Abstract
Progesterone and progesterone receptors (PR) have a storied albeit controversial history in breast cancers. As endocrine therapies for breast cancer progressed through the 20th century from oophorectomy to antiestrogens, it was recognized in the 1970s that the presence of estrogen receptors (ER) alone could not efficiently predict treatment responses. PR, an estrogen regulated protein, became the first prognostic and predictive marker of response to endocrine therapies. It remains in clinical use today as the gold standard for predicting the existence of functional, targetable ER, in breast malignancies. PRs were subsequently identified as highly structured transcription factors that dictate a variety of physiological processes in breast cancer cells. In the early 2000s, the somewhat surprising finding that prolonged use of synthetic progestin-containing menopausal hormone therapies increase breast cancer incidence raised new questions about the role of PR in “tumorigenesis”. Most recently, PR have been linked to expansion of cancer stem cells, and postulated to be the principal cells reactivated in occult or dormant disease. Other studies establish PR as dominant modulators of ER activity. Taken together these findings mark PR as bona fide targets for progestin- or antiprogestin-based therapies, yet their diverse actions have confounded that use. Here we summarize the early history of PR in breast cancer; debunk the theory that progesterone causes cancer; discuss recent discoveries implicating PR in regulation of cell heterogeneity; attempt to unify theories describing PR as either good or bad actors in tumors; and discuss emerging areas of research that may help explain this enigmatic hormone and receptor.
Keywords: progesterone, progesterone receptor, progestins, cancer stem cells, biomarker
Introduction
Progesterone is a small lipophilic hormone that plays a fundamental role in normal female biology and medicine. In premenopausal women, progesterone is primarily synthesized in a cyclical manner in the ovaries, with additional synthesis in peripheral tissues including adrenal glands, the nervous system and brain (Africander and Storbeck 2018; Giatti, et al. 2015). At menopause, circulating progesterone levels decline sharply, but whether local tissue production continues is unknown. The breast is a major target of progesterone where it regulates development of the branched ductal epithelium and expansion of milk-secreting alveoli during lactation. Medically, bioidentical progesterone formulations or synthetic compounds termed progestins are taken by women of different ages for reasons that span birth control, to menopausal hormone therapies (MHT), to treatment of Alzheimer’s disease. However, exposure to exogenous progestins is associated with increased breast cancer incidence and/or disease progression. Nevertheless, progestins continue to be tested as treatments or preventatives for breast cancers. Some of the breast effects are clearly paradoxical and much remains to be learned about this hormone.
The major effector of progesterone and the target of progestins are the progesterone receptors (PR). PR are highly structured multi-domain proteins that upon ligand binding transmit their signals primarily through regulation of gene transcription. In humans there are two main PR isoforms expressed from a single gene located at chromosome 11q22.1 -- a 933 amino acid PR-B and a truncated 769 amino acid PR-A transcribed from an internal start-site (Kastner, et al. 1990). PR share conserved functional domains with other members of the steroid/nuclear receptor family of transcription factors. These include an N-terminal domain that is highly modified post-translationally and contains transcriptional activation functions, a central DNA binding domain consisting of two cysteine-anchored zinc fingers, and a C-terminal ligand binding domain (Mangelsdorf, et al. 1995; Takimoto, et al. 2003). The PR gene is activated by estrogens, with both PR-A and PR-B expressed in approximately one third of luminal epithelial cells of the normal breast, though there is some evidence for PR expression in basal epithelial cells as well (Hilton, et al. 2012). The two PR isoforms are co-expressed in breast cancer cells, but often unequally, with a heightened PR-A:PR-B ratio correlating with poor prognosis (Graham, et al. 1995; Hopp, et al. 2004; Rojas, et al. 2017). For the purposes of this review we focus mainly on the collective activity of PR, recognizing that most PR+ breast cancers contain both PR-A and PR-B in varying ratios.
While early studies focused on PR structure and function, the last decade has seen analyses of PR-regulatory activities and biological endpoints. The demonstration that PR regulate breast cancer cell heterogeneity coincided with the reemergence of the cancer stem cell (CSC) theory that proposed a hierarchical mechanism by which rare pre-existing cancer cells avoid drug killing and perpetuate the re-population of bulk tumor cells (Reya, et al. 2001). CSCs are currently recognized as a plastic state that can be achieved by genetic and/or phenotypic adaptation, and can be influenced by the cellular microenvironment and environmental signals (Meacham and Morrison 2013). Multiple groups have described progestin and PR regulation of populations of cells with CSC properties (reviewed in Axlund and Sartorius 2012; Cenciarini and Proietti 2019; Finlay-Schultz and Sartorius 2015; Simoes, et al. 2015). The consequence of PR regulation of CSCs is unclear. It has been suggested that CSCs contribute to the long-term dormancy of estrogen receptor (ER)+PR+ breast cancers; accelerate tumor progression upon development of endocrine therapy resistance; or conversely, that they impart cytostasis on estrogen-driven cells. Here we discuss the transition of PR as an important factor in breast cancer prognosis, to its role as a regulator of tumor cell plasticity and heterogeneity, and its future as a target of therapy.
Past: PR as a biomarker and prognostic factor in breast cancer
Hormonal control of breast cancers was first demonstrated in the late 19th century when metastatic tumors of patients regressed following ovariectomy (Beatson 1896) (see Figure 1 for a time-line of major discoveries relevant to progesterone and PR). By the early 1970’s it was known that approximately 30% of tumors were responsive to therapies involving either ablation of endocrine glands or addition of a variety of hormones or their inhibitors (Dao 1972; McGuire, et al. 1974). Such tumors were classified generically as “hormone responsive”. The major ovarian hormones upon which studies focused were the estrogens, which accumulate in reproductive organs (Glascock and Hoekstra 1959; Jensen and Jacobson 1960). Experimental rat mammary tumors induced by the carcinogen DMBA were found to be estrogen target tissues (King, et al. 1966). Development of the MCF-7 human breast cancer cell line at the Michigan Cancer Foundation and the demonstration of ER therein (Soule, et al. 1973), laid the foundations for human ER research. Clinically, hormone dependent breast cancers were shown to accumulate more radioactive estrogens than autonomous ones, and this uptake was due to the presence of ER (McGuire 1973; McGuire, et al. 1976). Elwood Jensen postulated that ER marked the hormone dependent tumor subset, and indeed, an international workshop convened in 1974 correlated the data from several trials in 380 patients, which showed that regardless of treatment type, 55–60% of ER+ tumors regressed in response to endocrine therapies, while only 8% of ER– tumors did so (McGuire and Chamness 1973).
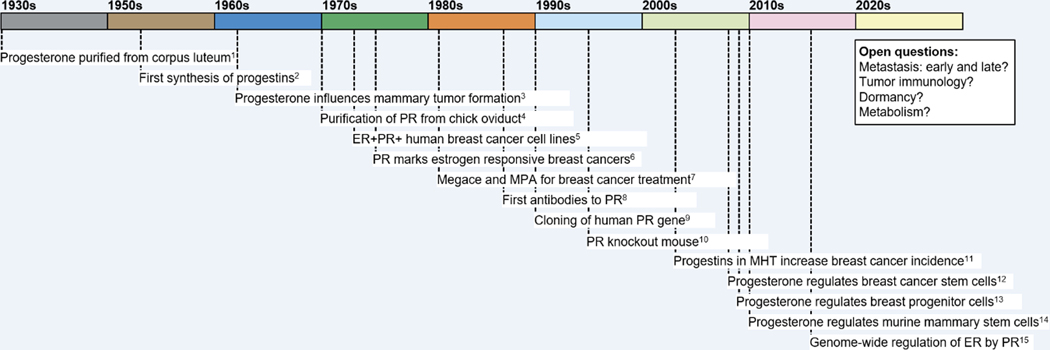
Figure 1. Time-line of discoveries related to progesterone, progestins, and PR in breast cancer.
Time-line indicates key discoveries in the modern history of progesterone, progestins and PR in breast cancer. References are as follows and as discussed in the text. 1. (Allen 1930, 2005); 2. (Djerassi 1966); 3. (Huggins, et al. 1962); 4. (O’Malley, et al. 1970); 5. (Brooks, et al. 1973); 6. (Horwitz and McGuire 1975); 7. (reviewed in Santen, et al. 1990); 8. (Estes, et al. 1987; Sullivan, et al. 1986); 9. (Kastner, et al. 1990); 10. (Lydon, et al. 1995); 11. (Collaborative Group on Hormonal Factors in Breast 2019); 12. (Horwitz, et al. 2008); 13. (Graham, et al. 2009a); 14. (Asselin-Labat, et al. 2010; Joshi, et al. 2010); 15. (Mohammed, et al. 2015; Singhal, et al. 2016).
