Abstract
Despite reports on poor survival outcomes after hepatectomy for colorectal liver metastases (CRLM) with BRAF V600E mutation (mBRAF) exist, the role of mBRAF testing for technically resectable cases remains unclear. A single‐center retrospective study was performed to investigate the survival outcomes of patients who underwent upfront hepatectomy for solitary resectable CRLM with mBRAF between January 2005 and December 2017 and to compare them with those of unresectable cases with mBRAF. Of 172 patients who underwent initial hepatectomy for solitary resectable CRLM, mBRAF, RAS mutations (mRAS), and wild‐type RAS/BRAF (wtRAS/BRAF) were observed in 5 (2.9%), 73 (42.4%), and 93 (54.7%) patients, respectively. With a median follow‐up period of 72.8 months, mBRAF was associated with a significantly shorter OS (median, 14.4 months) than wtRAS/BRAF (median, not reached [NR]) (hazard ratio [HR], 27.6; p < 0.001) and mRAS (median, NR) (HR, 9.9; p < 0.001), and mBRAF had the highest HR among all the indicators in the multivariable analysis (HR, 17.0; p < 0.001). The median OS after upfront hepatectomy for CRLM with mBRAF was identical to that of 28 unresectable CRLM with mBRAF that were treated with systemic chemotherapy (median, 17.2 months) (HR, 0.78; p = 0.65). When technically resectable CRLM are complicated with mBRAF, its survival outcome becomes as poor as unresectable cases; therefore, those with mBRAF should be considered as oncologically unresectable. Patients with CRLM should undergo pre‐treatment mBRAF testing regardless of technical resectability.
Clinical trial registration number: UMIN000034557.
Keywords: BRAF V600E, colorectal liver metastases, hepatectomy, resectable, surgery
A single‐center retrospective study was performed to investigate the survival outcomes of patients who underwent upfront hepatectomy for solitary resectable CRLM with BRAF V600E mutation between January 2005 and December 2017 and to compare them with those of unresectable cases with the mutation. When technically resectable CRLM are complicated with BRAF V600E, its survival outcome becomes as poor as unresectable cases; therefore, those with the mutation should be considered as oncologically unresectable.

1. INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer‐related deaths worldwide. 1 The liver is the most common site of metastatic CRC (mCRC), with approximately 30% of patients with CRC developing liver metastases over the course of their disease. 2 Since both the median recurrence‐free survival (RFS) and overall survival (OS) rates after hepatectomy for CRLM are high at approximately 20 and 60 months, respectively, 3 hepatectomy is generally recommended for curative intent. 4 , 5 However, even apparently resectable CRLM are a heterogeneous disease in which approximately 70%–80% of patients eventually experience recurrence. 6 Despite the definition of multiple CRLM ≥5 lesions as oncologically unresectable in the consensus guidelines by the European Society for Medical Oncology (ESMO), 5 additional biomarkers to define the oncological resectability remain warranted.
The RAS–RAF–MEK–ERK–MAP kinase pathway regulates cellular growth and is activated in many human cancers. 7 BRAF is one of the three RAF genes that code for serine/threonine kinases, and V600E is the most dominant type of its mutation in which disruption of the A‐loop and the P‐loop of the BRAF mutant protein directly phosphorylates downstream MEK activity and BRAF kinase activity increases up to 700‐fold of the wild type to stimulate rapid cell growth of the tumor. 8 In today's era of personalized precision medicine, the evaluation of RAS/BRAF V600E mutations at the time of diagnosis of unresectable mCRC has been strongly recommended in clinical practice guidelines due to their prognostic significance and their utility in guiding the selection of optimal chemotherapeutic regimens. 4 , 5 For resectable CRLM, RAS/BRAF mutations are associated with poor prognosis, with BRAF V600E mutation (mBRAF) as a poorer prognostic factor for OS. 9 , 10 , 11 , 12 , 13 When resectable CRLM were complicated with mBRAF, OS after hepatectomy for CRLM was reported to be 22–31 months, while that of unresectable mCRC treated with systemic chemotherapy was reported to be 19 months. 14 Although mBRAF is recognized as negatively associated with poor survival after hepatectomy, the role of mBRAF testing before hepatectomy remains unclear due to the absence of studies that directly compare the survival outcome of patients treated with hepatectomy with those treated with systemic chemotherapy.
The purpose of this study was to investigate the survival outcomes of surgical patients who underwent upfront hepatectomy for CRLM without neoadjuvant chemotherapy depending on RAS/BRAF mutational status, to compare the survival outcomes of patients with BRAF V600E‐mutant CRLM depending on technical resectability, and to clearly define the role of the mBRAF testing before hepatectomy for CRLM. This study focused solely on patients who had undergone hepatectomy for solitary resectable CRLM without neoadjuvant chemotherapy in order to clarify the impact of mBRAF on survival outcomes and to elucidate the natural history of patients with mBRAF after hepatectomy. We opted to focus on this clearly resectable cohort as other factors including the number of tumors, use of neoadjuvant chemotherapy, and initial resectability may act as confounding factors for the effect of mBRAF.
2. METHODS
2.1. Study design
Patients who were treated for CRLM between January 2005 and December 2017 at the National Cancer Center Hospital East, Kashiwa, Japan, were included in the study. In the first analysis, patients who underwent upfront hepatectomy without neoadjuvant chemotherapy for solitary resectable liver‐limited tumors at preoperative diagnosis were included. Inclusion criteria were those CRLM cases diagnosed preoperatively by ultrasound, computed tomography, and magnetic resonance imaging and histologically proven as metastatic adenocarcinoma of CRC origin. CRLM that required the resection of >70% of the entire liver, all three major hepatic veins, or bilateral branches of the hepatic artery/portal vein were considered technically unresectable and were excluded from the analysis. Since guidelines by the ESMO and the Japanese Society for Cancer of the Colon and Rectum have not yet established the efficacy and feasibility of neoadjuvant chemotherapy for technically resectable solitary CRLM, 5 , 15 neoadjuvant chemotherapy has not been offered to patients with solitary resectable CRLM in Japan. Survival outcomes including RFS, time to surgical failure (TSF), and OS after initial hepatectomy were analyzed in relation to RAS/BRAF V600E mutations and other clinicopathological factors.
In the second analysis, patients with mBRAF among those who received systemic chemotherapy for initially unresectable CRLM were included. OS after the start of the first‐line chemotherapy was analyzed and compared with that of patients who underwent upfront hepatectomy without neoadjuvant chemotherapy for BRAF V600E‐mutant solitary resectable liver‐limited tumors at preoperative diagnosis. Because of the retrospective nature of the study and the absence of invasive interventions, patients` personal written consents were waived. The study protocol was approved by the review board of the National Cancer Center Hospital East (approval number: 2018‐272) and was registered in the clinical trial registration system (UMIN000034557). This study received financial support from the National Cancer Center Research Development Fund (Research number: 30‐A‐8, Principal investigator: Shinichiro Takahashi).
