Abstract
The incidence of pancreatic cancer is increasing significantly and will soon become the 2nd leading cause of cancer-related deaths in the USA. We have previously shown that the gastrointestinal peptide gastrin, which is only expressed in the fetal pancreas and not in the adult pancreas, is activated during pancreatic carcinogenesis where it stimulates growth in an autocrine fashion. In this investigation we used transgenic LSL-KrasG12D/+; P48-Cre mice that develop precancerous pancreatic intraepithelial neoplasia (PanIN) lesions and pancreatic cancer over time. Starting at 3 months of age, mice were either left untreated (control) or were treated with a gastrin-targeted vaccine, Polyclonal Antibody Stimulator (PAS; 250μg) followed by a monthly booster until the mice reached 8-months of age when pancreata were excised, and analyzed by histology for PanIN grade in a blinded fashion. High grade PanIN-3 lesions were significantly less in PAS-treated mice (P=0.0077), and cancers developed in 33% of the control mice but only in 10% of the PAS-treated mice. Compared to the control mice, fibrosis was reduced by >50%, arginase positive M2 macrophages were reduced by 74%, and CD8+ T-cells were increased by 73% in the pancreas extracellular matrix in PAS-treated mice.
Keywords: Carcinogenesis, PanINs, microenvironment, cancer vaccine, gastrin
Introduction
Cancer prevention is the best approach for improving survival from cancers, and this strategy has proven to be an effective method in several malignancies. Surveillance screening procedures such as colonoscopy or mammography have significantly improved survival from colon (1) and breast cancer (2), respectively. Vaccinations targeted at preventing viral infections such as hepatitis B (3) and the human papilloma virus (4) have also significantly decreased the risk of hepatocellular and cervical cancer. For the prevention of pancreatic cancer, guidelines have been recommended from the International Cancer of the Pancreas Screening (CAPS) Consortium for examination according to age, family history and germline mutation status (5). This consortium recommends using imaging with MRI / magnetic retrograde cholangiopancreatography (MRCP) and/or endoscopic ultrasound (EUS) in high risk populations. About 15% of pancreatic cancers arise from pancreatic cystic lesions such as intraductal papillary mucinous neoplasm (IPMN) and mucinous cystic neoplasm (MCN) of the pancreas, and guidelines have been published on surveillance of subjects with these cystic lesions using radiographic imaging and endoscopic ultrasound (6). However, the majority (~85%) of pancreatic cancers arises from a histologic lesion called pancreatic intraepithelial neoplasia (PanIN), and these precancerous lesions are not identified on routine radiographic imaging or endoscopic ultrasound (7). With the exception of life style modification such as avoiding smoking and obesity (8), there are no other strategies to prevent pancreatic cancer that develops from the PanIN lesions.
One of the initiatives of the Cancer Moonshot Blue Ribbon panel report was to develop vaccinations for non-viral mediated cancers (9). A review on immunotherapy and prevention of pancreatic cancer stated that one of the obstacles to overcome in developing a vaccine for pancreatic cancer would be the immunosuppressive microenvironment and paucity of infiltrating T-lymphocytes characteristic of this tumor (10). A promising preclinical investigation (11) showed that vaccination with an attenuated intracellular Listeria monocytogenes vaccine targeting mutant KRAS, in KrasG12D/+; Trp53R172H/+;Pdx-1-Cre (KPC) mice could decrease PanIN progression and improve survival when mice were concomitantly treated with cyclophosphamide and an anti-CD25 antibody to deplete immunosuppressive T-regulatory cells. Our study supports the idea that pancreatic cancer could potentially be prevented with a vaccine if the tumor microenvironment is also modified rendering it less immunosuppressive.
Gastrin has been shown to stimulate growth of pancreatic cancer in an autocrine fashion (12). In the adult, gastrin is synthesized and secreted from the G-cells of the stomach and is not found in the pancreas. During pancreatic carcinogenesis, gastrin (13) and its receptor, the cholecystokinin B receptor (CCK-BR) (14), become expressed in PanINs. Evidence to support the importance of gastrin in pancreatic carcinogenesis was published in a study where gastrin-knockout (KO) transgenic mice (15) were crossed with LSL-KrasG12D/+; P48-Cre (KC) transgenic mice, and PanIN progression was arrested. Cancer did not occur in the absence of gastrin (16).
Polyclonal Antibody Stimulator (PAS) is a cancer vaccine comprised of a 9-amino acid epitope derived from the N-terminal sequence of gastrin-17 conjugated to diphtheria toxoid in an oil-based adjuvant. PAS vaccination activates humoral and cellular immunity by eliciting both the production of specific, high-affinity polyclonal anti-gastrin neutralizing antibodies and the activation of T-cells (17). In mice bearing pancreatic tumors, vaccination with PAS decreased primary tumor size and prevented metastases (18). A unique feature of PAS found in these murine studies (17), was that the vaccine also altered the tumor microenvironment. PAS increased the influx of CD8+ T-lymphocytes and decreased intratumoral immunosuppressive T-regulatory cells thus changing an immunologically ‘cold’ tumor into an immune responsive tumor. In fact, PAS monotherapy modified the immune cells of the tumor microenvironment without the addition of cyclophosphamide or an anti-CD-25 antibody. The purpose of our investigation was to examine whether PAS vaccination could arrest PanIN progression and alter the pancreas tumor microenvironment in LSL-KrasG12D/+; P48-Cre mice.
Methods
Vertebrate Animals
All studies in mice were performed in an ethical fashion and with the approval of the Georgetown University IACUC. Both male and female littermates from a transgenic LSL-KrasG12D/+; P48-Cre murine colony were used in this study. This model has previously been characterized (19) and shown to develop precancerous PanIN lesions by 3 months and pancreatic cancer over time. Mice were weaned and genotyped by 30 days of age and those with the LSL-KrasG12D/+; P48-Cre genotypes were randomized into one of two groups: controls-untreated (N=9) and PAS-treated (N=10).
Treatment
Nineteen age-matched littermates (males and females) were divided into two groups: control/ untreated (N=9) and PAS-treated (N=10). There were 2 female and 7 male mice in the control group and 4 female and six male mice in the PAS-treatment group. Starting at 3 months of age the PAS-mice received 3 induction doses of PAS 250μg administered subcutaneously (sc) at baseline, week 1 and week 3. Following this induction, the PAS-treated mice received a booster dose of PAS 250μg sc every 4 weeks until the mice reached 8 months of age for a total of 4 boosters. All mice, PAS-treated mice and controls, were ethically euthanized at 8 months of age.
Histology and PanIN scoring
For all mice, the pancreas was dissected, fixed in para-formaldehyde, and paraffin embedded. Tissue sections (5μm) were mounted and stained with hematoxylin and eosin. Histologic sections were scored by a pathologist, blinded to the treatment, for PanIN grade and percentage of PanINs replacing the pancreas. Pancreas sections were scored according to grade of PanINs, fibrosis and inflammation in the extracellular matrix as previously described (14).
Fibrosis analysis of pancreas microenvironment
Fibrosis within the pancreatic tissue was evaluated by Masson’s trichrome stain. Images (N=5) were taken of each sample from the slides using an Olympus BX61 microscope with a DP73 camera such that a total of N=45 images were taken in the control group and a total of N=50 images were taken of the PAS-treated pancreas. The mean densitometry value per slide was calculated using Image-J computer software between the mean values for fibrosis of the controls (N=9) to the mean values for fibrosis of the PAS-treated mouse pancreas (N=10).
