ABSTRACT
Acanthocephala is a phylum of parasitic pseudocoelomates that infect a wide range of vertebrate and invertebrate hosts and can cause zoonotic infections in humans. The zoologic literature is quite rich and diverse; however, the human-centric literature is sparse, with sporadic reports over the past 70 years. Causal agents of acanthocephaliasis in humans are reviewed as well as their biology and life cycle. This review provides the first consolidated and summarized report of human cases of acanthocephaliasis based on English language publications, including epidemiology, clinical presentation, treatment, and diagnosis and identification.
KEYWORDS: acanthocephalans, diagnosis, parasitology, epidemiology, helminths
INTRODUCTION
The acanthocephalans, or thorny-headed worms, are a diverse group of parasitic worms with an estimated 1,100 species described within the phylum Acanthocephala. Because they are highly modified parasites, the relationships of acanthocephalans to other animals remains problematic, but molecular systematics suggests that they are related to the rotifers or possibly nestled within Rotifera proper (1). These parasites can be found in reptiles, amphibians, birds, and mammals. The organism sizes and gross morphologies are quite different across the various members of the phylum, with some as small as 1 mm and others reaching more than 60 cm. These worms are characterized broadly by an elongated tube-like body with an anterior end containing an eversible, hooked proboscis which resides within a proboscis receptacle until contact with host tissue is made. The acanthocephalans, like cestodes, lack a mouth, intestine, circulatory system, and excretory system. Akin to nematodes, Acanthocephala are dioecious and sexually dimorphic (2).
Human cases of acanthocephaliasis are seemingly rare in medical literature; however, there have been a growing number of cases reported in the last 10 years (3–13). Previous reports have also described additional cases which were not published in full detail, which suggests that these parasites may be more frequently encountered than the medical community may appreciate (10, 14) (B. A. Mathison, unpublished data). Many popular medical microbiology textbook resources (conventionally used by diagnostic laboratories) do not include content related to the acanthocephalans. This omission from conventional text likely leads to their limited or inaccurate reporting, with many labs likely considering these worms nonparasites or identifying them inaccurately as Ascaris lumbricoides (7, 10).
This work represents the first consolidated review of the English language literature for the acanthocephalans, specifically focused on those species associated with human infections. Table 1 provides a summary of cases reports of human acanthocephaliasis published in English language literature.
TABLE 1.
Summary of cases reports of human acanthocephaliasis published in English language literature
| Speciesb | Location | Symptom(s) | Treatment | Reference |
|---|---|---|---|---|
| Acanthocephalan, NOS (nr. Plagiorhynchus) | United Kingdom | Eye discomfort | NAa | 16 |
| Bolbosoma cf. capitum | Japan | Found incidentally on colonoscopy (colon cancer) | NA | 3 |
| Bolbosoma nipponicum | Japan | Found incidentally on routine enteroscopy (Crohn’s disease) | NA | 13 |
| Bolbosoma sp. | Japan | Right lower quadrant pain mimicking appendicitis | NA | 42 |
| Bolbosoma sp. | Japan | Severe abdominal pain, nausea, ileus | NA | 8 |
| Bolbosoma sp. | Japan | Right lower quadrant pain mimicking appendicitis, abdominal perforation | NA | 41 |
| Corynosoma cf. validum | Japan | Abdominal pain, ileal ulcerations | Pyrantel pamoate | 12 |
| Corynosoma villosum | Japan | Abdominal pain, bloody stool (not loose) | NA | 6 |
| Macracanthorhynchus hirudinaceus | China | 2 pediatric cases: acute abdominal pain, abdominal perforations, surgical removal | NA | 23 |
| Macracanthorhynchus hirudinaceus | China | Multicase review | NA | 24 |
| Macracanthorhynchus hirudinaceus | Thailand | Abdominal pain/tenderness, anorexia, nausea; progression to severe abdominal pain before admission and diarrhea | NA | 19 |
| Macracanthorhynchus hirudinaceus | Thailand | Abdominal pain, anorexia, nausea, intestinal perforation, ovarian cysts found during surgery | NA | 20 |
| Macracanthorhynchus hirudinaceus | Thailand | Abdominal pain, vomiting, fever, intestinal perforations | NA | 21 |
| Macracanthorhynchus ingens | USA (Florida) | Eosinophilia (8%) | Pyrantel pamoate (11 mg/kg) | 10 |
| Macracanthorhynchus ingens (as M. hirudinaceus) | USA (Louisiana) | NA | Pyrantel pamoate | 7 |