2.2. Study outcomes
Standard clinicopathological data related to patient factors (age and sex), primary CRC factors (location, depth, lymph node metastases, pathology, and adjuvant chemotherapy after primary CRC resection), CRLM factors (timing, largest diameter, location, extrahepatic metastases status, preoperative chemotherapy for CRLM, adjuvant chemotherapy after hepatectomy, systemic chemotherapy regimens for unresectable tumors, CEA, carbohydrate antigen 19‐9 [CA19‐9], and residual tumor after hepatectomy), mismatch repair (MMR) protein status, microsatellite instability (MSI) status, and survival outcomes (RFS, TSF, and OS) along with information on tissue RAS/BRAF V600E mutations were retrospectively obtained. RFS was defined as the time from the date of hepatectomy to the date of the first radiological recurrence of the disease or to the date of death due to any cause. TSF was defined as either the time from the date of hepatectomy to the date of the radiological recurrence of unresectable disease or to the date of death due to any cause. OS was calculated either from the date of hepatectomy in the first analysis or the date of initiation of the first‐line chemotherapy in the second analysis until either the date of death due to any cause or the last follow‐up. Survival analysis was updated as of February 2021. As for the surveillance schedule, current Japanese guidelines recommend performing serial measurements of CEA and CA19‐9 levels and thoracoabdominal computed tomography scans every 3 and 6 months, respectively, after the resection of Stage I to III CRC. 15 The same schedule or an even more intensive schedule is recommended after the resection of Stage IV CRC or recurrent metastases. Generally, patients in this study were followed up every 3 months.
2.3. RAS and BRAF V600E mutational analysis
DNA was extracted from formalin‐fixed paraffin‐embedded tissue specimens which were selected from CRLM, primary CRC, or metastatic lung tumor in 159, 12, and 1 patients, respectively, and mutational analysis of RAS/BRAF V600E was centrally performed using a multiplex PCR‐based mutation detection kit (MEBGEN RASKET Kit; Medical and Biological Laboratories). RAS mutations (mRAS) were defined as mutations in codons 12, 13, 59, 61, 117, or 146 of KRAS or NRAS. Wild‐type RAS/BRAF (wtRAS/BRAF) was defined as those cases in which both RAS/BRAF V600E mutations were absent. In patients with BRAF V600E mutation, MMR protein status was determined by subjecting tumor samples to immunohistochemical analyses. RAS/BRAF V600E testing was retrospectively performed for this study and did not affect the clinical judgment of perioperative treatment in the first analysis. Meanwhile, it was performed as a part of clinical practice to decide chemotherapy regimens in the second analysis.
2.4. Statistical analyses
Statistical analyses of categorical variables were performed using the chi‐squared test or Fisher's exact test. Analyses of numerical variables were performed using the Mann–Whitney test. Survival curves were estimated and compared using the Kaplan–Meier method and the log‐rank test. Univariable and multivariable risk analyses were performed using Cox proportional hazards regression analysis. All p values are reported as two‐sided values, and p values < 0.05 were considered statistically significant. All statistical analyses were performed using the software EZR (ver. 1.37). 16
3. RESULTS
3.1. Clinicopathological characteristics
In the first analysis, 172 patients who underwent upfront hepatectomy for solitary resectable CRLM at preoperative diagnosis without neoadjuvant chemotherapy were eligible for this study (Figure 1). mBRAF, mRAS, and wtRAS/BRAF were identified in 5 (2.9%), 73 (42.4%), and 93 (54.7%) patients, respectively. Additionally, details of KRAS mutations were as follows: codon 12, 52 (30.2%); codon 13, 5 (2.9%); codon 59, 1 (0.6%); codon 117, 1 (0.6%); codon 146, 5 (2.9%); unspecified, 1 (0.6%). Also, details of NRAS mutations were codon 12 in 4 (2.3%) and codon 61 in 3 (1.7%). No preoperative baseline characteristics were significantly correlated with mutational status except for the pre‐hepatectomy CA19‐9 level, which was significantly higher in patients with mBRAF than in those with mRAS or wtRAS/BRAF (median, 137.2 IU/ml vs. 17.3 IU/ml vs. 14.2 IU/ml; p = 0.03; Table 1). Incidental peritoneal metastases were intraoperatively identified in one patient with mBRAF (20.0%), none among those with mRAS (0%), and two among those with wtRAS/BRAF (2.1%; p = 0.04). Meanwhile, incidental multiple CRLM were identified in four patients among those with mRAS (5.5%), one among those with wtRAS/BRAF (1.1%), and none among those with mBRAF (0%; p = 0.28). The extent of hepatectomy was not different among the three groups and all lesions including incidental ones were macroscopically completely resected.
FIGURE 1.
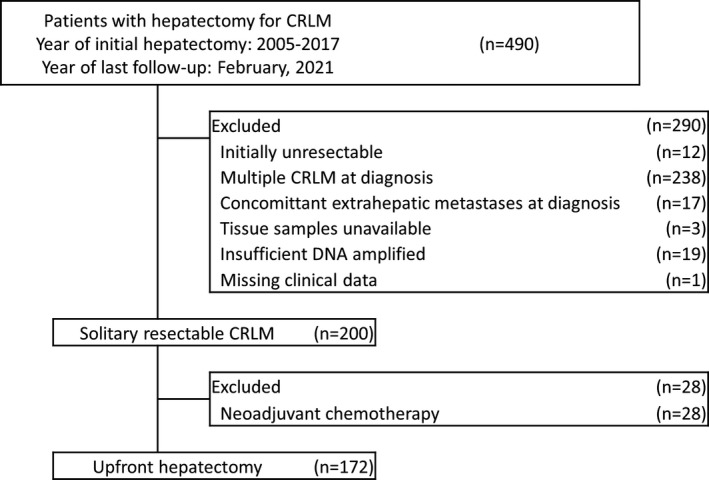
Consort diagram of eligible patients
TABLE 1.
