Abstract
Background: Mild cognitive impairment (MCI) represents a transitional state between normal aging and dementia disorders, especially Alzheimer's disease (AD). The disruption of the default mode network (DMN) is often considered to be a potential biomarker for the progression from MCI to AD. The purpose of this study was to assess MRI-specific changes of DMN in MCI patients by elucidating the convergence of brain regions with abnormal DMN function.
Methods: We systematically searched PubMed, Ovid, and Web of science for relevant articles. We identified neuroimaging studies by using amplitude of low frequency fluctuation /fractional amplitude of low frequency fluctuation (ALFF/fALFF), regional homogeneity (ReHo), and functional connectivity (FC) in MCI patients. Based on the activation likelihood estimation (ALE) algorithm, we carried out connectivity modeling of coordination-based meta-analysis and functional meta-analysis.
Results: In total, this meta-analysis includes 39 articles on functional neuroimaging studies. Using computer software analysis, we discovered that DMN changes in patients with MCI mainly occur in bilateral inferior frontal lobe, right medial frontal lobe, left inferior parietal lobe, bilateral precuneus, bilateral temporal lobe, and parahippocampal gyrus (PHG).
Conclusions: Herein, we confirmed the presence of DMN-specific damage in MCI, which is helpful in revealing pathology of MCI and further explore mechanisms of conversion from MCI to AD. Therefore, we provide a new specific target and direction for delaying conversion from MCI to AD.
Keywords: mild cognitive impairment, default mode network, amplitude of low frequency fluctuation/fractional amplitude of low frequency fluctuation, regional homogeneity, functional connectivity
Introduction
Mild cognitive impairment (MCI) is thought to be an intermediary stage between normal aging and AD (Wang et al., 2013; Giau et al., 2019). MCI patients present with memory impairment, but are able to maintain normal activities of daily living (Wang et al., 2018). To the best of our knowledge, one of the main AD symptoms is dysfunction of integrating semantic information into long-term effective memory, and this dysfunction is known to be correlated with MCI (Brueggen et al., 2016). It is important to note that AD pathology begins prior to clinical manifestations, and is irreversible (Lu et al., 2020). For this reason, carrying out a correct disease diagnosis and early intervention during MCI can effectively delay or even prevent occurrence of clinical dementia (Wang et al., 2015). In recent years, resting-state functional magnetic resonance imaging (rs-fMRI) has emerged as a powerful non-invasive to study internal brain functional connectivity as it does not require specific external tasks and is reliable (Wang et al., 2018). Therefore, our study utilized this approach to explore the presence of specific neuroimaging markers of DMN during MCI.
The human brain has many complex functional regions, separate and connected, that process different types of information (Horwitz, 2003). There are many networks in the human brain, including the DMN, the executive control network and the sensorimotor network. Several previous studies have summarized the brain regions of DMN (Yuan et al., 2016; Bi et al., 2018), which roughly includes the precuneus, posterior cingulate cortex (PCC), inferior parietal cortex, medial prefrontal cortex and hippocampus (Kang et al., 2018). Generally speaking, there are also abnormal changes that develop in large-scale network activity in AD. In the current functional neuroimaging research networks, DMN is known to be easily affected by the AD process (Weiler et al., 2014). Furthermore, activity in the DMN increased during quiet rest with eyes closed or simple visual fixations, while activity in the DMN continued to decrease when performing a variety of novel, attention-requiring, and non-self-referential tasks (Raichle, 2015). The discovery of the DMN has rekindled a long-standing interest in the importance of the brain's ongoing, or intrinsic, activity. Thus, we focused on DMN in this study.
As is known, AD is a disconnection syndrome that can be detected prior to the onset of cognitive impairment (Zhou et al., 2008), rather than just causing damage to isolated areas of the brain (Wang et al., 2018). AD affects the brain across many different levels, including molecular functions and neurotransmitter systems, which eventually leads to cognitive dysfunction (Kang et al., 2018). Currently, structural imaging markers, molecular imaging markers and functional imaging fractional markers can be useful diagnostic factors that play a role in the conversion of MCI to AD (Teipel et al., 2017). In recent years, fMRI has become increasingly important with regards to exploring neurological diseases of the brain, such as AD (Weiler et al., 2014). In AD patients, DMN is susceptible to neurodegeneration and is involved in early pathological changes (Wang et al., 2018). Aβ protein deposition and neurofibrillary tangles are both pathological characteristics of MCI that can further lead to degeneration of neuronal activity and functional impairment (Matura et al., 2020). According to the amyloid hypothesis, amyloid deposition is the main cause of AD (Matura et al., 2020). Several studies have suggested that Aβ42, total brain volume, and Tau protein are all predictors of conversion from MCI to AD, and that amyloid beta protein distribution overlaps with DMN in specific brain regions (Adriaanse et al., 2014). Furthermore, several studies suggest that DMN may be a biomarker for early prediction of AD. Currently, research of DMN has been applied to several diseases (Eyler et al., 2019). Therefore, it is reasonable to hypothesize that functional-specific changes in DMN can be utilized as evidence to support a diagnosis of MCI.
FC can often be seen as an indirect indicator of cross-synaptic activity, as it demonstrates connections between neural activity in different regions of the brain space (Balachandar et al., 2015). In this article, we focus on papers that evaluate connectivity through the correlation of BOLD (blood oxygenation level dependent) time series, although there are several other imaging methods that can evaluate functional connectivity. In MCI patients, FC decreased mainly in PCC, precuneus, and left PHG (Liu et al., 2012). Interestingly, reduction of PCC and precuneus is synchronous (Liu et al., 2012). A simultaneous increase of activity in some brain regions in MCI individuals is thought to compensate for defects in other areas (Farras-Permanyer et al., 2019). As far as we know, the ALFF technique has been shown to be reliable and useful in studying intrinsic or spontaneous brain activity among patients with MCI or AD (Zhen et al., 2018). The results of ALFF appearing in MCI are very interesting. Changes in the frontal lobes, temporal lobes, and parietal lobes are variable and can be either increased or decreased (Kang et al., 2018). Some have suggested that a decrease in ALFF in the temporal region may be the result of neurotangles, which is a claim that cannot be denied (Postema et al., 2019). ReHo is a reliable rs-fMRI analysis algorithm to explore local functional connectivity (Li et al., 2015). It has been shown that the ReHo of the left inferior parietal lobule, the medial prefrontal cortex, and the PCC/PCU are altered among patients with MCI (Zhang et al., 2012), and that in patients with AD, ReHo of some regions of the DMN decreases with disease severity (Wang et al., 2015). Changes of different indexes may represent different sensitivities, and the changes of multiple indexes in the same brain region at the same period can help improve sensitivity of diagnosis.
Hence, our aim was to comprehensively evaluate specific changes in DMN among patients with MCI. Additionally, we believe that three indicators of DMN will demonstrate special imaging anomaly markers. Similarly, we can have a deeper understanding of functional changes of DMN in MCI patients, further understand its pathological mechanism, and provide novel ideas to explore novel treatment directions.
Method
Search Strategy
We systematically and comprehensively searched PubMed, Ovid, and Web of science. The search terms were as follows: (1) “functional magnetic resonance imaging” OR “resting state” AND “mild cognitive impairment” AND “default mode network” OR “default network” AND “Functional connectivity.” (2) “functional magnetic resonance imaging” OR “resting state” AND “mild cognitive impairment” AND “regional homogeneity.” (3) “functional magnetic resonance imaging” OR “resting state” AND “mild cognitive impairment” AND “amplitude of low frequency fluctuation” OR “fractional amplitude of low frequency fluctuation.”
Inclusion and Exclusion Criteria
Our entry criteria included (1) an original article published in a peer-reviewed journal. (2) Subjects were only recruited if they met the diagnostic criteria of MCI. (3) The study included an analysis of DMN in the resting state. (4) Results were obtained in standard stereotactic space using the Montreal Neurological Institute (MNI) or Talairach/Tournoux template. (5) The study was a cross-sectional and case-control design. When the study was a longitudinal study, we used baseline patient imaging data. Our exclusion criteria were as follows: (1) patients were diagnosed with cerebrovascular dementia, Lewy body's dementia, and other diseases such as Parkinson, (2) meta-analysis and review, (3) a lack of normal control group and coordinates, and (4) missing data in the literature.
