Abstract
Excessive lung inflammation and airway epithelial damage are hallmarks of human inflammatory lung diseases, such as cystic fibrosis (CF). Enhancement of innate immunity provides protection against pathogens while reducing lung-damaging inflammation. However, the mechanisms underlying innate immunity–mediated protection in the lung remain mysterious, in part because of the lack of appropriate animal models for these human diseases. TLR5 (Toll-like receptor 5) stimulation by its specific ligand, the bacterial protein flagellin, has been proposed to enhance protection against several respiratory infectious diseases, although other cellular events, such as calcium signaling, may also control the intensity of the innate immune response. Here, we investigated the molecular events prompted by stimulation with flagellin and its role in regulating innate immunity in the lung of the pig, which is anatomically and genetically more similar to humans than rodent models. We found that flagellin treatment modulated NF-κB signaling and intracellular calcium homeostasis in airway epithelial cells. Flagellin pretreatment reduced the NF-κB nuclear translocation and the expression of proinflammatory cytokines to a second flagellin stimulus as well as to Pseudomonas aeruginosa infection. Moreover, in vivo administration of flagellin decreased the severity of P. aeruginosa–induced pneumonia. Then we confirmed these beneficial effects of flagellin in a pathological model of CF by using ex vivo precision-cut lung slices from a CF pigz model. These results provide evidence that flagellin treatment contributes to a better regulation of the inflammatory response in inflammatory lung diseases such as CF.
Keywords: flagellin, Pseudomonas aeruginosa, cystic fibrosis, TLR5, inflammation
Clinical Relevance
Cystic fibrosis lung disease is characterized by excessive lung inflammation and recurrent Pseudomonas aeruginosa infection. Our findings, using a highly valuable translational animal model, show that flagellin administration can modulate TLR (Toll-like receptor) signaling to reduce excessive inflammation in cystic fibrosis. We open an avenue for novel alternative therapies that can specifically target lung inflammation, which would have clear implications for this devastating disease.
TLRs (Toll-like receptors) are the main family of PRRs (pattern recognition receptors) of the innate immune system. Most cell types, including airway epithelial cell (AECs), express TLRs. These PRRs are part of the first line of defense against pathogens. TLRs trigger an inflammatory response upon recognition of microbe-associated molecular patterns (1). The host must tightly regulate this inflammatory response. It has to be strong enough to clear a pathogen, but it must avoid excessive tissue damage. Indeed, defective TLR signaling and a detrimental inflammatory response are linked to inflammatory lung diseases, such as chronic obstructive pulmonary disease and cystic fibrosis (CF) (2, 3). This persistent inflammation is often the ultimate cause of lung injury and death.
Among the different TLRs, TLR5 has emerged as a candidate target for immune therapies. Activation of TLR5 by the bacterial protein flagellin can provide protection against infection by different bacterial species at the respiratory level (4). Furthermore, TLR5-mediated immune stimulation does not lead to increased risks of exacerbated inflammation and tissue destruction, unlike agonists for TLR3 and TLR9 (5). Flagellin, on the contrary, seems to improve the general health status, promoting a more rapid and efficient resolution of inflammation. This positive effect even led to full restoration of the lung architecture in experimental models of acute lung infection (4). However, the mechanisms underlying flagellin-mediated changes in the inflammatory response to a pathogen are not completely understood.
TLR5 stimulation by flagellin activates TRAF6 (TNFR-associated factor 6). This process is required to trigger a signaling cascade that activates NF-κB and the MAPK (mitogen-activated protein kinases) (6), thus releasing proinflammatory cytokines. This proinflammatory signaling at the mucosal interfaces promotes the recruitment and maturation of immune cells, orchestrating an enhanced protection against pathogens (4, 7). The flagellin-mediated proinflammatory response is believed to be short-lived because of the strong regulatory mechanisms that involve transcriptional and posttranscriptional regulators such as TNFAIP3, IκBα, or IκBε (8). Recent reports also suggest that flagellin treatment elicits innate immune memory in bronchial epithelial cells, changing the immune response to a second, nonrelated inflammatory stimulus (9). Moreover, Pseudomonas aeruginosa flagellin seems to alter Ca2 + homeostasis in CF airways (10), potentially modifying the intensity and quality of the innate immune response (11). However, little is known regarding the effect of flagellin activation of TLR5 signaling in the innate immune response to a second inflammatory stimulus or whether flagellin stimulation has a longer-term impact on the airways’ immune response to a pathogen.
Here, we employed a pig model to study the airways innate immune response to flagellin and P. aeruginosa infection. Although mouse models represent one of the main tools in research, recent data suggest that new preclinical models with closer resemblance to humans are needed to study inflammatory diseases (12). Porcine lungs share many anatomical, histological, biochemical, and physiological features with those of humans, being a good experimental model for P. aeruginosa infections (13). Moreover, the generation of the CFTR−/− pigs (14, 15), which mimic human CF, make them a perfect candidate to study the regulation of the inflammatory response in the CF airways. We characterized the effect of flagellin stimulation in pig primary AECs. We evaluated the impact of flagellin preexposure on the response to a second inflammatory stimulus and to P. aeruginosa, the dominant pathogen in chronic obstructive pulmonary disease and CF (16, 17). In addition, we employed CFTR−/− pigs to study the regulation of the inflammatory response in the CF airways. We found that flagellin activates NF-κB signaling in AECs in a switch-like manner, leading to the expression of proinflammatory cytokine genes. Treatment of AECs with flagellin also modified calcium signaling and reduced the innate immune responses after a second flagellin stimulation or after P. aeruginosa infection. Preventive respiratory administration of flagellin in vivo improved the lung architecture after P. aeruginosa infection. Finally, we showed that the effect of flagellin on the inflammatory response to P. aeruginosa was conserved in a CF context.
Methods
Animals
In vivo experimental infections with P. aeruginosa were performed at the Plateforme d’Infectiologie Expérimentale (Unité Expérimentale 1277, Centre de Recherche Val de Loire, Institut National de Recherche pour l'Agriculture, l'Alimentation et l'Environnement Tours, France; https://doi.org/10.15454/1.5535888072272498e12). Pigs were inoculated with either flagellin or P. aeruginosa, as described in Reference 13. Newborn CFTR+/+ and CFTR−/− piglets were allowed to suckle colostrum, and the genotype was confirmed by using multiplex PCR analysis, as described by Guillon and colleagues (18). Samples were collected within 6 hours after birth.
Image Analysis
AECs were stained with a mouse monoclonal anti-P65 antibody (sc-8008, Santa Cruz Biotechnology) at a 1:100 dilution in 10% FBS and were incubated overnight at 4°C. Nuclei were stained with 500-ng/ml DAPI in PBS for 15 minutes. Samples were imaged using a Leica TCS SP8 confocal microscope with the Leica Application Suite AF software. P65 nuclear translocations were analyzed as described by Tudelska and colleagues (19).
Ca2 + Imaging of AECs
Ca2 + imaging was performed in air–liquid interface cultures of primary AECs. Cells were loaded with fura-2/AM (5μM; Invitrogen) for 60 minutes. Peak signals were calculated from the temporal profiles of the image ratio/fluorescent values as described by Trouillet and colleagues (20). Averaged results are based on recordings from three different animals for each condition.
Gene Expression Analysis
To monitor the TLR5 inflammatory response on the airway epithelia, air–liquid interface cultures of AECs were stimulated in the apical side with different concentrations of the recombinant flagellin FliCΔ174–400 or the vehicle (control) for 5 hours. To determine the role of the P38 pathway in the flagellin-mediated AEC transcriptional response, AECs were preincubated or not with the P38 inhibitor SB203580 (20μM, Tocris Bioscience) for 1 hour and were then stimulated with 100-ng/ml FliCΔ174–400 for 2 hours. To monitor the effect of flagellin prestimulation on the airway immune response, AECs and precision-cut lung slices (PCLS) were pretreated or not with 100-ng/ml FliCΔ174–400 for 24 hours and then stimulated with either 100-ng/ml FliCΔ174–400 or the P. aeruginosa strain K (PAK) (21) (multiplicity of infection = 0.2) for 5 hours. RNA was collected for the evaluation of gene expression by using quantitative PCR analysis or RNA sequencing (RNA-seq).
Bronchial Administration of Flagellin and Infection with P. aeruginosa in Wild-Type Pigs
One-month-old wild-type pigs were administered 10 ml of a suspension of 0.012 mg/kg FliCΔ174–400 or PBS without calcium or magnesium. Twenty-four hours after the flagellin treatment, pigs were inoculated with 10 ml of the P. aeruginosa strain PAK (21) (107 cfu/ml) in sterile conditions.