| Macracanthorhynchus ingens | USA (Ohio) | NA | Pyrantel pamoate (11 mg/kg) | 5 |
| Macracanthorhynchus ingens | USA (Texas) | NA | Pyrantel pamoate (11 mg/kg) | 4 |
| Macracanthorhynchus ingens | USA (Texas) | NA | Niclosamide + mebendazole | 27 |
| Macracanthorhynchus sp. | Papua New Guinea | Pediatric death associated with dysentery; worm found postmortem | NA | 25 |
| Moniliformis moniliformis | Australia | NA | Niclosamide | 26 |
| Moniliformis moniliformis | Iran | Diarrhea, vomiting, abdominal pain, coinfection with Giardia duodenalis | Levamisole | 32 |
| Moniliformis moniliformis | Iran | NA | Levamisole | 9 |
| Moniliformis moniliformis | Iran | Irritability, diarrhea, pallor, stunted growth, coinfection with Giardia duodenalis | Thiabendazole | 33 |
| Moniliformis moniliformis | Iran | Abdominal distention, anorexia, vomiting, diarrhea, stunted growth, eosinophilia (23%) | Niclosamide | 34 |
| Moniliformis moniliformis | Iran | Mild abdominal discomfort | Piperazine citrate | 35 |
| Moniliformis moniliformis | Iraq | Anorexia, diarrhea, weight loss, abdominal distention, coinfection with Giardia | NA | 36 |
| Moniliformis moniliformis | Nigeria | 19 cases: diarrhea, abdominal discomfort | Ivermectin | 14 |
| Moniliformis moniliformis | Nigeria | Weakness, giddiness, burning sensation in umbilicus, loose stools, abdominal discomfort | Niclosamide | 29 |
| Moniliformis moniliformis | Zimbabwe (as Rhodesia) | Anorexia, abdominal discomfort, irritability | Mebendazole | 30 |
| Moniliformis moniliformis | Saudi Arabia | Anorexia | Mebendazole | 31 |
| Moniliformis moniliformis | Australia (Tasmania) | Cough, irritability | Paracetamol | 37 |
| Moniliformis moniliformis | USA (Florida) | NA | Pyrantel pamoate (11 mg/kg) | 28 |
| Moniliformis moniliformis | USA (Florida) | NA | Pyrantel pamoate (11 mg/kg) | 11 |
NA, not available.
NOS, not otherwise specified; nr., near.
CAUSAL AGENTS
Most documented cases of human acanthocephaliasis are caused by Macracanthorhynchus hirudinaceus, Macracanthorhynchus ingens, and Moniliformis moniliformis. The true prevalence of these species in nature is not well understood, but M. hirudinaceus and M. moniliformis probably occur nearly worldwide wherever pigs and rats occur, respectively. Macracanthorhynchus ingens is endemic to eastern and midwestern North America where raccoons occur (10). Other species that have been only very rarely implicated in human infection (with their natural distributions in parentheses) include Acanthocephalus rauschi (Alaska), Pseudoacanthocephalus bufonis (Southeast Asia), Corynosoma species (worldwide, marine), and Bolbosoma species (worldwide, marine) (15). There is one report of an avian acanthocephalan (Plagiorhynchus or related) from the eye of a groundskeeper in England; however, it is believed that the presence of the parasite in the eye was incidental after accidental inoculation rather than true infection following consumption of an infected host (16).
BIOLOGY AND LIFE CYCLE
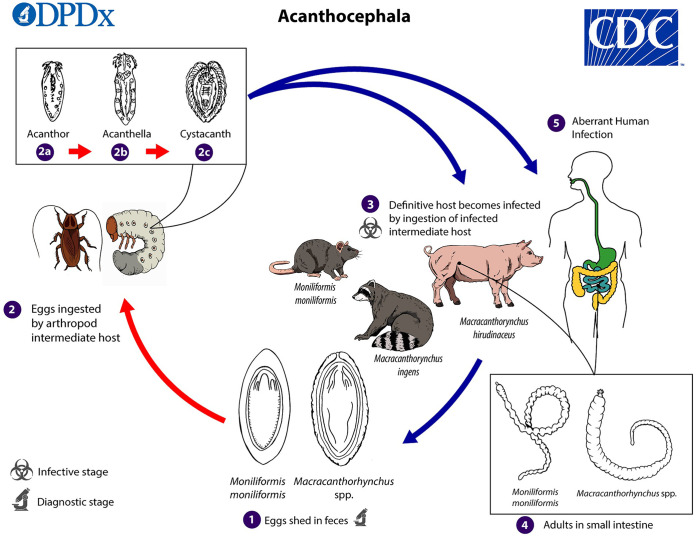
Acanthocephalans have a complex multihost life cycle, and only a few species have had their entire life cycles described. The three most common species implicated in human disease, M. hirudinaceus, M. ingens, and M. moniliformis, use pigs, raccoons, and rodents as their primary definitive hosts, respectively, although other carnivores may function as definitive hosts. Fully embryonated eggs containing an infective first-stage larva (acanthor) are shed in the feces of the definitive host. The eggs are ingested by an arthropod intermediate host. The primary groups of arthropods that serve as intermediate hosts include scarabaeoid beetles for M. hirudinaceus, millipedes for M. ingens, and various beetles and cockroaches for M. moniliformis; however, specificity for the arthropod host is believed to be low. Within the arthropod host, the parasite goes through multiple stages, from acanthor to acanthella to cystacanth, the last of which is the infective stage for the definitive host. The definitive host, including humans, becomes infected after ingesting arthropods containing infective cystacanths. In some cases, infection can occur from the ingestion of paratenic hosts. In the definitive host, liberated parasites attach to the mucosa of the small intestine, where they mature into adults and mate in about 8 to 12 weeks (17) (Fig. 1). The preponderance of human cases of Macracanthorhynchus and Moniliformis involving children may be due to children’s habit of putting objects, including insects, in their mouths. Human infection has also been documented in communities that use insects and other arthropods for medicinal purposes (10).
