Abstract
High oxalate consumption has been recognized as a risk factor for renal calcium oxalate stones in companion animals (dogs and cats). However, the cellular signaling involved in oxalate-induced dysfunction in renal tubular epithelial cells remains not fully elucidated. In this study, Mardin–Darby canine kidney (MDCK) cells, an epithelial cell line derived from canine kidney tubule, were tested for cell proliferation activity and barrier function after being exposed to sodium oxalate (NaOx). Further, the involvement of Wnt/β-catenin in NaOx-induced renal epithelial barrier dysfunction was evaluated. MDCK cells treated with NaOx exhibited reduction in cell proliferation and migration. Besides, NaOx exposure led to a decrease in transepithelial electrical resistance and an increase in paracellular permeability. The deleterious effects of NaOx on epithelial barrier function were related to the suppressed abundance of tight junction proteins including zonula occludens, occludin, and claudin-1. Of note, protein levels of β-catenin and phosphorylated (p)-β-catenin (Ser552) in MDCK cells were repressed by NaOx, indicating inhibitory effects on Wnt/β-catenin signaling. An inhibition of glycogen synthase kinase-3β (GSK-3β) by SB216763 enhanced the abundance of β-catenin and p-β-catenin (Ser552), and protected against epithelial barrier dysfunction in NaOx-treated MDCK cells. The results revealed a critical role of Wnt/β-catenin signaling in the epithelial barrier function of MDCK cells. Activation of Wnt/β-catenin signaling might be a potential therapeutic target for the treatment of oxalate-linked renal stones.
Keywords: barrier function, canine, cell proliferation, oxalate, renal epithelial cell, Wnt/β-catenin
Introduction
Nephrolithiasis, also known as renal stones, is a complicated pathological condition in which crystals or stones are deposited inside the kidney (Yasui et al., 2017). Nephrolithiasis has become a frequent problem in humans and animals. It was generally believed that renal stones formed by calcium oxalate are the second most prevalent type of nephrolithiasis occurring in companion animals (e.g., dogs and cats; Syme, 2012; Alford et al., 2020). High intake of oxalate has been a risk factor expediting the formation of calcium oxalate stones. Also, exposure to oxalate or calcium oxalate crystals induces cellular injury and impairment in the renal tubular epithelium (Wang et al., 2018). In response to renal injury, epithelial cells undergo proliferation for regeneration and tissue repair. Of particular note, the epithelial tight junction proteins, such as zonula occludens (ZOs), occludin, claudins, and junctional adhesion molecules, are critical determinants for the paracellular transport in renal tubular epithelial cells (Günzel and Yu, 2013), which may be disrupted following the renal stone-induced renal damage. Thus, exploring the mechanism relating to the function of the renal epithelial barrier is of significance for prevention or treatment of nephrolithiasis in humans and companion animals including dogs and cats.
The canonical Wnt/β-catenin signaling is critical for embryonic development, wound healing, and malignancies due to implication in a myriad of biological processes, such as proliferation, differentiation, migration, polarity, and cell death (Ponce et al., 2011; Steinhart and Angers, 2018). In the absence of Wnt ligands, the transcriptional activity of β-catenin is maintained at a low level by the β-catenin destruction complex, which is composed of the scaffold protein Axin, adenomatous polyposis coli, and two kinases, including glycogen synthase kinase-3 (GSK-3) and casein kinase 1 (CK1) (Stamos and Weis, 2013). In response to Wnt ligands, the activity of β-catenin destruction complex is suppressed, resulting in β-catenin accumulation and translocation to the nucleus, where it activates downstream targets via binding to and interacting with the T cell factor/lymphoid enhancer-binding factor family of transcription factors (Gewin, 2018). Over the past years, Wnt/β-catenin signaling has been identified to be involved in the modulation of multiple kidney diseases (Zhou et al., 2016; Nusse and Clevers, 2017; Malik et al., 2020). Despite these findings, the functional role of Wnt/β-catenin signaling and its contribution to oxalate-induced disorder in renal epithelial barrier remain unknown. The present study examined the cell proliferation activity, cell cycle profile, barrier integrity, and proteins implicated in barrier function of Mardin–Darby canine kidney (MDCK) cells exposed to sodium oxalate (NaOx). Further, the underlying mechanisms linking Wnt/β-catenin signaling and renal epithelial barrier integrity were validated.
Materials and Methods
Reagents
Dulbecco’s modified eagle medium (DMEM), Ca2+-free DMEM, and fetal bovine serum (FBS) were purchased from Gibco (Carlsbad, CA, USA). Sodium oxalate, trypan blue, propidium iodide (PI), and fluorescein isothiocyanate (FITC)-dextran (20 kDa) were bought from Sigma-Aldrich (St. Louis, MO, USA). TRIzol reagent was obtained from Mei5 Biotechnology (Beijing, China). Complementary DNA (cDNA) Synthesis SuperMix was produced by Yeasen Biotech (Shanghai, China). SYBR Green quantitative polymerase chain reaction (qPCR) Mix was purchased from Aidlab Biotechnologies (Beijing, China). LDH Release Assay Kit and EdU Cell Proliferation Kit were obtained from Beyotime Biotechnology (Shanghai, China). Annexin V-FITC Apoptosis Detection Kit was purchased from JIAMAYBIOTECH (Beijing, China). Antibodies against claudin-1, occludin, ZO-1, ZO-2, and ZO-3 were products of Sangon Biotech (Shanghai, China). Antibodies against cleaved-caspase-3, β-catenin, and phospho-β-catenin (Ser552) were obtained from Cell Signaling Technology (Beverly, MA, USA). Antibodies against PARP1 and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). SB216763 was obtained from Tocris Bioscience (Bristol, Avon, UK).
Cell culture
MDCK cells, a canine-derived renal epithelial cell line obtained from the American Type Culture Collection (ATCC, CCL-34), were cultured in DMEM medium supplemented with 10% FBS and 1% penicillin-streptomycin at 37 °C in a humidified 5% CO2 atmosphere. Cells were passaged once 80% to 90% confluence was reached.