Data Extraction and Quality Assessment
Two researchers in our group extracted data from literature. First, we included patients with MCI. Second, we read each study to determine whether it had valid coordinates and outcomes, and whether it was a study of FC, ReHo, and ALFF/fALFF in the DMN. Finally, we extracted coordinates of the DMN in literature, transformed the T coordinates, and then worked with the method in the form of MNI coordinates. In case of disagreement between the two current researchers on the adoption of the article, a third researcher will vote on the decision. ALFF and fALFF were utilized to measure the amplitude of brain activity in spontaneous regions. ReHo has been shown to be highly reliable when studying the local consistency of the brain. FC is often used to indicate whether connections between brain regions have been disrupted or are compensatory. Hence, we divided the data into two groups based on the results of FC, ReHo and ALFF/fALFF (MCI > HC Group and MCI < HC Group), and then analyzed them using a computer computing software.
The ALE Algorithm used in this meta-analysis is available to the neuroimaging community in the form of the GINGERALE desktop application (http://brainmap.org/ale) (Zhang et al., 2012). ALE is a coordinate-based meta-analysis method that can reduce the bias of laboratory results. ALE does not consider the activation points in neuroimaging studies as single activation points, but rather as the spatial probability distribution with given coordinates as the center, and then calculates the activation probability of each coordinate and draws an ALE map (Zhang et al., 2012). It has been widely utilized in rs-fMRI studies (Eickhoff et al., 2012).
Data Analysis Procedures
First, we divided subjects into the normal control group and MCI patient group. Then, three indexes (ALFF, FC, ReHo) were compared between the two groups. Finally, results from the comparison of the three indicators were divided into two groups: the ascending group and the descending group. The results of the two groups are also discussed. We utilized a software to calculate increased ALFF/fALFF (n = 75; foci14), decreased ALFF/fALFF (n = 99; foci16), increased ReHo (n = 148; foci18), decreased ReHo (n = 202; foci25), increased FC (n = 604; foci85), and decreased FC (n = 388; foci169).
Results
Search Results
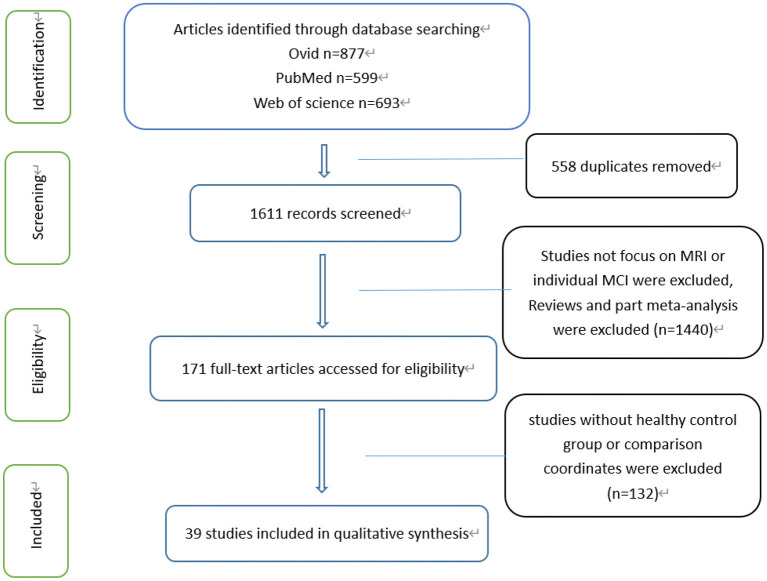
Overall, 1,839 articles were identified across three different databases, from which 511 were duplicated and 1,328 were removed. In total, 39 articles were included, all of which included comparisons between the MCI patient group and a normal control group. Each of the 39 articles has complete data coordinates and meaningful results (Figure 1). In addition, there were 27 references included in FC, 27 references with declining FC and 17 references with rising FC. Fifteen references had both rising and declining results. There were seven references included in ReHo, seven references with declining ReHo and five references with rising ReHo. Five references had both rising and declining results. There were five references included in ALFF, three references with a decline in ALFF and five references with rise in ALFF, among which three had both rising and declining results. This meta-analysis incorporates literature and summary of relevant information as shown in Table 1.
Figure 1.
Flow of information through different phases of a systematical review.
Table 1.
Demographic characteristics of the included studies.
| References | N | Age (SD) | Gender (M/F) | MMSE (SD) | Group contrasts | Foci |
|---|---|---|---|---|---|---|
| FC | ||||||
| Cai et al. (2015) | MCI 55 | 73.385 (7.61) | 29/26 | 26.895 (1.73) | MCI>HC | 0 |
| HC 30 | 75.8 (7.14) | 13/17 | 29.63 (1.52) | MCI < HC | 20 | |
| Joo et al. (2017) | MCI 50 | 72.1 (3.8) | 23/27 | 24.4 (3.3) | MCI>HC | 19 |
| HC 50 | 71.2 (4.3) | 22/28 | 28.4 (1.5) | MCI < HC | 3 | |
| Su et al. (2017) | MCI 80 | 69.9 (7.3) | 38/42 | 26.2 (1.7) | MCI>HC | 3 |
| HC 127 | 68.3 (6.6) | 64/63 | 28.6 (1.4) | MCI < HC | 5 | |
| Li et al. (2017) | MCI 25 | 64.56 (4.98) | 9/16 | 26.88 (1.856) | MCI>HC | 2 |
| HC 25 | 62.84 (2.79) | 11/14 | 27.60 (1.732) | MCI < HC | 1 | |
| Balachandar et al. (2015) | MCI 15 | 67.33 (6.6) | 6/9 | / | MCI>HC | 2 |
| HC 15 | 64.4 (8.9) | 6/9 | / | MCI < HC | 3 | |
| Wang et al. (2011) | MCI 14 | 69.64 (6.88) | 8/6 | 26.64 (1.01) | MCI>HC | 5 |
| HC 14 | 68.07 (7.46) | 8/6 | 28.57 (0.65) | MCI < HC | 28 | |
| Yi et al. (2015) | MCI 20 | 70.95 (2.105) | 2/8 | 23.55 (0.83) | MCI>HC | 5 |
| HC 12 | 71.75 (1.21) | 3/9 | 27.40 (0.45) | MCI < HC | 1 | |
| Han et al. (2012) | MCI 40 | 86.26 (4.49) | 33/7 | 27.10 (1.96) | MCI>HC | 4 |
| HC 40 | 86.28 (4.39) | 25/15 | 28.68 (1.23) | MCI < HC | 8 | |
