Introduction
In clinical medicine, assessment of mechanical properties of the biological tissue is an age-old practice to diagnose various diseases. It is a well-known fact that the biomechanical properties of specific tissue types vary significantly at different stages of physiological and pathological conditions. For instance, a malignant tumor in the breast is stiffer than a benign tumor or the normal tissue[1]. Palpation remains the time-tested and gold standard diagnostic tool to feel the difference between normal and abnormal tissues. However, non-invasive palpation is only applicable to superficial organs and cannot be applied to internal organs without surgery. Especially palpation is impossible to apply to the brain due to the presence of skull bone. Another limitation of palpation is that it is qualitative and may potentially bias the diagnosis. However, radiology medicine has technologically advanced, making it possible to detect tumors in the brain using computed tomography (CT), Magnetic resonance imaging (MRI), and Ultrasound Imaging (US) non-invasively. Unfortunately, none of these conventional techniques provide any information about their biomechanical properties, which is very useful while planning for surgery.
Decades of research on brain development in the pediatric and adolescent population using MRI have provided us rich information regarding changes in Grey matter (GM) and white matter (WM) volume [2], CSF content ([3], and myelination [4] and functional parameters such as cerebral blood flow during different activities and in resting condition, etc. In humans, the neural and behavioral changes during childhood and adolescent years are as rapid as the early postnatal development [5]–[7]. So far, these structural and functional changes and their correlation with respect to biomechanical properties of brain tissues were not fully explained. Extensive studies on biomechanical properties could potentially lead to new biomarkers for pathological conditions that could not be explained with the help of existing techniques. For example, neurodevelopmental disorders such as cerebral palsy (CP) caused by brain trauma during fetal development or shortly after birth may not display characteristic lesions in their structural MRI scans[8]. Therefore, the structural or functional images of conventional imaging are not well correlated to the functional ability of the organs [9], [10] and make them insufficient to diagnose and fully understand.
Therefore, a detailed understanding of mechanical properties, such as stiffness of the pediatric brain, plays paramount importance in clinical and diagnostic medicine. However, the availability of such data in the literature is very limited, and only a few studies are conducted with a limited age group and number of subjects [10]–[12]. The most established way to assess internal organs’ stiffness in a non-invasive method is based on elastographic techniques with the help of MRI and US systems. These methods rely on the propagation of mechanical motion waves in the tissues. In particular, the MR-based method called magnetic resonance elastography (MRE) is gaining a lot of attention from the research community due to its ability to make quantitative measurements of tissue elasticity non-invasively and three-dimensionally (3D).
Elasticity of Soft Tissue
In material science, an object/body’s elasticity is defined as “its ability to resist deformation when subjected to a mechanical force and return to its original shape and size when the force is removed.” The force applied to the object is called stress, and the displacement of an object in response is called strain. The slope of the stress-strain curve is the elastic modulus, a physical parameter that reflects the stiffness of a material. According to Hook’s law, pure elastic material has linear stress and strain relation and thereby behaves like a spring. Soft tissues in the human body usually have an inhomogeneous structure that is viscoelastic in nature and displays both elastic and viscous properties. Therefore, the elastic modulus of these tissues comprises “a storage modulus” representing the elasticity component and a “loss modulus” representing the viscosity component. The human brain is a soft tissue composed of neurons, neural stem cells, glial cells, and blood vessels, and its viscous property arises from the geometric organization of these tissue components and their network complexity.
Magnetic Resonance Elastography
There were many static, quasi-static and dynamic MR elastographic techniques developed over the years [13]–[17]. Dynamic MRE is the most commonly used technique due to its efficiency and simplicity. Dynamic MRE is a non-invasive and in-vivo diagnostic technique to assess the biological tissues’ stiffness using either a 1.5T or 3T MR scanner with additional hardware and software [17]–[21]. MRE measures oscillating shear wave displacements induced by an externally applied harmonic motion.
Measurement of shear wave displacements was obtained by modulating the MR gradients at the frequency of applied harmonic motion. Repeated measurements were performed with a linear phase difference between the externally applied motion and the MR gradient fields. This phase difference results in varying phase contrast across the MR images.
In MRE, there are three steps involved:
Inducing mechanical motion in soft tissues
Encoding waves in the phase of MR images
Generating an elastogram using an inversion algorithm
We describe the overview of MRE here to give an abstract understanding of the technique, whereas a detailed review of all the brain MRE methods can be found in Hiscox et al., 2016 [22].
Mechanical Tissue Harmonic Motion
To estimate the elasticity of an object, it is essential to apply the external load/force into the object of interest before the deformation measurements are obtained. In MRE, a continuous, single frequency, and stable mechanical shear wave motion is induced in soft tissues using a special hardware setup that is in synchronization with the MR acquisition pulse sequence. The complete MRE setup is shown in figure 1. This motion inducer consists of two components called an active driver and a passive driver. The active driver is an electromechanical device (such as a subwoofer) able to generate mechanical vibrations at a controllable frequency and amplitudes. This Active driver is placed outside the scanner room so that its electro-magnets will not be interfered with the scanner magnetic field and controlled through the signal generator.
Fig. 1.
The MRE technique: the patient’s head is vibrated in the MR imaging scanner with a pneumatic actuator, displacement wavefields are acquired, and the inversion algorithm calculates viscoelastic shear stiffness. (Reproduced from G. McIlvain et al., “Brain Stiffness Relates to Dynamic Balance Reactions in Children With Cerebral Palsy,” J. Child Neurol., vol. 35, no. 7, pp. 463–471, 2020, https://doi.org/10.1177/0883073820909274.)
Motion waves are transmitted via MR compatible plastic tube and delivered to tissue using a passive driver. This passive driver is a soft pillow-like structure placed inside the receiver coil for brain application. It’s important to position the passive driver in contact with the body surface and as close as possible to the region of interest. The frequencies generated from this driver are in the audible range (40–200Hz)[23] and are safer for the patient and do not interfere with the MR scanner. One sized passive driver may not fit all the patients and regions of interest; therefore, the passive drivers in different sizes and shapes are developed to optimize the shear wave delivery and increase the patient comfort. For the pediatric and smaller patients, small-sized soft drivers are available similar to the one shown in figure 2.
