SUMMARY
Objective:
Memory dysfunction is prevalent in many neurological disorders and can have a significant negative impact on quality of life. The genetic contributions to memory impairment in epilepsy, particularly temporal lobe epilepsy (TLE), remains poorly understood. Here, we compare the brain transcriptome between TLE patients with and without verbal memory impairments to identify genes and signaling networks important for episodic memory.
Methods:
Brain tissues were resected from 23 adults who underwent dominant temporal lobectomy for treatment of pharmacoresistant epilepsy. To control for potential effects of APOE on memory, only those homozygous for the APOE ε3 allele were included. A battery of memory tests was performed and patients were stratified into two groups based on preoperative memory performance. The groups were well-matched on demographic and disease-related variables. Total RNA-Seq and small RNA-Seq were performed on RNA extracted from the brain tissues. Pathway and integrative analyses were subsequently performed.
Results:
We identified 1092 differentially expressed transcripts (DETs), with the majority (71%) being underexpressed in brain tissues from patients with impaired memory compared to those from patients with intact memory. Enrichment analysis revealed overrepresentation of genes in pathways pertaining to brain-related neurological dysfunction, including a subset associated with neurodegenerative diseases, memory, and cognition (APP, MAPT, PINK1). Despite including patients with identical APOE genotypes, we identify APOE as a differentially expressed gene associated with memory status. Small RNA-Seq identified four differentially expressed microRNAs (miRNAs) that were predicted to target a subset (22%) of all DETs. Integrative analysis showed that these miRNA-predicted DET targets impact brain-related pathways and biological processes, also pertinent to memory and cognition.
Significance:
TLE-associated memory status may be influenced by differences in gene expression profiles within the temporal lobe. Upstream processes influencing differential expression signatures, such as miRNAs, could serve as biomarkers and potential treatment targets for memory impairment in TLE.
Keywords: temporal lobe epilepsy, epilepsy surgery, memory, transcriptome, RNA-Seq
INTRODUCTION
Temporal lobe epilepsy (TLE) is a leading cause of seizure disorders in adults and is associated with a high risk of memory deficits.1 In fact, up to 80% of patients with pharmacoresistant TLE demonstrate episodic memory impairments on neuropsychological testing,2 which often negatively impact day-to-day functioning and quality of life.3 While a host of demographic and disease-related variables have been associated with episodic memory dysfunction in patients with TLE [e.g., age,4 sex,5 age at seizure onset,6 duration of epilepsy,4 seizure frequency,7 hippocampal atrophy4], a substantial proportion of the variance in memory performance remains unexplained by the factors identified to date.
Twin studies have demonstrated that episodic memory ability is highly heritable, with heritability estimates as high as 64%.8 While a number of candidate genes have been found to be associated with episodic memory function in healthy adults and select patient populations,9, 10 it is estimated that only about 7% of the variance in episodic memory is accounted for by the candidate genes that have been identified to date.11 Despite the high prevalence of memory impairment in TLE, very little is known about the potential contribution of genetic factors to memory dysfunction in this population.10 In fact, the only gene that has been associated with memory impairment in TLE in more than one study is apolipoprotein E (APOE). Specifically, among patients with TLE, carriers of the APOE ε4 allele demonstrate poorer episodic memory performance than those without this allele, particularly in those with long disease duration.12
Learning and memory have been a major focus of cognitive neuroscience research over the past several decades, and it is now widely accepted that hippocampal synaptogenesis, along with remodeling and growth of existing synapses, play a critical role in learning and memory processes in adults, including consolidation and long-term memory storage.13, 14 There is also evidence to suggest that aberrations in synaptic development and plasticity are associated with age-related memory decline, as well as memory and other cognitive impairments in psychiatric, neurological, and neurodegenerative disorders.15 While the mechanisms of synaptogenesis and synaptic plasticity have yet to be completely elucidated, it is clear that genetic and epigenetic factors play an important role in these processes.14, 16–18 Recent research suggests that gene regulation, including that through microRNAs (miRNAs), lies at the crux of long-term potentiation and synaptic alterations.19, 20 However, the brain transcriptome, including mRNA-miRNA interactions, has not been explored in patients with TLE to examine its role in memory dysfunction.
The objective of this study was, for the first time, to compare the brain transcriptome between patients with and without verbal memory impairments to identify genes, signaling networks, and mRNA-miRNA interactions important for episodic memory in TLE.
METHODS
Participants
Brain tissue samples were obtained from 23 adults with pharmacoresistant TLE who underwent left (language dominant) temporal lobe resection for treatment of their seizures. Given the known association between APOE polymorphisms and memory performance [i.e., APOE ε4 detrimental, APOE ε2 protective21], only patients who were homozygous for APOE ε3 allele (wildtype) were included in the study. Tissue and clinical data used in this study were obtained from an IRB-approved epilepsy tissue bank and clinical data registry at Cleveland Clinic. Patients with a history of prior resective neurosurgery were excluded.
Memory measures and classification
All patients completed a comprehensive neuropsychological evaluation approximately 2 months (SD=3 months) prior to surgery that included assessment of verbal episodic memory. Specifically, all patients completed measures of both story recall [Logical Memory subtest of the Wechsler Memory Scale – Third or Fourth Edition] and word-list learning [Rey Auditory Verbal Learning Test or Wechsler Memory Scale – Third Edition]. Tests were administered, scored, and normed according to their respective test manuals resulting in standard scores (mean=100; SD=15) for each measure. Patients were then separated into two memory groups based on a mean composite delayed memory score (combined delayed story recall and word-list learning tasks).22, 23 Patients with scores less than 85 were classified as having “impaired” memory (n=10) and those with mean standard scores of 85 or above were classified as having “intact memory” (n=13).
