ABSTRACT
The coronavirus disease 2019 (COVID-19) pandemic reduced the sexually transmitted infection (STI) testing volume due to social-distancing and stay-at-home orders, among other reasons. These events highlighted previously known benefits of at-home STI self-testing or specimen self-collection and accelerated testing demand via telemedicine. We review testing outside traditional clinical settings. We focus on three curable bacterial STIs among the top 10 U.S. nationally notifiable conditions with screening recommendations: syphilis, gonorrhea (Neisseria gonorrhoeae, also known as the gonococcus [GC]), and chlamydia (Chlamydia trachomatis). At least 19 million GC/C. trachomatis (GC/CT) screening or diagnostic tests are performed annually, presenting a considerable challenge during the pandemic. Unlike for HIV, STI at-home tests are currently not commercially available. However, innovative telemedicine providers currently offer services where specimen self-collection kits are mailed to patients at home who then ship them to laboratories for processing. We discuss technical and regulatory aspects of modifications for home-based specimen self-collection. The telemedicine provider typically manages and communicates results, provides linkage to care, and is responsible for billing and case reporting. We also describe rapid testing devices in development that may present an opportunity for future self-testing. In summary, COVID-19 has accelerated the evaluation and development of STI self-tests and specimen self-collection. The remaining obstacles are high price, regulatory approval, support for laboratories offering the service, and uncertainty regarding whether target populations with the greatest need are reached effectively. However, increased testing, convenience, and privacy are potential benefits that may enhance uptake and outlast the pandemic.
KEYWORDS: self-testing, specimen self-collection, laboratory-developed test, rapid test
INTRODUCTION
INTRODUCTION: THE NEED IS GREAT
During the coronavirus disease 2019 (COVID-19) pandemic, many sexually transmitted infection (STI) providers have become interested in telemedicine care models. They can reduce potential patient and staff exposure to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) while allowing the provision of STI services. One obstacle is the need for laboratory testing and specimen collection. Before the pandemic, this typically necessitated a visit to a medical facility, even as fast and convenient STI care by express services (as described by the National Association of County and City Health Officials [1]) or in pharmacies was already advancing. This minireview provides a laboratory perspective and description of available technologies that could potentially be adapted to telemedicine models. Of note, the term “at-home” testing or collection has been perceived as excluding the homeless or incarcerated. In the context of laboratory diagnostic device terminology used by the FDA (Food and Drug Administration), it is meant to distinguish self-testing or self-collection under medical supervision from testing without such direct, in-person supervision. This is relevant as STI testing has advanced into nontraditional, often “nonclinical” settings like mobile vans, booths at health fairs, or other communal sites. There, specimen self-collection can take place, but a medical professional is still present.
STI testing before and during COVID-19.
More than 2.4 million U.S. cases of syphilis, gonorrhea, and chlamydia were reported to the Centers for Disease Control and Prevention (CDC) in 2018 (2). The number included 1,758,668 cases of chlamydia (Chlamydia trachomatis), 583,405 cases of gonorrhea (Neisseria gonorrhoeae, also known as the gonococcus [GC]), and 115,045 cases of all stages of syphilis (causative agent, Treponema pallidum), including 35,063 cases of primary and secondary syphilis. Most alarmingly, congenital syphilis cases are rising and reached 1,306 cases in 2018. These statistics reflect not only diagnostic testing of symptomatic individuals but also U.S. screening recommendations for these STIs, which may often be present without symptoms. Asymptomatic screening reduces harmful sequelae such as adverse pregnancy outcomes, infertility, pelvic inflammatory disease (PID), and risk of HIV acquisition, among others. It is an integral component of HIV prevention. There are other viral, parasitic, and bacterial STIs of concerning proportions and impacts, e.g., herpes simplex virus 1 (HSV-1), HSV-2, Trichomonas vaginalis, human papillomavirus (HPV), the emerging pathogen Mycoplasma genitalium, and others. However, for bacterial STIs, the CDC (3) and the U.S. Preventive Services Task Force (USPSTF) (4) currently recommend asymptomatic screening only for chlamydia, gonorrhea, and syphilis. Populations targeted for screening and screening frequency vary depending on risk. The screening recommendations cover, but are not limited to, sexually active young women under 25 years of age, pregnant women, men who have sex with men (MSM), and sexually active persons living with HIV (3). The CDC recommends screening for GC and C. trachomatis by nucleic acid amplification tests (NAATs) using urine from men or vaginal swabs from women (5); additional screening at extragenital sites (throat and rectum) may be recommended depending on sexual history. Many commercial test products consist of dual GC/C. trachomatis (GC/CT) NAATs. Syphilis screening by serology is recommended, as further discussed below (3). Here, we focus on screening of asymptomatic individuals; telemedicine management of symptomatic persons with other suspected STIs is not within the scope of this review.
Data on actual annual tests performed are scarce. The CDC receives data only on positive laboratory tests, not on negative tests. In 2016, Association of Public Health Laboratories (APHL) members responding to a survey reported performing 2,242,728 C. trachomatis and 2,298,596 GC tests; this resulted in 12% and 16.8% of the nationally reported C. trachomatis and GC cases that year, respectively (6). This broadly suggests that on a national level with testing by other types of laboratories (commercial, academic, other public health, or nonprofit laboratories), between 14 million and 19 million tests were performed for each disease in 2016. This is likely a substantial underestimation of the current testing volume because test positivity may be considerably lower in commercial laboratories that often work with private providers rather than public sexually transmitted disease (STD) clinics. Data on syphilis testing volume are even more difficult to estimate due to testing algorithms that require multiple sequential tests before a case report to the CDC and due to the use of different algorithms (see “Considerations for modifications of syphilis testing with home self-collected blood,” below).
The final COVID-19 impact on U.S. STI surveillance case counts is still unknown. The National Coalition of STD Directors (NCSD) recently reported (7) that 83% of STD programs deferred STD services or field visits, 66% of clinics reported a decrease in sexual health screening and testing, 60% reported a reduced capacity to treat STDs, and some sexual health clinics closed altogether for some period during the pandemic. This caused many providers to explore contactless ways to deliver STI testing; the first reports of their experiences are emerging, including home-based testing (8, 9).
Evidence review for STI self-testing.