Baseline characteristics
| Factor | Group | Genomic mutational status | |||
|---|---|---|---|---|---|
| Wild‐type RAS/BRAF | RAS mutations | BRAF V600E mutation | |||
| n = 94 | n = 73 | n = 5 | p value | ||
| Patient factor | |||||
| Sex, No. (%) | Female | 29 (30.9) | 34 (46.6) | 2 (40.0) | 0.12 |
| Male | 65 (69.1) | 39 (53.4) | 3 (60.0) | ||
| Age at hepatectomy, median [range] | Years | 67 [32, 84] | 67 [27, 87] | 71 [43, 76] | 0.64 |
| Primary colorectal tumor factor | |||||
| Location, No. (%) | Right‐sided | 13 (13.8) | 18 (24.7) | 2 (40.0) | 0.10 |
| Left‐sided | 81 (86.2) | 55 (75.3) | 3 (60.0) | ||
| Depth of invasion, No. (%) | T1–3 | 73 (77.7) | 55 (77.5) | 4 (80.0) | 0.99 |
| T4 | 21 (22.3) | 16 (22.5) | 1 (20.0) | ||
| Lymph node metastases, No. (%) | Absent | 44 (46.8) | 28 (38.9) | 0 (0.0) | 0.09 |
| Present | 50 (53.2) | 44 (61.1) | 5 (100.0) | ||
| Pathology of the primary tumor, No. (%) | Well diff. adenocarcinoma | 26 (27.7) | 19 (26.0) | 1 (20.0) | 0.92 |
| Others | 68 (72.3) | 54 (74.0) | 4 (80.0) | ||
| Adjuvant chemotherapy after original tumor resection, No. (%) | No | 21 (58.3) | 18 (62.1) | 2 (100.0) | 0.50 |
| Yes | 15 (41.7) | 11 (37.9) | 0 (0.0) | ||
| CRLM factors | |||||
| Timing of CRLM, No. (%) | Synchronous | 33 (35.1) | 25 (34.2) | 2 (40.0) | 0.50 |
| Early metachronous <1 year | 27 (28.7) | 22 (30.1) | 3 (60.0) | ||
| Late metachronous ≥1 year | 34 (36.2) | 26 (35.6) | 0 (0.0) | ||
| Diameter of CRLM at diagnosis, median [range] | mm | 26 [4, 80] | 23 [8, 68] | 19 [11, 46] | 0.13 |
| CEA at diagnosis, median [range] | ng/ml | 7.3 [1.0, 417.8] | 8.6 [1.0, 2165.0] | 4.0 [3.0, 93.3] | 0.72 |
| CA19‐9 at diagnosis, median [range] | IU/ml | 14.0 [0.6, 3710.0] | 17.3 [0.1, 1432.0] | 137.2 [10.2, 563.1] | 0.03 |
| Extent of hepatectomy a | <1 sectorectomy | 76 (80.9) | 58 (79.5) | 3 (60.0) | 0.395 |
| 1 sectorectomy | 10 (10.6) | 10 (13.7) | 2 (40.0) | ||
| ≥2 sectorectomy | 8 (8.5) | 5 (6.8) | 0 (0.0) | ||
| Residual tumor after hepatectomy, No. (%) | R0 | 90 (95.7) | 69 (94.5) | 4 (80.0) | 0.30 |
| R1 | 4 (4.3) | 4 (5.5) | 1 (20.0) | ||
| Adjuvant chemotherapy, No. (%) | No | 55 (58.5) | 36 (49.3) | 2 (40.0) | 0.41 |
| Yes | 39 (41.5) | 37 (50.7) | 3 (60.0) | ||
Abbreviations: CA19‐9, carbohydrate antigen 19–9; CEA, carcinoembryonic antigen; CRLM, colorectal liver metastases.
The extent of hepatectomy was defined according to the Couinaud's sector.
3.2. Survival analysis after hepatectomy according to RAS/BRAF V600E mutational status
The median duration of post‐hepatectomy follow‐up was 72.8 months (range, 0.6–179.2). Recurrences were identified in 5 (100%), 40 (54.8%), and 38 (40.4%) patients among those with mBRAF, mRAS, and wtRAS/BRAF, respectively (p = 0.01). The median RFS was 4.8 months (95% confidence interval [CI], 2.6–not available [NA]) and 21.4 months (95% CI, 11.9–NA) for mBRAF and mRAS, respectively. Additionally, the median RFS was not reached (NR) (95% CI, 29.1–NA) in patients with wtRAS/BRAF (Figure 2). The 1‐year and 3‐year RFS rates were both 0% for patients with mBRAF, and 60.9% and 45.4%, respectively, for those with mRAS, and 76.7% and 58.9%, respectively, for those with wtRAS/BRAF. The risk of recurrence was significantly higher in patients with mBRAF than those with wtRAS/BRAF (hazard ratio [HR], 10.9; 95% CI, 4.1–29.0; p < 0.001) and mRAS (HR, 5.8; 95% CI, 2.1–15.5; p < 0.001), and all patients with mBRAF developed early systemic unresectable recurrences within 8 months after surgery. A representative case is presented in Figure 3A. Sites of recurrence (liver‐limited vs. systemic) in patients with mBRAF (0% vs. 100%), mRAS (25.0% vs. 75.0%), and wtRAS/BRAF (39.5% vs. 60.5%; p = 0.14), rates of repeat surgery for recurrences (mBRAF vs. mRAS vs. wtRAS/BRAF, 0% vs. 50.0% vs. 52.6%; p = 0.08), and rates of systemic chemotherapy for recurrences (mBRAF vs. mRAS vs. wtRAS/BRAF, 80.0% vs. 35.0% vs. 42.1%; p = 0.15) were not statistically different regardless of the status of the mutations. Regimens of systemic chemotherapy included FOLFOX/CAPOX (oxaliplatin/capecitabine, folinic acid, and fluorouracil, n [%], 21 [61.8]), FOLFIRI (irinotecan, folinic acid, and fluorouracil, n [%], 10 [29.4]), irinotecan (n [%], 2 [5.9]), and UFT plus leucovorin (n [%], 1 [2.9]) while target agents were added in 23 patients (67.6%), and rates of each regimen were not statistically different among the three groups (p = 0.18). Details of treatment for recurrences are described in the Table S1.
FIGURE 2.
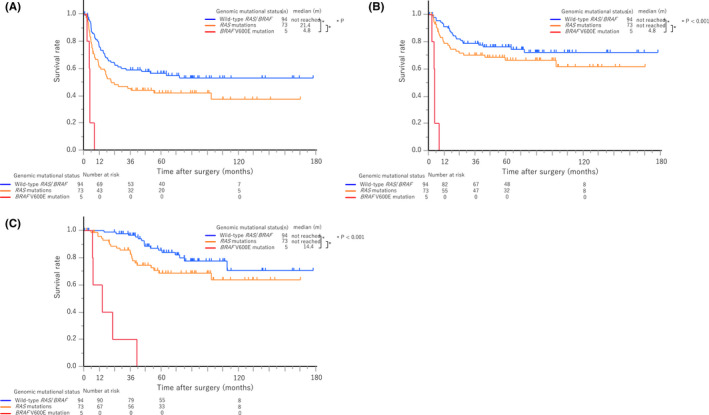
Recurrence‐free survival, time to surgical failure, and overall survival after hepatectomy according to genomic mutational status. A, Recurrence‐free survival. B, Time to surgical failure. C, Overall survival
FIGURE 3.