Trypan blue staining
MDCK cells were seeded in 6-well plates (6 × 104 cells per well). As cells expanded to 70% confluency, they were treated with 0, 0.5, 1, 2, and 4 mmol/L NaOx for 24 h. Cells were harvested with 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA) and then incubated with 0.4% trypan blue solution for 2 min at room temperature. Using an inverted microscope (Olympus, Japan), the stained cells were observed and counted with a hemocytometer.
Scratch assay
Briefly, MDCK cells were seeded in 6-well plates, which were labeled by markers for wound location. Once confluence reached >90%, the cell monolayer was scratched by using 200 µL sterile pipette tips. The magnitude and direction of the force applied to pipette tips were as consistent as possible. After washing three times with PBS to remove debris, cells were treated with a Ca2+-free medium containing various concentrations of NaOx. Cell migration was observed under an inverted microscope with images acquisition device at 12 and 24 h, respectively. Wound areas were measured using the Image J software (NIH, USA). Cell migration was calculated following the equation: cell migration rate (%) = (Original scratch area − current scratch area)/Original scratch area × 100%.
Lactate dehydrogenase release determination
The concentration of lactate dehydrogenase (LDH) in supernatant was measured using the LDH Release Assay Kit following the manufacturer’s instructions. MDCK cells were seeded onto 96-well plates (3,000 cells per well) and then incubated with an FBS-free medium containing various concentrations of NaOx for 24 h. The supernatant and working solution were transferred into new 96-well plates by a ratio of 2:1 and incubated for 30 min at room temperature in the dark. Subsequently, the absorbance was measured at 490 nm using a SpectraMax M3 Multi-Mode Microplate Reader (Molecular Devices, USA).
Cell cycle analysis
After a 6-h starvation in FBS-free medium, MDCK cells were incubated with various concentrations of NaOx for 24 h. Cells were collected and washed twice with ice-cold PBS, followed by 70% ethanol fixation at −20 °C overnight. The ethanol was removed completely by washing twice with PBS. After incubation with 100 µg/mL RNase A for 20 min, cells were stained with 50 µg/mL of PI for 30 min at room temperature in the dark. The fluorescence intensity of cells was analyzed by a CytoFLEX flow cytometer (Beckman Coulter, USA), and the data were presented as percentages of cells in G1, S, and G2/M phases by the CytExpert software (Beckman Coulter, USA).
5-Ethynyl-2′-deoxyuridine assay
The effect of NaOx on cell proliferation was further confirmed by using an EdU Cell Proliferation Kit, following the protocol provided by manufacturer. MDCK cells were seeded in 24-well plates with a density of 3 × 104 cells/well and treated with NaOx for 24 h. Then, prewarmed 5-ethynyl-2′-deoxyuridine (EdU) solution was added and the plates were incubated for 2 h in an incubator at 37 °C, following which the cells were stained by Hoechst 33342 (5 μg/mL) for 10 min away from light. The stained cells were observed and photographed under a fluorescence microscope (Zeiss, Germany).
Real-time quantitative PCR
Following treatment with NaOx for 24 h, MDCK cells were subjected to total RNA extraction by using the TRIzol reagent and reverse transcribed into cDNA by means of the cDNA Synthesis SuperMix according to the manufacturer’s instructions. In brief, 10 μL of 2 × SuperMix, 2.5 μg of total RNA, and RNase-free ddH2O up to 20 μL were included in the reverse transcription reaction mixture. The reverse transcription procedure was as follows: 25 °C, 5 min; 42 °C, 30 min; and 85 °C, 5 min. Real-time quantitative PCR (RT-qPCR) was performed with the SYBR Green qPCR Mix using the ABI 7500 Real-Time PCR system (Life Technologies, USA). Quantitative PCR mixture comprised 10 μL of 2 × SYBR qPCR Mix; 2 μL of 5- to 10-fold diluted cDNA template; 0.4 μL of forward primer (10 μmol/L); 0.4 μL of reverse primer (10 μmol/L); and sterilized double distilled H2O (ddH2O) up to 20 μL. Primer sequences used in this experiment are listed in Supplementary Table 1. Amplifications were performed following the procedure of a two-step method (95 °C, 15 min, 1 cycle; 95 °C 10 s followed by 60 °C 32 s, 40 cycles; and melting curve stage). GAPDH was used as a reference gene in the calculation of the relative expression level of a target gene by the 2−ΔΔCT method.
Monolayer transepithelial electrical resistance determination
Briefly, MDCK cells were seeded in the apical side of transwell inserts (polycarbonate membrane; membrane area, 0.33 cm2; pore size, 0.4 μm) in 24-well plates with a density of 5 × 104 cells per well. The transepithelial electrical resistance (TEER) of cells was measured by using a Millicell ERS-2 Volt-Ohm Meter (Millipore, USA) every day. Cells were challenged with 0, 0.5, 1, 2, and 4 mmol/L NaOx once the TEER readings had reached a plateau. Within 48 h following treatment, TEER was determined and recorded every 12 h.
Monolayer paracellular permeability measurement
The monolayer cellular barrier was constructed following the aforementioned steps. 1 mg/mL FITC-dextran and NaOx were added into the apical side of monolayer. The fluorescence intensity of medium obtained from the basolateral side was measured at 0, 12, 24, and 48 h using a SpectraMax M3 Multi-Mode Microplate Reader at excitation and emission wavelengths of 490 and 520 nm, respectively.