The idea of specimen self-collection or self-STI testing is not new. In 2019, the World Health Organization (WHO) published the WHO Consolidated Guideline on Self-Care Interventions for Health as a first installment in a planned series for various diseases (10). The first document focused on sexual and reproductive health and rights. Self-care, including self-testing, has the readily apparent benefits of privacy, confidentiality, speed, convenience, and access if the price is affordable. It is “people centered” (10) and enables active participation in one’s own health. It is also a health system approach as it can reduce the burden on stretched systems with worldwide shortages in medical personnel or other barriers to health care access. Potential risks include low specimen return rates, uncertain follow-up (linkage to care, including treatment; repeat testing, including test of cure; partner notification; and counseling on risk reduction), unintended/unnecessary use (resulting in false-positive results, with their own set of associated problems), incorrect use, a lack of understanding of window periods (resulting in false-negative results), and a lack of surveillance data generation, among other issues (10). The WHO systematically reviewed the evidence for self-testing or specimen self-collection for GC, C. trachomatis, and syphilis, including U.S. studies, and published a meta-analysis of available evidence (11). Programs offering self-collection of samples increased the overall uptake of STI testing services (relative risk [RR], 2.941 [95% confidence interval {CI}, 1.188 to 7.281]) and case finding (RR, 2.166 [95% CI, 1.043 to 4.498]), prior to the pandemic (11). U.S. laboratory research on the equivalence and/or superiority of self-collected versus provider-collected specimens for test sensitivity was reported by Gaydos (summarized or referenced in reference 12). Based on this evidence, the WHO issued a new recommendation in 2019, “Self-collection of samples for Neisseria gonorrhoeae and Chlamydia trachomatis should be made available as an additional approach to deliver STI testing services for individuals using STI testing services” (10). In addition, the WHO issued a new and conditional recommendation, “Self-collection of samples for Treponema pallidum (syphilis) and Trichomonas vaginalis may be considered an additional approach to deliver STI testing services for individuals using STI testing services” (10). Thus, even before the COVID-19 pandemic, substantial expert agreement existed concerning the benefits of this approach.
OVERVIEW OF DIFFERENT TELEMEDICINE MODELS DURING COVID-19
Self-testing at home.
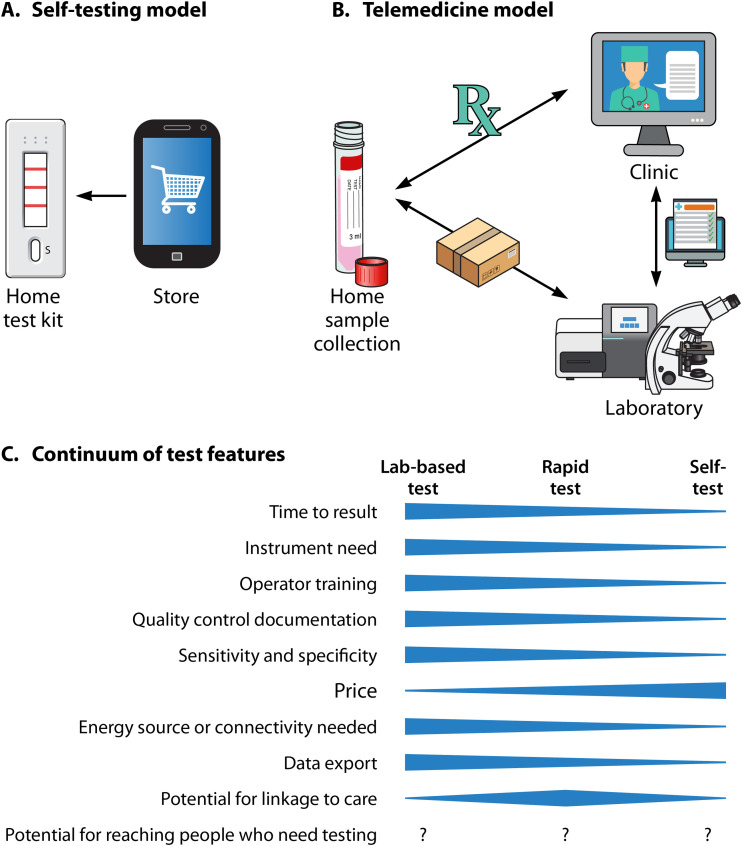
Figure 1A schematically depicts two main STI self-care and testing models. In a self-testing model, the patient or consumer decides what test they want. They buy it commercially “over the counter” (OTC) or online without a prescription or medical provider order. They collect the specimen using instructions and materials packaged with the test. Next, they perform the actual test and read and interpret the result. Follow-up happens only if the patient decides to contact a clinic. This setup is currently available for self-HIV testing but not for GC, C. trachomatis, or syphilis testing as there are no such tests commercially available; current regulatory and technical hurdles are discussed below. This model may be best suited for diseases where individuals have definitive symptoms and can reasonably guess what test they may need. Numerous STIs cause overlapping symptoms such as itching, penile or vaginal discharge, anogenital ulcers, or other symptoms that may prompt a desire for testing, as does exposure or risk behaviors. A consumer may order unnecessary tests at a considerable cost (often more than $100). A variation of this model is laboratories that operate independently, i.e., not in a telemedicine model. Rather, they accept self-collected specimens and testing requests directly from patients. A problem with such models is that some states have laws permitting only medical providers to order diagnostic STI tests and/or dispense laboratory results (13). There are also self-tests that require a prescription. Since none are available for STIs, they are not further discussed here.
FIG 1.
(A) Self-testing setup. There are currently no over-the-counter syphilis, gonorrhea, or chlamydia tests available, but one exists for HIV testing. (B) Telemedicine model for STI testing as commonly developed during the COVID-19 pandemic. In this model, the patient typically does not pay out of pocket for laboratory testing. The laboratory accepts home self-collected specimens because it has conducted a laboratory-developed test (LDT) modification, i.e., test validation under the supervision of the clinical laboratory director. (C) Continuum of test features of laboratory-based tests, rapid point-of-care (POC) tests, or self-tests.
Specimen self-collection at home and submission to clinical laboratories after laboratory-developed test validation.
In a telemedicine model (Fig. 1B), a patient receives remote assessment and evaluation. The provider may use questionnaires or previously provided patient information to determine if the individual falls under an STI screening category before the visit. The facility then sends the patient an at-home specimen collection kit with instructions. The patient ships the specimen to a laboratory that offers a test accepting self-collected specimens (further described below). Results are reported back to the clinic or directly to the patient. When the patient subsequently interacts with the medical provider, laboratory results are typically already available, and he/she is already linked to care. Alternatively, testing can be ordered during the telemedicine visit as needed. Many versions of this setup currently exist. For example, billing can be handled by the laboratory or clinic, and collection kits could be sent by the clinic, the laboratory, or even a third-party commercial entity.