| Lee et al. (2016) | MCI 87 | 71.4 (7.45) | 43/44 | 27.85 (1.7) | MCI>HC | 0 |
| HC 43 | 74.5 (5.8) | 18/25 | 28.7 (1.4) | MCI < HC | 19 | |
| Li et al. (2020) | MCI 30 | 68.53 (2.97) | 13/17 | 25.10 (0.66) | MCI>HC | 0 |
| HC 30 | 68.67 (3.19) | 14/16 | 28.20 (0.92) | MCI < HC | 1 | |
| Xue et al. (2019) | MCI 48 | 64.95 (8.57) | 17/21 | 27.575 (1.8685) | MCI>HC | 5 |
| HC 21 | 57.52 (8.07) | 7/14 | 28.81 (1.209) | MCI < HC | 0 | |
| Wang et al. (2015) | MCI 18 | 73.7 (9.1) | 8/10 | 27.9 (1.2) | MCI>HC | 4 |
| HC 16 | 70.7 (6.0) | 4/12 | / | MCI < HC | 0 | |
| Barban et al. (2017) | MCI 23 | 70.45 (6.2) | 14/9 | / | MCI>HC | 3 |
| HC 25 | 72.1 (6.15) | 7/18 | / | MCI < HC | 0 | |
| Bharath et al. (2017) | MCI 48 | 67.22 (8.00) | 13/35 | / | MCI>HC | 0 |
| HC 48 | 65.89 (7.20) | 13/35 | / | MCI < HC | 1 | |
| Agosta et al. (2012) | MCI 12 | 69.1 (7.4) | 6/6 | 26 (1) | MCI>HC | 0 |
| HC 13 | 68.5 (6.9) | 5/8 | 29 (1) | MCI < HC | 1 | |
| Cha et al. (2013) | MCI 34 | 68.4 (7.9) | 18/16 | 27.1 (2.1) | MCI>HC | 0 |
| HC 62 | 68.5 (8.0) | 17/45 | 28.6 (1.9) | MCI < HC | 3 | |
| De Vogelaere et al. (2012) | MCI 16 | 67.2 (7.9) | 8/8 | 24.4 (3.1) | MCI>HC | 7 |
| HC 16 | 62.1 (6.8) | 10/6 | 28.6 (1.3) | MCI < HC | 16 | |
| Qi et al. (2010) | MCI 14 | 71.8 (7.3) | 6/8 | 26.6 (0.3) | MCI>HC | 5 |
| HC 14 | 70.4 (5.8) | 8/6 | 28.5 (0.2) | MCI < HC | 7 | |
| Bai et al. (2009) | MCI 30 | 72.5 (4.4) | 15/15 | 27.0 (1.5) | MCI>HC | 5 |
| HC 26 | 71.6 (5.3) | 12/14 | 28.2 (1.4) | MCI < HC | 0 | |
| Krajcovicova et al. (2017) | MCI 17 | 73.56 (6.64) | 11/6 | 26.94 (1.68) | MCI>HC | 0 |
| HC 18 | 68.22 (8.78) | 5/13 | 29.17 (0.71) | MCI < HC | 1 | |
| Bosch et al. (2010) | MCI 15 | 74.63 (6.85) | 6/9 | 25.50 (2.03) | MCI>HC | 3 |
| HC 15 | 72.20 (5.75) | 5/10 | 27.67 (1.49) | MCI < HC | 3 | |
| Yao et al. (2014) | MCI 13 | 75.5 (8.7) | 8/5 | 25.8 (3.4) | MCI>HC | 1 |
| HC 13 | 74.5 (8.7) | 8/5 | 27.3 (1.8) | MCI < HC | 5 | |
| Conwell et al. (2018) | MCI 15 | 71.1 (6.0) | 9/6 | 25.0 (3.4) | MCI>HC | 0 |
| HC 15 | 67.3 (8.4) | 9/6 | 29.5 (0.6) | MCI < HC | 1 | |
| Gardini et al. (2015) | MCI 21 | 70.62 (4.66) | 13/8 | / | MCI>HC | 3 |
| HC 21 | 69.75 (6.45) | 7/14 | / | MCI < HC | 0 | |
| Wang et al. (2011) | MCI 14 | 69.64 (6.88) | 8/6 | 26.64 (1.01) | MCI>HC | 0 |
| HC 14 | 68.07 (7.46) | 8/6 | 28.57 (0.65) | MCI < HC | 20 | |
| Li et al. (2016) | MCI 14 | 67.9 (9.5) | 14/17 | 23.5 (2.9) | MCI>HC | 0 |
| HC 14 | 65.6 (7.1) | 15/27 | 28.0 (2.3) | MCI < HC | 9 | |
| Yan et al. (2013) | MCI 18 | 66.7 (8.9) | 11/7 | 24.3 (1.5) | MCI>HC | 9 |
| HC 18 | 64.9 (8.4) | 10/8 | 29.5 (0.5) | MCI < HC | 7 | |
| ALFF/fALFF | ||||||
| Yin et al. (2014) | MCI 11 | 66.6 (8.7) | 2/9 | 24.6 (3.2) | MCI>HC | 2 |
| HC 22 | 62.1 (8.1) | 12/10 | 29.2 (1.1) | MCI < HC | 4 | |
| Wang et al. (2011) | MCI 16 | 69.38 (7.00) | 7/9 | 26.50 (1.03) | MCI>HC | 8 |
| HC 22 | 66.55 (7.67) | 7/15 | 28.59 (0.59) | MCI < HC | 10 | |
| Cai et al. (2015) | MCI 39 | 72.4 (5.01) | 20/19 | 25.51 (2.88) | MCI>HC | 2 |
| HC 38 | 73.92 (3.90) | 19/19 | 29.28 (0.88) | MCI < HC | 3 | |
| Jing et al. (2012) | MCI 10 | 75.35 (6.45) | 5/5 | / | MCI>HC | 4 |
| HC 8 | 78.42 (9.65) | 3/5 | / | MCI < HC | 0 | |
| Zhao et al. (2015) | MCI 34 | 68.0 (7.6) | 14/20 | 25.5 (1.6) | MCI>HC | 0 |
| HC 34 | 66.9 (6.7) | 18/16 | 29.2 (0.9) | MCI < HC | 2 | |
| ReHo | ||||||
| Cai et al. (2015) | MCI 102 | 72.015 (6.475) | 52/50 | 24.27 (2.7) | MCI>HC | 2 |
| HC 53 | 76.08 (6.45) | 29/24 | 28.2 (2.13) | MCI < HC | 2 | |
| Luo et al. (2018) | MCI 64 | 73.665 (4.76) | 34/30 | 27.75 (1.695) | MCI>HC | 2 |
| HC 49 | 73.33 (4.60) | 18/31 | 29.02 (1.20) | MCI < HC | 2 | |
| Wang et al. (2015) | MCI 32 | 69.1 (5.8) | 18/12 | 26.2 (2.2) | MCI>HC | 6 |
| HC 30 | 70.1 (5.5) | 15/17 | 28.1 (1.5) | MCI < HC | 5 | |
| Bai et al. (2008) | MCI 20 | 71.3 (3.8) | 10/10 | 28.3 (1.4) | MCI>HC | 1 |
| HC 20 | 69.4 (3.8) | 9/11 | 27.2 (1.6) | MCI < HC | 5 | |
| Kang et al. (2018) | MCI 34 | 76.1 (4.7) | 7/27 | 22.7 (3.9) | MCI>HC | 0 |
| HC 38 | 74.0 (5.4) | 19/19 | 26.3 (2.3) | MCI < HC | 2 | |
| Min et al. (2019) | MCI 10 | 69.80 (2.658) | 5/5 | 25.90 (0.738) | MCI>HC | 3 |
| HC 10 | 69.90 (2.601) | 5/5 | 29.30 (0.823) | MCI < HC | 5 | |
| Yuan et al. (2016) | MCI 36 | 66.8 (9.5) | 17/19 | 24.9 (3.4) | MCI>HC | 6 |
| HC 46 | 64.3 (7.8) | 19/27 | 28.5 (2.0) | MCI < HC | 8 | |
Meta-Analysis Results
Altered ALFF/fALFF in MCI
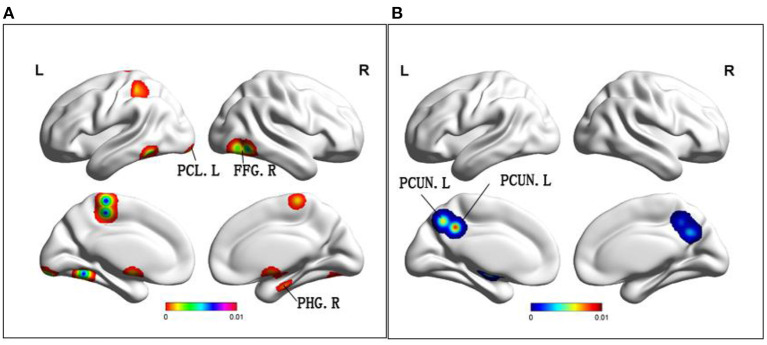
Compared to HC, MCI patients demonstrated increased ALFF/fALFF in the bilateral cerebellum posterior lobe, right parahippocampal gyrus and bilateral fusiform gyrus (Table 2 and Figure 2). In addition, MCI patients had decreased ALFF/fALFF in the bilateral precuneus (Table 2 and Figure 2).
Table 2.