Western blot analysis
MDCK cells incubated with NaOx were collected for protein extraction by using radioimmunoprecipitation assay (RIPA) lysis buffer (10 mmol/L Tris-HCl, pH 7.4; 10 mmol/L EDTA; 150 nmol/L NaCl; 1% NP-40; and 0.1% sodium dodecyl sulfate [SDS]) supplemented with protease and phosphatase inhibitor cocktail (Roche, Switzerland). The concentration of protein was determined by bicinchoninic acid (Reagent A: 0.1 g sodium bicinchoninate, 2.0 g Na2CO3·H2O, 0.16 g sodium tartrate [dehydrate], 0.4 g NaOH, and 0.95 g NaHCO3 in 100 mL of ddH2O; Reagent B: 0.4 g CuSO4·5H2O in 10 mL of ddH2O = 50:1) assay. About 25 μg denatured protein of each sample was separated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) gels (10 mL of Resolving gel: 4 mL of 30% acrylamide, 2.5 mL of 1.5 mol/L Tris-HCl (pH 8.8), 0.1 mL of 10% SDS, 0.1 mL of 10% ammonium persulfate (APS), 4 μL of tetramethylethylenediamine (TEMED), and 4 mL of ddH2O; 4 mL of Stacking gel: 0.67 mL of 30% acrylamide, 0.5 mL of 1 mol/L Tris-HCl (pH 6.8), 40 μL of 10% SDS, 40 μL of 10% APS, 4 μL of TEMED, and 2.7 mL of ddH2O), and then transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked by 5% nonfat milk for 1 h at 25 °C, subsequently incubated with primary antibodies (1: 2,000) overnight at 4 °C. Thereafter, the membranes were incubated with appropriate secondary antibodies (1:5,000) for 1 h at 25 °C. The bands of target protein were visualized by the Image Quant LAS 4000 mini system (GE Healthcare, Sweden) after incubation with the enhanced chemiluminescence reagent (Reagent A:B = 1:1; Huaxingbio Biotechnology Co., China). The intensity of blots was quantified by Image J software (NIH, USA) and normalized to GAPDH. All results were indicated as the relative value of the control group.
Statistical analysis
Data were represented as mean ± SEM. One-way analysis of variance (ANOVA) and Duncan’s post hoc tests were used for statistical analysis by SAS 9.1 (SAS Institute, USA). Graphs were generated by using GraphPad Prism 7.02 (GraphPad Software, USA). P < 0.05 indicated significant difference.
Results
Sodium oxalate inhibited cell proliferation activity and migration in MDCK cells
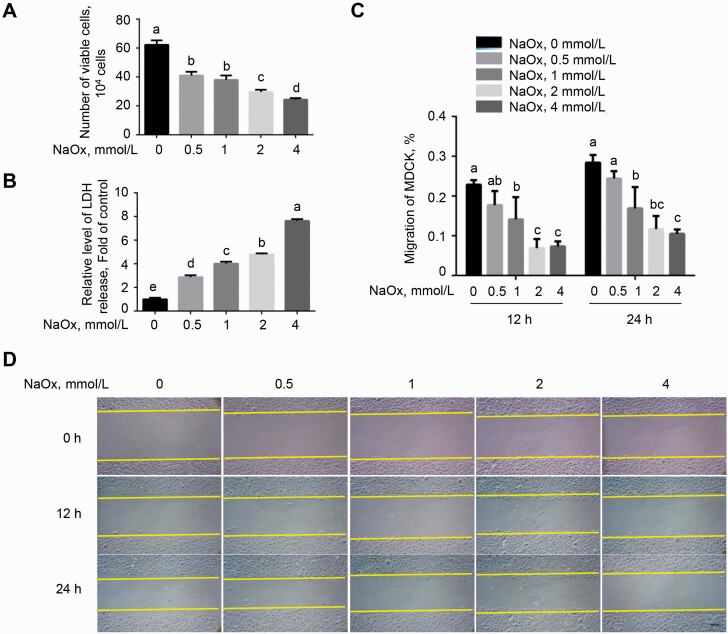
As shown in Figure 1A, MDCK cells incubated with NaOx for 24 h resulted in a decreased cell proliferation activity (P < 0.05) in a dose-dependent manner, compared with the control. Besides, the levels of LDH release in medium were significantly elevated in cells exposed to NaOx treatment (P < 0.05; Figure 1B), indicating a loss of membrane integrity in response to NaOx. Thereafter, we performed a wound-healing assay as shown in Figure 1C and D to evaluate the rate of cell migration. Treatment with NaOx dose-dependently impeded the distance traveled by MDCK cells at 12 and 24 h (P < 0.05), suggesting that the cell migration rate was blocked by NaOx.
Figure 1.
Effect of NaOx exposure on proliferation activity of MDCK cells. (A) Cells were treated with various doses (0, 0.5, 1, 2, and 4 mmol/L) of NaOx for 24 h, followed by a determination of cell proliferation activity assessed by trypan blue staining. (B) The activity of lactate dehydrogenase (LDH) released into medium from cell treated with NaOx for 24 h was detected. (C and D) Following treatment with NaOx for 24 h, the confluent monolayers were wounded with pipette tips. The cell migration rate was recorded at 0, 12, and 24 h, respectively. Images are representative of three observations. Scale bar indicates 50 μm. Values are means ± SEM. n = 3. Means without common superscripts differ (P < 0.05). Abbreviations: MDCK, Mardin–Darby canine kidney; NaOx, sodium oxalate.