Figure 2:
Soft pillow-like passive driver used with head-coil for brain MRE applications, during the image acquisition the driver positioned beneath the head to induce shear waves in the brain. Active driver connects the passive driver via a plastic tube to induce vibrations. (Reproduced from K.M Pepin et al., “MR Elastography Analysis of Glioma Stiffness and IDH1-Mutation Status” AJNR Am J Neuroradiol 10.3174/ajnr.A5415, http://dx.doi.org/10.3174/ajnr.A5415)
Imaging the Shear Waves
The fundamental concept of MRE, measuring propagating motion (i.e, harmonic waves) in soft tissue, stemmed from the conventional MR imaging technique called phase-contrast MRI. In MR imaging, the steady-state motion of nuclear spins in the presence of magnetic field gradients causes a phase shift in the MR image. In MRI phase-contrast imaging, flow-encoding gradients are deployed in the MR pulse sequence to track the moving spins inside the blood vessels. During the image readout, the moving spins of blood and surrounding static tissues accumulate different phase shifts in MR phase images. Similarly, propagation of shear waves in the target tissue is captured and visualized using modified phase-contrast MR sequences where the motion encoding gradients (MEG) are synchronized to the vibrational input. Imaging of the shear waves can be performed using a wide range of readouts such as gradient echo (GRE), spin-echo (SE), echo-planar imaging (EPI) (Figure 3), or balanced steady-state free precession (ibSSFP) imaging.
Fig. 3.
MRE pulse sequence diagrams depicting the radiofrequency (RF) pulses, gradients in the frequency encoding, phase encoding, and sliceselect directions, and the applied external motion. On the left is a GRE MRE sequence for imaging 60-Hz mechanical motion using 16-millisecond gradient-moment-nulled (GMN) MEG applied along Slice encoding direction. On the right is an SE-EPI MRE sequence for imaging 60-Hz motion using 2 bipolar 6.5-m MEGs, 1 on each side of the refocusing pulse and synchronized to the motion. Both sequences are shown with GMN imaging gradients and spatial presaturation pulses. (Adapted from R. L. E. Matthew C Murphy et al.,, “Phase Correction for Interslice Discontinuities in Multislice EPI MR Elastography,” vol. 269, no. 5232, p. 5232, 2012.)
The MEGs are applied in all three orthogonal planes of motion to measure the 3-dimensional wave vector. The frequency of MEGs applied is typically the same as the mechanical frequency and synchronized using the trigger pulses. Several wave images were acquired at different time points by varying the phase offset between the MEGs and mechanical motion. Each MR image produces a vector component of the tissue motion in the direction of MEGs. Therefore, the adjusted relative phase offset between driver and MEGS in multiple acquisitions provides the full vector motion within the tissue.
Mechanical Parameter Mapping
The proportionality relationship between the transverse stress (shear stress) and shear strain (deformation) in an isotropic linear elastic material can be described by the mechanical property called shear modulus (μ). According to Hook’s law, the shear modulus is directly proportional to the speed of propagation of planar shear waves in a medium and the density of the medium [24].
| (1) |
Shear wave propagation speed can be calculated from the frequency (f) and wavelength (λ) of the externally applied mechanical motion as:
| (2) |
Therefore, complex-valued displacement filed maps (wave images) obtained are inverted using equations 1 and 2 providing complex modulus where real part represents storage modulus and imaginary part represents loss modulus. In MRE, for stiffness calculation, most of the soft tissues’ density is assumed to be equivalent to that of water (ρ=1 g cm−3) [25]. Further, the propagating wave field is a complex phenomenon comprised of geometric complexities such as reflections, refractions, and interferences which are minimized using band-pass and directional filters. Different inversion algorithms are currently being used to estimate the stiffness of soft tissue[26]. The most common of these is a direct inversion and is being applied to estimate the brain’s stiffness. Direct inversion assumes that the waves are propagating in a uniform, infinite, isotropic, and homogeneous medium. However, we understand that the brain is anisotropic in nature; but anisotropic stiffness estimation requires information of fiber orientation in the brain. A few studies have already demonstrated anisotropic brain stiffness estimation [27]–[29]; however, most of the current brain applications use isotropic stiffness estimation for simplicity.
MRE Applications in Brain
As an emerging diagnostic tool, the use of MRE in routine clinical diagnosis is still limited. Though MRE is considered the most accurate non-invasive tool for quantifying liver fibrosis [30], [31], its usage in other organs is still under investigation. Over the last two decades, a number of studies established that the MRE is capable of measuring 2D and 3D [32]–[34] shear wave fields in the human brain. The stiffness estimation in the brain is sensitive to experimental choices such a vibrational frequency, acquisition strategy, and post-processing pipelines which makes it challenging to quantitatively assess the reproducibility of stiffness across sites. Therefore, keeping all the MRE acquisition parameters consistent is essential to attribute any stiffness changes to the underlying biological process [35].
Many studies have quantified the shear modulus of the brain in healthy and patient populations, and the values are ranging from 2.8 to 12.9 kPa for grey matter and 2.5 to 15.2 kPa for white matter [33], [34], [36], [37]. Though most of the studies are consistent with the fact that the white matter is significantly stiffer than grey matter, some of the studies reported otherwise [38]. This variability among the studies points to the fact that there is no proven definite method to measure brain tissue elasticity in vivo. However, these studies reestablish the fact that there is a clear elasticity difference between the GM and WM [36], [39].
A Number of MRE studies on a healthy aging population were conducted, and a consensus has been formed that the stiffness of the brain tissue decreases with age (Figure 5) [29], [40]–[42]. Regarding the effect of sex on stiffness, initial studies were equivocal, female population exhibiting significantly higher global stiffness [41] than the male counterparts, while recent studies further concluded that the effect of sex is significant only in the temporal and occipital lobes[40].
Fig. 5.
Summary of the relationship between global brain (shear modulus) viscoelasticity and age. The shear modulus from this model decreases significantly with age and is larger in women compared with men. (From I. Sack et al., “The impact of aging and gender on brain viscoelasticity,” Neuroimage, vol. 46, no. 3, pp. 652–657, 2009, https://doi.org/10.1016/j.neuroimage.2009.02.040.)
The first MRE study that demonstrated the sensitivity of brain stiffness to the pathology was performed in 2010 by Wuerfel et al. in multiple sclerosis (MS) patients and illustrated that brain stiffness is decreased in MS patients compared to age-matched control subjects[43]. A subsequent study on the effect of MS disease course also concluded that the chronic form of this disease would lead to altered cerebral geometry and thereby stiffness[44]. There are few MRE studies reported contradictory results on global and regional brain stiffness in a given pathological condition. One study reported as stiffness increased in parietal and occipital lobes in patients with normal hydrocephalus (NPH) [45], while the other study reported increased global stiffness in NPH [44]. Therefore, multicenter studies with same experimental setup is essential to report uniform stiffness measurements.