All clusters from ALE analysis.
| Cluster | Volume (mm3) | MNI | Anatomical regions | Maximum ALE value | Side | BA | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| FC | ||||||||
| MCI>HC | ||||||||
| 1 | 848 | 4 | 58 | −14 | Medial Frontal Gyrus | 0.015601669 | Right | 10 |
| 1 | 848 | −6 | 56 | −12 | Anterior Cingulate | 0.01257849 | Left | 10 |
| MCI < HC | ||||||||
| 1 | 9,168 | 2 | −68 | 56 | Precuneus | 0.009993 | Left | 7 |
| 1 | 9,168 | 0 | −66 | 50 | Precuneus | 0.009981 | Left | 7 |
| 1 | 9,168 | 12 | −54 | 48 | Precuneus | 0.009978 | Right | 7 |
| 1 | 9,168 | −4 | −62 | 38 | Precuneus | 0.009745 | Left | 7 |
| 1 | 9,168 | 12 | −68 | 60 | Superior Parietal Lobule | 0.009298 | Right | 7 |
| 1 | 9,168 | 0 | −80 | 42 | Cuneus | 0.008475 | Left | 19 |
| 1 | 9,168 | 4 | −56 | 40 | Precuneus | 0.008349 | Right | 7 |
| 1 | 9,168 | 2 | −72 | 42 | Precuneus | 0.008181 | Left | 7 |
| 1 | 9,168 | 0 | −46 | 40 | Precuneus | 0.00759 | Left | 31 |
| 1 | 9,168 | 10 | −42 | 48 | Cingulate Gyrus | 0.007052975 | Right | 31 |
| 2 | 6,776 | −46 | −74 | 8 | Middle Occipital Gyrus | 0.015483191 | Left | 19 |
| 2 | 6,776 | −56 | −62 | 24 | Superior Temporal Gyrus | 0.011498914 | Left | 39 |
| 2 | 6,776 | −54 | −64 | 28 | Middle Temporal Gyrus | 0.010231528 | Left | 39 |
| 2 | 6,776 | −54 | −58 | 36 | Superior Temporal Gyrus | 0.0014 | Left | 39 |
| 2 | 6,776 | −36 | −78 | 24 | Middle Temporal Gyrus | 0.00157 | Left | 19 |
| 2 | 6,776 | −48 | −74 | 24 | Middle Temporal Gyrus | 0.007693 | Left | 39 |
| 2 | 6,776 | −46 | −72 | 36 | Angular Gyrus | 0.00742211 | Left | 39 |
| 3 | 5,960 | −56 | 2 | −28 | Middle Temporal Gyrus | 0.014750081 | Left | 21 |
| 3 | 5,960 | −64 | −20 | −18 | Middle Temporal Gyrus | 0.010508 | Left | 21 |
| 3 | 5,960 | −52 | 12 | −12 | Superior Temporal Gyrus | 0.008636334 | Left | 22 |
| 3 | 5,960 | −46 | 12 | −18 | Superior Temporal Gyrus | 0.008573441 | Left | 38 |
| 3 | 5,960 | −60 | −10 | −28 | Inferior Temporal Gyrus | 0.007971286 | Left | 20 |
| ALFF/fALFF | ||||||||
| MCI>HC | ||||||||
| 1 | 19,336 | −6 | −30 | 60 | Paracentral Lobule | 0.001944255 | Left | 5 |
| 2 | 14,864 | −30 | −78 | −30 | Tuber | 0.006901412 | Left | / |
| 2 | 14,864 | −18 | −93 | −15 | Declive | 0.006519429 | Left | / |
| 3 | 14,656 | −42 | −54 | −15 | Fusiform Gyrus | 0 | Left | 37 |
| 4 | 13,168 | 16 | −14 | −22 | Parahippocampal Gyrus | 0.006627638 | Right | 34 |
| 4 | 13,168 | −1 | −2 | −18 | Hypothalamus | 0.006446497 | / | / |
| 5 | 12,656 | 51 | −60 | −12 | Fusiform Gyrus | 0.007649368 | Right | 37 |
| 5 | 12,656 | 39 | −69 | −9 | Declive | 0.007187633 | Right | / |
| MCI < HC | ||||||||
| 1 | 27,400 | −8 | −48 | 38 | Precuneus | 0.008772571 | Left | 31 |
| 1 | 27,400 | 12 | −60 | 34 | Precuneus | 0.007666224 | Right | 31 |
| 1 | 27,400 | −6 | −60 | 44 | Precuneus | 0.006712989 | Left | 7 |
| 1 | 27,400 | 14 | −52 | 44 | Precuneus | 0.006358274 | Right | 7 |
| 2 | 13,736 | −20 | −10 | −10 | Medial Globus Pallidus | 0.008748008 | Left | / |
| 2 | 13,736 | −18 | 6 | −4 | Putamen | 0.007649428 | Left | / |
| ReHo | ||||||||
| MCI>HC | ||||||||
| 1 | 688 | −59 | 17 | 11 | Inferior Frontal Gyrus | 0.015190582 | Left | 44 |
| 2 | 560 | 56 | 14 | 14 | Inferior Frontal Gyrus | 0.016221888 | Right | 44 |
| MCI < HC | ||||||||
| 1 | 640 | −58 | −16 | −6 | Superior Temporal Gyrus | 0.015689934 | Left | 21 |
| 2 | 640 | −51 | −42 | 45 | Inferior Parietal Lobule | 0.015689865 | Left | 40 |
BA, Brodmann Area; ALE, Anatomical/Activation Likelihood Estimation; MNI, Montreal Neurologic Institute; MCI, amnestic mild cognitive impairment; HCs, healthy controls; ALFF, the amplitude of low frequency fluctuation; ReHo, regional homogeneity; FC, functional connectivity.
Figure 2.
(A) Brain regions showing increased ALFF in MCI patients compared to HCs. (B) Brain regions showing decreased ALFF in MCI patients compared to HCs. MCI, amnestic mild cognitive impairment; HCs, healthy controls; ALFF, the amplitude of low frequency fluctuation; PCL, paracentral lobule; FFG, fusiform gyrus; PHG, parahippocampal gyrus; PCUN, precuneus; R, right; L, left.
Altered ReHo in MCI
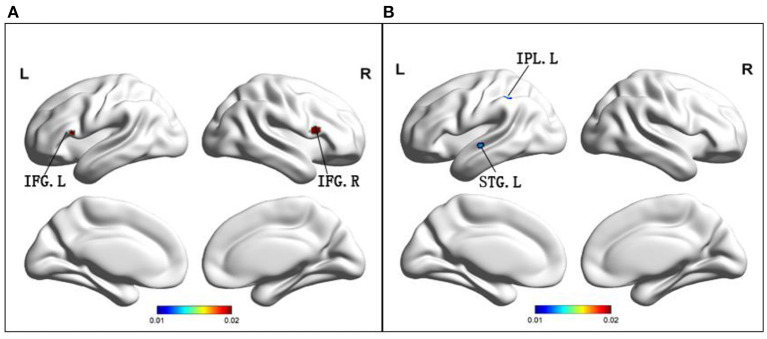
Compared to HC, MCI patients demonstrated increased ReHo in the bilateral inferior frontal gyrus (Table 2 and Figure 3), while MCI patients showed decreased ReHo in the left superior temporal gyrus and left inferior parietal lobule (Table 2 and Figure 3).
Figure 3.
(A) Brain regions showing increased ReHo in MCI patients compared to HCs. (B) Brain regions showing decreased ReHo in MCI patients compared to HCs. MCI, amnestic mild cognitive impairment; ReHo, regional homogeneity; HCs, healthy controls; IFG, inferior frontal gyrus; IPL, inferior parietal lobule; STG, superior temporal gyrus; R, right; L, left.
Altered FC in MCI
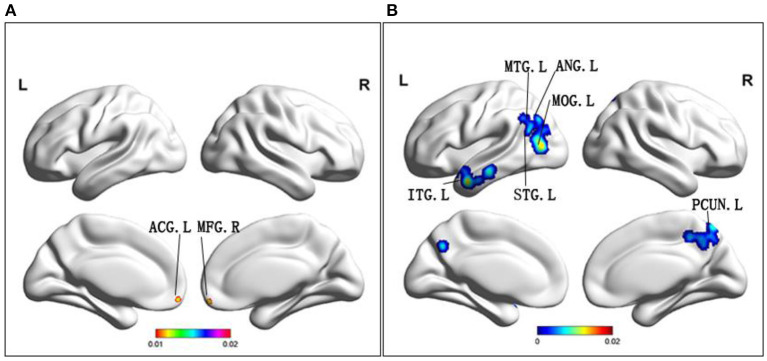
Compared to HC, MCI patients demonstrated increased FC in the right medial frontal gyrus, left limbic lobe and left anterior cingulate cortex (ACC) (Table 2 and Figure 4). Conversely, MCI patients showed decreased FC in the bilateral precuneus, left middle occipital gyrus, right cingulate gyrus, left superior temporal gyrus, left middle temporal gyrus, left inferior temporal gyrus, left middle occipital gyrus, and left angular gyrus (Table 2 and Figure 4).