Statistical Analysis
Time-series figures are presented as the mean ± SEM. Statistical analyses were performed by using R version 3.6.0 (R Foundation for Statistical Computing) and the packages ggplot2, drc, and ggpubr. Intergroup differences were analyzed by using the Kruskal-Wallis test followed by a pairwise Mann-Whitney test with Benjamini-Hochberg false discovery rate correction for multiple comparisons and by using two-way ANOVA followed by the Tukey honestly-significant-difference post hoc test. Differences in the severity of the histological lesions were determined by using a contingency chi-square test. A P value of <0.05 was considered to indicate statistical significance.
Detailed materials and methods are described in the data supplement.
Results
Flagellin Triggers a Switch-like Inflammatory Response in the AECs, Leading to a Transient Proinflammatory Response
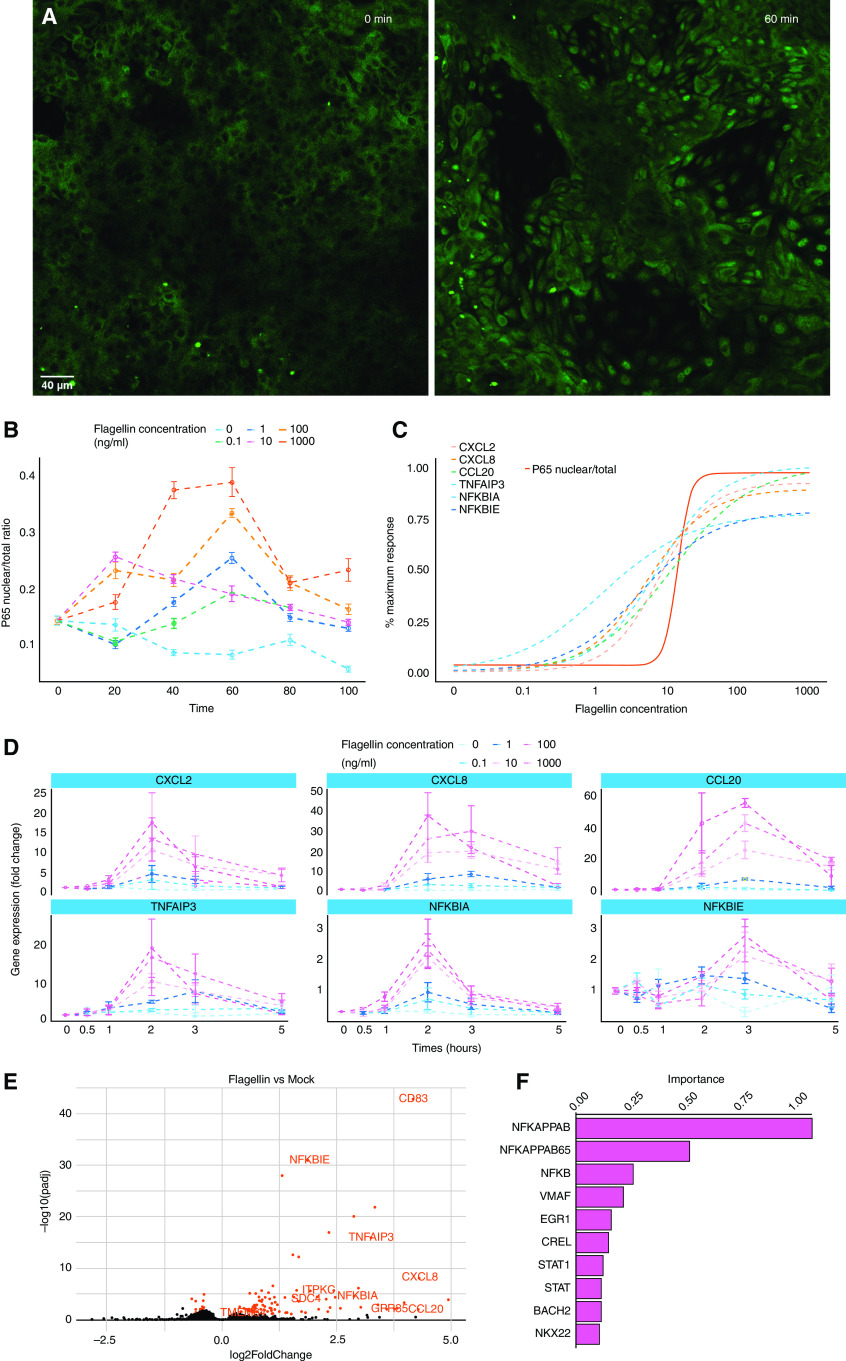
To better understand how AECs integrate flagellin stimulation, we performed quantitative analysis of the nuclear translocation of p65, a subunit of the NF-κB family (6). First, we examined the dose-dependent activation of TLR5 signaling. Porcine AECs were stimulated with the recombinant FliCΔ174–400 over a concentration range of five orders of magnitude (0.1–1,000 ng/ml). Flagellin induced NF-κB activation and subsequent p65 nuclear translocation with a peak nuclear/total p65 ratio at 60 minutes after stimulation (Figures 1A and 1B; see also Figure E1 in the data supplement). Next, we calculated the area under the curve of p65 nuclear translocation for each of the different flagellin concentrations. We observed an ultrasensitive response (Hill coefficient > 1) to flagellin with a half maximal effective concentration (EC50) of 13.68 ng/ml and a maximum concentration of 100 ng/ml (Figure 1C). To evaluate how flagellin drives the transcriptional program of the inflammatory response, we performed a gene expression analysis of three proinflammatory cytokines (CXCL2, CXCL8, and CCL20) and three regulatory factors of the NF-κB signaling pathway (TNFAIP3 [tumor necrosis factor, alpha-induced protein 3], NFκBIA [nuclear factor-κ-B-inhibitor α], and NFκBIE [nuclear factor-κ-B-inhibitor ε]) in response to different flagellin concentrations (0.1–1,000 ng/ml). Gene expression kinetics showed EC50s between 1.14- and 11.29-ng/ml flagellin, which are similar to the EC50 observed for p65 translocation (13.68 ng/ml) (Figures 1C and 1D). Consistent with a transcriptional process, the gene expression increase was less sharp than the NF-κB response (Hill coefficient = 1). Gene expression reached a maximum at 100 ng/ml, which also elicited a maximal p65 translocation responses (Figure 1C). Next, we analyzed the time course of gene expression over 5 hours. We observed maximum transcription for most genes >2 hours after stimulation (Figure 1D). Expression decreased after 2 hours for all genes except CCL20 and NFκBIE, which displayed peak expression at 3 hours (Figure 1D). Altogether, our data show that primary AECs respond to flagellin in a switch-like manner, in which a certain flagellin dose needs to be exceeded to induce the activation of the NF-κB pathway and the expression of proinflammatory cytokines.
Figure 1.
Flagellin triggers a switch-like TLR5 (Toll-like receptor 5) response. Evaluation of P65 nuclear translocation in differentiated airway epithelial cells treated with 200 μl of 0 (vehicle), 0.1-, 1-, 10-, 100-, or 1,000-ng/ml apical FliCΔ174–400 for 0, 20, 40, 60, 80, and 100 minutes was conducted. (A) Representative confocal images showing cells before (0 min; left) and after (60 min; right) stimulation with 100-ng/ml FliCΔ174–400. Scale bar, 40 μm. (B) Time course of P65 nuclear translocation. (C) Dose responses for the expression of the CXCL2, CXCL8, CCL20, TNFAIP3, NFκBIA, and NFκBIE genes (dashed lines) and P65 nuclear translocation (solid line, half maximal effective concentration = 13.68 ng/ml). The area under the curve was extracted for the time course data and plotted over the dose response. (D) Time course of airway epithelial cells’ inflammatory response to flagellin. Quantitative PCR analysis of differentiated airway epithelial cells from wild-type pigs treated with 0- (vehicle), 0.1-, 1-, 10-, 100-, or 1,000-ng/ml apical FliCΔ174–400 for 0, 0.5, 1, 2, 3, and 5 hours was conducted. Gene expression is shown relative to the mock group. (E) Volcano plot of RNA-sequencing data, in which the −log10 of the padj is plotted against log2 fold change. Differentiated airway epithelial cells were stimulated or not with 100-ng/ml FliCΔ174–400 for 2 hours. Transcripts that are highlighted in red are discussed in the text. (F) Distant Regulatory Elements of Co-regulated Genes transcription factor motif analysis for the differentially expressed genes after flagellin stimulation; the importance is a product of the motif occurrence (the fraction of regulatory elements containing the motif) and weight score (motif prevalence compared with background gene list). Data are representative of three to four experiments. NFκBIA = nuclear factor-κ-B-inhibitor alpha; NFκBIE = nuclear factor-κ-B-inhibitor ε; padj = adjusted P value; TNFAIP3 = tumor necrosis factor, α-induced protein 3.