Progesterone is of course the other major ovarian hormone. Its importance in experimental mouse mammary tumors was documented by the early studies of Huggins et al. (Huggins, et al. 1962). However, to this day, the issue of whether progesterone is stimulatory or inhibitory in breast disease remains controversial with observations that vary depending on the models used, study of physiological vs. pharmacologic doses, use of progesterone vs. synthetic progestins, the presence or absence of estrogens or carcinogens, clinical data from MHT, and the like. We discuss this further below. The first conclusive evidence that progesterone bound to PR used the estrogen-primed chick oviduct (O’Malley, et al. 1970). In the early 1970s B. O’Malley and his key collaborators including M. Sherman, W. Schrader, D. Toft, T. Spelsberg and A. Means, showed in a series of elegant studies and multiple publications that the liganded receptors exist as dimers, compartment into both cytoplasm and nucleus, bind chromatin at specific sites, and regulate transcription (reviewed in Schrader and O’Malley 1978). Similar studies in mammals including human tissues proved to be difficult however, due to progesterone’s relative low receptor binding affinity, rapid metabolism, and lack of specificity. This problem was solved by the synthesis and tritium labeling of the progestin R5020 at Roussel-Uclaf (Philibert and Raynaud 1974). [3H]R5020 in ligand binding assays (LBA) of human tumor biopsy extracts detected PR when radiolabeled progesterone failed to do so (Horwitz and McGuire 1975b). The availability of MCF-7 cells allowed for the first demonstration that ER and PR can coexist in one tumor; possibly in the same cell (Horwitz, et al. 1975).
The clinical utility of these facts was readily apparent. As discussed above, it was noted in the 1970s that at best, 50–60% of ER+ tumors respond to endocrine therapies. Response failures were ascribed to flawed ER proteins or to errors in downstream ER signaling or transcription. It was learned in both the chick oviduct and the guinea pig uterus (Freifeld, et al. 1974) that estrogen regulates a progesterone “receptor”. Horwitz et al. (Horwitz and McGuire 1975a) reasoned that an ideal marker of hormone responsiveness in ER+ tumors would be a measurable product of estrogen action, and PR filled that need. We postulated that PR would be rare in tumors that lacked or failed ER signaling, but that PR-positivity would mark an ER+ tumor capable of regulating at least one end-product and would be hormone sensitive. Initial analysis of 50 tumor cytosols by LBA using [3H]R5020 found 0/14 (0%) to be ER−/PR+, but 20/36 (56%) to be ER+/PR+; a number close to the expected responders. We reported response to hormone therapies in 9 patients. Objective remissions were restricted to tumors that were ER+PR+. Patients with ER+PR− or ER−PR− tumors failed to respond. A larger set of 521 random tumors were 7% ER−PR−, 9% ER−PR+ and 74% ER+PR+, with a markedly higher likelihood of response associated with PR-positivity (McGuire, et al. 1977). Even before official publication of the PR paper (1975a), the White House learned of it in September 1974 when First Lady Betty Ford was diagnosed with breast cancer (Wu 2012, Sept 27). She underwent a radical mastectomy and her tumor was sent to us for analysis. We found it to be exceptionally PR-rich (disclosed with Mrs. Ford’s permission). In retrospect, she would have been an excellent candidate for minimal surgery and hormone therapies; the standard of care today. PR analysis was quickly adopted. Since 1975 millions of patient tumor samples have been assessed for ER and PR, which has spared many women extensive mastectomies in favor of lumpectomies and hormone therapies. LBAs have been replaced by simple and reliable immunohistochemical assays, and the predictive value of well-validated PR assays in both the adjuvant setting and for advanced disease has been solidly documented (Hammond, et al. 2010; Osborne 1998).
Progestin drugs and breast “tumorigenesis”
Because of its essential role in controlling human reproduction bioidentical progesterone formulations and synthetic progestins that bind and activate PR have been developed for more than half a century. The first chemically synthesized progestin was norethindrone in the 1950s (Djerassi 1966), which became the first FDA-approved oral contraceptive in combination with estrogen in the US (1960) and Europe (1961). Today, progesterone or progestins are widely and safely used for contraception, treatment of infertility, endocrine disorders, and menopausal hormone therapy (MHT) with several on the WHO list of essential medicines (progesterone, medroxyprogesterone acetate (MPA), norethisterone). The benefits of progestins are however, counterbalanced by their negative impact on rates of breast cancer diagnoses, particularly by users of MHT.
It was originally assumed that for MHT, progestins would counteract any tumor-promoting effects of estrogens in the breast, akin to their protective effects in the uterus (Gambrell, et al. 1983). This was despite studies in rodents which suggested otherwise (Huggins 1965; Lydon, et al. 1999). For instance, mice given chronic long-term MPA develop mammary tumors with high frequency (Lanari, et al. 2009). The protective hypothesis was debunked by two large studies in the US (Women’s Health Initiative) and UK (Million Women Study) that reported a significant increase in breast cancer incidence in women taking combined estrogen plus progestin compared to women taking estrogen alone (Beral, et al. 2011; Chlebowski, et al. 2010). A 2019 meta-analysis by the Collaborative Group on Hormonal Factors in Breast Cancer confirmed the increased risk of breast cancer for MHT containing MPA, norethindrone acetate, or levonorgestrol, compared to never users or estrogen-only users (Collaborative Group on Hormonal Factors in Breast 2019). This was especially pronounced for long term (>10 year) progestin users, who had twice the risk of developing breast cancer. Notably, this meta-analysis did not include bioidentical progesterone formulations, which had either no additional risk or even decreased breast cancer risk (discussed in Piette 2018). This has led to speculation that the androgenic and glucocorticoid activity of MPA and other progestins are responsible for the increased breast cancer risk (Carroll, et al. 2017; Piette 2018). Other hypotheses argue that it is the expansion of occult malignant cells, pre-existent in the breasts of some women of menopausal age, that is stimulated by the progestins (Horwitz and Sartorius 2008). In this scenario, progestins promote occult disease or activate dormant tumor cells, while perhaps suppressing established disease. Furthermore, it is important to distinguish between progestins and natural progesterone. Currently these tend to be lumped together leading to the view that progesterone is “carcinogenic” (i.e. cancer causer). It is our opinion that natural progesterone does not “cause” breast cancer but can expand it (see below). Hence despite widespread linkage between the terms “progestins” and “carcinogenesis” we suggest that care must be taken with these ideas, as with the term “bioidentical”, until solid data are available, in women, differentiating between the natural hormone and any biosynthetic ones.
PR as therapeutic targets in advanced breast cancers
In the century following the discovery that oophorectomy slowed progression of breast cancers (Beatson 1896; Boyd 1900), it became clear that estrogens are the main mitogens for about three quarters of tumors. Accordingly, most endocrine therapies in use today target the ER signaling axis in one manner or another. This has evolved from surgical and/or pharmacological blockade of ovarian estrogen production; to development of antiestrogens or “selective estrogen receptor modulators” (SERMs) such as tamoxifen that bind to and alter the activity of ER; to newer agents such as fulvestrant that degrade or down-regulate ER (SERDs) (Palmieri, et al. 2014). Another approach is to inhibit tissue estrogen production in postmenopausal women by use of aromatase inhibitors (AI) such as anastrozole, exemestane or letrozole. Tamoxifen and other SERMs are now usually reserved for premenopausal women with ER+ disease, for cancer prevention in high risk women, and for patients intolerant of AI. Adjuvant endocrine therapies can be curative or provide long-term stabilization for many patients. Unfortunately, it is estimated that between 10% to more than 40% of women will experience a recurrence depending on initial disease stage and grade, and this risk persists for more than 20-years following successful initial treatment (Pan, et al. 2017). Newer inhibitors that target CDK4/6 or mTOR used in combination with endocrine therapies improve progression-free but not overall survival (Echavarria, et al. 2017; Schettini, et al. 2017). Alternative endocrine therapies utilizing progestins, androgens, and glucocorticoids have been tested and used since the 1940s to supplement the estrogen inhibitors (Henderson and Canellos 1980).
The most common progestins for breast cancer are megestrol acetate (Megace) and MPA. Multiple clinical studies through the 1990s found that they were as effective as tamoxifen at improving progression-free survival of ER+ disease (Carroll et al. 2017). Megace can be used at lower doses than MPA with equal or better efficacy and fewer toxicities (Santen, et al. 1990), and is therefore the preferred progestin in use for late stage disease refractory to estrogen/ER targeted therapies. Mifepristone (RU486), a compound that binds both PR and glucocorticoid receptors (GR) with both antiprogestin and antiglucocorticoid activity, has also been tested, but it achieved only partial remission in some patients and has unacceptable side effects (reviewed in Santen et al. 1990). Two ongoing window of opportunity trials in the UK and Australia are testing prometrium (micronized progesterone) in combination with either tamoxifen or letrozole in ER+PR+ disease (ISRCTN23662758; ACTRN12618000928213). The goal of these studies is to see if progesterone reduces proliferation below that obtained with ER targeted therapies. Onapristone (Apristor), a PR antagonist, is currently in Phase II trials in combination with fulvestrant for women who fail on CDK4/6 inhibitors (Context Therapeutics). Thus, whether targeting PR has efficacy in treating advanced breast cancer remains an open question, with ligands that both activate and deactivate the receptors in clinical use or trials.