Evaluation of CCK-B receptors in mouse pancreas tissues
Tissue sections (5μm) were prepared from the paraffin embedded mouse pancreas tissues. The tissues were reacted with a goat polyclonal CCK-BR primary antibody (Abcam, cat # ab7707) at 1:200 overnight at 4°C after deparaffination and antigen retrieval procedures. Slides were incubated with biotinylated secondary antibody (R&D systems cat # CTS008) for 60 minutes then reacted with DAB Chromogen (R&D systems cat # CTS008), and counterstained for 1 min at RT with Hematoxylin (Fisher, Harris Modified Hematoxylin), blued in 1% ammonium hydroxide and mounted with Permount (Fisher Chemical, cat # UN1249). Images were captured using an Olympus BX61 microscope with a DP73 camera.
CCK-B receptor mRNA expression by Real-time qRT-PCR was performed to compare the expression in control versus PAS-treated pancreas tissues. RNA was extracted (Qiagen) from control and PAS-treated mouse pancreata. cDNA was generated and subjected to qRT-PCR using SYBR® Green (Life Technologies) in an Applied Biosystems 7300 thermal cycler with the following conditions: initial incubation for 10 minutes at 95°C followed by 40 cycles of 95°C x 30 seconds, 60°C x 1 minutes, and 72°C for 30 seconds with selective CCK-BR primers as follows: 5’GATGGCTGCTACGTGCAACT3’ (forward) and 5’CGCACCACCCGCTTCTTAG3’ (reverse). HPRT was used as a reference gene and HPRT murine primers were as follows: 5’TCAGTCAACGGGGGACATAAA 3’ (forward); and 5’GGGGCTGTACTGCTTAACCAG3’ (reverse).
Immunohistochemical staining for immune cells
To study the effects of PAS vaccination on cancer promoting M2-polarized macrophages in the pancreas microenvironment, tissue sections (5μm) were prepared from the paraffin embedded mouse pancreas tissues. These sections were deparaffinized and hydrated in xylene and descending grades of alcohol. After rinsing in PBS, heat induced epitope retrieval (HIER) was performed by immersing the tissue sections in Target Retrieval Solution, Low pH (DAKO) in the PT Link (DAKO). The endogenous peroxidase activity was blocked by incubating the slides in 3% hydrogen peroxide for 10 min. Another 10 minutes block with 10% normal goat serum was performed to reduce the background, and then the slides were washed in buffer. This procedure was followed by incubation with rabbit polyclonal antibody against arginase-1 (ThermoFisher, cat # PA5–29645) at a dilution 1:1,800 for 1 hour at room temperature. Slides were exposed to the appropriate horseradish peroxidase (HRP) labeled polymer for 30 minutes. The antibody reaction was detected using diaminobenzidine (DAB) as chromogen. Sections were counterstained with Hematoxylin (Sigma, Harris Modified Hematoxylin). Negative controls were obtained by omitting the primary antibody in the above procedure on consecutive tissue sections.
Immunohistochemical staining of mouse tissue was performed for total number of macrophages in the pancreas microenvironment on tissue sections (5μm) using F4/80 antibody (e-Biosciences, cat # 14–4801-85) against mouse, made in Rat at a titer of 1:40 overnight at 4°C. HIER was performed by immersing the tissue sections at 37°C for 14 minutes in pronase. Immunohistochemical staining was performed using a HRP-labeled polymer from Vector MP-7444, according to manufacturer’s instructions. Slides were exposed to the Impress Anti-Rat IgG (mouse adsorbed) labeled polymer for 30min and DAB chromagen (Dako) for 5 minutes. Slides were counterstained with Hematoxylin (Sigma, Harris Modified Hematoxylin) at 1:10 dilution for 2 minutes at room temperature, blued in 1% ammonium hydroxide for 1 minute at room temperature, dehydrated, and mounted with Acrymount. Sections with the omitted primary antibody were used as negative controls.
For analysis of CD8+ T-lymphocytes in the pancreas microenvironment, tissue sections (5μm) were treated with 3% hydrogen peroxide and 10% normal goat serum for 10 minutes each, and exposed to primary antibodies for CD8, (1:25, Cell Signaling, cat # 98941) overnight at 4°C. Slides were exposed to the appropriate HRP labeled polymer for 30 minutes and DAB chromagen (Dako) for 5 minutes. Slides were counterstained with Hematoxylin (Sigma), blued in 1% ammonium hydroxide, dehydrated, and mounted with Acrymount.
Tissue sections (5μm) were reacted with a rabbit monoclonal antibody for Ki67 (Biocare, cat# CRM325; 1:80) to determine the proliferation activity in the pancreas of the control versus PAS-treated mice.
F4/80, CD8, and Ki67 stained slides were scanned using an Aperio GT450 machine and images analyzed with software from Aperio Image Scope. The number of total macrophages was analyzed by densitometry with Image-J software corrected for area of tissue examined. CD8+ stained cells were counted manually and normalized for area of tissue for each pancreas section.
Serum gastrin measurement by ELISA
Blood was collected at the time of euthanasia and serum separated and frozen at −80°C until analyzed for serum gastrin with an ELISA kit (Enzo Life Sciences, Inc., cat # ADI-900–149). Samples were diluted 1:2 and the assay performed in duplicate.
Western Blot
Protein was extracted from mouse pancreas with protein lysis buffer (1 mM EDTA, 0.5% Triton X-100, 5 mM NaF, 6 M Urea, 1 mM activated Sodium Orthovanadate) containing multiple proteinase inhibitors (ThermoFisher, cat # A32955). NuPAGE LDS Sample Buffer (Invitrogen, cat # NP0007) was added to each protein lysate at a 4:1 ratio. Samples of equal protein (50 μg) were loaded onto NuPAGE 4–12% Bis-Tris gels (Invitrogen, cat # NP0321BOX) and were separated by electrophoresis at voltage 200, then transferred to a nitrocellulose membrane (ThermoFisher, cat # 88018) with a voltage 50 for 2 hours. The membrane was blocked in 5% non-fat milk for 1 hour at RT, then blotted using the mouse anti-PCNA monoclonal antibody (Proteintech, cat # 60097–1-Ig) at a dilution of 1:5,000 overnight. Then the blots were incubated with anti-mouse IgG at RT for 1 hour, conjugated to HRP, and developed by WesternBright ECL-Spray (Advansta, cat # K-12049-D50). The Spectra Multicolor Broad Range Protein Ladder (ThermoFisher, cat # 26634) was used for molecular weight determinations. For loading normalization, the same membrane was blotted with mouse monoclonal anti-β actin antibody (Invitrogen, cat # MA1–140) at a dilution of 1:1,000 overnight, incubated with anti-mouse IgG at RT for 1 hour, and developed by ECL. The western blot bands were quantified using image-J software, then statistically analyzed using the student’s T-test method.
Statistical Analysis
Differences on pancreas PanIN grade, fibrosis density, M2-polarized macrophages, F4/80 macrophages, CD8+ T-cells, and serum gastrin values between control-untreated and PAS-treated mice were determined using GraphPad Prism version 9 statistical analysis programs. Mean values were compared by Student’s T-test between the two groups and significance was set at a confidence level of 95% or P<0.05. For gastrin levels between controls and PAS-treated mice with and without cancer and wild-type mouse, an ANOVA two-way comparison was performed between the groups. Differences in the incidence of cancer in each group were analyzed using a Fisher Exact Test.
