Abstract
Sensory impairments are a core feature of autism spectrum disorder (ASD). These impairments affect visual perception and have been hypothesized to arise from imbalances in cortical excitatory and inhibitory activity. There is conflicting evidence for this hypothesis from several recent studies of transgenic mouse models of ASD; crucially, none have measured activity from identified excitatory and inhibitory neurons during simultaneous impairments of sensory perception. Here, we directly recorded putative excitatory and inhibitory population spiking in primary visual cortex (V1) while simultaneously measuring visual perceptual behavior in CNTNAP2−/− knockout (KO) mice. We observed quantitative impairments in the speed, accuracy, and contrast sensitivity of visual perception in KO mice. During these perceptual impairments, stimuli evoked more firing of inhibitory neurons and less firing of excitatory neurons, with reduced neural sensitivity to contrast. In addition, pervasive 3–10 Hz oscillations in superficial cortical layers 2/3 (L2/3) of KO mice degraded predictions of behavioral performance from neural activity. Our findings show that perceptual deficits relevant to ASD may be associated with elevated cortical inhibitory activity along with diminished and aberrant excitatory population activity in L2/3, a major source of feedforward projections to higher cortical regions.
Keywords: autism, excitation, inhibition, mouse, perception, visual cortex
Introduction
Impaired sensory perception is a core feature of autism spectrum disorder (ASD) (American Psychiatric Association 2013; Robertson and Baron-Cohen 2017). Sensory disturbances occur in the majority of individuals with ASD, and across lifetimes (Tomchek and Dunn 2007; Tavassoli et al. 2014). Early sensory symptoms can also predict later disease severity (Estes et al. 2015). Since there is detailed knowledge about the neural circuit basis of mammalian sensory processing, understanding impaired sensory perception in autism models provides an entry point for identifying neural circuit dysfunctions underlying core symptoms of ASD.
A prominent theory of ASD proposes that imbalanced excitatory–inhibitory activity ratios in cortex generate behavioral deficits (Rubenstein and Merzenich 2003; Nelson and Valakh 2015; Sohal and Rubenstein 2019). However, little direct evidence for this hypothesis has been measured from identified excitatory and inhibitory neurons during quantifiable sensory perceptual impairments. One recent study of several ASD mouse models (Antoine et al. 2019) observed that deficits of excitatory and inhibitory activity undergo homeostatic adjustment, resulting in overall preserved sensory responses; remarkably, responses showed little difference between anesthetized or awake conditions (Haider et al. 2013), and there were no measurements during sensory impairments. Another study in awake Fmr1−/− ASD model mice found reduction of inhibitory but not excitatory activity in superficial cortical layers 2/3 (L2/3); this reduced inhibitory activity correlated with sensory and behavioral deficits, but again without measuring activity during perceptual impairments (Goel et al. 2018). Another study of awake ASD model mice (CNTNAP2−/− knockout [KO]) instead found reduced and disorganized excitatory activity (Lazaro et al. 2019), but also did not measure sensory responses during perceptual impairments. It thus remains unresolved how different brain states and task engagement impact excitatory and inhibitory activity in ASD models, and whether excitatory or inhibitory neural activity deficits can explain simultaneous trial-by-trial impairments of sensory perception.
There is extensive mechanistic knowledge about the excitatory and inhibitory basis of visual processing (Douglas and Martin 2004; Isaacson and Scanziani 2011; Priebe and Ferster 2012), providing an ideal framework for resolving questions about neural activity deficits and perceptual impairments in ASD model mice. Remarkably, in individuals with ASD, deficits of visual processing arise as early as primary visual cortex (V1) (Robertson et al. 2014), but there remains considerable debate about how these deficits in V1 activity might relate to perceptual impairments. We recently established that the state of activity in V1 plays a decisive role for trial-by-trial visual spatial perception in mice (Speed et al. 2019, 2020). Here, we directly record putative excitatory and inhibitory neuron spiking in V1 of CNTNAP2−/− KO mouse model of ASD, while measuring the speed, accuracy, and contrast dependence of perceptual behavior. We found that KO mice showed multiple quantitative deficits in visual perception, and these were simultaneously associated with elevated inhibitory and reduced excitatory neuron activity along with aberrant low-frequency network oscillations in the superficial layers of V1.
Materials and Methods
Experimental Model and Subjects
All procedures were approved by the Institutional Animal Care and Use Committee at the Georgia Institute of Technology and conformed to guidelines by the National Institutes of Health.
Subjects
Male C57BL6J (RRID: IMSR_JAX:000664) and CNTNAP2−/− (RRID: IMSR_JAX:017482) mice (5–8 weeks old; reverse light cycle individual housing; bred in house) were used for this study. C57BL6J mice are referred to as wildtype (WT), and CNTNAP2−/− referred to as KO. As in prior studies of ASD mice (Goel et al. 2018), we used separate litters of WT and KO mice (rather than littermate controls) because littermates with genotypes different than the dam tend to receive unequal attention (Zupan and Toth 2008), potentially systematically biasing comparisons between WT and KO mice littermates. To further ensure adequate parenting, we cross-fostered litters of KO mice pups with experienced and attentive dams, as in our previous studies (Sato et al. 2016).
Surgery
Mice were chronically implanted with a stainless steel headplate with a recording chamber during isoflurane (1–2%) anesthesia. The headplate was affixed to the skull using thin layer of veterinary glue (Vetbond) and secured using dental cement (Metabond). The recording chamber was sealed with a removable polymer (KwikCast). After implant surgery, mice were allowed to recover for 3 days before experimentation. During recovery, mice were habituated to experimenter handling.
Behavior
Water Restriction
Following recovery from surgery, mice were placed under a restricted water schedule (to provide motivation) and trained to detect visual stimuli for water reward. Mice received a daily minimum amount of water of 40 mL/kg/day (Burgess et al. 2017; Speed et al. 2019). If mice did not receive their daily minimum water in task, they received supplemental hydration (Hydrogel).
Training
Mice first learned to associate visual stimuli with water reward through passive instrumental conditioning. For naïve mice to learn this association, water reward was delivered 0.7 s after the onset of a visual stimulus (see “Visual stimuli”). Following reward consumption, mice then had to withhold from licking for a mandatory period of time (exponentially distributed intervals from 0.5–6 s, randomly selected per trial) in order for visual stimuli to appear on subsequent trials. Lick times were measured with custom built contactless lick detectors (Williams et al. 2018). Typically, within 3–7 days of training, mice began licking shortly after stimulus onset and prior to reward delivery (anticipatory licking), indicating behavioral responses to the onset of the visual stimulus. Mice were then transitioned to an active paradigm where they only received rewards contingent upon licking during the stimulus presentation (typically 1 s long). On 20% of trials, 0% contrast stimuli were presented in order to measure the probability of licking to the absence of visual stimuli (false alarms). When detection performance was above chance for 2 consecutive days (d'>0, see `Behavioral Metrics'), the contrast and/or size of stimuli were decreased to maintain task difficulty. The main conclusions of this study involve detection of stimuli at a single position centered at the vertical meridian in the binocular visual field. Once performance was above chance for a range of low and high contrasts on binocular trials (2–33% contrast), we performed acute extracellular recordings.
Behavioral Metrics
Detection performance was quantified with the psychometric sensitivity index (d’ [Green and Swets 1974], which was calculated as
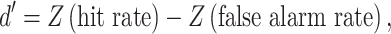 |
where Z represents the inverse of the normal cumulative distribution (MATLAB function norminv). Response bias or criterion (c) was calculated using the formula:
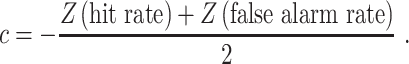 |
Higher criterion indicates more conservative response bias (withholding responses).
Recordings
Surgical Preparation
A small craniotomy (100–400 microns) was opened over binocular V1 (0.5 mm anterior from lambda, 3 mm lateral from midline) during isoflurane anesthesia. Mice were allowed ≥3 h of recovery before awake acute recordings. There was no difference in behavioral performance in WT mice during recordings (d’: 1.7 ± 0.5) versus the previous day (1.7 ± 0.2, P = 0.6, signed rank test). To remove any potential effect of anesthesia or surgery on perceptual performance in KO mice, craniotomies were performed 12–24 h prior to recordings to ensure equally robust behavioral performance during recordings (d’: 1.7 ± 0.2 Vs. 1.5 ± 0.3, P = 0.4). For recordings during anesthesia, mice were given a combination of sedative chlorprothixene (0.1 mg/kg) and isoflurane (0.5–1%), as in our previous studies (Haider et al. 2016).
Electrophysiology
Single shank linear 32 site silicon probes (Neuronexus, A1x32) were used to record neural activity across cortical layers. The electrode was typically advanced to 1000 microns below the dura, and the site was covered in sterile artificial cerebrospinal fluid (aCSF). Recordings typically lasted 90 min, whereupon the probe was removed and the site cleaned with sterile aCSF and covered with polymer (KwikCast). Typically, we were able to record 3 consecutive days from the same craniotomy.
Visual Stimuli
During behavior, mice detected Gabor gratings (0.05–0.1 cycles/°, σ = 10–20°, horizontal orientation, phase randomized per trial) presented on a linearized LCD monitor (DELL U2417H). Low-contrast (5%) task-irrelevant bars (9° wide, 0.1 s duration, interstimulus interval of 0.3 s, vertical orientation) were also presented during the intertrial intervals to measure receptive fields; these brief and faint bars did not affect behavioral performance and they are not analyzed here. At the completion of the behavioral session, these bars were also presented at a range of contrasts (5–100%) to map the receptive field. These same stimuli were used to measure visual responses in anesthetized and awake mice (that were not performing the behavioral task). The bar at the center of the receptive field and the adjacent ±1 bars were used in all analyses of visually evoked responses.
