Abstract
Objectives:
The aim of the study was to determine whether pediatric patients with nonalcoholic fatty liver disease (NAFLD) exposed to psychotropic medications have more severe liver disease compared to their counterparts who are not on these medications. We hypothesize that use of psychotropic agents is associated with liver disease severity.
Methods:
Children and adolescents with biopsy-confirmed NAFLD were included in this study. Histology data, detailed clinical information, and results of serum biochemistries performed within 3 months of the liver biopsy were collected retrospectively. Univariate and multivariate modeling was used to determine differences between the groups and to control for confounders.
Results:
A total of 228 patients were included, 17 (8%) of whom where on psychotropic medications at the time of the liver biopsy. Patients on psychotropic medications were more likely to also be on metformin (53% vs 18%, P<0.01) and antihypertensive medications (29% vs 8%, P<0.01) compared to children with NAFLD who were not on psychotropic agents. There were no differences in regards to biochemical evidence of liver injury, insulin resistance, and dyslipidemia between the groups. On histology, however, the use of psychotropic medications was associated with increased steatosis severity (score 2.4 vs 1.9, P=0.04) and increased likelihood of having an NAFLD Activity Score ≥5 (seen in 59% vs 35% or patients; P=0.05, respectively).
Conclusions:
In this large cohort of children with biopsy-confirmed NAFLD, the use of psychotropic medications was associated with increased liver disease severity. Exposure to psychotropic agents should be considered when risk stratifying children with NAFLD.
Keywords: antidepressants, antipsychotics, hepatic steatosis, pediatric obesity
Psychotropic medications are defined as drugs that have the ability to modify a subject’s mood or behavior. Antipsychotics, antidepressants, and mood stabilizers are considered psychotropic agents. Use of these medications has an impact on body weight and metabolic risk factors, with antipsychotics and antidepressants often contributing to weight gain, hypertension, dyslipidemia, and type 2 diabetes mellitus (1–3). The metabolic side effects of certain psychotropic medications are common. For instance, when considering a complication such as weight gain of ≥7%, for olanzapine the number needed to harm is only 3 (4). This is particularly concerning given epidemiologic data that suggest that in the past several decades the use of psychotropic medications in pediatrics (single or combinations of drugs) has been increasing (5,6).
In addition to the aforementioned metabolic complications, certain psychotropic agents, particularly the atypical antipsychotics, can directly affect lipid handling within the liver leading to increased de novo lipogenesis (7) or decreased fatty acid oxidation (8). Through these mechanisms, psychotropic medications can contribute to the development and possible progression of hepatic steatosis (9). Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease in pediatrics (10). It typically occurs in the context of excess adiposity and is characterized by hepatic steatosis, with or without necroinflammation and fibrosis (11,12). Based on recent animal data, exposure to atypical antipsychotics is associated with increased severity of NAFLD activity and fibrosis compared to nonexposed controls (13). Thus, one can postulate that exposure of patients with NAFLD to atypical antipsychotics has the potential to alter the natural history of this disease (13).
Although it has been shown that adults with more severe depressive symptoms have more significant hepatocellular ballooning in the context of NAFLD (14), there are limited data on the impact of antidepressants on the development and progression of fatty liver disease. Commonly used antidepressants, such as serotonin reuptake inhibitors, lead to increases in serotonin levels. Serotonin has been shown to contribute to oxidative stress in murine models of nonalcoholic steatohepatitis (NASH) (15). Other agents, such as tricyclic antidepressants, have previously been linked to more significant portal inflammation in adults with NASH (14). In spite of the aforementioned literature, the impact of psychotropic medications (single or combinations) on liver disease severity of children with NAFLD has not yet been investigated.
The objective of this study was to determine the prevalence of psychotropic agent use in a pediatric cohort with NAFLD and to investigate whether these patients have more severe liver disease than their counterparts who are not exposed to psychotropic medications.
METHODS
This was a retrospective study performed at the Steatohepatitis Center of Cincinnati Children’s Hospital Medical Center. Inclusion criteria were patients with histologically confirmed NAFLD followed at the Steatohepatitis Center from August 2010 to November 2017. Exclusion criteria were secondary causes of hepatic steatosis (eg, medication use, endocrine disorders, genetic/metabolic conditions) or other liver diseases in addition to NAFLD (eg, autoimmune hepatitis, Wilson disease, viral hepatitis). Approval by the Institutional Review Board was obtained before data collection.
