Abstract
Introduction:
Auditory attention is a critical foundation for successful language comprehension, yet is rarely studied in individuals with acquired language disorders.
Methods:
We used an auditory version of the well-studied Attention Network Test to study alerting, orienting, and executive control in 28 persons with chronic stroke (PWS). We further sought to characterize the neurobiology of each auditory attention measure in our sample using exploratory lesion-symptom mapping analyses.
Results:
PWS exhibited the expected executive control effect (i.e., decreased accuracy for incongruent compared to congruent trials), but their alerting and orienting attention were disrupted. PWS did not exhibit an alerting effect and they were actually distracted by the auditory spatial orienting cue compared to the control cue. Lesion-symptom mapping indicated that poorer alerting and orienting were associated with damage to the left retrolenticular part of the internal capsule (adjacent to the thalamus) and left posterior middle frontal gyrus (overlapping with the frontal eye fields), respectively.
Discussion:
The behavioral findings correspond to our previous work investigating alerting and spatial orienting attention in persons with aphasia in the visual modality and suggest that auditory alerting and spatial orienting attention may be impaired in PWS due to stroke lesions damaging multi-modal attention resources.
Keywords: auditory attention, Attention Network Test, stroke, alerting, spatial orienting, multi-modal resources
Introduction
Deficits in attention are common following a left hemisphere stroke (LaCroix, Tully, et al., 2020; Lee & Pyun, 2014; Murray, 2012; Villard & Kiran, 2017). Yet, attention is not a homogenous construct (Mirsky et al., 1991; Posner & Petersen, 1990; Sohlberg & Mateer, 2010) and can be divided into at least three distinct components: alerting, orienting, and executive control (Posner & Petersen, 1990). Alerting is a measure of an individual’s readiness to perceive information, orienting is a measure of an individual’s ability to select specific information from a stimulus, and executive control is a measure of how efficiently a correct response is achieved when task relevant information conflicts with task irrelevant information (Fan & Posner, 2004; Posner & Petersen, 1990).
The classic Attention Network Test (ANT) uses a single cued-flanker task in the visual modality to simultaneously measure alerting (cued vs. non-cued trials), orienting (spatially vs. non-spatially cued trials), and executive control (incongruent vs. congruent flanker trials; Fan et al., 2002). In a recent study using the classic ANT in persons with aphasia, we found that persons with aphasia exhibited the expected executive control effect (i.e., reduced accuracy and longer reaction times for incongruent trials compared to congruent trials), however, the magnitude of this effect did not differ between the aphasia and control groups. These executive control findings replicate previous work using the flanker task in persons with aphasia (Kuzmina & Weekes, 2017). However, unlike what has been previously reported, we found that persons with aphasia and controls differ in their visual alerting and spatial orienting attention abilities. More specifically, the aphasia group did not demonstrate the expected alerting effect (i.e., increased accuracy and/or faster reaction times in response to cued versus non-cued trials). We also found that the aphasia group was actually distracted by the spatial orienting cue rather than having it facilitate their task performance (LaCroix, Tully, et al., 2020). These results suggest that alerting and spatial orienting attention may be disrupted in persons with aphasia because of the manner in which stroke lesions affect the supporting neural resources.
Distinct brain regions support alerting, orienting, and executive control attention (Fan et al., 2005; Petersen & Posner, 2012; Posner & Petersen, 1990). Alerting attention is consistently associated with the thalamus, brainstem, and right fronto-parietal cortices (Petersen & Posner, 2012; Rinne et al., 2013; Sturm & Willmes, 2001); orienting with the right temporal-parietal junction, interparietal sulcus, superior parietal lobe, and frontal eye fields (Petersen & Posner, 2012; Rinne et al., 2013); and executive control with bilateral prefrontal cortex (Rinne et al., 2013) as well as the fronto-parietal and cingulo-opercular networks (Dosenbach et al., 2008; Petersen & Posner, 2012). However, the exact neural resources supporting attention vary depending on sensory modality (Anderson et al., 2010; Fritz et al., 2007; Kong et al., 2014; Petersen & Posner, 2012). For example, in neurotypical controls, both visual and auditory alerting cues activate the right posterior superior temporal gyrus (Fan et al., 2005; Thiel & Fink, 2007). However, visual alerting cues activate bilateral inferior occipital gyri and posterior parietal cortices more than auditory alerting cues. Auditory alerting cues activate bilateral superior temporal gyri, right middle frontal gyrus, and left inferior and superior frontal gyri more than visual alerting cues (Thiel & Fink, 2007). Similar findings have also been observed for orienting attention: visual spatial orienting attention activates bilateral frontal and parietal cortices including the precentral and postcentral gyri, superior frontal gyri and sulci, fusiform gyri, and superior parietal lobule (Corbetta, 1998; Fan et al., 2005). Auditory spatial orienting attention activates similar, yet distinct regions, within this bilateral fronto-parietal network, as well as the bilateral superior temporal gyri (Alho et al., 2014; Kong et al., 2014). These modality-specific effects on the neural resources supporting attention indicate the need for reliable assessments of each subtype of attention within both the auditory and visual modalities as stroke lesions may differentially impact visual and auditory attention, with the latter being particularly important for auditory language.