Tissue preparation and RNA isolation
Total RNA was extracted from flash-frozen brain tissue samples from temporal lobe resections, as we have previously reported24. Tissues were homogenized in 1ml Trizol (Life Technologies, Carlsbad, CA, USA) using 5 mm stainless-steel beads for 2 minutes at 25 Hz with a TissueLyser II (Qiagen, Valencia, CA, USA). Total RNA was then isolated using Trizol standard extraction protocol. We utilized the Ambion PureLink RNA Mini Kit (Life Technologies) for RNA clean-up and DNase treatment. We utilized the RNeasy Lipid Tissue Kit (Qiagen) to extract total RNA, including small RNAs. We evaluated RNA quality using a 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). RNA Integrity Number (RIN) scores ≥ 7 were considered acceptable for downstream sequencing.
Library preparation and RNA sequencing
For the bulk RNA-Seq experiment, sequencing libraries were prepared using the Illumina TruSeq Stranded Total RNA with Ribo Zero kit (Illumina, San Diego, CA, USA). Libraries were sequenced using the Illumina HiSeq 2000 platform (100 base pairs, paired-end) at the Case Western Reserve University School of Medicine Genomics Core (Cleveland, OH, USA). For the small RNA-Seq experiment, sequencing libraries were prepared using the NEXTFLEX® Small RNA-Seq Kit v3 (PerkinElmer, Waltham, MA, USA). Libraries were sequenced using the Illumina NovaSeq 6000 platform (100 base pairs, single-end) at the Genomics Core of the Lerner Research Institute at Cleveland Clinic (Cleveland, OH, USA).
RNA-Seq read mapping and quantification of transcript expression
Raw sequencing FASTQ files were quality controlled using FastQC analysis, version 0.11.2, (http://www.bioinformatics.babraham.ac.uk/projects/fastqc) to identify base quality profiles, inefficient removal of ribosomal RNA (rRNA), k-mer analysis to identify problems such as adapter contamination, and abundance of fragments that are shorter than target read length. Adapter trimming was performed using the Trimmomatic tool (version 0.32) prior to alignment and downstream analysis to remove adaptor sequences used during library preparation. Pseudoalignment and transcript-based quantification were conducted using Kallisto (version 0.44.0). The R Bioconductor package NOISeq (version 2.26.1) was used to perform upper quartile normalization and to adjust for batch effects. We used the NOISeq-sim pipeline to compute differential expression. The standard probability threshold of q=0.9 was used as a cut-off for differential expression. Differentially expressed transcripts (DETs) between the two memory groups were identified using NOISeq (P<0.05 following Benjamini-Hochberg multiple testing correction).
miRNA-Seq read mapping and quantification of transcript expression
Raw sequence FASTQ files underwent read quality assessment with FASTQC (version 0.11.8). We used TrimGalore (https://github.com/FelixKrueger/TrimGalore, version 0.6.3) for Adapter removal (3’ sequence: TGGAATTCTCGGGTGCCAAGG) and quality trimming (4 bases at 5’ and 3’ ends). We aligned resultant reads with Bowtie (version 1.2.2) to mature miRNA sequences from miRBase25 (GRCh37 or hg19 genome build). Reads aligning to mature miRNA sequences were counted with SAMtools (version 1.9). miRNA read counts were filtered for low expression with function filterByExpr from R package edgeR (version 3.26.8). We performed normalization and differential expression analysis with DESeq2 (version 1.26.0). Differentially expressed transcripts (DETs) between the two memory groups were identified with P<0.05 following Benjamini-Hochberg multiple testing correction.
Pathway and gene enrichment analyses
We performed pathway analysis and enrichment for diseases and functions using Ingenuity Pathway Analysis (IPA, Qiagen Bioinformatics, Redwood City, CA, USA). We examined for association of Gene Ontology (GO, version 2018) terms and Mouse Genome Informatics (MGI) Mammalian Phenotypes (Level 4, version 2019) using Enrichr enrichment analysis tool. We analyzed miRNA target pathways using miRNA Pathway Dictionary Database (miRPathDB, version 2.0). All outputs are reported with Benjamini-Hochberg corrected P values, with P<0.05 considered as statistically significant.
Integrative mRNA-miRNA analyses
We performed miRNA target analysis using Ingenuity Pathway Analysis (IPA, Qiagen Bioinformatics). IPA utilizes Ingenuity Expert Findings, miRecords, TarBase, and TargetScan Human databases to identify experimentally observed moderate (predicted) or high (predicted) mRNA targets. Differentially expressed transcripts (DETs) from the RNA-Seq dataset were paired with each of the differentially expressed miRNAs. We then performed pathway enrichment analysis for all DETs that are predicted to be targeted by at least one of the differentially expressed miRNAs using IPA, as described above.
Covariate analyses
There are a number of factors that may impact transcript expression26–28 and/or memory performance,6, 29, 30 most notably sex, age at time of testing, age at seizure onset, and pathology. While there were no significant group differences on any of these variables, to ensure no undue influence of these factors on the transcriptome, all analyses were performed after collectively controlling for these variables. For the RNA-Seq pipeline through NOISeq, we used sex, age at surgery, age at seizure onset, duration of epilepsy, and pathology as covariates and the memory group (intact versus impaired) as the outcome. For the small RNA-Seq pipeline through DESeq2, miRNA differential expression was tested for association with memory group (intact versus impaired) while controlling for sex, age at surgery, duration of epilepsy, pathology, and memory scores.