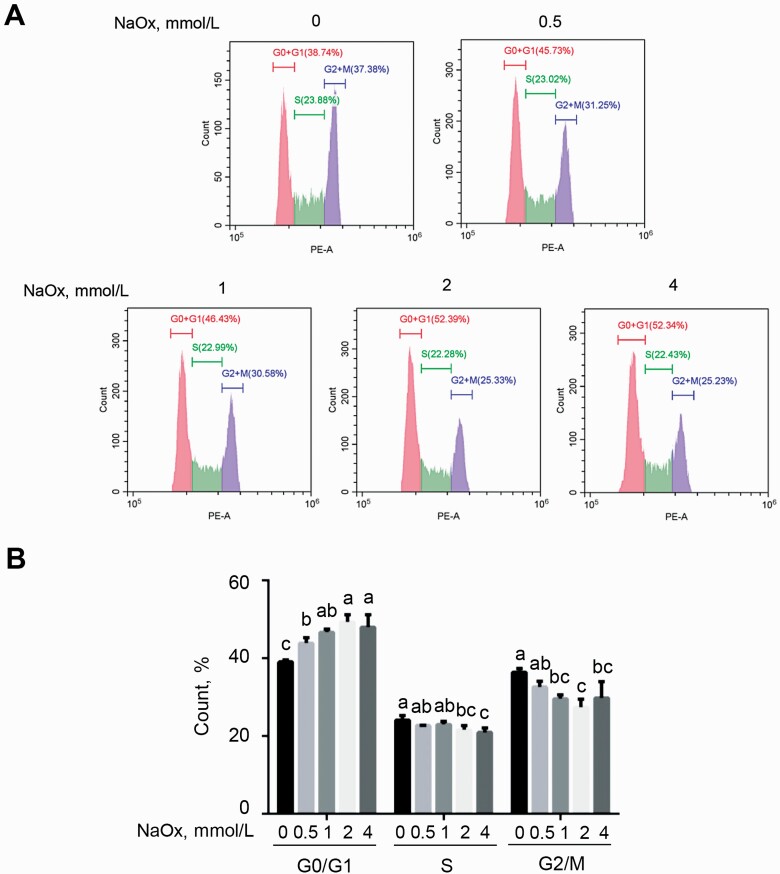
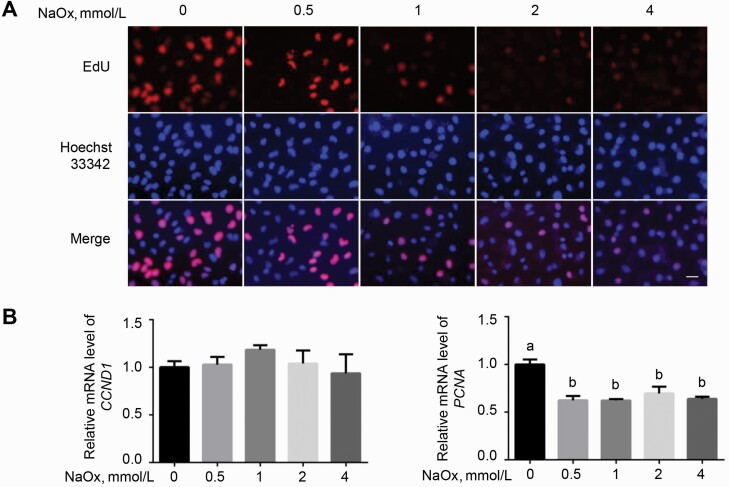
Cell cycle arrest induced by NaOx in MDCK cells
Compared with the control, NaOx treatment increased the proportion of cells in G0/G1 phase and decreased the proportion of cells in S and G2/M phases (P < 0.05), suggesting that the cell cycle shifted from S phase and G2/M phases toward G0/G1 phase (Figure 2A and B). EdU staining further verified the inhibition of NaOx on cell proliferation (Figure 3A). To confirm the cell proliferation at molecular level, mRNA level of genes involved in cell cycle was detected. As shown by RT-qPCR assay, cells incubated with NaOx displayed a downregulated mRNA level of proliferating cell nuclear antigen (PCNA) (P < 0.05). No significant difference was observed in terms of the mRNA level of cyclin D1 between NaOx-treated MDCK cells and the control (P > 0.05; Figure 3B).
Figure 2.
Cell cycle analysis of MDCK cells exposed to NaOx treatment. (A) MDCK cells were subjected to a 6-h serum starvation and then a 24-h treatment of NaOx, following which cell cycle distribution was measured by flow cytometry. (B) The percentage of MDCK cells in G0/G1, S, and G2/M phases. Data are presented as means ± SEM (n = 3). Bars labeled with different letters mean significant difference (P < 0.05). Abbreviations: MDCK, Mardin–Darby canine kidney; NaOx, sodium oxalate.
Figure 3.
Sodium oxalate-induced stagnation in cell proliferation evaluated by EdU assay and cell cycle-related gene expression. (A) MDCK cells were allowed to proliferate for 24 h in the presence of different doses of NaOx, followed by EdU and Hoechst 33342 staining. Scale bar: 20 μm. (B) Cells were treated with NaOx for 24 h before RNA extraction, after which RT-qPCR was performed to detect the relative mRNA level of CCND1 and PCNA. GAPDH was used as a reference gene (n = 3). Means without a common letter differ (P < 0.05). Abbreviations: Edu, 5-ethynyl-2′-deoxyuridine; MDCK, Mardin–Darby canine kidney; NaOx, sodium oxalate; RT-qPCR, real-time quantitative PCR.
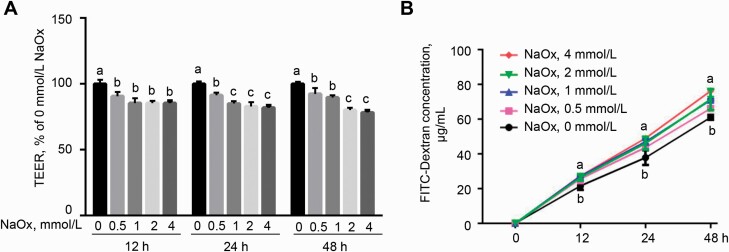
Sodium oxalate exposure led to epithelial barrier dysfunction in MDCK cells
To explore the effect of NaOx on epithelial barrier function, TEER and paracellular permeability of monolayer were measured, respectively. Following the exposure of cells to NaOx, monolayer TEER was markedly reduced (P < 0.05) at 12, 24, and 48 h (Figure 4A), in comparison with the control. Consistent with the decreased TEER in MDCK cells, 4 mmol/L of NaOx treatment significantly increased the concentration of FITC-dextran (P < 0.05) in the basolateral of transwell insert at 12, 24, and 48 h (Figure 4B), indicating an increase in paracellular permeability of monolayer.
Figure 4.