The purpose of this study was to explore auditory alerting, orienting, and executive control attention and the underlying neurobiology in persons with left hemisphere chronic stroke (PWS). It was hypothesized that PWS would demonstrate altered auditory alerting (i.e., no differences in accuracy for double cued versus non-cued trials) and spatial orienting attention (i.e., decreased accuracy for spatially cued versus center cued trials) as previously seen in the visual domain. We additionally expected to observe typical executive control effects (i.e., decreased accuracy for incongruent versus congruent trials), aligning with what has been previously observed in PWS using other executive control tasks (e.g., Green et al., 2010; Kuzmina & Weekes, 2017; Pompon et al., 2015). It was further hypothesized that PWS with damage to the left superior temporal, inferior frontal, and/or superior frontal gyri would demonstrate poorer auditory alerting attention than those without damage to these regions. Auditory spatial orienting attention was expected to be reduced in PWS with lesions to the left superior temporal gyrus, prefrontal cortex, intraparietal sulci, and/or supramarginal gyri.
Method
Participants
Participants were 28 chronic PWS (15 females) who experienced a single left hemisphere cerebral stroke1 at least 6 months prior to testing (Table 1). PWS ranged in age from 28 to 80 years (M= 54.68, sd= 12.57), were pre-morbidly right-handed, native speakers of American English, with no self-reported history of neurological disease, head trauma, or psychiatric disturbances prior to their stroke. Aphasia classification was determined using the Boston Diagnostic Aphasia Evaluation-III (Goodglass et al., 2000); each stroke participant’s aphasia diagnosis is reported in Table 1. We additionally recruited 20 neurotypical controls as part of this study. The control group had near ceiling performance, therefore we only report their data in Supplementary Tables 1 and 2. All participants were monetarily compensated for their participation. Arizona State University’s Institutional Review Board approved all procedures.
Table 1.
Participant characteristics.
| Gender | Age | Months Post Stroke |
Years of Ed. |
HearingΨ |
BDAE-III Auditory Single Word Comp. |
Aphasia Diagnosis |
|
|---|---|---|---|---|---|---|---|
| AZ1001 | F | 57 | 77 | 18 | 5.00 | 16/16 | None |
| AZ1003* | F | 48 | 110 | 19 | 15.00 | 16/16 | Broca’s |
| AZ1006* | M | 60 | 138 | 14 | 26.25 | 14/16 | Broca’s |
| AZ1011* | F | 73 | 53 | 16 | 18.75 | 16/16 | Anomic |
| AZ1012* | M | 77 | 85 | 16 | 53.75 | 10/16 | Wernicke’s |
| AZ1013* | F | 47 | 258 | 17 | −3.75 | 11/16 | Broca’s |
| AZ1016* | M | 37 | 142 | 14 | −2.50 | 16/16 | Broca’s |
| AZ1018* | F | 43 | 29 | 14 | 16.25 | 15/16 | Broca’s |
| AZ1022* | F | 46 | 79 | 14 | 10.00 | 15/16 | Broca’s |
| AZ1026 | M | 70 | 50 | 16 | 22.50 | 16/16 | None |
| AZ1028* | F | 80 | 19 | 24 | 21.25 | 14/16 | Wernicke’s |
| AZ1029 | F | 34 | 174 | 14 | −5.00 | 16/16 | None |
| AZ1030* | M | 56 | 32 | 16 | 23.75 | 16/16 | Broca’s |
| AZ1031* | F | 40 | 63 | 20 | 21.25 | 16/16 | Broca’s |
| AZ1032* | M | 28 | 20 | 13 | 5.00 | 15/16 | Anomic |
| AZ1033* | M | 57 | 180; 60 | 14 | 20.00 | 8/16 | Global |
| AZ1034* | F | 59 | 110 | 15 | 8.75 | 16/16 | Anomic |
| AZ1035* | F | 41 | 72 | 17 | 6.25 | 15/16 | Broca’s |
| AZ1036* | M | 65 | 158 | 15 | 12.50 | 15/16 | Broca’s |
| AZ1037* | M | 57 | 13 | 16 | 16.25 | 16/16 | Broca’s |
| AZ1038* | M | 54 | 155 | 14 | 12.50 | 16/16 | Broca’s |
| AZ1039* | F | 66 | 48 | 14 | 13.75 | 16/16 | Anomic |
| AZ1040* | F | 54 | 45 | 14 | 15.00 | 16/16 | Broca’s |
| AZ1041* | F | 59 | 24 | 12 | 35.00 | 15/16 | Anomic |
| AZ1042* | M | 55 | 37 | 14 | 18.75 | 13/16 | Broca’s |
| AZ1043 | F | 57 | 25 | 12 | 18.75 | 16/16 | None |
| AZ1045 | M | 61 | 18 | 20 | 6.25 | 16/16 | Conduction |
| AZ1046 | M | 50 | 16 | 232 | 8.75 | 14/16 | Broca’s |
Indicates participant was included in the visual ANT study (LaCroix, Tully, et al., 2020).
Pure Tone Average (PTA) better ear for 500-4000 Hz.
Auditory Attention Network Test (ANT)
In this task, participants heard the words “high,” “low,” and “day” spoken in either a high-pitched or a low-pitched voice. Participants were instructed to ignore the semantic content (i.e., the spoken word “high,” “low,” or “day”) and indicate via button press whether the speaker's voice was high or low in pitch; participants pressed the up and down arrows on the keyboard if the word was spoken in a high-pitched or low-pitched voice, respectively. A congruent trial occurred when the semantic content of the word corresponded with the vocal pitch, an incongruent trial when the semantic content of the word conflicted with the vocal pitch, and a neutral trial when the control word “day” was presented in either a high or low-pitched voice. The auditory Stroop targets were recorded by a single female speaker in American English. The average fundamental frequency of the high-pitch words was 356.67 Hz (sd=5.96); the average for the low-pitch words was 211.17 Hz (sd=5.73).