Figure 4.
(A) Brain regions showing increased FC in MCI patients compared to HCs. (B) Brain regions showing decreased FC in MCI patients compared to HCs. MCI, amnestic mild cognitive impairment; FC, functional connectivity; HCs, healthy controls; ACG, anterior cingulate gyrus; MFG, middle frontal gyrus; ITG, inferior temporal gyrus; MTG, middle temporal gyrus; STG, superior temporal gyrus; ANG, angular gyrus; MOG, middle occipital gyrus; PCUN, precuneus; R, right; L, left.
Main Voxel-Wise Meta-Analysis
Among the different brain regions with FC changes, the precuneus was the largest, and the middle frontal gyrus and anterior angular gyrus were the smallest. The precuneus was shown to have largest changes in ALFF, and the smallest change occurred in the fusiform gyrus. Interestingly, the largest and smallest groups of changes in ReHo were in the inferior frontal gyrus, with a larger group on the left. The size of the point in the figure represents size of the cluster, and size of the cluster is consistent with its effect. This explanation applies to graphs with three different indicators.
Discussion
In the past, there have been separate studies on three different indicators (ReHo, ALFF, FC) for DMN in MCI (Bai et al., 2008; Cai et al., 2015; Zhao et al., 2015). However, this was the first meta-analysis to conduct a comprehensive analysis of all three indicators. In our meta-analysis, the superior temporal gyrus, inferior temporal gyrus, parahippocampal gyrus, and precuneus were consistent with prior findings (Robinson et al., 2012). These results highlight the importance of altered DMN function in the pathophysiology of MCI. Furthermore, some of the altered brain regions have not yet been reported as belonging to DMN. Thus, perhaps the specific brain regions that are related to DMN remain to be explored. There is no denying that the brain regions that have specific changes may serve as biomarkers for diagnosis of MCI patients. Additionally, these brain regions can serve as biomarkers for predicting AD.
Firstly, it is known that changes in FC shown by patients with MCI are both complex and varied, depending on the study as well as method of use (Eyler et al., 2019). Previously, we found no evidence that changes in DMN connectivity were effective predictors of transition from MCI to AD (Xue et al., 2019). In our results, areas of FC increased mainly included the right medial frontal lobe and the left ACC. Some scholars propose that the increase of FC in the medial frontal lobe may be related to semantic memory deficiency (Su et al., 2017). However, maintenance of normal daily living functions during MCI may be the result of compensating for a disconnection after early hyperconnectivity of the medial frontal lobe (Wiepert et al., 2017). Another area of the brain that presents with increased FC was the left ACC. As is known, the PCC is the core area of DMN (Fransson and Marrelec, 2008). In AD, FC of PCC was severely damaged. In this study, the area where FC increased was ACC. On the other hand, previous studies have shown that the association between Theta activity in ACC and response time in the planning period may reflect the high cognitive demands associated with this task (Domic-Siede et al., 2021). Research supports that the anterior cingulate gyrus is well-suited to regulate behavioral selection and learning on multiple time scales, and to respond differently to environmental uncertainty and volatility (Monosov et al., 2020). Furthermore, increased ACC functional connectivity may be the result of making up for decreased PCC activity, thus maintaining relatively normal ability of daily living.
The increase or decrease of FC alone cannot be an effective diagnostic factor for the transition of MCI to AD. A combination of increase and decreased FC can improve sensitivity (Eyler et al., 2019). In our results, a decline in FC was mainly present in the bilateral precuneus and superior parietal lobes, both of which are responsible for visual, sensory, and motor integration. Previous studies have suggested that the precuneus is the “core node” of the DMN, and has been associated with high levels of amyloid deposition in early AD (Tao et al., 2017). As far as we know, deposition of protein and shrinkage of gray matter volume make DMN known to be a susceptible area of AD. However, whether early changes of FC in the precuneus, the core of DMN, can be regarded as a specific biomarker for the transition to AD, is unknown (Eyler et al., 2019). Another noteworthy area of FC decline is the middle temporal gyrus. The middle temporal gyrus (MTG) is understood to play a role in language-related tasks such as lexical comprehension and semantic cognition (Briggs et al., 2021). Furthermore, an increase of FC in the middle frontal gyrus may also be allowed to compensate for a decrease of FC in the medial temporal gyrus (Gardini et al., 2015). Previous literature has suggested that a FC decline during MCI and PCC-temporal cortex may be the central role of cognitive deficits in MCI patients (Fransson and Marrelec, 2008). However, the fact that the meta-analyses results are not clearly presented is also a limitation (Wiepert et al., 2017). In conclusion, changes in the FC of medial frontal gyrus, precuneus and middle temporal gyrus may be utilized as effective biomarkers to predict AD conversion.
The presence of ALFF reflects regional characteristics of brain activity. The study demonstrated that not all changed brain structures had a drop in ALFF, and there was no correlation between the changed brain and ALFF changes (Yin et al., 2014). One interesting result is that while the volume of gray matter in the frontal lobes, temporal lobes, and parietal lobes decrease, ALFF values can go up as well as down (Zou et al., 2015). Although results of ALFF remain controversial, their significance cannot be denied. The areas that demonstrate ALFF are noteworthy in the posterior lobe of the cerebellum, as well as the parahippocampal gyrus. The cerebellum is involved in cognitive, emotional, and sensory processing (Yin et al., 2014). In addition, a worthy increase in cerebellar ALFF may be a complement to cognitive deficits (Stoodley and Schmahmann, 2009; Wang et al., 2011). Parahippocampal gyrus and hippocampus located in the medial temporal lobe are the main brain regions that develop pathological change of AD. In addition, they play a crucial role in memory function, and may compensate for neurodegenerative changes following injury to MCI (Pan et al., 2017). Another area with a significant increase in ALFF is the fusiform gyrus. It is well-known that the fusiform gyrus is primarily involved in memory processing (Zhao et al., 2014). It has been found that the FC in the fusiform gyrus is also extensively altered when MCI patients perform facial-matching tasks (Xuan et al., 2012). The related memory and cognitive functions of MCI patients remain normal, which could not be ruled out as a compensation for increasing ALFF values in these specific regions.
A decrease of ALFF was mainly found in the bilateral precuneus. The precuneus acts as an intermediary between the semantic network, as well as the hippocampal memory system, encoding meaningful events into episodic memories (Schmahmann et al., 2007). Different from the ALFF decrease in the precuneus proposed in our study, most of the previous studies showed a decrease of FC in precuneus (Stoodley and Schmahmann, 2009; Wang et al., 2011). The exact mechanism, however, remains to be explored, which may enhance the credibility of the presence of abnormal neuroimaging markers in DMN during MCI. In conclusion, ALFF changes in the posterior cerebellar lobe, parahippocampal gyrus fusiform gyrus and precuneus may be utilized as predictors of AD conversion.
ReHo mainly explored differences of spontaneous activity in the whole brain. Both an increase and decrease in ReHo indicate changes in brain activity. With increase or decrease of ReHo value, regional metabolic rate constantly changes, and cerebral blood flow also increases or decreases (Bokde et al., 2006). The areas where ReHo dropped were mainly the superior temporal gyrus and the inferior parietal lobule. It is well-known that the parietal cortex has extensive connections with frontal lobes and controls sensory information for movement (Fransson and Marrelec, 2008). It has been shown that the left inferior parietal lobule is damaged among patients with MCI. Therefore, we hypothesize that a reduction of ReHo in the left inferior parietal lobule provides evidence for presence of DMN as a predictor of the conversion to AD.
Our meta-analysis demonstrated that the region where ReHo was elevated was the inferior frontal gyrus, which is one of the key regions of DMN (Huang et al., 2016), and is largely responsible for declarative long-term memory function (Sakurai and Gamo, 2019; Gao et al., 2020). Previous studies have demonstrated that the atrophy of the inferior frontal gyrus can predict conversion of MCI to AD (Yuan et al., 2013), so we may assume that elevation of ReHo value in the inferior frontal gyrus also has an important function in the conversion process of AD.