Statistical analyses
Baseline descriptive statistics stratified by memory group (intact versus impaired) were calculated. Statistics are presented as means with standard deviations for normally-distributed variables and medians with interquartile ranges for non-normal variables. Wilcoxon rank-sum and Fisher’s Exact tests were used to examine group differences on demographic and disease-related variables. P values of < 0.05 were considered to be statistically significant.
Data availability
Individual-level clinical and transcriptomic data that support the findings of this study are not publicly available because of IRB-based restricted access. However, further information about these datasets is available from the corresponding author on reasonable request.
RESULTS
Participants
On average, patients were 43 years old (mean and median; range 20–71) and had 13 years of education (range 11–18) at baseline assessment. Age at seizure onset was 26 years on average (range 1–50), and mean duration of epilepsy was 17 years (range 2–49). All patients in this sample were White and non-Hispanic, and over half the sample was female (57%). The two memory groups were well-matched on demographic and disease-related variables. As intended, the impaired memory group demonstrated significantly lower delayed verbal memory scores (median 8th percentile) than the intact memory group (median 32nd percentile) (Table 1).
Table 1.
Demographic and epilepsy-related data for study patients
| Intact Memory (n=13) |
Impaired Memory (n=10) |
P | |
|---|---|---|---|
|
| |||
| M (IQR) | M (IQR) | ||
| Age | 43 (31–51) | 46 (34–53) | 0.804 |
| Education | 12 (12–16) | 12 (12–13) | 0.545 |
| Age at Seizure Onset | 24 (14–35) | 25.5 (16–45) | 0.456 |
| Duration of Epilepsy (years) | 18 (14–24) | 10.5 (10–16) | 0.640 |
| Seizure Frequency (number/year) | 78 (24–208) | 52 (18–78) | 0.100 |
| Full Scale IQ | 91 (88–99) | 88 (82–99) | 0.335 |
| Mean Verbal Delayed Memory | 93 (90–98) | 79 (75–81) | <0.0001 |
| Number (%) | Number %) | ||
| Sex (Male) | 5 (38%) | 5 (50%) | 0.685 |
| History of Febrile Seizures | 5 (38%) | 3 (30%) | 1.000 |
| History of GTCs | 9 (69%) | 9 (90%) | 0.339 |
| History of TBI | 3 (33%) | 2 (20%) | 0.629 |
| Preoperative AEDs | |||
| One | 1 (8%) | 1 (10%) | 0.402 |
| Two | 9 (69%) | 4 (40%) | |
| Three | 3 (23%) | 5 (50%) | |
| Pathology | |||
| HS | 9 (70%) | 5 (50%) | 0.199 |
| Other (tumor, FCD) | 2 (15%) | 0 (0%) | |
| No Lesion | 2 (15%) | 5 (50%) | |
Abbreviations: M, median; IQR, interquartile range; IQ, intelligence quotient; GTCs, generalized tonic-clonic seizures; TBI, traumatic brain injury; AEDs, anti-epileptic drugs; HS, hippocampal sclerosis; FCD, focal cortical dysplasia
Differentially expressed total RNA transcripts and associated pathways
To determine whether memory function in TLE is associated with transcriptional differences, we first analyzed gene expression from temporal lobe brain resections derived from well-matched individuals with impaired memory scores (n=10) or with intact memory scores (n=13). We identified 1092 differentially expressed transcripts (DETs), with the majority (775 or 71%) being underexpressed in brain tissues from patients with impaired memory compared to those from patients with intact memory (Table S1).
Pathway enrichment analysis revealed a strong expression signature of brain-associated processes that are impacted between the two memory groups (Figure 1A and Table S2). Analysis of the diseases and functions associated with the gene expression differences between brains from patients with and without memory impairments revealed a number of neurological phenotypes associated with brain development and memory function, including development of neurons (P=3.5×10−36), synaptic transmission (P=1.9×10−24), cognition (P=5.7×10−22), tauopathy (P=1.1×10−16), long-term potentiation of hippocampus (P=2.1×10−11), and memory (P=5.5×10−7) (Table S3). Relevant to our clinical phenotype, the ‘Memory’ annotation identified multiple relevant genes that are differentially expressed in brain tissues of individuals with impaired memory compared to those from individuals with intact memory. These genes include APOE (encoding apolipoprotein E), APP (encoding amyloid beta precursor protein), GABBR1 (encoding gamma-aminobutyric acid type B receptor subunit 1), GABBR2 (encoding gamma-aminobutyric acid type B receptor subunit 2), MAPT (encoding microtubule associated protein tau), and others (Figure 1B). Gene Ontology (GO) and MGI Mammalian Phenotypes enrichment analyses corroborated the pathway enrichment analyses (Figure 1C–D).
Figure 1. Differentially expressed genes between brains from patients with and without verbal memory impairments converge on brain-related pathways and processes.
