ABSTRACT
Lyme disease is commonly diagnosed by serologic response to Borrelia burgdorferi and related species, but the relationship between serologic targets and clinical features is unknown. We developed a multiantigen Luminex-based panel and evaluated IgG responses in 527 children 1 to 21 years of age assessed for Lyme disease across 4 Pedi Lyme Net emergency departments, including 127 Lyme cases defined by either an erythema migrans (EM) lesion or positive C6 enzyme immunoassay followed by immunoblotting and 400 patients considered clinical mimics. Of 42 antigens tested, 26 elicited specific reactivity in Lyme patients without marked age-dependent variation. Children with single EM lesions typically lacked Borrelia-specific IgG. By principal-component analysis, children with early disseminated and late Lyme disease clustered separately from clinical mimics and also from each other. Neurological disease and arthritis exhibited distinct serologic responses, with OspC variants overrepresented in neurological disease and p100, BmpA, p58, and p45 overrepresented in arthritis. Machine learning identified a 3-antigen panel (VlsE_Bb, p41_Bb, and OspC_Bafz) that distinguished Lyme disease from clinical mimics with a sensitivity of 86.6% (95% confidence interval [CI], 80.3 to 92.1) and a specificity of 95.5% (95% CI, 93.4 to 97.4). Sensitivity was much lower in early Lyme disease (38.5%; 95% CI, 15.4 to 69.2). Interestingly, 17 children classified as Lyme mimics had a positive 3-antigen panel, suggesting that more comprehensive serologic analysis could help refine Lyme diagnosis. In conclusion, multiplex antigen panels provide a novel approach to understanding the immune response in Lyme disease, potentially helping to facilitate accurate diagnosis and to understand differences between clinical stages.
KEYWORDS: Lyme disease, children, serology
INTRODUCTION
Acute Lyme disease is conventionally divided into three clinical stages based on time from infection (1). Early Lyme disease typically presents as an erythema migrans (EM) lesion, developing days to weeks after a causative tick bite. Early disseminated Lyme disease presents weeks to months after a tick bite, with manifestations such as multiple EM lesions, carditis, meningitis, or cranial nerve palsy. Late Lyme disease presents months to years after infection, typically with arthritis (2). Although untreated patients can develop neurological manifestations first and arthritis later (3), most patients develop only one clinical manifestation, raising the possibility that differences in host immune response contribute to disease phenotype.
Multiplexed antigen assays could replace conventional two-tier testing for the diagnosis of Lyme disease (4). A panel of 10 surface antigens demonstrated improved sensitivity compared to conventional two-tier testing in adults with Lyme disease compared to healthy controls (5). In 2019, the Federal Drug Administration approved a 4-antigen multiplex immunoassay as a first-tier Lyme disease diagnostic (6), and multiantigen panels have been developed as a point-of-care diagnostics for early Lyme disease (7, 8). In this study of children and young adults evaluated for Lyme disease, we sought to identify antigens that differentiate patients with Lyme disease from those with similar symptoms but ultimately determined to have an alternate diagnosis and to determine whether serologic response differs by age (9, 10).
MATERIALS AND METHODS
Study design.
We performed a prospective cohort study between June 2015 and October 2016 at four emergency departments located in Lyme disease areas of endemicity within the Pedi Lyme Net collaborative network (11): A. I. Dupont Children’s Hospital (Wilmington, DE), Boston Children’s Hospital (Boston, MA), Children’s Hospital of Wisconsin (Milwaukee, WI), and Hasbro Children’s Hospital (Providence, RI). The institutional review board of each participating institution approved the study protocol, including permission for data and sample sharing.
Patients.
We included patients 1 to 21 years of age whose treating clinicians obtained Lyme disease testing as part of the diagnostic evaluation. Study participation included collection of clinical phenotype as well as research biosamples. We excluded children who did not have a research serum sample obtained or who did not have symptoms consistent with acute Lyme disease.
Data collection.
We collected the following clinical data at enrollment: demographics, tick bite and Lyme disease history, clinical symptoms and duration, and physical examination findings. We classified a patient as pretreated if any antibiotics were administered within the 72 h preceding research biosample collection (12). One month after enrollment, study staff abstracted laboratory test results and clinical outcome through medical record review and direct patient contact. We used clinical history and physical examination to determine Lyme disease stage: early (single EM lesion), early disseminated (multiple EM lesions, fever, headache, facial palsy, or carditis), or late (arthritis) (13). Research serum samples collected at the time of enrollment were processed as soon as possible, typically within 12 h, and kept frozen at −80°C until research testing was performed.
Outcome measures.
We defined a case of Lyme disease based either on a physician-diagnosed EM rash or a positive two-tier Lyme disease serology: a first-tier C6 enzyme immunoassay (EIA) with an index value of ≥0.91 (Oxford Immunotec, Marlborough, MA) followed by a positive immunoblot (MarDx IgG/IgM Marblot; Trinity Biotech), interpreted using standardized criteria (2). We considered a positive IgM alone to indicate Lyme infection only if symptom duration was <30 days (14). Symptomatic patients who did not meet the Lyme disease case definition were classified as “clinical mimics.”
Multiplex antigen testing.
Patient serum was tested for antibodies against 42 antigens (22 antigens from Borrelia burgdorferi, 9 from other Borrelia species, 9 from other tick-borne pathogens, and 2 from Ixodes species ticks; see Table S1 in the supplemental material) using a Luminex 200 instrument with standardized dilution and plate incubation procedures (5). The string “mV” indicates linkage to a modified short sequence of the C6 peptide (15, 16). Biotinylated antigen was purchased from Bio-Rad (Hercules, CA, USA) and bound to streptavidin-coated beads. Pooled beads were incubated with serum followed by anti-human IgG-phycoerythrin. Mean fluorescence intensity (MFI) minus fluorescence background was quantified (5).
Selection of potentially informative antigens.
Antigens were excluded from further analysis if no meaningful overall variance was detected (standard error of MFI of <50 over all samples; n = 14) or if Luminex failed to calculate a fluorescence in fewer than 90% of samples (n = 1). The remaining 27 antigens were further considered. For all subsequent analyses except the assessment of diagnostic test performance, only patients with results in all 27 antigens were analyzed.
Variation of serological reactivity.
We explored antigen expression in two a priori comparisons, (i) age of patient and (ii) disease stage (early versus early disseminated versus late Lyme disease). For age of patient, reactivity was assessed by Pearson’s r (unadjusted P values), and patients were divided into three age groups: 1 to 7, 8 to 12, and 13 to 21 years. An expression score was calculated as the MFI of the 27-antigen panel. Distribution differences in the expression scores of different age ranges were assessed by a 3-sample Anderson-Darling test. For disease stage, expression was separated by stage, and the Kruskal-Wallis test was then used per antigen, followed by post hoc Dunn’s test for the pairwise comparisons; P values were adjusted by Holm’s correction. was used as an estimate of effect size for the Kruskal-Wallis test: (k, number of groups; N, number of observations; H, H statistic) (17). Stage dependency was further assessed by principal component (PC) analysis with reactivity against the 27 antigens calculated and plotted in both 2 and 3 dimensions. Quality of representation (cos2) was calculated for the 2-dimension scenario and compared with the 3-dimension scenario. Intercorrelation of variables with PC1 and PC2 was visualized in a correlation circle, and the relative contribution of variables to each PC was calculated as cos2 divided by the total cos2 of each PC. Contributions of antigens to the first three PCs were compared with a hypothetical uniform variable contribution scenario. Nonnormality was confirmed by Shapiro-Wilk. We then used the Mann-Whitney U test with P value adjustment by Holm’s correction, followed by a calculation of (Z, Z statistic; n, number of observations), as a measure of effect size (18) for significant comparisons.