To gain a better insight of AECs’ transcriptional response to flagellin, we performed RNA-seq in AECs stimulated with 100-ng/ml FliCΔ174–400 for 2 hours. This is the time and concentration that gives a maximum transcriptional response in our cells for the immune response–related genes (Figure 1E). RNA-seq analysis identified a total of 106 differentially expressed genes (DEGs). Consistent with our previous quantitative PCR results, most of the DEGs were genes encoding proinflammatory cytokines or genes involved in the NF-κB pathway but also included DEGs with a potential role in calcium signaling, such as CD83 (22, 23), GPR35 (24, 25), ITPKC (26), SDC4 (27, 28), and TMEM64 (29) (Figure 1E).
We used the Distant Regulatory Elements of Co-regulated Genes web server to identify the transcription factors linked to the observed response to FliCΔ174–400 by analyzing proximal promoters and distant regulatory elements in the input genes to calculate their occurrence and importance (30). Analysis showed that the DEGs were enriched in genes harboring NF-κB motifs, indicating an important role of this transcription factor in the TLR5-mediated inflammatory response (Figure 1F). Moreover, RNA-seq analysis showed that only 12 genes were differentially expressed in flagellin-stimulated AECs when p38 was inhibited in the AECs, suggesting that p38 did not have a major effect in the AEC response to flagellin (Figure E2). Altogether, we show that flagellin activates NF-κB signaling in the airway epithelium, triggering the transcription of a set of genes with the potential to affect the cell response to an incoming pathogen.
Flagellin Treatment Modulates Cytosolic Calcium Homeostasis
Our RNA-seq results that highlighted several DEGs involved in calcium signaling prompted us to investigate a possible effect of FliCΔ174–400 in calcium homeostasis. We evaluated Ca2 + responses in AECs pretreated with 100-ng/ml FliCΔ174–400 for 24 hours in response to 100μM histamine. Histamine-receptor activation causes an increase in inositol 1,4,5-triphosphate and thus causes the release of Ca2 + from the endoplasmic reticulum (ER) (10). We measured cytosolic Ca2 + dynamics in AECs loaded with the Ca2 + dye fura-2 and found that histamine-induced Ca2 + increase was sevenfold higher in cells pretreated with FliCΔ174–400 (Figures 2A and 2B).
Figure 2.
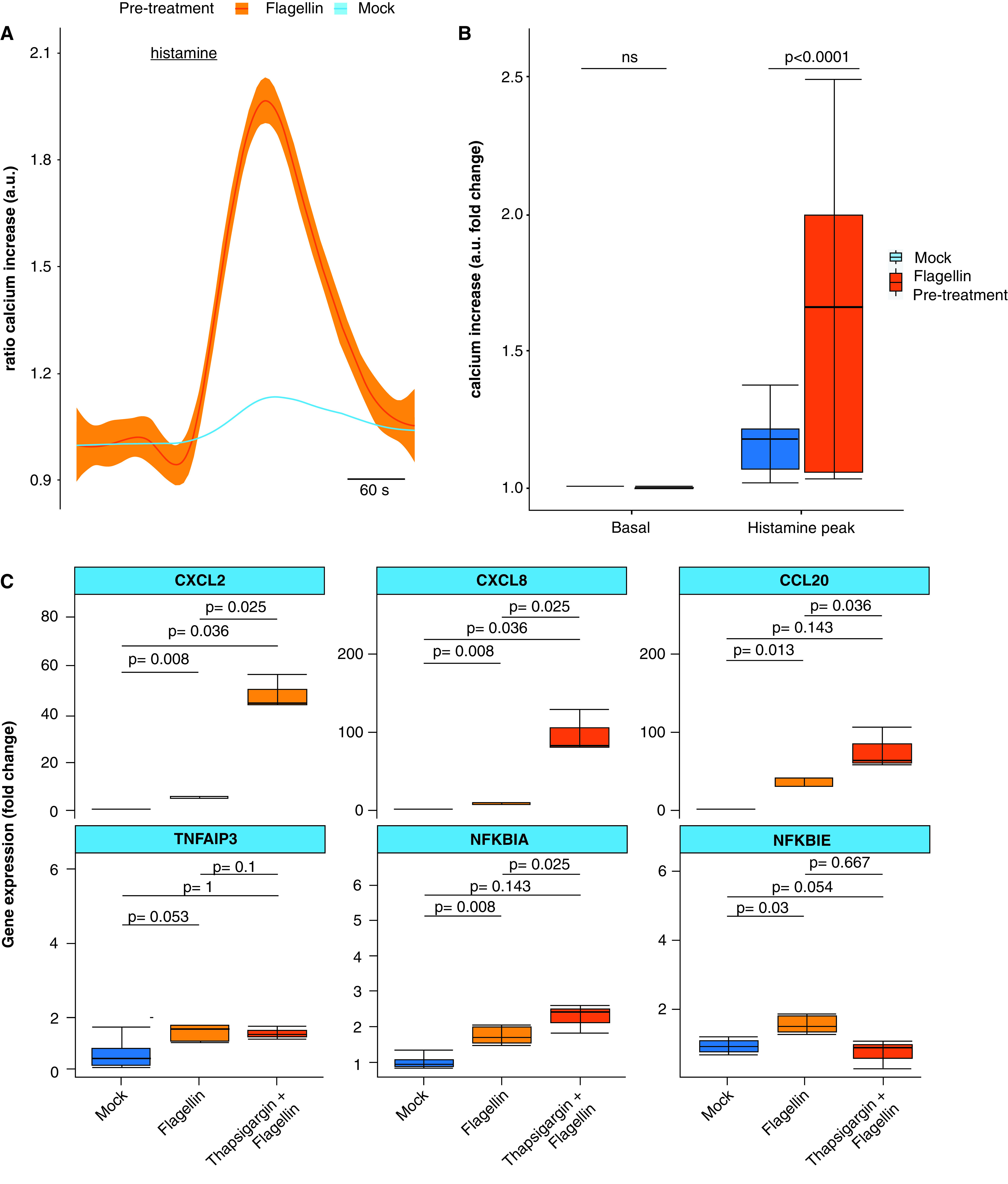
Calcium homeostasis modulates the TLR5 response. (A) Histamine-dependent cytosolic Ca2 + responses in differentiated airway epithelial cells pretreated (red) or not (blue) with 100-ng/ml apical FliCΔ174–400 for 24 hours and stimulated with histamine (100μM) for 1 minute. Differences were evaluated by using the Kolmogorov-Smirnov (K-S) test. (B) Boxplot showing the fold change increase in the histamine-induced peak Ca2 + response in cells pretreated or not with FliCΔ174–400 for 24 hours. “Basal” indicates the mean value before histamine stimulation. (C) Quantitative PCR analysis of differentiated airway epithelial cells from wild-type pigs pretreated or not with 1μM thapsigargin for 24 hours and later stimulated or not with flagellin (100 ng/ml) for 5 hours. “Mock” indicates PBS-treated and nonstimulated airway epithelial cells. Gene expression is shown relative to the mock group. Intergroup differences were analyzed by using the Kruskal-Wallis test followed by a pairwise Mann-Whitney test with Benjamini-Hochberg false discovery rate correction. Data are representative of three experiments. a.u.= arbitrary unit; ns = not significant.
To investigate the role of ER Ca2 + in TLR5 signaling, we pretreated AECs with the SERCA inhibitor thapsigargin (1μM) for 24 hours and then stimulated them with 100-ng/ml FliCΔ174–400. Thapsigargin leads to Ca2 + depletion from the ER, inducing ER stress, and may trigger exaggerated inflammatory responses (31). We observed that thapsigargin treatment in AECs induced an increase in the expression of the CXCL2, CXCL8, CCL20, and NFKBIA genes, compared with the nonpretreated AECs in response to FliCΔ174–400 (Figure 2C). These data suggest that intracellular Ca2 + signaling is actively implicated in regulating TLR5 signaling. Therefore, although FliCΔ174–400 induces a short-lived transient activation of TLR5 signaling (Figure 1D), the effect of FliCΔ174–400 pretreatment in Ca2 + signaling suggests that FliCΔ174–400 could modulate the immune response of AECs to protect against a subsequent infection.
Flagellin Treatment Decreases Airway Epithelial Inflammatory Response to a Second Inflammatory Stimulus
Next, we aimed to determine whether flagellin treatment may influence the response to a second inflammatory stimulus. We hypothesized that flagellin pretreatment may produce a reduced TLR5-dependent inflammatory response to a second FliCΔ174–400 stimulus. To test this, we pretreated AECs for 24 hours with 100-ng/ml FliCΔ174–400 and then stimulated AECs with a second, acute 100-ng/ml FliCΔ174–400 administration. Flagellin pretreatment significantly decreased the gene expression of six proinflammatory cytokines and regulators of NF-κB activity (Figure 3A). In addition, P65 translocation into the nucleus was significantly reduced (P < 0.0001) in pretreated samples, with a lower number of cells being activated after FliCΔ174–400 stimulation (Figures 3B and 3C). These results were confirmed by using an immortalized porcine bronchial epithelial cell line (Figure E3). Thus, our data suggest that flagellin pretreatment decreases inflammatory responses in AECs.