TERMINOLOGY FOR DIAGNOSTIC SELF-TESTING AND SPECIMEN SELF-COLLECTION
Regulatory terms for clinical laboratory diagnostics.
Brief definitions of relevant terms are also summarized in Table 1. Some confusion exists around the terms “self-testing” and “specimen self-collection.” In the United States, the FDA regulates diagnostic testing and defines test labels. A “self-test” refers to a test that is FDA approved/cleared for patient performance in its entirety, meaning not just specimen collection and send-off to a laboratory but also performing the actual test and reading the result. This would be clearly indicated as “intended use” in the product’s package insert. Reviewing the package insert and all other packaging inclusions such as specimen collection materials is an integral part of the FDA’s clearance process. A prescription may or may not be required to obtain the self-test. The provider can also send the test home to their client. The consumer then collects a specimen and conducts the test without direct medical supervision. The public is most familiar with home pregnancy self-tests, available in the United States since the 1970s.
TABLE 1.
Test descriptions with relevance for STI at-home testing or specimen collection
| Term | Definition |
|---|---|
| FDA-cleared test | A testing device that has been cleared by the FDA for use in the U.S.; all FDA-cleared tests are manufactured with a package insert that specifies their use, including acceptable specimens and their collection methods |
| Self-test | A device that an individual can obtain with a prescription or over the counter, possibly by mail; the package insert indicates self-testing as the intended use; the individual then conducts specimen collection and the test on their own using instructions from the insert and obtains results without shipping anything to a laboratory or waiting for results |
| Provider-instructed specimen self-collection kit | A collection kit/device sold with a package insert that specifies that a medical provider will instruct an individual on how to use the kit at a medical facility; many GC/CT NAATs are FDA cleared for this type of specimen self-collection, e.g., for urine and vaginal swab self-collection; the facility’s staff then typically submits the specimen to an associated laboratory or performs a test |
| CLIA-regulated test | The CLIA are regulations governed by the CMS; their purpose is to establish quality standards for clinical laboratory testing to ensure that patient test results are accurate and reliable; clinical laboratory testing is governed by the CLIA and involves activities such as documentation of staff training and proficiency |
| Laboratory-developed test | A test that has not gone through regular FDA clearance (with the exception of emergency uses) but can nevertheless be performed at local laboratories if the local clinical laboratory director reviews and approves test performance data according to CLIA regulations |
| POC and/or rapid test | Point-of-care test, a test that is designed to be performed rapidly at a medical provider facility while the patient waits; the term implies that results are available for treatment decisions, as opposed to a test performed after specimen submission to a laboratory; some rapid tests can be performed at the POC but also outside medical provider facilities |
| CLIA-waived test | A test device maker can apply for the label “CLIA waived” during FDA clearance review; it indicates that the test can be safely performed by nonlaboratorians, typically due to its simplicity; CLIA-waived tests can be good candidates for self-testing but are not automatically approved for self-testing; some CLIA-waived tests still require equipment that cannot be transported to a home and thus cannot be adapted for home self-testing |
Furthermore, the distinction between “provider-instructed” and “at-home” self-collection is in part explained above. There are available STI tests where nuances are particularly relevant: many available GC and C. trachomatis NAATs are FDA cleared for specimen self-collection at a provider facility under medical supervision but not at home. This is stated in package inserts. During provider-instructed collection, the medical provider explains the process and potential risks in person, possibly with visual materials packaged with the kit, in accordance with the intended use of the kit. The patient then goes to a private clinic area to collect urine or a vaginal swab. Numerous studies have demonstrated that some patient-collected samples have test performances similar to those of provider-collected samples (reviewed and summarized in reference 12). In the STI field, many GC/CT NAAT diagnostic devices include a provider-instructed specimen self-collection kit. Alternatively, the kit is available for purchase separately but clearly labeled for use with the diagnostic test. Typically, there is a collection instrument (e.g., a swab or a urine container) and a corresponding test tube with buffers/solutions that stabilize the collected material until testing. However, even such collection kits may not simply be used at home since this would be outside their intended use. If the materials were to be used at home, it has so far been necessary to conduct a laboratory-developed test (LDT) validation study, as further detailed below.
The situation is slightly different for syphilis testing. Typical nonrapid laboratory-based serological syphilis tests accept blood, serum, or plasma, and the kits do not typically contain specimen collection materials. They are designed for venipuncture by a medical professional. Universally available OTC blood self-collection kits, e.g., dried blood spots (DBSs) and BD Microtainers, can be purchased separately or provided by the LDT manufacturer and are discussed below.
The Clinical Laboratory Improvement Amendments of 1988 (CLIA) are regulations promulgated by the Centers for Medicare and Medicaid Services (CMS) (14). Their purpose is to establish quality standards for clinical laboratory testing to ensure that patient test results are accurate and reliable. In the United States, clinical laboratory testing is governed by CLIA and requires activities such as documentation of staff training and proficiency, equipment calibration and maintenance, and other quality measures. Laboratories are regularly inspected for their compliance with the amendments and may operate only if they have a current CLIA certificate. CLIA federal regulations apply unless the state has enacted laws relating to laboratory requirements that are equal to or more stringent than CLIA requirements, e.g., the Clinical Laboratory Evaluation Program in New York State. Compliance is achieved by implementing all aspects of cleared tests as described in the package insert and by verifying that the test performs as expected in one’s local laboratory that is inspected by the CMS and has a valid CLIA certificate. “CLIA waivers” are discussed in the following paragraph. An LDT is a test that has not gone through regular FDA review (with the exception of tests that received emergency use authorization). The CLIA have so far permitted such tests if the local clinical laboratory director approves test performance data according to CLIA regulations. The regulation of LDTs may change in the future. Laboratory-developed tests have so far used FDA-cleared components, e.g., a cleared GC/CT NAAT diagnostic device, and modified them, e.g., by accepting self-collected specimens. However, it is currently unclear if the FDA will issue further communication on its regulation of self-collection devices and LDTs. The current process of laboratory-developed test validation for STIs in local laboratories is described below.
Terms for rapid tests, point-of-care tests, and CLIA-waived tests.