FIG 1.
Life cycle of acanthocephalans. Eggs are shed in the feces of the definitive hosts (1). The eggs contain a fully developed acanthor when shed in feces. The eggs are ingested by an intermediate host (2), which is an arthropod. Within the hemocoel of the insect, the acanthor (2a) molts into a second larval stage, called an acanthella (2b). After 6 to 12 weeks, the worm reaches the infective stage, called a cystacanth (2c). The definitive host becomes infected upon ingestion of intermediate hosts containing infective cystacanths (3). In the definitive host, liberated juveniles attach to the wall of the small intestine, where they mature image and mate in about 8 to 12 weeks. In humans (4), the worms seldom develop to full maturity and produce eggs. (Courtesy of the CDC-DPDx.)
EPIDEMIOLOGY OF HUMAN ACANTHOCEPHALIASIS
Acanthocephaliasis may be an ancient disease of humans. Analysis of fossilized feces from prehistoric humans in Utah have revealed the eggs of Moniliformis; however, it is unknown whether this represented true or spurious infections (18).
Macracanthorhynchus hirudinaceus is a nearly cosmopolitan parasite of pigs. Human infection has been documented in Thailand (19–22), Bulgaria (15), the Czech Republic (15), Vietnam (15), China (23, 24), Russia (15), Papua New Guinea (25), Australia (26), Madagascar (15), and Brazil (15). It has also been documented once from a patient in the United States (Louisiana) (7); however, two of us (M.R.C. and B.A.M.) had the opportunity to reexamine the specimen and determined it to be M. ingens. Human infection is most commonly seen in young children. Two cases were reported in children in China, ages 2.5 and 7 years old, presenting with acute abdominal colic (23). In a case from Thailand, M. hirudinaceus was recovered postmortem in a 32-year-old female who had died of rheumatic heart disease (22). In another case from Thailand, an adult worm was removed from the duodenum of a 26-year-old male patient by laparotomy (19). In the 1800s human infection was commonly reported in the Volga Valley in Russia, where the cockchafer beetle (Melolontha melolontha) was commonly consumed raw (23). The cases from Brazil were believed to be spurious following consumption of infected pig intestines (15).
Macracanthorhynchus ingens is a raccoon parasite endemic to eastern North America. To date there have been four documented human cases (exclusive of the aforementioned case from Louisiana) from Texas (4, 27), Florida (10), and Ohio (5). The reported cases in the United States have all been in children under the age of 18 months and diagnosed by the finding of adult worms in stool. The two cases in Texas were reported for infants of the ages 10 months and 15 months (4, 27). The case from Florida was reported for an 18-month-old female. The patient was asymptomatic but presented with eosinophilia. There were no known animal contacts, but the parents reported millipedes, the natural intermediate host for M. ingens, around the yard and garden (10). The infection in Ohio occurred in a 17-month-old female who was examined by her primary care physician after a parent noticed the worm in her stool. This Ohio case was novel in that eggs of M. ingens were also detected by ova-and-parasite (O&P) examination, indicating patent infection. The patient had no history of gastrointestinal symptoms or changes in bowel habit. She lived in rural Ohio and had no travel history outside the region (5). In the case from Louisiana, the patient, a 17-month-old male, had no documented animal exposures but regularly played outside, where he was reportedly seen putting objects in his mouth (7).
Moniliformis moniliformis is a cosmopolitan parasite of rats and other rodents. Human infection has been reported from the United States (11, 28), Belize (15), Colombia (15), Italy (15), Egypt (15), Nigeria (14, 29), Sudan (15), Zimbabwe (as Rhodesia) (30), Zambia (15), Saudi Arabia (31), Iran (9, 32–35), Iraq (36), Israel (15), Russia (15), Bangladesh (15), and Australia (26). Most cases are in children at or under 2 years of age (5, 10). Moniliformis moniliformis has been reported from Iran at least seven times, and in two cases, the patients were coinfected with Giardia duodenalis (35, 36). In one of those two, the patient was diagnosed with pica and the mother indicated that the child had been observed putting cockroaches in her mouth (35). A case diagnosed from Tasmania in a 14-month-old male was believed to have been acquired on mainland Australia (37). In 2006, a 20-month-old female from the Eastern Province of Saudi Arabia was diagnosed with M. moniliformis. The parents reported that one or two worms were seen daily in the patient’s stool for the prior 2 months; the mother also noted the patient had been observed on several occasions ingesting cockroaches (31).