Diagnostic marker optimization.
To identify the optimal diagnostic biomarker panel, we excluded children who had been pretreated with antibiotics prior to enrollment. We used penalized regression analysis using Least Absolute Shrinkage and Selection Operator (LASSO) to select predictors. For regularization, λ was tuned by machine learning (with caret version 6.0–86) (19), using 10-fold cross-validation with a high area under the receiver operating characteristic (ROC) curve as the target metric. Internal validity was assessed by repeating the 10-fold cross-validation 100 times. The Youden’s J statistic (J = sensitivity + specificity – 1) was employed as the optimal cut point for the estimated probability to maximize both sensitivity and specificity. Sensitivity and specificity were reported on the cohort that received valid results in the antigens that were not dropped by the regression. Confidence intervals were calculated with 2,000 stratified bootstrap replicates.
We used Fisher’s exact test to compare proportions. We used R (v4.0.4) and BioRender.com for statistical analyses and visualization (19–28).
RESULTS
Patient population.
We enrolled 583 children across the 4 Pedi Lyme Net sites between June 2015 and October 2016, of whom 527 (90%) met initial study inclusion criteria (Table 1). Overall, 127 (24%) had Lyme disease and 400 (76%) were clinical mimics. Among the 127 children with Lyme disease, 13 had a single EM lesion and were considered early Lyme, whereas 55 had early disseminated and 59 late Lyme disease.
TABLE 1.
Clinical characteristics of enrolled children with and without Lyme disease
| Parameter | Value(s) for children with or without Lyme disease |
|
|---|---|---|
| With (N = 127) | Without (N = 400) | |
| Median age, yr (interquartile range) | 9 (6, 14) | 9 (6, 13) |
| Male gender [no./total no. (%)] | 86/127 (68) | 202/400 (51) |
| Female gender [no./total no. (%)] | 41/127 (32) | 198/400 (49) |
| Race [no./total no. (%)] | ||
| White | 118/126 (93) | 313/388 (81) |
| Black | 5/126 (4) | 44/388 (11) |
| Asian | 2/126 (2) | 12/388 (3) |
| Other | 1/126 (1) | 19/388 (5) |
| Hispanic | 11/126 (9) | 68/397 (17) |
| Presentation during peak Lyme seasona [no./total no. (%)] | 103/127 (81) | 320/400 (80) |
| Early (single EM lesion) [no./total no. (%)] | 13/127 (10) | NAb |
| Early disseminated [no./total no. (%)] | 55/127 (44) | 212/400 (53) |
| Multiple EM lesions (no.) | 2 | NA |
| Facial palsy (no.) | 21 | 28 |
| Meningitis (no.) | 26 | 181 |
| Carditis (no.) | 6 | 3 |
| Late (arthritis) [no./total no. (%)] | 59/127 (46) | 167/400 (42) |
| Nonspecific symptoms [no./total no. (%)] | NA | 21/400 (5) |
Peak Lyme season defined as June to October.
NA, not applicable.
Application of a multiplex antigen Luminex panel.
The overall design of the multiplex Luminex assessment of Lyme patients and controls is depicted in Fig. 1a. We limited the remainder of our analyses to the 468 patients (89% of enrolled) with data for all antigens. For the 27 informative antigens, we performed hierarchical clustering of raw MFI values (Fig. 1b), observing clear separation of cases from symptomatic controls. Further, by k-means clustering (k = 2), we observed that 27/55 (49%) of early disseminated Lyme patients and 46/59 (78%) of late Lyme disease patients clustered together.
FIG 1.
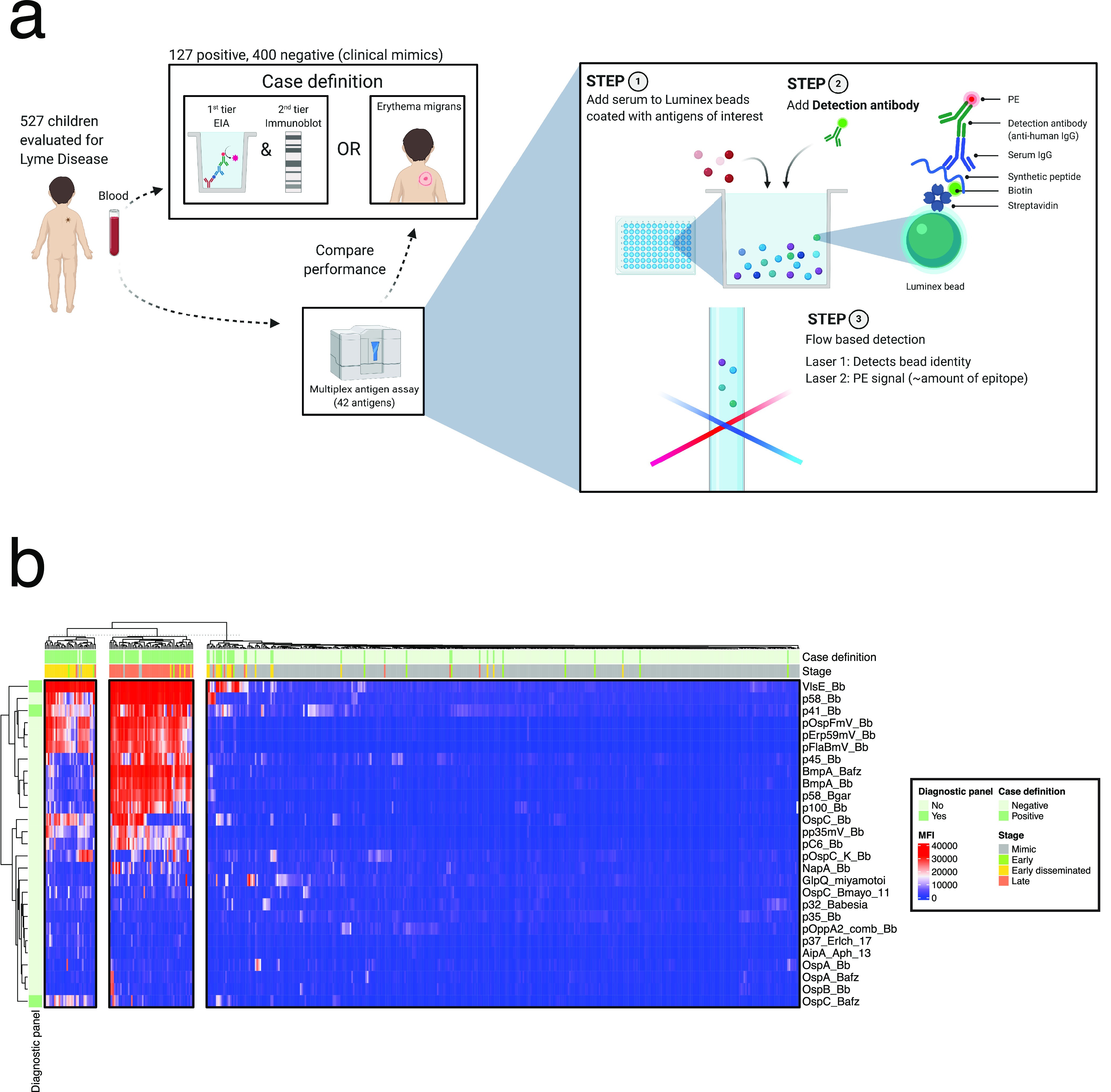
Multiplexed determination of serologic reactivity to Lyme disease in children. (a) Overview of case definition and the diagnostic procedure. (b) Hierarchical clustering of MFI among the 468 patients. Heatmap columns were partitioned by k-means clustering (k = 3). Row annotation indicates antigens that were part of the derived diagnostic panel (Fig. 5).
