Abstract
Introduction:
The recommended treatment for intermediate and high-risk nonmuscle invasive bladder cancer (NMIBC) is adjuvant intravesical bacillus Calmette–Guerin (BCG) instillation. However, up to 50% experience tumor recurrences even after adjuvant BCG, and many patients develop local or systemic adverse effects. Our study compared adverse effects, short-term recurrence rates, and cost-implications of BCG therapy to Hyperthermic Intra-VEsical Chemotherapy (HIVEC) with Mitomycin-C (MMC) in these patients.
Materials and Methods:
Retrospective analysis of intermediate and high-risk NMIBC patients who received either intravesical BCG or HIVEC® after transurethral resection of bladder tumor in our institute (January 2017 to March 2020) was done. Twenty-two patients who received HIVEC and 29 who received BCG were analyzed. We used SPSS Statistics v20.0 (IBM Corp., Armonk, NY, USA) software for the statistical analysis.
Results:
Nineteen (86.4%) patients in the HIVEC group had no adverse effects. Two (9.1%) patients had Grade I lower urinary tract symptoms (LUTS) treated symptomatically. One patient developed UTI after HIVEC, and further cycles were stopped (Grade II). BCG group had a higher rate of Grade III adverse effects in six (20.7%) patients. Median follow-up was 10.5 and 22 months. The tumor recurred in one (4.5%) and six (20.7%) patients in HIVEC and BCG groups, respectively. There was no difference in recurrence-free survival at 18 months and the cost for the HIVEC therapy was more.
Conclusions:
HIVEC with MMC is a reasonable adjuvant treatment option in NMIBC, which is well tolerated, albeit increased cost of the treatment. Randomized trials with more follow-up are required for further conclusion.
Keywords: Adjuvant bacillus Calmette–Guerin, adverse effects, bladder cancer, Hyperthermic Intra-VEsical Chemotherapy, nonmuscle invasive bladder cancer, outcomes
INTRODUCTION
Bladder cancer is the tenth most common cancer worldwide[1] and the second most common genitourinary malignancy with 18,296 newly diagnosed cases in India alone in 2018.[2] Nonmuscle invasive bladder cancer (NMIBC) accounts for 75% of bladder cancer cases.[3] The standard treatment for NMIBC is transurethral resection of the bladder tumor (TURBT) and adjuvant intravesical therapy. Despite the use of adjuvant treatment, high-risk NMIBC has a 52% risk of recurrence and a 20% risk of progression in 5 years.[4]
NMIBC is stratified into low-risk, intermediate-risk, and high-risk categories based on clinical and histopathological features. Guidelines recommend adjuvant intravesical bacillus Calmette–Guerin (BCG) instillation in intermediate and high-risk NMIBC disease. BCG is related to about 65% of local toxic effects, and about 12% of patients do not opt for maintenance therapy.[5] Approximately 50% of patients experience recurrent disease even after adequate BCG treatment.[6] The scarcity of BCG is noted in many countries due to the stoppage of the production of BCG-Connaught strain. BCG-Danish 1331 strain, which was earlier used extensively in India, is not available and has been replaced by more reactogenic BCG Moscow-I strain.[7]
Other treatment options available are intravesical chemotherapy, chemohyperthermia (CHT), and systemic chemotherapy. Intravesical CHT therapy has been widely studied as an active alternate treatment for intermediate and high-risk NMIBC.
Mitomycin-C (MMC) alone is less effective than BCG in intermediate-risk tumors and use in the high-risk tumor is limited,[8] due to low absorption of the drug (<1% of the instilled drug).[9,10] Studies have shown, the use of CHT has more efficacy, a 59% relative reduction in NMIBC recurrence when compared to MMC alone.[11]
CHT with Hyperthermic Intra-VEsical Chemotherapy (HIVEC®), delivered by COMBAT BRS (COMBined Antineoplastic Thermotherapy-Bladder Recirculation System, Wheathampstead, UK) device is the newer modality, which has shown to be safe, effective, with 2-year cumulative incidence of recurrence is 12.5% in the adjuvant setting.[9] The primary objective of this study is to compare the adverse effects, short-term outcomes of HIVEC with MMC, and intravesical BCG instillation in the management of intermediate and high-risk NMIBC; a secondary objective is to check the cost implications of HIVEC.