There are also “CLIA-waived tests.” They are simple to perform, carry a low risk of an erroneous result, and can sometimes be candidates for further development into self-tests. The CLIA waiver designation is determined by the FDA and is based on a review of the manufacturer’s application and data submission. It indicates that the test can be safely performed by nonlaboratorians. The complexity of instrument operation and maintenance is considered, among other factors. They are often rapid tests, “point-of-care” (POC) tests, or “single-use devices” (SUDs), terms that are used interchangeably in this review and not further differentiated. However, not all rapid, POC, or SUD tests are CLIA waived, and CLIA-waived tests are not automatically approved for self-testing; generally, additional trials and data submission are required for a test to be approved for self-testing. For example, the Syphilis Health Check (SHC) syphilis rapid test is CLIA waived for testing using fingerstick whole-blood specimens, while the Chembio DPP HIV-syphilis test is currently not CLIA waived as of manuscript submission. Furthermore, some CLIA-waived tests may not be suitable for self-testing. A typical reason is that it would not be feasible for an associated instrument to be used at home. It is also possible that the manufacturer has not collected and submitted data for FDA review to obtain this indication, among other reasons.
Figure 1C shows a comparison of features often attributed to clinical laboratory tests, rapid tests, and self-tests. Typically, clinical laboratory-based tests have features such as a relatively long time to result, high instrumentation need, operator training need, quality control documentation need, high test performance metrics, high energy/Internet usage, and great potential for electronic data export. Self-testing and even rapid tests may rank lower on these, but their price can be high, at least initially, reducing access (further discussed below [see “Lessons learned from at-home HIV self-testing”]). The potential for linkage to care and for reaching people who may need testing is debatable and needs further evaluation. Since rapid tests are often designed as POC tests and have a quick turnaround, their potential for linkage appears high. However, it is unclear how many people can be tested in a timely fashion, particularly if simple instruments run only one test at a time. The COVID-19 pandemic or new express services are expected to change how people access STI screening and testing.
PRACTICAL EXAMPLES OF STI LABORATORY SERVICES DURING THE PANDEMIC: HOW HAVE SOME LABORATORIES MODIFIED EXISTING STI TESTS FOR ACCEPTANCE OF HOME-COLLECTED SPECIMENS?
When the COVID-19 pandemic unfolded, a few U.S. laboratories were already able to accept at-home self-collected specimens. These were mainly commercial laboratories that had introduced the technology in previous years as well as academic research centers. The CDC and partners made lists of available laboratories for communications with HIV or STD service providers during the pandemic (see https://www.cdc.gov/hiv/testing/self-testing.html, https://www.cdc.gov/std/prevention/disruptionGuidance.htm, and https://www.ncsddc.org/resource/covid-command-center-for-std-programs/). The goal was to give rapid technical assistance to partners; it did not constitute endorsement, completeness, or quality assurance. How was it possible for the offerors to receive approval for such LDTs by the CLIA director? We give examples of how LDT validation has been done for GC/CT NAATs or syphilis tests. The APHL/CDC STD Steering Committee published the General Checklist for Establishment of Performance Specifications for Tests Not FDA-Cleared or Approved (15). This 2009 document contains practical advice from STI public health laboratory experts for STI test modifications.
Considerations for modification of GC/CT NAATs for at-home specimen self-collection.
Laboratory-based GC/CT NAATs are typically done with high-throughput, automated instruments (16). Laboratories are likely interested in keeping their established instrument. There are precedents of LDT modifications for extragenital specimen acceptance, as older tests were initially not cleared for their acceptance (17). The overall validation purpose is to ensure that test performance specifications are not substantially reduced by modification. For GC/CT home self-collection and submission to a laboratory, deviations from the approved package inserts exist in two main areas: (i) the lack of medical oversight during at-home specimen self-collection and (ii) preservation of specimen integrity due to handling, temperature variation, or delays in transit to a distant laboratory. A laboratory has likely been required to show that test results are comparable in both clinic-based and home-based settings, among other local issues that the CLIA laboratory director wishes to address. It is possible that future FDA communication on regulation of self-collection devices will determine the LDT validation process.
For potential problems with obtaining the specimen at home, such as safety or incorrect specimen harvest, a remote provider can instruct a patient similarly over the phone or by video-assisted secure technology as could be done in person at a medical facility. Some successful providers have made videos (e.g., Emory University Center for AIDS Research [https://vimeo.com/138977095]) or included visual materials in the mailed kit or materials that may make sample collection more convenient, such as foldable urine cups or cardboard tube stands. They can establish a contact mechanism, e.g., a phone number or hotline, should unforeseen problems arise. A test developer can also reference the substantial literature demonstrating successful self-collection (12).
The second validation goal is to document test accuracy when the patient performs initial specimen processing and initiates transport. Below is an example of urine collection for the Aptima Combo 2 test (18), the most often used NAAT in public health laboratories (16). The patient obtains first-catch urine using the provided cup. For initial sample stabilization, the patient would then follow the package insert instructions, i.e., “transfer 2 ml of urine into the urine specimen transport tube using the disposable pipette provided” and then “transport the processed urine specimens … at 2°C to 30°C and store at 2°C to 30°C until tested. Processed urine specimens should be assayed with the Aptima assay within 30 days of collection.” It is also possible to give the patient easier-to-read materials that still convey the procedure. The laboratory preparing the test validation plan and data could conduct a research study. However, enrolling human subjects in research studies to collect two specimens in parallel (one at home and one at a medical facility), obtaining their consent, and other study provisions may not be feasible for every laboratory, particularly during the pandemic. It is important to not overinterpret requirements for test validation, as suggested by the APHL, who described examples of STI test validations (15). The document describes using spiked specimens for validation. Alternatively, leftover specimens from laboratories that have already obtained validation, or from commercial sources, can be used. When spiking specimens, “the matrices should be true specimens” (e.g., leftover urine or materials from swabs). Laboratories may overestimate the number of specimens needed for successful validation. The APHL document specifies, “A minimum of 10 positive and 10 negative previously characterized specimens should be tested … Ideally, a total of 30 specimens should be tested.” Therefore, a laboratory may expose 30 spiked specimens to local shipping conditions and compare their test results to that of a specimen aliquot without shipping. The laboratory may also ask nonlaboratorians to perform initial sample processing steps using spiked specimens, for comparison to aliquots handled by laboratory professionals. Furthermore, the laboratory can develop specimen integrity acceptance criteria; e.g., they can stipulate kit return within 30 days or sooner. Some laboratories include a checklist for the patient to mark that they shipped the specimen under climate-controlled conditions rather than leaving it in their home mailbox exposed to the weather. The greatest laboratory need is often for technical assistance with the provision of leftover or spiked samples. Due to protections of human subjects in research studies, there is a requirement that personal identifiable information (PII) is removed from leftover specimens. This requires review, approval, and documentation and may be beyond the capacity of some public health laboratories and the associated infrastructure, particularly during the pandemic. The CDC STD Laboratory has been contacted repeatedly to provide leftover samples and is working with partners to develop a panel of suitable specimens to assist with future validation needs.