(A) Top 20 canonical pathways impacted by DETs. Colors correspond to activation z-scores as predicted through IPA (NA, no activity pattern available). Color intensities reflect significance (-log10 adjusted P values). (B) Multiple differentially expressed genes converge on ‘Memory’ as predicted through IPA. (C) Enrichment analysis depicting top 10 gene ontology (GO) biological processes predicted to be impacted by the DETs. (D) Enrichment analysis depicting top 10 mammalian phenotypes predicted to be impacted by the DETs.
Differentially expressed miRNA transcripts and integrative analyses
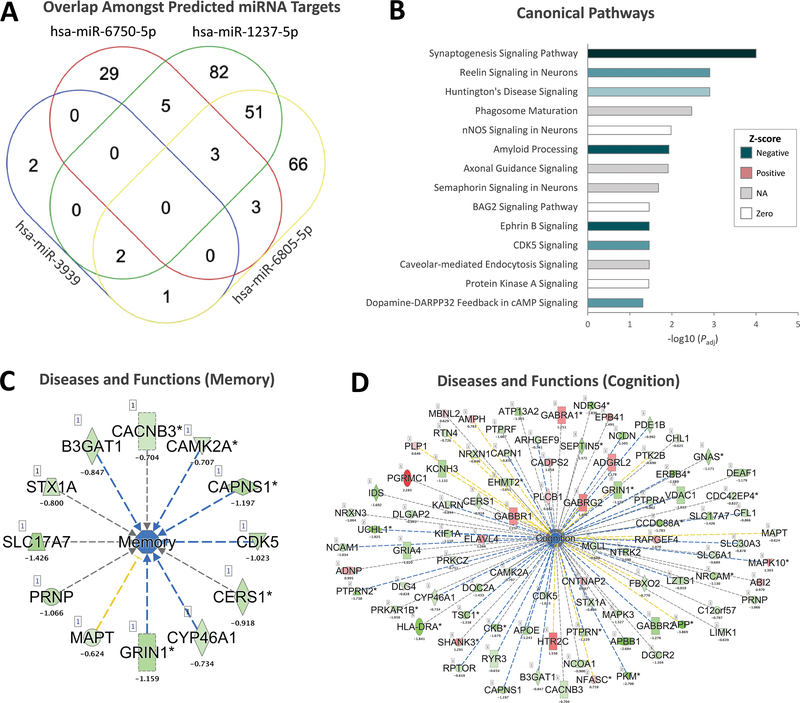
Since the majority of DETs belong to highly connected pathways and biological processes impacting brain-related functions, we hypothesized that miRNAs may act as upstream effectors of these observed gene expression differences. Accordingly, small RNA sequencing followed by differential transcript analysis identified four differentially expressed miRNAs in brain tissues of individuals with impaired memory compared to those from individuals with intact memory. These include hsa-miR-1237–5p (log2 fold change −21.85, P=1.4×10−4), hsa-miR-3939 (log2 fold change 18.42, P=5.8×10−3), hsa-miR-6805–5p (log2 fold change −14.01, P=1.2×10−2), and hsa-miR-6750–5p (log2 fold change 17.87, P=1.2×10−2). Both hsa-miR-1237–5p and hsa-miR-6805–5p were underexpressed, whereas hsa-miR-3939 and hsa-miR-6750–5p were overexpressed in brain tissues derived from individuals with impaired memory compared to those from individuals with intact memory. Significant pathways associated with these miRNAs include multiple neurological processes and biological functions (Figure 2). Importantly, these four differentially expressed miRNAs were predicted to target 244 of the 1092 (22%) of differentially expressed mRNA transcripts (Figure 3A). Pathway enrichment analysis of the latter DETs indicated enrichment of brain-related canonical pathways including the synaptogenesis signaling pathway (P=9.9×10−5), Huntington’s disease signaling (P=1.3×10−3), and Reelin signaling in neurons (P=1.3×10−3) (Figure 3B and Table S4).
Figure 2. Enrichment analysis for the four differentially expressed miRNAs.
Pathways and processes were derived from miRPathDB 2.0 for (A) hsa-miR-1237–5p, (B) hsa-miR-3939, (C) hsa-miR-6805–5p, and (D) hsa-miR-6750–5p. Top five pathways are depicted for each miRNA for each of Reactome pathways, KEGG pathways, GO cellular components, and GO biological pathways.
Figure 3. mRNA-miRNA integrative analysis.
(A) Predicted miRNA targets that also represent DETs in brains from patients with and without verbal memory impairments were extracted from IPA. Unique and shared predicted DET targets exist for each differentially expressed miRNA. (B) Canonical pathways impacted by DETs predicted to be targeted by the four differentially expressed miRNAs. Colors correspond to activation z-scores as predicted through IPA (NA, no activity pattern available). Color intensities reflect significance (-log10 adjusted P values). (C) Multiple target DETs converge on ‘Memory’ as predicted through IPA. (D) Multiple target DETs converge on ‘Cognition’ as predicted through IPA.
Analysis of the diseases and functions associated with the predicted miRNA target DETs between brains from patients with intact versus impaired memory similarly revealed a number of neurological phenotypes associated with brain development and memory function, including morphology of nervous system (P=1.2×10−15), synaptic transmission (P=1.2×10−11), cognition (P=1.1×10−7), tauopathy (P=2.2×10−5), long-term potentiation of hippocampus (P=2.9×10−3), and memory (P=1.6×10−2) (Table S5). Relevant to our clinical phenotype, the ‘Memory’ and ‘Cognition’ annotations identified multiple relevant genes that are differentially expressed in brain tissues of individuals with impaired memory compared to those from individuals with intact memory and that are predicted to be targeted by the differentially expressed miRNAs (Figure 3C–D).