MATERIALS AND METHODS
Study design
From January 2017 to March 2020, 72 patients of intermediate and high-risk NMIBC received intravesical adjuvant therapy with either BCG (Moscow-I Russia) or HIVEC with MMC at our institute based on the patient's choice, affordability, and understanding of adverse effect profile of different intravesical therapies. HIVEC therapy has been available from January 2019 at our institute. We retrospectively analyzed from our prospectively collected database about adverse effects, short term outcomes, and cost implications of HIVEC and compared them to those who had received standard BCG treatment.
Study criteria
The study included all patients who had complete resection of the bladder tumor, no residual disease, histopathological confirmation of urothelial carcinoma stage Ta, T1, and low-or high-grade disease. These patients were categorized into low-risk, intermediate-risk, and high-risk NMIBC (EORTC risk calculator) for recurrence and progression, based on the number of tumors (solitary vs. multiple) grades (low vs. high), size of the tumor (>3 cms as high risk), depth of invasion (Ta, T1), recurrent or primary tumor, and associated CIS. The intermediate and high-risk patients who received adjuvant therapy with HIVEC or BCG and had a minimum of one follow-up at 3 months with check cystoscopy were included in the study.
Overall 72 patients of intermediate and high-risk NMIBC received adjuvant therapy after TURBT. Eleven patients received immediate intravesical mitomycin after TURBT. Fifty-one patients fulfilled study criteria and had complete medical records available for analysis. Out of the 51 patients, 22 patients received HIVEC, and 29 patients received BCG during this period. In the study cohort five patients were post-BCG recurrence, two patients received HIVEC, and other three patients received a second induction course of BCG.
We collected the patient's baseline demographic, tumor, and TURBT details. Age, sex, history of LUTS, history of previous intravesical treatment, number of tumors, size of the tumor, HIVEC details, BCG details, adverse effects, short-term tumor recurrence rates were collected, and cost implications for both patient study groups were calculated.
All patients underwent TURBT and complete resection of the bladder tumor. Re-TURBT to rule out any residual disease was done in those who had high-grade disease. Adjuvant treatment was started 2 weeks after TURBT. After confirming the diagnosis and staging of bladder cancer, these patients received either 6 weekly injections of HIVEC with MMC 40 mg in 50 ml of saline or 120 mg of BCG (Moscow-I strain) in 50 ml of saline once a week for 6 weeks in the outpatient department of our institute. Only six patients accepted for maintenance therapy with BCG, out of which only two could complete 2 years of therapy.
Hyperthermic Intra-VEsical Chemotherapy Mitomycin therapy technique and follow-up protocol
We used the COMBAT-BRS HIVEC® CHT system for administering hyperthermic intravesical mitomycin. The temperature of mitomycin was increased to 43°C (±0.5°C) outside the body in the device and instilled into the bladder. The MMC re-circulated through pump between the bladder and the mitomycin bag, in a closed circuit through the heating system, delivering at constant temperature and pressure. Mitomycin was instilled in the bladder using 16Fr 3-way Foley's bladder catheter at a constant flow rate of 200 ml/min for 1 h. At the end of the procedure, the product was collected in a collection bag and discarded. Patients were informed about the sensation of warmth in the suprapubic area. Fluid intake was restricted for 4 h before therapy.
Patients were monitored for the occurrence of adverse effects during the therapy period in a specific format which is done routinely in all patients receiving intravesical therapy. Follow-up included urine cytology and cystoscopy starting from 6 weeks after the last instillation of HIVEC therapy and then every 3 months for the 1st year. Maintenance therapy with HIVEC was not advised as per our institute protocol due to lack of evidence in the literature. We assessed the cost to the patient for HIVEC therapy and compared it with the cost involved with BCG instillation in our institution.
