Abstract
Introduction
Obesity (body mass index [BMI] >35 kg/m2) remains a relative contraindication for kidney transplant, while patients after kidney transplantation (KTX) are predisposed to obesity. The present study aims to investigate the role of bariatric surgery in improving transplant candidacy in patients prior to KTX, as well its safety and efficacy in KTX patients postoperatively.
Methods
A systematic search was conducted up to March 2020. Both comparative and non-comparative studies investigating the role of bariatric surgery before or after KTX were considered. Outcomes included change in BMI, rates of mortality and complications, and the rate of patients who underwent KTX following bariatric surgery. Pooled estimates were calculated using the random effects meta-analysis of proportions.
Results
Twenty-one studies were eligible for final review; 11 studies investigated the role of bariatric surgery before KTX. The weighted mean BMI was 43.4 (5.7) kg/m2 at baseline and 33.9 (6.3) kg/m2 at 29.1 months followup. After bariatric surgery, 83% (95% confidence interval [CI] 57–99) were successfully listed for KTX and 83% (95% CI 65–97) of patients subsequently received successful KTX. Ten studies investigated the role of bariatric surgery after kidney transplant. Weighted mean baseline BMI was 43.8 (2.2) kg/m2 and mean BMI at 19.5 months followup was 34.2 (6.7) kg/m2. Overall, all-cause 30-day mortality was 0.5% for both those who underwent bariatric surgery before or after receiving a KTX. The results of this study are limited by the inclusion of only non-randomized studies, limited followup, and high heterogeneity.
Conclusions
Bariatric surgery may be safe and effective in reducing weight to improve KTX candidacy in patients with severe obesity and can also be used safely following KTX.
Introduction
The prevalence of obesity and its associated comorbidities, such as type 2 diabetes mellitus (T2DM), dyslipidemia, and hypertension, have been rising globally.1,2 Consequently, obesity and its associated comorbidities have been found to independently negatively affect kidney function.3,4 Considering this, the population is at higher risk for requiring transplantation; however, the effect of obesity on kidney transplant (KTX) outcomes is equivocal and controversial. Consensus guidelines released by the Canadian Society of Transplantation recommend that obesity alone should not be a contraindication for KTX, while also recommending that patients with obesity undergo a supervised weight loss program to achieve a target BMI <30 kg/m2 in order to mitigate the risks of transplantation.5
Numerous studies have demonstrated the negative effects of obesity on KTX outcomes, including significantly higher rates of delayed graft function.6,7 Obesity may also confer increased operative time, rates of acute graft rejection, rates of new-onset diabetes after transplant, and worse graft survival post-KTX.6–13 Thus, in the U.S., class II obesity or higher (body mass index [BMI] ≥35 kg/m2) is often used as a contraindication for KTX due to observed increased rates of perioperative and postoperative complications and poorer patient outcomes.14,15 Finally, obesity has been associated with increased all-cause mortality and cardiovascular disease-related deaths in the KTX population.6,16,17
In spite of these increased risks of graft failure, patients with obesity may still be evaluated for and subsequently undergo KTX, as the survival and quality of life benefits of KTX often outweigh the associated risks and the benefits of dialysis.15 Data from the United Network for Organ Sharing up to March 2011 demonstrated that 29.7% of 74 983 KTX recipients had obesity.18 Additionally, KTX predisposes the recipient to developing obesity owing to the chronic use of corticosteroids, alterations in leptin levels and appetite, and post-transplant lifestyle changes.19,20 The ensuing weight gain has been linked to poorer transplant outcomes, including graft rejection and increased mortality.4,19
Weight loss can improve glomerular filtration rate and proteinuria, resolve comorbidities, and reduce medication load.4,19 However, weight loss is slow and thus can be ineffective, especially in the kidney failure population.21,22 While bariatric surgery has been shown to be the most effective treatment for morbid obesity,22,23 it is only now starting to be recommended to patients with obesity who require transplantation and is not yet widely adopted as a treatment option for severe obesity in these populations.7,13,24 Differences exist in the literature on the utility of bariatric surgery to induce substantial weight loss prior to transplantation, resulting in improved suitability for KTX and the potential alleviated risks of high BMI on KTX outcomes.4,14 Finally, while bariatric surgery has been proven safe and effective, the benefits of bariatric surgery in the post-KTX patient have not been definitively shown.
In cases of chronic kidney disease and kidney failure, KTX offers longer survival and better quality of life compared to other treatment modalities, such as dialysis, and thus, it is paramount to optimize the accessibility to and outcomes of KTX in patients with and without obesity.25–30 The present study aimed to investigate the role of bariatric surgery in improving transplant candidacy in patients prior to KTX, as well as its safety and efficacy in KTX patients postoperatively.
Methods
Search strategy
We searched the following databases covering the period from database inception through March 2020: MEDLINE, EMBASE, CENTRAL, PubMed, and Web of Science. The search was designed and conducted by a medical librarian with input from study investigators. The search strategy included keywords relating to bariatric surgery and KTX. We also searched the references of published studies and searched grey literature manually to ensure that relevant articles were not missed. We did not discriminate full texts by language. This systematic review and meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).31
Outcomes
For studies that conducted bariatric surgery before KTX, primary outcomes were: 1) number of patients successfully listed for transplant after bariatric surgery; 2) number of patients that successfully received transplant; and 3) all-cause 30-day mortality after bariatric surgery. For studies that conducted bariatric surgery after transplant, the primary outcome was all-cause 30-day mortality after bariatric surgery.
Secondary outcomes for both types of studies were: 1) 30-day minor complication and 30-day major complications after bariatric surgery; 2) graft failure (i.e., return to dialysis) after bariatric surgery; 3) weight change after bariatric surgery; and 4) type remission of type 2 diabetes (i.e., discontinuing antidiabetic drugs or insulin) and/or hypertension (i.e., discontinuing antihypertensive drugs) after bariatric surgery as defined by individual study criteria. Major complications were classified as Clavien-Dindo complication classification grade III or above (conditions requiring surgical, radiological, and endoscopic intervention, organ dysfunction, or death) and minor complications were defined as conditions such as wound infections, diarrhea, and abdominal discomfort.32
Eligibility criteria and data abstraction
We included both non-comparative studies and comparative studies investigating the role of bariatric surgery before or after KTX in patients meeting standard criteria for bariatric surgery (BMI >40 or BMI >35 with obesity-related complications). Exclusion criteria were: 1) studies where patients did not meet standard criteria for bariatric surgery; 2) basic science, case reports, and review articles; 3) non-human studies; and 4) studies with no relevant outcome of interest.