Initial results of the studies that were focused on investigating brain stiffness as biomarkers for neurodegenerative disease reported that the global brain stiffness decreased in subjects with Alzheimer’s disease (AD)[46]. The variation of brain stiffness in AD patients was not only due to the amyloid deposition but also due to the degradation of extracellular matrix, loss of cytoskeletal structure, or altered synaptic connectivity that characterizes Alzheimer’s disease (figure 6). This study also demonstrated the specificity of stiffness changes (i.e. decrease in stiffness) in the heteromodal association cortices that are hardest hit by the disease and not throughout the brain. The brain stiffness changes in four classes of dementia was compared in a recent study, AD and frontotemporal dementia patients have showed decreased cerebral stiffness in frontal and temporal regions while AD patients additionally showed softening of the parietal lobe and sorimotor region. Patients with normal hydrocephalus showed decreased stiffness at parietal, occipital, and sorimotor regions whereas, patients with Lewy bodies did not show stiffness changes in any of the regions. These findings support the regional brain stiffness specificity [47] of previous studies. The summary of results is illustrated in figure 7.
Fig. 6.
Example brain MRE images collected with an SE-EPI pulse sequence (axial plane, repetition time/echo time (TR/TE) 5 1500/61.3 milliseconds). Images in the top row show the results from a cognitively normal control (CN) patient and the bottom row shows results from a male patient with AD. EPI magnitude images for each patient shown in the first column and the resulting stiffness maps shown in the second column. (From M. C. Murphy et al., “Decreased brain stiffness in Alzheimer’s disease determined by magnetic resonance elastography,” J. Magn. Reson. Imaging, vol. 34, no. 3, pp. 494–498, 2011, https://doi.org/10.1002/jmri.22707.)
Fig. 7.
Summary of stiffness changes caused by different forms of dementia. First from the left is a sagittal view of a lobar brain atlas with each region showing the mean stiffness in a group of CN control patients. Regions include frontal lobe (F), parietal lobe (P), temporal lobe (T), occipital lobe (O), deep gray and white matter (D), and cerebellum (C). The remaining panels show the difference between mean stiffness in the dementia group and the CN group. (Data from M. ElSheikh et al., “MR elastography demonstrates unique regional brain stiffness patterns in dementias,” Am. J. Roentgenol., vol. 209, no. 2, pp. 403–408, 2017, https://doi.org/10.2214/AJR.16.17455.)
Management of most of the brain pathological conditions such as tumors require surgery as a primary therapeutic tool. Generally, hard tumors are difficult to remove and most often requires a manual dissection while the soft tumor tissues can be removed by suction; therefore, the knowledge of tumor tissue stiffness property plays an important role during the pre-operative planning, determining surgical approach, and tumor resection strategy which is critical for safe and maximum post-therapeutic outcomes [48]–[50]. For this reason, among neuroradiologists, the interest to measure elasticity during pre-operative planning is growing.
To date, only MRI was relatively capable of qualitatively characterize the brain tumor either as softer or stiffer [51]. The requirement of the bone window is limiting the ultrasound-based techniques to measure the elasticity of the brain [52], and thereby MRE is emerging as a viable diagnostic method. Stiffness assessment of solid intracranial tumors was first performed in 2007 using MRE (Figure 9) and demonstrated the presence of large variability of viscoelasticity of the tumors [37]. The studies on meningiomas also reported that MRE stiffness assessment was well correlated with the surgeon’s evaluation of tumor consistency. However, the same studies highlighted challenges that remain to be addressed in small and heterogenous tumor cases [53], [54]. A study used MRE-based slip interface imaging (SII) to predict the degree of meningioma-brain adhesion and the results were compared with the surgery findings[55]. This study found that the significant correlation between SII predictions and the surgical findings, potentially an improved MRE tool for the prediction of surgical risk and tumor resectability.
Fig. 9.
Example MRE images from both a firm (top) and soft (bottom) meningioma (dotted outline). On the left is a T1-weighted anatomic image and their respective stiffness maps shown in the right column. (From M. C. Murphy et al., “Preoperative assessment of meningioma stiffness by magnetic resonance elastography,” J. Neurosurg., vol.118, no. 3, p. 643, 2013, https://doi.org/10.3171/2012.9.JNS12519.Preoperative.)
A reproducibility study of brain stiffness in pseudotumor patients was performed before and after lumbar puncture, and its results were compared with healthy volunteers[18]. The results showed a good reproducibility of brain stiffness estimation in all subjects and observed a significant stiffness difference between the patients and healthy volunteers. However, there was no significant correlation between the opening and closing of lumbar puncture pressure and the brain stiffness despite the CSF drainage. These findings corroborate with the previous studies [44] and emphasize that the CSF pressure may not be a significant factor influencing brain stiffness.
A recent review on MRE application in brain tumor diagnosis compared 19 original research studies, which were conducted on a population ranging from 4–34 patients each, including 184 metastatic brain tumor patients[35], [56], [57]. This study noted that the group mean tumor stiffness of meningiomas and pituitary adenomas correlated with the intra-operative findings while the pooled data analysis demonstrated significant overlap between shear modulus values across brain tumor types. These overlaps limit the applicability of MRE for diagnostic purposes and highlight the need for further rigorous studies.
MRE and Pediatric Brain Imaging
The mechanical properties of an organ are based on its tissue composition and structural arrangement; therefore, MR elastography is potentially a powerful tool to assess the microstructural changes in the brain that takes place during prenatal, postpartum, childhood, and adolescence. In humans and animals, only during these phases of life, the brain undergoes enormous anatomical, physiological and neurological changes. So far, there were no published MRE studies in the human prenatal and postpartum population, and only a postnatal study on the rat model was reported [58]. In recent years, there are only a few MRE brain stiffness studies in the children and adolescents were reported [10]–[12], [59].