As this was a retrospective study, an informed consent for inclusion in the study from participants was not taken. However, all the participants provided a written informed consent for undergoing HIVEC and we adhered to the principles of Helsinki Declaration, 1964 (amended in 2013). We confirm the availability and access to all the original data reported in this study.
Statistical analysis
We used SPSS Statistics v20.0 (IBM Corp., Armonk, NY, USA) software for the statistical analysis. Chi-square and Fischer's exact tests were used to compare patient and tumor characteristics. Survival functions were calculated using the Kaplan–Meier estimate, and a log-rank test was used to compare survival functions in different groups, and P value and confidence intervals were used to measure significance. A P < 0.05 was taken as statistically significant.
RESULTS
Baseline characteristics
The patient and tumor characteristics were equally balanced between the two groups. The median age was 62 years (range, 43–82) in the HIVEC group and 61 years in the BCG group (range, 38–80) with a P = 0.419. In HIVEC group, there were 20 (91%) male and two (9%) female patients and in BCG group 25 (86%) males and four (14%) female patients (P = 0.476) [Table 1].
Table 1.
Baseline demographic details and tumor characteristics of patients treated with adjuvant Hyperthermic Intra-VEsical Chemotherapy with mitomycin and intravesical Bacillus Calmette–Guerin (Moscow I strain) for nonmuscle invasive bladder cancer
| HIVEC (n=22) | BCG (n=29) | P | |
|---|---|---|---|
| Age (median, range) | 62 (4382) | 61 (3880) | 0.419 |
| Gender (n, %) | |||
| Male | 20 (91) | 25 (86) | 0.476 |
| Female | 2 (9) | 4 (14) | |
| T-stage (n, %) | |||
| Ta | 10 (45.5) | 10 (34.50 | 0.315 |
| T1 | 8 (36.4) | 14 (48.3) | |
| T1+CIS | 3 (13.6) | 2 (6.9) | |
| Ta+CIS | 1 (4.5) | 0 (0) | |
| CIS only | 0 (0) | 3 (10.3) | |
| Tumor grade (n, %) | |||
| Low grade | 12 (54.5) | 16 (55.2) | 0.964 |
| High grade | 10 (45.5) | 13 (44.8) | |
| Single or multiple tumors | |||
| Single | 11 (50) | 16 (55) | 0.591 |
| Multiple (≥2) | 11 (50) | 13 (45) | |
| Risk group (EORTC) | |||
| Intermediate risk | 12 (54.5) | 17 (58.6) | 0.784 |
| High risk | 10 (45.5) | 12 (41.4) |
HIVEC: Hyperthermic Intra-VEsical Chemotherapy, BCG: Bacillus Calmette-Guerin, EORTC: European Organization for Research and Treatment of Cancer
Among 22 patients who received HIVEC therapy, 12 (54.5%) were intermediate-risk, and 10 (45.5%) high-risk patients. In the BCG group, 16 (55.2%) had intermediate-risk, and 13 (44.8%) high-risk NMIBC (P = 0.784). The distribution of grades of the tumor and T stage was also similar in the two groups with P = 0.964 and 0.315, respectively [Table 1].
Adverse effects and tolerability
Adverse effects were grouped as Grade I, Grade II and Grade II based on the severity of symptoms (Cleveland Clinic grading system). Grade I adverse effects included irritative lower urinary tract symptoms (LUTS), which subsided within 48 h without medication or with symptomatic treatment. Grade II adverse effects included prolonged LUTS requiring medications and/or lasting for more than 2–5 days, urinary tract infection (UTI) requiring antibiotics, hematuria lasting more than 2–3 days, postponing of HIVEC, or BCG therapy, reducing the dose of BCG. Grade III side effects included patients needing admission for severe UTI, BCG sepsis, severe hematuria, stopping HIVEC or BCG therapy, BCG cystitis requiring anti-tubercular treatment, and bladder contracture requiring cystectomy.