Eye Tracking
We recorded the animal’s pupil during awake recordings. A high-speed camera (Imaging source DMK 21Bu04.H) with a zoom lens (Navitar 7000) and infrared filter (Mightex, 092/52x0.75) was placed ~22 cm from the animal’s right eye. A near-infrared LED (Mightex, SLS-02008-A) illuminated the eye. Video files were acquired and processed using the Image Acquisition Toolbox in MATLAB with custom code; 1 mm corresponded to ~74 pixels on each frame.
Electroretinography
We tested retinal photoreceptor function using full-field flash electroretinography (ERG) as previously described (Mees et al. 2019). Briefly, after overnight dark-adaptation, we anesthetized mice (ketamine 60 mg/kg, xylazine 7.5 mg/kg) under dim red light, anesthetized corneas with tetracaine (0.5%; Alcon), and dilated pupils with tropicamide (1%; Sandoz). Binocular retinal responses were measured via gold-loop corneal electrodes, with platinum needle electrodes serving as reference and ground in the cheeks and tail, respectively. Testing consisted of six scotopic flashes (−4.86–2.5 log cd*s/m2), followed by 10 min of light adaption (30 cd/m2) and three photopic flashes (−0.2–1.4 log cd*s/m2). Responses were differentially amplified (1–1500 Hz, 250 ms, 2 kHz) and stored (UTAS BigShot). We measured amplitude and implicit time for a and b waves (Penn and Hagins 1969) and averaged the traces from right and left eyes for statistical analysis.
Analysis
Spike Sorting
Electrical signals were acquired through a Cereplex Direct (Blackrock Microsystems). Raw neural signals were acquired at 30 kHz, and single-unit activity was isolated with a semi-automated sorting algorithm (Rossant et al. 2016), as detailed in our previous studies (Speed et al. 2019). We classified single units as fast spiking (FS, waveform peak-to-trough <0.57 ms) and regular spiking (RS, peak-to-trough >0.57 ms) based on their waveform widths (Supplementary Fig. S3). In mice, >85% of RS neurons are putative excitatory neurons, whereas FS neurons are nearly exclusively parvalbumin-positive inhibitory neurons (Pfeffer et al. 2013), as optogenetically verified in our prior study (Speed et al. 2019).
LFP Analysis
Local field potentials (LFP) were band pass filtered at 0.3–200 Hz. Layers were identified via current source density analysis (Niell and Stryker 2008; Speed et al. 2019) and laminar LFP responses were calculated by taking the average across channels spanning particular layers. We analyzed the residual LFP power in hit and miss trials in the low-frequency band (2–20 Hz). We calculated the residual LFP power by fitting the entire power spectrum with a single exponential that excluded the bandwidth of interest. In this bandwidth, residual LFP power is the difference between the measured power and power of the fit, normalized by the fit (Saleem et al. 2017; Speed et al. 2019).
LFP Receiver Operating Curve Analysis
Receiver operating curves were constructed to measure the discriminability of hit and miss trial based on low-frequency (3–10 Hz) residual power. The area under the receiver operating curve (AUROC) was calculated, and error bars were obtained by bootstrap resampling 100 times.
LFP–Behavior Correlations
Reaction times were split into quartiles within each recording. The average residual power or stimulus-evoked spiking activity was then averaged for each quartile. A linear regression model was then fit to the data to determine if there was a correlation between neural activity and reaction time. Error bars were obtained by bootstrap resampling and repeating the fitting procedure 50 times. Distributions of partial correlations controlling for contrasts were constructed in a similar fashion (Fig. 5C).
Figure 5 .
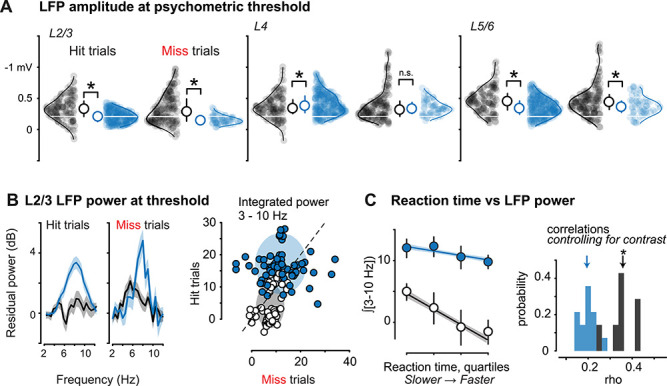
Aberrant neural activity in L2/3 correlates with perceptual impairments in KO mice. (A) L2/3 stimulus-evoked LFP amplitudes at discrimination threshold (see Fig. 1J–K) are reduced on both correct and incorrect trials (WT Hits: −0.33 ± 0.017 mV, mean ± SEM throughout figure, n = 533 trials, seven recordings in three mice; KO Hits: −0.21 ± 0.007 mV, n = 1402 trials, 15 recordings in 3 mice, P < 0.01, Wilcoxon rank sum; WT Misses: −0.33 ± 0.020 mV, n = 211 trials; KO Misses = −0.16 ± 0.009 mV, n = 423 trials, P < 0.01). Ordinate reversed for visualization (greater negativity indicates stronger LFP response). Middle, L4 responses on Hit (WT: −0.36 ± 0.017 mV; KO = −0.41 ± 0.011, P < 0.05) and Miss trials (WT: −0.38 ± 0.020 mV; KO: −0.35 ± 0.016 mV, P = 0.52). Right, L5/6 responses on Hit (WT: −0.46 ± 0.018 mV, KO = −0.36 ± 0.010 mV, P < 0.01) and Miss trials (WT: −0.48 ± 0.021.0, KO = −0.38 ± 0.021, P < 0.05). L4 and L5/6 responses during detection are not reduced to the same magnitude as L2/3 in KO mice (L2/3 median in KO mice indicated by white horizontal line). (B) Left, significantly elevated low-frequency (3–10 Hz) LFP power in L2/3 of KO versus WT mice on both Hit trials (KO: 13.27 ± 1.55; WT: 2.45 ± 1.78; P < 0.01) and Miss trials (KO: 11.98 ± 2.51; WT: 7.03 ± 1.57; P < 0.05). Mean ± SEM of integrated power 3–10 Hz at psychometric threshold. See Methods for residual power calculation. Right, integrated 3–10 Hz residual power was greater on Misses versus Hits in WT mice (Hits: 4.01 ± 0.69; Misses: 7.56 ± 0.48, P < 0.01, sign rank), but not in KO mice (Hits: 17.22 ± 0.76; Misses: 11.95 ± 0.93, P < 0.01, sign rank). Shaded regions show 2D Gaussian fit (±1σ). Power calculated per channel recorded in L2/3 (WT: n = 58, KO: 68). (C) Left: low-frequency LFP power was significantly and negatively correlated with reaction time in WT mice (linear regression model: 48 ± 12% variance explained within mouse, P < 0.05, r2 = 0.35; P < 0.05), but not in KO mice (31 ± 8% within mouse, P = 0.22, r2 = 0.19; P = 0.08). Single trial reaction times (Hit trials) were binned into quartiles, and single trial integrated 3–10 Hz LFP power was averaged within quartile for all WT and KO trials (mean ± SEM). Shaded regions are bootstrap error of fits (see Methods). Right: correlations between low frequency LFP power and reaction time are not explained by contrast. Partial correlations between the L2/3 power and reaction time, accounting for contrast, were significant and greater in WT mice than in KO mice (WT: 0.36 ± 0.06, P < 0.01; KO: 0.19 ± 0.04, P = 0.08; see Methods).
Pupil Analysis
Raw video frames were cropped to isolate the eye and pupil. Frames were smoothed with a 2D Gaussian filter. Based on pixel intensity, the pupil was identified and a least-squares error 2D ellipse was fit to the contours. The pupil area was determined by the number of pixels in the ellipse. ΔPupil area was calculated as the percent deviation from the mean:
Δ Pupil area = 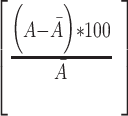 ,where A is the area in pixels and Ā is the average area across all frames. Similarly, the change in pupil position (in azimuth) was calculated by subtracting the average position across all frames.
,where A is the area in pixels and Ā is the average area across all frames. Similarly, the change in pupil position (in azimuth) was calculated by subtracting the average position across all frames.
Stimulus-Evoked Analysis
Visually evoked firing rates were calculated as the difference between prestimulus activity (0.1 s preceding the stimulus onset) and poststimulus activity (anesthetized: 0–0.25 s; awake, no task: 0–0.125 s; awake, grating responses during task: 0–0.2 s). These windows were chosen based upon the duration of the LFP responses in each condition (Supplementary Fig. S3). Violin plots of individual data points in all figures show 95% of the data range (±2.5% of range clipped for display). All statistics used full data ranges.
Psychometric Analysis
Weibull functions were fit to the hit rate and d’ data (Fig. 1).
Figure 1 .