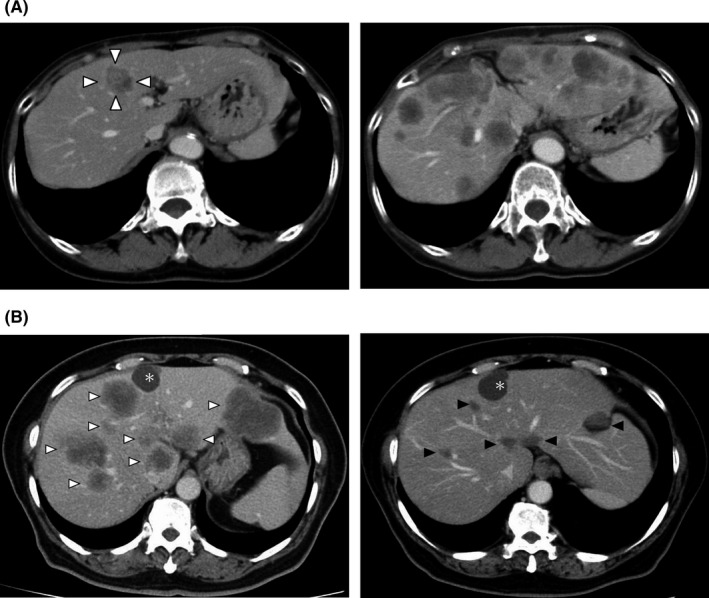
Computed tomography findings of representative patients with BRAF V600E mutation. A, A solitary resectable case. (left) A 71‐year‐old female patient who underwent hepatectomy for metachronous solitary CRLM (triangles). (right) However, the patient developed multiple liver metastases along with lung and peritoneal metastases 4.4 months after hepatectomy and died 6.8 months after surgery. B, An unresectable case. (left) A 61‐year‐old female patient developed metachronous multiple unresectable CRLM (white triangles) and peritoneal metastases along with a liver cyst (asterisk). (right) After receiving FOLFOXIRI plus bevacizumab for 9 months, the CRLM shrunk with good response (black triangles). Afterward, she received the BEACON CRC triplet regimen (encorafenib, binimetinib, and cetuximab) with other treatments and died 36.0 months after the start of the first‐line chemotherapy
The median TSF was 4.8 months (95% CI, 2.6–NA), not reached (95% CI, 99.0–NA), and not reached (NA–NA) in patients with mBRAF, mRAS, and wtRAS/BRAF, respectively. The 1‐year and 3‐year TSF rates were both 0% for patients with mBRAF, 78.8% and 70.2%, respectively, for those with mRAS, and 91.1% and 78.8%, respectively, for those with wtRAS/BRAF. TSF was significantly shorter in patients with mBRAF than those with wtRAS/BRAF (HR, 28.7; 95% CI, 9.6–86.1; p < 0.001) and mRAS (HR, 14.6; 95% CI, 4.8–44.5; p < 0.001).
The median OS was 14.4 months (95% CI, 6.7–NA) in patients with mBRAF, not reached (95% CI, 99.0–NA) in those with mRAS, and not reached (95% CI, NA–NA) in those with wtRAS/BRAF. The 1‐year, 3‐year, and 5‐year OS rates were 60.0%, 20.0%, and 0%, respectively, for patients with mBRAF, 95.8%, 85.7%, and 68.7%, respectively, for those with mRAS, and 100%, 96.6%, and 85.5%, respectively, for those with wtRAS/BRAF. Overall, OS was significantly shorter in patients with mBRAF than those with wtRAS/BRAF (HR, 27.6; 95% CI, 9.5–80.4; p < 0.001) and mRAS (HR, 9.9; 95% CI, 3.5–27.5; p < 0.001). The trend toward poorer survival of mBRAF was also consistent irrespective of the sidedness (right or left) of the primary CRC in RFS, TSF, and OS (Figures S1–S3).
Univariable and multivariable analyses for the risk factors of RFS, TSF, and OS are described in Table 2. mBRAF was strongly associated with a shorter survival and had the highest HR among all the indicators in terms of RFS (HR: 12.5, 95% CI, 4.3–35.8; p < 0.001), TSF (HR: 19.0, 95% CI, 6.1–59.4; p < 0.001), and OS (HR: 17.0, 95% CI: 5.2–55.9, p < 0.001) in the multivariable analysis.
TABLE 2.
Univariable and multivariable analyses of risk factors for recurrence‐free survival, time to surgical failure, and overall survival after hepatectomy for solitary resectable colorectal liver metastasis
| Characteristics | n | Recurrence‐free survival | ||||
|---|---|---|---|---|---|---|
| Univariable | Multivariable | |||||
| Median (m) (95% CI) | p value | HR (95% CI) | p value | |||
| (A) Recurrence‐free survival | ||||||
| Patient factors | ||||||
| Sex | Female | 65 | 23.7 (11.1–NA) | 0.33 | ||
| Male | 107 | 64.1 (21.1–NA) | ||||
| Age at hepatectomy (years) | <65 | 70 | 34.2 (17.1–NA) | 0.88 | ||
| ≥65 | 102 | 74.4 (17.2–NA) | ||||
| Primary colorectal tumor factors | ||||||
| Location | Right‐sided | 33 | 11.9 (5.6–99.0) | 0.008 | 1 [reference] | |
| Left‐sided | 139 | 74.4 (24.3–NA) | 0.8 (0.4–1.3) | 0.28 | ||
| Depth of invasion | T1–T3 | 132 | 36.8 (17.7–NA) | 0.69 | ||
| T4 | 38 | 51.6 (11.8–NA) | ||||
| Lymph node metastases | Absent | 72 | NR (64.1–NA) | <0.001 | 1 [reference] | |
| Present | 99 | 17.1 (11.7–26.9) | 1.9 (1.2–3.1) | 0.005 | ||
| Pathology | Well diff. adenocarcinoma | 46 | NR (26.9–NA) | |||
| Others | 126 | 24.3 (16.2–NA) | ||||
| CRLM factors | ||||||
| Timing of CRLM | Synchronous | 60 | 17.1 (10.7–NA) | 0.03 | 1 [reference] | |
| Early metachronous <1 year | 52 | 24.3 (11.5–NA) | 1.1 (0.7–1.9) | 0.66 | ||
| Late metachronous ≥1 year | 60 | NR (45.0–NA) | 0.6 (0.4–1.2) | 0.15 | ||
| Diameter of CRLM at diagnosis (mm) | <25.0 | 83 | 36.8 (16.3–NA) | 0.83 | ||
| ≥25.0 | 89 | 51.6 (18.3–NA) | ||||
| Incidental intraoperative multiple CRLM | Absent | 167 | 45.0 (18.7–NA) | 0.53 | ||
| Present | 5 | NR (5.6–NA) | ||||
| Incidental intraoperative peritoneal metastases | Absent | 169 | 54.5 (21.1–NA) | 0.03 | 1 [reference] | |
| Present | 3 | 11.8 (4.8–NA) | 2.5 (0.7–9.1) | 0.15 | ||
| CEA at diagnosis (ng/ml) | <5.0 | 65 | NR (32.9–NA) | 0.03 | 1 [reference] | |
| ≥5.0 | 107 | 24.3 (16.2–64.1) | 1.9 (1.1–3.1) | 0.02 | ||
| CA19‐9 at diagnosis (IU/ml) | <37.0 | 126 | 99.0 (23.7–NA) | 0.03 | 1 [reference] | |
| ≥37.0 | 46 | 18.7 (8.2–51.6) | 1.1 (0.7–1.8) | 0.75 | ||
| Residual tumor after hepatectomy | R0 | 163 | 64.1 (21.0–NA) | 0.03 | 1 [reference] | |
| R1 | 9 | 16.2 (1.6–NA) | 2.1 (0.9–4.6) | 0.07 | ||