Considerations for modifications of syphilis testing with home self-collected blood.
Currently recommended syphilis diagnosis consists of clinical evaluation supported by laboratory testing for treponemal and nontreponemal antibodies from whole blood, serum, or plasma (3). The tests are done in sequence with the traditional algorithm (nontreponemal test first) or reverse algorithm (treponemal test first). Once a person has had syphilis, treponemal tests typically stay positive for life, even after treatment. In contrast, nontreponemal antibodies wane over time after treatment, often indicate current infection, and are used to monitor treatment success. Treponemal tests in general have few technical challenges. They were the first to become widely available in an automated format. On the other hand, nontreponemal tests are more technically challenging, in part due to lipid antigen-antibody recognition, the manual nature of the tests, the subjectivity of result interpretation, and titer needs for reactive specimens. Given the efficiency afforded by automation and high throughput, some laboratories prefer to perform treponemal tests first. This may be beneficial in settings conducting high-volume screening of populations at low risk for syphilis, e.g., prenatal screening. However, in high-seroprevalence populations of people with previous syphilis and high treponemal antibody positivity, e.g., men who have sex with men, who may require frequent screening, beginning with nontreponemal testing would be preferred. FDA-cleared automated nontreponemal tests have recently been introduced in the United States and might help with expediting syphilis screening (19).
New technical issues are arising for self-collected specimens. DBSs have been considered due to easy transportation and the limited blood volume needed. The Treponema pallidum particle agglutination assay (TPPA) performs well from DBSs (20). Smit et al. (21) demonstrated sensitivity and specificity of over 95% using TPPA-DBS compared to TPPA-plasma specimens. There are currently commercial laboratories that offer complete DBS treponemal test kits for at-home self-collection and shipping to a central laboratory. They include all needed supplies (blood collection card, collection and handling instructions, single-use lancets for finger pricking, alcohol pads, gauze pad, bandages, biohazard bags, and supplier contact details). Unfortunately, however, DBSs are not easily suitable for nontreponemal tests and might yield reduced sensitivity (M. Shukla and E. Kersh, unpublished data). To our knowledge, no U.S. laboratory has successfully validated such tests. Titers are required for all reactive nontreponemal tests to monitor infections. Quantitative testing is a challenge when antibodies are not sufficiently eluted from DBSs. Some telemedicine providers have instead used blood finger-prick self-collection into OTC BD Microtainers to transport blood in liquid form before processing at a laboratory. However, a blood volume of 250 to 600 μl is needed. Self-collecting this high blood volume is a significant hurdle and may lead to a lack of kit return.
As with all syphilis test validations, the inclusion of specimens from all disease stages is a consideration when establishing performance characteristics to demonstrate equivalent results obtained using routine specimens. Validation studies exist for treponemal tests with paired testing of DBSs and venous blood (20) and could be expanded with BD Microtainers, preferably from the same subjects. Forty to 100 or more samples have been considered for similar DBS comparison experiments (22). It is important to establish and validate the stability of self-collected specimens under storage temperature and time, humidity (applicable to DBSs), and transport conditions (22). The CDC and APHL recently opened a characterized syphilis serum specimen bank (23). These available specimens can be mixed with freshly collected blood (nonreactive for syphilis), subjected to DBS or BD Microtainer handling and shipping conditions, and be tested in conjunction with regularly processed specimens.
During the pandemic, a few offerors have emerged: to our knowledge, some laboratories offer treponemal testing from DBSs but not nontreponemal tests. If reactive, they call persons in or refer them for further blood collection and nontreponemal testing. This may work for test populations where syphilis rates are low. Some STD programs are currently offering GC/CT testing only remotely, and if any syphilis test is indicated, the patient is asked to attend in person or is referred elsewhere.
A LOOK AHEAD: ARE NEW TESTS ON THE HORIZON THAT COULD BE ADAPTED FOR HOME USE, AND WHAT HURDLES EXIST?
Modification of existing laboratory-based tests.
Could laboratory-based GC/CT NAATs be FDA cleared to accept self-collected specimens permanently? This could be an attainable, near-term solution if a test developer takes the initiative to collect and submit data to the FDA and applies for clearance of at-home specimen self-collection as an intended use of the test. The benefit would be to render it unnecessary that each laboratory conduct LDT validation experiments. It would widely allow implementation for GC/CT testing. Laboratories without resources for LDT validations could then participate in telemedicine models and contribute to the nation’s testing needs in an era of persistently rising STI cases. After FDA clearance, each implementing laboratory would still conduct verification of test performance according to CLIA regulations, including documentation that a test ordering and result reporting process is in place. It is possible that data from laboratories with LDTs and collected during the pandemic could serve as a portion of such an application for FDA review, and additional data may be required. It is currently unclear whether additional performance quality controls would be needed when cleared laboratory-based or rapid tests are modified to accept such specimens, e.g., to mitigate the risk of false-negative test results. However, there is already evidence that self-collection of urine and genital swabs is on par with physician collection for STI testing (12). Experiences during the pandemic may increase available evidence further.
Modification of GC/CT rapid tests.
Rapid and POC tests sometimes have the potential for adaptation to self-testing. Table 2 provides a summary of the landscape of rapid STI test development for the U.S. market, as it is known to us. We discuss whether the tests are suitable for further development into self-tests. Several tests are in development and nearing the market for settings like express clinics, including pharmacies or outreach settings.
TABLE 2.