Out of 22 patients who received HIVEC, 19 (86.4%) patients did not encounter any adverse effects, and 2 (9.1%) patients had Grade I adverse effects of LUTS like urgency and frequency, were treated symptomatically. None of the patients had Grade II symptoms. One patient had traumatic catheterization; hence, mitomycin instillation was delayed for 1 week. One patient (4.5%) did not tolerate HIVEC therapy (Grade III), developed severe UTI requiring hospitalization after the first instillation, and the treatment was discontinued [Table 2]. There was no need for dose reduction or postponing of cycles due to adverse effects in this group.
Table 2.
Grading of adverse effects in adjuvant Hyperthermic Intra VEsical Chemotherapy with mitomycin group and intravesical Bacillus Calmette-Guerin (Moscow I strain) group
| Adverse effects | HIVEC n (%) | BCG n (%) | P |
|---|---|---|---|
| Nil | 19 (86.4) | 4 (13.8) | 0.001 |
| Grade I | 2 (9.1) | 6 (20.7) | 0.331 |
| Grade II | 0 (0) | 13 (44.8) | Significant |
| Grade III | 1 (4.5) | 6 (20.7) | 0.143 |
HIVEC: Hyperthermic Intra-VEsical Chemotherapy, BCG: Bacillus Calmette-Guerin
Compared to the HIVEC group, the BCG group had a significantly higher incidence of adverse effects (P = 0.003). Grade I adverse effects were seen in six (20.7%), and Grade II in 13 (44.8%) patients. A dose reduction of BCG to 80 mg and delaying the cycles was required in 11 (38%) patients. Grade III adverse events were seen in six (20.7%) patients, of which five (17%) patients required a stoppage of BCG treatment. Two of them required anti-tubercular therapy for BCG cystitis, and one patient required cystectomy due to severe bladder contracture.
Efficacy and recurrence rates
Median follow-up was 10.5 months (interquartile range [IQR], 7.75-14.00) for HIVEC group and 22 months (IQR; 19-26) in the BCG group. One patient who did not complete HIVEC therapy after the first cycle due to side effects had multiple high-grade tumor recurrence at the end of 3 months and was advised radical cystectomy and ileal conduit.
Due to a short follow-up period of the HIVEC group, 18-month recurrence-free survival was calculated for both groups, as it was the longest follow-up for HIVEC group. Tumor recurrence was noted in one patient (4.5%) in the HIVEC group (n = 22) and six patients (20.7%) in the BCG group (n = 29) till the last follow-up with a P = 0.091. There was no difference in 18-month recurrence-free survival of patients treated with HIVEC (94.1%: 95% confidence interval [CI], 93.5–94.7) when compared to BCG group (89.3: 95% CI; 87.6–91.0) (P = 0.666) [Figure 1].
Figure 1.
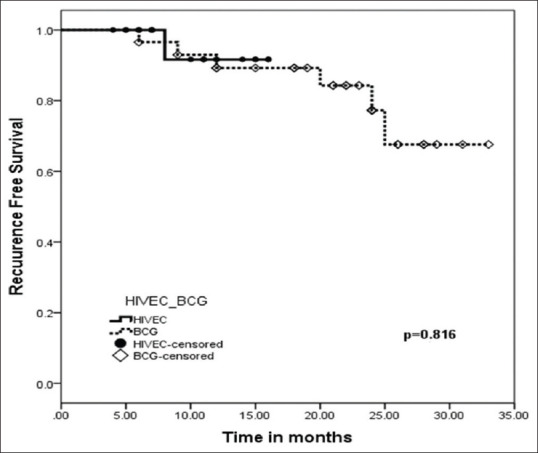
Estimated probability with Kaplan Meier analysis of recurrence-free survival in patients adequately treated with adjuvant HIVEC with mitomycin (black) and Intravesical BCG (Moscow-I strain) (dotted line) for Nonmuscle Invasive Bladder Cancer (HIVEC: Hyperthermic Intra-VEsical Chemotherapy, BCG: Bacillus Calmette-Guerin)
Cost implications
The upfront capital cost of the COMBAT BRS device is Rs. 1.5 million (£15,700), whereas BCG instillation is not associated with any capital cost. The total cost of the consumables per instillation in HIVEC is about Rs. 25,000–30,000 (£265-315). The average expenditure is around Rs. 150,000–180,000 (£1578–1890) for six cycles of HIVEC therapy compared to about Rs. 30,000–60,000 (£315–630) for six cycles of BCG with a cost of Rs. 5,000–10,000 (£52–105) per instillation.