| Adjuvant chemotherapy | No | 93 | 32.9 (10.7–NA) | 0.28 | ||
| Yes | 79 | 54.5 (21.1–NA) | ||||
| Genomic mutational status | Wild‐type RAS/BRAF | 94 | NR (29.1–NA) | <0.001 | 1 [reference] | |
| RAS mutations | 73 | 21.4 (11.9–NA) | 1.5 (1.0–2.3) | 0.08 | ||
| BRAF V600E mutation | 5 | 4.8 (2.6–NA) | 12.5 (4.3–35.8) | <0.001 | ||
| Characteristics | n | Time to surgical failure | ||||
|---|---|---|---|---|---|---|
| Univariable | Multivariable | |||||
| Median (m) (95% CI) | p value | HR (95% CI) | p value | |||
| (B) Time to surgical failure | ||||||
| Patient factors | ||||||
| Sex | Female | 65 | NA (NA–NA) | 0.54 | ||
| Male | 107 | NA (NA–NA) | ||||
| Age at hepatectomy (years) | <65 | 70 | NR (NA–NA) | 0.94 | ||
| ≥65 | 102 | NR (99.0–NA) | ||||
| Primary colorectal tumor factors | ||||||
| Location | Right‐sided | 33 | 99.0 (11.9–NA) | 0.12 | ||
| Left‐sided | 139 | NR (NA–NA) | ||||
| Depth of invasion | T1–T3 | 132 | NR (NA–NA) | 0.13 | ||
| T4 | 38 | 99.0 (42.5–NA) | ||||
| Lymph node metastases | Absent | 72 | NR (NA–NA) | 0.002 | 1 [reference] | |
| Present | 99 | NR (43.9–NA) | 2.2 (1.2–4.2) | 0.02 | ||
| Pathology | Well diff. adenocarcinoma | 46 | NR (NA–NA) | 0.32 | ||
| Others | 126 | NR (NA–NA) | ||||
| CRLM factors | ||||||
| Timing of CRLM | Synchronous | 60 | NR (42.5–NA) | 0.05 | ||
| Early metachronous <1 year | 52 | NR (74.4–NA) | ||||
| Late metachronous ≥1 year | 60 | NR (NA–NA) | ||||
| Diameter of CRLM at diagnosis (mm) | <25.0 | 83 | NR (NA–NA) | 0.21 | ||
| ≥25.0 | 89 | NR (99.0–NA) | ||||
| Incidental intraoperative multiple CRLM | Absent | 167 | NR (NA–NA) | 0.16 | ||
| Present | 5 | NR (NA–NA) | ||||
| Incidental intraoperative peritoneal metastases | Absent | 169 | NR (NA–NA) | 0.05 | ||
| Present | 3 | 11.8 (4.8–NA) | ||||
| CEA at diagnosis (ng/ml) | <5.0 | 65 | NR (NA–NA) | 0.09 | ||
| ≥5.0 | 107 | NR (99.0–NA) | ||||
| CA19‐9 at diagnosis (IU/ml) | <37.0 | 126 | NR (NA–NA) | 0.06 | ||
| ≥37.0 | 46 | NR (26.5–NA) | ||||
| Residual tumor after hepatectomy | R0 | 163 | NR (NA–NA) | 0.003 | 1 [reference] | |
| R1 | 9 | 23.7 (4.4–NA) | 2.6 (1.1–6.4) | 0.03 | ||
| Adjuvant chemotherapy | No | 93 | NR (NA–NA) | 0.92 | ||
| Yes | 79 | NR (NA–NA) | ||||
| Genomic mutational status | Wild‐type RAS/BRAF | 94 | NR (NA–NA) | <0.001 | 1 [reference] | |
| RAS mutations | 73 | NR (99.0–NA) | 1.4 (0.8–2.5) | 0.24 | ||
| BRAF V600E mutation | 5 | 4.8 (2.6–NA) | 19.0 (6.1–59.4) | <0.001 | ||
| Characteristics | n | Overall survival | ||||
|---|---|---|---|---|---|---|
| Univariable | Multivariable | |||||
| Median (m) (95% CI) | p value | HR (95% CI) | p value | |||
| (C) Overall survival | ||||||
| Patient factors | ||||||
| Sex | Female | 65 | NR (74.4–NA) | 0.33 | ||
| Male | 107 | NR (NA–NA) | ||||
| Age at hepatectomy (Years) | <65 | 70 | NR (111.2–NA) | 0.83 | ||
| ≥65 | 102 | NR (NA–NA) | ||||
| Primary colorectal tumor factors | ||||||
| Location | Right‐sided | 33 | 99.0 (39.9–NA) | 0.07 | ||
| Left‐sided | 139 | NR (NA–NA) | ||||
| Depth of invasion | T1–T3 | 132 | NR (NA–NA) | 0.05 | ||
| T4 | 38 | 99.0 (58.0–NA) | ||||
| Lymph node metastases | Absent | 72 | NR (NA–NA) | 0.002 | 1 [reference] | |
| Present | 99 | NR (78.1–NA) | 2.0 (0.9–4.2) | 0.07 | ||
| Pathology | Well diff. adenocarcinoma | 46 | NR (NA–NA) | 0.29 | ||
| Others | 126 | NR (111.2–NA) | ||||
| CRLM factors | ||||||
| Timing of CRLM | Synchronous | 60 | NR (111.2–NA) | 0.27 | ||
| Early metachronous <1 year | 52 | NR (78.1–NA) | ||||
| Late metachronous ≥1 year | 60 | NR (NA–NA) | ||||
| Diameter of CRLM at diagnosis (mm) | <25.0 | 83 | NR (NA–NA) | 0.19 | ||
| ≥25.0 | 89 | NR (99.0–NA) | ||||
| Incidental intraoperative multiple CRLM | Absent | 167 | NR (NA–NA) | 0.19 | ||
| Present | 5 | NR (NA–NA) | ||||
| Incidental intraoperative peritoneal metastases | Absent | 169 | NR (NA–NA) | 0.003 | 1 [reference] | |
| Present | 3 | 46.9 (7.3–NA) | 8.00 (1.59–40.33) | 0.01 | ||
| CEA at diagnosis (ng/ml) | <5.0 | 65 | NR (111.2–NA) | 0.60 | ||
| ≥5.0 | 107 | NR (NA–NA) | ||||
| CA19‐9 at diagnosis (IU/ml) | <37.0 | 126 | NR (NA–NA) | 0.001 | 1 [reference] | |
| ≥37.0 | 46 | 78.1 (51.6–NA) | 2.14 (1.11–4.12) | 0.02 | ||
| Residual tumor after hepatectomy | R0 | 163 | NR (NA–NA) | 0.001 | 1 [reference] | |
| R1 | 9 | 58.3 (6.7–NA) | 5.04 (1.93–13.18) | <0.001 | ||
| Adjuvant chemotherapy | No | 93 | NR (NA–NA) | 0.94 | ||
| Yes | 79 | NR (111.2–NA) | ||||
| Genomic mutational status | Wild‐type RAS/BRAF | 94 | NR (NA–NA) | <0.001 | 1 [reference] | |
| RAS mutations | 73 | NR (99.0–NA) | 1.97 (1.02–3.82) | 0.04 | ||
| BRAF V600E mutation | 5 | 14.4 (6.7–NA) | 17.02 (5.19–55.85) | <0.001 | ||
Abbreviations: CA19‐9, carbohydrate antigen 19–9; CEA, carcinoembryonic antigen; CI, confidence interval; CRLM, colorectal liver metastases; NA, not available; NR, not reached.