Patients were included if they were on psychotropic medications at the time of the liver biopsy, and for a minimum of 3 months immediately preceding the biopsy (details on these medications are included in the Supplementary Table 1, Supplemental Digital Content, http://links.lww.com/MPG/B658). Because stimulants are psychotropic medications associated with weight loss, which is not expected to have an adverse impact on NAFLD, they were not included in the analyses. Similarly, use of topiramate, which is a mood stabilizer also associated with weight loss, was excluded from the analyses. Other data collected for the purposes of this study included general cohort characteristics (age, sex, ethnicity, anthropometrics, other medication use including vitamin E, metformin or statins, diagnosis of type 2 diabetes mellitus), laboratory investigations obtained within 3 months of the liver biopsy, and the detailed liver histology data. In our cohort, vitamin E was used for the treatment of NASH, metformin was used to treat either impaired glucose tolerance or as prophylaxis in those prescribed certain psychotropic medications and statins were used for the treatment of dyslipidemia.
Severity of obesity was defined using established pediatric criteria (16). Severity of NAFLD was scored using validated criteria to calculate the NAFLD activity score (NAS), and was also assessed by comparing scores for steatosis, portal and lobular inflammation, ballooning degeneration, and fibrosis severity (12). In our institution, patients are likely to undergo a liver biopsy if there is suspicion for more severe NAFLD (eg, patients with persistently elevated liver enzymes [alanine transferase (ALT) >50 U/L for 3–6 months] and lack of BMI improvement in spite of healthy lifestyle counseling; a diagnosis of metabolic syndrome or obstructive sleep apnea; imaging evidence of splenomegaly and/or increased liver stiffness on magnetic resonance elastography) or if there is concern regarding another underlying liver disease (eg. Wilson disease due to a low ceruloplasmin level).
Descriptive statistics were used and data were presented as medians with ranges. Wilcoxon rank-sum test was used to compare continuous variables between the groups and chi square or Fisher exact testing was used to compare categorical variables. Proportional odds and logistic regression models were used to assess the risk of more advanced histologic scores after controlling for confounding variables. Graphic representation of the data was performed using box plots. Stata MP v13.0 for Mac (College St, TX) was the software used to perform the statistical analyses.
RESULTS
Of the 228 patients who had undergone a liver biopsy for the investigation of fatty liver disease, 17 (8%) were on concurrent psychotropic medications. The majority of the patients were on more than one of these agents at the time of the liver biopsy (44% were on 1 medicine, 50% on 2, and 6% on 3 psychotropic medications concurrently). The median duration of psychotropic medication use before the liver biopsy was 12 months (range: 3–53 months). The categories of medications used were mood stabilizers (26% of patients), antidepressants (42% of patients), atypical antipsychotics (63% of patients) and anxiolytics (21% of patients). Patients on psychotropic medications were of similar age, sex, ethnicity, and obesity severity as their counterparts who were not on these medications (Table 1). The proportion of patients with type 2 diabetes mellitus was not different between the groups (18% of those receiving psychotropic medications vs 9% in those not on these medications, P=0.25). The median (range) duration of treatment with vitamin E, statins, metformin and antihypertensives in this cohort was 8 (0.5–34) months, 17 (6–29) months, 9 (0.5–66) months, and 7 (0.5–58) months, respectively. None of the patients on psychotropic medications were receiving vitamin E or statins at the time of the liver biopsy; however, the proportion of patients receiving metformin was greater in the group of patients also on psychotropic medications (53% vs 18%, P<0.01). Similarly, use of antihypertensives was more frequent in the group of patients receiving psychotropic medications (29% vs 8%, P<0.01). The clinic visit frequency was not different between those taking compared to those not taking these drugs (8.8±1.1 vs 7.2±0.3, P=0.12).
TABLE 1.
