Abstract
Alectinib is the first-line targeted treatment for advanced ALK-positive non-small-cell lung cancer. Although it has a relatively mild toxicity profile, adverse events (AEs) do occur. We present a case of alectinib-induced bilateral pleural effusions and pericardial effusion that has not previously been reported. The patient developed severe dyspnea 3 months after starting alectinib. He underwent thorough clinical examination including evaluations of heart function. The heart function was normal. There was no sign of pneumonitis or progressive disease on the CT scans. Cytology samples of the pleural fluid from multiple thoracocenteses were examined and showed no malignant cells. Next-generation sequencing (NGS) analysis of circulating tumor DNA from sequential blood samples was also carried out. NGS identified no known driver mutations associated with the effusions. Hence, the effusions were suspected to be an alectinib-induced AE. Alectinib was withdrawn, and the patient commenced brigatinib. The effusions subsequently regressed.
Keywords: Alectinib, Pleural effusion, Pericardial effusion, Non-small-cell lung cancer
Introduction
ALK rearrangement occurs in 2–7% of all NSCLCs and is more prevalent among young patients, nonsmokers, and who have adenocarcinomas [1]. Standard treatment for patients with ALK-translocated advanced NSCLC is tyrosine kinase inhibitors (TKIs). With a 1-year survival of >80%, the prognosis for this group of patients is substantially better than that for other patients with stage IV NSCLC [2, 3]. Nonetheless, acquired treatment resistance develops in all patients [4].
Case Report/Case Presentation
Here, we present a 56-year-old, physically active, never-smoking male with dyspnea and chest pain during exercise. He was referred to a cardiologist. The electrocardiogram and echocardiogram were normal. A heart-CT showed normal heart structure but revealed lung infiltrations and possible lytic bone destruction in Th12. Supplementary CT and PET-CT scans confirmed these findings and also showed multiple enlarged thoracic lymph nodes. Malignant disease was suspected, and a lymph node biopsy confirmed metastases from lung adenocarcinoma, stage 4. NGS analysis of the lymph node biopsy revealed an EML4-ALK translocation. The patient started treatment with alectinib and zoledronic acid in October 2019. The dyspnea improved. He did not take any medication prior to or during this treatment.
Three months after alectinib treatment was started, the patient's dyspnea worsened. A CT scan in January 2020 showed bilateral pleural effusions and minor pericardial effusion. The patient was admitted to a local hospital. An echocardiogram was performed showing normal heart structure and function and a clinically nonsignificant pericardial effusion. There were no signs of infection. Cytological examination of the pleural fluid did not demonstrate malignant cells.
During the subsequent 4 months, the patient underwent multiple thoracocenteses with periodic improvement of dyspnea. Pleural fluid production was approximately 6,000 mL/week (right pleural cavity) and 2,500 mL/week (left pleural cavity) (Fig. 1). Left-sided talc pleurodesis was performed in April and May 2020 with regression of the pleural effusion and some relief of dyspnea. Because of massive fluid production in the right pleural cavity, a permanent pleural catheter was inserted in June with a daily fluid production of 800–900 mL. The patient remained unable to do physical exercise because of dyspnea.
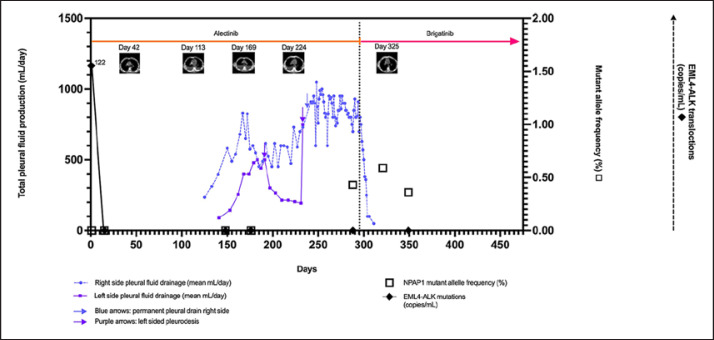
Fig. 1.