3.3. Comparison of overall survival between patients with solitary resectable CRLM and those with unresectable CRLM
In the second analysis, 28 patients with unresectable BRAF V600E‐mutant CRLM who received systemic chemotherapy were identified (Figure S4) and compared with those with solitary resectable BRAF V600E‐mutant CRLM identified in the first analysis. A representative case is presented in Figure 3B. Baseline CRLM characteristics were far more advanced in the unresectable group than in the solitary resectable group as reflected in the number of tumors (median, 18 vs. 1, p = 0.001) and the rate of concurrent extrahepatic metastases by diagnostic imaging (n [%], 20 [71.4] vs. 0 [0]; p = 0.005; Table 3). No patients were MSI‐high nor MMR protein‐deficient. FOLFOX/CAPOX (n [%], 23 [82.1]), FOLFOXIRI (oxaliplatin, irinotecan, folinic acid, and fluorouracil, n [%], 3 [10.7]), and FOLFIRI (n [%], 2 [7.1]) were included as first‐line chemotherapy regimens for patients with unresectable CRLM, and target agents were added in 21 patients (75.0%). An anti‐BRAF agent (encorafenib) was used in seven patients (25.0%) as second‐ or third‐line chemotherapy as a part of the BEACON CRC trial. 17 In the solitary resectable group, no patients underwent repeat surgery for recurrences after hepatectomy. FOLFIRI (n [%], 2 [40.0]), FOLFOX (n [%], 1 [20.0]), and irinotecan (n [%], 1 [20.0]) were included as chemotherapy regimens for the recurrences, and target agents were added in two patients (40.0%, Table S1). One patient did not receive any chemotherapy due to rapid deterioration of performance status. No patients received the anti‐BRAF agent (encorafenib). The median OS of patients with unresectable BRAF V600E‐mutant CRLM was 17.2 months (95% CI, 7.5–25.2), which was nearly identical to that of those with solitary resectable CRLM (HR, 0.78; 95% CI, 0.26–2.33; p = 0.65, Figure 4).
TABLE 3.
Clinicopathological characteristics of patients with BRAF V600E mutation according to the technical resectability
| Factor | Group | Technical resectability | p value | |
|---|---|---|---|---|
| Unresectable | Solitary resectable | |||
| n = 28 | n = 5 | |||
| Patient factors | ||||
| Age at the upfront treatment, median [range] | 61 [27, 73] | 71 [43, 76] | 0.06 | |
| Sex, No. (%) | Female | 16 (57.1) | 2 (40.0) | 0.64 |
| Male | 12 (42.9) | 3 (60.0) | ||
| Primary colorectal tumor factors | ||||
| Location, No. (%) | Right‐sided | 17 (60.7) | 2 (40.0) | 0.63 |
| Left‐sided | 11 (39.3) | 3 (60.0) | ||
| Pathology of the primary tumor, No. (%) | Well diff. adenocarcinoma | 2 (7.1) | 1 (20.0) | 0.40 |
| Others | 26 (92.9) | 4 (80.0) | ||
| CRLM factors | ||||
| Timing of CRLM, No. (%) | Synchronous | 24 (85.7) | 2 (40.0) | 0.05 |
| Metachronous | 4 (14.3) | 3 (60.0) | ||
| Number of CRLM at diagnosis a , median [range] | 18 [1, 102] | 1 [1, 1] | 0.001 | |
| Diameter of CRLM at diagnosis a , median [range] | mm | 31 [6, 82] | 19 [11, 46] | 0.24 |
| CEA at diagnosis, median [range] | 37.9 [1.3, 5,577.0] | 4.0 [3.0, 93.3] | 0.13 | |
| CA19‐9 at diagnosis, median [range] | 986.6 [1.0, 49,740.0] | 137.2 [10.2, 563.1] | 0.35 | |
| Concomitant extrahepatic metastases at diagnosis a , No. (%) | Yes | 20 (71.4) | 0 (0.0) | 0.005 |
| No | 8 (28.6) | 5 (100.0) | ||
| Extent of extrahepatic metastases at diagnosis a , No. (%) | Lung | 3 (10.7) | 0 (0.0) | |
| Peritoneum | 11 (39.3) | 0 (0.0) | ||
| Lymph node | 10 (35.7) | 0 (0.0) | ||
| Other organs | 1 (3.6) | 0 (0.0) | ||
| Microsatellite instability | High | 0 (0.0) | NA | |
| Mismatch repair protein | Deficient | NA | 0 (0.0) | |
| Treatment factors | ||||
| Initial therapy, No. (%) | Surgery | 0 (0.0) | 5 (100.0) | |
| FOLFOX/CAPOX | 23 (82.1) | 0 (0.0) | ||
| FOLFOXIRI | 3 (10.7) | 0 (0.0) | ||
| FOLFIRI | 2 (7.1) | 0 (0.0) | ||
| Use of target agents | 21 (75.0) | 0 (0.0) | ||
| Use of anti‐BRAF agents in the later therapy, No. (%) | No | 21 (75.0) | 5 (100.0) | 0.56 |
| Yes | 7 (25.0) | 0 (0.0) | ||
Abbreviations: CA19‐9, carbohydrate antigen 19–9; CEA, carcinoembryonic antigen; CRLM, colorectal liver metastases; NA, not available; NR, not reached.
Those diagnoses were made by pre‐treatment imaging modalities.
FIGURE 4.
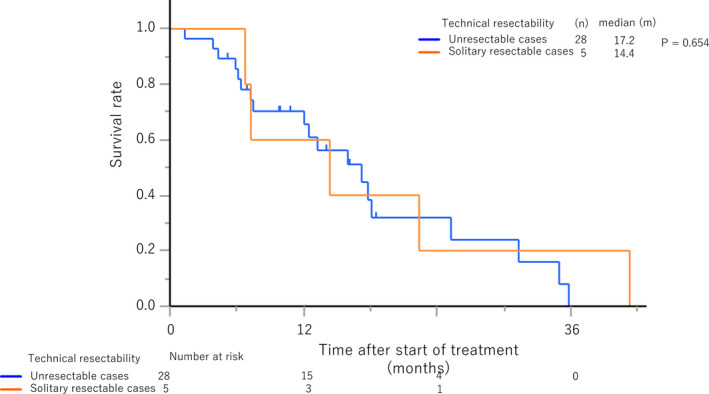
Overall survival of patients with BRAF V600E‐mutant colorectal liver metastases depending on the technical resectability
4. DISCUSSION
In this study, mBRAF was associated with intraoperative incidental peritoneal metastases and markedly shorter RFS, TSF, and OS in patients who underwent upfront hepatectomy without neoadjuvant chemotherapy for solitary resectable CRLM. Particularly, mBRAF status was the strongest predictor of survival beyond all conventional clinicopathological factors for RFS, TSF, and OS. All patients with mBRAF developed early systemic unresectable recurrences within 8 months after surgery. Interestingly, OS after upfront hepatectomy for CRLM associated with mBRAF was almost identical to that after systemic chemotherapy for unresectable ones, although baseline tumor characteristics were very different. Inherent tumor biology as assessed by the status of mBRAF seemed more important than conventional technical resectability as assessed by radiological findings in judging oncological resectability of CRLM. This study clearly demonstrated the deleterious natural history of patients with mBRAF who underwent upfront hepatectomy unselectively from a conventional perspective of technical resectability and elucidated the necessity to detect those with mBRAF preoperatively. Thus, CRLM with mBRAF can be recognized as oncologically unresectable irrespective of technical resectability, and we propose to perform pre‐treatment genetic testing for mBRAF not only for technically unresectable cases but also for resectable ones. To the best of our knowledge, this study is the first to directly compare the survival outcome of patients undergoing hepatectomy for BRAF V600E‐mutant CRLM with those treated with systemic chemotherapy for unresectable cases.