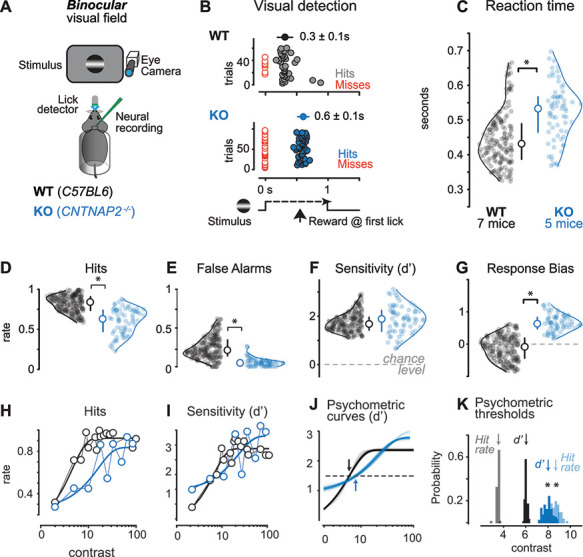
Visual perceptual behavior is impaired in the CNTNAP2−/− mouse model of ASD. (A) Head-fixed mice reported detection of visual stimuli in the binocular visual field by licking for water. Pupil activity, neural activity, and licking were recorded simultaneously with behavior. C57BL6J (Wildtype, WT) in black, CNTNAP2−/− (Knockout, KO) in blue throughout. (B) Example behavioral session shows detection latency (reaction time) was markedly slower for KO versus WT mice. Stimulus time course shown at bottom, with first lick times on correct trials (hits, colored circles) shown for individual consecutive trials (ordinate). Failures of detection (Misses) plotted in red. Average reaction times: WT, 0.3 ± 0.1 s; KO, 0.6 ± 0.1 s, mean ± SD reported throughout the figure. (C) KO mice detected stimuli significantly more slowly than WT mice (KO: 0.52 ± 0.08, 71 sessions, 5 mice; WT: 0.45 ± 0.08, 187 sessions, 7 mice; P < 0.01, Wilcoxon rank sum throughout the figure). Average stimulus contrast was not significantly different across KO and WT mice (WT: 23 ± 24%; KO: 23 ± 22%). Circles show reaction time average per session, smooth lines show kernel density estimates of distributions. Median ± IQR plotted inside the distributions. (D) KO mice showed significantly lower hit rates (KO: 0.6 ± 0.18; WT: 0.82 ± 0.12; P < 0.01). (E) KO mice showed significantly lower false alarm rates (KO: 0.06 ± 0.07; WT: 0.24 ± 0.15; P < 0.01). (F) Sensitivity index (d’) was not different between KO and WT mice (KO: 1.84 ± 0.71; WT: 1.74 ± 0.47; P = 0.1). (G) KO mice showed higher criterion (c), indicating increased bias to withhold from responding (WT: 0.12 ± 0.43; KO: 0.65 ± 0.29; P < 0.01). Criterion was significantly >0 for KO mice, but not for WT mice (WT: P = 0.06; KO: P < 0.01). (C–G) All during same behavioral trials and same mice. (H) Hit rate as a function of contrast. Dark line is a psychometric fit (see Methods). (I) Same as H for d’. (H–I) During same sessions in same mice as C–G. (J) Psychometric fit reliability from data in I (see Methods). Dashed line indicates d’ threshold (1.5) and arrows indicate mean contrast values at threshold. Curves show 100 overlaid fits by resampling (see Methods). (K) Contrast thresholds for hit rate (at 50% correct) and d’ (at 1.5), both significantly elevated in KO versus WT mice (contrast at hit rate threshold: WT = 3.5 ± 0.1%, KO = 8.7 ± 0.4%, P < 0.01; contrast at d’ threshold: WT = 6.0 ± 0.1%, KO = 8.0 ± 0.4%, P < 0.01). Arrows indicate means of resampled psychometric curve threshold distributions (see Methods).
Weibull Fit = 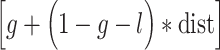 ,where g is the guess rate, l is the lapse rate, and dist is defined as the cumulative Gaussian distribution:
,where g is the guess rate, l is the lapse rate, and dist is defined as the cumulative Gaussian distribution:
dist = 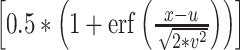 ,where erf is the error function (“erf” in MATLAB), u is the mean value, and v is the standard deviation (Wichmann and Hill, 2001).
,where erf is the error function (“erf” in MATLAB), u is the mean value, and v is the standard deviation (Wichmann and Hill, 2001).
Stimulus contrast values at hit rate threshold (0.5) and d’ threshold (1.5) were calculated from fits constructed by jackknife resampling (10% of the data left out, 100 iterations) and generating fits as in previous studies (Busse et al. 2011). A d’ threshold of 1.5 was chosen because it falls approximately halfway between the minimum and maximum points of the WT d’ psychometric curve and is consistent with high-quality perceptual performance, as detailed in our previous studies (Speed et al. 2019, 2020).
Contrast Sensitivity Analysis
Stimulus-evoked single-unit activity curves for passive awake recordings were constructed from responses to flashing bars at the center of the receptive field (5–100% contrast). Single-unit activity during behavior was binned between 5 and 35% (bin size = 10 ms; Fig. 3). A linear regression model was fit to the firing rate data as a function of contrast to calculate the slope. A resampling approach as described above (100 resamples) was used to construct distributions of estimated slopes.
Figure 3 .
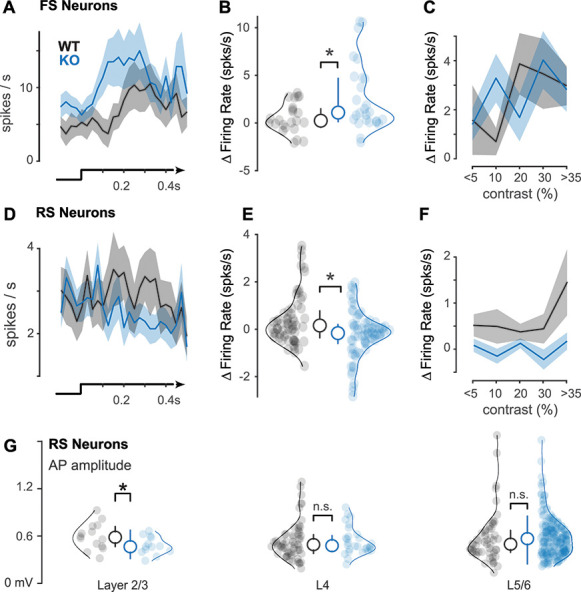
Enhanced FS and diminished RS visual responses at discrimination threshold. (A) Peristimulus time histograms of FS neurons during perceptual detection of visual stimuli (bottom) at contrasts defined by the discrimination threshold (~5% for WT mice, ~10% for KO mice, Fig. 1I–K). (B) KO mice have enhanced FS responses to visual stimuli during perceptual detection (WT: 1.58 ± 1.27 spikes/s, n = 20 neurons, mean ± SEM throughout figure; KO: 3.29 ± 1.15, n = 38 neurons, P < 0.05, one-tail Wilcoxon rank sum test; Δfiring rate calculated as difference from prestimulus baseline, see Methods) (C) FS neuron responses as a function of contrast (binned; see Methods). (D) Same as A, for RS neurons. (E) Same as B, for RS neurons. KO mice have diminished RS responses during perceptual detection (WT: 0.52 ± 0.21 spikes/s, n = 49 neurons; KO: −0.15 ± 0.18, n = 103 neurons, P < 0.01, one-tail Wilcoxon rank sum test). (F) Same as C, for RS neurons. (G) Action potential (AP) amplitudes significantly smaller in L2/3 RS neurons in KO mice (0.48 ± 0.03 mV; mean ± SEM, n = 13; 0.59 ± 0.04 mV, n = 14; P < 0.05, one-tail Wilcoxon rank sum test). No differences in L4 (KO: 0.54 ± 0.03; n = 28; WT: 0.54 ± 0.03, n = 59; P = 0.35) or L5/6 (KO: 0.59 ± 0.02; n = 178; WT: 0.58 ± 0.04, n = 53; P = 0.12). Neurons aggregated across awake recordings. Median ± IQR plotted inside distributions throughout figure.
Awake LFP Visual Responses Analysis
Weibull functions were fit to the LFP contrast response curves (Fig. 4). A resampling approach as described above was used to construct distributions of the difference in LFP amplitude between WT and KO mice.
Figure 4 .