Figure 3.
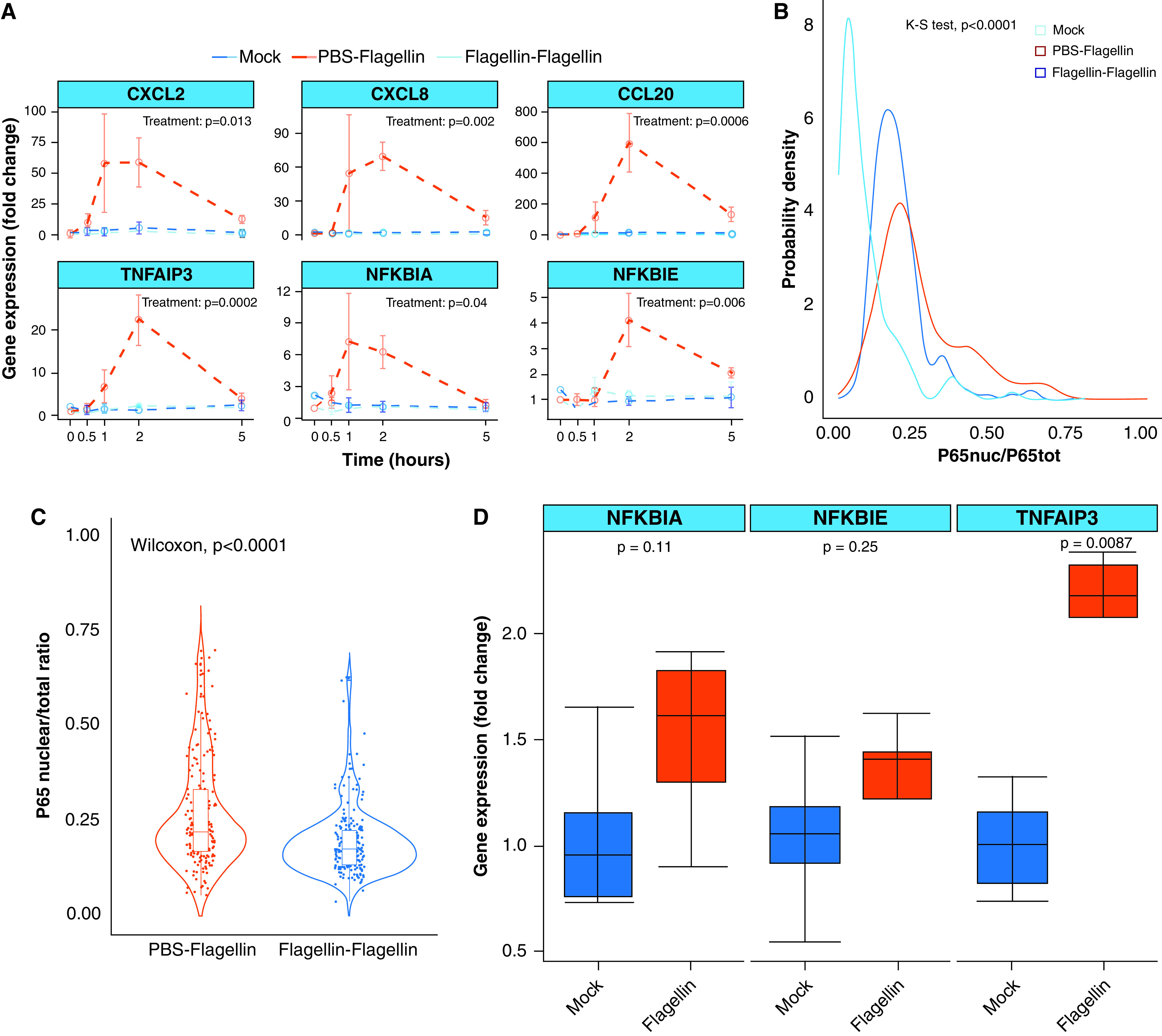
Flagellin treatment decreases the TLR5 response to a second flagellin input. (A) Quantitative PCR analysis of differentiated airway epithelial cells from wild-type pigs pretreated or not with 200 μl of 100-ng/ml apical FliCΔ174–400 for 24 hours and later stimulated or not with a second flagellin input (100 ng/ml) for 0, 0.5, 1, 2, and 5 hours. “Mock” indicates PBS-treated and nonstimulated airway epithelial cells. Gene expression is shown relative to the mock group. Intergroup differences were analyzed by using two-way ANOVA with time and treatment as factors, which was followed by a Tukey honestly-significant-difference (HSD) post hoc test. Data are representative of three experiments. (B) Gaussian probability density functions and (C) violin plots for P65 nuclear translocation responses in differentiated airway epithelial cells pretreated or not with 100-ng/ml apical FliCΔ174–400 for 24 hours and later stimulated with 100-ng/ml FliCΔ174–400 for 60 minutes. Differences were evaluated by using the K-S test and the Mann-Whitney Wilcoxon test. (D) Quantitative PCR analysis of differentiated airway epithelial cells from wild-type pigs pretreated or not with 200 μl of 100-ng/ml apical FliCΔ174–400 for 24 hours. Differences were evaluated by using the Mann-Whitney Wilcoxon test. Data are representative of four to six experiments.
Persistent exposure to the TLR ligand can induce tachyphylaxis and reduce TLR responses (32). To exclude that a lengthy exposure to flagellin caused the observed reduction in the response to a second FliCΔ174–400 stimulation, we exposed AECs to 100-ng/ml FliCΔ174–400 for a short 1-hour duration. Then cells were washed for 1 hour and treated with a second 100-ng/ml FliCΔ174–400 stimulus. We observed a decrease in the expression of proinflammatory cytokines in those samples that had been prestimulated for 1 hour with FliCΔ174–400. These results are similar to those obtained after a 24-hour flagellin pretreatment, excluding that the observed effect was related to flagellin overexposure (Figure E4). To determine whether the decreased TLR5 response to flagellin is rather the result of negative feedback on the NF-κB pathway, we evaluated whether there was any alteration in the expression of NF-κB regulatory genes after a 24-hour flagellin treatment. We observed a significant twofold increase in the expression of TNFAIP3 compared with the mock controls (Figure 3D). These results show that treating primary AECs with flagellin modifies their inflammatory response to a second stimulus of a similar nature, which could have important implications for the control of inflammatory exacerbations in the airways.
We further investigated whether flagellin plays a role in the immune response to a pathogenic challenge. To test this, we pretreated AECs for 24 hours with 100-ng/ml FliCΔ174–400, infected them with the P. aeruginosa PAK strain for 5 hours, and measured the inflammatory response by using quantitative PCR analysis. As expected, infection triggered an important upregulation of the CXCL2, CXCL8, CCL20, TNFAIP3, NFKBIA, and NFκBIE genes (Figure E5). A 24-hour preexposure to FliCΔ174–400 decreased the expression for all of these six genes in AECs (Figure 4). These data show that flagellin treatment modulates the immune response to P. aeruginosa.
Figure 4.