Human infection with Acanthocephalus rauschi has been reported in French language literature (38) but could not be reviewed by us. Acanthocephalus rauschi was described from the Alaskan grayling (Thymallus arcticus) and North American river otter from Alaska (39), but the source of human infection is unknown.
Pseudoacanthocephalus bufonis, a parasite of toads in Southeast Asia, has been implicated in human infection in Indonesia. The intermediate or paratenic hosts, which are the likely source of human infection, are unknown, and the single human case was diagnosed postmortem on autopsy (40).
Members of the genus Bolbosoma are parasites of whales. Marine planktonic crustaceans are believed to serve as the intermediate host and fish as paratenic hosts. Human infection is believed to be acquired from the consumption of infected fish. All human cases of Bolbosoma infection to date have been documented from Japan (3, 8, 13, 41, 42). In one case, the patient had reported eating sashimi as part of his diet; in that case the parasite was identified as Bolbosoma cf. capitatum by DNA sequencing (3). In another case, a worm was removed from the jejunum of a 27-year-old female with bowel obstruction and identified by DNA sequencing as Bolbosoma (8). In yet another case, Bolbosoma was recovered from a 37-year-old male with a history of Crohn’s disease; the worm was removed surgically from the jejunal mucosa and was identified by DNA sequencing as Bolbosoma nipponicum (13). A 16-year-old boy was diagnosed with a probable Bolbosoma species infection after the recovery of an acanthocephalan in a tumor in the serosa over the ileum (42).
Members of the genus Corynosoma use marine amphipod crustaceans as intermediate hosts and pinnipeds and whales as definitive hosts, with marine fish serving as paratenic hosts and the source of infection. Two human cases have been recorded from Japan (6, 12) and one has been recorded from Alaska (43). The case from Alaska was diagnosed by microscopy of the intact worm from an Inuit male; the parasite was not identified to the species level (43). In one case from Japan, a worm was removed from the ileum of a 73-year-old male with a 2-year history of abdominal pain and a history of consuming uncooked seafood; the parasite was identified as Corynosoma villosum by molecular methods (6). In the other case from Japan, a worm was removed from the ileum of a 70-year-old female and was identified by DNA sequencing as Corynosoma cf. validum (12).
CLINICAL PRESENTATION, PATHOLOGY, AND TREATMENT
The majority of diagnosed cases of acanthocephaliasis are associated with no overt symptomology (3–5, 7, 9–11, 13, 26–28). Passage of an adult worm is typically the trigger for clinical evaluation, as the worms are large and readily noticeable in a stool specimen. Cases have also been diagnosed incidentally during endoscopic examination for nondescript gastrointestinal symptoms or other preexisting medical conditions, such as colon cancer, Crohn’s disease, and ovarian cysts (3, 13, 20). The overall spectrum of symptoms associated with acanthocephaliasis can be quite wide, and peripheral eosinophilia may be present in only some cases despite eosinophilic infiltration in tissues (10, 34). Few published cases of asymptomatic shedding of adult worms include peripheral white blood cell count, and those that do rarely include eosinophil count in the differential. Mild symptoms can include anorexia, nausea, cramping, constipation, diarrhea, abdominal discomfort, and irritability in young children (14, 29–31, 33, 35, 37). More moderate symptoms can include vomiting, severe abdominal pain, abdominal distension, and weight loss (12, 19, 32, 34, 36, 42). Severe symptoms include bloody diarrhea and ileus, perforation, and/or ulceration of the intestinal wall (most common with Bolbosoma and Corynosoma) (6, 8, 20, 21, 23, 41). Rare extraintestinal manifestations have also been reported (described above) (16).
The pathology within the intestinal tract has been described in multiple cases to date, each with unique aspects depending on the clinical severity (8, 19, 21). The typical findings at the site of attachment of the proboscis are characterized by ulceration of the mucosal surface with profound white blood cell infiltration which may include neutrophils, eosinophils, and/or lymphocytes (15, 44). Lymphocytic and monocytic infiltration has also been described at sites of submucosal edema (8, 19). Lacteals in the lamina propria are dilated with hemorrhage in the ileal wall, which may resemble necrotizing enteritis (15, 44). Follicular hyperplasia may be seen in mesenteric lymph nodes, with eosinophils entering the lymph nodes resulting in dilatation.