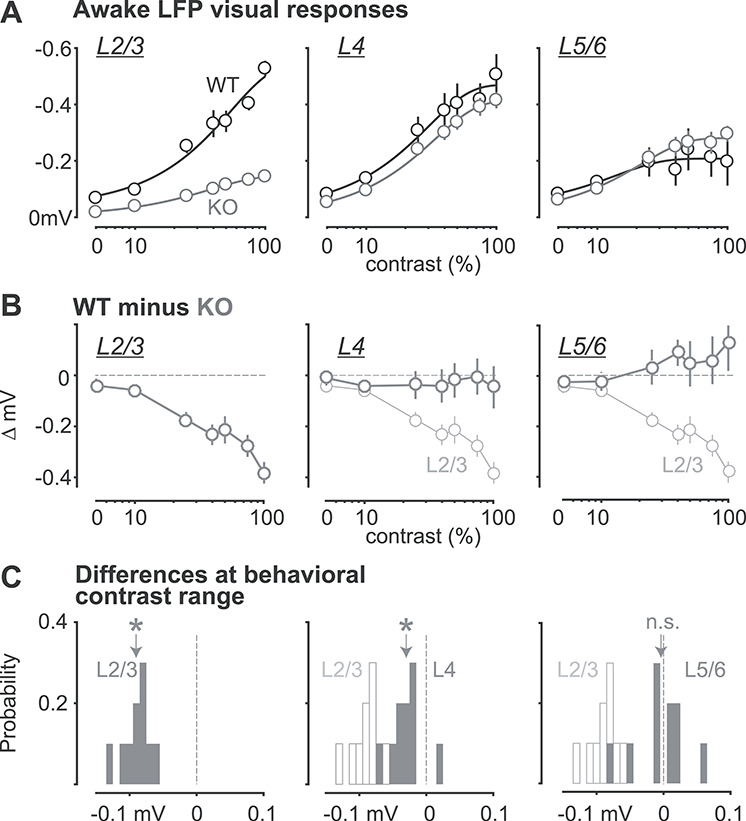
Strong reduction of LFP responses in L2/3 of KO mice. (A) Contrast dependence of awake LFP responses, split by layers (L2/3, L4, L5/6; left to right). Ordinate reversed for visualization (greater negativity indicates stronger LFP response). Dark lines are Weibull fits (see Methods). (B) Difference between WT and KO LFP contrast responses. L2/3 (left, gray) has the greatest reduction in stimulus-evoked LFP compared with L4 (middle), and L5/6 (right). L2/3: −0.19 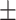 0.014 mV, mean ± SEM, P < 0.01; L4: −0.029 ± 0.007 mV, P = 0.23; L5/6: 0.046
0.014 mV, mean ± SEM, P < 0.01; L4: −0.029 ± 0.007 mV, P = 0.23; L5/6: 0.046 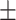 0.011 mV, P < 0.01, repeated measurements ANOVA followed by multiple comparisons, significant effect of layer. (C) Probability distributions of LFP amplitude differences at contrasts matching behavioral range (2–35%; see Methods). L2/3 responses (left) in KO mice significantly reduced (−0.09 ± 0.002 mV, P < 0.01, signed rank). L4 responses slightly but significantly reduced (−0.03 ± 0.003 mV, P < 0.05). L5/6 not different (−0.004 ± 0.004 mV, P = 0.77), distributions. Open histograms replot L2/3 distribution for comparison to L4 and L5/6. Arrows indicate means.
0.011 mV, P < 0.01, repeated measurements ANOVA followed by multiple comparisons, significant effect of layer. (C) Probability distributions of LFP amplitude differences at contrasts matching behavioral range (2–35%; see Methods). L2/3 responses (left) in KO mice significantly reduced (−0.09 ± 0.002 mV, P < 0.01, signed rank). L4 responses slightly but significantly reduced (−0.03 ± 0.003 mV, P < 0.05). L5/6 not different (−0.004 ± 0.004 mV, P = 0.77), distributions. Open histograms replot L2/3 distribution for comparison to L4 and L5/6. Arrows indicate means.
Spike–LFP Phase Locking Analysis
Phase locking was measured using a Hilbert phase transform of the spike triggered LFP in a 1 s window (−0.5–0.5) centered at each spike in the prestimulus period; 180° corresponds to an LFP trough. Statistics were calculated using the Circular Statistics Toolbox in MATLAB (Berens 2009).
Experimental Design and Statistical Analysis
Our experimental design centered on measuring neural activity and behavior during identical sensory conditions, and comparing these across KO and WT mice matched in age, sex, recording region, and methods. Experimenters were not blinded to the group identity of each subject, but this was not required for performing identical automated measurements of behavioral performance or identical electrophysiology data analysis. We performed these studies in comparable numbers of subjects and experiments across groups. Throughout this paper, unpaired comparisons utilized Wilcoxon rank sum tests (tails specified) or sign tests (for differences from scalar values), and paired comparisons utilized Wilcoxon signed rank tests, unless otherwise noted.
Results
We trained both C57BL6J (WT) and CNTNAP2−/− KO mice to report perception of spatially localized visual stimuli. Mice were head-fixed and rested in a semi-enclosed plastic tube, eliminating complicated interactions of visual responses and locomotion that can be detrimental for psychometric visual detection behavior (Neske et al. 2019). Mice learned to lick for water rewards when static visual stimuli (horizontally oriented Gabor gratings, σ = 10–20°, see Methods) appeared on a screen (Fig. 1A). Stimuli appeared only after a mandatory period of no licking (0.5–6 s, randomized per trial), and rewards were delivered only upon the first lick during the stimulus response window (typically 1–1.5 s). We quantified perceptual performance using signal detection theory (Green and Swets 1974). Our prior studies in WT mice showed that stimulus detection in the peripheral (monocular) visual field is more difficult than detection in the central (binocular) visual field (Speed et al. 2019). We found that KO mice had lower detection sensitivity than WT mice for stimuli appearing in these more difficult (monocular) spatial locations (P < 0.01, rank sum). Since the goal of this study was to examine neural activity deficits in KO mice during repeatable and robust measurements of perceptual performance, here we focused on examining neural correlates of visual detection in the binocular visual field (central ±20°; stimulus centered at vertical meridian). This allowed us to measure large numbers of correct and incorrect trials of stimulus detection in WT and KO mice at the exact same spatial locations, stimulus sizes, stimulus durations, and stimulus contrast ranges (see Methods).
We first examined overall detection speed and accuracy for stimuli aggregated across all contrasts. KO mice detected binocular visual stimuli less frequently and more slowly than WT mice. KO mice showed significantly slower reaction times (Fig. 1B,C), and significantly fewer correct (Hit) trials of stimulus detection (Fig. 1D) than WT mice. However, KO mice also made fewer false alarms, which led to overall detection sensitivity (d’) that did not differ significantly from WT mice (Fig. 1E,F, see Methods). These measurements also revealed that KO mice held a significantly more conservative response bias than WT mice (Fig. 1G). This conservative response bias was not simply explained by lower task engagement—in fact, KO mice showed slightly higher arousal before stimulus onset than WT mice (measured via pupil area; Supplementary Fig. S1). In addition, the rate of premature responses (stray licks) was significantly higher in WT than KO mice (2.42 ± 0.11; 1.41 ± 0.16 stray licks/trial; P < 0.01, rank sum test), suggesting that higher arousal in KO mice did not increase impulsivity or distractibility (Fonseca et al. 2015). Moreover, premature responses in KO mice were themselves not modulated by arousal (1.5 ± 0.1 stray licks/trial vs. 1.6 ± 0.1, sorted relative to median pupil area; P = 0.73, rank sum test), whereas response vigor (licking frequency) for reward was nearly equal for KO and WT mice (Supplementary Fig. S1). These results suggest that neither motor aspects of licking (Travers et al. 1997) nor lower motivation assessed via licking frequency (Berditchevskaia et al. 2016; Swanson et al. 2019; Amarante and Laubach 2020) explain impairments in the overall rate and speed of detection in KO mice. We next examined how trial-by-trial properties of the visual stimulus affected behavioral performance.
Psychometric detection of stimulus contrast was significantly impaired in KO mice. KO mice showed far lower hit rates for stimuli of low and medium contrast as compared with WT mice (Fig. 1H), with little reduction for high-contrast stimuli. Accordingly, psychometric detection sensitivity (d’) as a function of contrast was both reduced and less steep in KO mice (Fig. 1I). We fit psychometric functions (for both hit rates and d’) using a resampling approach across subsets of the data (Busse et al. 2011) and found that KO mice showed a significantly shallower slope of the psychometric function (Supplementary Fig. S2) and elevated threshold contrast (Fig. 1K; threshold defined at d’ ≥1.5). The weaker behavioral sensitivity to contrast in KO mice was also evident in reaction times during these same trials (Supplementary Fig. S2). Taken together, these results show that KO mice have impaired psychometric sensitivity when considering detection across a range of stimulus contrast.
We next found that excitatory and inhibitory neuron visual responses were altered in KO mice, but only in wakefulness. We measured FS (putative inhibitory) and RS (putative excitatory) neuron populations in primary visual cortex (V1) (Fig. 2 and Supplementary Fig. S3) with silicon probe recordings across layers of V1. We did not impose any exclusion criteria for firing rates or visual selectivity and assessed overall neuronal population responses. During anesthesia, we observed no differences between KO and WT mice in the overall distributions of visually evoked spiking in either RS or FS neurons (Fig. 2C,D). However, recordings during wakefulness (but in the absence of the behavioral task) revealed that visually evoked spiking of FS neurons in KO mice was significantly elevated versus WTs, whereas RS neuron responses were significantly reduced during the same recordings (Fig. 2E–H).
Figure 2 .