Each trial began with a 500 Hz fixation tone with an onset jittered between 400-1600 milliseconds. Following the offset of the fixation tone, an auditory cue was immediately presented for 50 milliseconds, followed by 600 milliseconds of silence, and then presentation of the auditory Stroop target. Auditory cues were 50 millisecond bursts of speech-shaped noise; the first and last 10 milliseconds of each cue was cosine gated. Auditory cue conditions were as follows: (1) center cue (correlated noise bursts designed to be perceived in the center of the head), (2) double cue (uncorrelated noise bursts designed to be perceived as separate signals in each ear), (3) spatial cue (single noise burst) presented in the left or right ear (the auditory spatial cue always predicted the location of the auditory Stroop task), and (4) no cue. Participants completed a total of 180 trials where all cue types and Stroop conditions were presented equally via Panasonic over-the-ear headphones at a sound-level considered comfortable by the participant. Reaction time and accuracy data was collected for each trial. Trial presentation was randomized for each participant. Verbal and written instructions, examples of all stimuli, and 10 practice trials preceded the start of the experiment. Note, the auditory ANT was modeled after the one used by Roberts and colleagues (2006) with the procedures and cues being the same, however, the auditory Stroop stimuli were recorded in American English.
Boston Diagnostic Aphasia Examination-III (BDAE-III) Short Form
To ensure all participants had adequate single word comprehension to complete the auditory ANT, auditory single word comprehension was assessed using the Basic Word Discrimination subtest of the BDAE-III short form (Goodglass et al., 2000). Participants were instructed to point to 16 familiar objects/pictures (e.g., body parts, animals, vehicles, etc.) following a verbal prompt from the examiner (e.g., “point to the bear”). Participants could achieve a maximum raw score of 16. Raw scores were transformed into proportion correct and included as a covariate in the behavioral analyses.
Hearing
Hearing acuity was assessed using pure-tone audiometry with a GSI 18 Audiometer and supra-aural headphones in a quiet room using a pulsed tone and a two down, one up procedure in steps of 5 dB for each correctly and incorrectly detected tone. Hearing acuity was summarized as the pure tone average across 500-4000 Hz in the better ear. Participants’ pure tone average ranged from −5.0 to 53.75 dB (M=15.0, sd=11.92; Table 1). Each participant’s pure tone average was included as a covariate in the behavioral analyses.
Data Analysis
Behavioral measures of auditory attention
Overall, across all trials, PWS were slower and less accurate than a neurotypical control group (Supplementary Table 1). Our analyses therefore focus on accuracy as attention measures derived from accuracy difference scores differentiated PWS from a neurotypical control group (Supplementary Table 2), while attention measures from reaction time difference scores did not. Logistic regression was used to assess the effects of alerting, orienting, and executive control within accuracy using PROC GENMOD in SAS software, Version 9.4. The fixed effects were target congruency (congruent, incongruent, neutral) and cue (no cue, double cue, center cue, spatial cue). The dependent variable was response accuracy (correct vs. incorrect). Age, hearing, and auditory single word comprehension (Table 1) were included as covariates in the model. Pairwise comparisons were specified a priori for the alerting (double cue vs. no cue), orienting (spatial cue vs. center cue), and executive control effects (incongruent trial vs. congruent trial). Multiple comparisons were corrected for using the Bonferroni correction (p=.05/3=.017).
ROI-based lesion symptom mapping
Twenty-three PWS (Figure 1) additionally underwent MRI scanning on a 3T Phillips Ingenia MRI scanner equipped with a 32 channel radiofrequency head coil located at the Keller Center for Imaging Innovation at the Barrow Neurological Institute in Phoenix, Arizona. The remaining five participants (AZ1013, AZ1035, AZ1036, AZ1042, AZ1046) were excluded due to scanning contraindications. A T1 image (FOV = 270 × 252, TR = 6.7, flip angle = 9, voxel size = 1 × 1 × 1 mm) was collected for the present study’s lesion analyses in addition to other imaging collected for other studies. Lesion mapping procedures have previously been reported in LaCroix, Blumenstein, et al., (2020) and are briefly described here: Lesion maps were first demarcated on the T1 image in MRIcron (Rorden & Brett, 2000), then smoothed with a 3mm full-width half maximum Gaussian kernel before enantiomorphic normalization (Nachev et al., 2008) was conducted in SPM12 using the procedures developed by Rorden et al., (2012 ) (i.e., NiiStat’s “nii_harvest”). Each lesion map was then transformed into standard space using SPM12's unified segmentation-normalization procedure (Ashburner & Friston, 2005). The normalized lesion maps were then binarized using a 50% probability threshold.