Treatment for acanthocephalans is largely unstandardized. For mild or asymptomatic cases, many patients have been treated successfully with pyrantel pamoate (11 mg/kg of body weight per dose) orally in a three-dose regimen separated by 2 weeks per dose (4, 5, 10, 11, 28). Typically, patients ceased to shed worms, ceased to have symptoms, or were negative on fecal exam for eggs after treatment with pyrantel pamoate. Previous reports have also described treatment success with levamisole (9, 32), niclosamide (26, 29, 34), mebendazole (30, 31), ivermectin (14), paracetamol (37), thiabendazole (33), and piperazine citrate (35). For more invasive infections involving bowel perforations, ileus, or ulceration, endoscopic removal of the worm without concomitant antihelminth therapy is common (8, 20, 21, 23, 41, 42).
DIAGNOSIS AND IDENTIFICATION
Acanthocephaliasis is usually diagnosed by the identification of adult parasites in stool specimens, usually in diaper-aged children. The number of cases with this particular demographic is probably largely due to (i) the habits of children to eat insects and (ii) the fact that a diaper may serve as a good collection apparatus, especially with astute parents who are likely to observe the worms when changing diapers. In children who are toilet trained, any evidence may be inconveniently flushed away. Patent acanthocephalan infections in humans are rare but do occur, and in those instances identification can be made by the finding of eggs in concentrated wet mounts of stool (5). Many technologists may not be trained to immediately identify the eggs; however, the eggs have a distinctive morphology that should draw attention and warrant further scrutiny.
Adult acanthocephalans are large, pseudocoelomate worms. Female specimens of M. moniliformis measure 10 to 27 cm; males are smaller, at 4 to 10 cm (Fig. 2A). Female specimens of Macracanthorhynchus measure 12 to 32 cm; males are smaller, at 7 to 8 cm (Fig. 2B). Adults of both genera are long and slender. They superficially resemble adults of Ascaris lumbricoides but are usually heavily wrinkled (Macracanthorhynchus) or exhibit pseudosegmentation (Moniliformis), and they do not exhibit a consistent length-to-width ratio along the length of the body. The anterior ends of both genera have readily visible proboscis receptacles which are hollow round openings, compared to the three “lips” seen with ascarids. The armed proboscis may be extended or retracted, and when retracted, some dissection may be necessary for confirmation and identification (10). Separation of M. hirudinaceus from M. ingens requires morphometric analysis of the proboscis and hooklets (45) or molecular methods such as PCR followed by sequencing analysis of the cytochrome c oxidase subunit I (COX-I) gene (5).
FIG 2.
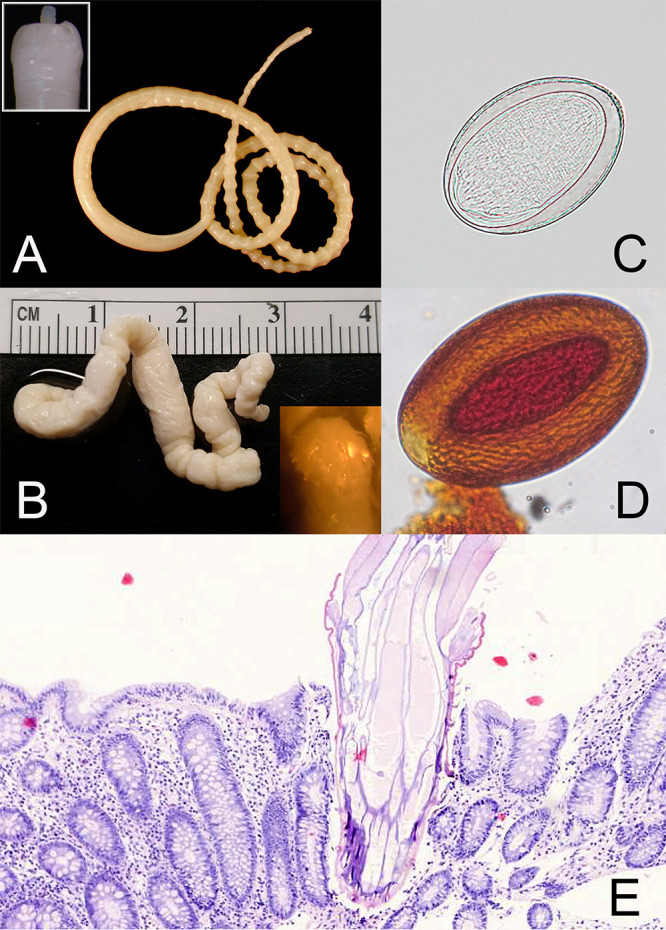
Adults and eggs of acanthocephalans. (A) Moniliformis moniliformis, adult; inset shows closeup of the extended proboscis. (B) Macracanthorhynchus ingens, adult; inset shows closeup of retracted proboscis after dissection. (C) Unstained wet mount of an egg of Moniliformis moniliformis. (Courtesy of the CDC-DPDx.) (D) Iodine-stained wet mount of an egg of a Macracanthorhynchus sp. (E) Proboscis of an acanthocephalan embedded in human intestinal epithelium. (Courtesy of Ramon Sandin.)