Finally, we discussed the brain regions that changed over the same time period and had overlapping indicators. The region of the brain where the FC and ALFF overlap was the precuneus. Both of the indexes were decreased in the precuneus. The areas where FC and ReHo overlap were the superior temporal gyrus, as well as the inferior parietal lobule. Interestingly, FC and ReHo decreased in both the superior temporal gyrus, and the inferior parietal lobule. It is well-known that impairment of the precuneus and inferior parietal lobule can cause memory impairment. The posterior superior temporal gyrus belongs to the Wernicke's area and may be associated with visuo-spatial function of MCI patients. Therefore, we suspect that there is a decrease in functioning of these regions, which may partially support occurrence of clinical symptoms.
In conclusion, a relationship between the three factors in predicting AD conversion remains to be considered. Although literature on neuroimaging studies of MCI is abundant, it is necessary to analyze and evaluate these studies. The significance of this meta-study is to try and identify consistent differential regions in DMN and provide specific biomarkers in a diagnosis of MCI. In this study, the middle frontal gyrus, cingulate gyrus, precuneus, temporal lobe, fusiform gyrus, parietal lobule and parahippocampal gyrus were utilized as biomarkers to predict the occurrence of AD. There is no doubt that our meta-analysis produced interesting and meaningful results, such as TMS therapy and drug therapy, which can be used to select the appropriate targets for optimal treatment. Our findings provide a specific imaging feature for diagnosis of MCI and is the basis for further research.
Limitations
Although this meta-analysis has produced some interesting results, it has also some limitations. First of all, there is some heterogeneity in the subject's age, sex, years of education and other factors. However, none of these factors had a material effect on the outcomes. Next, we selected and included literature from different data sources, using different pre-processing, statistical and imaging methods, which led to some differences. In this case, we tried to select abundant data sources in the database in order to improve coverage of the literature, and select several researchers to identify the screening work during the extraction process. This further reduced difference in outcomes that resulted from this cause. Finally, we do not rule out the literature based on seed method, and selection of the seed is subject to the operator's subjective influence. The different seed selection point will influence results to some extent. Although seed-based algorithms are not excluded, inclusion of literature on such algorithms also enriched our results.
Conclusion
Herein, we performed ALE meta-analysis in patients with MCI in order to determine functional changes in DMN. In our results, we found that function of altered brain regions were mainly in the precuneus, ACC, frontotemporal parietal lobe, some putamen and marginal lobe, among which the damage and compensatory mechanisms coexisted. Changes in these specific brain regions can help identify potential imaging biomarkers for MCI. It also explains the pathology of MCI, to a certain extent, and provides a novel specific target and direction for clinical diagnosis and treatment.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
QY, WQ, CX, and JC designed the study. HG, GH, SC, WX, YS, and XZ screened the literature. QY, WQ, CX, and SC collected the data. QY and WQ: analyzed the data and prepared the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81701675); the Key Project supported by Medical Science and technology development Foundation, Nanjing Department of Health (No. JQX18005); the Cooperative Research Project of Southeast University-Nanjing Medical University (No. 2018DN0031); the Key Research and Development Plan (Social Development) Project of Jiangsu Province (No. BE2018608); the Innovation and Entrepreneurship Training Program for College Students in Jiangsu Province (No.201810312061X; 201910312035Z); Jiangsu Provincial Natural Science Foundation-Youth Foundation Projects (BK20180370).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Adriaanse S. M., Sanz-Arigita E. J., Binnewijzend M. A., Ossenkoppele R., Tolboom N., van Assema D. M., et al. (2014). Amyloid and its association with default network integrity in Alzheimer's disease. Hum. Brain Mapp. 35, 779–791. 10.1002/hbm.22213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F., Pievani M., Geroldi C., Copetti M., Frisoni G. B., Filippi M. (2012). Resting state fMRI in Alzheimer's disease: beyond the default mode network. Neurobiol. Aging. 33, 1564–1578. 10.1016/j.neurobiolaging.2011.06.007 [DOI] [PubMed] [Google Scholar]
- Bai F., Watson D. R., Yu H., Shi Y., Yuan Y., Zhang Z. (2009). Abnormal resting-state functional connectivity of posterior cingulate cortex in amnestic type mild cognitive impairment. Brain Res. 1302, 167–174, 10.1016/j.brainres.2009.09.028 [DOI] [PubMed] [Google Scholar]
- Bai F., Zhang Z., Yu H., Shi Y., Yuan Y., Zhu W., et al. (2008). Default-mode network activity distinguishes amnestic type mild cognitive impairment from healthy aging: a combined structural and resting-state functional MRI study. Neurosci. Lett. 438, 111–115. 10.1016/j.neulet.2008.04.021 [DOI] [PubMed] [Google Scholar]
- Balachandar R., John J. P., Saini J., Kumar K. J., Joshi H., Sadanand S., et al. (2015). A study of structural and functional connectivity in early Alzheimer's disease using rest fMRI and diffusion tensor imaging. Int. J. Geriatr. Psychiatry 30, 497–504. 10.1002/gps.4168 [DOI] [PubMed] [Google Scholar]
- Barban F., Mancini M, Cercignani M., Adriano F, Perri R., Annicchiarico R., et al. (2017). A pilot study on brain plasticity of functional connectivity modulated by cognitive training in mild Alzheimer's disease and mild cognitive impairment. Brain Sci. 7:50. 10.3390/brainsci7050050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharath S., Joshi H., John J. P., Balachandar R., Sadanand S., Saini J., et al. (2017). A multimodal structural and functional neuroimaging study of amnestic mild cognitive impairment. Am. J. Geriatr. Psychiatry. 25, 158–169. 10.1016/j.jagp.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Bi X. A., Sun Q., Zhao J., Xu Q., Wang L. (2018). Non-linear ICA analysis of resting-state fMRI in mild cognitive impairment. Front. Neurosci. 12:413. 10.3389/fnins.2018.00413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokde A. L., Lopez-Bayo P., Meindl T., Pechler S., Born C., Faltraco F., et al. (2006). Functional connectivity of the fusiform gyrus during a face-matching task in subjects with mild cognitive impairment. Brain 129, 1113–1124. 10.1093/brain/awl051 [DOI] [PubMed] [Google Scholar]
- Bosch B., Bartrés-Faz D., Rami L., Arenaza-Urquijo E. M., Fernández-Espejo D., Junqué C., et al. (2010). Cognitive reserve modulates task-induced activations and deactivations in healthy elders, amnestic mild cognitive impairment and mild Alzheimer's disease. Cortex 46, 451–461. 10.1016/j.cortex.2009.05.006 [DOI] [PubMed] [Google Scholar]
- Briggs R. G., Tanglay O., Dadario N. B., Young I. M., Fonseka R. D., Hormovas J., et al. (2021). The unique fiber anatomy of middle temporal gyrus default mode connectivity. Oper. Neurosurg. 21, E8–E14. 10.1093/ons/opab109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggen K., Kasper E., Dyrba M., Bruno D., Pomara N., Ewers M., et al. (2016). The primacy effect in amnestic mild cognitive impairment: associations with hippocampal functional connectivity. Front. Aging Neurosci. 8:244. 10.3389/fnagi.2016.00244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S., Huang L., Zou J., Jing L., Zhai B., Ji G., et al. (2015). Changes in thalamic connectivity in the early and late stages of amnestic mild cognitive impairment: a resting-state functional magnetic resonance study from ADNI. PLoS ONE 10:e0115573. 10.1371/journal.pone.0115573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J., Jo H. J., Kim H. J., Seo S. W., Kim H. S., Yoon U., et al. (2013). Functional alteration patterns of default mode networks: comparisons of normal aging, amnestic mild cognitive impairment and Alzheimer's disease. Eur. J. Neurosci. 37, 1916–1924. 10.1111/ejn.12177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conwell K., von Reutern B., Richter N., Kukolja J., Fink G. R., Onur O. A. (2018). Test-retest variability of resting-state networks in healthy aging and prodromal Alzheimer's disease. NeuroImage Clin. 19, 948–962. 10.1016/j.nicl.2018.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vogelaere F., Santens P., Achten E., Boon P., Vingerhoets G. (2012). Altered default-mode network activation in mild cognitive impairment compared with healthy aging. Neuroradiology 54, 1195–1206. 10.1007/s00234-012-1036-6 [DOI] [PubMed] [Google Scholar]