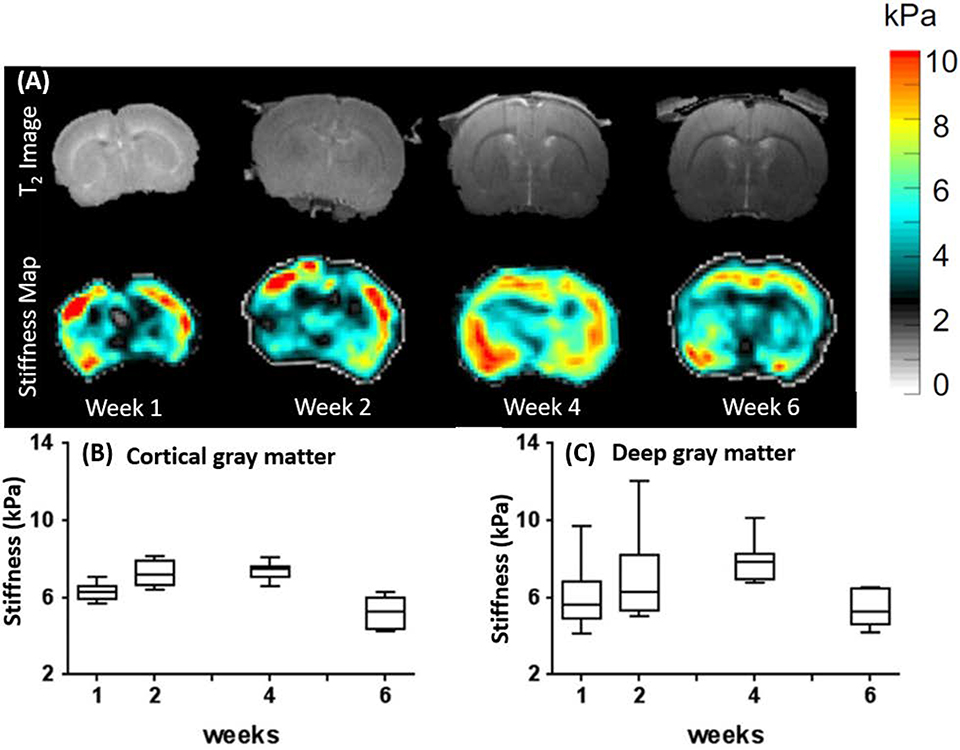
The animal brain MRE study using rats was performed between 9 – 42 days (6 weeks) after birth that represents neonatal to juvenile age in animals [58]. During this 6 weeks study period, animals were placed with their mothers in the initial 2 weeks, housed in a standard cage during the 3rd and 4th week, and then set free within a large area and given free access to food and water during 4–6 weeks. Mechanical properties (stiffness) of brain tissue were measured using MRE at every developmental process stage as shown in Figure 10.
Fig. 10.
(A) Representative T2-weighted coronal MR images at the level of foramina of Monro shown in the upper row (images acquired week 1 to 6), and respective MRE stiffness maps of atypical animal with the shear modulus in kilopascals (Middle row). Complex shear modulus, for (B) cortical gray matter and (C) deep gray matter shown in plots B and C in the bottom row. Longitudinal changes assessed using a generalized linear model with pairwise comparisons of means between weeks. Images are reproduced from A. C. Pong et al., “Longitudinal measurements of postnatal rat brain mechanical properties in-vivo,” J. Biomech., vol. 49, no. 9, pp.1751–1756, 2016, https://doi.org/10.1016/j.jbiomech.2016.04.005.)
This study found that there was a rapid increase in the brain size between weeks one and two followed by a slower growth thereafter even though there was overall growth (i.e. size) in the animal (weight increase). There was a spatial variability in shear modulus between different brain regions in the same animal, and also there was a considerable variability among the young animals for the same brain regions, specifically in the deep gray matter region. The shear modulus in the cortical gray matter region increased during the first two weeks and remained stable for the next four weeks, followed by a significant decrease during the 6th week. Shear modulus of deep gray matter gradually increased from week one to four and was significantly lower in week six compared to week three and four.
The measured shear modulus at different time points is shown below figure 11:
Fig. 11.
Scatter plots of pediatric patients (7–18 years old) showing age against the frequency dependence stiffness for white matter (WM) and gray matter Spearman correlations show no significant age dependence. The adult data plotted as the mean. (Adapted from J.Yeung et al., “Paediatric brain tissue properties measured with magnetic resonance elastography” Biomechanics and Modeling in Mechanobiology (2019) 18:1497–1505 https://doi.org/10.1007/s10237-019-01157-x)
This study concluded that the stiffness of the brain tissue changes between the two brain regions was dissimilar. The rate of stiffness change was high during the early postnatal period, which was equivalent to postpartum and early childhood in humans, then the postnatal (week six) (approximately equivalent to adolescence in humans).
Over the last two decades, through a large number of research studies and technological advancements, we improved our understanding of the brain’s structural and functional developmental changes. For example, earlier, it was believed that the brain undergoes most developmental changes until the adolescent years, but now the medical and research communities agree that these changes are protracted till the third decade of life [59]–[61]. Studies that examine brain structural changes in cortical and sub-cortical regions reported significant heterogeneity in terms of developmental timings of each brain region [60], [62], [63].
A multi-frequency MRE study to characterize the brain tissue stiffness in pediatric subjects compared with the adult brain was performed by Yeung et al. [12]. This study aimed to determine the correlation between brain developmental changes in the early years and corresponding changes in biomechanical properties. This study reported that the stiffness values did not correlate with age in grey matter and white matter regions. There were no significant differences in global brain stiffness between the adolescent and adult brains. However, they found that caudate and putamen regions of the adolescent brain were significantly stiffer than the adults while other regions were softer. In pediatric subjects, the stiffness of white matter and grey matter was very similar, which was contrast to the earlier studies that have found white matter was stiffer than grey matter [22].
Another MRE study on healthy children population using multi-frequency stimulation to identify age and sex discrepancies in the brain’s mechanical properties [11].
This study concluded that there were no significant difference in stiffness measured for the whole brain over the age range, while there was a significant increase in structural parameters of white matter with age. This might be because of an increased geometrical alignment of the material’s microstructure. This study also found that the female children and adolescents’ brains were much stiffer than the male counterparts, which is in line with earlier studies [10], [12].
The brain MRE study on children with cerebral palsy was recently published [10]. This study reported a direct relationship between brain stiffness and the child’s dynamic balance reactions. This study identified 12 distinct regions in the brain to compare the tissue stiffness, in which 10 of them have been identified as important for balance and locomotion functions[64], [65]. The correlation between the tissue stiffness in these regions and balance reactions when the child was stepping anterior or posterior was compared and noted that higher brain stiffness in locomotive control regions as shown in Figure 15, which was an indication of higher balance recovery.