Effect of NaOx treatment on barrier function of MDCK cells. Cells were subjected to NaOx for up to 48 h, during which TEER (A) and paracellular permeability (B) were determined. Values are shown as mean ± SEM (n = 3). Different letters represent significant differences (P < 0.05). Abbreviations: FITC, fluorescein isothiocyanate; MDCK, Mardin–Darby canine kidney; NaOx, sodium oxalate; TEER, transepithelial electrical resistance.
A reduction in expression of tight junction proteins in response to NaOx
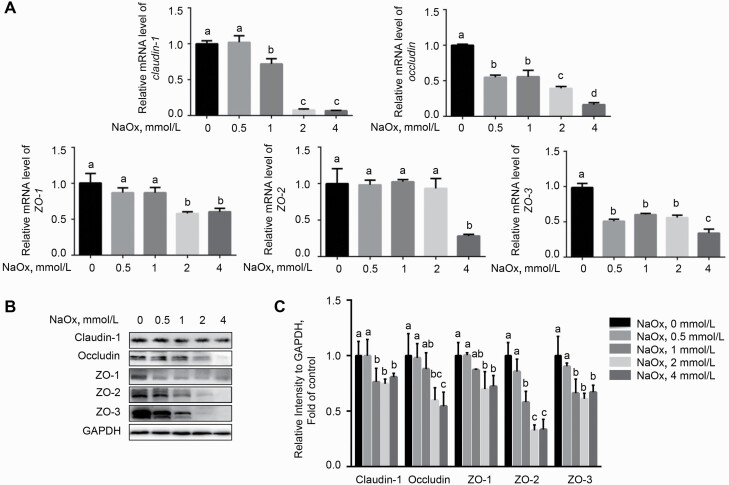
To investigate whether tight junction proteins are involved in NaOx-induced epithelial barrier dysfunction, we examined the expression of tight junction proteins by RT-qPCR assay and Western blot. Cells exposure to NaOx showed lower mRNA levels of claudin-1, occludin, ZO-1, ZO-2, and ZO-3 (P < 0.05) than the control (Figure 5A). Likewise, the protein abundances of tight junction proteins (P < 0.05), including claudin-1, occludin, ZO-1, ZO-2, and ZO-3, were prominently dose-dependently declined in response to NaOx treatment (Figure 5B).
Figure 5.
The addition of NaOx inhibits the expression of tight junction proteins at both transcriptional and protein levels. Cells treated with 0, 0.5, 1, 2, and 4 mmol/L of NaOx for 24 h were harvested for RT-qPCR (A) or Western blot analysis (B and C). GAPDH was regarded as a reference gene or loading control (n = 3). Means without a common letter differ (P < 0.05). Abbreviations: NaOx, sodium oxalate; RT-qPCR, real-time quantitative PCR.
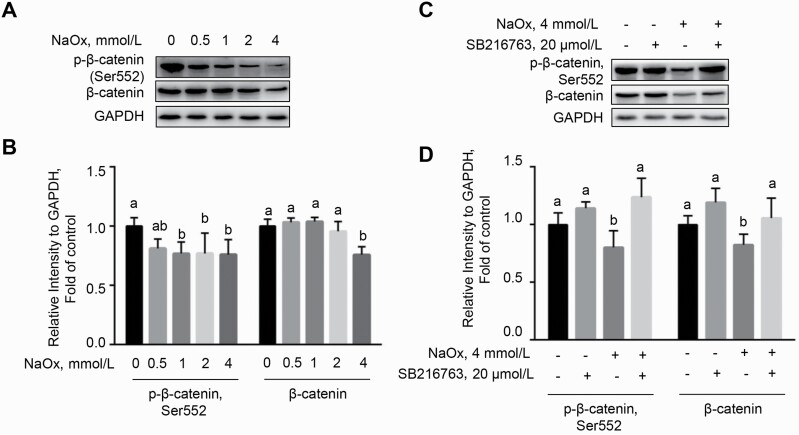
Sodium oxalate repressed Wnt/β-catenin signaling in MDCK cells
Since Wnt/β-catenin signaling plays a vital role in repairing injured renal tubular epithelial cells (Zhou and Liu, 2016), we next detected the abundance of β-catenin and phosphorylated-β-catenin. As shown, compared with the control cells, NaOx treatment led to decreased levels of β-catenin and p-β-catenin (Ser552) (P < 0.05) which are known as determinants for the activation of Wnt/β-catenin signaling (Figure 6A and B). Given that cells treated with 4 mmol/L of NaOx exhibited reduction in abundances of both β-catenin and p-β-catenin (Ser552) (P < 0.05), this dose of NaOx was designated in the following experiments.
Figure 6.
GSK3β inhibitor (SB216763) blocks NaOx-induced decrease in the levels of p-β-catenin (Ser552) and β-catenin. (A and B) MDCK cells were challenged with various concentrations of NaOx before Western blot analysis. (C and D) The reduction in the protein abundance of p-β-catenin (Ser552) and β-catenin was impeded in the presence of SB216763 (n = 3). Bars without a common letter denote significant difference (P < 0.05). Abbreviations: GSK, glycogen synthase kinase; MDCK, Mardin–Darby canine kidney; NaOx, sodium oxalate.