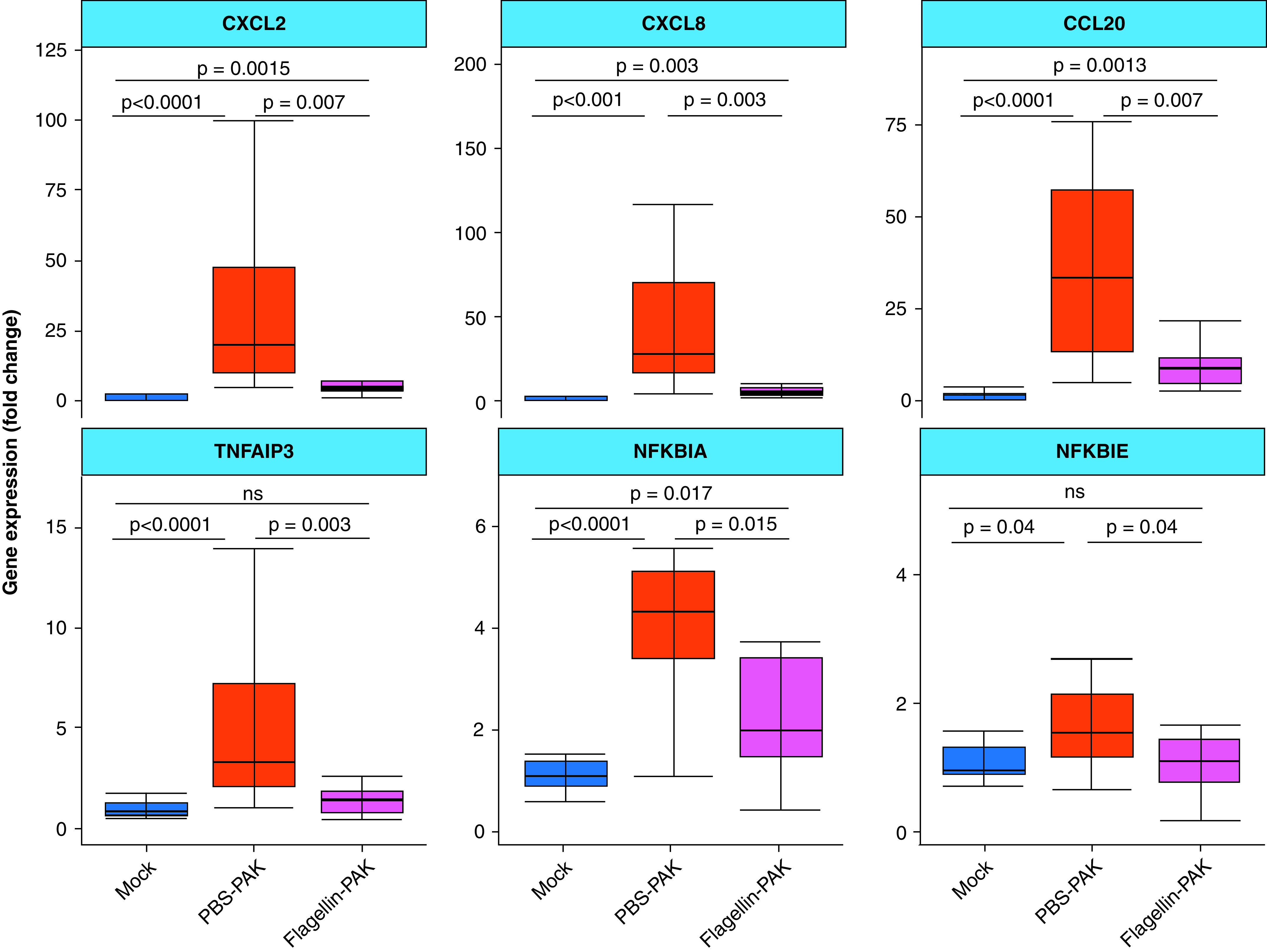
Flagellin treatment decreases the immune response to Pseudomonas aeruginosa strain K (PAK) in wild-type airway epithelial cells. Quantitative PCR analysis of wild-type airway epithelial cells pretreated (Flagellin–PAK) or not (PBS–PAK) with 200 μl of 100-ng/ml apical FliCΔ174–400 or left untreated for 24 hours and later infected with the P. aeruginosa strain PAK (multiplicity of infection = 0.2) for 5 hours. Gene expression is shown relative to the mock group (treated with the vehicle). Intergroup differences were analyzed using the Kruskal-Wallis test followed by a pairwise Mann-Whitney test with Benjamini-Hochberg false discovery rate correction. Data are representative of 12 to 15 experiments.
Preventive Respiratory Administration of Flagellin In Vivo Decreases the Lung Inflammatory Response to P. aeruginosa Infection in Pigs
Our data show that flagellin treatment alters the airway epithelial innate immune response to P. aeruginosa in vitro. However, whether flagellin treatment influences the inflammatory response to this pathogen in vivo remains to be elucidated. To answer this question, we performed bronchial administration of 10 ml of a suspension of 0.012 mg/kg FliCΔ174–400 in 1-month-old pigs. We observed a mild inflammatory response to flagellin in the lungs within 3 hours after administration, as shown by an increase in the lung CXCL8 expression and the secretion of IL-8 into the conducting airways (Figure E6). There was also a tendency for peripheral blood neutrophils increase, with recruitment of inflammatory cells into the lungs being shown (Figures E7 and E8). The response remained highly heterogeneous, with it being higher in the middle lung lobe (Figure E9). We next tested whether preexposure to flagellin also modulated the inflammatory response to P. aeruginosa in vivo. Pigs previously treated with FliCΔ174–400 were inoculated with 10 ml of the P. aeruginosa strain PAK (107 cfu/ml) in the bronchi 24 hours after the flagellin treatment. Histological analysis of the lungs showed dilated interlobular septa and cellular infiltration into the alveolar spaces after P. aeruginosa infection in PBS-treated control animals. Notably, flagellin treatment before the infection significantly prevented extensive dilated interlobular septa and cellular infiltration (Figures 5A–5D and Table 1). We also observed lower CXCL8 and CCL20 gene expression when pigs were treated with FliCΔ174–400 before P. aeruginosa infection (Figures 5E and E10). Altogether, these data suggest that flagellin treatment can reduce the inflammatory response to P. aeruginosa in pig lungs in vivo.
Figure 5.
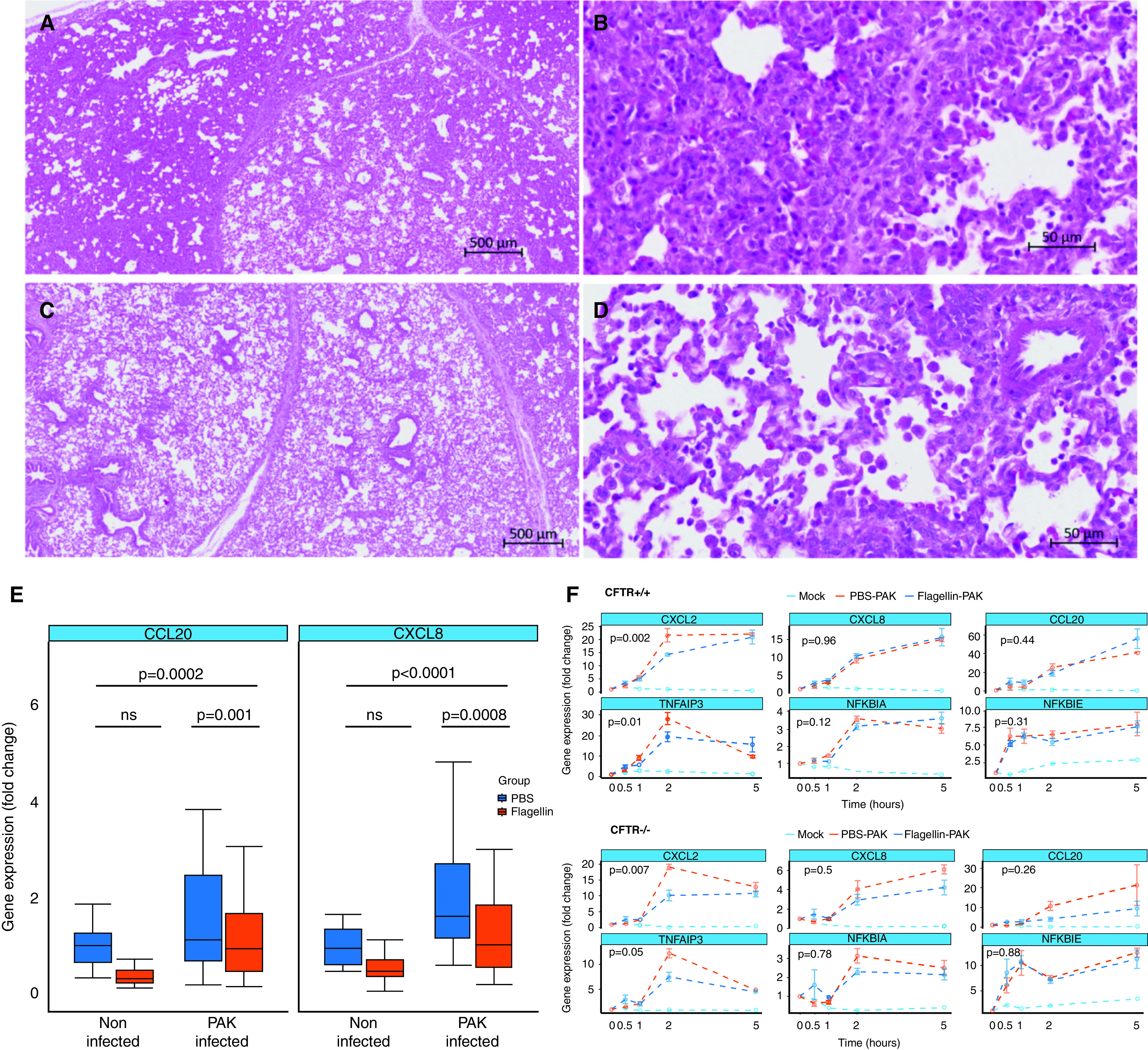
Flagellin treatment before P. aeruginosa infection dampens lung inflammation in wild-type and cystic fibrosis pigs. (A–D) Lung sections were stained 24 hours after infection with hematoxylin and eosin. Images are shown of PBS-treated and infected pig (PBS–PAK) (A and B) and flagellin-treated and infected pig (Flagellin–PAK) (C and D). (E) Wild-type pigs (n = 5) received a bronchial administration of 0.012-mg/kg FliCΔ174–400 or PBS and were infected or not with 10 ml of a 107-cfu/ml suspension of the P. aeruginosa strain PAK 24 hours later. The inflammatory response was evaluated 24 hours after infection. CCL20 and CXCL8 gene expression amounts were evaluated by using quantitative PCR analysis. Gene expression is shown relative to the PBS-treated, noninfected group. Data were analyzed by two-way ANOVA, using flagellin administration and P. aeruginosa infection as factors, which was followed by a Tukey HSD post hoc test. (F) Quantitative PCR analysis of precision-cut lung slices from newborn CFTR+/+ and CFTR−/− piglets treated or not with 100-ng/ml FliCΔ174–400 for 24 hours and later infected with the P. aeruginosa strain PAK (multiplicity of infection = 0.2) for 0, 0.5, 1, 2, and 5 hours. Gene expression is shown relative to the mock group (nontreated and noninfected samples). Intergroup differences were analyzed by two-way ANOVA using time and treatment as factors, which was followed by a Tukey HSD post hoc test. Data are representative of three experiments. Scale bars: A and C, 500 μm; B and D, 50 μm.