Serologic discrimination of patients from clinical mimics.
Corrected for multiple comparisons, we observed differential expression of the MFI for 26 of the 27 antigens for children with Lyme disease compared to clinical mimics (Fig. 2). A large effect size (r ≥ 0.50) was observed for 15 antigens (pFlaBmV_Bb, VlsE_Bb, p41_Bb, pOspFmV_Bb, OspC_Bb, pErp59mV_Bb, p58_Bb, pC6_Bb, pp35mV_Bb, BmpA_Bafz, BmpA_Bb, OspC_Bafz, p100_Bb, p58_Bgar, and p45_Bb), and a small to moderate effect size (0.10 ≤ r < 0.50) was observed for the remaining 11 antigens (OspC_Bmayo_11, pOspC_K_Bb, NapA_Bb, AipA_Aph_13, p32_Babesia, p37_Erlch_17, OspA_Bafz, OspA_Bb, GlpQ_miyamotoi, OspB_Bb, and pOppA2_comb_Bb). Findings from uninformative antigens excluded from further analysis are shown in Fig. S1 in the supplemental material.
FIG 2.
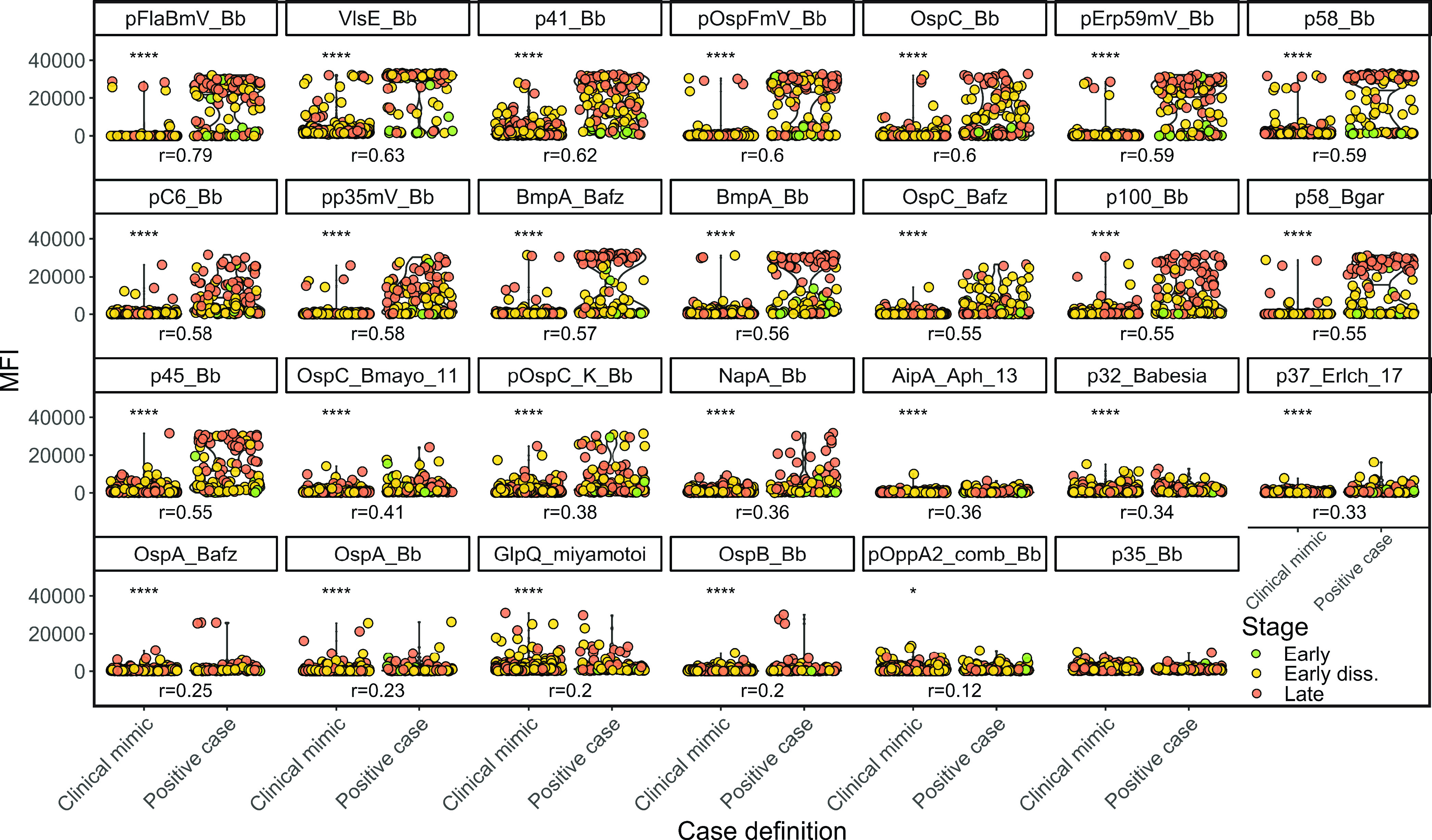
Capability of Borrelia antigens to discriminate Lyme patients from clinical mimic. Uninformative antigens (Fig. S1) were filtered as stated in Materials and Methods. Asterisks indicate significance. Early diss., early disseminated. Distribution of antigen expression as a function of case definition. For significant comparisons (P ≤ 0.05) in the Mann-Whitney U test, r is shown as a measure of effect size. Asterisks indicate statistical significance according to the respective P value (*, P ≤ 0.05, **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001).
Serologic reactivity to Borrelia antigens does not differ by age.
To assess whether patient age correlated with antigen response, we calculated Pearson’s r for each antigen and observed no significant bivariate correlation with age (Fig. S2a). After separating positive cases into three age groups (1 to 7, 8 to 12, and 13 to 21 years), the calculated MFI scores were similar across age groups (Fig. S2b). Finally, we represented the distribution of expression frequencies in a density plot (Fig. S2c and d) and calculated a 3-sample Anderson-Darling test, finding no significant difference with age. Taken together, these data identified no marked age-specific differences in seroreactivity to Borrelia antigens in children with Lyme disease, allowing us to pool data for subsequent comparisons.