Longitudinal measurements of the patient's pleural effusions and ctDNA. The x-axis indicates time in days. Hence, day 1 corresponds to the date of alectinib treatment start. The left y-axis indicates the total daily pleural fluid production in milliliter/day. The right solid y-axis depicts the mutant allele frequency of NPAP1 (%). The dashed right y-axis depicts the EML4-ALK translocations (copies/mL) with 122 copies/mL found in the ctDNA at treatment start. The horizontal orange line is the timeline for alectinib, and the magenta horizontal line is the timeline for brigatinib treatment. The dashed vertical line shows the time point where alectinib was withdrawn and brigatinib was commenced. The 5 pictures in the top of the graph are the CT scans performed during the treatment course. These CT scans are shown in an enlarged version in Figure 2.
Cytological examination of the fluid from the pleural cavities was performed on 17 occasions with no signs of malignancy. CT scans of the thorax every 8th week all showed stable disease according to RECIST version 1.1 criteria. There was no sign of pneumonitis, and the minor pericardial effusion remained stable. Lung scintigraphy in July 2020 showed mildly reduced perfusion in the left lung without signs of pulmonary embolism. NGS analysis of ctDNA isolated from blood was performed 4 times during alectinib treatment (shown in Fig. 1). The EML4-ALK translocation was found in the ctDNA before alectinib treatment began. It was cleared from the blood 3 weeks later and remained cleared during the rest of the treatment course. An NPAP1 mutation (p.Ala80Thr) was found in the ctDNA sample immediately before brigatinib treatment at an allelic frequency of 0.43%. The NPAP1 mutation is oncogenic and reported in COSMIC but has not been reported in lung cancers. However, the same NPAP1 mutation was present in the 2 subsequent blood samples with approximately the same frequency (0.59% and 0.36%, respectively). As the allelic frequency remained stable, this was not suggestive of progression caused by this mutation. The mutation was therefore interpreted as not clinically significant.
Hence, the pleural and pericardial effusions were suspected to be alectinib induced, and alectinib was withdrawn in July 2020. The patient subsequently started brigatinib treatment. Two weeks later, the pleural effusions regressed. Figure 1 depicts the volume of pleural effusions evolving over time illustrating the reduction in pleural effusion after stopping alectinib and starting brigatinib. The permanent right-sided lung catheter was removed by the end of August 2020. The subsequent CT scan (shown in Fig. 2) showed stable disease and remarkable regression of the pleural effusion and a mild cardiac decompensation successfully treated with 20 mg furosemide. The patient's dyspnea improved considerably, and he was able to resume physical exercise. As of June 2021, his condition remains stable.
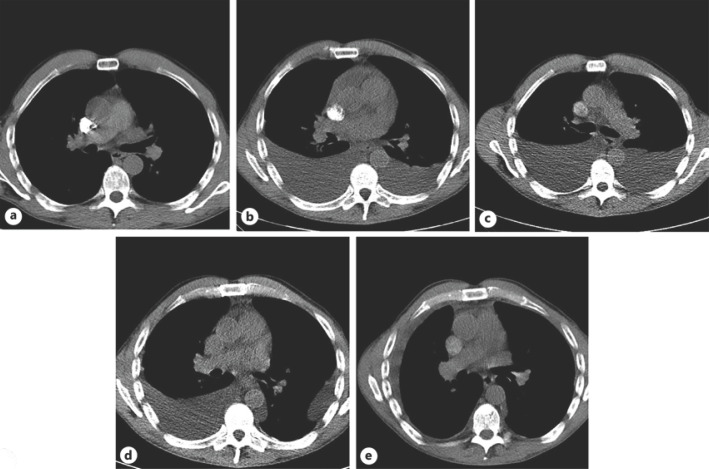
Fig. 2.
CT scans of the lungs. A Day 42. Seven weeks after alectinib start. B Day 113. Debut of pleural effusions 3 months after alectinib start. C Day 169. After repeated bilateral thoracentesis. D Day 224. After left-sided talc pleurodesis. E Day 325. Four weeks after brigatinib start. Removal of the permanent right-sided pleural drain was performed on day 322.