Two reviewers independently screened the titles and abstracts of articles identified through the search strategy. Discrepancies in the title and abstract stage were resolved by automatic inclusion to ensure that all relevant articles were not missed. Discrepancies at the full-text or data abstraction stage were resolved by consensus between two reviewers, and if disagreement persisted, a third reviewer was consulted. Two reviewers independently extracted relevant data on patient characteristics and outcomes at the full-text level. Data was inputted on a standardized spreadsheet designed a priori. Disagreements between reviewers were also solved by consensus or by a third reviewer.
We extracted the following variables from each study: year of publication, study design, number of patients, type of bariatric procedure, characteristics of patients before and after bariatric surgery (gender, age, BMI, percent excess weight loss (%EWL), comorbidities of patients before and after bariatric surgery (e.g., type 2 diabetes, hypertension, dyslipidemia, obstructive sleep apnea), any reported complications, mortality after bariatric surgery, and KTX-related information (e.g., indications for KTX, time from bariatric surgery to KTX, complications). Patient comorbidities and anthropometric data were captured at multiple time points after bariatric surgery in the event the study reported on patients at multiple followup time intervals.
Statistical analysis
All statistical analysis and meta-analysis were performed on STATA, version 14 (StataCorp, College, TX, U.S.) and Cochrane Review Manager 5.3 (London, U.K.). The threshold for statistical significance was set a priori at alpha=0.05. The pooled proportion of KTX outcomes before and after bariatric surgery was calculated using the Freeman-Tukey double arcsine transformation of proportions. DerSimonian and Laird random effects meta-analysis of proportions was used to generate the overall effect size of each outcome. We performed pairwise meta-analyses using a DerSimonian and Laird random effects model for continuous variables before and after bariatric surgery. Pooled effect estimates for continuous variables were obtained by calculating the mean difference, and dichotomous variables were obtained by calculating risk ratios along with their respective 95% confidence intervals (CI) to confirm the effect size estimation. Assessment of heterogeneity was completed using the inconsistency (I2) statistic. We considered I2 higher than 50% to represent considerable heterogeneity.30 Herein, pooled means are reported with standard deviation unless otherwise specified.
Quality assessment of included studies
Risk of bias assessment for individual studies was conducted using the Methodological Index for Non-Randomized Studies (MINORS) tool.33 Two reviewers independently graded the articles based on the 12-point framework. Discrepancies between reviewers were resolved by consensus and a discussion with a third reviewer if discrepancies persisted.
Results
Study characteristics
In total, initial query of electronic databases yielded 3155 potentially eligible studies. Included studies were divided into two groups based on bariatric surgery timing with respect to KTX: bariatric surgery before KTX and bariatric surgery after KTX. Based on study inclusion and exclusion criteria, 11 studies34–44 were included for the subgroup of bariatric surgery before KTX and 10 studies4,14,15,20,36,45–49 were included for bariatric surgery after KTX (Fig. 1). The characteristics of the included studies are shown in Table 1.
Fig. 1.

PRISMA flow diagram.
Table 1.
Characteristics of studies with patients that underwent bariatric surgery before and after kidney transplantation
| Study | Country | Study design | Type of bariatric surgery | n | % F | Age (SD) | FU (mo, SD) | Pre-bariatric Sx BMI, kg/m2 (SD) | Post-bariatric Sx BMI, kg/m2 (SD) | % EWL (SD) |
|---|---|---|---|---|---|---|---|---|---|---|
| Before kidney transplant | ||||||||||
| Bouchard, 2019 | Canada | Retrospective cohort | LSG | 32 | 44 | 51 (11) | 14 | 42.3 (5.2) | 31 (9) | 56 (27) |
| Yemini, 2019 | Israel | Retrospective cohort | LSG, n=17 RYGB, n=7 |
24 | 33 | 54 (3.1) | 47 (6.5) | 41.5 (0.79) | 29 (1.3) | 66 (6.3) |
| Cohen, 2019 | U.S. | Retrospective cohort | RYGB, n=27 SG, n=9 LAGB, n=6 Unknown, n=1 |
43 | 41 | 50 | 60 | 43 | 36 | 69 |
| Thomas, 2018 | U.S. | Retrospective cohort | RYGB | 31 | 54.8 | 45 (2.2) | – | 43.5 (0.7) | 28.1 | 72.8 (3) |
| Kim, 2020 | U.S. | Retrospective cohort | LSG | 41 | 46.3 | 56 (9.3) | 22.2 (16.4) | 41.1 (4.6) | 32.1 (4.7) | – |
| Newcombe, 2005 | Australia | Case series | LAGB | 3 | 0 | 44 | 16.3 (8.3) | 44.6 | 33.7 (4.95) | – |
| Koshy, 2008 | U.S. | Case series | LAGB | 3 | 33 | 41 | 13.6 (1.24) | 40.1 | 34.1 (2.76) | 37 |
| Kassam, 2019 | U.S. | Retrospective cohort | LSG | 243 | 58 | 54 (11.1) | 28.3 (18.3) | 44 (6.3) | 36.7 (6.6) | 38.2 (20.3) |
| Al-Bahri, 2017 | U.S. | Case series | LSG, n=1 RYGB, n=12, LAGB, n=3 |
16 | 62.5 | 55 (7) | 48 (36) | 48 (8) | 31.7 (7) | 62 (24) |
| Kienzl-Wagner, 2017 | Austria | Case series | LSG | 8 | 62 | 48 | 38.4 (16.8) | 38 (3.8) | 1yr=30.4 (10.7) 2yr=30.7 (4.4) 3yr=30.7 (6.0) |
– |
| Carandina, 2017 | France | Case series | LSG | 9 | 88.9 | 53.2 | 15.6 | 45.9 | 6mo=36.2 18mo=28.3 |