Table 1.
Histological Evaluation of Pig Lungs Treated or Not Treated with Flagellin before Pseudomonas aeruginosa strain K (PAK) Infection
| None or Mild (%) | Medium (%) | Severe (%) | P Value | |
|---|---|---|---|---|
| PBS–PAK | 30.43 | 43.47 | 26.8 | <0.021 |
| Flagellin–PAK | 71.42 | 21.42 | 7.14 |
Definition of abbreviations: P. aeruginosa = Pseudomonas aeruginosa; PAK = Pseudomonas aeruginosa strain K. Flagellin was administered in the respiratory tract of pigs 24 hours before P. aeruginosa infection. Lung sections from the accessory, left caudal, left cranial, middle, right caudal, and right cranial lobes were stained 24 hours after infection with hematoxylin and eosin. This table shows the percentages of lung lobes classified according to lesion severity (n = 5 pigs). None or mild: histological score lower than 2; medium: histological scores of 2 or 3; severe: histological score higher than 3. P values were calculated by using the contingency chi-square test.
Flagellin Treatment Decreases Airway Epithelial Inflammatory Response to P. aeruginosa in CF Pig Lungs
Next, we asked whether a similar protective effect of flagellin would be observed in an inflammatory lung disease such as CF (3).
CFTR+/+ and CFTR−/− PCLS were treated with 100-ng/ml flagellin for 24 hours before being infected with the P. aeruginosa strain PAK (multiplicity of infection = 0.2). As expected, P. aeruginosa infection upregulated the transcription of the CXCL2, CXCL8, CCL20, TNFAIP3, NFKBIA, and NFKBIE genes in both CFTR+/+ and CFTR−/− samples (Figure 5F). Importantly, flagellin treatment before P. aeruginosa infection significantly reduced the expression of the CXCL2 and TNFAIP3 genes in both CFTR+/+ and CFTR−/− lung PCLS (Figure 5F). When compared with AECs, flagellin treatment affected only a subset of the tested genes, likely because of differences in tissue structure and cell composition (33). However, it is remarkable that flagellin treatment had the same effect on both genotypes, despite the important alterations in the inflammatory response in CF lungs (3). Therefore, flagellin may induce a protective effect in CF lungs by preventing a pathogen-induced excessive inflammatory response.
Discussion
In this study, we show that flagellin stimulation of TLR5 signaling represents an important mechanism for improving the immune response against P. aeruginosa and reducing the excessive inflammation observed in CF lungs. This is consistent with the known role played by TLR5 as a modifier gene in CF (34). We observed that a single administration of flagellin to the airways (in vitro and in vivo) generated a transient expression of proinflammatory cytokines. Furthermore, flagellin stimulation significantly dampened the response against a second inflammatory stimulus (both flagellin and P. aeruginosa). Notably, flagellin pretreatment improved the lung status after infection with P. aeruginosa, suggesting a better response to infection after flagellin therapy, as indicated by Muñoz and colleagues (4).
In this study, we employed a recombinant flagellin, FliCΔ174–40, that lacks its antigenic domain. This molecule has a lower intrinsic antigenicity than native flagellin, and it has been demonstrated to be safe and effective against different pathogens after several flagellin administrations (7). Flagellin stimulation has been shown to provide protection in several disease backgrounds (e.g., Salmonella spp., Burkholderia cepacia, and Yersinia pseudotuberculosis) (35). This protection seems to depend on the secretion of proinflammatory cytokines, which are essential for the recruitment of immune cells, the secretion of antimicrobial molecules and mucins, and the maturation of dendritic cells and type 3 innate lymphoid cells (35). However, it is unclear how the airway epithelium processes the different microbial signals that it receives to generate an appropriate inflammatory response. The respiratory tract is not a sterile environment. There is a continuous exchange of microorganisms (commensal and/or pathogenic) and, the airway epithelium must therefore be able to discriminate between “dangerous” and “nondangerous” signals to decide whether or not to activate the inflammatory response (36–38). A better understanding of these processes is needed to develop efficient immune therapies that target TLRs to fight disease. A recent study in macrophages suggested that a switch-like (or digital) p38 MAPK activation may serve as the critical signal triggering the TLR4-mediated inflammatory response (37). Our data showed that the AECs’ inflammatory response to flagellin was preferentially mediated through digital activation of the NF-κB pathway. This indicates that in the airway epithelium, a certain flagellin concentration needs to be exceeded to activate NF-κB and trigger the expression of inflammatory genes (37). Otherwise, cells remain in a “resting” steady state. Similar results have been observed in fibroblasts, in which switch-like NF-κB responses are used to integrate information on the timing and intensity of an LPS stimulus (38).
We observed that a single administration of flagellin to the airways (in vitro and in vivo) generated a transient expression of proinflammatory cytokines. Interestingly, flagellin stimulation significantly dampened the response against a second flagellin stimulus or to P. aeruginosa, both in vitro and in vivo (9). The mechanisms through which flagellin treatment could diminish the response to P. aeruginosa are unclear. It is possible that flagellin induces epigenetic changes and trained immunity in the AECs (39). In this regard, Bigot and colleagues (9) showed that flagellin reduced bronchial epithelial cells’ response to P. aeruginosa in vitro by modifying their epigenetic regulation. The observed decreased inflammatory response could also be related to TLR5 signaling desensitization due to a decrease in receptor availability or tachyphylaxis, as observed when repeated doses of TLR ligands are administered to mice (32). However, it has been reported that flagellin degrades rapidly in the airways (in less than 12 h). Thus, it is unlikely that flagellin was present in the pig lungs at the moment of infection during our in vivo experiments (40) because we analyzed the lungs 48 hours after a single flagellin administration.
There are several other mechanisms through which flagellin could modulate the inflammatory response. Calcium signaling has been linked to the regulation of the nature and intensity of TLR responses to an inflammatory stimulus (11, 41), and altered mitochondrial Ca2 + homeostasis has been reported in several pathological conditions, including CF (10). Moreover, we and others observed that thapsigargin increased the stimulatory effect of flagellin (42). We also observed that flagellin stimulation induced the expression of several genes that link calcium signaling with the immune response. For instance, the CD83 gene (upregulated 17-fold), which is a member of the immunoglobulin family and is mostly expressed in matured dendritic cells (22). This gene plays important roles in the immune response by enhancing calcium response in T lymphocytes, driving T-cell proliferation (23). GPR35, which encodes for an orphan G protein–coupled receptor, elevates intracellular calcium upon activation and has been associated with the regulation of the immune response (24). There were also other genes with a potential impact on calcium regulation, such as ITPKC, which forms part of the ITP3K (inositol-trisphosphate-kinase) and phosphorylate inositol 1,4,5-triphosphate to inositol 1,3,4,5-tetraphosphate, acting as a direct regulator of calcium signaling (26), as well as SDC4 (28) and TMEM64 (29). Finally, although previous reports indicated that flagellin stimulation does not trigger an increase in cytosolic Ca2 + (42), we observed that a 24-hour flagellin pretreatment modulated the histamine-dependent Ca2 + increase. This result suggests that flagellin can have an effect on Ca2 + homeostasis and storage within the ER, which could alter the intensity of the TLR response to a ligand.