- Domic-Siede M., Irani M., Valdés J., Perrone-Bertolotti M., Ossandón T. (2021). Theta activity from frontopolar cortex, mid-cingulate cortex and anterior cingulate cortex shows different roles in cognitive planning performance. Neuroimage 226:117557. 10.1016/j.neuroimage.2020.117557 [DOI] [PubMed] [Google Scholar]
- Eickhoff S. B., Bzdok D., Laird A. R., Kurth F., Fox P. T. (2012). Activation likelihood estimation meta-analysis revisited. Neuroimage 59, 2349–2361. 10.1016/j.neuroimage.2011.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyler L. T., Elman J. A., Hatton S. N., Gough S., Mischel A. K., Hagler D. J., et al. (2019). Resting state abnormalities of the default mode network in mild cognitive impairment: a systematic review and meta-analysis. J. Alzheimers. Dis. 70, 107–120. 10.3233/JAD-180847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farras-Permanyer L., Mancho-Fora N., Montala-Flaquer M., Gudayol-Ferre E., Gallardo-Moreno G. B., Zarabozo-Hurtado D., et al. (2019). Estimation of brain functional connectivity in patients with mild cognitive impairment. Brain Sci. 9:30. 10.3390/brainsci9120350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P., Marrelec G. (2008). The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage 42, 1178–1184. 10.1016/j.neuroimage.2008.05.059 [DOI] [PubMed] [Google Scholar]
- Gao S., Ming Y., Wang J., Gu Y., Ni S., Lu S., et al. (2020). Enhanced prefrontal regional homogeneity and its correlations with cognitive dysfunction/psychopathology in patients with first-diagnosed and drug-naive schizophrenia. Front Psychiatry 11:580570. 10.3389/fpsyt.2020.580570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardini S., Venneri A., Sambataro F., Cuetos F., Fasano F., Marchi M., et al. (2015). Increased functional connectivity in the default mode network in mild cognitive impairment: a maladaptive compensatory mechanism associated with poor semantic memory performance. J. Alzheimers. Dis. 45, 457–470. 10.3233/JAD-142547 [DOI] [PubMed] [Google Scholar]
- Giau V. V., Bagyinszky E., An S. S. A. (2019). Potential fluid biomarkers for the diagnosis of mild cognitive impairment. Int. J. Mol. Sci. 20:4149. 10.3390/ijms20174149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S. D., Arfanakis K., Fleischman D. A., Leurgans S. E., Tuminello E. R., Edmonds E. C., et al. (2012). Functional connectivity variations in mild cognitive impairment: associations with cognitive function. J. Int. Neuropsychol. Soc. 18, 39–48. 10.1017/s1355617711001299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B. (2003). The elusive concept of brain connectivity. Neuroimage 19, 466–470. 10.1016/S1053-8119(03)00112-5 [DOI] [PubMed] [Google Scholar]
- Huang T., Zhao Z., Yan C., Lu J., Li X., Tang C., et al. (2016). Altered Spontaneous Activity in Patients with Persistent Somatoform Pain Disorder Revealed by Regional Homogeneity. PLoS ONE 11:e0151360. 10.1371/journal.pone.0151360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing L.-L., Huang L.-Y., Huang D.-F., Niu J., Zhong Z. (2012). Amplitude of low frequency fluctuation at different frequency bands in early amnestic mild cognitive impairment: results from ADNI. J. Innov. Opt. Health Sci. 05:1150003. 10.1142/s1793545811500039 [DOI] [Google Scholar]
- Joo S. H., Lee C. U., Lim H. K. (2017). Apathy and intrinsic functional connectivity networks in amnestic mild cognitive impairment. Neuropsychiatr. Dis. Treat. 13, 61–67. 10.2147/ndt.S123338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D. W., Lim H. K., Joo S. H., Lee N. R., Lee C. U. (2018). Alterations in intra- and interregional intrinsic brain connectivity are differentially associated with memory performance in amnestic mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 46, 229–242. 10.1159/000493167 [DOI] [PubMed] [Google Scholar]
- Krajcovicova L., Barton M., Elfmarkova-Nemcova N., Mikl M., Marecek R., Rektorova I. (2017). Changes in connectivity of the posterior default network node during visual processing in mild cognitive impairment: staged decline between normal aging and Alzheimer's disease. J. Neural Transm. (Vienna, Austria: 1996) 124, 1607–1619. 10.1007/s00702-017-1789-5 [DOI] [PubMed] [Google Scholar]
- Lee E. S., Yoo K., Lee Y. B., Chung J., Lim J. E., Yoon B., et al. (2016). Default mode network functional connectivity in early and late mild cognitive impairment: results from the Alzheimer's disease neuroimaging initiative. Alzheimer Dis. Assoc. Disord. 30, 289–296. 10.1097/wad.0000000000000143 [DOI] [PubMed] [Google Scholar]
- Li H. J., Dai X. J., Gong H. H., Nie X., Zhang W., Peng D. C. (2015). Aberrant spontaneous low-frequency brain activity in male patients with severe obstructive sleep apnea revealed by resting-state functional MRI. Neuropsychiatr. Dis. Treat. 11, 207–214. 10.2147/NDT.S73730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Zheng G., Zheng Y., Xiong Z., Xia R., Zhou W., et al. (2017). Alterations in resting-state functional connectivity of the default mode network in amnestic mild cognitive impairment: an fMRI study. BMC Med. Imaging. 17:48. 10.1186/s12880-017-0221-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Wang F., Liu X., Cao D., Cai L., Jiang X., et al. (2020). Changes in brain function networks in patients with amnestic mild cognitive impairment: a resting-state fMRI study. Front. Neurol. 11:554032. 10.3389/fneur.2020.554032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wang X., Li Y., Sun Y., Sheng C., Li H., et al. (2016). Abnormal resting-state functional connectivity strength in mild cognitive impairment and its conversion to Alzheimer's disease. Neural Plast. 2016:4680972. 10.1155/2016/4680972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Bai L., Dai R., Zhong C., Wang H., You Y., et al. (2012). Exploring the effective connectivity of resting state networks in mild cognitive impairment: an fMRI study combining ICA and multivariate Granger causality analysis. Annu Int Conf IEEE Eng Med Biol Soc 2012, 5454–5457. 10.1109/embc.2012.6347228 [DOI] [PubMed] [Google Scholar]
- Lu X., Chen J., Shu H., Wang Z., Shi Y. M., Yuan Y. G., et al. (2020). Predicting conversion to Alzheimer's disease among individual high-risk patients using the Characterizing AD Risk Events index model. CNS Neurosci. Ther. 26, 720–729. 10.1111/cns.13371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X., Jiaerken Y., Huang P., Xu X. J., Qiu T., Jia Y., et al. (2018). Alteration of regional homogeneity and white matter hyperintensities in amnestic mild cognitive impairment subtypes are related to cognition and CSF biomarkers. Brain Imaging Behav. 12, 188–200. 10.1007/s11682-017-9680-4 [DOI] [PubMed] [Google Scholar]
- Matura S., Köhler J., Reif A., Fusser F., Karakaya T., Scheibe M., et al. (2020). Intrinsic functional connectivity, CSF biomarker profiles and their relation to cognitive function in mild cognitive impairment. Acta Neuropsychiatr. 32, 206–213. 10.1017/neu.2019.49 [DOI] [PubMed] [Google Scholar]
- Min J., Zhou X. X., Zhou F., Tan Y., Wang W. D. (2019). A study on changes of the resting-state brain function network in patients with amnestic mild cognitive impairment. Braz. J. Med. Biol. Res. 52:e8244. 10.1590/1414-431x20198244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monosov I. E., Haber S. N., Leuthardt E. C., Jezzini A. (2020). Anterior cingulate cortex and the control of dynamic behavior in primates. Curr. Biol. 30, R1442–R1454. 10.1016/j.cub.2020.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P., Zhu L., Yu T., Shi H., Zhang B., Qin R., et al. (2017). Aberrant spontaneous low-frequency brain activity in amnestic mild cognitive impairment: A meta-analysis of resting-state fMRI studies. Ageing Res. Rev. 35, 12–21. 10.1016/j.arr.2016.12.001 [DOI] [PubMed] [Google Scholar]