DISCUSSION
More than 60% of high-risk NMIBC patients develop recurrent tumors within 1 year after treatment.[12,13] Newer adjuvant therapies are formulated and tested over time. MMC is a cytotoxic antibiotic that induces cell death by alkylation and cross-linking of DNA. Due to higher molecular mass, the risk of systemic absorption and toxic effects are less.[14] Previous studies have shown that hyperthermia is tumoricidal. It causes alteration of intracellular metabolism, causing DNA damage, and finally induces apoptosis of the tumor cells.[15] Heat also activates immune response by mimicking fever; heat shock protein (HSP) activates dendritic cells, T-cells, NK cells, causing anti-tumor response.[16] MMC, in the presence of heat, has a synergistic effect, becomes more effective at a higher temperature, and an increase in temperature causes enhanced blood perfusion and increases cell permeability.[15] Studies have shown that CHT to be more effective than MMC alone.[11]
Alejandro Sousa et al., in their adjuvant group with HIVEC treatment in intermediate and high risk patients, reported a 2-year cumulative incidence of recurrence of 12.5% (95% CI, 7.8%–19.3%) with the earliest recurrence at 7 months in one patient, who was successfully retreated with HIVEC.[9]
In the pre-HIVEC era, around 20 studies on the efficacy of radiofrequency induced hyperthermia (RITE) have been published.[3] Studies conducted in the adjuvant setting showed recurrence-free survival ranging from 53% to 91% with 10–24 months’ follow-up duration.[17,18,19,20] Three RCTs compared RITE with either MMC or BCG alone. Colombo et al. reported RFS at 24 months, which was significantly improved in patients who receive RITE– 82.9% versus passive MMC (42.5%) (P = 0.002).[21] Arends et al., in their RCT of 190 patients, although they could not complete the trial, suggested significantly higher RFS in RITE arm than BCG arm for the patient with the papillary disease (81.8% vs. 64.8%, P = 0.02).[17] Tan et al.,[18] in their 104 patients in the HYMN trial, demonstrated a nonsignificant increased disease-free survival in patients with the papillary only disease when treated with RITE and compared with control (BCG arm).
Results of our study comparing the HIVEC with MMC and BCG as the adjuvant therapy in intermediate and high-risk NMIBC demonstrated equal efficacy at short-term follow-up. Recurrence was noted in 1/22 patients in HIVEC arm (follow-up 18 months) and 6/29 patients in the BCG group (follow-up 33 months). There was no significant difference in 18 months RFS in HIVEC arm, which was (94.1%: 95% CI, 93.5–94.7) when compared with BCG group 89.3 (95% CI– 87.6–91.0) with a P = 0.666. However, a longer follow-up study is required for further validation of the efficacy of HIVEC [Table 2].
In the HIVEC group of patients, we observed the persistence of necrotic tissue over the resected area, even at the end of 6 months in 10 patients, which needed resection and biopsy. However, final histopathology showed only inflammatory tissue, and this requires further study to confirm the cause of delay in healing of the resected area.
The results of ongoing prospective randomized phase II study HIVEC I (EudraCT 2013-002628-18) and HIVEC II (ISRCTN 23639415), with a larger sample size of 303 and 259 patients will allow us to better assess the efficacy of this thermo-chemotherapy compared to passive mitomycin in NMIBC intermediate-risk disease patients.[22,23]
Three different techniques are available for causing hyperthermia in the bladder (1) Microwave induced heating: Intravesical radiofrequency (RF) emitting antenna incorporated in a catheter, (2) Conductive heat when chemotherapy fluid is externally heated, (3) Using externally placed RF energy.[24] In India, the available modality of CHT is HIVEC (COMBAT-BRS System), which is a conductive-based hyperthermia; the re-circulating fluid causes an increase in temperature inside the bladder. The main advantage of this system is a smaller unit that can be easily transported, easier to use, and easily reproducible.