Landscape of STI tests with relevance for future home testing pending further development
| Test parameter | Description |
|
|---|---|---|
| GC/CT | Syphilis | |
| CDC-recommended main diagnostic or screening test | GC/CT NAAT from urine (men) and vaginal swabs (women) | Sequential treponemal or nontreponemal antibody detection in blood |
| Is a self-testa currently available in the U.S.? | No | No |
| Existing CLIA-waived tests | Binx io NAAT; 30 min (as of March 2021); near-patient test with instrument need | Syphilis Health Check, a rapid qualitative test for detection of antibodies to T. pallidum; 10–15 min; visual reading of results |
| Visby Medical Sexual Health Test NAAT; 30 min (as of August 2021), uses portable, handheld, disposable device, accepts vaginal swabs | ||
| Existing POC or rapid tests without a CLIA waiverb | Cepheid GeneXpert NAAT; 90 min; near-patient test with instrument need | Chembio HIV-syphilis (treponemal antibody), a rapid qualitative multiplex test for the detection of antibodies to HIV/T. pallidum; 15–25 min; reader and test device holder assembly are required for reading results |
“Self-test” refers to a test that an individual can use to obtain, perform, and receive results without shipping a specimen back to the laboratory and waiting for results.
The GeneXpert technology has been adapted to a CLIA-waived “Xpress” instrument; however, to our knowledge, this is currently not available for GC/CT testing. Criteria used here are a turnaround time of 90 min or less and up to moderate complexity.
The Cepheid GC/CT GeneXpert test (Cepheid, Sunnyvale, CA) was the first rapid NAAT to receive U.S. clearance in 2012. Since 2019, its intended use is also cleared for extragenital specimens (17). U.S. “express clinic” models (1) have almost exclusively used this test, as it was the only same-day testing system available for many years. The test is typically performed on-site, e.g., at an STD clinic, with a 90-min turnaround time. The FDA cleared it as “moderately complex,” meaning that it requires trained staff and generally some laboratory equipment such as precision measuring devices (pipettes). It is currently not available as a CLIA-waived test. For other disease diagnostics, however, Cepheid has adopted its technology to “GeneXpert Xpress” status as CLIA-waived tests, i.e., for influenza virus, respiratory syncytial virus (RSV), and group A Streptococcus. It may become available for STIs in the future. Its adaptation to home usage is unlikely in its current formats since the instrument cannot easily be sent home.
The Binx io (Binxhealth, Cambridge, MA) molecular rapid point-of-care test was FDA cleared in 2019 and takes only 30 min (https://mybinxhealth.com/news/binx-health-receives-fda-510(k)-clearance-for-rapid-point-of-care-platform-for-womens-health). It requires a small, movable, desktop instrument, which was initially not readily available for sale. The test needs single-use GC/CT cartridges that contain all reagents. It accepts male and female urine and vaginal swabs, and no preprocessing is needed. The test is currently not cleared for extragenital specimens unless an additional laboratory-developed test modification is done. On 30 March 2021, the FDA allowed the use of the test under a CLIA certificate of waiver, allowing use in near-patient point-of-care settings (24). CLIA waiver certificates can be obtained from the Centers for Medicare and Medicaid Services (CMS) (25). Adaptability to self-testing at home faces the challenge that the instrument is not suitable to be sent home.
Of note, an additional rapid GC/CT NAAT by Visby Medical (26) was cleared in August 2021 and includes detection of Trichomonas vaginalis. It consists of a handheld, disposable, low-cost device and may thus be suitable for home use or nonclinical settings. Other devices may also be nearing the market; of note, data submission to the FDA for clearance review is not public information.
Modification of rapid syphilis testing.
The Syphilis Health Check (SHC) test is a rapid test cleared for use in the United States. It detects treponemal antibodies and can be performed on whole blood as well as plasma or serum. It is CLIA waived for fingerstick whole-blood specimens and can thus be used in outreach settings. It is not currently approved for self-testing. Test kits do not include blood collection or fingerstick materials like a lancet. There are currently no published studies evaluating self-usage to our knowledge. The test requires three drops of blood: the first is contaminated with alcohol and is discarded, and the next drops are collected in a pipette and applied to the device. Reading the test result precisely at the prescribed time, i.e., 10 min, not exceeding 15 min, is critical for the accuracy of results and has caused false-positive results even when performed by trained laboratorians (27). Chembio’s DPP HIV-syphilis (treponemal) test has recently received FDA clearance (28). This rapid test detects antibodies to HIV (types 1 and 2) and T. pallidum bacteria in fingerstick whole blood, venous whole blood (potassium EDTA), or plasma specimens (potassium EDTA). It requires a small, handheld optical reader and is currently not CLIA waived or approved for self-testing.
Internationally, several other rapid antibody tests are in use, as recently reviewed by Toskin et al. (29). Some syphilis tests include both a treponemal and a nontreponemal component; other popular tests are HIV-syphilis dual tests. The latter tests are most commonly implemented internationally for antenatal testing for the prevention of mother-to-child transmission of both infections, where long wait periods and potential loss to follow-up would be disastrous to the infant.
DISCUSSION
Lessons learned from at-home HIV self-testing.
HIV self-testing uptake increased during the pandemic according to some reports (30), due to the one available HIV self-test, i.e., OraQuick from OraSure Technologies. The long road to test uptake, reviewed by Stevens et al. (31), might offer insights for STI self-test development and marketing. A precursor test was first introduced in 1996 as an OTC home self-blood collection kit followed by submission to a laboratory. It was modified to a fingerstick blood rapid test but gained acceptability only when oral fluids were shown to be an acceptable sample type in 2004. Finally, in 2012, the OraQuick test was approved for in-home use as a self-test but had slow uptake. Thus, the development timeline spanned decades. This suggests that interim solutions are necessary for STI testing. It also provides examples of how to prevent a similarly long timeline for STI testing. Issues around pricing and reimbursement are important and numerous and can be addressed only superficially in this laboratory review. Suffice it to say that the current OTC price (approximately $40) is a major hurdle to the use of the OraQuick test. One conclusion is that avoiding direct costs to patients is an important consideration, perhaps by requiring a prescription or by sending the test home. A discussion of insurance coverage and reimbursement by insurance companies is beyond the scope of this article but is nonetheless essential.
Blood self-collection is another considerable hurdle. Therefore, developing an oral or urine syphilis test would be ideal. Molecular methods can be used to detect Treponema pallidum subsp. pallidum in the mouth (32), and protein antigens are detectable in urine (33), albeit more technical development is required.
Areas of greatest need for additional knowledge on STI testing.
For STI screening, telemedicine models emerged as workable models. In addition to the above-discussed benefits, cost to patients and proper test selection are mitigated when the providers select testing as indicated and bill patients’ insurance plans, reducing potential out-of-pocket costs. Finally, syphilis, gonorrhea, and chlamydia are nationally notifiable diseases; diagnosing them at home would likely result in nonreporting. Telemedicine providers, on the other hand, have developed procedures for mandated public health reporting.