Figure 15:

Maps of the voxel-wise correlation coefficient, rs, reveals clusters of regions in which brain stiffness significantly correlated with (A) anterior and (B) single-step threshold balance reaction task.
While the MRE technique is becoming an increasingly attractive method for clinical professionals, researchers are expanding the scope of MRE into other unexplored territories. For example, recent research using stiffness to study the relationship between brain structure and function in a healthy population is fascinating [66]. Initial results of this study demonstrated that the hippocampus’s damping ratio is a significant predictor of performance on a relational memory task and was also able to predict behavioral performance.
Conclusions:
As an emerging diagnostic technique, initial pediatric brain MRE studies (though there are only a few) so far are promising to provide additional information about tissue characteristics. In the adult population, MRE studies have demonstrated a strong test-retest repeatability in measuring stiffness with typical errors of 1% for the global brain, 2% for measuring lobes of the brain, and 3–7 % for measuring subcortical grey matter[35]. However, MRE is still investigational, particularly in diagnosing small tumors due to limited image resolution on elasticity maps; and elasticity of tumors with cystic changes also cannot be diagnosed effectively because of the fact that shear waves cannot propagate through cystic components [37], [57].
MRE is used in the adult population to study various brain pathological conditions, and the results are promising though some limitations are yet to be addressed, while studies in the young population are facing some additional technical challenges as well. Some of the major challenges are listed below.
Similar to conventional MR imaging, MRE cannot be performed in patients with cerebral aneurysms and cochlear implants, and claustrophobic patients. Since MRE is being performed with additional hardware, obese patients may have problems fitting into the MR scanner and also in the head coil. A major challenge in pediatric brain MRE is that the image acquisition time typically takes 4–10 minutes which is too long for children to stay still. Since MRE is a motion-sensitive technique, subject motion during image acquisition would introduce phase errors and image artifacts resulting in errors in stiffness maps. Though small motions can be corrected using existing techniques [67], [68], large motions expected towards the end of the scan once the child becomes restless cannot be corrected and leads to errors in the final image. Having a short acquisition protocol with motion navigator and correction techniques would be one solution to address this challenge. Furthermore, in order to resolve stiffness in small tumors, higher frequency vibrations should be induced in the brain. However, this might lead to attenuation of waves in deep brain structures. Using lower frequency vibrations generates longer wavelengths which might violate infinite medium assumption when inverting the wave data to produce robust stiffness estimates. However, an appropriate frequency which could overcome above limitations should be chosen.
The same standard mechanical drivers used for adult subjects cannot be used in young children because 1) standard driver may not provide optimal coupling between the patient body surface and the driver leading to ineffective power delivery 2) due to comparatively high power can potentially cause mechanical or thermal injury. Therefore, it’s essential to use a separate passive driver with reduced power up to 50% from what is used for the adult subjects.
Fig. 4.
(a) Axial T2-weighted MR imaging showing the tumor and associated edema and (b) MRE stiffness map showing the tumor is substantially stiffer, whereas edema is softer, in relation to unaffected tissue in a woman brain with meningioma. (c) And (d) are data from man with a glioma (grade IV). Where (c) is T2-weighted MR imaging showing the tumor mass; and (d) stiffness map showing the tumor to be softer compared with unaffected brain tissue. (Adapted from L. V. Hiscox et al., “Magnetic resonance elastography (MRE) of the human brain: Technique, findings and clinical applications,” Phys. Med. Biol., vol. 61, no. 24, pp. R401–R437, 2016, https://doi.org/10.1088/0031-9155/61/24/R401.)
Fig. 8.
Concordant patient with a complete slip interface at imaging and no adhesion at surgery. (A) T1- weighted image with contrast enhancement shows a large left medial sphenoid wing meningioma. The tumor-brain interface clearly defined with the (B) shear line image and (C) octahedral shear strain (OSS) map the arrows indicating the presence of the slip interface and no adhesion. Surgical findings showed that the dissection plane was nearly completely extrapial with no meningioma-brain adhesion encountered. (From Z. Yin et al., “Slip interface imaging based on MR-elastography preoperatively predicts meningioma–brain adhesion,” J. Magn. Reson. Imaging, vol. 46, no. 4, pp. 1007–1016, 2017, https://doi.org/10.1002/jmri.25623.)
Fig. 12.
Storage modulus and. loss modulus estimated from the healthy children at vibrating frequency of 40 Hz, 60 Hz, and 80 Hz shown in the figure. (Adapted from E.Ozkaya et al., “Viscoelasticity of children and adolescent brains through MR elastography” journal of the mechanical behavior of biomedical materials 115 (2021) 104229, https://doi.org/10.1016/j.jmbbm.2020.104229).
Fig. 13.
Maps of the voxel-wise correlation coefficient (rs) reveals clusters of regions in which brain stiffness significantly correlated with (A) anterior and (B) single-step threshold balance reaction task. (Images adapted from G.McIlvain et al., “Brain Stiffness Relates to Dynamic Balance Reactions in Children With Cerebral Palsy” Journal of Child Neurology 2020, Vol. 35(7) 463–471. DOI: 10.1177/0883073820909274)
Figure 14:

A) Sketch of the MRE actuation setup: A vibrating pillow below the subject’s head causes shear wave propagation in the brain. B) T1-w anatomical image of a brain (subject age: 13 yrs). C) Gray matter mask. D) White matter mask. Wave propagation image for E) 40 Hz, F) 60 Hz, and G) 80 Hz. Storage modulus (G′ ) for H) 40 Hz, I) 60 Hz, and J) 80 Hz. Loss modulus (G′′ ) for K) 40 Hz, L) 60 Hz, and M) 80 Hz.
Key Points.
MRE is a non-invasive and non-irradiation technique alternative to palpation.
Quantitative stiffness estimation of internal organs is possible without surgery.
A preferred method for diagnosing chronic liver diseases and hepatic fibrosis.
MRE applications in other organs is still under investigation.
Results of initial pediatric studies are promising to deliver clinically valuable information.
Synopsis.
Magnetic resonance elastography (MRE) is an emerging non-invasive technique, alternative to palpation for quantitative assessment of biomechanical properties of tissue. In MRE, tissue stiffness information is obtained by a three-step process, propagating mechanical waves in the tissues, measuring the wave propagation using modified MR pulse sequences, and finally generate the quantitative stiffness maps from the MR images. While, MRE is clinically used in patients with liver diseases, whereas its applications in other organs are still in the investigational stage. Currently, the pediatric studies are in the initial stage and the preliminary results are promising to provide additional information about tissue characteristics.