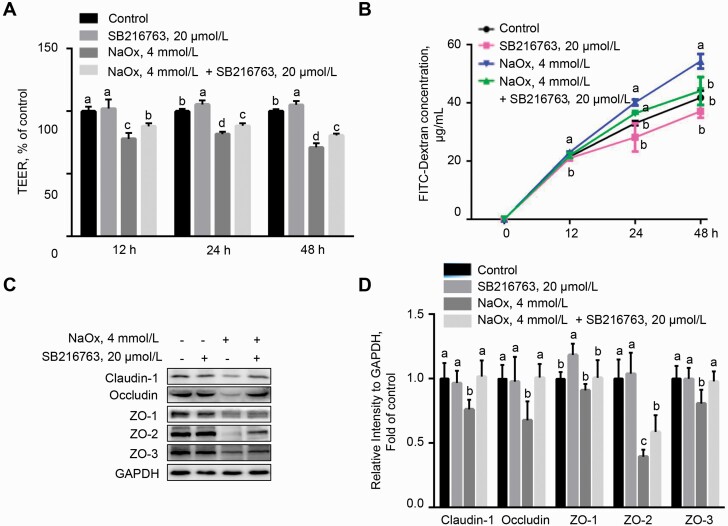
Sodium oxalate-induced barrier dysfunction was reversed by GSK-3β inhibitor in MDCK cells
It has been reported that Wnt/β-catenin signaling regulates barrier function in vascular endothelial cells, particularly the blood–brain barrier (Cong and Kong, 2020). To ascertain the functional role of Wnt/β-catenin signaling in cellular injury, MDCK cells were incubated with NaOx in the presence or absence of GSK-3β inhibitor SB216763 (20 μmol/L), a specific agonist of Wnt/β-catenin signaling. Sodium oxalate-induced downregulation of β-catenin and p-β-catenin (Ser552) was significantly reversed by SB216763 (P < 0.05), indicating that Wnt/β-catenin signaling was reactivated by SB216763 (Figure 6C and D). Intriguingly, the decreased TEER of monolayer induced by NaOx was alleviated by SB216763 (P < 0.05) compared with NaOx treatment alone at 12, 24, and 48 h (Figure 7A). Consistently, the elevated paracellular permeability in MDCK cells exposed to NaOx was repressed by SB216763 treatment, as evidenced by a lower concentration of FITC-dextran (P < 0.05) in the basolateral of transwell insert at 48 h (Figure 7B). These results indicated that Wnt/β-catenin signaling is critical for NaOx-induced epithelial barrier dysfunction in MDCK cells. We next examined the effect of activation of Wnt/β-catenin on the NaOx-induced reduction in the expression of tight junction (TJ) proteins by Western blot. As shown in Figure 7C and D, NaOx treatment led to downregulation in the protein abundance of claudin-1, occludin, ZO-1, ZO-2, and ZO-3, which was abrogated by SB216763 (P < 0.05).
Figure 7.
Sodium oxalate-induced disruption in barrier function of MDCK cells is revered by treatment of SB216763. (A) TEER measurement of MDCK monolayer treated with NaOx in the presence or absence of SB216732. (B) Evaluation of paracellular permeability by FITC-Dextran assay. (C and D) Western blot analysis of tight junction proteins (claudin-1, occluding, ZO-1, ZO-2, and ZO-3) in MDCK cells exposed to NaOx, SB216763, or NaOx in combination with SB216763 (n = 3). Means with no common letters indicate significant difference (P < 0.05). Abbreviations: FITC, fluorescein isothiocyanate; MDCK, Mardin–Darby canine kidney; NaOx, sodium oxalate; TEER, transepithelial electrical resistance; ZO, zonula occluden.
Discussion
The renal epithelial cell is a pivotal cell type that participates in reabsorption, excretion, and selective transport of nutrients and metabolic waste (Blanchard et al., 2009). Renal stone has become one of the most common diseases affecting the health of humans and animals (O′Kell et al., 2017; Hunprasit et al., 2019). However, the mechanism responsible for renal epithelial cell injury during the development of stones has not been fully illuminated. High oxalate intake has been a critical dietary factor actuating renal stones (Marsh et al., 2019). In the present study, we found that NaOx treatment inhibited the proliferation and migration of MDCK cells by inducing cell cycle arrest. Notably, a reduction in TEER and an increase in paracellular permeability were observed in NaOx-treated epithelial cells, which is attributed to the decreased expression of tight junction proteins, including claudin-1, occludin, ZO-1, ZO-2, and ZO-3. These effects of NaOx were significantly blocked by GSK-3β inhibitor SB216763, indicating a crucial role of Wnt/β-catenin signaling in NaOx-induced barrier damage in MDCK cells.
Renal epithelial cells compensate for the injured kidney through cell proliferation and migration. However, oxalate emerges as a factor inhibiting the proliferation of renal epithelial cells, which may exacerbate the progression of kidney disease. It has been reported that oxalate inhibits the viability of human proximal tubular epithelial cells in a dose- and time-dependent manners (Bhandari et al., 2002). The mechanism by which oxalate inhibits the proliferation of rabbit renal proximal tubule cells has been relevant to oxidative stress, cytosolic phospholipaseA2 (cPLA(2)), p38 mitogen activated protein kinase (MAPK), and Jun N-terminal kinase (JNK) signaling pathways (Han et al., 2004). The reduction in cell proliferation activity and cell migration rate was observed in MDCK cells exposed to NaOx in our study. Previous reports have shown that high concentrations of oxalate or calcium oxalate block the cell cycle progression in renal tubule epithelial cells of humans and monkeys (Sun et al., 2017; Han et al., 2019). Consistently, our results indicated that NaOx-treated MDCK cells displayed G1-phase cell cycle arrest and a decreased cell population in the S and G2 phases, suggesting that NaOx inhibits cell growth in MDCK, at least in part, by triggering G1 arrest.
The transmembrane resistance of renal tubular epithelial cells is well-known indicator for the barrier integrity (Srinivasan et al., 2015). We found that NaOx decreased the transmembrane resistance of MDCK monolayer cells in both dose- and time-dependent manners. In agreement with our study, results from the study of Schepers et al. (2005) corroborated that the transmembrane resistance presents a significant decrease in proximal renal tubular epithelial cells exposed to calcium oxalate crystals. In addition, NaOx treatment results in a corresponding increase in the permeability of monolayer cells, indicating that NaOx presents destructive effects on the barrier function of MDCK cells. The structural and functional integrity of the renal tubular epithelial cell barrier is extremely important for the reabsorption of water and nutrients and the maintenance of a stable internal environment. Oxygen-free radicals are known to decrease the expression of tight junction proteins in renal tubular epithelial cells (Peerapen and Thongboonkerd, 2013). In addition, exposure of MDCK cells to hydrogen peroxide significantly reduced the transmembrane resistance and the expression of tight junction proteins ZO-1 and occludin (Bilal et al., 2018). Peerapen and Thongboonkerd (2011) have demonstrated that calcium oxalate crystals decreased the expression of tight junction proteins ZO-1 and occludin by promoting the production of reactive oxygen species (ROS) and pro-inflammatory cytokines, which in turn leads to the destruction of the tight junctions of renal tubular epithelial cells. In the present study, we found that NaOx treatment decreased the expression of multiple tight junction proteins, including claudin-1, occludin, ZO-1, ZO-2, and ZO-3, in a dose-dependent manner at both transcriptional and protein levels. The tight junction proteins are inversely correlated to the paraepithelial cell permeability. Sodium oxalate reduced the levels of tight junction proteins, thereby contributing to the dysfunction of the barrier function in MDCK cells.