Marquette et al. reported that patients on HIVEC showed an excellent tolerance profile, and no patient had adverse effects more than grades-3 or 4, and 2/22 patients required early stoppage of treatment due to insufficient bladder capacity and leakage of instillation fluid.[25] Sousa et al., in their novel paper on HIVEC using the COMBAT BRS system, reported that 158/160 instillations were completed (98.7%) successfully. The majority of the adverse effects were mild and self-limiting. Toxicity of more than grade-3 was not reported during the entire study. The most common adverse events were, MMC induced cystitis with irritative LUTS (two cases), bladder spasm (two cases), pelvic pain, and hematuria in one case.[9] In the present study, the BCG group had a significantly higher incidence of adverse effects compared to the HIVEC group. Grade I adverse effects were noted in two cases (9.1%) in HIVEC arm compared to six cases (20.7%) in the BCG arm. Whereas, grade III adverse effect was noted in one patient in the HIVEC group compared to six patients (20.7%) in BCG group. Two patients required anti-tubercular treatment, and one patient required cystectomy due to BCG toxicity in patients treated with BCG therapy. One patient in the HIVEC arm developed severe UTI with fever, which required hospitalization and discontinuation of treatment. Due to the lesser incidence of adverse effects in HIVEC arm, more patients completed the instillation cycles of MMC, which may lead to better oncological outcomes, better RFS, which has to be validated by longer follow-up.
The cost-effectiveness of HIVEC therapy is not much discussed in the literature. The calculated average total cost in our institute for the 6-week induction cycle, including the mitomycin and consumables, was Rs163,200 (£ 1717). The only other article in literature which has mentioned the cost-effectiveness of different hyperthermia systems, estimated the total end user-cost (£) for 6 weeks induction treatment of HIVEC as £1650 (approx. Rs. 158,000) which is cost-effective when compared to other hyperthermia systems such as Synergo (£ 4380) or EMDA (£ 2040).[3] The calculated average total cost for six cycles of BCG induction treatment, including the consumables, is about Rs. 28,500 (£300) in our institute. However, considering the side effects profile, cost, and time involved with the treatment of moderate and severe adverse effects of BCG, which are significantly higher, HIVEC with mitomycin therapy scores better in terms of tolerance and cost-benefit [Table 3.
Table 3.
Cost Implications of adjuvant Hyperthermic Intra-VEsical Chemotherapy with mitomycin and Bacillus Calmette-Guerin treatment
| HIVEC* (£/INR) | BCG* (£/INR) | HIVEC[3] (£/INR) | Synergo[3] (£/INR) | |
|---|---|---|---|---|
| Estimated cost of MMC/BCG | 23/2200 | 24/2250 | 80/7600 | 80/7600 |
| Estimated cost of disposables per treatment | 265/25,000 | 26/2500 | 195/18,500 | 650/61,700 |
| Number of treatments | 6 | 6 | 6 | 6 |
| Total cost of MMC/BCG | 140/13,200 | 142/13,500 | 480/45,600 | 480/45,600 |
| Total cost of disposables | 1578/150,000 | 160/15,000 | 1170/111,000 | 3900/370,000 |
| Total estimated cost of induction treatment | 1717/163,200 | 300/28,500 | 1650/158,000 | 4380/416,000 |
*Our study. 1 Pound Sterling (£) = 95 Indian Rupee (INR). HIVEC: Hyperthermic Intra-VEsical Chemotherapy, BCG: Bacillus Calmette-Guerin, MMC: Mitomycin-C
The limitations of the present study are, this is a non-randomized retrospective study with limited patients, included from different time periods. However, matched samples were used for analysis, and HIVEC group has a shorter follow-up period.
