Abstract
Pseudoprogression, defined as the radiographic false appearance of disease progression, is not frequently observed in patients with malignant peripheral nerve sheath tumor (MPNST). We report on a case of a patient with neurofibromatosis type 1 (NF1) MPNST pseudoprogression that presented as suspected local recurrence 9.5 years after last treatment. The patient underwent surgical resection following growth of a mass on sequential MRI imaging; surgical pathology, however, showed skeletal muscle with atrophy, fibroadipose tissue, and fat necrosis, without any evidence of tumor. As MPNST survival rates increase, physicians should consider pseudoprogression as a potential presentation after prior treatment.
Keywords: Malignant peripheral nerve sheath tumors, Pseudoprogression, Neurofibromatosis, Nerve tumor, MRI
Introduction
Malignant peripheral nerve sheath tumors (MPNSTs) are aggressive soft tissue sarcomas that account for 10% of soft tissue sarcomas [1]. MPNSTs are more common in patients with neurofibromatosis (NF1) [1], with 23–51% of MPNST cases occurring in these patients [1, 2]. Survival rates for MPNST are worse than most other soft tissue sarcomas [2], with NF1-related MPNSTs having worse 5-year survival (∼49.5%) than sporadic MPNSTs (∼63.1%) [3]. MPNSTs that present with heterologous elements, such as rhabdomyoblastic differentiation, also known as malignant triton tumors, are associated with worse survival [4]. Pseudoprogression, defined as the radiographic false appearance of disease progression or recurrence, has been described in soft tissue sarcomas, primarily in the context of enlargement of a lesion treated with preoperative radiation with or without chemotherapy where lesion enlargement occurs while the lesion is undergoing cystic necrosis [5]. Fat necrosis after surgery and radiation therapy appears to be most common for patients treated for breast cancer [6]. Most recently, pseudoprogression has complicated response assessment in patients treated with immunotherapy, where some responding lesions initially enlarge from infiltration of immune effector cells prior to subsequent shrinkage [7]. Recognition of the possibility of pseudoprogression is necessary to providing best care. Here, we describe, to the best of our knowledge, the first published case of pseudoprogression of a MPNST.
Case Report/Case Presentation
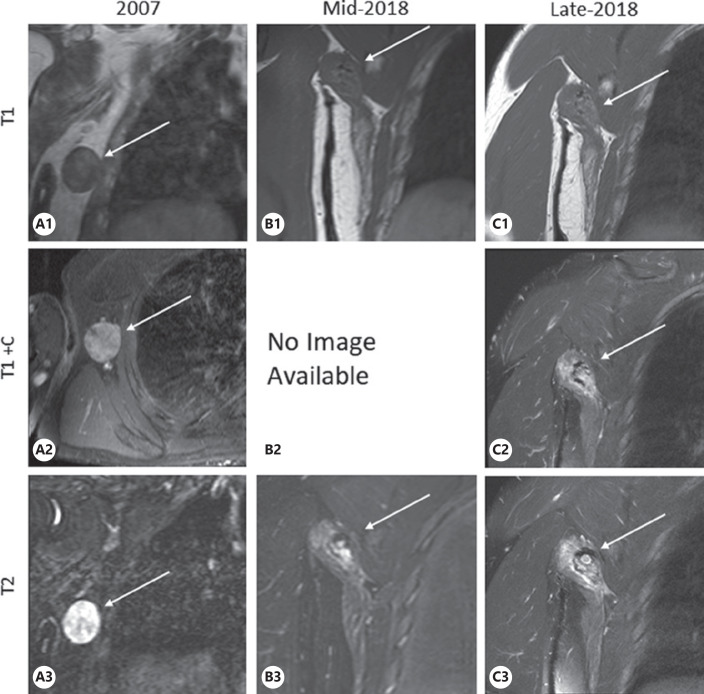
A 39-year-old man with NF1 presented with an asymptomatic T2 hyperintense lesion on MRI in 2018 (Fig. 1C). Patient was initially diagnosed with a 4.0 × 3.5 × 1.5 cm malignant triton tumor (a subtype of MPNST) of the right axilla in 2007 (Fig. 1A). He received 50 Gy/25 daily fractions of external beam radiation followed by surgery to resect the tumor bed (along with a latissimus dorsi flap), and 7 cycles of adjuvant vincristine, actinomycin D (Dactinomycin) and cyclophosphamide chemotherapy. One year later, in 2008, a recurrent 2.1 × 1.6 × 1.6 cm MPNST in the right axilla was confirmed via biopsy and surgically removed followed by additional 45 Gy radiation delivered via interstitial, low-dose rate brachytherapy over 100 h. A second 1.2 × 0.9 × 0.9 cm recurrence in the right axilla was surgically removed 6 months later in 2009 with no additional radiation delivered. Patient was followed in Sarcoma Clinic and monitored with regular surveillance chest CT scans and later chest radiographs without signs of further tumor recurrence or new primary malignancy. Nine and a half years later, during an investigational whole-body MRI as part of a research study involving surveillance for patients with NF1, a T2 hyperintense lesion measuring approximately 2.1 × 1.3 cm (Fig. 1B) with adjacent edema was noted next to the right latissimus dorsi flap. Three months later, a diagnostic MRI focused on this area showed measurable growth and identified a T2 hyperintense infiltrative enhancing mass measuring 4.1 × 2.0 × 2.4 cm (Fig. 1C). As this growing mass was at the site of the original tumor and subsequent recurrences, strong suspicion was raised for recurrent MPNST. After consent was obtained, the patient underwent surgical removal of the area, including resection of the latissimus dorsi flap, teres major, and portion of the scapula. Pathology post-surgery showed skeletal muscle with atrophy, fibroadipose tissue, and fat necrosis, without any malignant cells. The patient has remained without evidence of local or distant tumor, now 2 years after this most recent surgery.