Studies have shown that the Wnt/β-catenin signaling pathway is crucial for the formation and maintenance of tight junctions in endothelial cells (Peerapen and Thongboonkerd, 2013; Tran et al., 2016; Laksitorini et al., 2019). Depletion of Apcdd1, one of the Wnt/β-catenin signaling pathway inhibitors, leads to an acceleration in barrier maturation of the retinal endothelial cells, which can be rescued by overexpression of Apcdd1 (Mazzoni et al., 2017). In vitro study confirms an essential role of β-catenin signaling for the maintenance of the integrity of brain endothelial cells (Tran et al., 2016). Despite these studies, the regulation of Wnt/β-catenin signaling on the tight junctions of renal tubular epithelial cells remains unknown. Our findings present relevance of a blockage of β-catenin signaling with abnormally increased transmembrane resistance and reduced expression of claudin-1, occludin, ZO-1, ZO-2, and ZO-3 induced by NaOx in MDCK cells. In contrast, these effects were reversed by activation of β-catenin, suggesting a protective role of Wnt/β-catenin in renal epithelial cells of canine. It should be mentioned here that, despite the mechanisms of renal tubular epithelial barrier dysfunction induced by NaOx that we validated using a canine cell line, kidneys from humans and mammals possess parallel structures and functions, with internal cell types exhibiting identical properties. Hence, this study also provides reference for the response mechanism of renal tubular epithelial cells of other species to NaOx, though the sensitivity to chemicals may differ.
Conclusions
In summary, NaOx exposure led to reduction in cell proliferation viability, G1 arrest in cell cycle progression, disruption in epithelial barrier integrity, and inhibition of Wnt/β-catenin signaling in MDCK cells. SB216763, a GSK-3 inhibitor, restored the impaired barrier function induced by NaOx, validating the importance of Wnt/β-catenin signaling in NaOx-induced renal epithelial injury. The data presented here provided a scientific foundation for the therapy of oxalate-related renal stones in canine animals.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31625025), the Zhengzhou 1125 Talent Program, and the Jinxinnong Animal Science Development Foundation.
Glossary
Abbreviations
- GSK-3
glycogen synthase kinase-3
- LDH
lactate dehydrogenase
- NaOx
sodium oxalate
- PCNA
proliferating cell nuclear antigen
- PI
propidium iodide
- RT-qPCR
real-time quantitative PCR
- TEER
trans epithelial electrical resistance
- ZOs
zonula occludens
Author Contributions
Conceptualization: Z.W., methodology: Y.J., formal analysis: S.F. and Y.Y., investigation: S.F., writing—original draft preparation: Y.J., writing—review and editing: Y.J. and Z.W., visualization: S.F., supervision: Z.W., and funding acquisition: Z.W. All authors approved the final manuscript.
Conflict of interest statement
The authors declare no conflict of interest.
Literature Cited
- Alford, A., Furrow E., Borofsky M., and Lulich J.. . 2020. Animal models of naturally occurring stone disease. Nat. Rev. Urol. 17:691–705. doi: 10.1038/s41585-020-00387-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari, A., Koul S., Sekhon A., Pramanik S. K., Chaturvedi L. S., Huang M., Menon M., and Koul H. K.. . 2002. Effects of oxalate on HK-2 cells, a line of proximal tubular epithelial cells from normal human kidney. J. Urol. 168:253–259. doi: 10.1016/S0022-5347(05)64903-8 [DOI] [PubMed] [Google Scholar]
- Bilal, S., Jaggi S., Janosevic D., Shah N., Teymour S., Voronina A., Watari J., Axis J., and Amsler K.. . 2018. ZO-1 protein is required for hydrogen peroxide to increase MDCK cell paracellular permeability in an ERK 1/2-dependent manner. Am. J. Physiol. Cell Physiol. 315:C422–C431. doi: 10.1152/ajpcell.00185.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard, A., Poussou R., and Houillier P.. . 2009. [Exploration of renal tubular functions]. Nephrol. Ther. 5:68–83. doi: 10.1016/j.nephro.2008.03.004 [DOI] [PubMed] [Google Scholar]
- Cong, X., and Kong W.. . 2020. Endothelial tight junctions and their regulatory signaling pathways in vascular homeostasis and disease. Cell. Signal. 66:109485. doi: 10.1016/j.cellsig.2019.109485 [DOI] [PubMed] [Google Scholar]
- Gewin, L. S. 2018. Renal tubule repair: is Wnt/β-catenin a friend or foe? Genes (Basel) 9(2):8. doi: 10.3390/genes9020058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günzel, D., and Yu A. S.. . 2013. Claudins and the modulation of tight junction permeability. Physiol. Rev. 93:525–569. doi: 10.1152/physrev.00019.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, J., Guo D., Sun X. Y., Wang J. M., Ouyang J. M., and Gui B. S.. . 2019. Repair effects of astragalus polysaccharides with different molecular weights on oxidatively damaged HK-2 cells. Sci. Rep. 9:9871. doi: 10.1038/s41598-019-46264-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, H. J., Lim M. J., and Lee Y. J.. . 2004. Oxalate inhibits renal proximal tubule cell proliferation via oxidative stress, p38 MAPK/JNK, and cPLA2 signaling pathways. Am. J. Physiol. Cell Physiol. 287:C1058–C1066. doi: 10.1152/ajpcell.00063.2004 [DOI] [PubMed] [Google Scholar]