DISCUSSION
Episodic memory dysfunction is one of the most common cognitive comorbidities of TLE, particularly in patients whose seizures arise from the dominant temporal lobe.2, 31 Results of the current study provide evidence that alterations in the temporal lobe transcriptome may be related to these memory impairments. We identified over 1000 DETs and four differentially expressed miRNAs in brain tissues from patients with impaired memory compared to those with intact memory function. Notably, the majority of DETs were underexpressed in brains from patients with impaired memory performance. Importantly, we observed an overrepresentation of impacted pathways and biological processes pertaining to brain-related neurological functions.
Pathway analyses also revealed an overrepresentation of genes involved in pathways associated with neurotransmitters and nervous system signaling (Table S2), including the synaptogenesis signaling pathway and GABA receptor signaling. Both of these pathways play an essential role in synaptic plasticity. Importantly, burgeoning data suggest that the expression of genes affecting synaptic function is implicated in impaired memory performance.16, 17, 32 It is now widely accepted that hippocampal synaptogenesis, along with remodeling and growth of existing synapses, plays a crucial role in learning and memory processes in adults, including consolidation and long-term memory storage.13, 14, 33 There is also evidence to suggest that aberrations in synaptic development and plasticity are associated with age-related memory decline as well as memory and other cognitive impairments in psychiatric, neurological, and neurodegenerative disorders.13, 34 While the mechanisms of synaptogenesis and synaptic plasticity are yet to be completely understood,14 it is intriguing that there was an overrepresentation of genes in pathways associated with these processes in this series of patients with and without episodic memory impairments.
Notably, we also identified DETs involved in cognitive impairment and neurodegenerative processes (e.g., progressive neurological disorder, neuronal cell death, and tauopathy). Indeed, several of the implicated genes among these diseases/functions, including APOE, APP, and MAPT, have been associated with cognitive impairment and neuropathological changes in neurodegenerative conditions, such as Alzheimer’s disease.35, 36 As noted previously, memory impairment is prevalent in patients with TLE,2 and longitudinal studies suggest that at least a subset of TLE patients (20–25%) demonstrate cognitive declines over time, particularly in vulnerable domains like memory.37 Further, longitudinal imaging studies have shown progressive cortical thinning38 and ipsilateral hippocampal volume loss39 in patients with TLE. These findings, together with recent evidence of an association between chronic TLE, memory impairment/decline, and tau-related proteins,40, 41 have led to speculation that hippocampal sclerosis-related TLE may represent a novel tauopathy.42
Remarkably, despite a uniform genetic background with regards to APOE genotype (i.e., all patients were homozygous for APOE ε3), APOE agnostically was identified as a differentially expressed gene in brains from patients with compared to those without memory impairments (Table S1). More specifically, APOE was underexpressed in the memory impaired group. APOE plays a critical role in cholesterol and lipid transport throughout the body and is involved in neuronal repair following injury or stress in the brain.43 Of the three APOE isoforms, APOE ε4 is the least stable, has a number of known neuropathological effects (e.g., impaired neurite outgrowth, increased amyloid β production, altered amyloid β clearance), and has been associated with memory impairment and the development of late-onset Alzheimer’s disease.44 Indeed, our prior work has demonstrated that the APOE ε4 genotype is associated with impaired memory in patients with longstanding, pharmacoresistant temporal lobe epilepsy.12 Because our objective in the current study was to identify new genes and pathways associated with memory in TLE, we intentionally restricted the sample to patients homozygous for the APOE ε3 allele. Thus, the differential expression of APOE between our memory groups was an unexpected finding suggesting a final common pathway converging on APOE regardless of genotype.
While identifying the precise mechanism of APOE underexpression is beyond the scope of this study, recent studies have demonstrated changes in APOE expression independent of the epsilon genotype, and there is evidence to suggest that DNA methylation in the APOE genomic region is associated with altered gene expression and memory performance.45 This becomes particularly important for APOE because it is involved not only in cholesterol transport but in β amyloid aggregation and clearance, and may be involved in tau phosphorylation, synaptic plasticity and neuroinflammation.46 In addition to APOE, a number of other genes that play an important role in synaptic plasticity and learning and memory as well as accumulation of β amyloid and development of neurodegenerative disease were underexpressed in the temporal lobe of patients with impaired memory (e.g., APP, MAPT, PINK1) Collectively, our observations corroborate that the subset of TLE associated with memory impairment may represent a novel tauopathy, with implications for patient stratification and individualized management based on risk of memory impairment.