Results
Effects of PAS vaccination on PanINs
Treatment with PAS was started when the mice were 3 months of age, when the pancreas already has established low-grade PanINs (Supplementary data Fig. S1). The results of the PanIN grades between the eight month old pancreata of each group are shown in Fig. 1. High grade PanIN-3 lesions were significantly reduced in the pancreas of PAS-treated mice compared to controls (Fig. 1A; P=0.0077). The number of low grade PanIN-2 lesions and PanIN-1 lesions are shown between the PAS-treated mice and controls in Fig 1B and Fig 1C, respectively. There was no statistical difference in the number of low-grade PanIN lesions between PAS-treated mice and controls. To correct for any variability in tissue size from sectioning, the PanIN grade and number was represented as a percentage of PanIN grade per area of tissue (Fig. 1D). Control mice had 55% more total (N=22774) PanIN lesions per area of tissue than the PAS-treated mice (N=12425). When euthanized at 8-months of age, 62.3% of the PanINs in control mice were high grade PanINs (PanIN-3 lesions, i.e., carcinoma in situ) compared to 39.3% in the PAS-treated mice (Fig. 1D). Although the numbers were small, 33.3% of control mice developed invasive carcinoma by month-8 and only 1 out of the10 PAS-treated mice developed cancer (Fig 1E). Due to the small numbers this value did not reach statistical significance (P=0.3).
Figure 1.
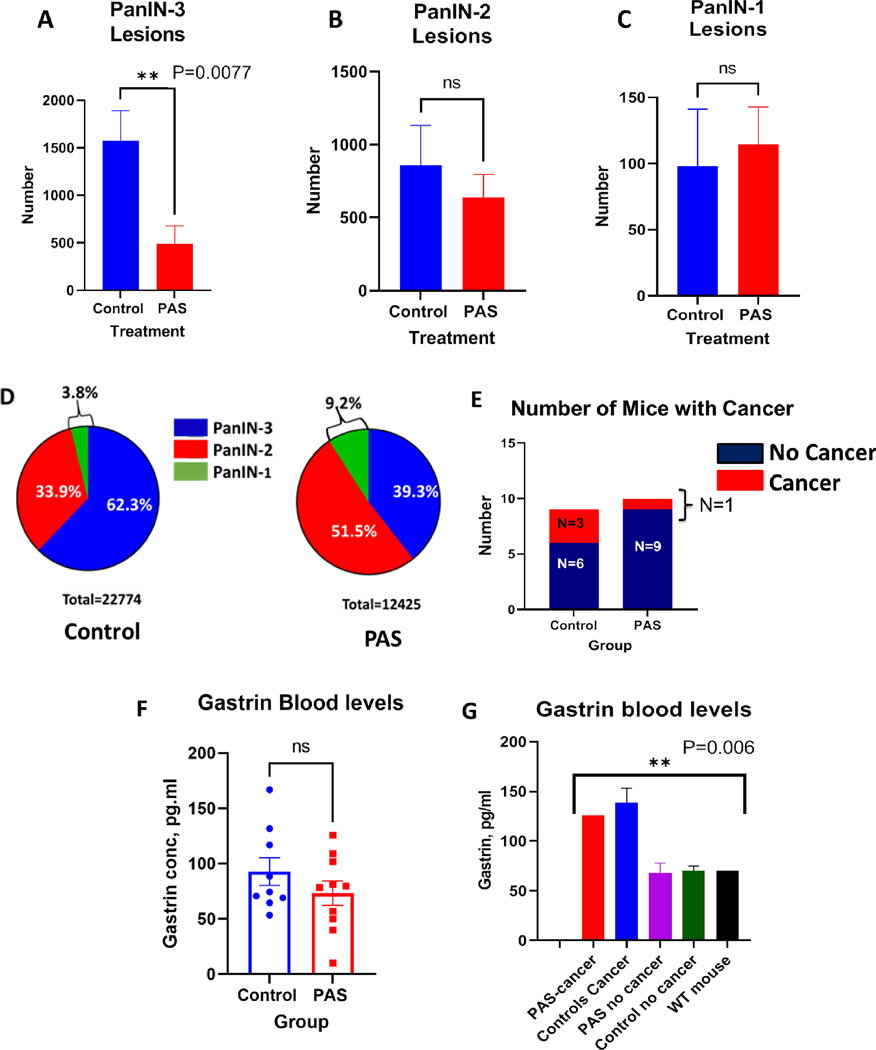
PAS-treated mice have fewer high grade PanIN-3 lesions in the pancreas. A, The number of PanIN-3 lesions was significantly lower in the pancreas of PAS-treated mice compared to control mice (P=0.0077). B, PanIN-2 lesions were 25% less in the pancreas of PAS-treated mice but this was not significant. C, No significant difference was found in the number of PanIN-1 lesions between the controls and PAS-treated mice. D, Grade of PanIN lesions is shown as a percentage for each group normalized for the area of the tissue. Control mouse pancreas (left) exhibited a greater percentage of PanIN-3 lesions compared to that of the PAS-treated mouse pancreata (right). E, The total number of mice in each group with cancer (red) and without cancer (blue) is shown in each stacked column. Three mice in the control group developed cancer at 8 months while only one in the PAS-treated group developed cancer, (P=0.3). F, Individual mouse serum gastrin levels in pg/ml are shown in each column. PAS-treated mice had gastrin levels that were 22% lower (73±11 pg/ml vs 93±13 pg/ml) that control mice but this difference did not reach significance. G, Gastrin serum levels are shown according to those with and without cancer and a wild-type C57BL/6 control mouse (Black column). Gastrin levels were higher in the mice with cancer compared to the mice without cancer (P=0.006).
Serum blood gastrin levels were measured by ELISA. PAS-treated mice had mean gastrin levels of 73±11 pg/ml compared to a mean value of 93±13 pg/ml in the untreated control mice (Fig. 1F). This 22% decrease in serum gastrin levels in PAS-treated mice did not reach statistical significance. Gastrin levels are shown for the mice with and without cancer compared to gastrin levels of an age-matched wild type C57BL/6 mouse (Fig. 1G); and those mice with cancer had significantly higher gastrin levels (P=0.006). When we examined the gastrin levels of the mice with cancer, we found that the one PAS-treated mouse with cancer had an increased gastrin level of 126 pg/ml, a value similar to the mean gastrin level (139 ± 14.8 pg/ml) of the 3 control mice that also developed cancer (Fig. 1G). This finding would suggest that this particular PAS-treated mouse did not respond to the PAS vaccination.
Representative H&E histologic images of the pancreata from untreated control mice showed high grade PanINs with complete disruption of the normal pancreatic architecture and extensive fibrosis (Fig. 2A, B). An image taken from a control mouse with invasive pancreatic cancer is shown in Fig. 2C. In contrast, representative images of pancreata from PAS-treated mice demonstrate fewer PanIN-3 lesions and preservation of much of the normal pancreas architecture (Fig. 2D–F). The pancreas from PAS-treated mice showed 59% more normal pancreatic acinar cells compared to controls. A representative image taken at a lower magnification of the pancreas from a control mouse shows distortion of the normal pancreas architecture and near complete replacement of the pancreas tissue with PanIN lesions and fibrosis (Fig. 2G). A representative image of the pancreas from a PAS-treated mouse at the same magnification exhibits fewer PanINs with some preservation of the normal pancreatic architecture (Fig. 2H).
Figure 2.