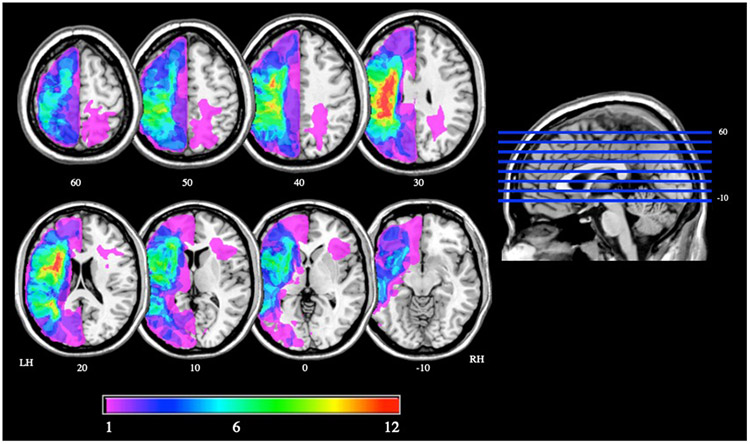
Figure 1.
Lesion overlap map for 23 participants with MRI scans who were included in the LSM analyses. Color bar denotes the number of participants with a lesion to each brain region. LH=left hemisphere; RH=right hemisphere.
An exploratory ROI-based lesion symptom mapping (LSM) analysis was conducted to identify regions of interest (ROI) within the left hemisphere that when damaged result in significantly poorer alerting (double – no cue), orienting (spatial – center cue), or executive control attention (incongruent – congruent). ROIs were based on the Johns Hopkins University (JHU) atlas (Faria et al., 2012). The proportion of damage in each ROI was computed for each participant and entered into separate general linear models that predicted the three measures of attention; results are output as z-scores (Findlater et al., 2016; Rorden et al., 2007). Lesion volume was included in the analyses as a covariate. Multiple comparisons were controlled for using a permutation method in which the data were permutated 3000 times (Kimberg et al., 2007). This approach allowed us to establish the permutation threshold of p=.05, which we refer to as p=.05 corrected below. The LSM results should be interpreted with caution due to the relatively small sample size which may lead to inadequate power (Lorca-Puls et al., 2018).
Results
Behavioral measures of auditory attention
Alerting, orienting, and executive control are known to be independent subtypes of attention (Fan et al., 2002; Petersen & Posner, 2012; Posner & Petersen, 1990). Therefore, we focus on the pairwise comparisons specified a priori here. However, full model results are reported in Table 2. The alerting effect was not significant (χ2(1)=0.09, p=.76, odds ratio=1.04; no cue: M=.88, sd=.18; double cue: M=.88, sd=.17). Both the orienting (χ2(1)=5.81, p=.016, odds ratio=1.29) and executive control (χ2(1)=14.87, p<.001, odds ratio=4.04) effects were significant and both survived Bonferroni correction (p<.017). The executive control effect was as expected: PWS demonstrated poorer accuracy on incongruent trials (M=.79, sd=.23) compared to congruent trials (M=.93, sd=.15). The orienting effect was in the opposite direction to what was expected: PWS were less accurate on the spatial orienting cue (M=.87, sd=.17) than the center cue (M=.89, sd=.17). Interestingly, the congruency x cue interaction was also significant (Table 2) and indicated that PWS benefited equally from the spatial and center cues for a congruent trial (z=1.87, p=.06, odds ratio=1.42; congruent spatial cue: M=.92, sd=.16; congruent center cue: M=.94, sd=.14), but were less accurate on the spatial orienting cue than the center cue for an incongruent trial (z=2.99, p=.003, odds ratio=1.47; incongruent spatial cue: M=.76, sd=.25; incongruent center cue: M=.82, sd=.23).
Table 2.
Full logistic regression model results.
| Statistic | |
|---|---|
| Congruency | χ2(2)=15.08, p<.001* |
| Cue | χ2(3)=6.56, p=.09 |
| Congruency*Cue | χ2(6)=13.46, p=.04* |
| Age | χ2(1)=0.14, p=.71 |
| Hearing (PTA better ear) | χ2(1)=1.02, p=.31 |
| Single Word Comprehension | χ2(1)=0.50, p=.48 |
Significant at p<.05
ROI-based lesion symptom mapping
The LSM analyses identified a significant relationship between alerting attention and damage to the left retrolenticular part of the internal capsule (z=2.94, p=.05 corrected; Figure 2A): PWS with poorer alerting attention (i.e., decreased accuracy for double cue trials compared to no cue trials) had damage to the left retrolenticular part of the internal capsule after controlling for lesion volume. The LSM analysis for orienting attention identified the left posterior middle frontal gyrus (pMFG) as significant (z=3.32, p=.05 corrected; Figure 2A): PWS with poorer orienting attention (i.e., decreased accuracy for spatially cued trials compared to center cued trials) had damage to the left pMFG. However, this result should be interpreted with caution as it does not survive correction for lesion volume. The LSM for executive control did not identify any significant ROIs.