Discussion/Conclusion
Serious adverse events (SAEs) represent an important clinical issue in targeted treatment of metastatic ALK-translocated NSCLC. Alectinib is a second-generation TKI and is highly selective for ALK inhibition. It is the first-line treatment in stage 4 ALK-translocated NSCLC as it is generally well tolerated and has a beneficial effect on progression-free survival and brain metastasis [5, 6, 7].
Pleural effusions have previously been reported in a limited number of patients treated with second-generation ALK-TKIs. Gettinger et al. [8] found brigatinib-induced treatment-emergent pleural effusions in 2 patients out of 137. The Ascend-5 study [9] of 115 ceritinib-treated patients reported 5 patients with grade 3 pleural effusion and 3 patients with grade 3 pericardial effusion.
To our knowledge, this is the first reported case of alectinib-induced bilateral pleural effusions. We demonstrate that this SAE can be reversed by switching to another ALK-TKI, which in our case was brigatinib.
The strengths of this study are that we monitored the patient's treatment course with regular CT scans, NGS analyses of blood-derived ctDNA, and cytological examinations of the pleural fluid. None of these showed signs of progressive disease. The EML4-ALK translocation was present before treatment but did not reappear in the blood after alectinib treatment began. This highlights that the effusions were not caused by progressive disease. Furthermore, this indicates that brigatinib was as effective on the lung cancer as alectinib.
In conclusion, clinically significant bilateral pleural effusions can occur as an SAE to alectinib treatment. This condition can be restored by the withdrawal of alectinib and commencement of another ALK-TKI.
Statement of Ethics
This case report was conducted in accordance with the World Medical Association of Helsinki. The patient is participating in the research project entitled “Clinical Use of Circulating Tumor DNA in Patients with Metastatic ALK-Translocated NSCLC.” The project is approved by the Danish National Committee on Health Research Ethics, approval ref. 1-10-72-37-19. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding sources were designated to the work presented here.
Author Contributions
Maiken Parm Ulhoi collected the data and performed the graphics and drafted the manuscript under guidance from the 2 co-authors. The idea for the manuscript was conceived and designed by Peter Meldgaard. Boe Sandahl Sorensen contributed to the data analyses.
Data Availability Statement
All data are available with exception to data protected according to the Danish Health Legislations.
Acknowledgment
The authors thank Birgit Mortensen for professional technical assistance with laboratory work.
References
- 1.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010 Oct 28;363((18)):1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014 Dec 4;371((23)):2167–77. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 3.Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018 Oct 1;29((Suppl 4)):iv192–237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 4.Lin JJ, Riely GJ, Shaw AT. Targeting ALK: precision medicine takes on drug resistance. Cancer Discov. 2017 Feb;7((2)):137–55. doi: 10.1158/2159-8290.CD-16-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hida T, Nokihara H, Kondo M, Kim YH, Azuma K, Seto T, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017 Jul 1;390((10089)):29–39. doi: 10.1016/S0140-6736(17)30565-2. [DOI] [PubMed] [Google Scholar]
- 6.Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017 Aug 31;377((9)):829–38. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 7.Novello S, Mazières J, Oh IJ, de Castro J, Migliorino MR, Helland Å, et al. Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: results from the phase III ALUR study. Ann Oncol. 2018 Jun 1;29((6)):1409–16. doi: 10.1093/annonc/mdy121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gettinger SN, Bazhenova LA, Langer CJ, Salgia R, Gold KA, Rosell R, et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. Lancet Oncol. 2016 Dec;17((12)):1683–96. doi: 10.1016/S1470-2045(16)30392-8. [DOI] [PubMed] [Google Scholar]
- 9.Shaw AT, Kim TM, Crinò L, Gridelli C, Kiura K, Liu G, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017 Jul;18((7)):874–86. doi: 10.1016/S1470-2045(17)30339-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available with exception to data protected according to the Danish Health Legislations.