Therapeutic targeting of PR in advanced breast cancers has both benefits and limitations. Formulations of natural progesterone, and to a lesser extent progestins, are generally well tolerated. Overall, PRs are expressed to varying degrees in ~75% of ER+ breast cancers at diagnosis (McGuire, et al. 1986; Yi et al Ann Onc 25:1004–2014). However, up to 30% of advanced ER+ breast cancers have loss of heterozygosity at the PR locus, with many of these tumors losing PR expression {Tomlinson, 1996 #2096}. ER+ tumors that have completely lost PR during tamoxifen therapy have worse prognoses (Cui, et al. 2005). This suggests that despite ER-positivity, PR loss is associated with development of resistance. However threshold PR expression levels for efficient targeting are unknown. Tumors with no or low (<10%) ER+ cells that retain sufficient PR positivity (>10% PR+; ~9% of tumors) (Yi et al 2016) may benefit from progestin or antiprogestin therapies. Some advanced breast cancers express high PR levels including those harboring mutations in the ER gene (ESR1), in which ER target genes such as PR are constitutively upregulated (Dustin, et al. 2019). Whether PR could be co-targeted in these ER mutant cancers, is unknown. There is also speculation that if PR are retained, they can be an alternative driver in endocrine resistant tumors (Knutson and Lange 2014). Recent studies suggest that progestins could compromise immune surveillance by decreasing expression of interferon stimulated genes (Goodman, et al. 2019; Walter, et al. 2017). Thus, deciphering the context dependent actions of PR in breast cancer is the next step in deciding whether to positively or negatively target the receptors.
(The below section edited entirely…per reviewer request to tighten it up; also added section titles to clarify
PR as drivers of normal breast differentiation and tumor-cell heterogeneity
Normal mouse mammary stem cells (MaSCs).
Normal breast epithelium is maintained by a hierarchy of self-renewing MaSCs giving rise to progenitor cells that produce the bulk of terminally differentiated luminal and basal/myoepithelial cells (Fu, et al. 2014; Villadsen, et al. 2007). Progesterone is a key hormone controlling MaSC subpopulations. As this has been reviewed previously (Axlund and Sartorius 2012; Hilton, et al. 2018), we highlight here the major findings. Two studies in mice showed that progesterone is necessary for regulating the number and function of MaSCs (Asselin-Labat, et al. 2010; Joshi, et al. 2010). Using flow cytometry with defined markers, Asselin-Labat et al (2010) observed that while ovariectomy did not reduce total MaSC numbers, it impaired their ability to repopulate a functional mammary gland upon transplantation. Restoration of fully functional MaSCs required supplementation with both estrogens and progesterone. Joshi et al (2010) reported that murine MaSCs are located in a specialized niche in the basal epithelium. Their numbers are highest at diestrous when progesterone levels peak, and higher in pregnant than in nulliparous animals. Estrogen appears to be necessary to induce PR, and progesterone then stimulates MaSC self-renewal. Since murine MaSCs are ER– PR–, progesterone upregulates them via paracrine factors such as Wnt4 and RANKL secreted from the luminal PR+ cells, which then drive expansion of the basal MaSCs (Asselin-Labat et al. 2010; Joshi et al. 2010). RANK receptor inhibitors or targeted RANK antibodies impair MaSC function (Gonzalez-Suarez, et al. 2010; Schramek, et al. 2010). Overall, during the reproductive cycle of mice, progesterone plays a dynamic role in activating adult MaSCs within the mammary stem cell niche.
Normal human MaSCs.
Hormonal regulation of epithelial cell hierarchy in the normal human breast has been studied in ex vivo organoid cultures from reduction mammoplasties. These form lobular structures that retain progenitor cells, plus committed luminal and myoepithelial cells (Graham, et al. 2009b). In these models, progesterone increases proliferation indices, total cell number of organoids (Graham, et al. 2009a), mammosphere formation, and aldehyde dehydrogenase (ALDH); all measures of stem/progenitor cell activity (Dontu, et al. 2003; Ginestier, et al. 2007). RANKL is an important PR target gene. In ex vivo organoids, well defined lineage markers show that progesterone increases the levels of RANKL expression in PR+ luminal cells, and this is associated with an increase in ER−PR− progenitor cells (Tanos, et al. 2013). RANKL’s role in regulating human MaSCs, in “tumorigenesis”, and in tumor cell expansion is under extensive study.
Single cell RNA sequencing of normal human breast epithelial cells detects ER and PR transcripts in two luminal epithelial cell types termed “secretory” and “hormone responsive” (Nguyen, et al. 2018). However, immunohistochemical studies of cells isolated by flow cytometry detect PR transcripts in the basal epithelial cell fraction, and in occasional PR+ myoepithelial cells (SMA+p63+) as well (Hilton et al. 2018; Hilton et al. 2012), suggesting that progesterone may also have direct autocrine effects on a subset of PR+ myoepithelial cells (Hilton et al. 2018). Given these heterogeneities, a definitive picture of progesterone’s role in generating the physiological and complex cellular architecture of the normal human breast requires further study.
Luminal breast CSCs in cell lines.
The term “CSC” has undergone evolving definitions. It was originally thought that tumors, from their origin, contain small populations of cells that share properties of normal stem cells including quiescence, self-renewal capacity, and the ability to generate differentiated progeny (Reya et al. 2001). Studies in multiple cancer types redefined CSCs as a functional state, that can exist de novo and/or be acquired through environmental cues or therapeutic pressure as tumors evolve (Meacham and Morrison 2013). Generically, for breast disease, CSCs were initially defined (Al-Haaj et al, 2003) as having the surface marker signature CD44+CD24−/lowEpCAM+, with increased ALDH activity (Ginestier et al. 2007). It is unclear whether these factors define the luminal ER+PR+ CSCs subtype. It has been postulated that phenotypic adaptation through transcriptional and epigenetic modulation may be the dominant force dictating ER+ tumor heterogeneity and evolution (Patten, et al. 2018), and that, as in the normal tissues, hormones are key factors controlling CSC number and function in luminal disease. Liganded PR, especially PR-A (Truong et al. 2019), increase populations of cells with CSC properties and tumorsphere formation capacity (reviewed in Axlund and Sartorius 2012; Cenciarini and Proietti 2019; Finlay-Schultz and Sartorius 2015; Simoes et al. 2015) (Cittelly, et al. 2013; Finlay-Schultz, et al. 2015; Hilton, et al. 2014; Knutson, et al. 2017; Truong, et al. 2019). Additionally, several genes implicated in CSC number and function are targets of liganded PR, including the pluripotent transcription factor KLF4 (Cittelly et al. 2013), the co-activator FOXO1 (Truong et al. 2019), the transcriptional repressor BCL6 (Sato, et al. 2014), and micro-RNAs (miR)-29 and miR141 (Cittelly et al. 2013; Finlay-Schultz et al. 2015). Thus, progesterone and progestins increase populations of self-renewing CSCs in all models tested and the signaling intermediates are being defined since they could serve as therapeutic targets.
CK5+ Luminal CSCs.
Molecular profiling of breast cancers, led to their classification into 4 major subtypes (Perou, et al. 2000), including Luminal A (ER and PR rich); Luminal B (trending to lower ER, low or no PR, high proliferation rate); mainly HER2+ER−; and an ER–PR– basal-like or TNBC group (Prat and Perou 2011). Interestingly, luminal tumors have relatively low or no CD44+ and ALDH+ CSCs compared to TNBC, leading to speculation that luminal tumors have alternate CSCs. Analyses of luminal breast cancer cell xenografts treated with progestins show increased transcript levels of basal cytokeratins (CK) (CK5, CK6, and CK17), and decreased levels of luminal CKs (CK8, CK18, and CK19) (Sartorius et al., 2005). CK5 is especially interesting since it is a marker of luminal progenitor cells (Lim, et al. 2009) and a well-established indicator of poor prognosis (Cheang, et al. 2008; Malzahn, et al. 1998; van de Rijn, et al. 2002). Of note, progestin-induced breast cancer cells lose ER and PR while gaining CK5. Such cellular heterogeneity is not surprising since clinically, one-third to one-half of all ER+ breast cancers contain ER−PR− CK5+ cell subpopulations (Haughian, et al. 2012; Horwitz, et al. 2008; Joensuu, et al. 2013). Compared to CK5– cells, CK5+ cells possess all the hallmarks of CSCs including tumor initiation capacity, quiescence, and resistance to chemo- and endocrine therapies (Axlund, et al. 2013; Goodman, et al. 2016; Horwitz et al. 2008; Kabos, et al. 2011; Sato et al. 2014). Further, as CK5+ cells lose hormone responsiveness, they gain expression of mesenchymal transcription factors such as Slug and Twist, and increase Wnt and Notch signaling (Haughian et al. 2012). Progestins target the CK5 gene directly by rapidly recruiting PR to the proximal CK5 promoter at two progesterone response elements (Fettig, et al. 2017).