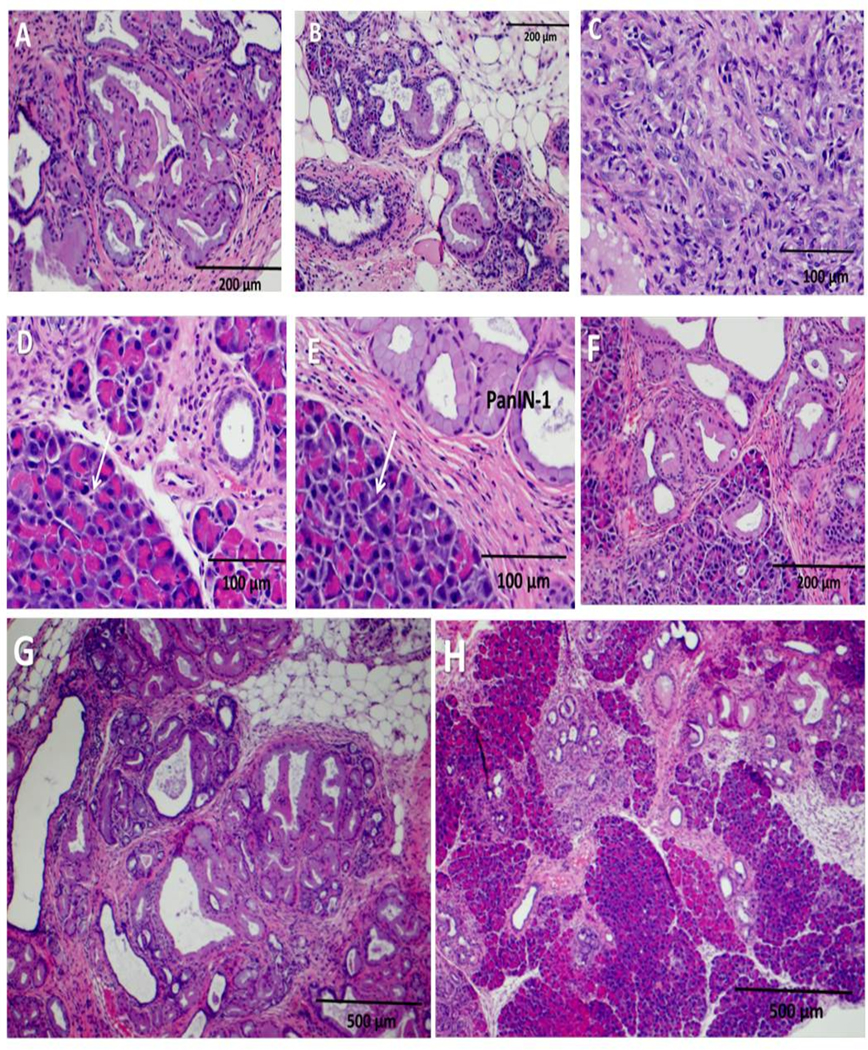
Hematoxylin & Eosin stain of mouse pancreas showing PanIN grade. A, and B, Images of control mouse pancreas with high grade PanIN-3 lesions and loss of normal pancreatic acinar cells are shown. C, Control mouse pancreas with invasive pancreatic cancer is shown. D, E, F, Images from the pancreas from a PAS-treated mouse shows fewer high grade PanINs with normal pancreatic acinar cells (arrows). G, Pancreas from control mouse at low magnification (4X) demonstrates that the pancreas tissue is replaced with extensive PanIN lesions and fibrosis. H, Pancreas from a PAS-treated mouse at lower magnification (4X) shows fewer PanINs and preservation of normal pancreas acinar cells.
Effects of PAS on pancreatic fibrosis
One of the characteristics of pancreatic cancer is the formation of dense fibrotic tissue (20) surrounding the tumor, rendering the tumor less permeable to chemotherapeutic agents (21) and immune cells (22, 23). Extensive fibrosis was revealed by Masson’s trichrome stain in the pancreas extracellular matrix of control mice (Fig. 3A), while there was significantly less fibrosis observed in PAS-treated mice (Fig. 3B). Quantitation of the fibrosis density by morphometric computerized analysis showed that the amount of pancreatic tissue fibrosis was 50% less in the mice vaccinated with PAS as compared to the pancreas of control mice (Fig. 3C), and this difference was significant (P=0.0001).
Figure 3.
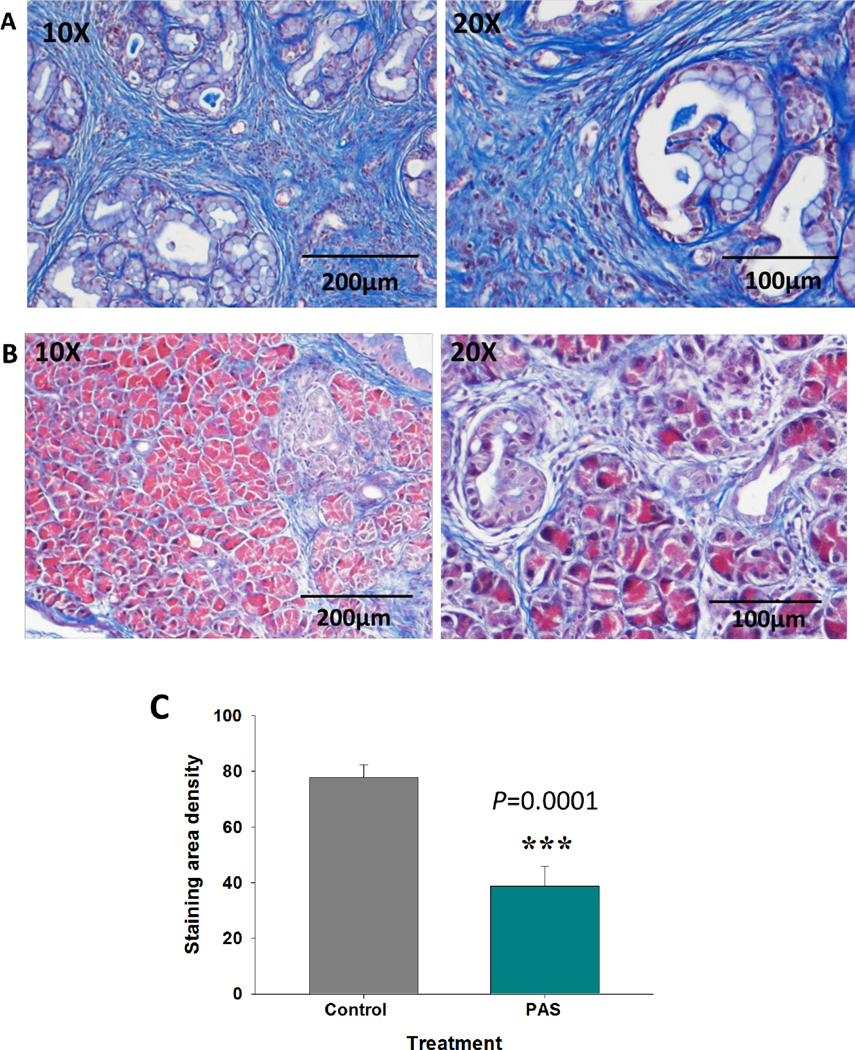
Pancreatic fibrosis in the pancreas microenvironment by Masson’s trichrome stain. A, Representative images of pancreas from control KC mice at 8-months of age showing extensive fibrosis (blue stain) at magnifications 10X (left) and 20X (right). B, Representative Masson trichrome images of pancreas from age-matched 8-month old mice vaccinated with PAS show significantly less intra-pancreatic fibrosis, magnifications 10X (left) and 20 X (right). C, Morphometric computerized analysis of fibrosis density shows significantly less fibrosis in the PAS-treated pancreas (P=0.0001).