miRNAs represent a class of small noncoding RNAs that regulate gene expression post-transcriptionally by destabilizing, degrading, or silencing mRNAs. miRNAs have been shown to play a pivotal role in nervous system development,47 synaptic plasticity,19 learning and memory,19, 20, 48 as well as in the etiology of neurological disorders like Alzheimer’s disease and Parkinson disease.20 Our findings suggest that miRNAs are upstream regulators of many of the observed DETs between brains from patients with and without memory impairment. We hypothesize that this highly correlated miRNA-mRNA network resulted from the robust transcriptional changes that converge on interconnected brain-related pathways and processes. Importantly, 3 of the 4 differentially expressed miRNAs may be detectable in blood plasma49 highlighting their potential as peripheral biomarkers for memory dysfunction in TLE. Because miRNA activities can be modulated using miRNA mimics or anti-miRs, they may also hold promise as therapeutic targets for treatment of memory impairment in TLE in the future.50
There are some study limitations that deserve mention. First, the sample size is relatively small, and findings will need to be replicated in a larger patient cohort. However, it should be noted that our sample series is currently the largest. Notably, we are encouraged by the large effect size despite the sample size. Furthermore, the pathways identified are biologically relevant to the clinical phenotype and overlap with those identified in other populations with a memory impairment phenotype (e.g., Alzheimer’s disease). Second, given the retrospective nature of this study, fresh frozen brain tissue samples available for analyses were limited to neocortical regions of the dominant temporal lobe. Given the primary role of the hippocampus in new memory formation, future studies should seek to determine if similar expression changes are apparent in the hippocampus and adjacent mesial temporal structures. Finally, the majority of patients in this study had pathologically confirmed hippocampal sclerosis (ILAE Type I). Thus, results may not be generalizable to patient groups with other neuropathological substrates. Nevertheless, it is interesting to note that 50% of the patients with decreased memory did not have hippocampal sclerosis, and 70% of the patients with intact memory had hippocampal sclerosis. In light of our results, these findings suggest that transcriptomic changes may play a more prominent role in memory impairment than hippocampal sclerosis.
In summary, our observations indicate that TLE-associated memory function/dysfunction is influenced by differences in gene expression profiles within the temporal lobe, particularly in genes implicated in neuronal-related pathways, as well as those associated with neurodegenerative processes that affect memory. Importantly, there appear to be a series of integrated and overlapping networks that contribute to memory impairment in TLE independent of demographic factors, disease-related variables, and epilepsy severity markers, and these networks appear to be regulated by upstream miRNAs. This suggests that network changes are the primary contributors to memory impairment, and epilepsy may simply be a “second hit” in this subset of patients. Future research will focus on identification of peripheral biomarkers for these gene expression regulatory factors, which will serve as a crucial first step toward personalized prognostication models and evidence-based clinical trials for memory impairment in TLE.
Supplementary Material
Table S2. Canonical pathways associated with DETs between brains from patients with and without verbal memory impairments
Table S3. Diseases and functions associated with DETs between brains from patients with and without verbal memory impairments
Table S1. Differentially expressed transcripts (DETs) between brains from patients with and without verbal memory impairments
Table S4. Canonical pathways associated with DETs predicted to be targeted by the differentially expressed miRNAs
Table S5. Diseases and functions associated with DETs predicted to be targeted by the differentially expressed miRNAs
KEY POINTS.
TLE-associated memory dysfunction is influenced by differences in gene expression profiles within the temporal lobe that are independent of disease severity
A large subset of differentially expressed transcripts are associated with neurodegenerative diseases, memory, and cognition (e.g., APOE, APP, MAPT, PINK1)
The observed differential transcript expression appears largely related to upstream microRNA regulators
TLE associated with memory impairment may represent a novel tauopathy with implications for patient stratification and individualized management
MicroRNAs may serve as peripheral biomarkers and potential treatment targets for memory impairment in TLE
ACKNOWLEDGMENTS
This study was supported, in part, by the Clinical and Translational Science Collaborative of Cleveland (KL2TR000440, UL1TR000439) from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research (to R.M.B.); Cleveland Clinic Epilepsy Center (to R.M.B.) and the Lerner Research Institute Center of Excellence for Epilepsy and Co-morbidities Research (to R.M.B., I.N. and C.E.). L.Y. is an Ambrose Monell Foundation Cancer Genomic Medicine Fellow at the Cleveland Clinic Genomic Medicine Institute. C.E. is the Sondra J. and Stephen R. Hardis Endowed Chair of Cancer Genomics Medicine at the Cleveland Clinic, and an ACS Clinical Research Professor. The authors are grateful to all the patients who participated in this study and thank Charissa Peterson, Jessica Altemus, and Phyllis Harbor for technical assistance with RNA extraction for this project.