That CK5 contributes functionally to the luminal CSCs phenotype is suggested by its knockdown (with shRNA) or knockout (with CRISPR), both of which impair progestin-induced tumorsphere formation (Fettig et al. 2017; McGinn, et al. 2020). Chronically hormone-withdrawn luminal cells constitutively upregulate CK5 (Haughian et al. 2012). Such cells were used to identify non-cytoskeletal interactors of CK5 that influence cell phenotype (McGinn et al. 2020). One is β-catenin, a downstream effector of the Wnt signaling factor, an essential component of adherens junctions, and an important regulator of normal and malignant stem cells (Reya and Clevers 2005). CK5+ luminal cancer cells, whether progestin-induced or constitutive, have reduced β-catenin and E-cadherin at the cell membrane; factors that influence cell polarity, migration, and invasion in association with poor prognosis. This agrees with the fact that in breast cancers, invasive leader cells express basal CKs including CK5 (Cheung, et al. 2013). Our current working hypothesis is that by paracrine signaling, progestins transition a minor subpopulation of malignant cells from a proliferative but non-aggressive hormone sensitive ER+PR+CK5− state, to a more invasive hormone resistant ER−PR−CK5+ state (see Figure 2). If so, antiprogestins could serve as potent preventatives in moderate to high risk premenopausal women; a theory currently in Phase I clinical investigation using ulipristal acetate (Ref.)
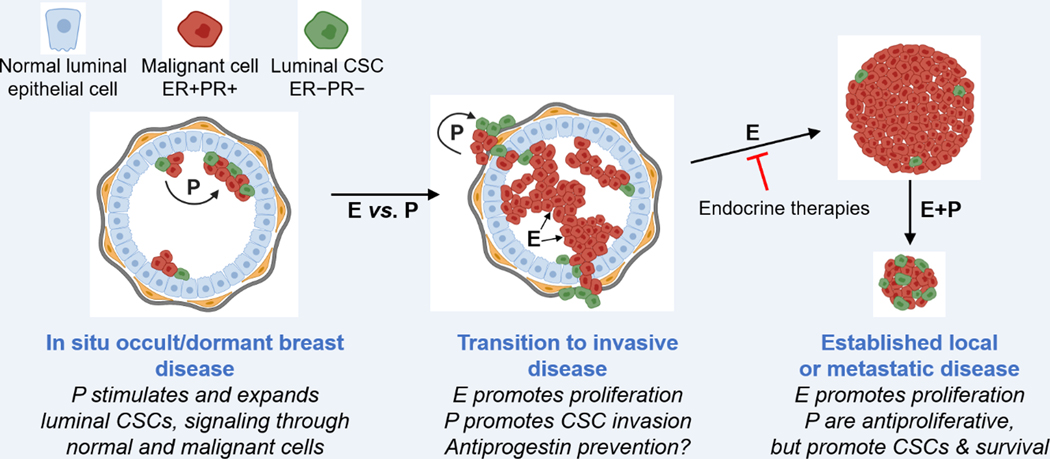
Figure 2. Effects of progesterone and progestins in early and late luminal breast cancer.
Progesterone and progestins are collectively termed “P”. LEFT. In localized, occult ER+PR+ (red) tumors such as DCIS or at distant metastatic sites, P upregulate ER−PR− cancer stem cells (CSCs; green) by signaling from the malignant ER+PR+ cells via paracrine factors. CENTER. In invasive carcinomas, P upregulation of CSCs promotes tumor invasiveness over proliferation. RIGHT. In established local or metastatic disease endocrine therapies target estrogen (E)-driven growth. Addition of P suppress growth but upregulate CSCs. Such tumors may be indolent or enter long-term dormancy but remain capable of reactivation by P or other signals. Created, in part, using Biorender.com.
Progesterone, PR, tumor dormancy and metastasis
In the absence of a chemical carcinogen it is difficult if not impossible to “cause” breast cancer with progesterone. Yet we and others remain puzzled by the clear evidence that women prescribed MHT have a heightened incidence of breast cancer if a progestin is added to the estrogen. What is the progestin doing if not “causing” disease? In the seminal studies of Huggins (Huggins 1965; Huggins et al. 1962) and others (Sivaraman, et al. 2001) on effects of progesterone or pregnancy on tumor “induction”, animals were always pre-treated with a carcinogen, followed weeks later by endogenous or exogenous estrogen plus progesterone. Tumor development was recorded by palpation. Such studies found that heightened progestational states decreased the time to tumor formation, or increased tumor number. Notably, when progesterone was included as a control without the carcinogen no tumors developed (Jabara 1967). In hindsight, these studies document the failure of progesterone alone to “induce” palpable disease, and were technically incapable of detecting carcinogen-induced micro-disease prior to hormone addition. Instead, we suggest that they point to progesterone as a promoter of pre-established disease caused by the carcinogen, and postulate that undetected pre-existing occult, possibly dormant, micro-disease explains the promoting effects of progesterone in women prescribed MHT. Figure 2 depicts how progesterone and progestins may differentially impact early and late breast cancer.
We developed models to test these theories using multicolor fluorescence to track malignant cells in mice (Ogba, et al. 2014). We showed that for weeks after hormone dependent ER+PR+CK5− luminal breast cancer cells are injected into ovariectomized mice there is no evidence of disease. However, some apparently disease-free mice harbor a reservoir of luminal CK8/18+ micro-metastases in lymph nodes and other sites that have low or no ER, PR or CK5. If mice are re-exposed to physiological hormones, fulminant disease emerges rapidly. And if the regimen includes progesterone this is accompanied by extensive upregulation of CK5+ CSC-like cells. Clearly, in these models, hormones trigger expansion of microscopic occult cells into overt disease. Given this, it is not unreasonable to propose that some women of menopausal age unknowingly harbor pre-existent minimal disease, which expands upon hormone supplementation leading to a diagnosis of breast cancer. This scenario also argues strongly against prescribing MHT to breast cancer survivors. It is of considerable interest that it is in such early, clinically hidden cancers, in which progesterone increases CSCs, it also triggers cell migration leading to widespread metastatic dissemination in mouse models of early HER2-driven breast cancer (Hosseini, et al. 2016).
PR modulation of ER action
The prevalence of ER+PR+ breast cancers increases with age; they constitute >80% of diagnoses in postmenopausal women. In this environment, locally produced estrogens are the major mitogens and the role of endogenous and exogenous progestins remains unclear (Carroll et al. 2017; Diep, et al. 2015; Giulianelli, et al. 2013). In general, there are two types of studies that either test progesterone and progestins in the absence of estrogens or assess their impact on estrogen mediated growth. Studies in breast cancer cells initially recognized that treatment with the progestin R5020 alone decreases two dimensional cell growth (Horwitz and Freidenberg 1985). This was later found to be due to inhibition of the cell cycle after a transient round of proliferation (Musgrove et al., 1991, Groshong et al., 1997). Other studies found that progestins increase growth of BT-474 breast cancer xenografts (Liang, et al. 2010). Regarding PR isoforms, cells expressing PR-B increase proliferation in response to progestin, and this is attenuated by PR-A expression (Tung, et al. 1993). Following short term treatment of ex vivo breast tumor explants, progestins have variable effects on proliferation (Knutson et al. 2017), confirming that the impact of progestins on breast cancer growth is both tumor and context-dependent.
In breast cancer, PR are co-expressed with ER and their activities are intertwined. This was first recognized using simple promoter-reporter constructs, where PR were found to repress ER transcriptional activity (Wen, et al. 1994). Other studies found that exogenously expressed PR abolish estrogen induced growth in MCF-7 cells (Zheng, et al. 2005). Various immunoprecipitation assays have demonstrated direct interactions between ER and PR (Ballare, et al. 2003; Daniel, et al. 2015; Giulianelli, et al. 2012; Mohammed et al. 2015), finding the two receptors in multi-protein complexes and/or co-localized in proximity on chromatin. Two more recent studies in breast cancer cell lines discovered that short term (3–24 h) treatment with progesterone or a progestin reprograms ER DNA binding (Mohammed et al. 2015; Singhal, et al. 2016). Mohammed et al (2015) found that PR are present at ER target genes in the absence of ligand and act as cofactors. However, in the presence of progesterone, PR redirect ER to a cistrome with decreased occupancy of oncogenic genes coinciding with reduced cell or tumor growth. Singhal et al (2016) noted that while there was some coordinate activity of the agonist R5020 and estrogen at target genes, the overall phenotypic effect was a progestin-induced reduction in estrogen-driven cell and tumor growth. Notably, they also found that the selective PR antagonist CDB4124 (telapristone acetate) suppressed estrogen-induced tumor growth. That is, either agonist- or antagonist-liganded PR were additive with tamoxifen..This could partially explain why both PR agonists and antagonists have seen some success in clinical trials. Overall, most studies find that liganded, activated PR attenuate ER activity and have a net repressive effect on estrogen mediated growth. Taken together the data confirm the long-held notion that progesterone is a schizophrenic hormone with regard to breast disease: it is harmful in occult, early, low density lesions where it upregulates stemness and promotes cell migration, proliferation and metastases via paracrine signals that involve WNT4 and RANKL; it is beneficial in high density palpable tumors where it suppresses these activities by directly suppressing ER proliferative effects, at the expense however of expanding the CSC reservoir (see Figure 2).