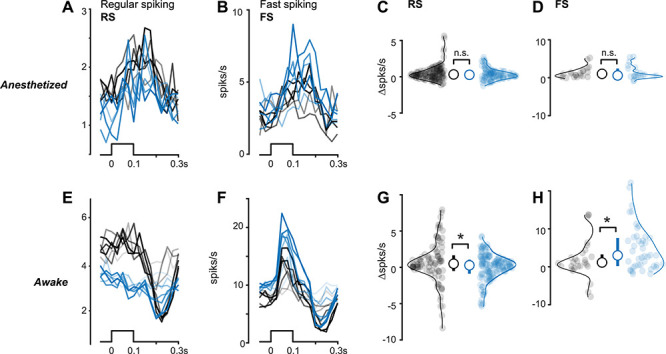
Reduced excitatory and elevated inhibitory neuron activity in KO mice depends upon brain state. (A) Regular spiking (RS) putative excitatory neuron firing to black or white bars (bottom) presented from 5 to 100% contrast during anesthesia. Stimulus contrast level indicated by line transparency. Spikes binned at 25 ms. (B) Same as A for fast spiking (FS) putative inhibitory neurons. (C) No significant difference between stimulus-evoked activity in RS neurons during anesthesia (WT: 0.51 ± 0.12 spikes/s, n = 129 neurons; mean ± SEM throughout figure; KO: 0.32 ± 0.09, n = 96 neurons, P = 0.27, one-tail Wilcoxon rank sum test throughout the figure). (D) Same as C for FS neurons (WT: 1.44 ± 0.44 spikes/s, n = 23 neurons; KO: 1.43 ± 0.45, n = 22 neurons, P = 0.49). Median ± IQR plotted inside distributions. (E) Same as A, during wakefulness. (F) Same as B, during wakefulness. (G) Same as C, during wakefulness. RS neuron responses are significantly reduced in KO mice during wakefulness (WT: 0.49 ± 0.37, n = 95 neurons; KO: 0.03 ± 0.25, n = 131 neurons, P < 0.05). (H) Same as D, during wakefulness. FS neuron responses are significantly elevated in KO mice during wakefulness (WT: 1.90 ± 1.19, n = 29 neurons; KO: 5.10 ± 1.04, n = 45 neurons, P < 0.05).
Consistent with behavioral impairments, neural responses in awake KO mice also showed aberrant contrast dependence. We plotted spiking as a function of contrast (spaced from 5 to 100% contrast; Supplementary Fig. S4) and found that the contrast dependence of RS neuron firing in KO mice was significantly weaker than WT mice (slope of firing rate vs. contrast: WT, 0.5 ± 0.05; KO, −0.21 ± 0.02; P < 0.01; Wilcoxon rank sum; mean ± standard deviation [SD]); during these same experiments; FS neurons in KO mice had steeper contrast dependence than WT mice (linear fits, slope of firing rate vs. contrast: KO, 6.1 ± 0.3; WT, 4.3 ± 0.3; P < 0.01; Wilcoxon rank sum; mean ± SD). These differences in KO mice were not explained by differences in excitability or visual selectivity: Spontaneous firing rates (no stimulus) were not different in awake WT and KO mice (RS: WT = 2.55 ± 0.32; KO = 2.96 ± 0.32; P = 0.39; FS: WT = 6.16 ± 1.35; KO = 6.53 ± 1.14; P = 0.43, single-tail rank sum test), and receptive field properties (spatial profile, amplitude, and latency) were comparable in WT and KO mice (Supplementary Figs S3 and S4). Lastly, perceptual impairments were not explainable by slower or diminished visual responses starting at the level of photoreceptors— ERG responses were nearly identical in KO and WT mice across a wide range of light intensities (Supplementary Fig. S4).
During perceptual behavior, KO mice showed elevated FS activity and reduced RS activity, along with weaker contrast dependence. Recordings in binocular V1 (Supplementary Fig. S3) during binocular stimulus detection revealed an overall greater number of visually evoked spikes in FS neurons in KO versus WT mice on correct (Hit) trials at perceptual threshold (Fig. 3B) and simultaneously fewer visually evoked spikes in RS neurons (Fig. 3E). These effects were observed in the first 0.2 s following stimulus onset, but were evident even when including spikes up to 0.3 s after stimulus onset (not shown). Reduced RS neuron firing in KO mice was not simply explained by poorer orientation tuning for the detected stimulus (Supplementary Fig. S3). During behavior, the relationship between stimulus contrast and firing rate was significantly weaker in KO versus WT mice, in both FS (contrast vs. firing rate linear fit slopes: WT, 5.6 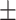 0.20; KO, 3.6 ± 0.01, P < 0.01, Wilcoxon rank sum test) and RS neurons (linear fit slopes: WT, 1.8
0.20; KO, 3.6 ± 0.01, P < 0.01, Wilcoxon rank sum test) and RS neurons (linear fit slopes: WT, 1.8 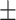 0.04; KO, 0.1 ± 0.01, P < 0.01, Wilcoxon rank sum test). Overall, the weakest relationship between stimulus contrast and firing rate was in RS neurons of KO mice (P < 0.01, Kruskal–Wallis test followed by multiple comparisons correction). Again, these changes in neural activity in KO mice during behavior were not due to lower task engagement: prestimulus pupil dilation in KO mice resulted in significantly higher (not lower) firing rates (Supplementary Fig. S5, r = 0.98, P < 0.01). Reduced contrast modulation of RS neurons during the task was consistent with weaker contrast modulation outside of the task (Supplementary Fig. S4), even though the stimulus contrast range was far lower during behavior (5–35% contrast).
0.04; KO, 0.1 ± 0.01, P < 0.01, Wilcoxon rank sum test). Overall, the weakest relationship between stimulus contrast and firing rate was in RS neurons of KO mice (P < 0.01, Kruskal–Wallis test followed by multiple comparisons correction). Again, these changes in neural activity in KO mice during behavior were not due to lower task engagement: prestimulus pupil dilation in KO mice resulted in significantly higher (not lower) firing rates (Supplementary Fig. S5, r = 0.98, P < 0.01). Reduced contrast modulation of RS neurons during the task was consistent with weaker contrast modulation outside of the task (Supplementary Fig. S4), even though the stimulus contrast range was far lower during behavior (5–35% contrast).
Deficits in RS neuron activity in KO mice were most pronounced in layers 2/3 (L2/3). We first examined spiking responses across layers (identified using current source density analysis; Supplementary Fig. S3) and found that we isolated far fewer neurons in L2/3 of KO versus WT mice. We aggregated across all awake recordings (both inside and outside of the task) and found that L2/3 excitatory neurons in KO versus WT mice showed slightly but significantly smaller action potential waveform amplitudes (Fig. 3G), consistent with prior reports (Scott et al. 2019). Importantly, in these same recording sessions, RS and FS neurons in L4 and L5/6 did not show significant differences in action potential (AP) amplitudes or activity profiles (L4: FS, P = 0.27; RS, P = 0.35; L5/6: FS, P = 0.29, RS, P = 0.12; rank sum tests), arguing against a global deficit in action potentials (APs) in KO mice. We performed several control measures to assess if the lack of active L2/3 RS neurons was due to experimental conditions in KO mice. First, unresolvable background activity levels were significantly lower in KO versus WT mice, whereas AP amplitudes in all layers were several orders of magnitude larger than the background (Supplementary Fig. S6). Second, the smallest fraction of RS neurons were isolated in L2/3 of both WT and KO mice, (WT: 4.2 ± 3.0%, n = 15 recordings; KO: 0.5 ± 0.4%, n = 24 recordings; mean ± standard error of the mean [SEM], P = 0.13, one-tail rank sum test). Lastly, the fraction of L2/3 RS neurons isolated across multiple consecutive recording sessions (spanning 1–2 days after the initial craniotomy) was not different in WT and KO mice (WT, 66.7% of total isolated after initial craniotomy; KO, 51% of total), suggesting that the quality and longevity of experimental preparations were comparable in WT and KO mice.
Neural population responses were strongly reduced in L2/3 of KO mice. In awake, nontask conditions, the L2/3 LFP is directly related to aggregate synaptic activity in the local network (Haider et al. 2016). Here, we found that L2/3 LFP visual responses in KO mice were specifically and significantly reduced at all contrasts compared with responses in WT mice (Fig. 4A,B). Using the range of contrasts presented during the behavioral task (5–35%), we measured the difference in LFP response amplitude between WT and KO mice within layers and found the largest reductions in L2/3 (Fig. 4C; P < 0.01, repeated measures analysis of variance followed by correction for multiple comparisons, significant effect of layer).
Similarly, L2/3 LFP responses in KO mice were also weaker during perceptual behavior. On both correct and incorrect detection trials (at psychometric threshold contrasts), L2/3 LFP responses to gratings were significantly smaller than those in WT mice (Fig. 5A), but with no significant delay in response latency (L2/3: P = 0.65; L4; P = 0.8; L5/6; P = 0.7), consistent with prior findings (Bertone et al. 2005). These weaker LFP responses in L2/3 of KO mice were evident despite psychometric threshold stimuli appearing at higher contrast in KO versus WT mice.
During perceptual behavior, L2/3 LFP responses in KO mice also displayed aberrant low-frequency oscillations that degraded the relationship of neural activity to performance. Several prior studies in WT mice have shown that low frequency LFP power impairs stimulus detection (Einstein et al. 2017; McBride et al. 2019; Speed et al. 2019), as we also observed in WT mice here. Remarkably, in KO mice, stimulus-evoked 3–10 Hz LFP power was significantly elevated, both preceding (Supplementary Fig. S7) and during the stimulus for both Hit and Miss trials (Fig. 5B). When examined within recordings and within behavioral sessions, stimulus-evoked low-frequency LFP power in L2/3 was significantly greater in KO mice on Hit rather than Miss trials (Fig. 5B; integrated 3–10 Hz power in KO mice: Hits, 17.2 ± 0.8; Misses, 12.0 ± 0.9, P < 0.01 Wilcoxon signed rank test; in WT mice: Hits, 4.0 ± 0.7; Misses, 7.6 ± 0.5, P < 0.01, Wilcoxon signed rank test; mean ± SEM) and provided poorer decoding of perceptual outcome at threshold (AUROC for Hits vs. Misses: WT = 0.541 ± 0.003, mean ± SEM, KO = 0.525 ± 0.002, P < 0.01, Wilcoxon rank sum). Moreover, in WT mice, higher L2/3 LFP power was significantly correlated with slower reaction times (Fig. 5C, linear regression model: 48 ± 12% variance explained within mouse; 18 ± 1% variance explained across mice, P < 0.05), but this relationship was weaker in KO mice (31 ± 8% within mouse; 3 ± 1% across mice, P = 0.22). Elevated 3–10 Hz power in KO mice was uncorrelated with RS neuron firing rates (Supplementary Fig. S5) and resulted in poorer spike–LFP phase locking in KO mice (Supplementary Fig. S7), consistent with previous reports (Lazaro et al. 2019). Importantly, even when we controlled for the effect of contrast on reaction times (partial correlation), there remained a significant predictive relationship between stimulus-evoked L2/3 LFP power and reaction times in WT mice, but this relationship between LFP power and reaction time was significantly diminished in KO mice (Fig 5C).