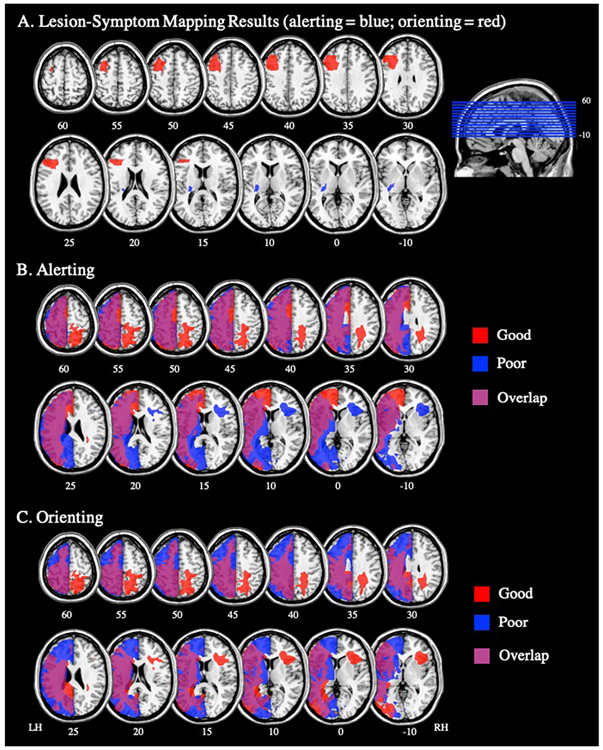
Figure 2.
(A) Left pMFG ROI (red) and left retrolenticular part of the internal capsule ROI (blue). (B) Lesion overlap map for PWS with good alerting attention (red; n=10) and poor alerting attention (blue; n=13). (C) Lesion overlap map for PWS with good orienting attention (red; n=10) and poor orienting attention (blue; n=13). LH=left hemisphere; RH=right hemisphere.
To further explore the LSM results, we identified PWS with good and poor alerting and orienting attention (Table 3). PWS were classified as having poor alerting or orienting attention if they demonstrated the opposite effect to what was expected (i.e., alerting: decreased accuracy for double cued trials compared to no cue trials; orienting: decreased accuracy for spatially cued trials compared to center cued trials). We then generated lesion overlap maps for PWS with good and poor alerting, and good and poor orienting, respectively (Figure 2B,C). Our visual inspection of these overlap maps supported the LSM results. For alerting attention, PWS with poor alerting attention, but not PWS with good alerting, had lesions that included subcortical nuclei and white matter tracts including the left retrolenticular part of the internal capsule (Figure 2B; blue). For orienting attention, those with poor orienting attention, but not those with good orienting, had lesions that included the left frontal cortices including the left pMFG (Figure 2C; blue).
Table 3.
Alerting and orienting accuracy for PWS included in the LSM analyses.
| Participant | Alerting Accuracy (double cue – no cue) |
Orienting Accuracy (spatial cue – center cue) |
|---|---|---|
| AZ1001 | 0 | 0 |
| AZ1003 | 0.05 | 0.04 |
| AZ1006 | −0.13* | −0.13* |
| AZ1011 | 0.02 | 0.04 |
| AZ1012 | 0.03 | −0.04* |
| AZ1016 | 0.14 | −0.10* |
| AZ1018 | 0 | 0 |
| AZ1022 | −0.03* | −0.11* |
| AZ1026 | 0 | −0.02* |
| AZ1028 | −0.02* | −0.07* |
| AZ1029 | −0.06* | 0 |
| AZ1030 | 0.12 | −0.01* |
| AZ1031 | −0.06* | −0.02* |
| AZ1032 | −0.02* | 0.05 |
| AZ1033 | −0.07* | −0.09* |
| AZ1034 | −0.02* | 0.02 |
| AZ1037 | 0.02 | 0 |
| AZ1038 | −0.01* | −0.06* |
| AZ1039 | 0.02 | −0.09* |
| AZ1040 | −0.09* | 0.04 |
| AZ1041 | −0.07* | 0 |
| AZ1043 | 0.02 | 0 |
| AZ1045 | 0 | 0 |
PWS with poor alerting or orienting attention.
Discussion
Auditory attention is not well-characterized in PWS despite attention being a fundamental resource for successful auditory language abilities (LaCroix, Blumenstein, et al., 2020; Murray, 2012; Villard & Kiran, 2017). In the present study, we measured three distinct subtypes of attention in PWS using an auditory ANT. Our results indicate that PWS demonstrate the expected executive control effects (i.e., poorer performance on incongruent trials compared to congruent trials), but that their auditory alerting and orienting attention abilities are disrupted. The implications of these findings are discussed below.
PWS did not demonstrate the expected alerting effect (i.e., more accurate responses following a double cued compared to a non-cued trial). This finding corresponds to previous work demonstrating that persons with aphasia have reduced auditory alerting (or vigilance) compared to neurotypical controls (Erickson et al., 1996; Laures, 2005). The lack of an auditory alerting effect also corresponds to our previous work using the ANT to measure alerting attention in persons with aphasia in the visual modality (LaCroix, Tully, et al., 2020). Persons with left hemisphere stroke may demonstrate reduced alerting attention (in both the visual and auditory domains) due to a disruption in the norepinephrine system that is known to support alerting attention (Oberlin et al., 2005; Petersen & Posner, 2012; Samuels & Szabadi, 2008; Schwarz & Luo, 2015). Norepinephrine is produced in the locus coeruleus with major projections to the thalamus (Samuels & Szabadi, 2008; Schwarz & Luo, 2015), and the thalamus’ role in arousal and vigilance is well established (Fan et al., 2005; Petersen & Posner, 2012; Rinne et al., 2013; Sturm & Willmes, 2001). While we do not find a relationship between thalamic damage and alerting attention in the present study, we do find damage to the left retrolenticular part of the internal capsule to be associated with reduced auditory alerting attention. Notably, of the 14 PWS with damage to this ROI, nine were classified as having poor alerting attention (i.e., decreased accuracy for double cue vs. no cue trials). Given known projections between the retrolenticular portion of the internal capsule and the thalamus (Moini & Piran, 2020; Wiegell et al., 2003), it is possible that structural damage to the retrolenticular part of the internal capsule may impact the function of the thalamus, possibly leading to reduced alerting attention.