Differences in demographic and clinical characteristics between those who were and those who were not on psychotropic medications at the time of the liver biopsy
| Variable | No psychotropic medications (n=211) | Receiving psychotropic medications (n=17) | P |
|---|---|---|---|
| Age | 14 (2–21) | 15 (8–17) | 0.82 |
| Sex; n (% male) | 136 (65%) | 13 (76%) | 0.33 |
| Ethnicity | 0.61 | ||
| N (%) Hispanic | 45 (21%) | 2 (12%) | |
| N (%) non-Hispanic | 164 (78%) | 15 (88%) | |
| Obesity categories | |||
| Overweight; n (%) | 6 (3%) | 0 (0%) | 0.48 |
| Class I obesity; n (%) | 42 (20%) | 2 (12%) | 0.41 |
| Class II obesity; n (%) | 67 (32%) | 5 (29%) | 0.84 |
| Class III obesity; n (%) | 93 (44%) | 9 (53%) | 0.48 |
| Unknown; n (%) | 3 (1%) | 1 (6%) | |
| Body mass index, kg/m2 | 36 (31–40) | 36 (35–42) | 0.36 |
| Type 2 diabetes mellitus n(%) | 19(9%) | 3 (18%) | 0.25 |
| Other treatments | |||
| Vitamin E; n (%) | 9 (4%) | 0 (0%) | 0.38 |
| Metformin; n (%) | 55 (26%) | 9 (53%) | 0.02 |
| Statins; n (%) | 2 (1%) | 0 (0%) | 0.69 |
| Anti-HTN drugs; n (%) | 16 (8%) | 5 (29%) | 0.01 |
Data are presented as medians (ranges) unless otherwise indicated.
HTN = hypertension.
The results of the laboratory investigations that had been performed within 3 months of the liver biopsy are shown in Table 2. In summary, biochemical evidence of liver injury was not different between the groups. Similarly, the lipid profile and HbA1c levels were not significantly different between the groups.
TABLE 2.
Results of laboratory investigations performed within 3 months of the liver biopsy
| Variable | No psychotropic medications (n=208) | Receiving psychotropic medications (n=17) | P |
|---|---|---|---|
| ALT (U/L) | 85 (26–429) | 79 (35–199) | 0.68 |
| AST (U/L) | 49 (15–240) | 41 (21–132) | 0.18 |
| GGT (U/L) | 47 (12–710) | 52 (17–115) | 0.84 |
| Alkaline Phosphatase (U/L) | 171 (55–465) | 196 (78–408) | 0.59 |
| HbA1c (%) | 5.2 (4.3–8.8) | 5.1 (4.4–10.1) | 0.90 |
| HOMA-IR | 6.0 (0.8–69) | 6.3 (1.4–14.8) | 0.92 |
| LDL (mg/dL) | 91 (33–182) | 109 (20–202) | 0.09 |
| HDL (mg/dL) | 38 (19–64) | 35 (15–51) | 0.11 |
| Triglycerides (mg/dL) | 142 (49–495) | 149 (88–721) | 0.12 |
| Steatosis | 2 (0–3) | 3 (1–3) | 0.039 |
| Lobular inflammation | 1 (0–3) | 1 (0–2) | 0.53 |
| Ballooning degeneration | 1 (0–2) | 1 (0–2) | 0.94 |
| NAFLD activity score | 4 (1–8) | 5 (2–6) | 0.17 |
| Portal inflammation | 1 (0–2) | 1 (0–1) | 0.53 |
| Fibrosis severity | 1 (0–3) | 1 (0–3) | 0.70 |
Data are presented as medians (ranges).
In terms of histology, the severity of steatosis (average score 2.4 vs 1.9, respectively; P=0.04) was higher in patients on psychotropic medications compared to the patients not receiving these medications. In addition, the proportion of patients with NAS ≥5 was higher in patients receiving psychotropic medications compared to those who were not (n patients [%]: 10 [59%] vs 74 [35%], respectively; P=0.05). The median NAS, the severity of lobular and portal inflammation, and the fibrosis score were not different between the groups (Fig. 1). There were no differences in the severity of steatosis (P=0.41), lobular inflammation (P=0.41), or the risk of having an NAS ≥5 (P=0.62) in patients receiving 1 versus more psychotropic medications. Logistic regression with multiple variables (each variable was tested individually along with psychotropic medication use) suggested that log-transformed ALT and psychotropic medication use predicted steatosis. These variables were jointly significant (logALT odds ratio [OR] 6.69, 95% confidence intervals 2.56–17.20; absence of psychotropic medication use OR 0.33, 95% confidence intervals 0.12–0.89). When psychotropic medication use was modeled jointly with other variables to predict lobular inflammation the findings were not significant. LogALT and psychotropic agent use were also jointly predictive of an NAS ≥5 (logALT OR 6.8, 95% confidence intervals 2.3–20.0; absence of psychotropic medication use OR 0.4, 95% confidence intervals 0.1–1.0).