PAS vaccination decreases pro-tumorigenic M2 macrophages
During pancreatic carcinogenesis, the number of pro-tumorigenic macrophages increases in number in the extracellular matrix of the microenvironment surrounding the PanIN lesions (22, 23). The pro-carcinogenic macrophages stain arginase positive and polarize as M2 macrophages (24). The arginase positive macrophages were abundant in the pancreas microenvironment of the control mice (Fig. 4A), while the pancreas microenvironment of PAS-treated mice had significantly fewer M2 macrophages (Fig. 4B). Computer analysis of M2 macrophage number revealed that PAS-treated mice had 4-fold fewer arginase positive macrophages (Fig. 4C) than control mice, suggesting that PAS vaccination rendered the pancreas less tumorigenic (P<0.001).
Figure 4.
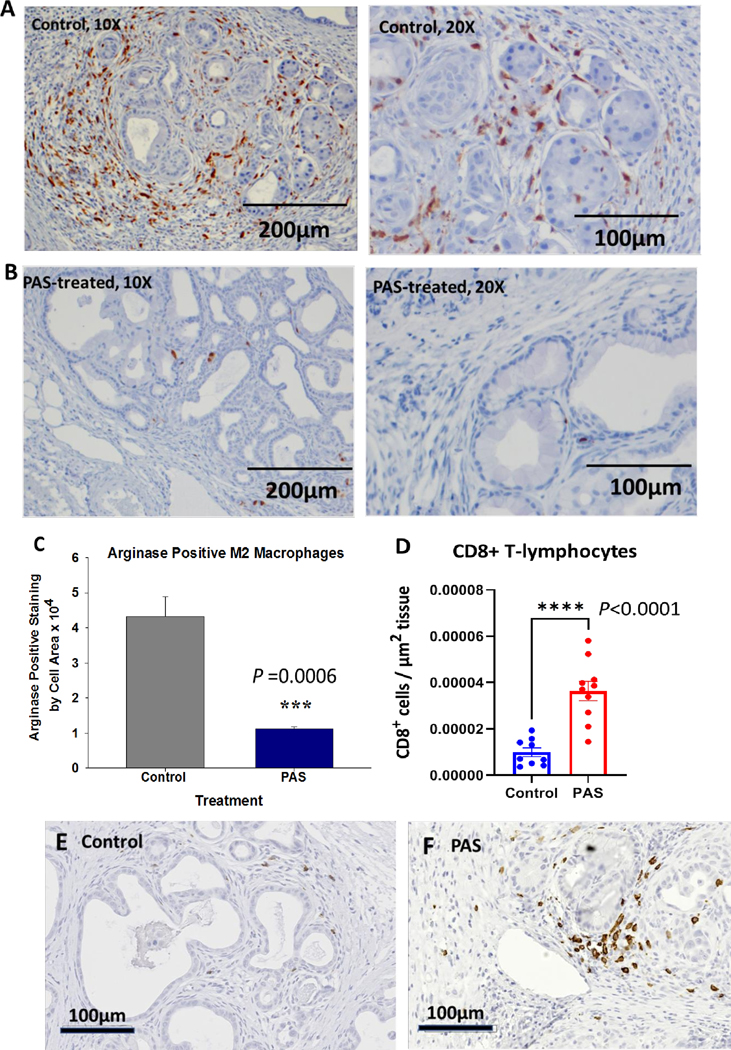
PAS-treatment changes the immune cell signature in the pancreas microenvironment A, Section from representative control mouse pancreas shows numerous arginase positive M2 polarized macrophages (10X, left). Photo of a control mouse pancreas (20X; right) shows arginase positive macrophages surrounding PanIN lesions. B, Photo from a pancreas from a PAS-treated mouse shows few arginase positive macrophages (10X; left). Higher magnification (20X) of a pancreas from a PAS-treated mouse shows decreased M2 arginase positive macrophages compared to control mice. C, Computer analysis of arginase positive M2 macrophages shows a 4-fold decrease in tumor associated macrophages in the pancreas of PAS-treated mice, (P=0.0006). D. CD8+ T lymphocytes per area of tissue are shown for each individual sample analyzed. PAS-treated mice exhibited higher numbers of CD8+ immunoreactive T-cells in the pancreas compared to controls (P<0.0001). E, A representative photo from a control mouse pancreas shows few CD8+ cells in the pancreas extracellular matrix. F, Image taken from a the pancreas of a PAS-treated mouse shows increased number of CD8+ cells.
To determine whether PAS-therapy decreased the total number of macrophages in the pancreas extracellular matrix, tissues were reacted with an F4/80 antibody and analyzed by densitometry. No statistical difference was found between control and the PAS-treated mice for the immunoreactivity of total macrophages (Supplemental data, Fig. S2). Representative images from control mouse pancreata reacted with the F4/80 antibody are shown (Supplemental data, Figs. S2A, B). Representative images from a PAS-treated mouse pancreas are shown in Supplemental data Figs. S2C, D. Densitometry analysis comparing the F4/80 staining macrophages was compared to that of the PAS-treated pancreas F4/80 reacting macrophages (Supplemental data, Fig. S2E), and no statistical differences were observed between the total F4/80 staining macrophages in these groups. These results suggest that PAS-treatment changes the macrophage polarization in the pancreas microenvironment and not the total number of macrophages.
PAS vaccination increases the influx of CD8+ T-lymphocytes
The total number of CD8+ cells were evaluated by immunohistochemistry and manually counted. The number of CD8+ T-cells was significantly increased per area of tissue in mice vaccinated with PAS compared to untreated controls (Fig. 4D). A representative image taken from the pancreas of a control mouse reacted with the CD8 antibody, shows few CD8+ T-lymphocytes (Fig. 4E). In contrast, a representative image taken from the pancreas of a PAS-treated mouse (Fig. 4F) showed increased numbers of CD8+ T-lymphocytes.
Effects of PAS on Cell Proliferation
Protein analysis was performed to determine if PAS decreased PanIN grade by decreasing cellular proliferation or increased apoptosis. Western blot revealed decreased proliferation, as determined by PCNA, in the pancreas tissue of PAS-treated mice compared to controls (Fig. 5A). Densitometry of the immunoreactive PCNA band normalized by actin shows the significant difference in the groups consistent with decreased proliferation in the pancreas of PAS-treated mice (P=0.028) (Fig. 5B). Proliferation index was also determined by cellular reactivity to a Ki67 antibody. The number of Ki67 positive cells was numerous in the pancreas of control mice (Fig. 5C). Immunoreactivity for Ki67 positive cells was decreased in the pancreas of mice treated with PAS (Fig. 5D). Quantitative morphometric analysis of Ki67 positive cells was analyzed and determined to be 5.5-fold less (P<0.0001) in the pancreata of mice treated with PAS (Fig. 5E). When the pancreas protein from control mice and PAS-treated mice was compared by ELISA for apoptosis and increased cleaved caspase-3, no statistical difference was observed (Fig. 5F). These results indicate that the decreased number of high grade PanIN-3 lesions in PAS-treated mice was due to a decrease in proliferation rather than by increasing apoptosis.
Figure 5.