New ER+PR+ human tumor models
Basic and preclinical studies on PR in breast cancer have relied heavily on several human breast cancer cell lines that were developed in the 1970s including MCF-7, T47D, ZR75, and BT-474 (Brooks, et al. 1973; Engel, et al. 1978; Keydar, et al. 1979; Lasfargues, et al. 1978). Among these, T47D cells have traditionally been the go-to cell line for PR research, due primarily to their unique constitutively high PR expression levels independent of exogenous estradiol supplementation (Horwitz, et al. 1982), allowing analysis of the autonomous actions of progestins. While numerous other cell line collections have been subsequently developed, the majority lack ER and/or PR at the protein level (Neve, et al. 2006). A great deal of information regarding PR structure and function has been dissected using cell line models. They have also been extremely useful for initial studies of solid tumors since, with estrogen supplementation, ER+PR+ cell lines grow readily as xenografts in immune compromised mice (Clarke 1996). There are also numerous transgenic murine mammary tumor models. However, these are commonly ER−PR− (or express ER/PR only transiently early in tumor development) and are not estrogen dependent (Pfefferele et al Genome Biol 2013, 14:R125). With improved immune compromised mouse strains such as NOD-scid IL2Rgnull (NSG), a series of patient-derived xenografts (PDX) have been developed by direct transplantation of tumor fragments into mouse mammary glands. More than 500 such PDX are available worldwide (Dobrolecki, et al. 2016), but only one third are ER+ due to intrinsic limitations in generating them. Furthermore, in many ER+ PDX, PR are either not expressed or only expressed in a small cell fraction. We have generated several ER+PR+ PDX that are well-suited for study of ER and PR action (Kabos, et al. 2012). Grafting into milk ducts may also be promising for maintaining ER-positivity (Sflomos et al). Breast cancer specimens can also be adapted to patient-derived organoids (PDO) (Sachs, et al. 2018) or patient-derived explants, in which tumor tissue is partitioned into a sponge culture and cultured with hormones for up to 72 h (Centenera, et al. 2018). Each of these models has limitations including the fact that they are difficult to manipulate genetically. To get around this, we recently generated three ER+ PDX-derived breast cancer cell lines, two of which express PR, which are amenable to manipulations such as viral transduction (C. Sartorius, unpublished data). Collectively, the expanding repertoire of human breast cancer models is allowing the study of PRs in heterogeneous tumors and will broaden our understanding of their actions.
PR regulation of RNA polymerase III
We have used our ER+PR+ PDX tumor models to define how progestins influence tumor behavior and PR action in heterogeneous disease, and find that chronic treatment with estrogen plus either progesterone or MPA slows estrogen-driven tumor growth (Finlay-Schultz, et al. 2017), supporting earlier cell line data and reflecting clinical data. Using gene expression analyses we noted that progesterone or MPA reverse expression of approximately half of estrogen-regulated genes by decreasing upregulated ones and increasing downregulated ones. In contrast, few transcripts were regulated by estrogen and progesterone/MPA in the same direction. We assessed ER chromatin occupancy by ChIP-seq and found that progesterone caused a ~20% loss or gain of estrogen-induced ER binding sites. Thus, akin to data in cell lines, in PDXs, progestins impact ER gene regulation and attenuate estrogen driven growth. However, the ER chromatin shift is not as striking as in cell lines. This may be due to longer in vivo treatment times, a more complex hormone environment, and greater cellular heterogeneity.
Somewhat surprisingly, by assessing chromatin occupancy, we found that PR were localized at approximately 50% of tRNA genes, unlike ER which bind there only sparsely. We confirmed that progestin co-treatment decreased unprocessed pre-tRNAs and their corresponding mature tRNAs. Furthermore, rapid IP-mass spectrometry of endogenous protein analysis displayed PR in association with RNA polymerase III (Pol III); the polymerase that transcribes all tRNA genes. Pol III transcription is a key target of the dynamic balance between cell growth and quiescence. Its direct or indirect regulation is necessary for breast cancer targeted therapies such as mTOR inhibitors that push cells into cytostasis. Interestingly, direct repression of Pol III is a survival mechanism for cells undergoing stress, inducing a quiescent state like one that maintains the longevity of normal tissue stem cells. Pol III can even increase organismal lifespan (Filer, et al. 2017; Moir and Willis 2013). tRNAs are the most abundant product of Pol III and changes in their levels and type regulates cell phenotype through reduced and/or selective mRNA translation (Frenkel-Morgenstern, et al. 2012). Thus, PR regulation of Pol III could simultaneously contribute to both growth inhibition and expansion of CSCs. Furthermore, this mechanism could impact ER+ tumors independent of direct effects on ER transcription.
Future: where do progesterone and PR go from here?
It is indisputable that in luminal breast cancers, estrogens acting through functional ER are the major mitogenic drivers, explaining the success of drugs that target ER at several signaling stages. Therefore it has long been clinically important to assess for “functional ER”, and PR expression, an end-point of functional ER signaling, continues to serve that purpose. The issue before us is not just whether PR proteins, but whether functioning progesterone-liganded PR proteins, play a role in breast cancers. There is no question that progesterone is a key hormone required for maturation of the normal breast, where it drives paracrine signaling from PR+ cells to PR– MaSCs, which then generate the organ’s complex cellular architecture and function. In short, in the normal breast, progesterone promotes differentiation at the expense of growth. Is it possible that in breast malignancies progesterone’s functions are similar? That progesterone targets stem cells and promotes cellular heterogeneity in cancers like it does in the normal breast? We address questions raised by this hypothesis, below.
First, we need to debunk the notion that progesterone “causes” breast cancers. There is considerable experimental and clinical evidence that alone and at physiological levels, progesterone is incapable of causing breast cancers so that its reputation as a “tumorigenic” or “carcinogenic” hormone is undeserved. It would be useful to have definitive proof of this once and for all, and to eliminate use of these terms in reference to progesterone and the breast.
Further, we review data leading us to postulate that progesterone behaves in breast disease like it does in the normal gland -- it targets stem cells. But in the case of breast cancers these are “cancer” stem cells located in pre-existing disease previously induced by other mechanisms; a carcinogen perhaps. Such a role for progesterone in transformed cells is of course highly consequential, and it is our contention that following up on the myriad possible pathways is where the future lies. From these ideas flow the likelihood that progesterone plays a role in activating dormant disease; in generating tumor-cell heterogeneity; in enhancing aggressivenes of one or more tumor-cell subpopulations; and in promoting tumor-cell dissemination and metastasis. Extensive research is required in human models and the clinic with regard to progesterone’s role in these areas: 1. Stem cells. What are the molecular markers of luminal CSCs (and are there several such cell types); how do they arise; how are they transformed, induced or activated; what is the role of progesterone therein; how are CSCs metabolically fueled and maintained; how do they receive and send signals; can CSCs or their signals be pharmacologically or immunologically suppressed; what is their role in resistance to hormone therapies? 2. Aggressiveness: How and what heterogenous cell subpopulation(s) are induced or activated by progesterone; is this at an early or late stage of disease; are there leading-edge cell markers; how are cells targeted to one or more distant organs; can they be pharmacologically or immunologically suppressed at early and/or late stages? 3. Minimal vs. established disease: Can minimal disease be diagnosed; are CSCs identifiable in minimal disease; is tumor dormancy real and can it be sustained; what signals activation of dormant tumor-cells; is there a role for progesterone or antiprogestins therein; is progesterone a growth suppressor or a mitogen in established cancers; does this differ across disease stages; are progesterone and synthetic progestins similar or not; do synthetic progestins “cause” cancer; if so, how? As new human breast cancer models are developed and tested, and existing or unimagined new technologies are applied, these questions can be answered. We believe that we are entering an important and exciting period in research on progesterone and PR in breast cancers and their future as targets of therapy.
Acknowledgments
Funding
This work was supported by National Institutes of Health grant 2R01 CA140985 (CAS) and the Breast Cancer Research Foundation (CAS, KBH).
Footnotes
Declaration of interests
The authors declare they have no competing interests.