The pandemic caused an STI test kit and reagent shortage (34) in the 3rd and 4th quarters of 2020 and stretched the limits of public health and other laboratory workforces. It affected STI testing volume and limited test format choices in all settings. The shortage is not further discussed in this article. It is acknowledged that it may have caused STI testing after home specimen collection through online offerors out of necessity; e.g., laboratories typically associated with STD clinics were overburdened with COVID-19 testing.
Many other aspects of STI telemedicine and testing need further evaluation; some policy areas are out of the scope of this review. For example, studies on access in rural areas are needed. Low kit return rates may be an issue; however, it is currently unclear if blood self-collection is the main cause. Previous studies of kit return rates and associated factors may inform improvements (35). Even so, unreturned devices are inexpensive, and laboratory services are not actually billed if no specimen is shipped. Staffing of shipping, phone, or other hotlines for people using at-home collection kits and other patient services can be an issue, particularly during the pandemic, when health provider systems are stretched. Shipping delays occurred during the height of the pandemic and during holidays. Another concern is that at-home receipt of materials suggesting STI infection may not work for people with the need for privacy from their family or cohabitants, possibly due to partner violence and fear of family members who are unaware of sexual activities or preferences. Workarounds like pickup locations are possible. The pandemic has also reignited conversations about the digital divide in this country: some populations may not have access to technologies that enable telemedicine, i.e., computers, the Internet, and cell phones. Therefore, a remaining question is whether people with the greatest need for testing are reached with telemedicine provision of services. Other open questions remain about the need for confirmatory testing, timely care, and impacts on rescreening rates.
SUMMARY
Even before the COVID-19 pandemic, there was a growing movement in the STI field to work toward specimen self-collection at a minimum and ideally also toward complete self-testing. Perhaps the greatest benefit is that it allows convenience and privacy and gives the patient an opportunity to avoid embarrassment while still accessing STI care. Increasing access to STI testing is a key strategy to mitigate continuously rising STI rates, particularly congenital syphilis. The pandemic and the need for social distancing have accelerated and increased the urgency of this need. While some laboratory offerings have emerged for GC/CT testing, syphilis testing remains challenging and will require additional research.
ACKNOWLEDGMENTS
We thank members of the COVID-19 NCHHSTP HIV, STI, Hepatitis Self-Testing TIGER team for insightful discussions. We thank members of the HHS COVID response Joint Commissioned Cell Testing and Diagnostic Work Group for sharing their knowledge of CLIA waivers.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
We have no conflicts of interest. This work was funded by the CDC.
Contributor Information
Ellen N. Kersh, Email: ekersh@cdc.gov.
Romney M. Humphries, Vanderbilt University Medical Center
REFERENCES
- 1.National Association of County and City Health Officials. 2019. Issue brief. STI express services: increasing access and testing while maximizing resources. National Association of County and City Health Officials, Washington, DC. https://www.naccho.org/uploads/downloadable-resources/issue-brief_STI-Express-Services.pdf. [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2019. 2018 STD surveillance report. Centers for Disease Control and Prevention, Atlanta, GA. https://www.cdc.gov/nchhstp/newsroom/2019/2018-STD-surveillance-report.html#:∼:text=According%20to%20the%20annual%20Sexually%20Transmitted%20Disease%20Surveillance,baby%20during%20pregnancy%E2%80%93%20increased%2040%20percent%20from%202017-2018. Accessed 7 September 2021. [Google Scholar]
- 3.Workowski KA. 2015. Centers for Disease Control and Prevention sexually transmitted diseases treatment guidelines. Clin Infect Dis 61(Suppl 8):S759–S762. 10.1093/cid/civ771. [DOI] [PubMed] [Google Scholar]
- 4.LeFevre ML, US Preventive Services Task Force. 2014. Screening for Chlamydia and gonorrhea: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 161:902–910. 10.7326/M14-1981. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2014. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recommend Rep 63:1–19. [PMC free article] [PubMed] [Google Scholar]
- 6.Davis A, Gaynor A. 2020. Testing for sexually transmitted diseases in US public health laboratories, 2016. Sex Transm Dis 47:122–127. 10.1097/OLQ.0000000000001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Coalition of STD Directors. 2020. COVID-19 & the state of the STD field. National Coalition of STD Directors, Washington, DC. https://www.ncsddc.org/wp-content/uploads/2020/05/STD-Field.Survey-Report.Final_.5.13.20.pdf. [Google Scholar]
- 8.Carnevale C, Richards P, Cohall R, Choe J, Zitaner J, Hall N, Cohall A, Whittier S, Green DA, Sobieszczyk ME, Gordon P, Zucker J. 2021. At-home testing for sexually transmitted infections during the COVID-19 pandemic. Sex Transm Dis 48:e11–e14. 10.1097/OLQ.0000000000001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melendez JH, Hamill MM, Armington GS, Gaydos CA, Manabe YC. 2021. Home-based testing for sexually transmitted infections: leveraging online resources during the COVID-19 pandemic. Sex Transm Dis 48:e8–e10. 10.1097/OLQ.0000000000001309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. 2019. WHO consolidated guideline on self-care interventions for health: sexual and reproductive health and rights. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 11.Ogale Y, Yeh PT, Kennedy CE, Toskin I, Narasimhan M. 2019. Self-collection of samples as an additional approach to deliver testing services for sexually transmitted infections: a systematic review and meta-analysis. BMJ Glob Health 4:e001349. 10.1136/bmjgh-2018-001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaydos CA. 2018. Let’s take a “selfie”: self-collected samples for sexually transmitted infections. Sex Transm Dis 45:278–279. 10.1097/OLQ.0000000000000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herbin SR, Klepser DG, Klepser ME. 2020. Pharmacy-based infectious disease management programs incorporating CLIA-waived point-of-care tests. J Clin Microbiol 58:e00726-19. 10.1128/JCM.00726-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Code. 1988. Title 42. The public health and welfare. Chapter 6A. Subchapter II. Part F. Subpart 2. Clinical laboratories. § 263a. Certification of laboratories. https://www.govinfo.gov/content/pkg/USCODE-2011-title42/pdf/USCODE-2011-title42-chap6A-subchapII-partF-subpart2-sec263a.pdf.