Footnotes
Disclosure Statement
“The Authors have nothing to disclose.”
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Manjunathan Nanjappa, Department of Radiology, The Ohio State Wexner Medical Center, Columbus, Ohio USA-43210.
Arunark Kolipaka, Department of Radiology, The Ohio State Wexner Medical Center, Columbus, Ohio USA-43210.
References:
- [1].Moran PR, “A Flow Velocity Zeugmatographic Interlace for NMR Imaging in Humans.” Magnetic Resonance Imaging Vol.1, pp. 197–203, 1982. [DOI] [PubMed] [Google Scholar]
- [2].Lenroot RK and Giedd JN, “Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging,” Neurosci. Biobehav. Rev, vol. 30, no. 6, pp. 718–729, 2006, doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- [3].Hedner J, Lundell KH, and Breese GR, “Development variations in CSF monoamine metabolites during childhood,” Biol. Neonate, vol. 49, no. 4, pp. 190–197, 1986. [DOI] [PubMed] [Google Scholar]
- [4].Klingberg T, Vaidya CJ, Gabrieli JDE, Moseley ME, and Hedehus M, “Myelination and organization of the frontal white matter in children: A diffusion tensor MRI study,” Neuroreport, vol. 10, no. 13, pp. 2817–2821, 1999, doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- [5].Jernigan TL, Baaré WFC, Stiles J, and Madsen KS, “Postnatal brain development,” pp. 77–92, 2011, doi: 10.1016/b978-0-444-53884-0.00019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schmithorst VJ and Yuan W, “White matter development during adolescence as shown by diffusion MRI,” Brain Cogn, vol. 72, no. 1, pp. 16–25, 2010, doi: 10.1016/j.bandc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- [7].Toledo E et al. , “The young brain and concussion: Imaging as a biomarker for diagnosis and prognosis,” Neurosci. Biobehav. Rev, vol. 36, no. 6, pp. 1510–1531, 2012, doi: 10.1016/j.neubiorev.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Himmelmann K et al. , “MRI classification system (MRICS) for children with cerebral palsy: development, reliability, and recommendations,” Dev. Med. Child Neurol, vol. 59, no. 1, pp. 57–64, 2017, doi: 10.1111/dmcn.13166. [DOI] [PubMed] [Google Scholar]
- [9].Robinson MN, Peake LJ, Ditchfield MR, Reid SM, Lanigan A, and Reddihough DS, “Magnetic resonance imaging findings in a population-based cohort of children with cerebral palsy,” Dev. Med. Child Neurol, vol. 51, no. 1, pp. 39–45, 2009, doi: 10.1111/j.1469-8749.2008.03127.x. [DOI] [PubMed] [Google Scholar]
- [10].McIlvain G et al. , “Brain Stiffness Relates to Dynamic Balance Reactions in Children With Cerebral Palsy,” J. Child Neurol, vol. 35, no. 7, pp. 463–471, 2020, doi: 10.1177/0883073820909274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ozkaya E et al. , “Viscoelasticity of children and adolescent brains through MR elastography,” J. Mech. Behav. Biomed. Mater, vol. 115, no. December 2019, p. 104229, 2021, doi: 10.1016/j.jmbbm.2020.104229. [DOI] [PubMed] [Google Scholar]
- [12].Yeung J, Jugé L, Hatt A, and Bilston LE, “Paediatric brain tissue properties measured with magnetic resonance elastography,” Biomech. Model. Mechanobiol, vol. 18, no. 5, pp. 1497–1505, 2019, doi: 10.1007/s10237-019-01157-x. [DOI] [PubMed] [Google Scholar]
- [13].Parker KJ, Doyley MM, and Rubens DJ, “Corrigendum: Imaging the elastic properties of tissue: the 20 year perspective,” Phys. Med. Biol, vol. 57, no. 16, pp. 5359–5360, 2012, doi: 10.1088/0031-9155/57/16/5359. [DOI] [PubMed] [Google Scholar]
- [14].Lewa CJ and de Certaines JD, “MR imaging of viscoelastic properties,” J. Magn. Reson. Imaging, vol. 5, no. 2, pp. 242–244, 1995, doi: 10.1002/jmri.1880050221. [DOI] [PubMed] [Google Scholar]
- [15].Lewa CJ and De Certaines JD, “Viscoelastic property detection by elastic displacement NMR measurements,” J. Magn. Reson. Imaging, vol. 6, no. 4, pp. 652–656, 1996, doi: 10.1002/jmri.1880060414. [DOI] [PubMed] [Google Scholar]
- [16].Sabet AA, Christoforou E, Zatlin B, Genin GM, and Bayly PV, “Deformation of the human brain induced by mild angular head acceleration,” J. Biomech, vol. 41, no. 2, pp. 307–315, 2008, doi: 10.1016/j.jbiomech.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Muthupillai R, Lomas DJ, Rossman PJ, Greenleaf JF, Manduca A, and Ehman RL, “Magnetic resonance elastography by direct visualization of propagating acoustic strain waves,” Science (80-. )., vol. 269, no. 5232, pp. 1854–1857, 1995, doi: 10.1126/science.7569924. [DOI] [PubMed] [Google Scholar]
- [18].Kolipaka A et al. , “Magnetic resonance elastography to estimate brain stiffness: Measurement reproducibility and its estimate in pseudotumor cerebri patients,” Clin. Imaging, vol. 51, no. January, pp. 114–122, 2018, doi: 10.1016/j.clinimag.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kenyhercz WE et al. , “Quantification of aortic stiffness using magnetic resonance elastography: Measurement reproducibility, pulse wave velocity comparison, changes over cardiac cycle, and relationship with age,” Magn. Reson. Med, vol. 75, no. 5, pp. 1920–1926, 2016, doi: 10.1002/mrm.25719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gandhi D, Kalra P, Raterman B, Mo X, Dong H, and Kolipaka A, “Magnetic resonance elastography-derived stiffness of the kidneys and its correlation with water perfusion,” NMR Biomed, vol. 33, no. 4, pp. 1–12, 2020, doi: 10.1002/nbm.4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fakhouri F, Dong H, and Kolipaka A, “Magnetic resonance elastography of the lungs: A repeatability and reproducibility study,” NMR Biomed, vol. 32, no. 7, pp. 1–10, 2019, doi: 10.1002/nbm.4102. [DOI] [PubMed] [Google Scholar]