Abbreviations:
- BP
biological processes
- CC
cellular components
- DETs
differentially expressed transcripts
- GO
gene ontology
- IPA
Ingenuity Pathway Analysis
- RIN
RNA integrity number
- TLE
temporal lobe epilepsy
Footnotes
DISCLOSURE OF CONFLICTS OF INTEREST
The authors report no competing interests relevant to this work.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1.Oyegbile TO, Dow C, Jones J, Bell B, Rutecki P, Sheth R, et al. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology. 2004;62(10):1736–42. [DOI] [PubMed] [Google Scholar]
- 2.Helmstaedter C. Effects of chronic epilepsy on declarative memory systems. Prog Brain Res. 2002;135:439–53. [DOI] [PubMed] [Google Scholar]
- 3.Giovagnoli AR, Parente A, Tarallo A, Casazza M, Franceschetti S, Avanzini G. Self-rated and assessed cognitive functions in epilepsy: impact on quality of life. Epilepsy Res. 2014;108(8):1461–8. [DOI] [PubMed] [Google Scholar]
- 4.Hermann BP, Seidenberg M, Dow C, Jones J, Rutecki P, Bhattacharya A, et al. Cognitive prognosis in chronic temporal lobe epilepsy. Ann Neurol. 2006;60:80–7. [DOI] [PubMed] [Google Scholar]
- 5.Berger J, Oltmanns F, Holtkamp M, Bengner T. Sex differences in verbal and nonverbal learning before and after temporal lobe epilepsy surgery. Epilepsy Behav. 2017;66:57–63. [DOI] [PubMed] [Google Scholar]
- 6.Rayner G, Jackson GD, Wilson SJ. Mechanisms of memory impairment in epilepsy depend on age at disease onset. Neurology. 2016;87(16):1642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voltzenlogel V, Vignal JP, Hirsch E, Manning L. The influence of seizure frequency on anterograde and remote memory in mesial temporal lobe epilepsy. Seizure. 2014;23(9):792–8. [DOI] [PubMed] [Google Scholar]
- 8.Finkel D, Pedersen N, McGue M. Genetic influences on memory performance in adulthood: comparison of Minnesota and Swedish twin data. Psychol Aging. 1995;10:437–46. [DOI] [PubMed] [Google Scholar]
- 9.Papassotiropoulos A, de Quervain DJ. Genetics of human episodic memory: dealing with complexity. Trends Cogn Sci. 2011;15(9):381–7. [DOI] [PubMed] [Google Scholar]
- 10.Busch RM, Najm I, Hermann BP, Eng C. Genetics of cognition in epilepsy. Epilepsy Behav. 2014;41:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabb FW, Burggren AC, Higier RG, Fo J, He J, Parker DS, et al. Challenges in phenotype definition in the whole-genome era: multivariate models of memory and intelligence. Neuroscience. 2009;164(1):88–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Busch RM, Lineweaver TT, Naugle RI, Kim KH, Gong Y, Tilelli CQ, et al. ApoE-epsilon4 is associated with reduced memory in long-standing intractable temporal lobe epilepsy. Neurology. 2007;68(6):409–14. [DOI] [PubMed] [Google Scholar]
- 13.Bruel-Jungerman E, Davis S, Laroche S. Brain plasticity mechanisms and memory: a party of four. The Neuroscientist. 2007;13(5):492–505. [DOI] [PubMed] [Google Scholar]
- 14.Nelson TJ, Alkon DL. Molecular regulation of synaptogenesis during associated learning and memory. Brain Research. 2015;1621:239–51. [DOI] [PubMed] [Google Scholar]
- 15.Bruel-Jungerman E, Davis S, Laroche S. Brain plasticity mechanisms and memory: a party of four. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2007;13(5):492–505. [DOI] [PubMed] [Google Scholar]
- 16.Minatohara K, Akiyoshi M, Okuno H. Role of immediate-early genes in synaptic plasticity and neuronal ensembles underlying the memory trace. Frontiers in molecular neuroscience. 2015;8:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richter JD, Klann E. Making synaptic plasticity and memory last: mechanisms of translational regulation. Genes & development. 2009;23(1):1–11. [DOI] [PubMed] [Google Scholar]
- 18.Woldemichael BT, Bohacek J, Gapp K, Mansuy IM. Epigenetics of memory and plasticity. Progress in molecular biology and translational science. 2014;122:305–40. [DOI] [PubMed] [Google Scholar]
- 19.Bredy TW, Lin Q, Wei W, Baker-Andresen D, Mattick JS. MicroRNA regulation of neural plasticity and memory. Neurobiology of learning and memory. 2011;96(1):89–94. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Kwon EJ, Tsai LH. MicroRNAs in learning, memory, and neurological diseases. Learn Mem. 2012;19(9):359–68. [DOI] [PubMed] [Google Scholar]
- 21.Small BJ, Rosnick CB, Fratiglioni L, Backman LA. Apolipoprotein E and cognitive performance: a meta-analysis. Psychol Aging. 2004;19(4):592–600. [DOI] [PubMed] [Google Scholar]
- 22.Banks SJ, Shifflett B, Berg J-L, Sundermann E, Peavy G, Bondi MW, et al. Sex-specific composite scales for longitudinal studies of incipient Alzheimer’s disease. Alzheimer’s & Dementia: Translational Research and Clinical Interventions. 2019;5:508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jonaitis EM, Koscik RL, Clark LR, Ma Y, Betthauser TJ, Berman SE, et al. Measuring longitudinal cognition: Individual tests versus composites. Cognitive and Behavioral Assessment. 2019;11:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jehi L, Yehia L, Peterson C, Niazi F, Busch R, Prayson R, et al. Preliminary report: Late seizure recurrence years after epilepsy surgery may be associated with alterations in brain tissue transcriptome. Epilepsia Open. 2018;3(2):299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. NAR. 2011;39 (Database issue):D152–D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weickert CS, Elashoff M, Richards AB, Sinclair D, Bahn S, Paabo S, et al. Transcriptome analysis of male-female differences in prefrontal cortical development. Mol Psychiatry. 2009;14(6):558–61. [DOI] [PubMed] [Google Scholar]
- 27.Xu H, Wang F, Liu Y, Yu Y, Gelernter J, Zhang H. Sex-biased methylome and transcriptome in human prefrontal cortex. Hum Mol Genet. 2014;23(5):1260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi L, Zhang Z, Su B. Sex Biased Gene Expression Profiling of Human Brains at Major Developmental Stages. Sci Rep. 2016;6:21181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berger J, Demin K, Holtkamp M, Bengner T. Female verbal memory advantage in temporal, but not frontal lobe epilepsy. Epilepsy Res. 2018;139:129–34. [DOI] [PubMed] [Google Scholar]