In addition to that, flagellin treatment may alter the activity of key negative regulators of the innate immune response to P. aeruginosa (6, 8). Flagellin upregulated TNFAIP3, which blocks TRAF6 ubiquitination and inhibits the activation of NF-κB (43), at least up to 24 hours after treatment. It is possible that a sustained elevated expression of TNFAIP3, even at low amounts, could affect the ability of AECs to respond to a second flagellin stimulation. Moreover, P. aeruginosa triggers innate immunity by activating TLR4 and TLR5 signaling (21). Both pathways share a similar topology. After activation, TLR4 and TLR5 recruit MyD88, forming a complex with the IRAK (IL-1R–associated kinase) family members to promote the ubiquitination of TRAF6 and NF-κB signaling (44). Therefore, an increase in TNFAIP3 concentration could potentially decrease both TLR4 and TLR5 signaling, thus modifying the general AEC response to P. aeruginosa.
Our results indicate that flagellin induces a transient inflammatory response in the airways that has a positive impact in the lung immune response to P. aeruginosa. This improved response could be related to an effect of flagellin on the airway epithelium as well as in the resident immune cells (35). This beneficial effect of flagellin would be especially important in the CF context, in which sequential pulmonary infections and excessive inflammation are common. Some authors also suggest that innate immunity is altered in patients with CF (3); therefore, it is unclear whether flagellin stimulation of the airways would have a similar effect in the CF lungs. To evaluate the effect of flagellin in CF, we employed a pig model of CF. This animal model shares many physiological and anatomical similarities with humans (15). This model also develops spontaneous lung infections as observed in patients with CF, which has allowed us to better understand the origins of CF lung disease (45, 46). We employed newborn CFTR−/− piglets to avoid interference of previous infections and/or chronic lung inflammation that could bring confounding effects to the airways’ response to an inflammatory stimulus (47). However, newborn CFTR−/− piglets have a very short lifespan (less than 24 hours) (18), which makes the in vivo administration of flagellin and subsequent P. aeruginosa infection unfeasible. To overcome this limitation, we employed PCLS as a surrogate system to study the lung innate immune response to P. aeruginosa (48). We show that flagellin’s effect on the immune response to P. aeruginosa was conserved in the CF lungs, indicating that modulation of TLR5 signaling could serve to reduce the excessive inflammatory response observed in CF. These data highlight the importance of TLR5 as a major mediator of inflammation and potential target for antiinflammatory therapies in CF (34).
In conclusion, we show that modulation of TLR5 signaling through flagellin administration can improve the innate immune response to P. aeruginosa, thus decreasing inflammation in the lungs. Modulation of the inflammatory response is crucial for patients with CF during pulmonary exacerbations, which are characterized by excessive neutrophilic inflammation. Our data open new leads toward the design of novel immunomodulatory therapies targeting TLR5 that could improve the management of lung inflammation in CF.
Acknowledgments
Acknowledgment
The authors thank Sandrine Melo and Nathalie Lallier from the Unité de Infectiologie et Santé Publique of the Institut National de Recherche pour l'Agriculture, l'Alimentation et l'Environnement (INRAE). They also thank Jérémy Pezant, Alain Deslis, and Alexis Pléau and the staff of the Plateforme d’Infectiologie Expérimentale (Unité d’Enseignement 1277, Centre de Recherche Val de Loire, INRAE, Nouzilly, France) as well as the Unité Expérimentale de Physiologie Animale de l’Orfrasière of INRAE–Centre de Recherche de Tours (France) for their technical support.
Footnotes
Supported by the Association Vaincre la Mucoviscidose (grants RF20150501357 [M.S.-T., P.B., and I.C.], RF20160501644 [J.-C.S. and I.C.], RF20170502036 [J.-C.S.], and RF20200502689 [I.C. and P.C.]), the Agence Nationale de la Recherche (grant ANR-18-CE20-0024-01 [I.C.]), and the European Union’s H2020 Research Infrastructures (grant VetBioNet/731014 [S.T. and T.M.]).
Author Contributions: R.L.-G.: data curation, formal analysis, investigation, and review and editing of writing. I.F.: data curation, formal analysis, and investigation. P.C.: conceptualization, investigation, resources, supervision, review and editing of writing, and funding acquisition. S.T.: conceptualization, resources, and funding acquisition. M.O.: investigation. C.C.: investigation. C.B.: investigation, methodology, and resources. M.R.: investigation, methodology, resources, and review and editing of writing. C.R.: investigation. A.G.: conceptualization and investigation. M.S.-T.: conceptualization, supervision, review and editing of writing, and funding acquisition. T.M.: investigation and resources. P.B.: investigation, supervision, review and editing of writing, and funding acquisition. A.B.: resources. N.K.: resources and review and editing of writing. J.-C.S.: conceptualization, project administration, resources, review and editing of writing, and funding acquisition; I.C. : conceptualization, data curation, formal analysis, investigation, project administration, supervision, validation, visualization, writing of original draft, and review and editing of writing.
This article has an online data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2021-0125OC on June 7, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 2. Lea SR, Reynolds SL, Kaur M, Simpson KD, Hall SR, Hessel EM, et al. The effects of repeated Toll-like receptors 2 and 4 stimulation in COPD alveolar macrophages. Int J Chron Obstruct Pulmon Dis. 2018;13:771–780. doi: 10.2147/COPD.S97071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bodas M, Vij N. The NF-κB signaling in cystic fibrosis lung disease: pathophysiology and therapeutic potential. Discov Med. 2010;9:346–356. [PMC free article] [PubMed] [Google Scholar]
- 4. Muñoz N, Van Maele L, Marqués JM, Rial A, Sirard JC, Chabalgoity JA. Mucosal administration of flagellin protects mice from Streptococcus pneumoniae lung infection. Infect Immun. 2010;78:4226–4233. doi: 10.1128/IAI.00224-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mifsud EJ, Tan AC, Jackson DC. TLR agonists as modulators of the innate immune response and their potential as agents against infectious disease. Front Immunol. 2014;5:79. doi: 10.3389/fimmu.2014.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caballero I, Boyd J, Almiñana C, Sánchez-López JA, Basatvat S, Montazeri M, et al. Understanding the dynamics of Toll-like Receptor 5 response to flagellin and its regulation by estradiol. Sci Rep. 2017;7:40981. doi: 10.1038/srep40981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Porte R, Van Maele L, Muñoz-Wolf N, Foligné B, Dumoutier L, Tabareau J, et al. Flagellin-mediated protection against intestinal Yersinia pseudotuberculosis infection does not require interleukin-22. Infect Immun. 2017;85:e00806-16. doi: 10.1128/IAI.00806-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ashall L, Horton CA, Nelson DE, Paszek P, Harper CV, Sillitoe K, et al. Pulsatile stimulation determines timing and specificity of NF-κB-dependent transcription. Science. 2009;324:242–246. doi: 10.1126/science.1164860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bigot J, Guillot L, Guitard J, Ruffin M, Corvol H, Chignard M, et al. Respiratory epithelial cells can remember infection: a proof-of-concept study. J Infect Dis. 2020;221:1000–1005. doi: 10.1093/infdis/jiz569. [DOI] [PubMed] [Google Scholar]
- 10. Rimessi A, Bezzerri V, Patergnani S, Marchi S, Cabrini G, Pinton P. Mitochondrial Ca2+-dependent NLRP3 activation exacerbates the Pseudomonas aeruginosa-driven inflammatory response in cystic fibrosis. Nat Commun. 2015;6:6201. doi: 10.1038/ncomms7201. [DOI] [PubMed] [Google Scholar]
- 11. Tang S, Chen T, Yang M, Wang L, Yu Z, Xie B, et al. Extracellular calcium elicits feedforward regulation of the Toll-like receptor-triggered innate immune response. Cell Mol Immunol. 2017;14:180–191. doi: 10.1038/cmi.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meurens F, Summerfield A, Nauwynck H, Saif L, Gerdts V. The pig: a model for human infectious diseases. Trends Microbiol. 2012;20:50–57. doi: 10.1016/j.tim.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chevaleyre C, Riou M, Bréa D, Vandebrouck C, Barc C, Pezant J, et al. The pig: a relevant model for evaluating the neutrophil serine protease activities during acute Pseudomonas aeruginosa lung infection. PLoS One. 2016;11:e0168577. doi: 10.1371/journal.pone.0168577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klymiuk N, Mundhenk L, Kraehe K, Wuensch A, Plog S, Emrich D, et al. Sequential targeting of CFTR by BAC vectors generates a novel pig model of cystic fibrosis. J Mol Med (Berl) 2012;90:597–608. doi: 10.1007/s00109-011-0839-y. [DOI] [PubMed] [Google Scholar]