Although this study focused on solitary, resectable, and thus, potentially curable CRLM, as opposed to previous studies that included heterogeneous cohorts of patients with variable numbers of metastases and different initial resectability statuses, 9 , 10 , 11 , 12 , 13 , 18 mBRAF was consistently associated with elevated levels of baseline serum CA19‐9 and shorter survival after hepatectomy. All but one previous study included patients who received preoperative chemotherapy before hepatectomy, and therefore, the survival outcomes of patients with mBRAF in those studies seemed better than they really were because only patients who responded to preoperative chemotherapy favorably were selected for hepatectomy. In contrast, Schirripa et al. reported that RFS and OS after upfront hepatectomy were 5.7 and 22.6 months, respectively. 9 , 19 The results of this study were consistent with those of Schirripa et al., and the OS of patients who underwent upfront hepatectomy was nearly identical to that of those with unresectable CRLM treated with systemic chemotherapy. It has been reported that elevated levels of serum CA19‐9 were associated with markedly impaired survival particularly in patients with mBRAF compared with those with mRAS or wtRAS/BRAF. 18 In this study, median level of serum CA19‐9 was elevated to 137.2 IU/ml and it might have augmented the aggressiveness of mBRAF compared with mRAS or wtRAS/BRAF. In addition, patient selection criteria of this study that solely included those who underwent upfront hepatectomy unselectively without neoadjuvant chemotherapy must have even enhanced the difference between mBRAF and mRAS or wtRAS/BRAF. The biological mechanisms of the increased kinase activity and the elevated levels of CA19‐9 as well as the clinical characteristics of the inclusion criteria elucidated the extremely poor natural history of surgical patients with BRAF V600E‐mutant CRLM. It seems that solitary resectable CRLM were just a short process of rapidly progressive systemic disease when complicated with mBRAF. The deleterious natural history of patients with mBRAF who underwent upfront hepatectomy unselectively from a conventional perspective of technical resectability indicates that CRLM with mBRAF can be considered as oncologically unresectable irrespective of technical resectability.
The evidence of upfront hepatectomy for BRAF V600E‐mutant CRLM resulting in unexceptionally detrimental outcomes warranted suggesting the proper selection of surgical candidates who my truly benefit from the invasive procedure. Survival outcomes of the previous studies seemed better as the rate of preoperative chemotherapy increases. 9 , 10 , 11 , 12 , 13 , 19 Cremolini C et al. even reported that prognosis of patients with initially unresectable CRLM who responded to chemotherapy (FOLFOXIRI plus bevacizumab) followed by conversion hepatectomy was not influenced by the presence of mBRAF, with RFS and OS reaching as long as 11.4 months and unreached, respectively. 19 We previously demonstrated that systemic chemotherapy should be offered to all surgical candidates with BRAF V600E‐mutant CRLM rather than upfront hepatectomy, 12 , 20 and this study confirmed that this same strategy should also be applied even for those with solitary resectable CRLM. The recent BEACON CRC trial demonstrated that the novel triplet regimen of encorafenib, binimetinib, and cetuximab, and the doublet one of encorafenib and cetuximab had superior efficacy compared to the standard therapy for patients with previously treated unresectable BRAF V600E‐mutant mCRC. 17 , 21 Although the efficacy of this triplet regimen is still under investigation in patients with previously untreated BRAF V600E‐mutant mCRC, 22 one potential treatment strategy for technically resectable BRAF V600E‐mutant CRLM is the upfront use of this novel triplet regimen followed by hepatectomy in eligible responders. 20 Therefore, mBRAF can be an actionable mutation even for technically resectable CRLM, and we propose to perform pre‐treatment genetic testing for mBRAF irrespective of technical resectability.
One potential problem of performing the pre‐treatment mBRAF testing might be the relatively low incidence of mBRAF among resectable CRLM. The incidence of mBRAF among unresectable mCRC was reported around 10% among Western countries and 5% among Asian countries, 23 , 24 , 25 , 26 , 27 while it was reported even lower among resectable CRLM. In this study, the incidence among solitary resectable cases was 2.9%. It might be argued that pre‐treatment mBRAF testing benefits only a minority of surgical candidates and that surgery should be offered for resectable cases irrespective of mBRAF status since it is the standard of care. However, because mBRAF is a rapidly progressive disease and accompanies rapid deterioration of performance status with relapse after surgery, not all patients can receive intensive systemic chemotherapy such as the BEACON triplet regimen or FOLFOXIRI plus bevacizumab for relapse after hepatectomy. In fact, one of the five patients who underwent upfront hepatectomy in this study could not receive any chemotherapy after recurrence. By performing pre‐treatment mBRAF testing even for resectable cases and providing upfront intensive chemotherapy followed by hepatectomy in responders, we can properly select surgical candidates, maximize the benefit of surgery, prolong the survival time, and might even cure the disease. A recent study regarding circulating tumor DNA (ctDNA) demonstrated that mBRAF can be detected before hepatectomy for CRLM. 28 Since turnaround time for ctDNA was reported as 7 days at median, 29 pre‐treatment rapid assessment for mBRAF status does not delay preoperative surgical management. We believe that mBRAF testing opens the door for precision onco‐surgery in resectable CRLM.
This study is limited primarily by its single‐center, retrospective nature with small sample size. Additionally, this study was limited to solitary, resectable CRLM. However, because this study is distinct in its comparison of survival outcomes of patients who were treated with systemic chemotherapy for unresectable CRLM with those who underwent upfront hepatectomy without neoadjuvant chemotherapy, which is not the clinical standard in Western literature, 4 , 5 we could shed light on the deleterious natural history of surgical patients with mBRAF. Our results convey important guidance to every clinician who takes care of surgical candidates with resectable CRLM. The detrimental impact of mBRAF on survival outcomes after surgery warrants its testing before consideration of hepatectomy.
5. CONCLUSIONS
Upon complication of technically resectable CRLM with mBRAF, survival outcome becomes as poor as those of unresectable cases. Cases with mBRAF should be considered as oncologically unresectable. Patients with CRLM should undergo pre‐treatment mBRAF testing regardless of technical resectability.
AUTHOR CONTRIBUTION
Shin Kobayashi, Shinichiro Takahashi, Hiroya Taniguchi, and Takayuki Yoshino: Designed and conceived this study. Shin Kobayashi: Patients enrollment, data acquisition, patient evaluation, and article preparation. Shinichiro Takahashi, Hiroya Taniguchi, and Takayuki Yoshino: Project administration and article review. Masashi Kudo, Motokazu Sugimoto, Masaru Konishi, and Naoto Gotohda: Patient enrollment, evaluation, and article review. Shogo Nomura: Statistical analysis and article review. Motohiro Kojima: Pathological diagnosis and article review. All authors read and approved the final manuscript.