Serological response varies with disease stage.
Since hierarchical clustering had suggested clustering not only by Lyme versus clinical mimic but also within Lyme disease by clinical stage (Fig. 1b), we examined the association between serologic response and clinical presentation in more detail. Plotting reactivity against each antigen against disease stage, we found that patients with early Lyme disease were negative for most antigens, likely because serologic expansion had not had time to occur, while serologic responses increased progressively from early disseminated to late Lyme disease (Fig. 3a). Intriguingly, not all antigens varied in parallel across stages (Fig. 3b). By principal component (PC) analysis, clinical mimics largely clustered with early Lyme disease, while early disseminated and late Lyme patients formed distinct clusters (Fig. 4a). PC1 differentiated cases from mimics, whereas PC2 distinguished early disseminated from late disease. These two PCs explained ∼59% of the total variance in seroreactivity among Lyme patients (Fig. S3a). A third PC contributed a further ∼8%, resolving the apparent overlap of several late Lyme patients with controls (Video S1, Fig. S3b). Two patients considered clinical mimics by our Lyme disease definition clustered with early disseminated disease and 3 clustered with late Lyme disease (Fig. 4a and Video S1); the clinical presentations of these 5 potentially misclassified patients are further described in Table S2.
FIG 3.
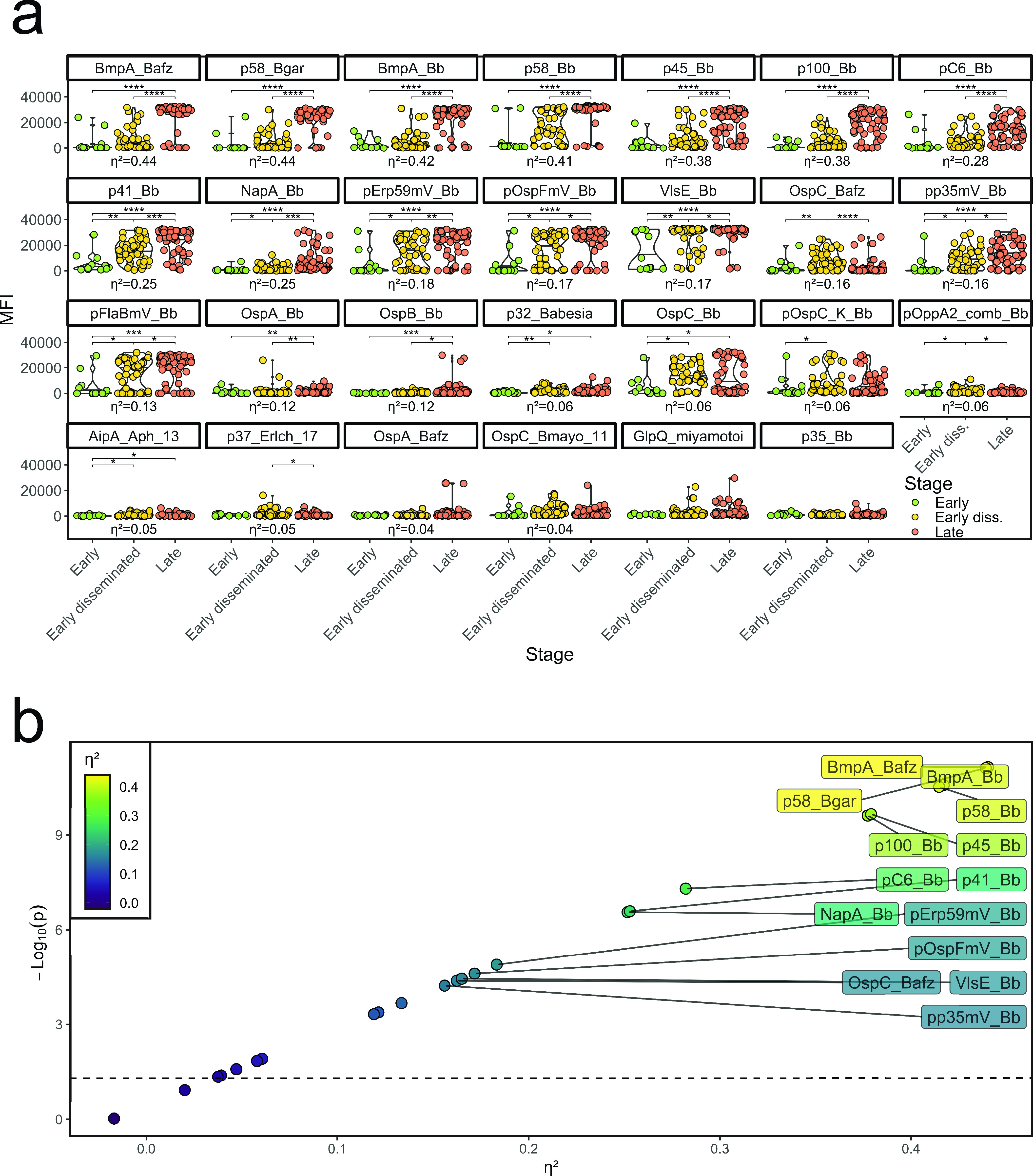
Stage-dependent expression. Uninformative antigens were filtered as stated in Materials and Methods. Asterisks indicate significance. (a) Distribution of antigen expression as a function of the clinical stage. Patients were filtered for positivity in the case definition. Only patients who did not receive antibiotics prior to the assay are shown. Kruskal-Wallis test followed by post hoc Holm’s-corrected Dunn’s test. For significant comparisons, indicated by asterisks (P ≤ 0.05), effect size is indicated by η2, based on Kruskal-Wallis’ H statistic (see Materials and Methods, “Statistical analysis”). (b) Scatterplot of effect size versus negative log of P value, obtained as described in panel a. Effect sizes that were considered large (η2 ≥ 0.14) were labeled. Asterisks indicate statistical significance according to the respective P value (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001).
FIG 4.