73.2 |
| After kidney transplant | ||||||||||
| Cohen, 2019 | U.S. | Retrospective cohort | RYGB, n=6 SG, n=10 LAGB, n=4 VBG, n=1 |
21 | 70 | 41 | 60 | 41 | 32 | 71 |
| Viscido, 2018 | Argentina | Case series | SG | 5 | 80 | - | 16.8 (14.5) | 42.18 (6.8) | 29.8 (7.3) | 58.0 (11.8) |
| Schindel, 2019 | Israel | Case-control | LSG, n=19 RYGB, n=10 BPD-DS, n=1 |
30 | 36.7 | 55.7 (10.7) | 7.2 (3.7) | 41.3 (3.7) | 29.5 (4.7) | 71.6 (28.7) |
| Gazzetta, 2017 | Italy | Case series | LSG | 6 | 33.3 | 50.3 (8.07) | 1 | 39.8 (5.29) | – | 27.6 |
| 3 | – | – | 44.1 | |||||||
| 6 | – | – | 74.2 | |||||||
| 12 | – | – | 75.9 | |||||||
| Gheith, 2017 | Kuwait | Prospective cohort study | - | 22 | 36.4 | 40.6 (12.3) | 6 | 38.5 (9.1) | 34.3 (7.6) | – |
| Golomb, 2014 | Israel | Case series | LSG | 10 | 40 | 55.3 (7.3) | 3 | 41.6 (596–152) | 33.0 | – |
| 6 | – | 31.2 (29.1–46.8) | 57.0 | |||||||
| 12 | – | 29.1 (28.1–48.4) | 75.5 | |||||||
| Szomstein, 2010 | U.S. | Case series | LSG, n=1 RYGB, n=4 |
5 | 100 | 39 (7.5) | 23 (2.53) | 52.2 (9.47) | 32.2 (2.99) | >50 |
| Arias, 2010 | Colombia | Case series | RYGB | 5 | 40 | 55.2 (10.6) | 26.4 (1.17) | 40.2 (1.47) | 29.2 (2.71) | – |
| Alexander, 2007 | U.S. | Case series | RYGB | 10 | – | 44 | 12 | 47.3 (6.8) | 32.3 (5.5) | – |
| Modanlou, 2009 | U.S. | Retrospective cohort | RYGB SL, n=50 RYGB SIR, n=20 VSG, n=10 GR, n=6 BPD-DS, n=1 |
87 | 59.8 | 45.2 (11.3) | 12 | 46.6 (4.6) | 40.2 (7.8) | 30.8 (8.7–48.3) |
BMI: body mass index; BPD-DS: biliopancreatic diversion with duodenal switch; ESRD: end-stage renal disease; F: female; FU: followup; GR: gastric restrictive; KTX: kidney transplant; LAGB: laparoscopic adjustable gastric band; LSG: laparoscopic sleeve gastrectomy; NIH: National Institute of Health; RYGB: Roux-en-Y gastric bypass; SD: standard deviation; SIR: small intestine reconstruction; SL: short limb; VBG: vertical banded gastroplasty.
Of the 11 studies examining bariatric surgery before KTX, six were retrospective cohort studies and five were case series. In total, there were 453 patients that received bariatric surgery before KTX. The mean age was 50.1 (5.1) years; 54.4% of this cohort was female. The included studies were conducted from 2005–2019, with a mean followup period of 30.3 (range 13.6–60) months after bariatric surgery. Indications for KTX were reported in 8/11 studies; common indications were diabetic nephropathy (n=65, 37.1%) and hypertension (n=23,13.4%) (Table 2). All 13 indications are listed in Table 2. Bariatric surgeries conducted in the studies were sleeve gastrectomy (SG; eight studies), laparoscopic adjustable gastric band (LAGB; four studies), and Roux-en-Y gastric bypass (RYGB; four studies) (number of patients in each surgery group can be found in Table 1).
Table 2.
Transplant indications and outcomes of patients that underwent bariatric surgery before kidney transplantation
| Author | n | Kidney transplant indications | Type of donor kidney | Successfully listed for transplant, n (%) | Successfully received transplant, n (%) | Time from Bari Sx to KTX in Mo (SD) | Graft survival 1 year after transplant, n (%) | Graft survival 5 years after transplant, n (%) |
|---|---|---|---|---|---|---|---|---|
| Bouchard, 2019 | 32 | - | - | 20 (63%) | 14 (44%) | 8 (12) | 14 (100%) | 14 (100%) |
| Yemini, 2019 | 24 | DM: 15 FSGS: 2 PCKD: 3 HUS: 1 Unknown: 3 |
DD: 5 (31.2%) LD: 11 (68.8%) |
21 (87.5%) | 16 (76.2%) | 18 | 16 (100%) | 16 (100%) |
| Cohen, 2019 | 43 | DM: 10 (23%) HTN: 5 (12%) Cystic disease: 5 (12%) Glomerular Disease: 2 (5%) Other: 21 (48%) |
DD: 25 (61.0%) LD: 16 (39.0%) |
41 (95.3%) | 41 (100%) | 72 | 41 (100%) | – |
| Thomas, 2018 | 31 | DM: 7 (50.0%) HTN: 3 (21.4%) Other: 4 (28.6%) |
DD: 13 (92.9%) LD: 1 (7.1%) |
25 (80.6%) | 14 (45.2%) | 33 | 10 (71.4%) | – |
| Kim, 2020 | 41 | DM: 22 (53%) HTN: 11 (27%) PCKD: 3 (7.3%) Other: 5 (12%) |
DD: 25 (61%) LD: 16 (39%) |
41 (100%) | 41 (100%) | 21.3 (16.4) | 40 (97.6%) | – |
| Newcombe, 2005 | 3 | – | – | 3 (100%) | 3 (100%) | 16 | – | – |
| Koshy, 2008 | 3 | DM: 2 (66.7%) FSGS: 1 (3.33%) |
DD: 1 (33%) LD: 2 (66%) |
3 (100%) | 3 (100%) | 13.5 | – | – |
| Kassam, 2019 | 243 | – | DD: 30 (66.7%) LD: 15 (33.3%) |
71 (29.2%) | 45 (63.4%) | 22.8 (15.6) | – | – |
| Al-Bahri, 2017 | 16 | FSGS: 1 (25%) IgA nephropathy: 1 (25%) DM: 1 (25%) HTN: 1 (25%) |
DD: 3 (75%) LD: 1 (25%) |
9 (56.3%) | 4 (25%) | 25 (14) | 4 (100%) | – |
| Kienzl-Wagner, 2017 | 8 | DM: 3 (37.5%) IgA nephropathy: 1 (12.5%) Analgesic Nephropathy: 1 (12.5%) Glomerulonephritis: 1 (12.5%) Reflux Nephritis: 1 (12.5%) Nephropathy: 1 (12.5%) Preeclampsia: 1 (12.5%) |
DD: 7 (100%) | 8 (100%) | 7 (87.5%) | 17 | 6 (87.5%) | – |
| Carandina, 2017 | 9 | DM: 5 (55.6%) HTN: 3 (33.3%) FSGS: 1 (11.1%) |
- | 5 (55.5%) | 1 (11.1%) | 21 | - | - |
ATN: acute tubular necrosis; DD: deceased donor; DM: diabetes mellitus; DNR: date not reported; FSGS: focal segmental glomerulosclerosis; HTN: hypertension; HUS: hemolytic uremic syndrome; KTX: kidney transplant; LAGB: laparoscopic adjustable gastric band; LD: living donor; LSG: laparoscopic sleeve gastrectomy; PCKD: polycystic kidney disease; RPGN: rapidly progressive glomerulonephritis; RYGB: Roux-en-Y gastric bypass; SD: standard deviation.