CONCLUSIONS
In this retrospective study, HIVEC CHT with mitomycin C treatment is well tolerated with lesser adverse effects that are short-lived. BCG therapy had a significantly higher incidence of Grade II to III adverse effects when compared to HIVEC treatment. The short-term outcomes of HIVEC are comparable to standard BCG therapy. HIVEC has better tolerance making it a potential treatment option for adjuvant therapy in intermediate and high-risk groups in NMIBC. However, HIVEC is associated with an increase in the initial cost of the treatment when compared to BCG. A larger prospective study with more extensive follow-up and maintenance therapy is required to validate the efficacy of HIVEC treatment in this era of BCG shortage and toxicity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Dr. Neha Sanwalka, for the support with the statistical analysis and Dr. Meenal Hastak and Dr. Bijal Kulkarni for their continued support and discussion of pathological aspects of disease.
REFERENCES
- 1. [[Last accessed on 2020 Apr 28]]. Available from: https://gco.iarc.fr/today/data/factsheets/cancers/30-Bladder-fact-sheet.pdf .
- 2. [[Last accessed on 2020 Apr 26]]. Available from: https://gco.iarc.fr/today/data/factsheets/populations/356-india-fact-sheets.pdf .
- 3.Tan WS, Kelly JD. Intravesical device-assisted therapies for non-muscle-invasive bladder cancer. Nat Rev Urol. 2018;15:667–85. doi: 10.1038/s41585-018-0092-z. [DOI] [PubMed] [Google Scholar]
- 4.Cambier S, Sylvester RJ, Collette L, Gontero P, Brausi MA, van Andel G, et al. EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1-3 years of maintenance bacillus Calmette-Guérin. Eur Urol. 2016;69:60–9. doi: 10.1016/j.eururo.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 5.Ojea A, Nogueira JL, Solsona E, Flores N, Gómez JM, Molina JR, et al. A multicentre, randomised prospective trial comparing three intravesical adjuvant therapies for intermediate-risk superficial bladder cancer: Low-dose bacillus Calmette-Guerin (27 mg) versus very low-dose bacillus Calmette-Guerin (13.5 mg) versus mitomycin C. Eur Urol. 2007;52:1398–406. doi: 10.1016/j.eururo.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 6.de Jong JJ, Hendricksen K, Rosier M, Mostafid H, Boormans JL. Hyperthermic intravesical chemotherapy for BCG unresponsive non-muscle invasive bladder cancer patients. Bladder Cancer. 2018;4:395–401. doi: 10.3233/BLC-180191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gan C, Mostafid H, Khan MS, Lewis DJ. BCG immunotherapy for bladder cancer – The effects of substrain differences. Nat Rev Urol. 2013;10:580–8. doi: 10.1038/nrurol.2013.194. [DOI] [PubMed] [Google Scholar]
- 8.Babjuk M, Burger M, Compérat EM, Gontero P, Mostafid AH, Palou J, et al. European association of urology guidelines on non-muscle-invasive bladder cancer (TaT1 and Carcinoma In Situ) – 2019 update. Eur Urol. 2019;76:639–57. doi: 10.1016/j.eururo.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Sousa A, Piñeiro I, Rodríguez S, Aparici V, Monserrat V, Neira P, et al. Recirculant hyperthermic IntraVEsical chemotherapy (HIVEC) in intermediate-high-risk non-muscle-invasive bladder cancer. Int J Hyperthermia. 2016;32:374–80. doi: 10.3109/02656736.2016.1142618. [DOI] [PubMed] [Google Scholar]
- 10.Logan C, Brown M, Hayne D. Intravesical therapies for bladder cancer – Indications and limitations. BJU Int. 2012;110(Suppl 4):12–21. doi: 10.1111/j.1464-410X.2012.11619.x. [DOI] [PubMed] [Google Scholar]