Eggs of M. moniliformis measure 90 to 125 μm long by 65 μm wide and have a thin shell; the mature acanthor is visible within the egg (Fig. 2C). Eggs of Macracanthorhynchus measure 80 to 100 μm long by 50 μm wide and possess a thick, wrinkly, and deeply pitted shell; internally a mature acanthor is visible (Fig. 2D). The eggs of M. hirudinaceus and M. ingens cannot be separated morphologically (10).
Acanthocephalans can also be identified by histologic examination (Fig. 2E). The tegument is thick and both circular and longitudinal muscles are present. There is no digestive tract, and the reproductive tract occupies most of the body cavity. The reproductive organs are contained within a connective tissue sheath called a ligament sac. Female worms may have one or two ovaries that are typically broken into sections called ovarian balls; males typically have two testes (44). The proboscis, when embedded in host tissue, usually displays hooklets (Fig. 2E).
Human infection and clinical symptomology due to acanthocephalans are rarely reported. This likely poses a problem in the appropriate detection and identification of these organisms. Due to the lack of familiarity of acanthocephalans among trained microscopists, expert consultation may be needed for these cases. Laboratorians may want to consider consulting their local and state public health laboratories, large reference laboratories with expert parasitologists on staff, or the Centers for Disease Control and Prevention, which offers a specialized service for parasite diagnostics.
Contributor Information
Blaine A. Mathison, Email: blaine.mathison@aruplab.com.
Romney M. Humphries, Vanderbilt University Medical Center
REFERENCES
- 1.García-Varela M, Pérez-Ponce de León G, de la Torre P, Cummings MP, Sarma SS, Laclette JP. 2000. Phylogenetic relationships of Acanthocephala based on analysis of 18S ribosomal RNA gene sequences. J Mol Evol 50:532–540. 10.1007/s002390010056. [DOI] [PubMed] [Google Scholar]
- 2.Goater TM, Goater CP, Esch GW. 2014. Parasitism: the diversity and ecology of animal parasites, 2nd ed. Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- 3.Arizono N, Kuramochi T, Kagei N. 2012. Molecular and histological identification of the acanthocephalan Bolbosoma cf. capitatum from the human small intestine. Parasitol Int 61:715–718. 10.1016/j.parint.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Brien JHF, Wehbe-Janek H. 2012. Successful treatment of Macracanthorhynchus ingens infection with mebendazole. J Pediatr Infect Dis 7:161–163. [Google Scholar]
- 5.Chancey RJ, Sapp SGH, Fox M, Bishop HS, Ndubuisi M, de Almeida M, Montgomery SP, Congeni B. 2021. Patent Macracanthorhynchus ingens infection in a 17-month-old child, Ohio. Open Forum Infect Dis 8:ofaa641. 10.1093/ofid/ofaa641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujita T, Waga E, Kitaoka K, Imagawa T, Komatsu Y, Takanashi K, Anbo F, Anbo T, Katuki S, Ichihara S, Fujimori S, Yamasaki H, Morishima Y, Sugiyama H, Katahira H. 2016. Human infection by acanthocephalan parasites belonging to the genus Corynosoma found from small bowel endoscopy. Parasitol Int 65:491–493. 10.1016/j.parint.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Hamula CL, Fang DC, Couturier MR. 2014. Photo Quiz: An unwelcome passenger in a 17-month-old male. J Clin Microbiol 52:2289; discussion, 2747. 10.1128/JCM.00280-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaito S, Sasaki M, Goto K, Matsusue R, Koyama H, Nakao M, Hasegawa H. 2019. A case of small bowel obstruction due to infection with Bolbosoma sp. (Acanthocephala: Polymorphidae). Parasitol Int 68:14–16. 10.1016/j.parint.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Maraghi SS, Rafiei A, Javaherizadeh H. 2014. Moniliformis moniliformis from Ahvaz southwest Iran. Hong Kong J Pediatrics 19:93–95. [Google Scholar]