Finally, these multiple L2/3 excitatory activity deficits in KO mice were not due to differences in perceptual threshold stimulus contrast. We re-analyzed neural activity in WT mice at the same contrasts that KO mice required for equivalent performance (Fig. 1K) and found that this did not eliminate the reductions in stimulus-evoked RS neuron activity or LFP responses in L2/3, nor did this explain the elevated 3–10 Hz LFP power and reduced decoding of perceptual performance (Supplementary Figs S7 and S8). Similarly, even for high stimulus contrasts, visual responses showed reduced RS neuron activity and aberrant L2/3 LFP power (Supplementary Fig. S8). These results indicate that impairments in both the speed and accuracy of visual perception in KO mice are correlated with and predictable from distinct network-level neural activity deficits in L2/3.
Discussion
Here, we revealed diminished excitatory and elevated inhibitory activity in cortex during impaired sensory perception in a genetically relevant mouse model of ASD. Using a well-controlled, head-fixed visual detection task, we quantified psychometric performance in CNTNAP2−/− KO mice while recording V1 neural activity driven by spatially localized visual stimuli. KO mice detected visual stimuli more slowly and less frequently than WT mice and displayed specific deficits in psychometric sensitivity to contrast. These deficits were accompanied by reduced contrast responses in excitatory neurons and LFP in L2/3, despite higher perceptual threshold contrast in KO mice. Moreover, L2/3 LFP activity in KO mice was strongly synchronized at low frequencies (3–10 Hz) across all trial types, and this was detrimental for predicting the speed and accuracy of perceptual performance from L2/3 neural activity. Our results establish that impairments of perceptual performance in a transgenic mouse model of ASD are accompanied by increased inhibitory activity along with laminar-specific reduction of excitatory activity and abnormal network rhythms.
A first aspect of diminished behavioral performance in KO mice was slower stimulus detection. KO mice took longer than WT mice to detect the same range of stimulus contrasts and exhibited less improvement of reaction time with higher contrasts; these effects were not explained by lower arousal (assessed via pupil) or by impaired motor vigor (assessed via licking dynamics). Slower stimulus detection was not due to longer neural response latencies in KO versus WT mice: These were nearly identical from photoreceptors up to L4 of V1. This suggests that areas downstream of V1 likely play a role in altered sensory–motor integration that leads to longer reaction times. Although individuals with ASD can also show slower and more variable reaction times (Karalunas et al. 2014), this remains debated (van der Geest et al. 2001; Ferraro 2016), and the brain areas involved remain unknown. Our study establishes a platform to launch further detailed interrogation of neural activity deficits across multiple brain areas and determine their roles in perceptual impairments and delayed reaction times in transgenic mouse models of ASD.
A second aspect of diminished performance in KO mice was less frequent stimulus detection. Overall, KO mice showed lower hit rates than WT mice, despite stimuli appearing at the same locations and same visual contrast ranges across all experiments. However, KO mice also showed lower false alarm rates, leading to overall detection sensitivity (d’) that was not statistically different than WT mice. These concerted changes lead to significantly higher response criterion in KO versus WT mice. Importantly, this lower tendency to respond was not explained by diminished arousal in KO mice. Both changes in d’ and response criterion contribute to visual perceptual performance, and these two components of perception can be dissociated with appropriate tasks (Luo and Maunsell 2018). Future studies in transgenic mouse models could identify and manipulate brain areas responsible for criterion effects (Sridharan et al. 2017; Crapse et al. 2018; Jin and Glickfeld 2019) versus those more related to stimulus coding (Goris et al. 2017) to determine contributions of cortical areas to perceptual impairments. Our results here identify that these two aspects of signal detection contribute to overall performance deficits in KO mice, in addition to trial-by-trial psychometric deficits in contrast detection, discussed next.
A third aspect of diminished performance in KO mice involved reduced psychometric and neural sensitivity to stimulus contrast. Threshold detection performance required significantly higher stimulus contrast in KO than WT mice, and the rate of rise to saturating performance was significantly shallower in KO mice. This weaker relationship between perceptual behavior and stimulus contrast in KO mice was mirrored by weaker contrast dependence of both RS and FS neuron firing during the task. These results in KO mice establish that altered neural sensitivity to stimulus contrast in V1 accompanies reduced psychometric sensitivity to contrast. Although contrast dependence was markedly reduced in both FS and RS neurons during task performance, FS neurons showed steeper contrast sensitivity in awake nontask conditions. It is conceivable that an “imbalance” of elevated FS activity and reduced RS activity, coupled with reduced sensitivity to contrast, contributes to the higher perceptual threshold observed in KO mice.
Excitatory and inhibitory neuron activity deficits in KO mice depended upon brain state; we found clear differences during wakefulness, but not anesthesia (Haider et al. 2013), an important consideration for studies of cortical deficits in mouse models of ASD. Furthermore, in awake mice performing the task, reduced RS firing in KO mice was evident despite using small, static stimuli at low threshold contrasts; these stimuli drove neural activity far less vigorously than full screen, drifting, high-contrast stimuli typical of many studies of mouse V1 (Durand et al. 2016; Michaiel et al. 2019). Even in these conditions where sensory responses appear weak overall, there was significant predictability of task performance and reaction times from neural population responses (Fig. 5C and Supplementary Fig. S8). Recent studies in mice show that weakly driven or even “non-sensory” neurons nonetheless contribute to stimulus decoding (Zylberberg 2018; Insanally et al. 2019). To the best of our knowledge, our results are the first to measure sensory responses in ASD mice across multiple brain states and during perceptual behavior around psychometric threshold. Future studies should consider these factors and compare neural circuit deficits across multiple brain states, stimulus strengths, and behavioral outcomes, and across multiple transgenic mouse models of ASD that may display distinct adjustments to excitatory and inhibitory sensory processing (Antoine et al. 2019; Chen et al. 2020).
Perceptual impairments in KO mice were accompanied by several L2/3 excitatory activity deficits. First, sensory-evoked L2/3 RS firing rates were significantly lower in a manner that depended upon brain state, behavioral outcome, and stimulus contrast. Second, L2/3 LFP responses in KO versus WT mice showed the greatest reduction in contrast sensitivity. Third, L2/3 LFP responses elicited by detected visual stimuli were significantly smaller in KO versus WT mice. These multiple deficits in L2/3 excitatory neurons dovetail with recent findings in humans showing that ASD individuals show specific alterations in L2/3 cortical excitatory neurons (Velmeshev et al. 2019). Importantly, in both primates and rodents, L2/3 RS neurons provide the major source of feedforward visual information to higher cortical areas (Rockland and Pandya 1979; Glickfeld et al. 2013). Future studies recording synaptic activity in L2/3 neurons could reveal greater insight into synaptic mechanisms underlying sensory and perceptual impairments in KO mice.
Our findings contribute to a vigorous and unresolved debate about cortical neuron deficits and their complicated relationship to behavioral impairments in ASD mouse models. These conflicting results may arise from several factors, including but not limited to (1) sensory modalities, (2) recording methods, and (3) heterogeneity across transgenic models. Our study of CNTNAP2−/− KO mice showed reduced excitatory neural responses in V1 during psychometric impairments of contrast detection; in the somatosensory system, several recent studies in genetically relevant ASD models (Fmr1−/−, Shank3B−/−, Mecp2−/−) instead show hypersensitivity to tactile somatosensory stimulation (He et al. 2017; Orefice et al. 2019; Chen et al. 2020). Even within the same mouse line, different recording methods can lead to different pictures of cortical circuit excitability; for example, patch-clamp recordings from excitatory neurons in primary somatosensory (S1) cortex of Fmr1−/− mice in vitro and in vivo show several correlates of hyperexcitability (Contractor et al. 2015); accordingly, awake juvenile Fmr1−/− mice show behavioral hyperreactivity to whisker stimulation, but Ca2+ imaging reveals lower pyramidal neuron excitability, along with a lack of sensory adaptation (He et al. 2017). Shank3B−/− mice also show hyperreactivity to whisker stimulation, but in these mice, this is associated with elevated cortical excitatory neuron Ca2+ responses and reduced inhibitory neuron activity (Chen et al. 2020). Yet, other models (Syngap1−/−) show both lower whisker-based task performance, along with reduced Ca2+ responses in both excitatory and inhibitory L2/3 neurons (Michaelson et al. 2018). These handful of recent studies in the somatosensory system using similar methodologies illustrate the challenges and complexities in defining a common circuit phenotype in ASD models even with a similar behavioral phenotype. Our findings of reduced excitatory and elevated inhibitory activity in CNTNAP2−/− mice are at odds with a recent study of the same mouse line showing reduced inhibitory neuron responses in S1 (Antoine et al. 2019). However, this same study measured synaptic responses in L2/3 excitatory neurons of S1 in four different ASD model transgenic lines and found that CNTNAP2−/− excitatory neurons showed the lowest overall levels of synaptic excitation, consistent with other in vitro findings of reduced synaptic excitation in L2/3 of CNTNAP2−/− mice (Sacai et al. 2020), and with our findings here of reduced excitatory neuron activity in L2/3 of V1 during perceptual impairments. Future studies could help provide clarity by measuring awake sensory responses in both S1 and V1 of CNTNAP2−/− mice or by extending our visual perceptual task to any one of the transgenic lines described above. Indeed, a recent study of visual discrimination in Fmr1−/− mice shows impaired orientation discrimination and reduced V1 inhibitory neuron activity, but used full screen 100% contrast stimuli, whereas our study used small, spatially localized threshold contrast stimuli (2–10%), providing opportunities to examine how stimulus size, contrast, and orientation drive visual perceptual impairments in multiple ASD models.