The orienting effect (i.e., spatial cue compared to center cue) was significant, however, in the opposite direction to what was expected. PWS were less accurate when cued with the spatial orienting cue compared to the center cue. This finding corresponds to similar results in visual spatial orienting attention in persons with aphasia: persons with aphasia trended towards being less accurate on spatially cued trials compared to center cued trials (LaCroix, Tully, et al., 2020). Previous work studying spatial orienting attention in neurotypical adults and other clinical populations has associated orienting attention with the frontal eye fields (FEF) in both the visual and auditory modalities (Corbetta, 1998; Fan et al., 2005; Garg et al., 2007; Mesulam, 1981). The FEF are located in Broadmann area 8, which is anatomically located at the posterior portion of the MFG and anterior portion of the precentral gyrus (Vernet et al., 2014). While the LSM analysis associating left pMFG damage with poorer auditory spatial orienting attention should be interpreted with caution, it is likely that the left pMFG, particularly the portion overlapping with the FEF, has some role in auditory spatial orienting attention and further indicates that the FEF have multi-modal control over spatial orienting attention.
In addition to the spatial orienting effect, we also found a cue x congruency interaction: PWS were less accurate on the spatially cued trials compared to the center cued trials for incongruent trials only, not congruent trials. This interaction is notable as it perhaps speaks to the relationship between the resources supporting orienting and executive control. The ANT contains two types of information that must be processed for successful task completion: cues and targets. Overall, human attention is known to have limited capacity (Kahneman, 1973) suggesting some shared neural substrates across different types of attention. PWS are also known to demonstrate an overall greater difficulty allocating attention resources than neurotypical controls (e.g., Hula & McNeil, 2008). Thus, we suggest that the combination of a spatial cue plus an incongruent target results in many PWS exceeding their shared attention resource capacity as the spatial cue utilizes a greater amount of shared attentional resources than a center cue, which results in less resources being available to process the incongruent target. This same pattern is not observed within congruent trials as the congruent trial requires less attention resources for successful completion than the incongruent trials, thus overall attention allocation remains below the participant’s resource capacity, even when a congruent trial is preceded by a spatial cue. This interaction is not present between alerting and executive control, suggesting some shared resources specifically for orienting and executive control in the left hemisphere that are damaged in our PWS sample.
In summary, we used an auditory version of the ANT to assess the efficiency of alerting, orienting, and executive control attention in PWS in the auditory modality; a topic that is largely understudied in this clinical population despite auditory attention being a fundamental component of communication, particularly auditory language abilities (LaCroix, Blumenstein, et al., 2020; Murray, 2012; Villard & Kiran, 2017). Here we find the expected executive control effects on the auditory Stroop component of the ANT, but that PWS demonstrate impaired auditory alerting and auditory spatial orienting attention. Our findings of disrupted auditory alerting and spatial orienting attention largely correspond to previous results in persons with aphasia (LaCroix, Tully, et al., 2020), suggesting that these attentional processes may be supported by multi-modal neural resources. Along these lines, an exploratory LSM analysis identified PWS with poorer auditory alerting and spatial orienting attention to have damage to the left retrolenticular portion of the internal capsule (located next to the thalamus, which has previously been associated with visual alerting attention) and left pMFG (which overlaps with the FEF and is associated with visual and auditory spatial orienting attention), respectively. While future work is certainly needed to further characterize auditory attention abilities and their associated neurobiology in PWS, the results from the present study suggest that auditory alerting and spatial orienting attention are impaired in PWS due to stroke lesions damaging multi-modal neural resources.
Supplementary Material
Acknowledgments
This work was supported by NIH DC009659 (PI: G. Hickok) and the American Heart Association pre-doctoral fellowship #18PRE33990328 (A. LaCroix).
Footnotes
One participant had two strokes ten years apart (AZ1033) and two other participants report a single stroke, but a bilateral lesion was evident on their MRI scans (AZ1001, AZ1040).
Disclosure Statement
The authors report no potential conflict of interest.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (A.L.), upon reasonable request.