FIGURE 1.
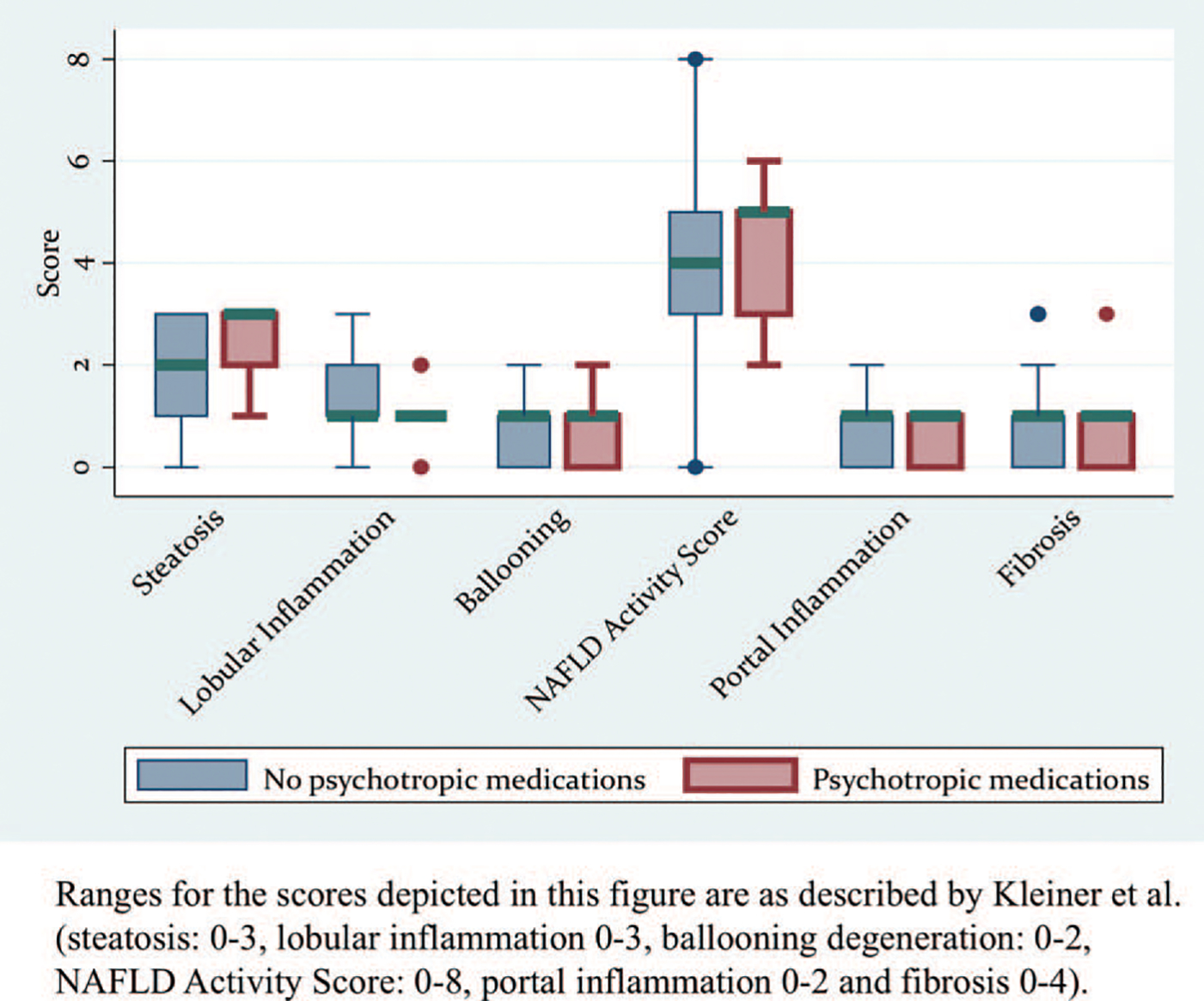
Box plots of histology scores of patients with NAFLD grouped by exposure to psychotropic medications. NAFLD = nonalcoholic fatty liver disease.