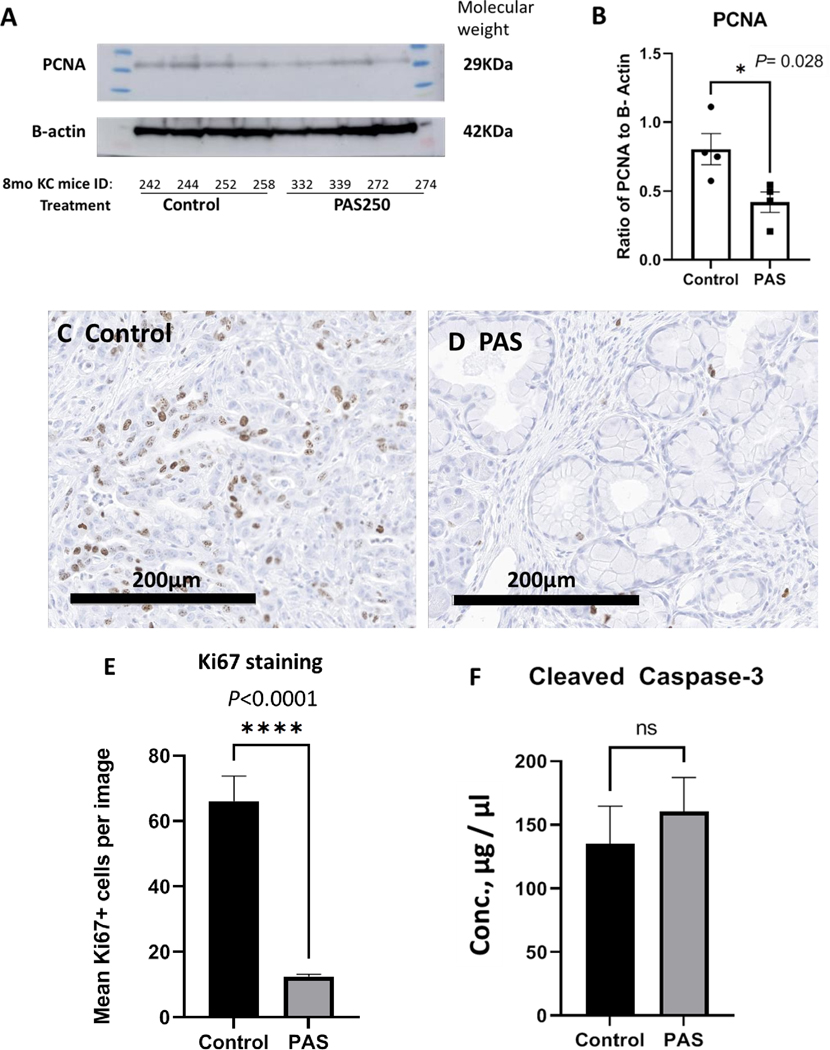
PAS- therapy decreases proliferation of the mouse KRAS pancreas. A, Western blot of protein extracts from mouse pancreas reacted with the antibody to the proliferating cell nuclear antigen (PCNA) is show at the expected 29KDa size. The protein is normalized to actin with the band shown at the expected 42KDa size. Mouse identification numbers are shown below the blot and protein ladder size exhibited on each side of the samples electrophoresed. B, The western blot was analyzed by densitometry and the individual samples (dots & squares) and mean ± SEM values (Columns, error bars) show a significant decrease in PCNA immunoreactivity in tissue from PAS-treated mice (P=0.028). C, Representative image from the pancreas of a control mouse reacted with a Ki67 antibody shows numerous immunoreactive proliferating cells. D, A representative image is shown from the pancreas of a PAS-treated mouse with Ki67 staining. E, Quantitative analysis of Ki67 immunoreactive cells is shown. Columns represent the mean number of Ki67+ cells counted per high power (200μm) image with N=10 images per slide. The number of Ki67+ proliferating cells is significantly increased in the pancreas tissue of control mice compared to the pancreas tissue from PAS-treated mice (P<0.0001). F, Columns represent the mean ± SEM of pancreatic protein cleaved caspase-3 levels in μg/μl as measured by ELISA revealed no significant difference between control and PAS-treated mice.
CCK-B receptor expression
Gastrin mediates its actions through the CCK-B receptor that becomes expressed in PanIN lesions during carcinogenesis (14). Immunoreactivity of the pancreas from the LSL-KrasG12D/+; P48-Cre mice at 8 months of age demonstrate the presence of CCK-B receptor staining in the PanIN epithelial cells (Supplemental data, Fig. S3A, B). Measurement of CCK-B receptor mRNA expression in the pancreas tissues was 5.6-fold lower in PAS-treated mice compared to controls, but this value did not reach statistical significance. (Supplemental data, Fig. S3C).
Discussion
In this investigation, we demonstrated that the progression of precancerous lesions of the pancreas can be prevented by a vaccine that targets gastrin. PAS-treated mice exhibited fewer high grade PanINs in the KRAS mice pancreas and also a decrease in the incidence of cancer. Proliferation of the pancreas tissue was decreased as demonstrated with less PCNA and fewer total number of PanIN lesions. In part, this anti-proliferative effect is mediated by the activation of B-cells that produce neutralizing antibodies to gastrin – a known trophic peptide (25) - in response to the vaccination. PAS vaccination has also been shown to elicit at T-cell response and activation of memory T-lymphocytes that respond when exposed to gastrin with the release of proteases such as granzyme and perforin and cytokines including interferon-γ and TNF-α (17). Since CCK-B receptors become expressed in early PanIN lesions (14) and gastrin activation of this receptor induces downstream signaling with epithelial cell proliferation (26), interruption of gastrin’s actions at the receptor interface most likely resulted in the PanIN arrest.
Another important finding of this investigation involves the alteration of the pancreas microenvironment in PAS-treated mice. During pancreatic carcinogenesis, the pancreatic stellate cells become activated myofibroblasts and deposit a collagenous desmoplasia in the pancreas (20). Stellate cells also have CCK-B receptors (27) and inhibiting gastrin activation of these receptors resulted in less fibrosis in the microenvironment. The fibroblasts of the pancreatic microenvironment communicate with the epithelial cells and immune cells resulting in the activation of cytokines (28). This immune activation leads to the destruction of normal pancreatic tissue and replacement with the precancerous PanIN lesions. Indeed, large sections of the normal pancreas and acinar cells were protected in the PAS- treated mice. Previously we had shown that intratumoral fibrosis was reduced in mice bearing established pancreatic tumors treated with a combination of PAS and a PD-1 antibody but that monotherapy with PAS or PD-1 antibody failed to reduce fibrosis (17, 18). One possible explanation for the marked decreased of fibrosis in the current investigation with PAS monotherapy could be that in the current investigation, PAS was administered over a longer duration in the KRAS mice compared to the tumor bearing mice of the previous study (months versus weeks) and several boosters were administered in the current investigation.
This investigation demonstrated that PAS therapy changes the immune cell signature of the pancreas extracellular matrix rendering it less carcinogenic. An important immune cell that promotes pancreatic carcinogenesis is the M2 macrophage (22, 23). The untreated control mouse pancreas microenvironment becomes infiltrated with abundant M2 arginase positive macrophages during carcinogenesis. PAS vaccination decreases the number of M2-polarized macrophages without decreasing the total number of tissue associated macrophages that are important for immune surveillance. This change in polarization of macrophages further contributes to decreasing the carcinogenic potential of the pancreas.
One reason pancreatic cancer has not responded to immune checkpoint antibody therapy (29) is related to the lack of CD8+ T-lymphocytes in the pancreas tumor microenvironment (30). Improved patient survival with pancreatic cancer has been directly correlated to the number of CD8+ T cells in the pancreas microenvironment (31). PAS therapy significantly increased the number of CD8+ immunoreactive T-cells in the pancreas microenvironment in the KRAS mutant mouse model. In a prior study in mice bearing pancreatic tumors, PAS-therapy increased intratumoral CD8+ T cells while also decreasing the immunosuppressive T-regulatory T cells (17).