Of the 10 studies examining bariatric surgery after KTX, there were six case series studies, two retrospective cohort studies, one case-control, and one prospective cohort study. These 10 studies include a total of 201 patients who received bariatric surgery after transplant, of whom 53.9% were female. The mean age of this cohort was 47.4 (10.8) years. The analyzed studies were all conducted from 2007–2019, with a mean followup period of 16.4 (range 1–60) months. In the included studies, indications for transplant were varied. Common indications were diabetic nephropathy (n=36, 17.9%), glomerulonephritis (n=17, 8.5%), and hypertension (n=13, 6.5%). Other indications are listed in Table 3. Bariatric surgeries conducted in the studies were LSG (seven studies), RYGB (six studies), biliopancreatic diversion and duodenal switch (BPD-DS; two studies), laparoscopic adjustable gastric banding (one study), vertical banded gastroplasty (one study), and gastric restrictive surgery (GR; one study) (Table 1).
Table 3.
Transplant indications and outcomes of patients that underwent bariatric surgery after kidney transplantation
| Author | n | Kidney transplant indications | Type of kidney transplant | Time from KTX to bariatric surgery, years (SD) | Graft failure or return to dialysis/rejection after bariatric surgery (n, %) |
|---|---|---|---|---|---|
| Cohen, 2019 | 21 | DM, n=6 (28.6%) HTN, n=4 (19.0%) Glomerular disease, n=4 (19.0%) Other, n=7 (33.3%) |
DD, n=14 (66.7%) LD, n =7 (33.3%) |
5 | 7 (33%) |
| Viscido, 2018 | 5 | T1DM, n=1 (20.0%) HTN, n=2 (40.0%) Congenital defects, n=1(20.0%) SLE, n=1 (20.0%) Glomerulonephritis, n=1 (20.0%) |
Unknown, n=5 (100%) | 15 (4.8) | - |
| Schindel, 2019 | 30 | T2DM, n=8 (26.7%) HTN, n=1 (3.3%) T2DM + HTN, n=4 (13.3%) Glomerulonephritis, n=4 (13.3%) Pyruvate kinase deficiency, n=1 (3.3%) Other, n=12 (40%) |
LD, n=16 (53.3%) Unknown, n=14 (46.7%) |
4.6 (3.1) | 1 (3.3%) |
| Gazzetta, 2017 | 6 | T2DM, n=3 (50.0%) IgA nephritis, n=1 (16.7%) Idiopathic, n=1 (16.7%) Obstructive congenital disorder, n=1 (16.7%) |
Unknown, n=6 (100%) | 7.6 | 0 (0%) |
| Gheith, 2017 | 22 | Glomerulonephritis, n=8 (36.4%) DM, n=5 (22.7%) HTN, n=1 (4.5%) Other, n=8 (36.4%) |
Living related, n=6 (27.2%) Living unrelated, n=12 (54.5%) DD, n=4 (18.2%) |
– | 1 (4.5%) |
| Golomb, 2014 | 10 | IgA nephropathy, n=1 (10.0%) DM, n=4 (40.0%) Chronic interstitial nephritis, n=1 (10.0%) Glomerulonephritis, n=1 (10.0%) Dicophenac sodium overdose, n=1(10.0%) DM + HTN, n=1 (10.0%) Unknown, n=1 (10.0%) |
DD, n=4 (40%) LD, n=6 (60%) |
5.27 (2.26) | 0 (0%) |
| Szomstein, 2010 | 5 | PCKD, n=1 (20.0%) DM, n=2 (40.0%) Glomerulonephritis, n=1 (20.0%) CKD, n=1 (20.0%) |
DD, n=1 (20%) LD, n=1 (20%) Unknown, n=3 (60%) |
– | 0 (0%) |
| Arias, 2010 | 5 | DM, n=2 (40.0%) Glomerulonephritis, n=2 (40.0%) Unknown, n=1 (20.0%) |
Unknown, n=5 (100%) | – | 0 (0%) |
| Alexander, 2007 | 10 | – | Unknown, n=10 (100%) | 5.3 | – |
| Modanlou, 2009 | 87 | – | Unknown, n=87 (100%) | 4.3 (2.6) | 1 (1.1%) |
DD: deceased donor; DM: diabetes mellitus; HTN: hypertension; KTX: kidney transplant; LAGB: laparoscopic adjustable gastric band; LD: living donor; LSG: laparoscopic sleeve gastrectomy; PCKD: polycystic kidney disease; RYGB: Roux-en-Y gastric bypass.
Bariatric surgery before transplant
In the studies investigating bariatric surgery prior to KTX, the mean age of the patients at the time of surgery was 52.7 years. The weighted mean BMI at baseline was 43.4 (5.7) kg/m2; at a weighted mean 29.1 months followup, weighted mean BMI was 33.9 (6.3) kg/m2, with an absolute percent reduction of 21.9% after surgery. The 30-day mortality after bariatric surgery was 0.2% (n=1). In total, 83% (95% CI 57–99%; 11 studies, I2=95%) of patients were successfully listed for KTX after bariatric surgery (Fig. 2). Of the 247 patients listed for transplant, 83% (95% CI 65–97%; 11 studies, I2=88%) of these patients successfully received a transplant (Fig. 3). The mean time between bariatric surgery to KTX was 24.3 (14.5) months. Deceased donor transplants accounted for 63.7% of transplanted kidneys. Subgroup analysis by donor type was not possible, as included studies did not report the outcomes separately.