DISCUSSION
In this study, we investigated the frequency of psychotropic medication usage in a large cohort of children and adolescents with biopsy-confirmed NAFLD and examined the association between treatment with psychotropic medications and liver disease severity. Nearly 1 in 10 patients were using psychotropic medications and the majority was taking >1 psychotropic agent at the time of liver disease assessment. Patients receiving these medications had more significant steatosis, and as a result a greater proportion of them had an NAS ≥5, in spite of similar obesity severity and comparable biochemical markers of liver injury and dyslipidemia. Histologic severity of inflammation, ballooning, and fibrosis was not different between those exposed and those not exposed to psychotropic medications. Although HbA1c levels did not differ, metformin use was almost twice as common in patients also receiving psychotropic agents. Use of antihypertensive medications was also more frequent in the group of patients exposed to psychotropic drugs. Collectively, these data support an association between psychotropic drug use and risk of metabolic comorbidities, including hepatic steatosis, independent of weight status.
The increased liver disease severity seen in patients exposed to psychotropic medications may be directly related to the drug use (as is the case with various other medications that can contribute to the development of hepatic steatosis, such as corticosteroids), or may be secondary to the metabolic dysregulation seen in these patients. The latter may have also been driven, at least in part, by a high proportion of patients taking atypical antipsychotics that are particularly known to contribute to impaired glucose tolerance and dyslipidemia. More frequent use of antihypertensive medications in those receiving psychotropic medications is one indicator of increased cardiometabolic risk in our cohort. Although our study was not designed to determine causation, there is substantial evidence from the literature that psychotropic medications can modify lipid handling within the liver (1,3,8,9,13). This could explain the higher steatosis scores seen in our patients; however, this remains to be investigated further.
The disproportionately more frequent use of metformin in the cohort of patients receiving psychotropic medications was not surprising and does not necessarily reflect more significant insulin resistance in this cohort. In fact, the proportion of patients with type 2 diabetes mellitus was not different between the groups. Often, metformin is started prophylactically in clinical practice when prescribing atypical antipsychotics, given its beneficial effects on weight and insulin sensitivity (17–19). Although impaired glucose tolerance is a known driver for the development of NAFLD and could have contributed to the higher steatosis scores, HbA1c levels were similar between the groups, rendering this hypothesis less likely. In summary, it should be highlighted that while use of metformin may have been beneficial in controlling psychotropic medication-induced weight gain and insulin resistance in our cohort, it was not associated with a protection against hepatic steatosis. A prior pediatric clinical trial of metformin in children with confirmed NAFLD likewise did not show a beneficial impact of metformin on the liver steatosis over a 2-year period (20).
Beyond direct effects, psychotropic medications could contribute to more severe liver disease in patients with NAFLD through indirect mechanisms, such as effects on appetite. The weight gain seen with the use of these drugs has in part been attributed to dysregulated satiety pathways (21). We did not have access to data quantifying appetite or caloric intake of patients included in this study. If appetite dysregulation were, however, a contributor to the more severe liver disease seen in patients on psychotropic agents, we would have expected to see more significant obesity in those on psychotropic medications than in the remaining NAFLD cohort, which was not the case in our study.
Apart from metabolic dysregulation and effects on appetite, the need for treatment with psychotropic medications may point to mental health comorbidities that complicate the care of patients with NAFLD. The mainstay of treatment for pediatric NAFLD is introduction of lifestyle changes through dietary and physical activity modifications (10). Implementing lifestyle changes may be more challenging in patients with concurrent underlying behavioral or psychological health issues (22). Similarly, another important aspect of the care of patients with NAFLD is the frequency with which they are followed in outpatient clinics. It has previously been shown that more frequent clinic visits were associated with more significant improvements in serum transaminases (23). Patients with behavioral or emotional comorbidities may be less likely to attend regular clinic visits either due to their underlying conditions or because they have multiple other providers that follow them (24). In our study, the clinic visit frequency was not lower in those taking psychotropic medications. Lastly, it is possible that those on psychotropic medications were more likely to consume alcohol; however, the literature on this topic is not clear (25,26).