- [22].Hiscox LV et al. , “Magnetic resonance elastography (MRE) of the human brain: Technique, findings and clinical applications,” Phys. Med. Biol, vol. 61, no. 24, pp. R401–R437, 2016, doi: 10.1088/0031-9155/61/24/R401. [DOI] [PubMed] [Google Scholar]
- [23].Yin M, Chen J, Glaser KJ, Talwalkar JA, and Ehman RL, “Abdominal magnetic resonance elastography,” Top. Magn. Reson. Imaging, vol. 20, no. 2, pp. 79–87, 2009, doi: 10.1097/RMR.0b013e3181c4737e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].K.F G, Wave Motion In Elastic Solids. Dover, New York, 1991. [Google Scholar]
- [25].Burlew M, “A new Ultrasound Tissue-Equivalent Material,” Radiology, vol. 134, no. 2, pp. 517–520, 1980. [DOI] [PubMed] [Google Scholar]
- [26].Manduca A et al. , “Magnetic resonance elastography: Non-invasive mapping of tissue elasticity,” Med. Image Anal, vol. 5, no. 4, pp. 237–254, 2001, doi: 10.1016/S1361-8415(00)00039-6. [DOI] [PubMed] [Google Scholar]
- [27].Romano A, Scheel M, Hirsch S, Braun J, and Sack I, “In vivo waveguide elastography of white matter tracts in the human brain,” Magn. Reson. Med, vol. 68, no. 5, pp. 1410–1422, 2012, doi: 10.1002/mrm.24141. [DOI] [PubMed] [Google Scholar]
- [28].Romano A et al. , “In vivo Waveguide elastography: Effects of neurodegeneration in patients with amyotrophic lateral sclerosis,” Magn. Reson. Med, vol. 72, no. 6, pp. 1755–1761, 2014, doi: 10.1002/mrm.25067. [DOI] [PubMed] [Google Scholar]
- [29].Kalra P, Raterman B, Mo X, and Kolipaka A, “Magnetic resonance elastography of brain: Comparison between anisotropic and isotropic stiffness and its correlation to age,” Magn. Reson. Med, vol. 82, no. 2, pp. 671–679, 2019, doi: 10.1002/mrm.27757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kaur H et al. , “ACR Appropriateness Criteria® Suspected Liver Metastases,” J. Am. Coll. Radiol, vol. 14, no. 5, pp. S314–S325, 2017, doi: 10.1016/j.jacr.2017.01.037. [DOI] [PubMed] [Google Scholar]
- [31].Kim DK, Choi JY, suk Park M, Kim MJ, and Chung YE, “Clinical feasibility of mr elastography in patients with biliary obstruction,” Am. J. Roentgenol, vol. 210, no. 6, pp. 1273–1278, 2018, doi: 10.2214/AJR.17.19085. [DOI] [PubMed] [Google Scholar]
- [32].Kruse SA, Dresner MA, Rossman PJ, Felmlee JP, Jack CR, and Ehman RL, “Palpation of the Brain Using Magnetic Resonance Elastography,” Int. Soc. Magn. Reson. Med, p. 43, 1999. [Google Scholar]
- [33].McCracken PJ, Manduca A, Felmlee J, and Ehman RL, “Mechanical transient-based magnetic resonance elastography,” Magn. Reson. Med, vol. 53, no. 3, pp. 628–639, 2005, doi: 10.1002/mrm.20388. [DOI] [PubMed] [Google Scholar]
- [34].Green L, Sinkus M, Cheng R, Bilston S, “3D MR-Elastography of the Brain at 3 tesla,” in ISMRM 13th Scientific Meeting & Exhibition, 2007, p. 427. [Google Scholar]
- [35].Murphy MC, Huston J, and Ehman RL, “MR elastography of the brain and its application in neurological diseases,” Neuroimage, vol. 187, no. August 2017, pp. 176–183, 2019, doi: 10.1016/j.neuroimage.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kruse SA et al. , “Magnetic resonance elastography of the brain,” Neuroimage, vol. 39, no. 1, pp. 231–237, 2008, doi: 10.1016/j.neuroimage.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Xu L, Lin Y, Han JC, Xi ZN, Shen H, and Gao PY, “Magnetic resonance elastography of brain tumors: Preliminary results,” Acta radiol, vol. 48, no. 3, pp. 327–330, 2007, doi: 10.1080/02841850701199967. [DOI] [PubMed] [Google Scholar]
- [38].Nagashima T, Shirakuni T, and Rapoport SI, “A two-dimensional, finite element analysis of vasogenic brain edema,” Neurol. Med. Chir. (Tokyo)., vol. 30, no. 1, pp. 1–9, 1990, doi: 10.2176/nmc.30.1. [DOI] [PubMed] [Google Scholar]
- [39].Kruse SA ER, “2D approximation of 3D wave propagation in MR elastography of the brain,” in International Society for Magnetic Resonance in Medicine, 2003, p. 1084. [Google Scholar]
- [40].Arani A et al. , “Measuring the effects of aging and sex on regional brain stiffness with MR elastography in healthy older adults,” Neuroimage, vol. 111, pp. 59–64, 2015, doi: 10.1016/j.neuroimage.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sack I et al. , “The impact of aging and gender on brain viscoelasticity,” Neuroimage, vol. 46, no. 3, pp. 652–657, 2009, doi: 10.1016/j.neuroimage.2009.02.040. [DOI] [PubMed] [Google Scholar]
- [42].Sack I, Streitberger KJ, Krefting D, Paul F, and Braun J, “The influence of physiological aging and atrophy on brain viscoelastic properties in humans,” PLoS One, vol. 6, no. 9, 2011, doi: 10.1371/journal.pone.0023451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wuerfel J et al. , “MR-elastography reveals degradation of tissue integrity in multiple sclerosis,” Neuroimage, vol. 49, no. 3, pp. 2520–2525, 2010, doi: 10.1016/j.neuroimage.2009.06.018. [DOI] [PubMed] [Google Scholar]
- [44].Streitberger KJ et al. , “Brain viscoelasticity alteration in chronic-progressive multiple sclerosis,” PLoS One, vol. 7, no. 1, pp. 1–7, 2012, doi: 10.1371/journal.pone.0029888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Perry DC et al. , “Reward deficits in behavioural variant frontotemporal dementia include insensitivity to negative stimuli,” Brain, vol. 140, no. 12, pp. 3346–3356, 2017, doi: 10.1093/brain/awx259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Murphy MC et al. , “Decreased brain stiffness in Alzheimer’s disease determined by magnetic resonance elastography,” J. Magn. Reson. Imaging, vol. 34, no. 3, pp. 494–498, 2011, doi: 10.1002/jmri.22707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].ElSheikh M et al. , “MR elastography demonstrates unique regional brain stiffness patterns in dementias,” Am. J. Roentgenol, vol. 209, no. 2, pp. 403–408, 2017, doi: 10.2214/AJR.16.17455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Itamura K, Chang KE, Lucas J, Donoho DA, Giannotta S, and Zada G, “Prospective clinical validation of a meningioma consistency grading scheme: Association with surgical outcomes and extent of tumor resection,” J. Neurosurg, vol. 131, no. 5, pp. 1356–1360, 2019, doi: 10.3171/2018.7.JNS1838. [DOI] [PubMed] [Google Scholar]