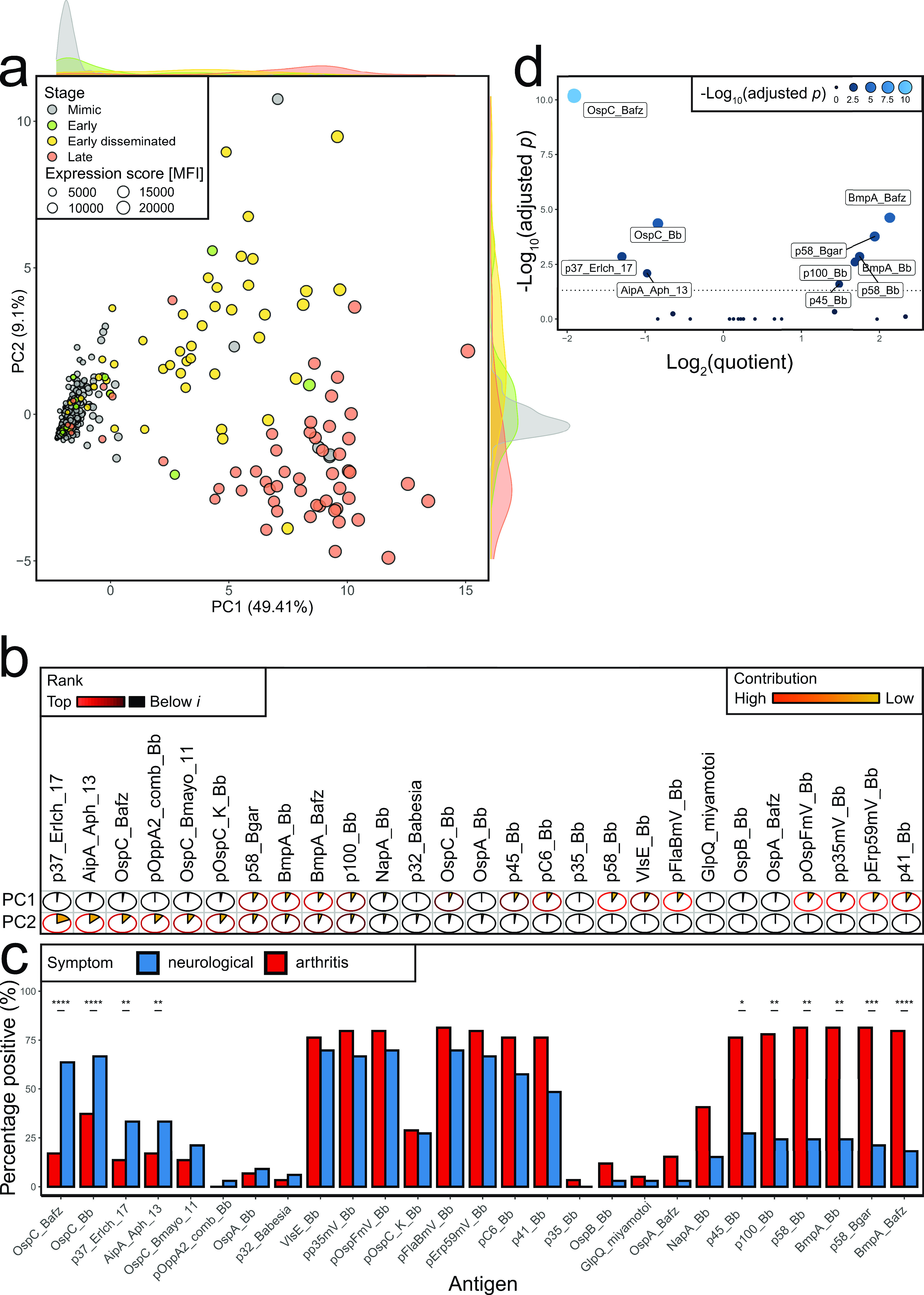
Stage dependency of serological response. Uninformative antigens (Fig. S1) were filtered as stated in Materials and Methods. Asterisks indicate significance. (a) Principal-component analysis of 27-antigen panel in 468 children (354 symptomatic controls, 13 early, 47 early disseminated, and 54 late-stage patients), colored by stage. To avoid overplotting, mimics were plotted first. Expression score was calculated as the mean MFI of the 27-antigen panel per patient. (b) Relative contribution of antigens per principal component. Pie charts and colors indicate the contribution to each individual principal component. Circle outline was colored in shades of red if expression was higher than in a hypothetical uniform variable contribution scenario with expression i (PC1, n = 14; PC2, n = 10), and brightness indicates rank position (Fig. S3c). (c) Antigen positivity was defined as described in Materials and Methods. Shown is the percentage of patients with arthritis (defined by swollen joint) and neurological symptoms (defined by cranial neuritis on exam or meningitis on LP [cerebrospinal fluid white blood cells, ≥10 cells/mm3]). Results were sorted by Holm’ adjusted P value of Fisher’s exact test, first increasing for antigens which were positive in more patients with arthritis than with neurological symptoms. The remaining antigens were sorted by decreasing level. (d) Quotient of patients with arthritis divided by patients with neurological symptoms per antigen versus adjusted P value. Dashed line indicates an adjusted P value of 0.05. Asterisks indicate statistical significance according to the respective P value (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001).
To examine the contribution of each antigenic target to the first 2 PCs, we compared the contribution of each antigen to that expected for a hypothetical uniform variable, finding that 14 antigens contributed significantly to PC1 and 10 to PC2 (Fig. S3c). This finding is illustrated in vector format in Fig. S3d, with the relative contribution of each antigen to PC1 and PC2 depicted in Fig. 4b. Serologic response to antigens such as p41_Bb, p58_Bb, and VlsE_Bb mainly distinguished cases from controls, whereas p37_Erlch_17, AipA_Aph_13, OspC_Bafz, and others mainly distinguished disease stage. PC3 reflected three antigens (OspB_Bb, OspA_Bafz, and p35_Bb) that otherwise contributed minimally to PC1 and PC2 (Fig. S3b and c).
Inspection of the differences between early disseminated and late Lyme disease with respect to PC2 antigens disclosed three patterns. For 4 antigens, responses in early disseminated disease were lower than in late disease (p58_Bgar, BmpA_Bb, BmpA_Bafz, and p100_Bb); 4 antigens exhibited more robust responses in early disseminated disease than late disease, although the magnitude of difference typically was modest (p37_Erlch_17, OspC_Bafz, pOppA2_comb_Bb, and pOspC_K_Bb); in a third group, no clear difference in reactivity was observed across stages (AipA_Aph_13 and OspC_Bmayo_11) (Fig. 3a).
Based on these findings, we examined whether distinct antibodies correlated with neurological or arthritic Lyme manifestations, focusing on the most unambiguous cases: neurological Lyme disease defined as cranial neuritis on exam or cerebrospinal fluid pleocytosis on lumbar puncture (n = 33) and arthritic Lyme disease manifested as overt joint swelling on physician examination (n = 59). Using the clinical mimics to define the upper limit of normal for each antigen at 98%, and after correction for multiple hypothesis testing, we found that the fraction of patients positive for certain antigens differed between neurological and arthritis groups for 10 antigens (Fig. 4c and d). Four antigens were overrepresented in patients with neurological Lyme, and 6 were overrepresented in arthritis; for example, seroreactivity for p100_Bb, p45_Bb, p58_Bb, and BmpA_Bb was present in over 70% of patients with arthritis symptoms but fewer than 50% of patients with neurological symptoms. The antigen with greatest ability to discriminate Lyme patients from clinical mimics, VlsE_Bb, was equally represented in both populations, as were related (mV) antigens.
Optimization of a Lyme serologic biomarker panel.