The use of psychotropic medications in this cohort was 8- to 10-fold higher than what would have been expected given the most recent pediatric literature from the United States (27,28). It is, however, within the expected range for a predominantly obese cohort of children and adolescents, as reported recently by Krawczyk et al (29). In this study, the rates of psychotropic medication use in children participating in weight-loss camps across the United States and the United Kingdom were not only higher than the general population, but they also ranged dramatically by geographic location. For instance, 3% of children attending weight loss camps in the United Kingdom were on psychotropic medications compared to 31% in Wisconsin. Regional approaches to psychotropic agent use and their impact on liver disease severity in children with NAFLD remains to be investigated further.
Limitations of the study include its retrospective design and the lack of data on dietary intake (including alcohol intake or substance abuse), physical activity, and medication compliance. The histology data included in this study had not been reviewed by a single pathologist. In addition, sample size precluded investigating independent effects of different classes of psychotropic medications on NAFLD severity. The impact of these drugs on metabolism varies and that effect may be further modified by altered drug metabolism driven by polypharmacy. Lastly, we did not have access to baseline data (ie, liver disease assessment or severity before the initiation of psychotropic medications).
CONCLUSIONS
This study provides preliminary evidence that the use of psychotropic medications in childhood is associated with an increased risk of more severe hepatic steatosis, in spite of similar obesity severity. It remains to be determined whether this association is causative and if it has an impact on the natural history of pediatric NAFLD. Other features of NASH, such as inflammation and fibrosis were not worse in those taking psychotropic medications in our study, a finding that should be explored further in larger cohorts. Given the increasing usage of these medications in children and the rising burden of NAFLD in childhood, the potential impact of these medications on clinical (eg, body composition, change in physical activity levels) and histological outcomes should be examined prospectively. In addition, further investigations of the impact of mood disorders alone or in conjunction with their comorbidities (eg, obstructive sleep apnea) on the severity of pediatric NAFLD are needed. Should our findings be validated in larger cohorts, exposure to psychotropic medications can be used to risk stratify patients at various stages of their care, such as when considering a liver biopsy, or when determining the optimal frequency of outpatient clinic visits, or lastly, when deciding on the use of treatments, such as vitamin E.
Supplementary Material
What Is Known
The use of psychotropic medications, namely antipsychotics and antidepressants, has been associated with weight gain and the development of metabolic dysregulation, such as insulin resistance.
Psychotropic medications can also affect lipid handling within the liver and thus predispose patients to nonalcoholic fatty liver disease.
What Is New
This study shows that in pediatric nonalcoholic fatty liver disease, there are obesity-independent associations between the use of psychotropic medications and liver disease severity.
Psychotropic medications are associated with more significant hepatic steatosis.
Footnotes
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jpgn.org).
REFERENCES
- 1.Jerrell JM, McIntyre RS, Tripathi A. Childhood treatment with psychotropic medication and development of comorbid medical conditions in adolescent-onset bipolar disorder. Hum Psychopharmacol 2011;26:451–9. [DOI] [PubMed] [Google Scholar]
- 2.McIntyre RS, Jerrell JM. Metabolic and cardiovascular adverse events associated with antipsychotic treatment in children and adolescents. Arch Pediatr Adolesc Med 2008;162:929–35. [DOI] [PubMed] [Google Scholar]
- 3.Andrade SE, Lo JC, Roblin D, et al. Antipsychotic medication use among children and risk of diabetes mellitus. Pediatrics 2011;128: 1135–41. [DOI] [PubMed] [Google Scholar]
- 4.Maayan L, Correll CU. Weight gain and metabolic risks associated with antipsychotic medications in children and adolescents. J Child Adolesc Psychopharmacol 2011;21:517–35. [DOI] [PubMed] [Google Scholar]
- 5.Rushton JL, Whitmire JT. Pediatric stimulant and selective serotonin reuptake inhibitor prescription trends: 1992 to 1998. Arch Pediatr Adolesc Med 2001;155:560–5. [DOI] [PubMed] [Google Scholar]