- 11.Lammers RJ, Witjes JA, Inman BA, Leibovitch I, Laufer M, Nativ O, et al. The role of a combined regimen with intravesical chemotherapy and hyperthermia in the management of non-muscle-invasive bladder cancer: A systematic review. Eur Urol. 2011;60:81–93. doi: 10.1016/j.eururo.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Gomez J, Madero R, Solsona E, Unda M, Martinez-Piñeiro L, Gonzalez M, et al. Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus Calmette-Guerin: The CUETO scoring model. J Urol. 2009;182:2195–203. doi: 10.1016/j.juro.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–5. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 14.Tomasz M. Mitomycin C: Small, fast and deadly (but very selective) Chem Biol. 1995;2:575–9. doi: 10.1016/1074-5521(95)90120-5. [DOI] [PubMed] [Google Scholar]
- 15.Zargar H, Aning J, Ischia J, So A, Black P. Optimizing intravesical mitomycin C therapy in non-muscle-invasive bladder cancer. Nat Rev Urol. 2014;11:220–30. doi: 10.1038/nrurol.2014.52. [DOI] [PubMed] [Google Scholar]
- 16.Rampersaud EN, Vujaskovic Z, Inman BA. Hyperthermia as a treatment for bladder cancer. Oncology (Williston Park) 2010;24:1149–55. [PubMed] [Google Scholar]
- 17.Arends TJ, Nativ O, Maffezzini M, de Cobelli O, Canepa G, Verweij F, et al. Results of a randomised controlled trial comparing intravesical chemohyperthermia with Mitomycin C versus bacillus Calmette-Guérin for adjuvant treatment of patients with intermediate- and high-risk non-muscle-invasive bladder cancer. Eur Urol. 2016;69:1046–52. doi: 10.1016/j.eururo.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Tan WS, Panchal A, Buckley L, Devall AJ, Loubière LS, Pope AM, et al. Radiofrequency-induced thermo-chemotherapy effect versus a second course of bacillus calmette-guérin or institutional standard in patients with recurrence of non-muscle-invasive bladder cancer following induction or maintenance bacillus Calmette-Guérin therapy (HYMN): A Phase III, open-label, randomised controlled trial. Eur Urol. 2019;75:63–71. doi: 10.1016/j.eururo.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Nativ O, Witjes JA, Hendricksen K, Cohen M, Kedar D, Sidi A, et al. Combined thermo-chemotherapy for recurrent bladder cancer after bacillus Calmette-Guerin. J Urol. 2009;182:1313–7. doi: 10.1016/j.juro.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 20.Gofrit ON, Shapiro A, Pode D, Sidi A, Nativ O, Leib Z, et al. Combined local bladder hyperthermia and intravesical chemotherapy for the treatment of high-grade superficial bladder cancer. Urology. 2004;63:466–71. doi: 10.1016/j.urology.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 21.Colombo R, Da Pozzo LF, Salonia A, Rigatti P, Leib Z, Baniel J, et al. Multicentric study comparing intravesical chemotherapy alone and with local microwave hyperthermia for prophylaxis of recurrence of superficial transitional cell carcinoma. J Clin Oncol. 2003;21:4270–6. doi: 10.1200/JCO.2003.01.089. [DOI] [PubMed] [Google Scholar]
- 22.ISRCTN - ISRCTN23639415: A Phase II, Open Label, Multicenter Randomised Controlled Trial Comparing Hyperthermia Plus Mitomycin To Mitomycin Alone, In Patients with Intermediate Risk Non-Muscle Invasive Bladder Cancer. [[Last accessed on 2020 Apr 26]]. Available from: http://www.isrctn.com/ISRCTN23639415 .
- 23.Clinical Trials Register – Search for 2013-002628-18. [[Last accessed on 2020 Apr 26]]. Available from: https://www.clinicaltrialsregister.eu/ctr-search/search?query=2013-002628-18 .
- 24.Liem EI, Crezee H, de la Rosette JJ, de Reijke TM. Chemohyperthermia in non-muscle-invasive bladder cancer: An overview of the literature and recommendations. Int J Hyperthermia. 2016;32:363–73. doi: 10.3109/02656736.2016.1155760. [DOI] [PubMed] [Google Scholar]
- 25.Marquette T, Walz J, Rybikowski S, Maubon T, Branger N, Fakhfakh S, et al. Tolérance de la thermo-chimiothérapie par HIVEC® chez les patients réfractaires au BCG. Prog En Urol. 2020;30:35–40. doi: 10.1016/j.purol.2019.11.001. [DOI] [PubMed] [Google Scholar]