Fig. 2.

Patients listed for kidney transplant.
Fig. 3.
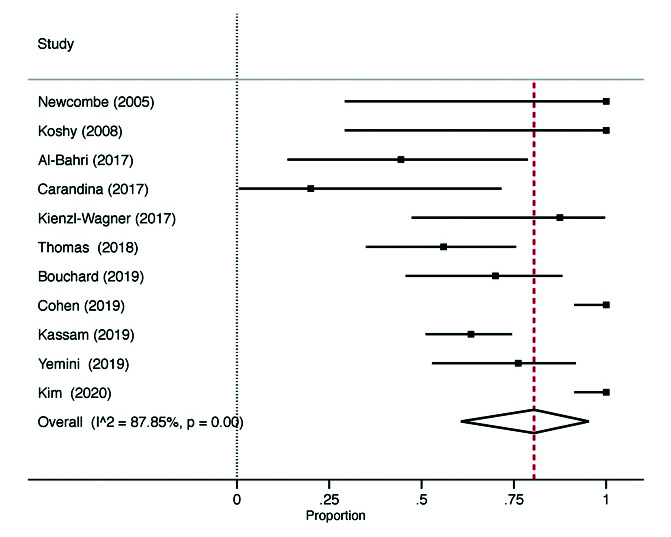
Patients with successful kidney transplant.
The most common baseline comorbidities were T2DM and hypertension (Supplementary Table 1; available at cuaj.ca). Overall, there was 56% (95% CI 38–74%) improvement or remission of T2DM after bariatric surgery (six studies, I2=62%). Improvement or remission of hypertension was noted in 46% (95% CI 33–59%) of the patients (six studies, I2=55%). There was one case of 30-day mortality; this was due to sleeve staple line leakage on postoperative day 21.35 Minor complications within 30 days of bariatric surgery occurred in seven patients (1.8%), and major complications occurring within 30 days happened in six patients (1.6%). Complications occurring more than 30 days following bariatric surgery were reported in 10 of the studies; overall, three complications occurred (0.71% of the patients who underwent bariatric surgery). These complications were mild gastritis,40 mild gastroesophageal reflux,35 and late dumping syndrome.40 The one-year graft survival rate was 98% (95% CI 91–100%; seven studies, I2=55%). Graft survival beyond one year was included in three studies, and was 100% in these studies.
Bariatric surgery after transplant
In the studies examining bariatric surgery after KTX, the mean time between KTX and bariatric surgery was 80.4 (38.4) months. At the time of surgery, the weighted mean age was 47.4 years. At baseline, the weighted mean BMI was 43.8 (2.2) kg/m2; at an average 19.5 months post-bariatric surgery, the weighted mean BMI was 34.2 (6.7) kg/m2, with an absolute percent reduction of 21.9% after surgery. All-cause 30-day mortality after bariatric surgery was 1.9% (n=2) across all included studies. Two patients (3.1%) sustained minor complications within 30 days of bariatric surgery (acute renal failure and wound infection) and one patient (1.6%) sustained a major complication (sleeve stricture requiring balloon dilatation). Complication rates at greater than 30 days post-bariatric surgery were included in 6/10 studies; two were reported. Alexander et al49 reported one death at 120 days following bariatric surgery related to cardiac causes, and Schindel et al reported one death from a bariatric surgery complication, though neither a date nor cause are listed.48 For the purposes of this study, it is classified as a complication after 30 days.
Graft failure was reported by 8/10 studies, with 11 patients out of 186 patients (5.9%) experiencing graft rejection or return to dialysis. Living donor transplants accounted for 23.9% of transplanted kidneys, deceased donor transplants accounted for 55.9%, and the remainder were not reported or unknown. Subgroup analysis by donor type was not possible, as studies did not report the outcomes separately.
T2DM and hypertension were the most identified comorbidities (Supplementary Table 1; available at cuaj.ca). Patients who received bariatric surgery after KTX had 38.9% (95% CI 15–100%; five studies, I2=87%) remission or improvement of T2DM after surgery. Hypertension improved or resolved in 45.2% of the patients (95% CI 19–100%; four studies, I2=90%) after bariatric surgery.
Quality assessment of studies
The mean MINORS score was 9.7 (0.99) for the 14 non-comparative studies and 17.7 (2.12) for the six comparative studies, representing fair quality of evidence.33 The MINORS result for individual studies are presented in Supplementary Table 2 (available at cuaj.ca). In summary, 15/20 (75.0%) studies included a clearly stated aim, 15/20 studies had prospectively collected the data (75.0%), all had followup time periods appropriate to the study (20/20, 100%), and all studies but one included consecutive patients (19/20, 95.0%). Only 12/20 (60.0%) studies reported adequate endpoints appropriate to the aim of the study. No study had unbiased assessment of the endpoints (0/20, 0%). Loss to followup less than 5% was both reported and achieved in 13/20 studies, reported but greater than 5% in 3/18 studies; loss to followup was not reported in 4/20 studies. For comparative studies, all included an adequate control group that received an optimal comparator intervention; 3/6 (50%) appropriately matched groups for a sufficient number of confounding variables. A single study (1/6, 16.7%) included a prospective calculation of sample size.20
Discussion
The present study showed that bariatric surgery is safe in patients with obesity and kidney failure prior to KTX, and safe in patients with obesity and a past history of KTX (30-day mortality rate after bariatric surgery being 0% for both groups). Additionally, the study demonstrated that bariatric surgery is effective in producing substantial weight loss that improves candidacy for KTX, as demonstrated by 83% of post-bariatric surgery patients qualifying for KTX, and 69% of these patients receiving bariatric surgery ultimately received KTX. This is a marked increase in KTX, considering that the United Network for Organ Sharing listed that only 29.7% of KTX recipients have any degree of obesity.18 Bariatric surgery is also effective in reducing prevalence of common comorbidities, such as T2DM, hypertension, and dyslipidemia, thereby reducing the comorbidity load and optimizing patients before KTX. Therefore, it appears that bariatric surgery before KTX may increase KTX candidacy and improve outcomes, and that previous KTX should not be a contraindication to bariatric surgery. Overall, bariatric surgery provides the same substantial weight loss and benefits to KTX candidates and recipients as it does in the non-obese, non-bariatric surgical population.