PAS is a peptide vaccine that selectively targets gastrin, a known trophic peptide that stimulates pancreatic cancer in both a paracrine (32) and autocrine (12) fashion. Gastrin and its related peptide, cholecystokinin (CCK) both mediate their proliferative effects through activation of the CCK-BR with equal affinity (33); however, only gastrin can stimulate growth in an autocrine fashion. Although CCK peptide expression may be found in pancreatic cancer cells, CCK does not stimulate growth in an autocrine fashion. Neither treatment of pancreatic cancer cells with selective CCK antibodies nor the down-regulation of CCK mRNA and peptide by shRNAs alters growth in vitro or in vivo (34). Conversely, when gastrin mRNA expression was down-regulated, the same pancreatic cancer cells failed to produce tumors in spite of having sustained levels of endogenous CCK (34). CCK blood levels may be increased by consumption of a diet high in saturated fats which in turn can stimulate pancreatic cancer growth in an exogenous fashion through the CCK receptor (35, 36). Epidemiologic studies have shown that the incidence of pancreatic cancer is increased in countries that consume high fat diets, especially saturated fat (37–39) and long-chain saturated fatty acids are effective releasers of CCK (40). Obesity has been associated with an increased risk for pancreatic cancer (41, 42) and upregulation of CCK in pancreatic islet cells has been associated with obesity in the Leptinob/ob mice mouse models (43, 44). Since both CCK and gastrin stimulate pancreatic carcinogenesis through the CCK-BR, the increased expression of CCK-BR in pancreatic carcinogenesis seems to be an important driver. Our prior work has demonstrated that diets high in saturated fat increase the expression of the CCK-BR by down regulating micro-RNA148a levels (45). And during pancreatic carcinogenesis in the mutant KRAS mouse models, levels of miR-148a decrease (46) as CCK-BR expression increases (16). Although PAS vaccination does not alter CCK levels, PAS therapy will neutralize endogenous gastrin levels and decrease signaling of the CCK-BR. In our KRAS model PAS vaccination slowed PanIN progression but did not completely arrest PanIN progression perhaps because CCK levels were unaltered in our model. Conceivably, combination therapy with PAS and a CCK-receptor antagonist, such as proglumide (47), would provide full protection for PanIN progression to pancreatic cancer.
Currently there are no therapies to prevent pancreatic cancer. Morrison et al. in their review on Immunotherapy and Prevention of Pancreatic Cancer commented that “Elimination of the precursor lesion might be enough to prevent the development of malignancy or at least ‘reset the clock’ (10).” Indeed, our findings support that immunization with PAS definitively decreases the progression low grade PanIN precursor lesions to high grade PanIN-3 lesions in this LSL-KrasG12D/+; P48-Cre mouse model that is destined to develop pancreatic cancer. Although we waited until 3 months of age to initiate therapy after establishment of PanIN lesions, one could speculate that administration of therapy with PAS at an earlier time point would be more protective. Populations that could benefit immediately from our research include those that are considered high risk for pancreatic cancer (5, 10), such as those with a family history of pancreatic cancer, chronic pancreatitis, or new onset diabetes. Those with BRCA2 germline alterations or hereditary pancreatitis may also benefit from PAS vaccination. Currently, these populations with high risks or family history of pancreatic cancer undergo MRI imaging surveillance and occasionally endoscopic ultrasound, but these techniques are only for surveillance and are not preventive. PAS vaccination could possibly offer a novel approach to prevent pancreatic cancer in these high risk populations. Another strategy for the clinical use of PAS could include vaccinating those that had a surgical resection / Whipple procedure for pancreatic cancer in order to prevent tumor recurrence. Although curative resection is attempted in up to 20% of those with pancreatic cancer, the 5-year survival for this group is still only 20–30% at best due to recurrence of microscopic disease. Treatment with PAS may help decrease recurrence after surgery. Since PAS has already been safely tested in human subjects with pancreatic cancer, strategies to develop PAS as a cancer vaccination in high risk subjects in order to prevent cancer (rather than just monitoring them) may improve survival from this devastating disease.
Supplementary Material
Prevention Relevance:
PAS vaccination significantly decreased high grade PanIN lesions and altered the pancreas microenvironment rendering it less carcinogenic.
Acknowledgments:
We thank the staff from the Lombardi Comprehensive Cancer Center Histology Core Facility for their assistance in experimental procedures and preparation of tissues. We also thank the staff in the Division of Comparative Medicine for their care and oversight of the animal experiments. We also thank Meghan Cato of Cancer Advances, Inc. for manually counting the CD8+ T-lymphocytes.
Funding: The study was funded in part by a grant from Cancer Advances, Inc., and its subsidiary Vaccicure, and NIH CA051008 to the Georgetown Lombardi Cancer Center Core facilities.
Footnotes
Disclosures: Cancer Advances, Durham NC has intellectual property rights for PAS. PAS drug substance was transferred to Georgetown University by a Material Transfer agreement with Vaccicure Limited, Liverpool, UK for this research project and work was done at Georgetown University under a sponsored research agreement. All authors work for Cancer Advances, Inc. or Georgetown University.