- Hunprasit, V., Schreiner P. J., Bender J. B., and Lulich J. P.. . 2019. Epidemiologic evaluation of calcium oxalate urolithiasis in dogs in the United States: 2010-2015. J. Vet. Intern. Med. 33:2090–2095. doi: 10.1111/jvim.15613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laksitorini, M. D., Yathindranath V., Xiong W., Hombach-Klonisch S., and Miller D. W.. . 2019. Modulation of Wnt/β-catenin signaling promotes blood-brain barrier phenotype in cultured brain endothelial cells. Sci. Rep. 9:19718. doi: 10.1038/s41598-019-56075-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik, S. A., Modarage K., and Goggolidou P.. . 2020. The role of Wnt signalling in chronic kidney disease (CKD). Genes (Basel) 11(5):496. doi: 10.3390/genes11050496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh, B. M., Sathianathen N., Tejpaul R., Albersheim-Carter J., Bearrick E., and Borofsky M. S.. . 2019. Public perceptions on the influence of diet and kidney stone formation. J. Endourol. 33:423–429. doi: 10.1089/end.2019.0010 [DOI] [PubMed] [Google Scholar]
- Mazzoni, J., Smith J. R., Shahriar S., Cutforth T., Ceja B., and Agalliu D.. . 2017. The Wnt inhibitor Apcdd1 coordinates vascular remodeling and barrier maturation of retinal blood vessels. Neuron 96:1055–1069.e6. doi: 10.1016/j.neuron.2017.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse, R., and Clevers H.. . 2017. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 169:985–999. doi: 10.1016/j.cell.2017.05.016 [DOI] [PubMed] [Google Scholar]
- O′Kell, A. L., Grant D. C., and Khan S. R.. . 2017. Pathogenesis of calcium oxalate urinary stone disease: species comparison of humans, dogs, and cats. Urolithiasis 45:329–336. doi: 10.1007/s00240-017-0978-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerapen, P., and Thongboonkerd V.. . 2011. Effects of calcium oxalate monohydrate crystals on expression and function of tight junction of renal tubular epithelial cells. Lab. Invest. 91:97–105. doi: 10.1038/labinvest.2010.167 [DOI] [PubMed] [Google Scholar]
- Peerapen, P., and Thongboonkerd V.. . 2013. p38 MAPK mediates calcium oxalate crystal-induced tight junction disruption in distal renal tubular epithelial cells. Sci. Rep. 3:1041. doi: 10.1038/srep01041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce, D. P., Maturana J. L., Cabello P., Yefi R., Niechi I., Silva E., Armisen R., Galindo M., Antonelli M., and Tapia J. C.. . 2011. Phosphorylation of AKT/PKB by CK2 is necessary for the AKT-dependent up-regulation of β-catenin transcriptional activity. J. Cell. Physiol. 226:1953–1959. doi: 10.1002/jcp.22527 [DOI] [PubMed] [Google Scholar]
- Schepers, M. S., van Ballegooijen E. S., Bangma C. H., and Verkoelen C. F.. . 2005. Crystals cause acute necrotic cell death in renal proximal tubule cells, but not in collecting tubule cells. Kidney Int. 68:1543–1553. doi: 10.1111/j.1523-1755.2005.00566.x [DOI] [PubMed] [Google Scholar]
- Srinivasan, B., Kolli A. R., Esch M. B., Abaci H. E., Shuler M. L., and Hickman J. J.. . 2015. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 20:107–126. doi: 10.1177/2211068214561025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamos, J. L., and Weis W. I.. . 2013. The β-catenin destruction complex. Cold Spring Harb. Perspect. Biol. 5:a007898. doi: 10.1101/cshperspect.a007898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhart, Z., and Angers S.. . 2018. Wnt signaling in development and tissue homeostasis. Development 145(11):dev146589. doi: 10.1242/dev.146589 [DOI] [PubMed] [Google Scholar]
- Sun, X. Y., Yu K., and Ouyang J. M.. . 2017. Time-dependent subcellular structure injuries induced by nano-/micron-sized calcium oxalate monohydrate and dihydrate crystals. Mater. Sci. Eng. C. Mater. Biol. Appl. 79:445–456. doi: 10.1016/j.msec.2017.05.081 [DOI] [PubMed] [Google Scholar]
- Syme, H. M. 2012. Stones in cats and dogs: what can be learnt from them? Arab J. Urol. 10:230–239. doi: 10.1016/j.aju.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran, K. A., Zhang X., Predescu D., Huang X., Machado R. F., Göthert J. R., Malik A. B., Valyi-Nagy T., and Zhao Y. Y.. . 2016. Endothelial β-Catenin signaling is required for maintaining adult blood-brain barrier integrity and central nervous system homeostasis. Circulation 133:177–186. doi: 10.1161/CIRCULATIONAHA.115.015982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., Zhou C. J., and Liu Y.. . 2018. Wnt signaling in kidney development and disease. Prog. Mol. Biol. Transl. Sci. 153:181–207. doi: 10.1016/bs.pmbts.2017.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui, T., Okada A., Hamamoto S., Ando R., Taguchi K., Tozawa K., and Kohri K.. . 2017. Pathophysiology-based treatment of urolithiasis. Int. J. Urol. 24:32–38. doi: 10.1111/iju.13187 [DOI] [PubMed] [Google Scholar]
- Zhou, L., and Liu Y.. . 2016. Wnt/β-catenin signaling and renin-angiotensin system in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 25:100–106. doi: 10.1097/MNH.0000000000000205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, D., Tan R. J., Fu H., and Liu Y.. . 2016. Wnt/β-catenin signaling in kidney injury and repair: a double-edged sword. Lab. Invest. 96:156–167. doi: 10.1038/labinvest.2015.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.