- 30.Hoppe C, Elger CE, Helmstaedter C. Long-term memory impairment in patients with focal epilepsy. Epilepsia. 2007;48 Suppl 9:26–9. [DOI] [PubMed] [Google Scholar]
- 31.Busch RM, Naugle RI. Pre-surgical Neuropsychological Workup: Risk Factors for Post-surgical Deficits. In: Lüders HE, editor. Textbook of Epilepsy Surgery. London: InformaHealtcare; 2008. p. 817–25. [Google Scholar]
- 32.Bungenberg J, Surano N, Grote A, Surges R, Pernhorst K, Hofmann A, et al. Gene expression variance in hippocampal tissue of temporal lobe epilepsy patients corresponds to differential memory performance. Neurobiol Dis. 2016;86:121–30. [DOI] [PubMed] [Google Scholar]
- 33.Bailey CH, Kandel ER, Harris KM. Structural components of synaptic plasticity and memory consolidation. Cold Spring Harb Perspect Biol. 2015;7:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurshan PT, Shen K. Synaptogenic pathways. Current Opinion in Neurobiology. 2019;57:156–62. [DOI] [PubMed] [Google Scholar]
- 35.Lashley T, Schott JM, Weston P, Murray CE, Wellington H, Keshavan A, et al. Molecular biomarkers of Alzheimer’s disease: progress and prospects. Disease models & mechanisms. 2018;11(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattsson N, Andreasson U, Zetterberg H, Blennow K. Association of Plasma Neurofilament Light With Neurodegeneration in Patients With Alzheimer Disease. JAMA neurology. 2017;74(5):557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hermann BP, Seidenberg M, Dow C, Jones J, Rutecki P, Bhattacharya A, et al. Cognitive prognosis in chronic temporal lobe epilepsy. Ann Neurol. 2006;60(1):80–7. [DOI] [PubMed] [Google Scholar]
- 38.Galovic M, van Dooren VQH, Postma T, Vos SB, Caciagli L, Borzi G, et al. Progressive cortical thinning in patients with focal epilepsy. JAMA Neurology. 2019;76(10):1230–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Briellmann RS, Berkovic SF, Syngeniotis A, King MA, Jackson GD. Seizure-associated hippocampal volume loss: a longitudinal magnetic resonance study of temporal lobe epilepsy. Ann Neurol. 2002;51(5):641–4. [DOI] [PubMed] [Google Scholar]
- 40.Tai XY, Koepp M, Duncan JS, Fox N, Thompson P, Baxendale S, et al. Hyperphosphorylated tau in patients with refractory epilepsy correlates with cognitive decline: a study of temporal lobe resections. Brain : a journal of neurology. 2016;139(Pt 9):2441–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kandratavicius L, Monteiro MR, Hallak JE, Carlotti CG Jr., Assirati JA Jr., Leite JP. Microtubule-associated proteins in mesial temporal lobe epilepsy with and without psychiatric comorbidities and their relation with granular cell layer dispersion. BioMed research international. 2013;2013:960126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tai XY, Bernhardt B, Thom M, Thompson P, Baxendale S, Koepp M, et al. Review: Neurodegenerative processes in temporal lobe epilepsy with hippocampal sclerosis: Clinical, pathological and neuroimaging evidence. Neuropathology and applied neurobiology. 2018;44(1):70–90. [DOI] [PubMed] [Google Scholar]
- 43.Mahley RW. Apolipoprotein E: from cardiovascular disease to neurodegenerative disorders. J Mol Med (Berl). 2016;94(7):739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahley RW, Huang Y. Apolipoprotein e sets the stage: response to injury triggers neuropathology. Neuron. 2012;76(5):871–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J, Zhao W, Ware EB, Turner ST, Mosley TH, Smith JA. DNA methylation in the APOE genomic region is associated with cognitive function in African Americans. BMC Med Genomics. 2018;11(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim J, Castellano JM, Jiang H, Basak JM, Parsadanian M, Pham V, et al. Overexpression of low-density lipoprotein receptor in the brain markedly inhibits amyloid deposition and increases extracellular A beta clearance. Neuron. 2009;64(5):632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho KHT, Xu B, Blenkiron C, Fraser M. Emerging roles of miRNAs in brain development and perinatal brain injury. Frontiers in physiology. 2019;10:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woldemichael BT, Mansuy IM. Micro-RNAs in cognition and cognitive disorders: Potential for novel biomarkers and therapeutics. Biochemical pharmacology. 2016;104:1–7. [DOI] [PubMed] [Google Scholar]
- 49.Aparicio-Puerta E, Jaspez D, Lebron R, Koppers-Lalic D, Marchal JA, Hackenberg M. liqDB: a small-RNAseq knowledge discovery database for liquid biopsy studies. Nucleic acids research. 2019;47(D1):D113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wen MM. Getting miRNA therapeutics into the target cells for neurodegenerative disease: a mini-review. Frontiers in molecular neuroscience. 2016;9:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S2. Canonical pathways associated with DETs between brains from patients with and without verbal memory impairments
Table S3. Diseases and functions associated with DETs between brains from patients with and without verbal memory impairments
Table S1. Differentially expressed transcripts (DETs) between brains from patients with and without verbal memory impairments
Table S4. Canonical pathways associated with DETs predicted to be targeted by the differentially expressed miRNAs
Table S5. Diseases and functions associated with DETs predicted to be targeted by the differentially expressed miRNAs
Data Availability Statement
Individual-level clinical and transcriptomic data that support the findings of this study are not publicly available because of IRB-based restricted access. However, further information about these datasets is available from the corresponding author on reasonable request.