Reference List
- 1.Niikura R, Hirata Y, Suzuki N, Yamada A, Hayakawa Y, Suzuki H, et al. Colonoscopy reduces colorectal cancer mortality: A multicenter, long-term, colonoscopy-based cohort study. PLoS One 2017;12:e0185294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabar L, Dean PB, Chen TH, Yen AM, Chen SL, Fann JC, et al. The incidence of fatal breast cancer measures the increased effectiveness of therapy in women participating in mammography screening. Cancer 2019;125:515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang MH. Hepatitis B virus and cancer prevention. Recent Results Cancer Res 2011;188:75–84. [DOI] [PubMed] [Google Scholar]
- 4.Brisson M, Kim JJ, Canfell K, Drolet M, Gingras G, Burger EA, et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet 2020;395:575–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goggins M, Overbeek KA, Brand R, Syngal S, Del CM, Bartsch DK, et al. Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut 2020;69:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka M, Fernandez-del CC, Kamisawa T, Jang JY, Levy P, Ohtsuka T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017;17:738–53. [DOI] [PubMed] [Google Scholar]
- 7.Lindquist CM, Miller FH, Hammond NA, Nikolaidis P. Pancreatic cancer screening. Abdom Radiol (NY) 2018;43:264–72. [DOI] [PubMed] [Google Scholar]
- 8.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013;144:1252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacks T, Jaffee E, Singer D. Cancer Moonshot Blue Ribbon Panel Report 2016. 2016. [Google Scholar]
- 10.Morrison AH, Byrne KT, Vonderheide RH. Immunotherapy and Prevention of Pancreatic Cancer. Trends Cancer 2018;4:418–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keenan BP, Saenger Y, Kafrouni MI, Leubner A, Lauer P, Maitra A, et al. A Listeria vaccine and depletion of T-regulatory cells activate immunity against early stage pancreatic intraepithelial neoplasms and prolong survival of mice. Gastroenterology 2014;146:1784–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith JP, Shih A, Wu Y, McLaughlin PJ, Zagon IS. Gastrin regulates growth of human pancreatic cancer in a tonic and autocrine fashion. Am J Physiol 1996;270:R1078–R1084. [DOI] [PubMed] [Google Scholar]
- 13.Prasad NB, Biankin AV, Fukushima N, Maitra A, Dhara S, Elkahloun AG, et al. Gene expression profiles in pancreatic intraepithelial neoplasia reflect the effects of Hedgehog signaling on pancreatic ductal epithelial cells. Cancer Res 2005;65:1619–26. [DOI] [PubMed] [Google Scholar]
- 14.Smith JP, Cooper TK, McGovern CO, Gilius EL, Zhong Q, Liao J, et al. Cholecystokinin receptor antagonist halts progression of pancreatic cancer precursor lesions and fibrosis in mice. Pancreas 2014;43:1050–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friis-Hansen L. Lessons from the gastrin knockout mice. Regul Pept 2007;139:5–22. [DOI] [PubMed] [Google Scholar]
- 16.Nadella S, Burks J, Huber M, Wang J, Cao H, Kallakury B, et al. Endogenous Gastrin Collaborates With Mutant KRAS in Pancreatic Carcinogenesis. Pancreas 2019;48:894–903. [DOI] [PubMed] [Google Scholar]
- 17.Osborne N, Sundseth R, Burks J, Cao H, Liu X, Kroemer AH, et al. Gastrin vaccine improves response to immune checkpoint antibody in murine pancreatic cancer by altering the tumor microenvironment. Cancer Immunol Immunother 2019;68:1635–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osborne N, Sundseth R, Gay MD, Cao HP, Tucker RD, Nadella S, et al. Vaccine Against Gastrin, Polyclonal Antibody Stimulator, Decreases Pancreatic Cancer Metastases. Am J Physiol Gastrointest Liver Physiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003;4:437–50. [DOI] [PubMed] [Google Scholar]
- 20.Apte MV, Park S, Phillips PA, Santucci N, Goldstein D, Kumar RK, et al. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas 2004;29:179–87. [DOI] [PubMed] [Google Scholar]
- 21.Waghray M, Yalamanchili M, di Magliano MP, Simeone DM. Deciphering the role of stroma in pancreatic cancer. Curr Opin Gastroenterol 2013;29:537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vonderheide RH, Bayne LJ. Inflammatory networks and immune surveillance of pancreatic carcinoma. Curr Opin Immunol 2013;25:200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng L, Xue J, Jaffee EM, Habtezion A. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology 2013;144:1230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol 2009;9:259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh JH. Role of gastrin as a trophic hormone. Digestion 1990;47 Suppl 1:11–6. [DOI] [PubMed] [Google Scholar]
- 26.Fino KK, Matters GL, McGovern CO, Gilius EL, Smith JP. Downregulation of the CCK-B receptor in pancreatic cancer cells blocks proliferation and promotes apoptosis. Am J Physiol Gastrointest Liver Physiol 2012;302:G1244–G1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berna MJ, Seiz O, Nast JF, Benten D, Blaker M, Koch J, et al. CCK1 and CCK2 receptors are expressed on pancreatic stellate cells and induce collagen production. J Biol Chem 2010;285:38905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas D, Radhakrishnan P. Tumor-stromal crosstalk in pancreatic cancer and tissue fibrosis. Mol Cancer 2019;18:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patnaik A, Kang SP, Rasco D, Papadopoulos KP, Elassaiss-Schaap J, Beeram M, et al. Phase I Study of Pembrolizumab (MK-3475; Anti-PD-1 Monoclonal Antibody) in Patients with Advanced Solid Tumors. Clin Cancer Res 2015;21:4286–93. [DOI] [PubMed] [Google Scholar]
- 30.Ajina R, Weiner LM. T-Cell Immunity in Pancreatic Cancer. Pancreas 2020;49:1014–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carstens JL, Correa de SP, Yang D, Barua S, Wang H, Rao A, et al. Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nat Commun 2017;8:15095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith JP, Fantaskey AP, Liu G, Zagon IS. Identification of gastrin as a growth peptide in human pancreatic cancer. Am J Physiol 1995;268:R135–R141. [DOI] [PubMed] [Google Scholar]
- 33.Dufresne M, Seva C, Fourmy D. Cholecystokinin and gastrin receptors. Physiol Rev 2006;86:805–47. [DOI] [PubMed] [Google Scholar]
- 34.Matters GL, McGovern C, Harms JF, Markovic K, Anson K, Jayakumar C, et al. Role of endogenous cholecystokinin on growth of human pancreatic cancer. Int J Oncol 2011;38:593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matters GL, Cooper TK, McGovern CO, Gilius EL, Liao J, Barth BM, et al. Cholecystokinin mediates progression and metastasis of pancreatic cancer associated with dietary fat. Dig Dis Sci 2014;59:1180–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nadella S, Burks J, Al-Sabban A, Inyang G, Wang J, Tucker RD, et al. Dietary fat stimulates pancreatic cancer growth and promotes fibrosis of the tumor microenvironment through the cholecystokinin receptor. Am J Physiol Gastrointest Liver Physiol 2018;315:G699–G712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghadirian P, Lynch HT, Krewski D. Epidemiology of pancreatic cancer: an overview. Cancer Detect Prev 2003;27:87–93. [DOI] [PubMed] [Google Scholar]
- 38.Heinen MM, Verhage BA, Goldbohm RA, van den Brandt PA. Meat and fat intake and pancreatic cancer risk in the Netherlands Cohort Study. Int J Cancer 2009;125:1118–26. [DOI] [PubMed] [Google Scholar]
- 39.Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol 2006;20:197–209. [DOI] [PubMed] [Google Scholar]
- 40.McLaughlin J, Grazia LM, Jones MN, D’Amato M, Dockray GJ, Thompson DG. Fatty acid chain length determines cholecystokinin secretion and effect on human gastric motility. Gastroenterology 1999;116:46–53. [DOI] [PubMed] [Google Scholar]
- 41.Bracci PM. Obesity and pancreatic cancer: overview of epidemiologic evidence and biologic mechanisms. Mol Carcinog 2012;51:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel AV, Rodriguez C, Bernstein L, Chao A, Thun MJ, Calle EE. Obesity, recreational physical activity, and risk of pancreatic cancer in a large U.S. Cohort. Cancer Epidemiol Biomarkers Prev 2005;14:459–66. [DOI] [PubMed] [Google Scholar]
- 43.Lavine JA, Raess PW, Stapleton DS, Rabaglia ME, Suhonen JI, Schueler KL, et al. Cholecystokinin is up-regulated in obese mouse islets and expands beta-cell mass by increasing beta-cell survival. Endocrinology 2010;151:3577–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung KM, Singh J, Lawres L, Dorans KJ, Garcia C, Burkhardt DB, et al. Endocrine-Exocrine Signaling Drives Obesity-Associated Pancreatic Ductal Adenocarcinoma. Cell 2020;181:832–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tucker RD, Ciofoaia V, Nadella S, Gay MD, Cao H, Huber M, et al. A Cholecystokinin Receptor Antagonist Halts Nonalcoholic Steatohepatitis and Prevents Hepatocellular Carcinoma. Dig Dis Sci 2020;65:189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LaConti JJ, Shivapurkar N, Preet A, Deslattes MA, Peran I, Kim SE, et al. Tissue and serum microRNAs in the Kras(G12D) transgenic animal model and in patients with pancreatic cancer. PLoS One 2011;6:e20687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith JP, Wang S, Nadella S, Jablonski SA, Weiner LM. Cholecystokinin receptor antagonist alters pancreatic cancer microenvironment and increases efficacy of immune checkpoint antibody therapy in mice. Cancer Immunol Immunother 2018;67:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


