menstrual TSS, although this needs to be confirmed by experimental and epidemiological studies.
In conclusion, S. aureus strains that produce both SEG and SEI may be associated with SSF and TSS (including menstrual cases), and the SEG and SEI determinants are close to each other on the S. aureus chromosome. The PCR amplification method used in this study is an efficient way of identifying strains harboring seg and sei.
ACKNOWLEDGMENTS
We are grateful to N. Viollant, C. Courtier, C. Gardon, and Y. Benito for technical assistance and to A. C. Wong for the gift of strain FRI-569.
REFERENCES
- 1.Arbuthnott J P, Billcliffe B. Qualitative and quantitative methods for detecting staphylococcal epidermolytic toxin. J Med Microbiol. 1976;9:191–201. doi: 10.1099/00222615-9-2-191. [DOI] [PubMed] [Google Scholar]
- 2.Bergdoll M S, Crass B A, Reiser R F, Robbins R N, Davis J P. A new staphylococcal enterotoxin, enterotoxin F, associated with toxic-shock-syndrome Staphylococcus aureus isolates. Lancet. 1981;i:1017–1021. doi: 10.1016/s0140-6736(81)92186-3. [DOI] [PubMed] [Google Scholar]
- 3.Berkley S F, Hightower A W, Broome C V, Reingold A L. The relationship of tampon characteristics to menstrual toxic shock syndrome. JAMA. 1987;258:917–920. [PubMed] [Google Scholar]
- 4.Chesney P J. Toxic shock syndrome. In: Crossley K B, Archer G L, editors. The staphylococci in human disease. New York, N.Y: Churchill Livingstone; 1997. pp. 509–525. [Google Scholar]
- 5.Chu M C, Kreiswirth B N, Pattee P A, Novick R P, Melish M E, James J F. Association of toxic shock toxin-1 determinant with a heterologous insertion at multiple loci in the Staphylococcus aureus chromosome. Infect Immun. 1988;56:2702–2708. doi: 10.1128/iai.56.10.2702-2708.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cone L A, Woodard D R, Byrd R G, Schulz K, Schlievert P M. A recalcitrant, erythematous, desquamating disorder associated with toxin-producing staphylococci in patient with AIDS. J Infect Dis. 1992;165:638–643. doi: 10.1093/infdis/165.4.638. [DOI] [PubMed] [Google Scholar]
- 7.Couch J L, Soltis M T, Betley M J. Cloning and nucleotide sequence of the type E staphylococcal enterotoxin gene. J Bacteriol. 1988;170:2954–2960. doi: 10.1128/jb.170.7.2954-2960.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crass B A, Bergdoll M S. Toxin involvement in toxic shock syndrome. J Infect Dis. 1986;153:918–926. doi: 10.1093/infdis/153.5.918. [DOI] [PubMed] [Google Scholar]
- 9.De Saxe M J, Hawtin P, Wieneke A A. Toxic shock syndrome in Britain: epidemiology and microbiology. Postgrad Med J. 1985;61(Suppl.):5–21. [PubMed] [Google Scholar]
- 10.Gaventa S, Reingold A L, Hightower A W, Broome C V, Schwartz B, Hoppe C, Harwell J, Lefkowitz L K, Makintubee S, Cundiff D R, Sitze S the Toxic-Shock Syndrome Study Group. Active surveillance for toxic shock syndrome in the United States, 1986. Rev Infect Dis. 1989;11:S28–34. doi: 10.1093/clinids/11.supplement_1.s28. [DOI] [PubMed] [Google Scholar]
- 11.Goswitz J J, Willard K E, Fasching C E, Peterson L R. Detection of gyrA gene mutations associated with ciprofloxacin resistance in methicillin-resistant Staphylococcus aureus: analysis by polymerase chain reaction and automated direct DNA sequencing. Antimicrob Agents Chemother. 1992;36:1166–1169. doi: 10.1128/aac.36.5.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson W M, Tyler S D, Ewan E P, Asthon F E, Pollard D R, Rozee K R. Detection of genes for enterotoxins, exfoliative toxins, and toxic shock syndrome toxin 1 in Staphylococcus aureus by the polymerase chain reaction. J Clin Microbiol. 1991;29:426–430. doi: 10.1128/jcm.29.3.426-430.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kokan N P, Bergdoll M S. Detection of low-enterotoxin-producing Staphylococcus strains. Appl Environ Microbiol. 1987;53:2675–2676. doi: 10.1128/aem.53.11.2675-2676.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee V T, Chang A H, Chow A W. Detection of staphylococcal enterotoxin B among toxic shock syndrome (TSS)- and non-TSS-associated Staphylococcus aureus isolates. J Infect Dis. 1992;166:911–915. doi: 10.1093/infdis/166.4.911. [DOI] [PubMed] [Google Scholar]