References
- Alho K, Rinne T, Herron TJ, & Woods DL (2014). Stimulus-dependent activations and attention-related modulations in the auditory cortex: A meta-analysis of fMRI studies. Hearing Research, 307, 29–41. 10.1016/j.heares.2013.08.001 [DOI] [PubMed] [Google Scholar]
- Anderson JS, Ferguson MA, Lopez-Larson M, & Yurgelun-Todd D (2010). Topographic maps of multisensory attention. Proceedings of the National Academy of Sciences, 107(46), 20110–20114. 10.1073/pnas.1011616107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, & Friston KJ (2005). Unified segmentation. NeuroImage, 26(3), 839–851. 10.1016/j.neuroimage.2005.02.018 [DOI] [PubMed] [Google Scholar]
- Corbetta M (1998). Frontoparietal cortical networks for directing attention and the eye to visual locations: Identical, independent, or overlapping neural systems? Proceedings of the National Academy of Sciences, 95(3), 831–838. 10.1073/pnas.95.3.831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, & Petersen SE (2008). A dual-networks architecture of top-down control. Trends in Cognitive Sciences, 12(3), 99–105. 10.1016/j.tics.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson RJ, Goldinger SD, & LaPointe LL (1996). Auditory Vigilance in Aphasic Individuals: Detecting Nonlinguistic Stimuli with Full or Divided Attention. Brain and Cognition, 30(2), 244–253. 10.1006/brcg.1996.0016 [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossela J, Flombaum J, & Posner M (2005). The activation of attentional networks. NeuroImage, 26, 471–479. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, & Posner MI (2002). Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience, 14(3), 340–347. [DOI] [PubMed] [Google Scholar]
- Fan J, & Posner M (2004). Human Attentional Networks. Psychiatrische Praxis, 31, 210–214. 10.1055/s-2004-828484 [DOI] [PubMed] [Google Scholar]
- Faria AV, Joel SE, Zhang Y, Oishi K, van Zjil PCM, Miller MI, Pekar JJ, & Mori S (2012). Atlas-Based Analysis of Resting-State Functional Connectivity: Evaluation for Reproducibility and Multi-Modal Anatomy-Function Correlation Studies. Neuroimage, 61(3), 613–621. 10.1016/j.neuroimage.2012.03.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlater SE, Desai JA, Semrau JA, Kenzie JM, Rorden C, Herter TM, Scott SH, & Dukelow SP (2016). Central perception of position sense involves a distributed neural network – Evidence from lesion-behavior analyses. Cortex, 79, 42–56. 10.1016/j.cortex.2016.03.008 [DOI] [PubMed] [Google Scholar]
- Fritz JB, Elhilali M, David SV, & Shamma SA (2007). Auditory attention—Focusing the searchlight on sound. Current Opinion in Neurobiology, 17(4), 437–455. 10.1016/j.conb.2007.07.011 [DOI] [PubMed] [Google Scholar]
- Garg A, Schwartz D, & Stevens AA (2007). Orienting Auditory Spatial Attention Engages Frontal Eye Fields and Medial Occipital Cortex in Congenitally Blind Humans. Neuropsychologia, 45(10), 2307–2321. 10.1016/j.neuropsychologia.2007.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E, & Barresi B (2000). Boston Diagnostic Aphasia Examination Record Booklet. Lippincott Williams & Wilkins. [Google Scholar]
- Green DW, Grogan A, Crinion J, Ali N, Sutton C, & Price CJ (2010). Language control and parallel recovery of language in individuals with aphasia. Aphasiology, 24(2), 188–209. 10.1080/02687030902958316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hula WD, & McNeil MR (2008). Models of Attention and Dual-Task Performance as Explanatory Constructs in Aphasia. Seminars in Speech and Language, 29(3), 169–187. 10.1055/s-0028-1082882 [DOI] [PubMed] [Google Scholar]
- Kahneman D (1973). Attention and Effort (Vol. 1063). Prentice-Hall. [Google Scholar]
- Kimberg DY, Coslett HB, & Schwartz MF (2007). Power in Voxel-based Lesion-Symptom Mapping. Journal of Cognitive Neuroscience, 19(7), 1067–1080. 10.1162/jocn.2007.19.7.1067 [DOI] [PubMed] [Google Scholar]
- Kong L, Michalka SW, Rosen ML, Sheremata SL, Swisher JD, Shinn-Cunningham BG, & Somers DC (2014). Auditory Spatial Attention Representations in the Human Cerebral Cortex. Cerebral Cortex (New York, NY), 24(3), 773–784. 10.1093/cercor/bhs359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmina E, & Weekes BS (2017). Role of cognitive control in language deficits in different types of aphasia. Aphasiology, 31(7), 765–792. 10.1080/02687038.2016.1263383 [DOI] [Google Scholar]
- LaCroix AN, Blumenstein N, Tully M, Baxter LC, & Rogalsky C (2020). Effects of prosody on the cognitive and neural resources supporting sentence comprehension: A behavioral and lesion-symptom mapping study. Brain and Language, 203, 104756. 10.1016/j.bandl.2020.104756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCroix AN, Tully M, & Rogalsky C (2020). Assessment of alerting, orienting, and executive control in persons with aphasia using the Attention Network Test. Aphasiology. 10.1080/02687038.2020.1795077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laures JS (2005). Reaction time and accuracy in individuals with aphasia during auditory vigilance tasks. Brain and Language, 95(2), 353–357. 10.1016/j.bandl.2005.01.011 [DOI] [PubMed] [Google Scholar]