To determine the optimal combination of serological markers to employ in the diagnosis of Lyme disease in children, we examined differences in expression of the 27 candidate antigens, beginning with the 420 children who had not been pretreated with antibiotics, given the potential effect of such pretreatment on serologic response (5). With 100 repeats of 10-fold cross-validation of a LASSO logistic regression model (Fig. S4), we found that 3 antigens provided the best discriminative ability, VlsE_Bb, p41_Bb, and OspC_Bafz (Fig. 5a), and determined a probability cutoff in our model using Youden’s J statistic. We applied the modified 3-antigen panel to our pediatric population (Table 2). In the full cohort, this panel had a sensitivity of 86.6% (95% confidence interval [CI], 80.3 to 92.1) and specificity of 95.5% (95% CI, 93.4 to 97.4) (Fig. 5b and c). Concordant with the PC analysis, these test characteristics were stage dependent. In the 55 patients presenting with symptoms compatible with early disseminated Lyme disease, sensitivity was 90.9% (95% CI, 83.6 to 98.2). In the patients presenting with symptoms compatible with late disease, sensitivity was 93.2% (95% CI, 86.4 to 98.3). The sensitivity of the 3-antigen panel in children with a single EM lesion (n = 13) was only 38.5% (95% CI, 15.4 to 69.2) (Table 2). Of the 17 Lyme mimics with a positive 3-antigen panel, 12 had clinical features suggestive of early disseminated and 5 of late Lyme disease (Fig. 5d and Table S2, Video S2).
FIG 5.
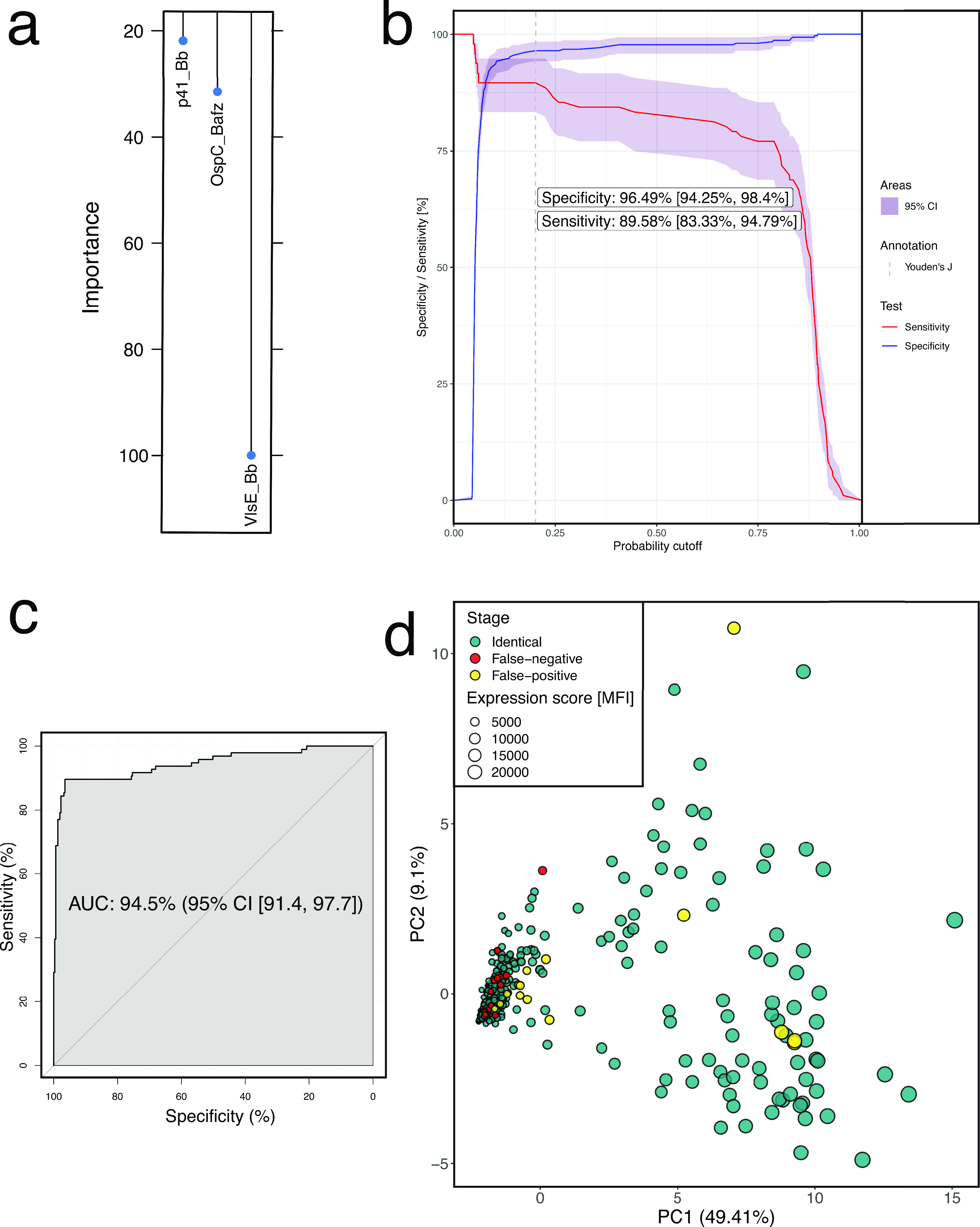
Optimizing a multiplexed antigen panel for Lyme diagnosis. (a) Relative importance of the predictors in the final logistic regression model. The regression coefficients were plotted relative to the strongest predictor. (b) Overall sensitivity and specificity as a function of probability cutoff. 95% CI was obtained by 2,000 stratified bootstrap replicates. The reported specificity and sensitivity were obtained by selecting the highest Youden’s J statistic as the probability cutoff. (c) ROC (receiver operating characteristic) curve of the logistic model using λ ≈ 0.042 after model tuning (Fig. S4a). (d) Principal component analysis from Fig. 4, colored by classification using the original case definition as the gold standard.
TABLE 2.
Test performance of the modified 3-antigen panel for the diagnosis of Lyme disease overall and by clinical stage
| Parameter | N | Performancea [n/N (%; 95% CI)] |
|||
|---|---|---|---|---|---|
| Sensitivity | Specificity | NPV | PPV | ||
| All patients | 506 | 110/127 (86.6; 80.3, 92.1) | 362/379 (95.5; 93.4, 97.4) | 362/379 (95.5; 93.5, 97.3) | 110/127 (86.6; 81.5, 92) |
| Clinical stage | |||||
| Early | 13 | 5/13 (38.5; 15.4, 69.2) | NA | NA | NA |
| Early disseminated | 267 | 50/55 (90.9; 83.6, 98.2) | 199/212 (93.9; 90.6, 96.7) | 199/204 (97.6; 95.6, 99.5) | 50/63 (79.4; 71.2, 88.3) |
| Late | 226 | 55/59 (93.2; 86.4, 98.3) | 163/167 (97.6; 95.2, 99.4) | 163/167 (97.6; 95.3, 99.4) | 55/59 (93.2; 87.1, 98.3) |
NA, not applicable.
DISCUSSION
We evaluated multiplex serology in children and young adults undergoing evaluation for Lyme disease, finding that seroreactivity against multiple antigens can effectively discriminate Lyme disease from its clinical mimics while also resolving differences across the clinical spectrum in a manner previously possible only through clinical and historical features of the presentation.