References
- Africander D & Storbeck KH 2018. Steroid metabolism in breast cancer: Where are we and what are we missing? Mol Cell Endocrinol 466 86–97. [DOI] [PubMed] [Google Scholar]
- Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, Yasuda H, Smyth GK, Martin TJ, Lindeman GJ, et al. 2010. Control of mammary stem cell function by steroid hormone signalling. Nature 465 798–802. [DOI] [PubMed] [Google Scholar]
- Axlund SD & Sartorius CA 2012. Progesterone regulation of stem and progenitor cells in normal and malignant breast. Mol Cell Endocrinol 357 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axlund SD, Yoo BH, Rosen RB, Schaack J, Kabos P, Labarbera DV & Sartorius CA 2013. Progesterone-inducible cytokeratin 5-positive cells in luminal breast cancer exhibit progenitor properties. Horm Cancer 4 36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballare C, Uhrig M, Bechtold T, Sancho E, Di Domenico M, Migliaccio A, Auricchio F & Beato M 2003. Two domains of the progesterone receptor interact with the estrogen receptor and are required for progesterone activation of the c-Src/Erk pathway in mammalian cells. Mol Cell Biol 23 1994–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatson GT 1896. On the treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment with illustrative cases. Lancet 2 104–107. [PMC free article] [PubMed] [Google Scholar]
- Beral V, Reeves G, Bull D & Green J 2011. Breast cancer risk in relation to the interval between menopause and starting hormone therapy. J Natl Cancer Inst 103 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd S 1900. On oophorectomy in cancer of the breast. BMJ 2 1161–1167. [Google Scholar]
- Brooks SC, Locke ER & Soule HD 1973. Estrogen receptor in a human cell line (MCF-7) from breast carcinoma. J Biol Chem 248 6251–6253. [PubMed] [Google Scholar]
- Carroll JS, Hickey TE, Tarulli GA, Williams M & Tilley WD 2017. Deciphering the divergent roles of progestogens in breast cancer. Nat Rev Cancer 17 54–64. [DOI] [PubMed] [Google Scholar]
- Cenciarini ME & Proietti CJ 2019. Molecular mechanisms underlying progesterone receptor action in breast cancer: Insights into cell proliferation and stem cell regulation. Steroids 152 108503. [DOI] [PubMed] [Google Scholar]
- Centenera MM, Hickey TE, Jindal S, Ryan NK, Ravindranathan P, Mohammed H, Robinson JL, Schiewer MJ, Ma S, Kapur P, et al. 2018. A patient-derived explant (PDE) model of hormone-dependent cancer. Mol Oncol 12 1608–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, Perou CM & Nielsen TO 2008. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res 14 1368–1376. [DOI] [PubMed] [Google Scholar]
- Cheung KJ, Gabrielson E, Werb Z & Ewald AJ 2013. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell 155 1639–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski RT, Anderson GL, Gass M, Lane DS, Aragaki AK, Kuller LH, Manson JE, Stefanick ML, Ockene J, Sarto GE, et al. 2010. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. Jama 304 1684–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cittelly DM, Finlay-Schultz J, Howe EN, Spoelstra NS, Axlund SD, Hendricks P, Jacobsen BM, Sartorius CA & Richer JK 2013. Progestin suppression of miR-29 potentiates dedifferentiation of breast cancer cells via KLF4. Oncogene 32 2555–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R 1996. Human breast cancer cell line xenografts as models of breast cancer. The immunobiologies of recipient mice and the characteristics of several tumorigenic cell lines. Breast Cancer Res Treat 39 69–86. [DOI] [PubMed] [Google Scholar]
- Collaborative Group on Hormonal Factors in Breast C 2019. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet 394 1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Schiff R, Arpino G, Osborne CK & Lee AV 2005. Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol 23 7721–7735. [DOI] [PubMed] [Google Scholar]
- Daniel AR, Gaviglio AL, Knutson TP, Ostrander JH, D’Assoro AB, Ravindranathan P, Peng Y, Raj GV, Yee D & Lange CA 2015. Progesterone receptor-B enhances estrogen responsiveness of breast cancer cells via scaffolding PELP1- and estrogen receptor-containing transcription complexes. Oncogene 34 506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao TL 1972. Ablation therapy for hormone-dependent tumors. Annu Rev Med 23 1–18. [DOI] [PubMed] [Google Scholar]
- Diep CH, Daniel AR, Mauro LJ, Knutson TP & Lange CA 2015. Progesterone action in breast, uterine, and ovarian cancers. J Mol Endocrinol 54 R31–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djerassi C 1966. Steroid oral contraceptives. Science 151 1055–1061. [DOI] [PubMed] [Google Scholar]
- Dobrolecki LE, Airhart SD, Alferez DG, Aparicio S, Behbod F, Bentires-Alj M, Brisken C, Bult CJ, Cai S, Clarke RB, et al. 2016. Patient-derived xenograft (PDX) models in basic and translational breast cancer research. Cancer Metastasis Rev 35 547–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ & Wicha MS 2003. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 17 1253–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin D, Gu G & Fuqua SAW 2019. ESR1 mutations in breast cancer. Cancer 125 3714–3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echavarria I, Jerez Y, Martin M & Lopez-Tarruella S 2017. Incorporating CDK4/6 Inhibitors in the Treatment of Advanced Luminal Breast Cancer. Breast Care (Basel) 12 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel LW, Young NA, Tralka TS, Lippman ME, O’Brien SJ & Joyce MJ 1978. Establishment and characterization of three new continuous cell lines derived from human breast carcinomas. Cancer Res 38 3352–3364. [PubMed] [Google Scholar]
- Estes PA, Suba EJ, Lawler-Heavner J, Elashry-Stowers D, Wei LL, Toft DO, Sullivan WP, Horwitz KB & Edwards DP 1987. Immunologic analysis of human breast cancer progesterone receptors. 1. Immunoaffinity purification of transformed receptors and production of monoclonal antibodies. Biochemistry 26 6250–6262. [DOI] [PubMed] [Google Scholar]
- Fettig LM, McGinn O, Finlay-Schultz J, LaBarbera DV, Nordeen SK & Sartorius CA 2017. Cross talk between progesterone receptors and retinoic acid receptors in regulation of cytokeratin 5-positive breast cancer cells. Oncogene 36 6074–6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filer D, Thompson MA, Takhaveev V, Dobson AJ, Kotronaki I, Green JWM, Heinemann M, Tullet JMA & Alic N 2017. RNA polymerase III limits longevity downstream of TORC1. Nature 552 263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay-Schultz J & Sartorius CA 2015. Steroid hormones, steroid receptors, and breast cancer stem cells. J Mammary Gland Biol Neoplasia 20 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay-Schultz J, Cittelly DM, Hendricks P, Patel P, Kabos P, Jacobsen BM, Richer JK & Sartorius CA 2015. Progesterone downregulation of miR-141 contributes to expansion of stem-like breast cancer cells through maintenance of progesterone receptor and Stat5a. Oncogene 34 3676–3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay-Schultz J, Gillen AE, Brechbuhl HM, Ivie JJ, Matthews SB, Jacobsen BM, Bentley DL, Kabos P & Sartorius CA 2017. Breast Cancer Suppression by Progesterone Receptors Is Mediated by Their Modulation of Estrogen Receptors and RNA Polymerase III. Cancer Res 77 4934–4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifeld ML, Feil PD & Bardin CW 1974. The in vivo regulation of the progesterone “receptor” in guinea pig uterus: dependence on estrogen and progesterone. Steroids 23 93–103. [DOI] [PubMed] [Google Scholar]
- Frenkel-Morgenstern M, Danon T, Christian T, Igarashi T, Cohen L, Hou YM & Jensen LJ 2012. Genes adopt non-optimal codon usage to generate cell cycle-dependent oscillations in protein levels. Mol Syst Biol 8 572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu N, Lindeman GJ & Visvader JE 2014. The mammary stem cell hierarchy. Curr Top Dev Biol 107 133–160. [DOI] [PubMed] [Google Scholar]
- Gambrell RD Jr.,, Bagnell CA & Greenblatt RB 1983. Role of estrogens and progesterone in the etiology and prevention of endometrial cancer: review. Am J Obstet Gynecol 146 696–707. [DOI] [PubMed] [Google Scholar]
- Giatti S, Romano S, Pesaresi M, Cermenati G, Mitro N, Caruso D, Tetel MJ, Garcia-Segura LM & Melcangi RC 2015. Neuroactive steroids and the peripheral nervous system: An update. Steroids 103 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, et al. 2007. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell 1 555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulianelli S, Molinolo A & Lanari C 2013. Targeting progesterone receptors in breast cancer. Vitam Horm 93 161–184. [DOI] [PubMed] [Google Scholar]
- Giulianelli S, Vaque JP, Soldati R, Wargon V, Vanzulli SI, Martins R, Zeitlin E, Molinolo AA, Helguero LA, Lamb CA, et al. 2012. Estrogen receptor alpha mediates progestin-induced mammary tumor growth by interacting with progesterone receptors at the cyclin D1/MYC promoters. Cancer Res 72 2416–2427. [DOI] [PubMed] [Google Scholar]
- Glascock RF & Hoekstra WG 1959. Selective accumulation of tritium-labelled hexoestrol by the reproductive organs of immature female goats and sheep. Biochem J 72 673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R, Pinkas J, Branstetter D & Dougall WC 2010. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature 468 103–107. [DOI] [PubMed] [Google Scholar]