Obesity actually confers a paradoxical survival benefit in patients receiving dialysis, despite deleterious effects on eventual KTX outcomes.50–53 The “obesity paradox” creates a challenge in managing obese kidney failure patients on dialysis who need to undergo weight reduction prior to being listed for transplant candidacy. The present study demonstrates a benefit of bariatric surgery in reducing wait and improving candidacy for KTX through decreased BMI, with most bariatric surgery recipients being listed for KTX. Thus, bariatric surgery may provide a more expeditious route to weight reduction, as well as time to KTX, in a population that may otherwise suffer from more longitudinal weight reduction efforts while on dialysis.
Graft survival can be increased beyond solely reducing one’s BMI by reducing the prevalence of comorbidities in obese patients with kidney failure. New-onset or existing DM persisting into the post-transplant period is strongly associated with poorer post-transplant graft and patient outcomes.54–56 The present study found that bariatric surgery is effective in reducing comorbidities in both pre-KTX and post-KTX patients, with 64% of patients with T2DM before bariatric surgery experiencing improvement or remission of T2DM, and 72% of patients who received bariatric surgery after KTX experiencing improvement or remission of their T2DM. Hence, bariatric surgery can play an important role in optimizing patients pre-transplant, as well as decreasing the rate of post-transplant diabetes and subsequent effects on graft function.
Inappropriate weight, including obesity, is responsible for 6% of the inactive status patients awaiting KTX in the U.S.; this is the fourth leading cause of inactivation in those previously listed for KTX.57 In addition, studies have demonstrated that patients with obesity who qualify for the active status list are less likely to receive a transplant than non-obese counterparts; in fact, the degree of obesity was directly related to likelihood of being overlooked for KTX.58,59 This study supports bariatric surgery as a successful tool for increasing access to KTX in the end-stage renal disease patient with obesity and for reducing comorbidity load in the patient post-KTX.
Our pooled estimates suggest that bariatric surgery can be performed safely in the pre-KTX and post-KTX patient as a weight-reduction intervention and as a tool to reduce comorbidity load. Hence, it may serve as an important intervention in this population to confer a survival benefit and augment graft outcomes, and should be appropriately presented to eligible patients. To our knowledge, this is the first systematic review and meta-analysis on the impact of bariatric surgery on KTX outcomes.
The findings in this study should be interpreted in light of the following limitations. First, all studies were observational or non-randomized control studies, leading to a low certainty of evidence in all outcomes. No randomized controlled trials examining the effect of bariatric surgery on pre-KTX and post-KTX outcomes were found in the literature. Heterogeneity between included studies was high (>50%) for many outcomes. Although we conducted sensitivity analyses to address this heterogeneity, our present results failed to explain why heterogeneity was present across pooled effect estimates. A source of significant heterogeneity was the wide range of followup time points across included studies. This highlights the need to create a standard set of patient-level markers and outcomes with clearly defined time intervals if the effect of bariatric surgery on KTX is to be explored further. In addition, there is a need for comprehensive reporting in the bariatric surgery population with data stratified by type of surgery patients received and indications for surgery, which limited comparisons in this review. Moreover, conducting a subgroup analysis by donor type (e.g., living vs. deceased donors) was not possible, and this may have impacted the graft failure outcomes post-KTX.60 Finally, there was no analysis of long-term followup for those receiving bariatric surgery pre-KTX to determine if weight loss was sustained in the post-transplant period in the long-term and whether outcomes were impacted. Ultimately, more robust evidence that directly compares KTX outcomes in bariatric surgery and non-bariatric surgery groups are needed before definitive conclusions can be made.
Conclusions
The present study demonstrates that bariatric surgery is a safe and effective intervention to reduce weight and comorbidities in both the pre-KTX and post-KTX patient. As demonstrated by the heterogeneity of our findings, there is no universally accepted BMI cutoff or time interval relative to KTX to warrant intervention with bariatric surgery in the pre-KTX and post-KTX patients. Research into optimal timing and criteria for bariatric surgery in this population should be considered in future studies.
Supplementary Information
Footnotes
Appendix available at cuaj.ca
Competing interests: The authors do not report any competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.WHO. Obesity and overweight: Fact sheet. WHO Media Cent; [Accessed March 18, 2021]. Modified June 9, 2021, Available at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. [Google Scholar]
- 2.Mendis S, Armstrong T, Bettcher D, et al. Global status report on noncommunicable diseases 2014. World Health Organisation; [Accessed on March 18, 2021]. Available at: http://apps.who.int/iris/bitstream/10665/148114/1/9789241564854_eng.pdf. [Google Scholar]
- 3.Bolignano D, Zoccali C. Effects of weight loss on renal function in obese CKD patients: A systematic review. Nephrol Dial Transplant. 2013;28:iv82–98. doi: 10.1093/ndt/gft302. [DOI] [PubMed] [Google Scholar]
- 4.Viscido G, Gorodner V, Signorini FJ, et al. Sleeve gastrectomy after renal transplantation. Obes Surg. 2018;28:1587–94. doi: 10.1007/s11695-017-3056-0. [DOI] [PubMed] [Google Scholar]