- 10.Mathison BA, Bishop HS, Sanborn CR, Dos Santos Souza S, Bradbury R. 2016. Macracanthorhynchus ingens infection in an 18-month-old child in Florida: a case report and review of acanthocephaliasis in humans. Clin Infect Dis 63:1357–1359. 10.1093/cid/ciw543. [DOI] [PubMed] [Google Scholar]
- 11.Messina AF, Wehle FJ, Intravichit S, Washington K. 2011. Moniliformis moniliformis infection in two Florida toddlers. Pediatr Infect Dis J 30:726–727. 10.1097/INF.0b013e31821e52e9. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi K, Ito T, Sato T, Goto M, Kawamoto T, Fujinaga A, Yanagawa N, Saito Y, Nakao M, Hasegawa H, Fujiya M. 2016. Infection with fully mature Corynosoma cf. validum causes ulcers in the human small intestine. Clin J Gastroenterol 9:114–117. 10.1007/s12328-016-0646-7. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto S, Yamada M, Matsumura K. 2018. Mimicking small intestinal anisakiasis. Gastroenterology 154:e9–e10. 10.1053/j.gastro.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Anosike JC, Njoku AJ, Nwoke BE, Okoro OU, Okere AN, Ukaga CN, Adimonye RN. 2000. Human infections with Moniliformis moniliformis (Bremser 1811) Travassos 1915 in south-eastern Nigeria. Ann Trop Med Parasitol 94:837–838. 10.1080/00034983.2000.11813608. [DOI] [PubMed] [Google Scholar]
- 15.Meyers WM, Neafie RC, Marty AM, Wear DJ (ed). 2000. Pathology of infectious disease, vol 1. Helminthiases. Armed Forces Institute of Pathology, Washington, DC. [Google Scholar]
- 16.Haustein T, Lawes M, Harris E, Chiodini PL. 2010. An eye-catching acanthocephalan. Clin Microbiol Infect 16:787–788. 10.1111/j.1469-0691.2009.02896.x. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt GD, Nickol BB. 1985. Development and life cycles, p 273–305. In Crompton DWT, Nickol BB (ed), Biology of the Acanthocephala. Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- 18.Moore JG, Fry GF, Englert E, Jr.. 1969. Thorny-headed worm infection in North American prehistoric man. Science 163:1324–1325. 10.1126/science.163.3873.1324. [DOI] [PubMed] [Google Scholar]
- 19.Kliks M, Tantachamrun T, Chaiyaporn V. 1974. Human infection by an acanthocephalan Macracanthorhynchus hirudinaceus in Thailand: new light on a previous case. Southeast Asian J Trop Med Public Health 5:303–309. [PubMed] [Google Scholar]
- 20.Radomyos P, Chobchuanchom A, Tungtrongchitr A. 1989. Intestinal perforation due to Macracanthorhynchus hirudinaceus infection in Thailand. Trop Med Parasitol 40:476–477. [PubMed] [Google Scholar]
- 21.Tesana S, Mitrchai J, Chunsuttwat S. 1982. Acute abdominal pain due to Macracanthorhynchus hirudinaceus infection: a case report. Southeast Asian J Trop Med Public Health 13:262–264. [PubMed] [Google Scholar]
- 22.Pradatsundarasar A, Pechranond K. 1965. Human infection with the acanthocephalan Macracanthorhynchus hirudinaceus in Bangkok: report of a case. Am J Trop Med Hyg 14:774–776. 10.4269/ajtmh.1965.14.774. [DOI] [PubMed] [Google Scholar]
- 23.Leng YJ, Huang WD, Liang PN. 1983. Human infection with Macracanthorhynchus hirudinaceus Travassos, 1916 in Guangdong Province, with notes on its prevalence in China. Ann Trop Med Parasitol 77:107–109. 10.1080/00034983.1983.11811681. [DOI] [PubMed] [Google Scholar]
- 24.Zhong HL, Feng LB, Wang CX, Kang B, Wang ZZ, Zhou GH, Zhao Y, Zhang YZ. 1983. Human infection with Macracanthorhychus hirudinaceus causing serious complications in China. Chin Med J (Engl) 96:661–668. [PubMed] [Google Scholar]
- 25.Barnish G, Misch KA. 1987. Unusual cases of parasitic infections in Papua New Guinea. Am J Trop Med Hyg 37:585–587. 10.4269/ajtmh.1987.37.585. [DOI] [PubMed] [Google Scholar]
- 26.Prociv P, Walker J, Crompton LJ, Tristram SG. 1990. First record of human acanthocephalan infections in Australia. Med J Aust 152:215–216. 10.5694/j.1326-5377.1990.tb125152.x. [DOI] [PubMed] [Google Scholar]
- 27.Dingley D, Beaver PC. 1985. Macracanthorhynchus ingens from a child in Texas. Am J Trop Med Hyg 34:918–920. 10.4269/ajtmh.1985.34.918. [DOI] [PubMed] [Google Scholar]