Fig. 1.
Historical coronal MRI images of right shoulder and axilla. Coronal MRI images of right shoulder and axilla. Images labeled TI are T1 weighted, images labeled T1+C are T1 weighted with contrast (gadolinium), images labeled T2 are T2 weighted. Images A are dedicated axilla MRI images from 2007 that show pathology-confirmed tumor mass, measuring 4.0 × 3.5 × 1.5 cm. Images B are from full-body MRI in mid-2018 that show artifact in the right axilla from prior tumor resection with interval increase in size of edema and a T2 hyperintense lesion adjacent to the right latissimus dorsi, measuring approximately 2.1 × 1.3 cm. Images C are MRI of the axilla 3 months later, showing a T2 hyperintense infiltrative enhancing mass with measurable growth, now measuring 4.1 × 2.0 × 2.4 cm.
Discussion/Conclusion
Pseudoprogression is the radiographic appearance of tumor progression in the absence of true tumor progression. Given this patient's prior local recurrences and the rarity of this entity in patients with sarcoma, pseudoprogression was not included in the differential diagnosis. It is important for clinicians to consider the possibility of pseudoprogression for patients who present with asymptomatic lesions in heavily pretreated regions of the body. We share this case − the first of its kind in the literature − with the hope that others may recognize this possibility in treatment decision-making.
Statement of Ethics
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images. The paper is exempt from Ethical Committee approval based on the Federal Policy for the Protection of Human Subjects (45 CFR 46.102[d]).
Conflict of Interest Statement
Mr. Andrés Lessing has no conflicts of interest to declare. Dr. Thomas DeLaney has no conflicts of interest to declare. Dr. Gregory M. Cote reports Advisory Board fees from Agios, Epizyme, and PharmaMar; Support provided to his institution for the conduct of clinical trials from Agios, Epizyme, PharmaMar, Eisai, Macrogenics, Boston Biomedical, Plexxicon, Merck KGaA/EMD Serono Research and Development Institute, CBA, SpringWorks Therapeutics, Bavarian-Nordic, and Bayer. Dr. Kevin Raskin has no conflicts of interest to declare. Dr. Scott Plotkin reports co-founding NFlection Therapeutics and NF2 Therapeutics and consulting for AstraZeneca, Akouos, and SonALAsense. Dr. Juan Lessing has no conflicts of interest to declare.
Funding Sources
No funding sources relevant to preparation of manuscript.
Author Contributions
Andrés J. Lessing, MBA: Contributed to design and conceptualization; drafting and revising the manuscript for intellectual content; major role in the acquisition of data. Gregory M. Cote, MD, PhD: Contributed to drafting and revising the manuscript for intellectual content. Thomas F. DeLaney, MD: Contributed to drafting and revising the manuscript for intellectual content; major role in the acquisition of data; analysis or interpretation of data. Scott R. Plotkin, MD, PhD: Contributed to design and conceptualization; drafting and revising the manuscript for intellectual content; major role in the acquisition of data; analysis and interpretation of data. Kevin A. Raskin, MD: Contributed to drafting and revising the manuscript for intellectual content; analysis and interpretation of data. Juan N. Lessing, MD FACP: Contributed to design and conceptualization; drafting and revising the manuscript for intellectual content.
Acknowledgements
The authors wish to thank Dr. Nick M. Mark and Dr. Eric J. Wannamaker for assistance selecting and reviewing the imaging studies.
References
- 1.Widemann BC. Current status of sporadic and neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Curr Oncol Rep. 2009;11((4)):322–8. doi: 10.1007/s11912-009-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin E, Coert JH, Flucke UE, Slooff WM, Ho VKY, van der Graaf WT, et al. A nationwide cohort study on treatment and survival in patients with malignant peripheral nerve sheath tumours. Eur J Cancer. 2020;124:77–87. doi: 10.1016/j.ejca.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Miao R, Wang H, Jacobson A, Lietz AP, Choy E, Raskin KA, et al. Radiation-induced and neurofibromatosis-associated malignant peripheral nerve sheath tumors (MPNST) have worse outcomes than sporadic MPNST. Radiother Oncol. 2019;137:61–70. doi: 10.1016/j.radonc.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Li G, Liu C, Liu Y, Xu F, Su Z, Wang Y, et al. Analysis of clinical features and prognosis of malignant triton tumor: a report of two cases and literature review. Oncol Lett. 2015;10((6)):3551–6. doi: 10.3892/ol.2015.3762. Epub 2015 Sep 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeLaney TF, Spiro IJ, Suit HD, Gebhardt MC, Hornicek FJ, Mankin HJ, et al. Neoadjuvant chemotherapy and radiotherapy for large extremity soft-tissue sarcomas. Int J Radiat Oncol Biol Phys. 2003 Jul 15;56((4)):1117–27. doi: 10.1016/s0360-3016(03)00186-x. [DOI] [PubMed] [Google Scholar]
- 6.Meric F, Buchholz TA, Mirza NQ, Vlastos G, Ames FC, Ross MI, et al. Long-term complications associated with breast-conservation surgery and radiotherapy. Ann Surg Oncol. 2002;9((6)):543–9. doi: 10.1007/BF02573889. [DOI] [PubMed] [Google Scholar]
- 7.Borcoman E, Nandikolla A, Long G, Goel S, Le Tourneau C. Patterns of response and progression to immunotherapy. Am Soc Clin Oncol Educ Book. 2018;38:169–78. doi: 10.1200/EDBK_200643. [DOI] [PubMed] [Google Scholar]