- 5.Knoll G, Cockfield S, Blydt-Hansen T, et al. Canadian Society of Transplantation consensus guidelines on eligibility for kidney transplantation. CMAJ. 2005;173:1181–4. doi: 10.1503/cmaj.051291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicoletto BB, Fonseca NKO, Manfro RC, et al. Effects of obesity on kidney transplantation outcomes: A systematic review and meta-analysis. Transplantation. 2014;98:167–76. doi: 10.1097/TP.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 7.Lafranca JA, IJermans JNM, Betjes MGH, et al. Body mass index and outcome in renal transplant recipients: A systematic review and meta-analysis. BMC Med. 2015;13:141. doi: 10.1186/s12916-015-0340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gore JL, Pham PT, Danovitch GM, et al. Obesity and outcome following renal transplantation. Am J Transplant. 2006;6:357–63. doi: 10.1111/j.1600-6143.2005.01198.x. [DOI] [PubMed] [Google Scholar]
- 9.Singh D, Lawen J, Alkhudair W. Does pretransplant obesity affect the outcome in kidney transplant recipients? Transplant Proc. 2005;37:717–20. doi: 10.1016/j.transproceed.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 10.Zrim S, Furlong T, Grace BS, et al. Body mass index and postoperative complications in kidney transplant recipients. Nephrology. 2012;17:582–7. doi: 10.1111/j.1440-1797.2012.01621.x. [DOI] [PubMed] [Google Scholar]
- 11.Furriel F, Parada B, Campos L, et al. Pre-transplantation overweight and obesity: Does it really affect kidney transplantation outcomes? Transplant Proc. 2011;43:95–9. doi: 10.1016/j.transproceed.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 12.Udgiri NR, Kashyap R, Minz M. The impact of body mass index on renal transplant outcomes: A significant independent risk factor for graft failure and patient death. Transplantation. 2003;75:249. doi: 10.1097/01.TP.0000043936.02412.23. [DOI] [PubMed] [Google Scholar]
- 13.Tran M-H, Foster CE, Kalantar-Zadeh K, et al. Kidney transplantation in obese patients. World J Transplant. 2016;6:135–43. doi: 10.5500/wjt.v5.i4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Modanlou KA, Muthyala U, Xiao H, et al. Bariatric surgery among kidney transplant candidates and recipients: Analysis of the United States renal data system and literature review. Transplantation. 2009;87:1167–73. doi: 10.1097/TP.0b013e31819e3f14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gazzetta PG, Bissolati M, Saibene A, et al. Bariatric surgery to target obesity in the renal transplant population: Preliminary experience in a single center. Transplant Proc. 2017;49:646–9. doi: 10.1016/j.transproceed.2017.02.032. [DOI] [PubMed] [Google Scholar]
- 16.Abbott KC, Glanton CW, Trespalacios FC, et al. Body mass index, dialysis modality, and survival: Analysis of the United States renal data system dialysis morbidity and mortality WAVE II study. Kidney Int. 2004;65:597–605. doi: 10.1111/j.1523-1755.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 17.Ahmadi SF, Zahmatkesh G, Streja E, et al. Body mass index and mortality in kidney transplant recipients: A systematic review and meta-analysis. Am J Nephrol. 2014;40:315–24. doi: 10.1159/000367812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cannon RM, Jones CM, Hughes MG, et al. The impact of recipient obesity on outcomes after renal transplantation. Ann Surg. 2013;257:978–84. doi: 10.1097/SLA.0b013e318275a6cb. [DOI] [PubMed] [Google Scholar]
- 19.Cashion AK, Hathaway DK, Stanfill A, et al. Pre-transplant predictors of one-year weight gain after kidney transplantation. Clin Transplant. 2014;28:1271–8. doi: 10.1111/ctr.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gheith O, Al-Otaibi T, Halim MA, et al. Bariatric surgery in renal transplant patients. Exp Clin Transplant. 2017;15:164–9. doi: 10.6002/ect.mesot2016.P35. [DOI] [PubMed] [Google Scholar]
- 21.Navaneethan SD, Yehnert H, Moustarah F, et al. Weight loss interventions in chronic kidney disease: A systematic review and meta-analysis. Clin J Am Soc Nephrol. 2009;4:1565–74. doi: 10.2215/CJN.02250409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of cardiology/American Heart Association task force on practice guidelines and the obesity society. Circulation. 2014;129:S102–38. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colquitt JL, Pickett K, Loveman E, et al. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;(8):CD003641. doi: 10.1002/14651858.CD003641.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai R, Wu M, Xing Y. Pretransplant metabolic syndrome and its components predict post-transplantation diabetes mellitus in Chinese patients receiving a first renal transplant. Ther Clin Risk Manag. 2019;15:497–503. doi: 10.2147/TCRM.S190185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–30. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 26.Rabbat CG, Thorpe KE, Russell JD, et al. Comparison of mortality risk for dialysis patients and cadaveric first renal transplant recipients in Ontario, Canada. J Am Soc Nephrol. 2000;11:917–22. doi: 10.1681/ASN.V115917. [DOI] [PubMed] [Google Scholar]
- 27.Meier-Kriesche HU, Ojo AO, Port FK, et al. Survival improvement among patients with end-stage renal disease: Trends over time for transplant recipients and wait-listed patients. J Am Soc Nephrol. 2001;12:1293–6. doi: 10.1681/ASN.V1261293. [DOI] [PubMed] [Google Scholar]
- 28.Oniscu GC, Brown H, Forsythe JLR. Impact of cadaveric renal transplantation on survival in patients listed for transplantation. J Am Soc Nephrol. 2005;16:1859–65. doi: 10.1681/ASN.2004121092. [DOI] [PubMed] [Google Scholar]
- 29.Port FK, Wolfe RA, Mauger EA, et al. Comparison of survival probabilities for dialysis patients vs. cadaveric renal transplant recipients. JAMA J Am Med Assoc. 1993;270:1339–43. doi: 10.1001/jama.1993.03510110079036. [DOI] [PubMed] [Google Scholar]
- 30.Snyder JJ, Kasiske BL, Gilbertson DT, et al. A comparison of transplant outcomes in peritoneal and hemodialysis patients. Kidney Int. 2002;62:1423–30. doi: 10.1111/j.1523-1755.2002.kid563.x. [DOI] [PubMed] [Google Scholar]
- 31.Moher D, Liberati A, Tetzlaff J, et al. The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Ann Intern Med. 2009;151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 32.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (Minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 34.Bouchard P, Tchervenkov J, Demyttenaere S, et al. Safety and efficacy of the sleeve gastrectomy as a strategy towards kidney transplantation. Surg Endosc. 2020;34:2657–64. doi: 10.1007/s00464-019-07042-z. [DOI] [PubMed] [Google Scholar]