- Postema M. C., De Marco M., Colato E., Venneri A. (2019). A study of within-subject reliability of the brain's default-mode network. MAGMA 32, 391–405. 10.1007/s10334-018-00732-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z., Wu X., Wang Z., Zhang N., Dong H., Yao L., et al. (2010). Impairment and compensation coexist in amnestic MCI default mode network. NeuroImage 50, 48–55. 10.1016/j.neuroimage.2009.12.025 [DOI] [PubMed] [Google Scholar]
- Raichle M. E. (2015). The brain's default mode network. Annu. Rev. Neurosci. 38, 433–447. 10.1146/annurev-neuro-071013-014030 [DOI] [PubMed] [Google Scholar]
- Robinson J. L., Laird A. R., Glahn D. C., Blangero J., Sanghera M. K., Pessoa L., et al. (2012). The functional connectivity of the human caudate: an application of meta-analytic connectivity modeling with behavioral filtering. Neuroimage 60, 117–129. 10.1016/j.neuroimage.2011.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T., Gamo N. J. (2019). Cognitive functions associated with developing prefrontal cortex during adolescence and developmental neuropsychiatric disorders. Neurobiol. Dis. 131:104322. 10.1016/j.nbd.2018.11.007 [DOI] [PubMed] [Google Scholar]
- Schmahmann J. D., Weilburg J. B., Sherman J. C. (2007). The neuropsychiatry of the cerebellum - insights from the clinic. Cerebellum 6, 254–267. 10.1080/14734220701490995 [DOI] [PubMed] [Google Scholar]
- Stoodley C. J., Schmahmann J. D. (2009). Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage 44, 489–501. 10.1016/j.neuroimage.2008.08.039 [DOI] [PubMed] [Google Scholar]
- Su F., Shu H., Ye Q., Xie C., Yuan B., Zhang Z., et al. (2017). Integration of multilocus genetic risk into the default mode network longitudinal trajectory during the Alzheimer's disease process. J. Alzheimers. Dis. 56, 491–507. 10.3233/JAD-160787 [DOI] [PubMed] [Google Scholar]
- Tao W., Li X., Zhang J., Chen Y., Ma C., Liu Z., et al. (2017). Inflection point in course of mild cognitive impairment: increased functional connectivity of default mode network. J. Alzheimers. Dis. 60, 679–690. 10.3233/JAD-170252 [DOI] [PubMed] [Google Scholar]
- Teipel S. J., Wohlert A., Metzger C., Grimmer T., Sorg C., Ewers M., et al. (2017). Multicenter stability of resting state fMRI in the detection of Alzheimer's disease and amnestic MCI. Neuroimage-Clinical 14, 183–194. 10.1016/j.nicl.2017.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Pan Y., Liu Y., Xu K., Hao L., Huang F., et al. (2018). Aberrant default mode network in amnestic mild cognitive impairment: a meta-analysis of independent component analysis studies. Neurol. Sci. 39, 919–931. 10.1007/s10072-018-3306-5 [DOI] [PubMed] [Google Scholar]
- Wang J., Zuo X., Dai Z., Xia M., Zhao Z., Zhao X., et al. (2013). Disrupted functional brain connectome in individuals at risk for Alzheimer's disease. Biol. Psychiatry 73, 472–481. 10.1016/j.biopsych.2012.03.026 [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhao X., Xu S., Yu L., Wang L., Song M., et al. (2015). Using regional homogeneity to reveal altered spontaneous activity in patients with mild cognitive impairment. Biomed Res. Int. 2015:807093. 10.1155/2015/807093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Yan C., Zhao C., Qi Z., Zhou W., Lu J., et al. (2011). Spatial patterns of intrinsic brain activity in mild cognitive impairment and Alzheimer's disease: a resting-state functional MRI study. Hum. Brain Mapp. 32, 1720–1740. 10.1002/hbm.21140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler M., Teixeira C. V., Nogueira M. H., de Campos B. M., Damasceno B. P., Cendes F., et al. (2014). Differences and the relationship in default mode network intrinsic activity and functional connectivity in mild Alzheimer's disease and amnestic mild cognitive impairment. Brain Connect. 4, 567–574. 10.1089/brain.2014.0234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiepert D. A., Lowe V. J., Knopman D. S., Boeve B. F., Graff-Radford J., Petersen R. C., et al. (2017). A robust biomarker of large-scale network failure in Alzheimer's disease. Alzheimers Dement. 6, 152–161. 10.1016/j.dadm.2017.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan Y., Meng C., Yang Y., Zhu C., Wang L., Yan Q., et al. (2012). Resting-state brain activity in adult males who stutter. PLoS ONE 7:e30570. 10.1371/journal.pone.0030570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue C., Yuan B., Yue Y., Xu J., Wang S., Wu M., et al. (2019). Distinct disruptive patterns of default mode subnetwork connectivity across the spectrum of preclinical Alzheimer's disease. Front. Aging Neurosci. 11:307. 10.3389/fnagi.2019.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H., Zhang Y., Chen H., Wang Y., Liu Y. (2013). Altered effective connectivity of the default mode network in resting-state amnestic type mild cognitive impairment. J. Int. Neuropsychol. Soc. 19, 400–409. 10.1017/s1355617712001580 [DOI] [PubMed] [Google Scholar]
- Yao H., Zhou B., Zhang Z., Wang P., Guo Y., Shang Y., et al. (2014). Longitudinal alteration of amygdalar functional connectivity in mild cognitive impairment subjects revealed by resting-state FMRI. Brain Connect. 4, 361–370. 10.1089/brain.2014.0223 [DOI] [PubMed] [Google Scholar]
- Yi D., Choe Y. M., Byun M. S., Sohn B. K., Seo E. H., Han J., et al. (2015). Differences in functional brain connectivity alterations associated with cerebral amyloid deposition in amnestic mild cognitive impairment. Front. Aging Neurosci. 7:15. 10.3389/fnagi.2015.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C., Yi L., Jia L., Wang J., Liu P., Guo Y., et al. (2014). Early morphological brain abnormalities in patients with amnestic mild cognitive impairment. Transl. Neurosci. 5, 253–259. 10.2478/s13380-014-0234-6 [DOI] [Google Scholar]
- Yuan R., Di X., Kim E. H., Barik S., Rypma B., Biswal B. B. (2013). Regional homogeneity of resting-state fMRI contributes to both neurovascular and task activation variations. Magn. Reson. Imaging 31, 1492–1500. 10.1016/j.mri.2013.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X., Han Y., Wei Y., Xia M., Sheng C., Jia J., et al. (2016). Regional homogeneity changes in amnestic mild cognitive impairment patients. Neurosci. Lett. 629, 1–8. 10.1016/j.neulet.2016.06.047 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Liu Y., Jiang T., Zhou B., An N., Dai H., et al. (2012). Altered spontaneous activity in Alzheimer's disease and mild cognitive impairment revealed by regional homogeneity. Neuroimage 59, 1429–1440. 10.1016/j.neuroimage.2011.08.049 [DOI] [PubMed] [Google Scholar]
- Zhao Z., Lu J., Jia X., Chao W., Han Y., Jia J., et al. (2014). Selective changes of resting-state brain oscillations in aMCI: an fMRI study using ALFF. Biomed Res. Int. 2014:920902. 10.1155/2014/920902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z. L., Fan F. M., Lu J., Li H. J., Jia L. F., Han Y., et al. (2015). Changes of gray matter volume and amplitude of low-frequency oscillations in amnestic MCI: An integrative multi-modal MRI study. Acta Radiol 56, 614–621. 10.1177/0284185114533329 [DOI] [PubMed] [Google Scholar]
- Zhen D., Xia W., Yi Z. Q., Zhao P. W., Zhong J. G., Shi H. C., et al. (2018). Alterations of brain local functional connectivity in amnestic mild cognitive impairment. Transl. Neurodegener. 7:26. 10.1186/s40035-018-0134-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Dougherty J. H., Hubner K. F., Bai B., Cannon R. L., Hutson R. K. (2008). Abnormal connectivity in the posterior cingulate and hippocampus in early Alzheimer's disease and mild cognitive impairment. Alzheimers. Dement. 4, 265–270. 10.1016/j.jalz.2008.04.006 [DOI] [PubMed] [Google Scholar]
- Zou F., Wu X., Zhai T., Lei Y., Shao Y., Jin X., et al. (2015). Abnormal resting-state functional connectivity of the nucleus accumbens in multi-year abstinent heroin addicts. J. Neurosci. Res. 93, 1693–1702. 10.1002/jnr.23608 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.