We identified a 3-antibody diagnostic panel that was reactive for Borrelia antigens (VlsE, p41, and OspC). Each of these synthetic antigens, derived from Borrelia surface proteins, have been included in previously derived multiplex Lyme disease antigen panels (5–8). The variable antigen VlsE is a surface lipoprotein from B. burgdorferi implicated in bacterial infection and immune evasion (29, 30). The C6 peptide, our first-tier Lyme disease diagnostic test, targets part of the VlsE protein, contributing to the ability of this antigen to discriminate between cases and mimics (15, 16). The p41 antigen forms part of the Borrelia flagellum. OspC (outer surface protein C) is a heterogenic class of lipoproteins in the outer membrane of spirochetes such as B. burgdorferi and B. afzelii that plays an important role during infection (31, 32). Although our antigen panel included recombinant OspC proteins from both Borrelia species, only B. afzelii’s OspC emerged from our unsupervised approach. However, Borrelia OspC exhibits an average homology of 74% between species, likely accounting for this serological response (33). Although these antigens may recognize an overlapping set of antibodies, the VslE-related antigens did not contribute substantially to the discrimination of specific disease manifestations compared to OspC antigens.
Of particular interest were the 17 children classified as clinical mimics but with a positive 3-antigen test. Twelve patients had symptoms compatible with early disseminated and 5 with late disease, raising concerns for false-negative two-tier Lyme disease serology for some children. Particularly intriguing are the 5 patients who clustered by PC analysis with early disseminated or late Lyme disease patients. These children exhibited reactivity to many Lyme antigens in characteristic patterns shared with confirmed Lyme patients but not observed in clinical mimics and, therefore, could represent misclassified Lyme disease cases. Since long-term follow-up was unavailable and cross-reactivity to antigens from non-Lyme organisms remains a possibility, we conclude only that multiantigen panels could prove a promising approach to improve the accuracy of conventional two-tier serology.
The serologic differences across clinical presentations of Lyme disease in children were especially intriguing. Early disseminated and late Lyme disease formed distinct serologic clusters. In part, this difference reflects the generally lower level of pathogen-specific IgG earlier in the disease course. However, comparison of neurologic and arthritic Lyme disease showed that some serological responses were strongest in patients with early disseminated disease, showing that reactivity differs in kind as well as in degree, suggesting that the host immune response could contribute to differences in clinical presentation (34–36).
Our study has several important limitations. First, our current Lyme disease diagnostic standard has well-described limitations, including false negatives in early infection. Second, the small number of children in clinically important subgroups limited our power to make comparisons. Third, we did not perform long-term follow-up for enrolled patients, and some children may have had Lyme disease or other conditions diagnosed more than 30 days after enrollment. Lastly, the specific 3-antigen panel remains to be validated in an independent population prior to clinical application.
In summary, we evaluated a multiplex antigen panel in a prospective cohort of children undergoing evaluation for Lyme disease that includes both confirmed Lyme cases and clinical mimics, the control population most relevant for clinical practice. We show that multiple antigens elicit serological responses in these patients, without marked age effect, and that serologic responses differ not only by Lyme disease status but also by clinical Lyme disease stage. A panel containing as few as 3 antigens is promising for diagnosis of early disseminated and late Lyme disease and might improve the current diagnostic standard. Differential serologic correlates of Lyme disease stage suggest a role for host immune response in clinical presentation.
ACKNOWLEDGMENTS
F.A.R. was funded by a medical fellowship from Boehringer Ingelheim Fonds and two Joint Biology Consortium microgrants from parent award U.S. National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) P30AR070253. P.A.N. was supported by P30AR070253. P.A.N. and L.E.N. were supported by a grant from the Peabody Foundation. L.E.N. was supported by the Global Lyme Alliance and the Bay Area Lyme Foundation.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material is available online only.
Contributor Information
Peter A. Nigrovic, Email: peter.nigrovic@childrens.harvard.edu.
Lise E. Nigrovic, Email: lise.nigrovic@childrens.harvard.edu.
Elitza S. Theel, Mayo Clinic
REFERENCES
- 1.Steere AC, Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J. 2018. Lyme borreliosis. McGraw-Hill Education, New York, NY. [Google Scholar]
- 2.Schwartz AM, Hinckley AF, Mead PS, Hook SA, Kugeler KJ. 2017. Surveillance for Lyme Disease—United States, 2008–2015. MMWR Surveill Summ 66:1–12. 10.15585/mmwr.ss6622a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steere AC, Schoen RT, Taylor E. 1987. The clinical evolution of Lyme arthritis. Ann Intern Med 107:725–731. 10.7326/0003-4819-107-5-725. [DOI] [PubMed] [Google Scholar]
- 4.Branda JA, Body BA, Boyle J, Branson BM, Dattwyler RJ, Fikrig E, Gerald NJ, Gomes-Solecki M, Kintrup M, Ledizet M, Levin AE, Lewinski M, Liotta LA, Marques A, Mead PS, Mongodin EF, Pillai S, Rao P, Robinson WH, Roth KM, Schriefer ME, Slezak T, Snyder J, Steere AC, Witkowski J, Wong SJ, Schutzer SE. 2018. Advances in serodiagnostic testing for Lyme disease are at hand. Clin Infect Dis 66:1133–1139. 10.1093/cid/cix943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lahey LJ, Panas MW, Mao R, Delanoy M, Flanagan JJ, Binder SR, Rebman AW, Montoya JG, Soloski MJ, Steere AC, Dattwyler RJ, Arnaboldi PM, Aucott JN, Robinson WH. 2015. Development of a multiantigen panel for improved detection of Borrelia burgdorferi infection in early Lyme disease. J Clin Microbiol 53:3834–3841. 10.1128/JCM.02111-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hahm JB, Breneman JW, Liu J, Rabkina S, Zheng W, Zhou S, Walker RP, Kaul R. 2020. A fully automated multiplex assay for diagnosis of Lyme disease with high specificity and improved early sensitivity. J Clin Microbiol 58:e01785-19. 10.1128/JCM.01785-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joung H-A, Ballard ZS, Wu J, Tseng DK, Teshome H, Zhang L, Horn EJ, Arnaboldi PM, Dattwyler RJ, Garner OB, Carlo DD, Ozcan A. 2020. Point-of-care serodiagnostic test for early-stage Lyme disease using a multiplexed paper-based immunoassay and machine learning. ACS Nano 14:229–240. 10.1021/acsnano.9b08151. [DOI] [PubMed] [Google Scholar]