- 35.Yemini R, Nesher E, Carmeli I, et al. Bariatric surgery is efficacious and improves access to transplantation for morbidly obese renal transplant candidates. Obes Surg. 2019;29:2373–80. doi: 10.1007/s11695-019-03925-1. [DOI] [PubMed] [Google Scholar]
- 36.Cohen JB, Tewksbury CM, Torres Landa S, et al. National postoperative bariatric surgery outcomes in patients with chronic kidney disease and end-stage kidney disease. Obes Surg. 2019;29:975–82. doi: 10.1007/s11695-018-3604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas IA, Gaynor JJ, Joseph T, et al. Roux-en-Y gastric bypass is an effective bridge to kidney transplantation: Results from a single center. Clin Transplant. 2018;32:e13232. doi: 10.1111/ctr.13232. [DOI] [PubMed] [Google Scholar]
- 38.Newcombe V, Blanch A, Slater GH, et al. Laparoscopic adjustable gastric banding prior to renal transplantation. Obes Surg. 2005;15:567–70. doi: 10.1381/0960892053723349. [DOI] [PubMed] [Google Scholar]
- 39.Kim Y, Bailey AJ, Morris MC, et al. Kidney transplantation after sleeve gastrectomy in the morbidly obese candidate: Results of a 2-year experience. Surg Obes Relat Dis. 2020;16:10–4. doi: 10.1016/j.soard.2019.09.069. [DOI] [PubMed] [Google Scholar]
- 40.Kienzl-Wagner K, Weissenbacher A, Gehwolf P, et al. Laparoscopic sleeve gastrectomy: gateway to kidney transplantation. Surg Obes Relat Dis. 2017;13:909–15. doi: 10.1016/j.soard.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Kassam A-F, Mirza A, Kim Y, et al. Long-term outcomes in patients with obesity and renal disease after sleeve gastrectomy. Am J Transplant. 2020;20:422–9. doi: 10.1111/ajt.15650. [DOI] [PubMed] [Google Scholar]
- 42.Koshy AN, Coombes JS, Wilkinson S, et al. Laparoscopic gastric banding surgery performed in obese dialysis patients prior to kidney transplantation. Am J Kidney Dis. 2008;52:15–7. doi: 10.1053/j.ajkd.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 43.Al-Bahri S, Fakhry TK, Gonzalvo JP, et al. Bariatric surgery as a bridge to renal transplantation in patients with end-stage renal disease. Obes Surg. 2017;27:2951–5. doi: 10.1007/s11695-017-2722-6. [DOI] [PubMed] [Google Scholar]
- 44.Carandina S, Genser L, Bossi M, et al. Laparoscopic sleeve gastrectomy in kidney transplant candidates: a case series. Obes Surg. 2017;27:2613–8. doi: 10.1007/s11695-017-2679-5. [DOI] [PubMed] [Google Scholar]
- 45.Golomb I, Winkler J, Ben-Yakov A, et al. Laparoscopic sleeve gastrectomy as a weight reduction strategy in obese patients after kidney transplantation. Am J Transplant. 2014;14:2384–90. doi: 10.1111/ajt.12829. [DOI] [PubMed] [Google Scholar]
- 46.Arias RH, Mesa L, Posada JG, et al. Kidney transplantation and gastric bypass: a better control of comorbidities. Obes Surg. 2010;20:851–4. doi: 10.1007/s11695-010-0165-4. [DOI] [PubMed] [Google Scholar]
- 47.Szomstein S, Rojas R, Rosenthal RJ. Outcomes of laparoscopic bariatric surgery after renal transplant. Obes Surg. 2010;20:383–5. doi: 10.1007/s11695-009-9969-5. [DOI] [PubMed] [Google Scholar]
- 48.Schindel H, Winkler J, Yemini R, et al. Survival benefit in bariatric surgery kidney recipients may be mediated through effects on kidney graft function and improvement of co-morbidities: a case-control study. Surg Obes Relat Dis. 2019;15:621–7. doi: 10.1016/j.soard.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 49.Alexander JW, Goodman H. Gastric bypass in chronic renal failure and renal transplant. Nutr Clin Pract. 2007;22:16–21. doi: 10.1177/011542650702200116. [DOI] [PubMed] [Google Scholar]
- 50.Navaneethan SD, Schold JD, Srinivas TR. Metabolic syndrome and mild to moderate chronic kidney disease among minorities. Semin Nephrol. 2010;30:51–8. doi: 10.1016/j.semnephrol.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 51.Armstrong KA, Campbell SB, Hawley CM, et al. Obesity is associated with worsening cardiovascular risk factor profiles and proteinuria progression in renal transplant recipients. Am J Transplant. 2005;5:2710–8. doi: 10.1111/j.1600-6143.2005.01073.x. [DOI] [PubMed] [Google Scholar]
- 52.Kramer HJ, Saranathan A, Luke A, et al. Increasing body mass index and obesity in the incident ESRD population. J Am Soc Nephrol. 2006;17:1453–9. doi: 10.1681/ASN.2005111241. [DOI] [PubMed] [Google Scholar]
- 53.Lassalle M, Fezeu LK, Couchoud C, et al. Obesity and access to kidney transplantation in patients starting dialysis: a prospective cohort study. PLoS One. 2017;12:e0176616. doi: 10.1371/journal.pone.0176616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vesco L, Busson M, Bedrossian J, et al. Diabetes mellitus after renal transplantation: Characteristics, outcome, and risk factors. Transplantation. 1996;61:1475–8. doi: 10.1097/00007890-199605270-00011. [DOI] [PubMed] [Google Scholar]
- 55.Kasiske BL, Snyder JJ, Gilbertson D, et al. Diabetes mellitus after kidney transplantation in the United States Bertram. Am J Transplant. 2003;3:178–85. doi: 10.1046/j.1600-6143.2003.00228.x. [DOI] [PubMed] [Google Scholar]
- 56.Cosio FG, Kudva Y, Van Der Velde M, et al. New-onset hyperglycemia and diabetes are associated with increased cardiovascular risk after kidney transplantation. Kidney Int. 2005;67:2415–21. doi: 10.1111/j.1523-1755.2005.00349.x. [DOI] [PubMed] [Google Scholar]
- 57.Matas A, Smith J, Skeans M, et al. OPTN/SRTR 2012 Annual Data Report: Kidney. Am J Transplant. 2014;14:11–44. doi: 10.1111/ajt.12579. [DOI] [PubMed] [Google Scholar]
- 58.Segev DL, Simpkins CE, Thompson RE, et al. Obesity impacts access to kidney transplantation. J Am Soc Nephrol. 2008;19:349–55. doi: 10.1681/ASN.2007050610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gill JS, Hendren E, Dong J, et al. Differential association of body mass index with access to kidney transplantation in men and women. Clin J Am Soc Nephrol. 2014;9:951–9. doi: 10.2215/CJN.08310813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Foroutan F, Friesen EL, Clark KE, et al. Risk factors for 1-year graft loss after kidney transplantation systematic review and meta-analysis. Clin J Am Soc Nephrol. 2019;14:1642–50. doi: 10.2215/CJN.05560519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