- Goodman CR, Sato T, Peck AR, Girondo MA, Yang N, Liu C, Yanac AF, Kovatich AJ, Hooke JA, Shriver CD, et al. 2016. Steroid induction of therapy-resistant cytokeratin-5-positive cells in estrogen receptor-positive breast cancer through a BCL6-dependent mechanism. Oncogene 35 1373–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman ML, Trinca GM, Walter KR, Papachristou EK, D’Santos CS, Li T, Liu Q, Lai Z, Chalise P, Madan R, et al. 2019. Progesterone Receptor Attenuates STAT1-Mediated IFN Signaling in Breast Cancer. J Immunol 202 3076–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JD, Roman SD, McGowan E, Sutherland RL & Clarke CL 1995. Preferential stimulation of human progesterone receptor B expression by estrogen in T-47D human breast cancer cells. J Biol Chem 270 30693–30700. [DOI] [PubMed] [Google Scholar]
- Graham JD, Mote PA, Salagame U, Balleine RL, Huschtscha LI & Clarke CL 2009b. Hormone-responsive model of primary human breast epithelium. J Mammary Gland Biol Neoplasia 14 367–379. [DOI] [PubMed] [Google Scholar]
- Graham JD, Mote PA, Salagame U, van Dijk JH, Balleine RL, Huschtscha LI, Reddel RR & Clarke CL 2009a. DNA replication licensing and progenitor numbers are increased by progesterone in normal human breast. Endocrinology 150 3318–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, et al. 2010. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28 2784–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughian JM, Pinto MP, Harrell JC, Bliesner BS, Joensuu KM, Dye WW, Sartorius CA, Tan AC, Heikkila P, Perou CM, et al. 2012. Maintenance of hormone responsiveness in luminal breast cancers by suppression of Notch. Proc Natl Acad Sci U S A 109 2742–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson IC & Canellos GP 1980. Cancer of the breast: the past decade (first of two parts). N Engl J Med 302 17–30. [DOI] [PubMed] [Google Scholar]
- Hilton HN, Clarke CL & Graham JD 2018. Estrogen and progesterone signalling in the normal breast and its implications for cancer development. Mol Cell Endocrinol 466 2–14. [DOI] [PubMed] [Google Scholar]
- Hilton HN, Santucci N, Silvestri A, Kantimm S, Huschtscha LI, Graham JD & Clarke CL 2014. Progesterone stimulates progenitor cells in normal human breast and breast cancer cells. Breast Cancer Res Treat 143 423–433. [DOI] [PubMed] [Google Scholar]
- Hilton HN, Graham JD, Kantimm S, Santucci N, Cloosterman D, Huschtscha LI, Mote PA & Clarke CL 2012. Progesterone and estrogen receptors segregate into different cell subpopulations in the normal human breast. Mol Cell Endocrinol. [DOI] [PubMed] [Google Scholar]
- Hopp TA, Weiss HL, Hilsenbeck SG, Cui Y, Allred DC, Horwitz KB & Fuqua SA 2004. Breast cancer patients with progesterone receptor PR-A-rich tumors have poorer disease-free survival rates. Clin Cancer Res 10 2751–2760. [DOI] [PubMed] [Google Scholar]
- Horwitz KB & McGuire WL 1975a. Predicting response to endocrine therapy in human breast cancer: a hypothesis. Science 189 726–727. [DOI] [PubMed] [Google Scholar]
- Horwitz KB & McGuire WL 1975b. Specific progesterone receptors in human breast cancer. Steroids 25 497–505. [DOI] [PubMed] [Google Scholar]
- Horwitz KB & Freidenberg GR 1985. Growth inhibition and increase of insulin receptors in antiestrogen-resistant T47DCO human breast cancer cells by progestins: implications for endocrine therapies. Cancer Res 45 167–173. [PubMed] [Google Scholar]
- Horwitz KB & Sartorius CA 2008. Progestins in hormone replacement therapies reactivate cancer stem cells in women with preexisting breast cancers: a hypothesis. J Clin Endocrinol Metab 93 3295–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz KB, Costlow ME & McGuire WL 1975. MCF-7; a human breast cancer cell line with estrogen, androgen, progesterone, and glucocorticoid receptors. Steroids 26 785–795. [DOI] [PubMed] [Google Scholar]
- Horwitz KB, Mockus MB & Lessey BA 1982. Variant T47D human breast cancer cells with high progesterone-receptor levels despite estrogen and antiestrogen resistance. Cell 28 633–642. [DOI] [PubMed] [Google Scholar]
- Horwitz KB, Dye WW, Harrell JC, Kabos P & Sartorius CA 2008. Rare steroid receptor-negative basal-like tumorigenic cells in luminal subtype human breast cancer xenografts. Proc Natl Acad Sci U S A 105 5774–5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini H, Obradovic MM, Hoffmann M, Harper KL, Sosa MS, Werner-Klein M, Nanduri LK, Werno C, Ehrl C, Maneck M, et al. 2016. Early dissemination seeds metastasis in breast cancer. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins C 1965. Two principles in endocrine therapy of cancers: hormone deprival and hormone interference. Cancer Res 25 1163–1167. [PubMed] [Google Scholar]
- Huggins C, Moon RC & Morii S 1962. Extinction of experimental mammary cancer. I. Estradiol-17beta and progesterone. Proc Natl Acad Sci U S A 48 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabara AG 1967. Effects of progesterone on 9,10-dimethyl-1,2-benzanthracene-induced mammary tumours in Sprague-Dawley rats. Br J Cancer 21 418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen EV & Jacobson HI 1960. Fate of seroid estrogens in target tissues. In Biologic activities of streoids in relation to cancer, pp 161–174: Academic Press. [Google Scholar]
- Joensuu K, Leidenius M, Kero M, Andersson LC, Horwitz KB & Heikkila P 2013. ER, PR, HER2, Ki-67 and CK5 in Early and Late Relapsing Breast Cancer-Reduced CK5 Expression in Metastases. Breast Cancer (Auckl) 7 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD & Khokha R 2010. Progesterone induces adult mammary stem cell expansion. Nature 465 803–807. [DOI] [PubMed] [Google Scholar]
- Kabos P, Haughian JM, Wang X, Dye WW, Finlayson C, Elias A, Horwitz KB & Sartorius CA 2011. Cytokeratin 5 positive cells represent a steroid receptor negative and therapy resistant subpopulation in luminal breast cancers. Breast Cancer Res Treat 128 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabos P, Finlay-Schultz J, Li C, Kline E, Finlayson C, Wisell J, Manuel CA, Edgerton SM, Harrell JC, Elias A, et al. 2012. Patient-derived luminal breast cancer xenografts retain hormone receptor heterogeneity and help define unique estrogen-dependent gene signatures. Breast Cancer Res Treat 135 415–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H & Chambon P 1990. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. Embo J 9 1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keydar I, Chen L, Karby S, Weiss FR, Delarea J, Radu M, Chaitcik S & Brenner HJ 1979. Establishment and characterization of a cell line of human breast carcinoma origin. Eur J Cancer 15 659–670. [DOI] [PubMed] [Google Scholar]
- King RJ, Gordon J, Cowan DM & Inman DR 1966. The intranuclear localization of [6,7–3H]-oestradiol-17-beta in dimethylbenzanthracene-induced rat mammary adenocarcinoma and other tissues. J Endocrinol 36 139–150. [DOI] [PubMed] [Google Scholar]
- Knutson TP & Lange CA 2014. Tracking progesterone receptor-mediated actions in breast cancer. Pharmacol Ther 142 114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson TP, Truong TH, Ma S, Brady NJ, Sullivan ME, Raj G, Schwertfeger KL & Lange CA 2017. Posttranslationally modified progesterone receptors direct ligand-specific expression of breast cancer stem cell-associated gene programs. J Hematol Oncol 10 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanari C, Lamb CA, Fabris VT, Helguero LA, Soldati R, Bottino MC, Giulianelli S, Cerliani JP, Wargon V & Molinolo A 2009. The MPA mouse breast cancer model: evidence for a role of progesterone receptors in breast cancer. Endocr Relat Cancer 16 333–350. [DOI] [PubMed] [Google Scholar]
- Lasfargues EY, Coutinho WG & Redfield ES 1978. Isolation of two human tumor epithelial cell lines from solid breast carcinomas. J Natl Cancer Inst 61 967–978. [PubMed] [Google Scholar]
- Liang Y, Benakanakere I, Besch-Williford C, Hyder RS, Ellersieck MR & Hyder SM 2010. Synthetic progestins induce growth and metastasis of BT-474 human breast cancer xenografts in nude mice. Menopause 17 1040–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, Asselin-Labat ML, Gyorki DE, Ward T, Partanen A, et al. 2009. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med 15 907–913. [DOI] [PubMed] [Google Scholar]
- Lydon JP, Ge G, Kittrell FS, Medina D & O’Malley BW 1999. Murine mammary gland carcinogenesis is critically dependent on progesterone receptor function. Cancer Res 59 4276–4284. [PubMed] [Google Scholar]
- Malzahn K, Mitze M, Thoenes M & Moll R 1998. Biological and prognostic significance of stratified epithelial cytokeratins in infiltrating ductal breast carcinomas. Virchows Arch 433 119–129. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. 1995. The nuclear receptor superfamily: the second decade. Cell 83 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinn O, Ward AV, Fettig LM, Riley D, Ivie J, Paul KV, Kabos P, Finlay-Schultz J & Sartorius CA 2020. Cytokeratin 5 alters beta-catenin dynamics in breast cancer cells. Oncogene 39 2478–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire WL 1973. Estrogen receptors in human breast cancer. J Clin Invest 52 73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire WL & Chamness GC 1973. Studies on the estrogen receptor in breast cancer. Adv Exp Med Biol 36 113–136. [DOI] [PubMed] [Google Scholar]