- Lee B, & Pyun S-B (2014). Characteristics of Cognitive Impairment in Patients With Post-stroke Aphasia. Annals of Rehabilitation Medicine, 38(6), 759–765. 10.5535/arm.2014.38.6.759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorca-Puls DL, Gajardo-Vidal A, White J, Seghier ML, Leff AP, Green DW, Crinion JT, Ludersdorfer P, Hope TMH, Bowman H, & Price CJ (2018). The impact of sample size on the reproducibility of voxel-based lesion-deficit mappings. Neuropsychologia, 115, 101–111. 10.1016/j.neuropsychologia.2018.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M (1981). A Cortical Network for Directed Attention and Unilateral Neglect. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 10(4), 309–325. [DOI] [PubMed] [Google Scholar]
- Mirsky AF, Anthony BJ, Duncan CC, Ahearn MB, & Kellam SG (1991). Analysis of the Elements of Attention: A Neuropsychological Approach. Neuropsychology Review, 2(2), 109–145. [DOI] [PubMed] [Google Scholar]
- Moini J, & Piran P (2020). Chapter 8 - Diencephalon: Thalamus and hypothalamus. In Moini J & Piran P (Eds.), Functional and Clinical Neuroanatomy (pp. 267–292). Academic Press. 10.1016/B978-0-12-817424-1.00008-2 [DOI] [Google Scholar]
- Murray LL (2012). Attention and Other Cognitive Deficits in Aphasia: Presence and Relation to Language and Communication Measures. American Journal of Speech-Language Pathology, 21(2), S51–S64. 10.1044/1058-0360(2012/11-0067) [DOI] [PubMed] [Google Scholar]
- Nachev P, Coulthard E, Jäger HR, Kennard C, & Husain M (2008). Enantiomorphic normalization of focally lesioned brains. NeuroImage, 39(3), 1215–1226. 10.1016/j.neuroimage.2007.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin B, Alford J, & Marrocco R (2005). Normal attention orienting but abnormal stimulus alerting and conflict effect in combined subtype of ADHD. Behavioural Brain Research, 165(1), 1–11. 10.1016/j.bbr.2005.06.041 [DOI] [PubMed] [Google Scholar]
- Petersen SE, & Posner MI (2012). The Attention System of the Human Brain: 20 Years After. Annual Review of Neuroscience, 35, 73–89. 10.1146/annurev-neuro-062111-150525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompon RH, McNeil MR, Spencer KA, & Kendall DL (2015). Intentional and Reactive Inhibition During Spoken-Word Stroop Task Performance in People With Aphasia. Journal of Speech, Language, and Hearing Research: JSLHR, 58(3), 767–780. 10.1044/2015_JSLHR-L-14-0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, & Petersen SE (1990). The attention system of the human brain. Annual Review of Neuroscience, 13(1), 25–42. [DOI] [PubMed] [Google Scholar]
- Rinne P, Hassan M, Goniotakis D, Chohan K, Sharma P, Langdon D, Soto D, & Bentley P (2013). Triple dissociation of attention networks in stroke according to lesion location. Neurology, 81(9), 812–820. 10.1212/WNL.0b013e3182a2ca34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts KL, Summerfield AQ, & Hall DA (2006). Presentation modality influences behavioral measures of alerting, orienting, and executive control. Journal of the International Neuropsychological Society, 12(04). 10.1017/S1355617706060620 [DOI] [PubMed] [Google Scholar]
- Rorden Chris, & Brett M (2000). Stereotaxic display of brain lesions. Behavioural Neurology, 12(4), 191–200. [DOI] [PubMed] [Google Scholar]
- Rorden Chris, Karnath H-O, & Bonilha L (2007). Improving Lesion-Symptom Mapping. Journal of Cognitive Neuroscience, 19(7), 1081–1088. 10.1162/jocn.2007.19.7.1081 [DOI] [PubMed] [Google Scholar]
- Rorden Christopher, Bonilha L, Fridriksson J, Bender B, & Karnath H-O (2012). Age-specific CT and MRI templates for spatial normalization. Neuroimage, 61(4), 957–965. 10.1016/j.neuroimage.2012.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels ER, & Szabadi E (2008). Functional Neuroanatomy of the Noradrenergic Locus Coeruleus: Its Roles in the Regulation of Arousal and Autonomic Function Part I: Principles of Functional Organisation. Current Neuropharmacology, 6(3), 235–253. 10.2174/157015908785777229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz LA, & Luo L (2015). Organization of the Locus Coeruleus-Norepinephrine System. Current Biology, 25(21), R1051–R1056. 10.1016/j.cub.2015.09.039 [DOI] [PubMed] [Google Scholar]
- Sohlberg MM, & Mateer CA (2010). APT-III: Attention process training: A direct attention training program for persons with acquired brain injury. Lash & Associates. [Google Scholar]
- Sturm W, & Willmes K (2001). On the Functional Neuroanatomy of Intrinsic and Phasic Alertness. NeuroImage, 14(1), S76–S84. 10.1006/nimg.2001.0839 [DOI] [PubMed] [Google Scholar]
- Thiel CM, & Fink GR (2007). Visual and Auditory Alertness: Modality-Specific and Supramodal Neural Mechanisms and Their Modulation by Nicotine. Journal of Neurophysiology, 97(4), 2758–2768. 10.1152/jn.00017.2007 [DOI] [PubMed] [Google Scholar]
- Vernet M, Quentin R, Chanes L, Mitsumasu A, & Valero-Cabré A (2014). Frontal eye field, where art thou? Anatomy, function, and non-invasive manipulation of frontal regions involved in eye movements and associated cognitive operations. Frontiers in Integrative Neuroscience, 8. 10.3389/fnint.2014.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villard S, & Kiran S (2017). To what extent does attention underlie language in aphasia? Aphasiology, 31(10), 1226–1245. 10.1080/02687038.2016.1242711 [DOI] [Google Scholar]
- Wiegell MR, Tuch DS, Larsson CHBW, & A VJW (2003). Automatic segmentation of thalamic nuclei from diffusion tensor magnetic resonance imaging. NeuroImage, 19(2), 391–401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (A.L.), upon reasonable request.