Finally, we found that aberrant 3–10 Hz LFP oscillations in L2/3 of KO mice correlated with perceptual impairments. Several recent studies in WT mice showed that 3–10 Hz LFP power impairs visual detection (McBride et al. 2019) and predicts perceptual failures (Speed et al. 2019), consistent with the detrimental influence of these oscillations for visual coding in L2/3 (Einstein et al. 2017). Here, we found that KO mice showed significantly elevated 3–10 Hz LFP power across both correct and incorrect trials; these oscillations worsened the predictability of behavioral outcome from LFP activity and obscured the relationship of LFP activity to reaction times on correct trials. One limitation of these findings is that we cannot rule out the role of feedback from higher visual areas in L2/3 of V1, but this seems readily investigated by recording simultaneously from V1 and higher visual areas. Similarly, our results are correlational and did not make use of causal experiments as in several recent studies of ASD-relevant mice (Banerjee et al. 2016; He et al. 2017; Goel et al. 2018; Orefice et al. 2019; Chen et al. 2020), providing interpretational constraints for the direct role of excitatory or inhibitory neuron activity underlying the perceptual impairments we measured. Future studies of L2/3 deficits in KO mice could be bolstered with direct optogenetic manipulations during behavioral performance, either by alleviating excitatory neuron activity deficits or by minimizing low-frequency oscillations. For example, one recent study has directly induced low-frequency oscillations in primate visual cortex by optogenetically manipulating excitatory neurons, and these oscillations caused visual perceptual impairments (Nandy et al. 2019). An intriguing possibility is that low-frequency oscillations in L2/3 reduce the dynamic range available to transmit feedforward sensory information to downstream areas during behavior. This suggests that directly attenuating low-frequency synchronization in L2/3 neurons may restore excitatory signaling bandwidth and remedy perceptual deficits, a hypothesis that now seems testable in mouse models of ASD.
Authors’ Contributions
J.D.R., H.A., and A.S. performed behavioral experiments; J.D.R. and A.S. performed electrophysiological experiments; C.M. performed ERG experiments and data analysis with M.P.; J.D.R., A.S., and B.H. performed data analysis; J.D.R. and B.H. wrote the manuscript with feedback from all authors.
Funding
Goizueta Foundation Fellowship (to J.D.R.); Alfred P. Sloan Foundation’s Minority Ph.D. (MPHD) Program Fellowship, awarded in 2018–19 (to J.D.R.); Georgia Tech Neural Engineering Center (1241384 to B.H.); the Whitehall Foundation (to B.H.); the Alfred P. Sloan Foundation Fellowship in Neuroscience (to B.H.); National Institutes of Health Neurological Disorders and Stroke (NS107968 to B.H.); National Institutes of Health BRAIN Initiative (NS109978 to B.H.). This work was supported by a grant from the Simons Foundation (SFARI 600343, B.H.). Department of Veterans Affairs Rehabilitation Research and Development Service Merit Award (I01RX002615 to M.P.); Research Career Scientist Award (IK6RX003134 to M.P.).
Data Availability
All data structures and code that generated each figure are available upon reasonable request.
Notes
Conflict of Interest: None declared.
Supplementary Material
Contributor Information
Joseph Del Rosario, Biomedical Engineering, Georgia Institute of Technology & Emory University, Atlanta, GA 30332, USA.
Anderson Speed, Biomedical Engineering, Georgia Institute of Technology & Emory University, Atlanta, GA 30332, USA.
Hayley Arrowood, Biomedical Engineering, Georgia Institute of Technology & Emory University, Atlanta, GA 30332, USA.
Cara Motz, Biomedical Engineering, Georgia Institute of Technology & Emory University, Atlanta, GA 30332, USA; Atlanta VA Center for Visual and Neurocognitive Rehabilitation, Decatur, GA 30033, USA.
Machelle Pardue, Biomedical Engineering, Georgia Institute of Technology & Emory University, Atlanta, GA 30332, USA; Atlanta VA Center for Visual and Neurocognitive Rehabilitation, Decatur, GA 30033, USA.
Bilal Haider, Biomedical Engineering, Georgia Institute of Technology & Emory University, Atlanta, GA 30332, USA.
References
- Amarante LM, Laubach M. 2020. Rhythmic activity in the medial and orbital frontal cortices tracks reward value and the vigor of consummatory behavior. bioRxiv, 2020.09.22.308809. doi: 10.1101/2020.2009.2022.308809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. D-TF . 2013. Diagnostic and statistical manual of mental disorders : DSM-5. 5th ed. Washington (DC): American Psychiatric Association. [Google Scholar]
- Antoine MW, Langberg T, Schnepel P, Feldman DE. 2019. Increased excitation-inhibition ratio stabilizes synapse and circuit excitability in four autism mouse models. Neuron. 101:648–661.e644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Rikhye RV, Breton-Provencher V, Tang X, Li C, Li K, Runyan CA, Fu Z, Jaenisch R, Sur M. 2016. Jointly reduced inhibition and excitation underlies circuit-wide changes in cortical processing in Rett syndrome. Proc Natl Acad Sci U S A. 113:E7287–E7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berditchevskaia A, Caze RD, Schultz SR. 2016. Performance in a GO/NOGO perceptual task reflects a balance between impulsive and instrumental components of behaviour. Sci Rep. 6:27389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens P. 2009. CircStat: a MATLAB toolbox for circular statistics. Journal of Statistical Software. 31:1–21. doi: 10.18637/jss.v031.i10. [DOI] [Google Scholar]
- Bertone A, Mottron L, Jelenic P, Faubert J. 2005. Enhanced and diminished visuo-spatial information processing in autism depends on stimulus complexity. Brain. 128:2430–2441. [DOI] [PubMed] [Google Scholar]
- Burgess CP, Lak A, Steinmetz NA, Zatka-Haas P, Bai Reddy C, Jacobs EAK, Linden JF, Paton JJ, Ranson A, Schroder S, et al. 2017. High-yield methods for accurate two-alternative visual psychophysics in head-fixed mice. Cell Rep. 20:2513–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse L, Ayaz A, Dhruv NT, Katzner S, Saleem AB, Scholvinck ML, Zaharia AD, Carandini M. 2011. The detection of visual contrast in the behaving mouse. J Neurosci. 31:11351–11361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Deister CA, Gao X, Guo B, Lynn-Jones T, Chen N, Wells MF, Liu R, Goard MJ, Dimidschstein J, et al. 2020. Dysfunction of cortical GABAergic neurons leads to sensory hyper-reactivity in a Shank3 mouse model of ASD. Nat Neurosci. 23:520–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contractor A, Klyachko VA, Portera-Cailliau C. 2015. Altered neuronal and circuit excitability in fragile X syndrome. Neuron. 87:699–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapse TB, Lau H, Basso MA. 2018. A role for the superior colliculus in decision criteria. Neuron. 97:181–194.e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RJ, Martin KA. 2004. Neuronal circuits of the neocortex. Annu Rev Neurosci. 27:419–451. [DOI] [PubMed] [Google Scholar]
- Durand S, Iyer R, Mizuseki K, Vries S, Mihalas S, Reid RC. 2016. A comparison of visual response properties in the lateral geniculate nucleus and primary visual cortex of awake and anesthetized mice. J Neurosci. 36:12144–12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein MC, Polack PO, Tran DT, Golshani P. 2017. Visually evoked 3-5 Hz membrane potential oscillations reduce the responsiveness of visual cortex neurons in awake behaving mice. J Neurosci. 37:5084–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes A, Zwaigenbaum L, Gu H, St John T, Paterson S, Elison JT, Hazlett H, Botteron K, Dager SR, Schultz RT, et al. 2015. Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. J Neurodev Disord. 7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro FR. 2016. No evidence of reaction time slowing in autism spectrum disorder. Autism. 20:116–122. [DOI] [PubMed] [Google Scholar]
- Fonseca MS, Murakami M, Mainen ZF. 2015. Activation of dorsal raphe serotonergic neurons promotes waiting but is not reinforcing. Curr Biol. 25:306–315. [DOI] [PubMed] [Google Scholar]
- Glickfeld LL, Andermann ML, Bonin V, Reid RC. 2013. Cortico-cortical projections in mouse visual cortex are functionally target specific. Nat Neurosci. 16:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Cantu DA, Guilfoyle J, Chaudhari GR, Newadkar A, Todisco B, Alba D, Kourdougli N, Schmitt LM, Pedapati E, et al. 2018. Impaired perceptual learning in a mouse model of fragile X syndrome is mediated by parvalbumin neuron dysfunction and is reversible. Nat Neurosci. 21:1404–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goris RLT, Ziemba CM, Stine GM, Simoncelli EP, Movshon JA. 2017. Dissociation of choice formation and choice-correlated activity in macaque visual cortex. J Neurosci. 37:5195–5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Swets JA. 1974. Signal detection theory and psychophysics. Huntington (NY): R. E. Krieger Pub. Co. [Google Scholar]