DISCLOSURE OF COMMERCIAL INTEREST
Shin Kobayashi, Masashi Kudo, Motokazu Sugimoto, Masaru Konishi, Naoto Gotohda, Shinichiro Takahashi, Hiroya Taniguchi, Motohiro Kojima, and Shogo Nomura have nothing to declare.
Takayuki Yoshino received research funding from Ono Pharmaceutical Co., Ltd.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICAL APPROVAL
The study conforms to the provisions of the Declaration of Helsinki and was approved by the hospital ethics committee (approval number: 2018‐272).
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Cancer Center Research Development Fund (Research number: 30‐A‐8).
Kobayashi S, Takahashi S, Nomura S, et al. BRAF V600E potentially determines “Oncological Resectability” for “Technically Resectable” colorectal liver metastases. Cancer Med. 2021;10:6998–7011. 10.1002/cam4.4227
Funding information
This work was supported by the National Cancer Center Research Development Fund (Research number: 30‐A‐8).
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Leporrier J, Maurel J, Chiche L, Bara S, Segol P, Launoy G. A population‐based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg. 2006;93(4):465‐474. [DOI] [PubMed] [Google Scholar]
- 3. Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long‐term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14(12):1208‐1215. [DOI] [PubMed] [Google Scholar]
- 4. NCCN Clinical Practice Guidelines in Oncology Colon Cancer. Version 1.2021 ‐ December 22, 2020 2021 https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
- 5. Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386‐1422. [DOI] [PubMed] [Google Scholar]
- 6. Beppu T, Sakamoto Y, Hasegawa K, et al. A nomogram predicting disease‐free survival in patients with colorectal liver metastases treated with hepatic resection: multicenter data collection as a Project Study for Hepatic Surgery of the Japanese Society of Hepato‐Biliary‐Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2012;19(1):72‐84. [DOI] [PubMed] [Google Scholar]
- 7. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949‐954. [DOI] [PubMed] [Google Scholar]
- 8. Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF‐ERK signaling pathway by oncogenic mutations of B‐RAF. Cell. 2004;116(6):855‐867. [DOI] [PubMed] [Google Scholar]
- 9. Schirripa M, Bergamo F, Cremolini C, et al. BRAF and RAS mutations as prognostic factors in metastatic colorectal cancer patients undergoing liver resection. Br J Cancer. 2015;112(12):1921‐1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Margonis GA, Buettner S, Andreatos N, et al. Association of BRAF mutations with survival and recurrence in surgically treated patients with metastatic colorectal liver cancer. JAMA Surg. 2018;153(7):e180996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gagniere J, Dupre A, Gholami SS, et al. Is Hepatectomy justified for BRAF mutant colorectal liver metastases? A multi‐institutional analysis of 1497 patients. Ann Surg. 2020;271(1):147‐154. [DOI] [PubMed] [Google Scholar]
- 12. Kobayashi S, Takahashi S, Takahashi N, et al. Survival outcomes of resected BRAF V600E mutant colorectal liver metastases: a multicenter retrospective cohort study in Japan. Ann Surg Oncol. 2020;27(9):3307‐3315. [DOI] [PubMed] [Google Scholar]
- 13. Umeda Y, Nagasaka T, Mori Y, et al. Poor prognosis of KRAS or BRAF mutant colorectal liver metastasis without microsatellite instability. J Hepatobiliary Pancreat Sci. 2013;20(2):223‐233. [DOI] [PubMed] [Google Scholar]
- 14. Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first‐line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open‐label, phase 3 TRIBE study. Lancet Oncol. 2015;16(13):1306‐1315. [DOI] [PubMed] [Google Scholar]
- 15. JSCCR Guidelines 2019 for the Treatment of Colorectal Cancer. Kanehara Publishing Co. Ltd; 2019. [Google Scholar]
- 16. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kopetz S, Grothey A, Yaeger R, et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E‐mutated colorectal cancer. N Engl J Med. 2019;381(17):1632‐1643. [DOI] [PubMed] [Google Scholar]
- 18. Thomsen M, Skovlund E, Sorbye H, et al. Prognostic role of carcinoembryonic antigen and carbohydrate antigen 19–9 in metastatic colorectal cancer: a BRAF‐mutant subset with high CA 19–9 level and poor outcome. Br J Cancer. 2018;118(12):1609‐1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cremolini C, Casagrande M, Loupakis F, et al. Efficacy of FOLFOXIRI plus bevacizumab in liver‐limited metastatic colorectal cancer: a pooled analysis of clinical studies by Gruppo Oncologico del Nord Ovest. Eur J Cancer. 2017;73:74‐84. [DOI] [PubMed] [Google Scholar]
- 20. Kobayashi S, Takahashi S, Yoshino T, Taniguchi H. ASO author reflections: the moment that BRAF V600E mutation starts evolving into "Precision Oncosurgery" in colorectal liver metastases. Ann Surg Oncol. 2020;27(9):3316‐3317. [DOI] [PubMed] [Google Scholar]
- 21. Tabernero J, Grothey A, Van Cutsem E, et al. Encorafenib plus cetuximab as a new standard of care for previously treated BRAF V600E‐mutant metastatic colorectal cancer: updated survival results and subgroup analyses from the BEACON study. J Clin Oncol. 2021;39(4):273‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Encorafenib, Binimetinib and Cetuximab in Subjects With Previously Untreated BRAF‐mutant ColoRectal Cancer (ANCHOR‐CRC). 2020. https://clinicaltrials.gov/ct2/show/NCT03693170
- 23. Pai RK, Jayachandran P, Koong AC, et al. BRAF‐mutated, microsatellite‐stable adenocarcinoma of the proximal colon: an aggressive adenocarcinoma with poor survival, mucinous differentiation, and adverse morphologic features. Am J Surg Pathol. 2012;36(5):744‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saridaki Z, Tzardi M, Sfakianaki M, et al. BRAFV600E mutation analysis in patients with metastatic colorectal cancer (mCRC) in daily clinical practice: correlations with clinical characteristics, and its impact on patients’ outcome. PLoS One. 2013;8(12):e84604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yokota T, Ura T, Shibata N, et al. BRAF mutation is a powerful prognostic factor in advanced and recurrent colorectal cancer. Br J Cancer. 2011;104(5):856‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ooki A, Akagi K, Yatsuoka T, et al. Combined microsatellite instability and BRAF gene status as biomarkers for adjuvant chemotherapy in stage III colorectal cancer. J Surg Oncol. 2014;110(8):982‐988. [DOI] [PubMed] [Google Scholar]
- 27. Won DD, Lee JI, Lee IK, Oh ST, Jung ES, Lee SH. The prognostic significance of KRAS and BRAF mutation status in Korean colorectal cancer patients. BMC Cancer. 2017;17(1):403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kobayashi S, Nakamura Y, Taniguchi H, et al. Impact of preoperative circulating tumor DNA status on survival outcomes after hepatectomy for resectable colorectal liver metastases. Ann Surg Oncol. 2021;28(8):4744‐4755. [DOI] [PubMed] [Google Scholar]
- 29. Nakamura Y, Taniguchi H, Ikeda M, et al. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM‐Japan GI‐SCREEN and GOZILA studies. Nat Med. 2020;26(12):1859‐1864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