- 16. Parameswaran GI, Sethi S. Pseudomonas infection in chronic obstructive pulmonary disease. Future Microbiol. 2012;7:1129–1132. doi: 10.2217/fmb.12.88. [DOI] [PubMed] [Google Scholar]
- 17. Sousa AM, Pereira MO. Pseudomonas aeruginosa diversification during infection development in cystic fibrosis lungs: a review. Pathogens. 2014;3:680–703. doi: 10.3390/pathogens3030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guillon A, Chevaleyre C, Barc C, Berri M, Adriaensen H, Lecompte F, et al. Computed tomography (CT) scanning facilitates early identification of neonatal cystic fibrosis piglets. PLoS One. 2015;10:e0143459. doi: 10.1371/journal.pone.0143459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tudelska K, Markiewicz J, Kochańczyk M, Czerkies M, Prus W, Korwek Z, et al. Information processing in the NF-κB pathway. Sci Rep. 2017;7:15926. doi: 10.1038/s41598-017-16166-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Trouillet AC, Keller M, Weiss J, Leinders-Zufall T, Birnbaumer L, Zufall F, et al. Central role of G protein Gαi2 and Gαi2+ vomeronasal neurons in balancing territorial and infant-directed aggression of male mice. Proc Natl Acad Sci USA. 2019;116:5135–5143. doi: 10.1073/pnas.1821492116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ramphal R, Balloy V, Jyot J, Verma A, Si-Tahar M, Chignard M. Control of Pseudomonas aeruginosa in the lung requires the recognition of either lipopolysaccharide or flagellin. J Immunol. 2008;181:586–592. doi: 10.4049/jimmunol.181.1.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Z, Ju X, Silveira PA, Abadir E, Hsu WH, Hart DNJ, et al. CD83: activation marker for antigen presenting cells and its therapeutic potential. Front Immunol. 2019;10:1312. doi: 10.3389/fimmu.2019.01312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pinho MP, Migliori IK, Flatow EA, Barbuto JA. Dendritic cell membrane CD83 enhances immune responses by boosting intracellular calcium release in T lymphocytes. J Leukoc Biol. 2014;95:755–762. doi: 10.1189/jlb.0413239. [DOI] [PubMed] [Google Scholar]
- 24. Maravillas-Montero JL, Burkhardt AM, Hevezi PA, Carnevale CD, Smit MJ, Zlotnik A. Cutting edge: GPR35/CXCR8 is the receptor of the mucosal chemokine CXCL17. J Immunol. 2015;194:29–33. doi: 10.4049/jimmunol.1401704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Divorty N, Mackenzie AE, Nicklin SA, Milligan G. G protein-coupled receptor 35: an emerging target in inflammatory and cardiovascular disease. Front Pharmacol. 2015;6:41. doi: 10.3389/fphar.2015.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dewaste V, Moreau C, De Smedt F, Bex F, De Smedt H, Wuytack F, et al. The three isoenzymes of human inositol-1,4,5-trisphosphate 3-kinase show specific intracellular localization but comparable Ca2+ responses on transfection in COS-7 cells. Biochem J. 2003;374:41–49. doi: 10.1042/BJ20021963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu Y, Echtermeyer F, Thilo F, Theilmeier G, Schmidt A, Schülein R, et al. The proteoglycan syndecan 4 regulates transient receptor potential canonical 6 channels via RhoA/Rho-associated protein kinase signaling. Arterioscler Thromb Vasc Biol. 2012;32:378–385. doi: 10.1161/ATVBAHA.111.241018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gopal S, Søgaard P, Multhaupt HA, Pataki C, Okina E, Xian X, et al. Transmembrane proteoglycans control stretch-activated channels to set cytosolic calcium levels. J Cell Biol. 2015;210:1199–1211. doi: 10.1083/jcb.201501060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim H, Kim T, Jeong BC, Cho IT, Han D, Takegahara N, et al. Tmem64 modulates calcium signaling during RANKL-mediated osteoclast differentiation. Cell Metab. 2013;17:249–260. doi: 10.1016/j.cmet.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gotea V, Ovcharenko I. DiRE: identifying distant regulatory elements of co-expressed genes. Nucleic Acids Res. 2008;36:W133–W139. doi: 10.1093/nar/gkn300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ni L, Zhou C, Duan Q, Lv J, Fu X, Xia Y, et al. β-AR blockers suppresses ER stress in cardiac hypertrophy and heart failure. PLoS One. 2011;6:e27294. doi: 10.1371/journal.pone.0027294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alfaro VY, Goldblatt DL, Valverde GR, Munsell MF, Quinton LJ, Walker AK, et al. Safety, tolerability, and biomarkers of the treatment of mice with aerosolized Toll-like receptor ligands. Front Pharmacol. 2014;5:8. doi: 10.3389/fphar.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu G, Betts C, Cunoosamy DM, Åberg PM, Hornberg JJ, Sivars KB, et al. Use of precision cut lung slices as a translational model for the study of lung biology. Respir Res. 2019;20:162. doi: 10.1186/s12931-019-1131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Blohmke CJ, Park J, Hirschfeld AF, Victor RE, Schneiderman J, Stefanowicz D, et al. TLR5 as an anti-inflammatory target and modifier gene in cystic fibrosis. J Immunol. 2010;185:7731–7738. doi: 10.4049/jimmunol.1001513. [DOI] [PubMed] [Google Scholar]
- 35. Vijayan A, Rumbo M, Carnoy C, Sirard JC. Compartmentalized antimicrobial defenses in response to flagellin. Trends Microbiol. 2018;26:423–435. doi: 10.1016/j.tim.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 36. Invernizzi R, Lloyd CM, Molyneaux PL. Respiratory microbiome and epithelial interactions shape immunity in the lungs. Immunology. 2020;160:171–182. doi: 10.1111/imm.13195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gottschalk RA, Martins AJ, Angermann BR, Dutta B, Ng CE, Uderhardt S, et al. Distinct NF-κB and MAPK activation thresholds uncouple steady-state microbe sensing from anti-pathogen inflammatory responses. Cell Syst. 2016;2:378–390. doi: 10.1016/j.cels.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kellogg RA, Tian C, Lipniacki T, Quake SR, Tay S. Digital signaling decouples activation probability and population heterogeneity. eLife. 2015;4:e08931. doi: 10.7554/eLife.08931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, et al. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352:aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Van Maele L, Fougeron D, Janot L, Didierlaurent A, Cayet D, Tabareau J, et al. Airway structural cells regulate TLR5-mediated mucosal adjuvant activity. Mucosal Immunol. 2014;7:489–500. doi: 10.1038/mi.2013.66. [DOI] [PubMed] [Google Scholar]
- 41.Shintani Y, Drexler HC, Kioka H, Terracciano CM, Coppen SR, Imamura H, et al. Toll-like receptor 9 protects non-immune cells from stress by modulating mitochondrial ATP synthesis through the inhibition of SERCA2. EMBO Rep. 2014;15:438–445. doi: 10.1002/embr.201337945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fu Z, Bettega K, Carroll S, Buchholz KR, Machen TE. Role of Ca2+ in responses of airway epithelia to Pseudomonas aeruginosa, flagellin, ATP, and thapsigargin. Am J Physiol Lung Cell Mol Physiol. 2007;292:L353–L364. doi: 10.1152/ajplung.00042.2006. [DOI] [PubMed] [Google Scholar]
- 43. Shembade N, Harhaj EW. Regulation of NF-κB signaling by the A20 deubiquitinase. Cell Mol Immunol. 2012;9:123–130. doi: 10.1038/cmi.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N Engl J Med. 2015;372:1574–1575. doi: 10.1056/NEJMc1502191. [DOI] [PubMed] [Google Scholar]
- 46. Bartlett JA, Ramachandran S, Wohlford-Lenane CL, Barker CK, Pezzulo AA, Zabner J, et al. Newborn cystic fibrosis pigs have a blunted early response to an inflammatory stimulus. Am J Respir Crit Care Med. 2016;194:845–854. doi: 10.1164/rccm.201510-2112OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Caballero I, Ringot-Destrez B, Si-Tahar M, Barbry P, Guillon A, Lantier I, et al. Evidence of early increased sialylation of airway mucins and defective mucociliary clearance in CFTR-deficient piglets. J Cyst Fibros. 2021;20:173–182. doi: 10.1016/j.jcf.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 48. Punyadarsaniya D, Liang CH, Winter C, Petersen H, Rautenschlein S, Hennig-Pauka I, et al. Infection of differentiated porcine airway epithelial cells by influenza virus: differential susceptibility to infection by porcine and avian viruses. PLoS One. 2011;6:e28429. doi: 10.1371/journal.pone.0028429. [DOI] [PMC free article] [PubMed] [Google Scholar]