- McGuire WL, Chamness GC, Costlow ME & Shepherd RE 1974. Hormone dependence in breast cancer. Metabolism 23 75–100. [DOI] [PubMed] [Google Scholar]
- McGuire WL, Horwitz KB, Chamness GC & Zava DT 1976. A physiological role for estrogen and progesterone in breast cancer. J Steroid Biochem 7 875–882. [DOI] [PubMed] [Google Scholar]
- McGuire WL, Horwitz KB, Pearson OH & Segaloff A 1977. Current status of estrogen and progesterone receptors in breast cancer. Cancer 39 2934–2947. [DOI] [PubMed] [Google Scholar]
- McGuire WL, Clark GM, Dressler LG & Owens MA 1986. Role of steroid hormone receptors as prognostic factors in primary breast cancer. NCI Monogr 19–23. [PubMed] [Google Scholar]
- Meacham CE & Morrison SJ 2013. Tumour heterogeneity and cancer cell plasticity. Nature 501 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed H, Russell IA, Stark R, Rueda OM, Hickey TE, Tarulli GA, Serandour AA, Birrell SN, Bruna A, Saadi A, et al. 2015. Progesterone receptor modulates ERalpha action in breast cancer. Nature 523 313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir RD & Willis IM 2013. Regulation of pol III transcription by nutrient and stress signaling pathways. Biochim Biophys Acta 1829 361–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, et al. 2006. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 10 515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen QH, Pervolarakis N, Blake K, Ma D, Davis RT, James N, Phung AT, Willey E, Kumar R, Jabart E, et al. 2018. Profiling human breast epithelial cells using single cell RNA sequencing identifies cell diversity. Nat Commun 9 2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley BW, Sherman MR & Toft DO 1970. Progesterone “receptors” in the cytoplasm and nucleus of chick oviduct target tissue. Proc Natl Acad Sci U S A 67 501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogba N, Manning NG, Bliesner BS, Ambler SK, Haughian JM, Pinto MP, Jedlicka P, Joensuu K, Heikkila P & Horwitz KB 2014. Luminal breast cancer metastases and tumor arousal from dormancy are promoted by direct actions of estradiol and progesterone on the malignant cells. Breast Cancer Res 16 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CK 1998. Steroid hormone receptors in breast cancer management. Breast Cancer Res Treat 51 227–238. [DOI] [PubMed] [Google Scholar]
- Palmieri C, Patten DK, Januszewski A, Zucchini G & Howell SJ 2014. Breast cancer: current and future endocrine therapies. Mol Cell Endocrinol 382 695–723. [DOI] [PubMed] [Google Scholar]
- Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, Peto R, Pritchard KI, Bergh J, Dowsett M, et al. 2017. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. N Engl J Med 377 1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten DK, Corleone G, Gyorffy B, Perone Y, Slaven N, Barozzi I, Erdos E, Saiakhova A, Goddard K, Vingiani A, et al. 2018. Enhancer mapping uncovers phenotypic heterogeneity and evolution in patients with luminal breast cancer. Nat Med 24 1469–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al. 2000. Molecular portraits of human breast tumours. Nature 406 747–752. [DOI] [PubMed] [Google Scholar]
- Philibert D & Raynaud JP 1974. Binding of progesterone and R 5020, a highly potent progestin, to human endometrium and myometrium. Contraception 10 457–466. [DOI] [PubMed] [Google Scholar]
- Piette P 2018. The history of natural progesterone, the never-ending story. Climacteric 21 308–314. [DOI] [PubMed] [Google Scholar]
- Prat A & Perou CM 2011. Deconstructing the molecular portraits of breast cancer. Mol Oncol 5 5–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T & Clevers H 2005. Wnt signalling in stem cells and cancer. Nature 434 843–850. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF & Weissman IL 2001. Stem cells, cancer, and cancer stem cells. Nature 414 105–111. [DOI] [PubMed] [Google Scholar]
- Rojas PA, May M, Sequeira GR, Elia A, Alvarez M, Martinez P, Gonzalez P, Hewitt S, He X, Perou CM, et al. 2017. Progesterone Receptor Isoform Ratio: A Breast Cancer Prognostic and Predictive Factor for Antiprogestin Responsiveness. J Natl Cancer Inst 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs N, de Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, Balgobind AV, Wind K, Gracanin A, Begthel H, et al. 2018. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell 172 373–386 e310. [DOI] [PubMed] [Google Scholar]
- Santen RJ, Manni A, Harvey H & Redmond C 1990. Endocrine treatment of breast cancer in women. Endocr Rev 11 221–265. [DOI] [PubMed] [Google Scholar]
- Sato T, Tran TH, Peck AR, Girondo MA, Liu C, Goodman CR, Neilson LM, Freydin B, Chervoneva I, Hyslop T, et al. 2014. Prolactin suppresses a progestin-induced CK5-positive cell population in luminal breast cancer through inhibition of progestin-driven BCL6 expression. Oncogene 33 2215–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schettini F, Buono G, Trivedi MV, De Placido S, Arpino G & Giuliano M 2017. PI3K/mTOR Inhibitors in the Treatment of Luminal Breast Cancer. Why, When and to Whom? Breast Care (Basel) 12 290–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader WT & O’Malley BW 1978. Molecular structure and analysis of progesterone receptors. In Receptors and Hormone Action, pp 189–224: Academic Press. [Google Scholar]
- Schramek D, Leibbrandt A, Sigl V, Kenner L, Pospisilik JA, Lee HJ, Hanada R, Joshi PA, Aliprantis A, Glimcher L, et al. 2010. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature 468 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes BM, Alferez DG, Howell SJ & Clarke RB 2015. The role of steroid hormones in breast cancer stem cells. Endocr Relat Cancer 22 T177–186. [DOI] [PubMed] [Google Scholar]
- Singhal H, Greene ME, Tarulli G, Zarnke AL, Bourgo RJ, Laine M, Chang YF, Ma S, Dembo AG, Raj GV, et al. 2016. Genomic agonism and phenotypic antagonism between estrogen and progesterone receptors in breast cancer. Sci Adv 2 e1501924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaraman L, Conneely OM, Medina D & O’Malley BW 2001. p53 is a potential mediator of pregnancy and hormone-induced resistance to mammary carcinogenesis. Proc Natl Acad Sci U S A 98 12379–12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule HD, Vazguez J, Long A, Albert S & Brennan M 1973. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst 51 1409–1416. [DOI] [PubMed] [Google Scholar]
- Sullivan WP, Beito TG, Proper J, Krco CJ & Toft DO 1986. Preparation of monoclonal antibodies to the avian progesterone receptor. Endocrinology 119 1549–1557. [DOI] [PubMed] [Google Scholar]
- Takimoto GS, Tung L, Abdel-Hafiz H, Abel MG, Sartorius CA, Richer JK, Jacobsen BM, Bain DL & Horwitz KB 2003. Functional properties of the N-terminal region of progesterone receptors and their mechanistic relationship to structure. J Steroid Biochem Mol Biol 85 209–219. [DOI] [PubMed] [Google Scholar]
- Tanos T, Sflomos G, Echeverria PC, Ayyanan A, Gutierrez M, Delaloye JF, Raffoul W, Fiche M, Dougall W, Schneider P, et al. 2013. Progesterone/RANKL is a major regulatory axis in the human breast. Sci Transl Med 5 182ra155. [DOI] [PubMed] [Google Scholar]
- Truong TH, Dwyer AR, Diep CH, Hu H, Hagen KM & Lange CA 2019. Phosphorylated Progesterone Receptor Isoforms Mediate Opposing Stem Cell and Proliferative Breast Cancer Cell Fates. Endocrinology 160 430–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung L, Mohamed MK, Hoeffler JP, Takimoto GS & Horwitz KB 1993. Antagonist-occupied human progesterone B-receptors activate transcription without binding to progesterone response elements and are dominantly inhibited by A-receptors. Mol Endocrinol 7 1256–1265. [DOI] [PubMed] [Google Scholar]
- van de Rijn M, Perou CM, Tibshirani R, Haas P, Kallioniemi O, Kononen J, Torhorst J, Sauter G, Zuber M, Kochli OR, et al. 2002. Expression of cytokeratins 17 and 5 identifies a group of breast carcinomas with poor clinical outcome. Am J Pathol 161 1991–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villadsen R, Fridriksdottir AJ, Ronnov-Jessen L, Gudjonsson T, Rank F, LaBarge MA, Bissell MJ & Petersen OW 2007. Evidence for a stem cell hierarchy in the adult human breast. J Cell Biol 177 87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter KR, Goodman ML, Singhal H, Hall JA, Li T, Holloran SM, Trinca GM, Gibson KA, Jin VX, Greene GL, et al. 2017. Interferon-Stimulated Genes Are Transcriptionally Repressed by PR in Breast Cancer. Mol Cancer Res 15 1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen DX, Xu YF, Mais DE, Goldman ME & McDonnell DP 1994. The A and B isoforms of the human progesterone receptor operate through distinct signaling pathways within target cells. Mol Cell Biol 14 8356–8364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C 2012, September 27 A leading lady: Betty Ford’s candor about her breast cancer diagnosis helped bring a private issue out of the shadows,. In Cancer today magazine. [Google Scholar]
- Zheng ZY, Bay BH, Aw SE & Lin VC 2005. A novel antiestrogenic mechanism in progesterone receptor-transfected breast cancer cells. J Biol Chem 280 17480–17487. [DOI] [PubMed] [Google Scholar]