- 6.Zito JM, Safer DJ, DosReis S, et al. Psychotropic practice patterns for youth: a 10-year perspective. Arch Pediatr Adolesc Med 2003;157: 17–25. [DOI] [PubMed] [Google Scholar]
- 7.Raeder MB, Ferno J, Vik-Mo AO, et al. SREBP activation by antipsychotic- and antidepressant-drugs in cultured human liver cells: relevance for metabolic side-effects? Mol Cell Biochem 2006;289:167–73. [DOI] [PubMed] [Google Scholar]
- 8.Oh KJ, Park J, Lee SY, et al. Atypical antipsychotic drugs perturb AMPK-dependent regulation of hepatic lipid metabolism. Am J Physiol Endocrinol Metab 2011;300:E624–32. [DOI] [PubMed] [Google Scholar]
- 9.Gracious BL, Bhatt R, Potter C. Nonalcoholic fatty liver disease and fibrosis in youth taking psychotropic medications: literature review, case reports, and management. J Child Adolesc Psychopharmacol 2015;25: 602–10. [DOI] [PubMed] [Google Scholar]
- 10.Vos MB, Abrams SH, Barlow SE, et al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr 2017;64:319–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology 2005;42:641–9. [DOI] [PubMed] [Google Scholar]
- 12.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–21. [DOI] [PubMed] [Google Scholar]
- 13.Soliman HM, Wagih HM, Algaidi SA, et al. Histological evaluation of the role of atypical antipsychotic drugs in inducing non-alcoholic fatty liver disease in adult male albino rats (light and electron microscopic study). Folia Biol (Praha) 2013;59:173–80. [DOI] [PubMed] [Google Scholar]
- 14.Youssef NA, Abdelmalek MF, Binks M, et al. Associations of depression, anxiety and antidepressants with histological severity of nonalcoholic fatty liver disease. Liver Int 2013;33:1062–70. [DOI] [PubMed] [Google Scholar]
- 15.Nocito A, Dahm F, Jochum W, et al. Serotonin mediates oxidative stress and mitochondrial toxicity in a murine model of nonalcoholic steatohepatitis. Gastroenterology 2007;133:608–18. [DOI] [PubMed] [Google Scholar]
- 16.Kelly AS, Barlow SE, Rao G, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation 2013;128:1689–712. [DOI] [PubMed] [Google Scholar]
- 17.De Silva VA, Suraweera C, Ratnatunga SS, et al. Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis. BMC Psychiatry 2016;16:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Generali JA, Cada DJ. Metformin: prevention and treatment of antipsychotic-induced weight gain. Hosp Pharm 2013;48:734–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu RR, Zhao JP, Jin H, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA 2008;299:185–93. [DOI] [PubMed] [Google Scholar]
- 20.Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA 2011;305:1659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruetsch O, Viala A, Bardou H, et al. Psychotropic drugs induced weight gain: a review of the literature concerning epidemiological data, mechanisms and management [in French]. Encephale 2005;31:507–16. [DOI] [PubMed] [Google Scholar]
- 22.Melnyk BM, Small L, Morrison-Beedy D, et al. Mental health correlates of healthy lifestyle attitudes, beliefs, choices, and behaviors in overweight adolescents. J Pediatr Health Care 2006;20:401–6. [DOI] [PubMed] [Google Scholar]
- 23.Lam C, Bandsma R, Ling S, et al. More frequent clinic visits are associated with improved outcomes for children with NAFLD. Can J Gastroenterol Hepatol 2016;2016:8205494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerkar N, D’Urso C, Van Nostrand K, et al. Psychosocial outcomes for children with nonalcoholic fatty liver disease over time and compared with obese controls. J Pediatr Gastroenterol Nutr 2013;56:77–82. [DOI] [PubMed] [Google Scholar]
- 25.Kandel DB, Johnson JG, Bird HR, et al. Psychiatric comorbidity among adolescents with substance use disorders: findings from the MECA Study. J Am Acad Child Adolesc Psychiatry 1999;38:693–9. [DOI] [PubMed] [Google Scholar]
- 26.Alessandrini G, Ciccarelli R, Battagliese G, et al. Treatment of alcohol dependence. Alcohol and the young: social point of view. Riv Psichiatr 2018;53:113–7. [DOI] [PubMed] [Google Scholar]
- 27.Olfson M, King M, Schoenbaum M. Treatment of young people with antipsychotic medications in the United States. JAMA Psychiatry 2015;72:867–74. [DOI] [PubMed] [Google Scholar]
- 28.Sultan RS, Correll CU, Schoenbaum M, et al. National patterns of commonly prescribed psychotropic medications to young people. J Child Adolesc Psychopharmacol 2018;28:158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krawczyk R, Kirschenbaum DS, Caraher KJ. Vast differences in psychotropic prescription rates, but not outcomes, for obese adolescents in immersion treatment across geographical regions. Child Obes 2018;14:165–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