- 28.Counselman K, Field C, Lea G, Nickol B, Neafie R. 1989. Moniliformis moniliformis from a child in Florida. Am J Trop Med Hyg 41:88–90. 10.4269/ajtmh.1989.41.88. [DOI] [PubMed] [Google Scholar]
- 29.Ikeh EI, Anosike JC, Okon E. 1992. Acanthocephalan infection in man in northern Nigeria. J Helminthol 66:241–242. 10.1017/s0022149x00014632. [DOI] [PubMed] [Google Scholar]
- 30.Goldsmith JM, Smith ME, Fleming F. 1974. Human infection with Moniliformis sp. in Rhodesia. Ann Trop Med Parasitol 68:363–364. 10.1080/00034983.1974.11686960. [DOI] [PubMed] [Google Scholar]
- 31.Sahar MM, Madani TA, Al Mohsen IZ, Almodovar EL. 2006. A child with an acanthocephalan infection. Ann Saudi Med 26:321–324. 10.5144/0256-4947.2006.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berenji F, Fata A, Hosseininejad Z. 2007. A case of Moniliformis moniliformis (Acanthocephala) infection in Iran. Korean J Parasitol 45:145–148. 10.3347/kjp.2007.45.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moayedi B, Izadi M, Maleki M, Ghadirian E. 1971. Human infection with Moniliformis moniliformis (Bremser, 1811) Travassos, 1915 (syn. Moniliformis dubius). Report of a case in Isfahan, Iran. Am J Trop Med Hyg 20:445–448. 10.4269/ajtmh.1971.20.445. [DOI] [PubMed] [Google Scholar]
- 34.Sahba GH, Arfaa F, Rastegar M. 1970. Human infection with Moniliformis dubius (Acanthocephala) (Meyer, 1932). (Syn. M. moniliformis, (Bremser, 1811) (Travassos, 1915) in Iran. Trans R Soc Trop Med Hyg 64:284–286. 10.1016/0035-9203(70)90137-9. [DOI] [PubMed] [Google Scholar]
- 35.Salehabadi A, Mowlavi G, Sadjjadi SM. 2008. Human infection with Moniliformis moniliformis (Bremser 1811) (Travassos 1915) in Iran: another case report after three decades. Vector Borne Zoonotic Dis 8:101–103. 10.1089/vbz.2007.0150. [DOI] [PubMed] [Google Scholar]
- 36.Al-Rawas AY, Mirza MY, Shafig A, Al-Kindy L. 1977. First finding of Moniliformis moniliformis (Bremser 1811) Travassos 1915 (Acanthocephala: Oligacanthorhynchidae) in Iraq from human child. J Parasitol 63:396–397. 10.2307/3280092. [DOI] [PubMed] [Google Scholar]
- 37.Bettiol S, Goldsmid JM. 2000. A case of probable imported Moniliformis moniliformis infection in Tasmania. J Travel Med 7:336–337. 10.2310/7060.2000.00090. [DOI] [PubMed] [Google Scholar]
- 38.Golvan YJ. 1969. Systématique des Acanthocéphales (Acanthocephala Rudolphi, 1801) Premiere partie L’Ordre des Palaecanthocephala Meyer 1931 premiere fascicule la super-damille des Echinorhynchoidea (Cobbold, 1876) Golvan et Houin, 1963. Memo Museum Natl Hist Naturelle A 57:291. [Google Scholar]
- 39.Schmidt GD. 1969. Paracanthocephalus rauschi sp. n. (Acanthocephala, Paracanthocephaliidae) from grayling, Thymallus arcticus (Pallas), in Alaska. Can J Zool 47:383–385. 10.1139/z69-071. [DOI] [Google Scholar]
- 40.Bush SE, Duszynski DW, Nickol BB. 2009. Acanthocephala from amphibians in China with the description of a new species of Pseudoacanthocephalus (Echinorhynchida). J Parasitol 95:1440–1445. 10.1645/GE-2101.1. [DOI] [PubMed] [Google Scholar]
- 41.Tada I, Otsuji Y, Kamiya H, Mimori T, Sakaguchi Y, Makizumi S. 1983. The first case of a human infected with an acanthocephalan parasite, Bolbosoma sp. J Parasitol 69:205–208. 10.2307/3281300. [DOI] [PubMed] [Google Scholar]
- 42.Beaver PC, Otsuji T, Otsuji A, Yoshimura H, Uchikawa R, Sato A. 1983. Acanthocephalan, probably Bolbosoma, from the peritoneal cavity of man in Japan. Am J Trop Med Hyg 32:1016–1018. 10.4269/ajtmh.1983.32.1016. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt GD. 1971. Acanthocephalan infections of man, with two new records. J Parasitol 57:582–584. 10.2307/3277920. [DOI] [PubMed] [Google Scholar]
- 44.Orihel TC, Ash LR. 1995. Parasites in human tissues. ASCP Press, Chicago, IL. [Google Scholar]
- 45.Richardson DJ. 2005. Identification of cystacanths and adults of Oligacanthorthynchus toruosa, Macracanthorhynchus ingens, and Macracanthorhynchus hirudinaceus based on proboscis and hook morphometrics. J Arkansas Acad Sci 59:205–209. [Google Scholar]