- 15.APHL/CDC STD Steering Committee. 2009. General checklist for establishment of performance specifications for tests not FDA-cleared or approved. Association of Public Health Laboratories, Silver Spring, MD. https://www.aphl.org/programs/infectious_disease/std/Documents/ID_2009Oct15_Checklist-STD-Performance-Specs.pdf. [Google Scholar]
- 16.Davis A, Gaynor A. 2021. A comparison of US clinical laboratory chlamydia and gonorrhea testing practices prior to and following the 2014 Centers for Disease Control and Prevention testing recommendations. Sex Transm Dis 48:e73–e76. 10.1097/OLQ.0000000000001299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doernberg SB, Komarow L, Tran TTT, Sund Z, Pandori MW, Jensen D, Tsalik EL, Deal CD, Chambers HF, Fowler VG, Jr, Evans SR, Patel R, Klausner JD, Antibacterial Resistance Leadership Group. 2020. Simultaneous evaluation of diagnostic assays for pharyngeal and rectal Neisseria gonorrhoeae and Chlamydia trachomatis using a master protocol. Clin Infect Dis 71:2314–2322. 10.1093/cid/ciz1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hologic, Inc. 2019. Aptima Combo 2 assay (Panther system). Hologic, Inc, San Diego, CA. https://www.hologic.com/sites/default/files/2019-12/502446-IFU-PI_009_01.pdf. [Google Scholar]
- 19.Tuddenham S, Katz SS, Ghanem KG. 2020. Syphilis laboratory guidelines: performance characteristics of nontreponemal antibody tests. Clin Infect Dis 71:S21–S42. 10.1093/cid/ciaa306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma J, Ren Y, He L, He X, Xing W, Jiang Y. 2020. An efficient method for simultaneously screening for HIV, syphilis, and HCV based on one dried blood spot sample. Antiviral Res 181:104775. 10.1016/j.antiviral.2020.104775. [DOI] [PubMed] [Google Scholar]
- 21.Smit PW, van der Vlis T, Mabey D, Changalucha J, Mngara J, Clark BD, Andreasen A, Todd J, Urassa M, Zaba B, Peeling RW. 2013. The development and validation of dried blood spots for external quality assurance of syphilis serology. BMC Infect Dis 13:102. 10.1186/1471-2334-13-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDade TW. 2014. Development and validation of assay protocols for use with dried blood spot samples. Am J Hum Biol 26:1–9. 10.1002/ajhb.22463. [DOI] [PubMed] [Google Scholar]
- 23.Shukla M, Sun Y, McCormick J, Hopkins A, Pereira L, Gaynor A, Kersh E, Fakile Y. 2020. Development of a syphilis serum bank to support research, development, and evaluation of syphilis diagnostic tests in the United States. Diagn Microbiol Infect Dis 96:114913. 10.1016/j.diagmicrobio.2019.114913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Food and Drug Administration. 2021. FDA allows for first point-of-care chlamydia and gonorrhea test to be used in more near-patient care settings. US Food and Drug Administration, Silver Spring, MD. https://www.fda.gov/news-events/press-announcements/fda-allows-first-point-care-chlamydia-and-gonorrhea-test-be-used-more-near-patient-care-settings. [Google Scholar]
- 25.Centers for Medicare and Medicaid Services. 2019. How to obtain a CLIA certificate of waiver. Centers for Medicare and Medicaid Services, Baltimore, MD. [Google Scholar]
- 26.Morris SR, Bristow CC, Wierzbicki MR, Sarno M, Asbel L, French A, Gaydos CA, Hazan L, Mena L, Madhivanan P, Philip S, Schwartz S, Brown C, Styers D, Waymer T, Klausner JD. 2021. Performance of a single-use, rapid, point-of-care PCR device for the detection of Neisseria gonorrhoeae, Chlamydia trachomatis, and Trichomonas vaginalis: a cross-sectional study. Lancet Infect Dis 21:668–676. 10.1016/S1473-3099(20)30734-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fakile YF, Brinson M, Mobley V, Park IU, Gaynor AM. 2019. Performance of the Syphilis Health Check in clinic and laboratory-based settings. Sex Transm Dis 46:250–253. 10.1097/OLQ.0000000000000974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chembio. 2018. DPP HIV-syphilis system. Chembio, Medford, NY. https://www.fda.gov/media/142615/download. [Google Scholar]
- 29.Toskin I, Govender V, Blondeel K, Murtagh M, Unemo M, Zemouri C, Peeling RW, Kiarie J. 2020. Call to action for health systems integration of point-of-care testing to mitigate the transmission and burden of sexually transmitted infections. Sex Transm Infect 96:342–347. 10.1136/sextrans-2019-054358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menza TW, Garai J, Ferrer J, Hecht J. 2021. Rapid uptake of home-based HIV self-testing during social distancing for SARS-CoV2 infection in Oregon. AIDS Behav 25:167–170. 10.1007/s10461-020-02959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevens DR, Vrana CJ, Dlin RE, Korte JE. 2018. A global review of HIV self-testing: themes and implications. AIDS Behav 22:497–512. 10.1007/s10461-017-1707-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang C-J, Chang S-Y, Wu B-R, Yang S-P, Liu W-C, Wu P-Y, Zhang J-Y, Luo Y-Z, Hung C-C, Chang S-C. 2015. Unexpectedly high prevalence of Treponema pallidum infection in the oral cavity of human immunodeficiency virus-infected patients with early syphilis who had engaged in unprotected sex practices. Clin Microbiol Infect 21:787.e1–787.e7. 10.1016/j.cmi.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 33.Osbak KK, Van Raemdonck GA, Dom M, Cameron CE, Meehan CJ, Deforce D, Ostade XV, Kenyon CR, Dhaenens M. 2018. Candidate Treponema pallidum biomarkers uncovered in urine from individuals with syphilis using mass spectrometry. Future Microbiol 13:1497–1510. 10.2217/fmb-2018-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. 2020. Dear colleague letter: DSTDP lab and drug shortages. Centers for Disease Control and Prevention, Atlanta, GA. https://www.cdc.gov/std/general/DCL-Diagnostic-Test-Shortage.pdf. [Google Scholar]
- 35.Ricca AV, Hall EW, Khosropour CM, Sullivan PS. 2016. Factors associated with returning at-home specimen collection kits for HIV testing among Internet-using men who have sex with men. J Int Assoc Provid AIDS Care 15:463–469. 10.1177/2325957416668579. [DOI] [PubMed] [Google Scholar]