- [49].Zada G, Du R, and Laws ER, “Defining the ‘edge of the envelope’: Patient selection in treating complex sellar-based neoplasms via transsphenoidal versus open craniotomy: Clinical article,” J. Neurosurg, vol. 114, no. 2, pp. 286–300, 2011, doi: 10.3171/2010.8.JNS10520. [DOI] [PubMed] [Google Scholar]
- [50].Zada G et al. , “A proposed grading system for standardizing tumor consistency of intracranial meningiomas,” Neurosurg. Focus, vol. 35, no. 6, pp. 1–6, 2013, doi: 10.3171/2013.8.FOCUS13274. [DOI] [PubMed] [Google Scholar]
- [51].Meyer F, Hoover J, and Morris J, “Use of preoperative magnetic resonance imaging T1 and T2 sequences to determine intraoperative meningioma consistency,” Surg. Neurol. Int, vol. 2, no. 1, p. 142, 2011, doi: 10.4103/2152-7806.85983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Chauvet M, Imbault D, Capelle M, Demene L, Mossad C, Karachi M, Boch C, Gennisson AL, Tanter J-L, “In Vivo Measurement of Brain Tumor Elasticity Using Intraoperative Shear Wave Elastography,” Ultraschall Med, vol. 37, pp. 584–590, 2016. [DOI] [PubMed] [Google Scholar]
- [53].Hughes JD et al. , “Higher-Resolution Magnetic Resonance Elastography in Meningiomas to Determine Intratumoral Consistency,” Neurosurgery, vol. 77, no. 4, pp. 653–659, 2015, doi: 10.1227/NEU.0000000000000892.Higher-Resolution. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Murphy MC et al. , “Preoperative assessment of meningioma stiffness by magnetic resonance elastography,” J. Neurosurg, vol. 118, no. 3, p. 643, 2013, doi: 10.3171/2012.9.JNS12519.Preoperative. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yin Z et al. , “Slip interface imaging based on MR-elastography preoperatively predicts meningioma–brain adhesion,” J. Magn. Reson. Imaging, vol. 46, no. 4, pp. 1007–1016, 2017, doi: 10.1002/jmri.25623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Bunevicius A, Schregel K, Sinkus R, Golby A, and Patz S, “REVIEW: MR elastography of brain tumors,” NeuroImage. Clin, vol. 25, no. November 2019, p. 102109, 2020, doi: 10.1016/j.nicl.2019.102109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Di Ieva A et al. , “Magnetic resonance elastography: A general overview of its current and future applications in brain imaging,” Neurosurg. Rev, vol. 33, no. 2, pp. 137–145, 2010, doi: 10.1007/s10143-010-0249-6. [DOI] [PubMed] [Google Scholar]
- [58].Pong AC, Jugé L, Cheng S, and Bilston LE, “Longitudinal measurements of postnatal rat brain mechanical properties in-vivo,” J. Biomech, vol. 49, no. 9, pp. 1751–1756, 2016, doi: 10.1016/j.jbiomech.2016.04.005. [DOI] [PubMed] [Google Scholar]
- [59].Johnson CL and Telzer EH, “Magnetic resonance elastography for examining developmental changes in the mechanical properties of the brain,” Dev. Cogn. Neurosci, vol. 33, no. February 2017, pp. 176–181, 2018, doi: 10.1016/j.dcn.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Mills KL et al. , “Structural brain development between childhood and adulthood: Convergence across four longitudinal samples,” Neuroimage, vol. 141, pp. 273–281, 2016, doi: 10.1016/j.neuroimage.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lebel C and Beaulieu C, “Longitudinal development of human brain wiring continues from childhood into adulthood,” J. Neurosci, vol. 31, no. 30, pp. 10937–10947, 2011, doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lebel C, Gee M, Camicioli R, Wieler M, Martin W, and Beaulieu C, “Diffusion tensor imaging of white matter tract evolution over the lifespan,” Neuroimage, vol. 60, no. 1, pp. 340–352, 2012, doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- [63].Raznahan A et al. , “Longitudinal four-dimensional mapping of subcortical anatomy in human development,” Proc. Natl. Acad. Sci. U. S. A, vol. 111, no. 4, pp. 1592–1597, 2014, doi: 10.1073/pnas.1316911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wittenberg E, Thompson J, Nam CS, and Franz JR, “Neuroimaging of human balance control: A systematic review,” Front. Hum. Neurosci, vol. 11, no. April, pp. 1–25, 2017, doi: 10.3389/fnhum.2017.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].la Fougère C et al. , “Real versus imagined locomotion: A [18F]-FDG PET-fMRI comparison,” Neuroimage, vol. 50, no. 4, pp. 1589–1598, 2010, doi: 10.1016/j.neuroimage.2009.12.060. [DOI] [PubMed] [Google Scholar]
- [66].Schwarb H, Johnson CL, McGarry MDJ, and Cohen NJ, “Medial temporal lobe viscoelasticity and relational memory performance,” Neuroimage, vol. 132, pp. 534–541, 2016, doi: 10.1016/j.neuroimage.2016.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Fehlner A et al. , “Increasing the spatial resolution and sensitivity of magnetic resonance elastography by correcting for subject motion and susceptibility-induced image distortions,” J. Magn. Reson. Imaging, vol. 46, no. 1, pp. 134–141, 2017, doi: 10.1002/jmri.25516. [DOI] [PubMed] [Google Scholar]
- [68].and Matthew C Murphy RLE, Huston John, , Glaser Kevin J, Manduca Armando, Felmlee Joel P, “Phase Correction for Interslice Discontinuities in Multislice EPI MR Elastography,” vol. 269, no. 5232, p. 5232, 2012. [Google Scholar]