- Haider B, Hausser M, Carandini M. 2013. Inhibition dominates sensory responses in the awake cortex. Nature. 493:97–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider B, Schulz DP, Hausser M, Carandini M. 2016. Millisecond coupling of local field potentials to synaptic currents in the awake visual cortex. Neuron. 90:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CX, Cantu DA, Mantri SS, Zeiger WA, Goel A, Portera-Cailliau C. 2017. Tactile defensiveness and impaired adaptation of neuronal activity in the Fmr1 knock-out mouse model of autism. J Neurosci. 37:6475–6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insanally MN, Carcea I, Field RE, Rodgers CC, DePasquale B, Rajan K, DeWeese MR, Albanna BF, Froemke RC. 2019. Spike-timing-dependent ensemble encoding by non-classically responsive cortical neurons. Elife. 8:e42409. doi: 10.7554/eLife.42409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M. 2011. How inhibition shapes cortical activity. Neuron. 72:231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Glickfeld LL. 2019. Contribution of sensory encoding to measured bias. J Neurosci. 39:5115–5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas SL, Geurts HM, Konrad K, Bender S, Nigg JT. 2014. Annual research review: reaction time variability in ADHD and autism spectrum disorders: measurement and mechanisms of a proposed trans-diagnostic phenotype. J Child Psychol Psychiatry. 55:685–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaro MT, Taxidis J, Shuman T, Bachmutsky I, Ikrar T, Santos R, Marcello GM, Mylavarapu A, Chandra S, Foreman A, et al. 2019. Reduced prefrontal synaptic connectivity and disturbed oscillatory population dynamics in the CNTNAP2 model of autism. Cell Rep. 28;27:2567–2578.e6. doi: 10.1016/j.celrep.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo TZ, Maunsell JHR. 2018. Attentional changes in either criterion or sensitivity are associated with robust modulations in lateral prefrontal cortex. Neuron. 97:1382–1393.e1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride EG, Lee SJ, Callaway EM. 2019. Local and global influences of visual spatial selection and locomotion in mouse primary visual cortex. Curr Biol. 29:1592–1605.e1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mees LM, Coulter MM, Chrenek MA, Motz CT, Landis EG, Boatright JH, Pardue MT. 2019. Low-intensity exercise in mice is sufficient to protect retinal function during light-induced retinal degeneration. Invest Ophthalmol Vis Sci. 60:1328–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson SD, Ozkan ED, Aceti M, Maity S, Llamosas N, Weldon M, Mizrachi E, Vaissiere T, Gaffield MA, Christie JM, et al. 2018. SYNGAP1 heterozygosity disrupts sensory processing by reducing touch-related activity within somatosensory cortex circuits. Nat Neurosci. 21:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaiel AM, Parker PRL, Niell CM. 2019. A hallucinogenic serotonin-2A receptor agonist reduces visual response gain and alters temporal dynamics in mouse V1. Cell Rep. 26:3475–3483.e3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandy A, Nassi JJ, Jadi MP, Reynolds J. 2019. Optogenetically induced low-frequency correlations impair perception. Elife. 8:e35123. doi: 10.7554/eLife.35123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SB, Valakh V. 2015. Excitatory/inhibitory balance and circuit homeostasis in autism spectrum disorders. Neuron. 87:684–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neske GT, Nestvogel D, Steffan PJ, McCormick DA. 2019. Distinct waking states for strong evoked responses in primary visual cortex and optimal visual detection performance. J Neurosci. 39:10044–10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. 2008. Highly selective receptive fields in mouse visual cortex. J Neurosci. 28:7520–7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orefice LL, Mosko JR, Morency DT, Wells MF, Tasnim A, Mozeika SM, Ye M, Chirila AM, Emanuel AJ, Rankin G, et al. 2019. Targeting peripheral somatosensory neurons to improve tactile-related phenotypes in ASD models. Cell. 178:867–886.e824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn RD, Hagins WA. 1969. Signal transmission along retinal rods and the origin of the electroretinographic a-wave. Nature. 223:201–204. [DOI] [PubMed] [Google Scholar]
- Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. 2013. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci. 16:1068–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe NJ, Ferster D. 2012. Mechanisms of neuronal computation in mammalian visual cortex. Neuron. 75:194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CE, Baron-Cohen S. 2017. Sensory perception in autism. Nat Rev Neurosci. 18:671–684. [DOI] [PubMed] [Google Scholar]
- Robertson CE, Thomas C, Kravitz DJ, Wallace GL, Baron-Cohen S, Martin A, Baker CI. 2014. Global motion perception deficits in autism are reflected as early as primary visual cortex. Brain. 137:2588–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockland KS, Pandya DN. 1979. Laminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkey. Brain Res. 179:3–20. [DOI] [PubMed] [Google Scholar]
- Rossant C, Kadir SN, Goodman DF, Schulman J, Hunter ML, Saleem AB, Grosmark A, Belluscio M, Denfield GH, Ecker AS, et al. 2016. Spike sorting for large, dense electrode arrays. Nat Neurosci. 19:634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. 2003. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2:255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacai H, Sakoori K, Konno K, Nagahama K, Suzuki H, Watanabe T, Watanabe M, Uesaka N, Kano M. 2020. Autism spectrum disorder-like behavior caused by reduced excitatory synaptic transmission in pyramidal neurons of mouse prefrontal cortex. Nat Commun. 11:5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem AB, Lien AD, Krumin M, Haider B, Roson MR, Ayaz A, Reinhold K, Busse L, Carandini M, Harris KD. 2017. Subcortical source and modulation of the narrowband gamma oscillation in mouse visual cortex. Neuron. 93:315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato TK, Haider B, Hausser M, Carandini M. 2016. An excitatory basis for divisive normalization in visual cortex. Nat Neurosci. 19:568–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R, Sanchez-Aguilera A, Elst K, Lim L, Dehorter N, Bae SE, Bartolini G, Peles E, Kas MJH, Bruining H, et al. 2019. Loss of Cntnap2 causes axonal excitability deficits, developmental delay in cortical myelination, and abnormal stereotyped motor behavior. Cereb Cortex. 29:586–597. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Rubenstein JLR. 2019. Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol Psychiatry. 24:1248–1257. doi: 10.1038/s41380-019-0426-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed A, Del Rosario J, Burgess CP, Haider B. 2019. Cortical state fluctuations across layers of V1 during visual spatial perception. Cell Rep 26:2868–2874.e2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed A, Del Rosario J, Mikail N, Haider B. 2020. Spatial attention enhances network, cellular and subthreshold responses in mouse visual cortex. Nat Commun. 11:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Steinmetz NA, Moore T, Knudsen EI. 2017. Does the superior colliculus control perceptual sensitivity or choice bias during attention? Evidence from a multialternative decision framework. J Neurosci. 37:480–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson K, Goldbach HC, Laubach M. 2019. The rat medial frontal cortex controls pace, but not breakpoint, in a progressive ratio licking task. Behav Neurosci. 133:385–397. [DOI] [PubMed] [Google Scholar]
- Tavassoli T, Miller LJ, Schoen SA, Nielsen DM, Baron-Cohen S. 2014. Sensory over-responsivity in adults with autism spectrum conditions. Autism. 18:428–432. [DOI] [PubMed] [Google Scholar]
- Tomchek SD, Dunn W. 2007. Sensory processing in children with and without autism: a comparative study using the short sensory profile. Am J Occup Ther. 61:190–200. [DOI] [PubMed] [Google Scholar]
- Travers JB, Dinardo LA, Karimnamazi H. 1997. Motor and premotor mechanisms of licking. Neurosci Biobehav Rev. 21:631–647. [DOI] [PubMed] [Google Scholar]
- Geest JN, Kemner C, Camfferman G, Verbaten MN, Engeland H. 2001. Eye movements, visual attention, and autism: a saccadic reaction time study using the gap and overlap paradigm. Biol Psychiatry. 50:614–619. [DOI] [PubMed] [Google Scholar]
- Velmeshev D, Schirmer L, Jung D, Haeussler M, Perez Y, Mayer S, Bhaduri A, Goyal N, Rowitch DH, Kriegstein AR. 2019. Single-cell genomics identifies cell type–specific molecular changes in autism. Science. 364:685–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. 2001. The psychometric function: I. Fitting, sampling, and goodness of fit. Perception & Psychophysics. 63:1293–1313. 10.3758/BF03194544. [DOI] [PubMed] [Google Scholar]
- Williams B, Speed A, Haider B. 2018. A novel device for real-time measurement and manipulation of licking behavior in head-fixed mice. J Neurophysiol. 120:2975–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupan B, Toth M. 2008. Wild-type male offspring of fmr-1+/− mothers exhibit characteristics of the fragile X phenotype. Neuropsychopharmacology. 33:2667–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylberberg J. 2018. The role of untuned neurons in sensory information coding. bioRxiv, 134379. doi: 10.1101/134379. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data structures and code that generated each figure are available upon reasonable request.