- 8.Arumugam S, Nayak S, Williams T, Maria FSDS, Guedes MS, Chaves RC, Linder V, Marques AR, Horn EJ, Wong SJ, Sia SK, Gomes-Solecki M. 2019. A multiplexed serologic test for diagnosis of Lyme disease for point-of-care use. J Clin Microbiol 57:e01142-19. 10.1128/JCM.01142-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng HD, Tirosh I, de Haan N, Stöckmann H, Adamczyk B, McManus CA, O'Flaherty R, Greville G, Saldova R, Bonilla FA, Notarangelo LD, Driessen GJ, Holm IA, Rudd PM, Wuhrer M, Ackerman ME, Nigrovic PA. 2020. IgG Fc glycosylation as an axis of humoral immunity in childhood. J Allergy Clin Immunol 145:710–713. 10.1016/j.jaci.2019.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agarwal S, Cunningham-Rundles C. 2007. Assessment and clinical interpretation of reduced IgG values. Ann Allergy Asthma Immunol 99:281–283. 10.1016/S1081-1206(10)60665-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nigrovic LE, Neville DN, Balamuth F, Levas MN, Bennett JE, Kharbanda AB, Thompson AD, Branda JA, Garro AC, Group PLNW, Pedi Lyme Net Working Group. 2020. Early release—pediatric Lyme Disease Biobank, United States, 2015–2020. Emerg Infect Dis 26:3099–3101. 10.3201/eid2612.200920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nigrovic LE, Malley R, Macias CG, Kanegaye JT, Moro-Sutherland DM, Schremmer RD, Schwab SH, Agrawal D, Mansour KM, Bennett JE, Katsogridakis YL, Mohseni MM, Bulloch B, Steele DW, Kaplan RL, Herman MI, Bandyopadhyay S, Dayan P, Truong UT, Wang VJ, Bonsu BK, Chapman JL, Kuppermann N. 2008. Effect of antibiotic pretreatment on cerebrospinal fluid profiles of children with bacterial meningitis. Pediatrics 122:726–730. 10.1542/peds.2007-3275. [DOI] [PubMed] [Google Scholar]
- 13.Solomon CG, Shapiro ED. 2014. Lyme disease. N Engl J Med 370:1724–1731. 10.1056/NEJMcp1314325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lantos PM, Lipsett SC, Nigrovic LE. 2016. False positive Lyme disease IgM immunoblots in children. J Pediatr 174:267–269. 10.1016/j.jpeds.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomes-Solecki MJC, Meirelles L, Glass J, Dattwyler RJ. 2007. Epitope length, genospecies dependency, and serum panel effect in the IR6 enzyme-linked immunosorbent assay for detection of antibodies to Borrelia burgdorferi. Clin Vaccine Immunol 14:875–879. 10.1128/CVI.00122-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dattwyler RJ, Gomes-Solecki MJC. 15 February 2011. Peptide diagnostic agent for Lyme disease. US patent US7887815B2.
- 17.Cohen BH. 2013. Explaining psychological statistics, 4th ed. Wiley, New York, NY. [Google Scholar]
- 18.Rosenthal R. 1994. Parametric measures of effect size, p 231–244. In The handbook of research synthesis. Russell Sage Foundation, New York, NY. [Google Scholar]
- 19.Kuhn M. 2008. Building predictive models in R using the caret package. J Stat Softw 28:1–26.27774042 [Google Scholar]
- 20.R Core Team. 2020. R: a language and environment for statistical computing. R Core Development Team, Vienna, Austria. [Google Scholar]
- 21.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, Müller M. 2011. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 12:77. 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wickham H. 2016. ggplot2: elegant graphics for data analysis. Springer-Verlag, New York, NY. [Google Scholar]
- 23.Kassambara A. 2020. ggpubr: 'ggplot2' based publication ready plots. R package, version 0.4.0. https://CRAN.R-project.org/package=ggpubr.
- 24.Le S, Josse J, Husson F. 2008. FactoMineR: an R package for multivariate analysis. J Statistical Software 25:1–18. 10.18637/jss.v025.i01. [DOI] [Google Scholar]
- 25.Kassambara A, Mundt F. 2020. factoextra: extract and visualize the results of multivariate data analyses. R package, version 1.0.7. https://CRAN.R-project.org/package=factoextra.
- 26.Wei T, Simko V. 2021. R package 'corrplot': visualization of a correlation matrix, version 0.90. https://github.com/taiyun/corrplot.
- 27.Weiner J. 2020. pca3d: three dimensional PCA plots. R package, version 0.10.2. https://CRAN.R-project.org/package=pca3d.
- 28.Kassambara A. 2021. rstatix: pipe-friendly framework for basic statistical tests. R package, version 0.7.0. https://CRAN.R-project.org/package=rstatix.
- 29.Liang FT, Philipp MT. 1999. Analysis of antibody response to invariable regions of VlsE, the variable surface antigen of Borrelia burgdorferi. Infect Immun 67:6702–6706. 10.1128/IAI.67.12.6702-6706.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Embers ME, Liang FT, Howell JK, Jacobs MB, Purcell JE, Norris SJ, Johnson BJB, Philipp MT. 2007. Antigenicity and recombination of VlsE, the antigenic variation protein of Borrelia burgdorferi, in rabbits, a host putatively resistant to long‐term infection with this spirochete. FEMS Immunol Med Microbiol 50:421–429. 10.1111/j.1574-695X.2007.00276.x. [DOI] [PubMed] [Google Scholar]
- 31.Lin Y-P, Tan X, Caine JA, Castellanos M, Chaconas G, Coburn J, Leong JM. 2020. Strain-specific joint invasion and colonization by Lyme disease spirochetes is promoted by outer surface protein C. PLoS Pathog 16:e1008516. 10.1371/journal.ppat.1008516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tilly K, Krum JG, Bestor A, Jewett MW, Grimm D, Bueschel D, Byram R, Dorward D, VanRaden MJ, Stewart P, Rosa P. 2006. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect Immun 74:3554–3564. 10.1128/IAI.01950-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theisen M, Borre M, Mathiesen MJ, Mikkelsen B, Lebech AM, Hansen K. 1995. Evolution of the Borrelia burgdorferi outer surface protein OspC. J Bacteriol 177:3036–3044. 10.1128/jb.177.11.3036-3044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nigrovic LE, Thompson AD, Fine AM, Kimia A. 2008. Clinical predictors of Lyme disease among children with a peripheral facial palsy at an emergency department in a Lyme disease–endemic area. Pediatrics 122:e1080–e1085. 10.1542/peds.2008-1273. [DOI] [PubMed] [Google Scholar]
- 35.Cohn KA, Thompson AD, Shah SS, Hines EM, Lyons TW, Welsh EJ, Nigrovic LE. 2012. Validation of a clinical prediction rule to distinguish lyme meningitis from aseptic meningitis. Pediatrics 129:e46–e53. 10.1542/peds.2011-1215. [DOI] [PubMed] [Google Scholar]
- 36.Deanehan JK, Nigrovic PA, Milewski MD, Tanny SPT, Kimia AA, Smith BG, Nigrovic LE. 2014. Synovial fluid findings in children with knee monoarthritis in Lyme disease endemic areas. Pediatr Emerg Care 30:16–19. 10.1097/PEC.0000000000000028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S4, Tables S1 to S3, and legends of Videos S1 and S2. Download JCM.01344-21-s0001.pdf, PDF file, 10.3 MB (10.3MB, pdf)
Video S1. Download JCM.01344-21-s0002.mp4, MP4 file, 0.7 MB (767.1KB, mp4)
Video S2. Download JCM.01344-21-s0003.mp4, MP4 file, 0.6 MB (575.3KB, mp4)