- 15.Lina G, Cozon G, Ferrandiz J, Greenland T, Vandenesch F, Etienne J. Detection of staphylococcal superantigenic toxins by a CD69-specific cytofluorimetric assay measuring T-cell activation. J Clin Microbiol. 1998;36:1042–1045. doi: 10.1128/jcm.36.4.1042-1045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lina G, Etienne J, Vandenesch F. Les syndromes toxiques staphylococciques en France de 1994 à 1997. Bull Epidémiol Hebd. 1998;17:69–70. [Google Scholar]
- 17.Lina G, Fleer A, Etienne J, Greenland T B, Vandenesch F. Coagulase-negative staphylococci isolated from two cases of toxic shock syndrome lack superantigenic activity, but induce cytokine production. FEMS Immunol Med Microbiol. 1996;13:81–86. doi: 10.1111/j.1574-695X.1996.tb00219.x. [DOI] [PubMed] [Google Scholar]
- 18.Lina G, Gillet Y, Vandenesch F, Jones M E, Floret D, Etienne J. Toxin involvement in staphylococcal scalded skin syndrome. Clin Infect Dis. 1997;25:1369–1373. doi: 10.1086/516129. [DOI] [PubMed] [Google Scholar]
- 19.Lindsay J A, Ruzin A, Ross H F, Kurepina N, Novick R P. The gene for toxic shock toxin is carried by a family of mobile pathogenicity islands in Staphylococcus aureus. Mol Microbiol. 1998;29:527–543. doi: 10.1046/j.1365-2958.1998.00947.x. [DOI] [PubMed] [Google Scholar]
- 20.Mahmood R, Khan S A. Role of upstream sequences in the expression of the staphylococcal enterotoxin B gene. J Biol Chem. 1990;265:4652–4656. [PubMed] [Google Scholar]
- 21.Marples R R, Wieneke A A. Enterotoxins and toxic-shock syndrome toxin-1 in non-enteric staphylococcal disease. Epidemiol Infect. 1993;110:477–488. doi: 10.1017/s0950268800050901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 23.Melconian A K, Fleurette J, Brun Y. Studies on staphylococci from toxic shock syndrome in France, 1981–1983. J Hyg Camb. 1985;94:23–29. doi: 10.1017/s002217240006109x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munson S H, Tremaine M T, Betley M J, Welch R A. Identification and characterization of staphylococcal enterotoxin types G and I from Staphylococcus aureus. Infect Immun. 1998;66:3337–3348. doi: 10.1128/iai.66.7.3337-3348.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Musser J M, Schlievert P M, Chow A W, Ewan P, Kreiswirth B N, Rosdahl V T, Naidu A S, Witte W, Selander R K. A single clone of Staphylococcus aureus causes the majority of cases of toxic shock syndrome. Proc Natl Acad Sci USA. 1990;87:225–229. doi: 10.1073/pnas.87.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Novick R P, Brodsky R. Studies on plasmid replication. I. Plasmid incompatibility and establishment in Staphylococcus aureus. J Mol Biol. 1972;68:285–302. doi: 10.1016/0022-2836(72)90214-8. [DOI] [PubMed] [Google Scholar]
- 27.Regassa L B, Couch J L, Betley M J. Steady-state staphylococcal enterotoxin type C mRNA is affected by a product of the accessory gene regulator (agr) and by glucose. Infect Immun. 1991;59:955–962. doi: 10.1128/iai.59.3.955-962.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren K, Bannan J D, Pancholi V, Cheung A L, Robbins J C, Fischetti V A, Zabriskie J B. Characterization and biological properties of a new staphylococcal exotoxin. J Exp Med. 1994;180:1675–1683. doi: 10.1084/jem.180.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlievert P M. Staphylococcal enterotoxin B and toxic-shock syndrome toxin-1 are significantly associated with non-menstrual TSS. Lancet. 1986;i:1149–1150. doi: 10.1016/s0140-6736(86)91859-3. [DOI] [PubMed] [Google Scholar]
- 30.Schlievert P M, Shands K N, Dan B B, Schmid G P, Nishimura R D. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J Infect Dis. 1981;143:509–516. doi: 10.1093/infdis/143.4.509. [DOI] [PubMed] [Google Scholar]
- 31.Su Y C, Wong A C. Identification and purification of a new staphylococcal enterotoxin, H. Appl Environ Microbiol. 1995;61:1438–1443. doi: 10.1128/aem.61.4.1438-1443.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Todd J, Fishaut M. Toxic-shock syndrome associated with phage-group-I staphylococci. Lancet. 1978;ii:1116–1118. doi: 10.1016/s0140-6736(78)92274-2. [DOI] [PubMed] [Google Scholar]
- 33.Williams R E O, Rippo J E. Bacteriophage typing of Staphylococcus aureus. J Hyg Camb. 1952;50:320–353. doi: 10.1017/s002217240001963x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang S, Iandolo J J, Stewart G C. The enterotoxin D plasmid of Staphylococcus aureus encodes a second enterotoxin determinant (sej) FEMS Microbiol Lett. 1998;168:227–233. doi: 10.1111/j.1574-6968.1998.tb13278.x. [DOI] [PubMed] [Google Scholar]