Abstract
Aim:
The aim of this study was to compare the efficacy of NovaMin™ (SHY-NM) and casein phosphopeptide-amorphous calcium phosphate fluoride (CPP-ACPF) (tooth mousse plus™) on enamel remineralization using DIAGNOdent® and scanning electron microscope (SEM).
Methodology:
Eighty-six natural permanent maxillary first premolars were selected and randomly divided into two groups of 43 each, Group A (NovaMin™) and Group B (CPP-ACPF). All the samples were assessed using DIAGNOdent® (KaVo) at the baseline, after demineralization, and remineralization after 7 days. Two samples were randomly selected from each group after remineralization to evaluate the surface changes using SEM at × 1000 and × 2000.
Results:
The mean value of remineralization was highest for Group A NovaMin™ (6.56 ± 0.93) compared to Group B, CPP-ACPF (tooth mousse plus™) (6.02 ± 1.09). The maximum demineralization to remineralization value within the groups showed that the mean values in Group B CPP-ACPF (7.02 ± 3.02) was higher than Group A NovaMin™ (6.42 ± 2.21). The difference in remineralizing potential between the groups and demineralization to remineralization value in within-group comparison was not found to be statistically significant.
Conclusion:
On comparing Group A NovaMin™ and Group B CPP-ACPF, Group B CPP-ACPF showed a higher amount of remineralization than Group A NovaMin™. From the present study, it can be inferred that both the experimental groups have the potential for remineralization.
Keywords: Casein phosphopeptide-amorphous calcium phosphate fluoride, Diagnodent®, NovaMin™, remineralization, scanning electron microscope
Introduction
Enamel remineralization has been studied for about 100 years, and it has been suggested that “the noninvasive treatment of early caries lesions by remineralization has the potential to be the major advancement in the clinical management of the disease.” Several remineralization techniques have been tried out in dentistry, among which the use of fluoride is well known. Some of the latest techniques involve the use of casein phosphopeptide stabilized-amorphous calcium phosphate (CPP-ACP; Recaldent™), unstabilized (ACP, Enamelon™) and a bioactive glass-containing calcium sodium phosphosilicate (NovaMin™).[1]
Milk and milk products such as cheese have been shown to exhibit anti-cariogenic properties in human and animal models.[2] Tooth mousse is a commercially available paste formula based on the nano complex of milk protein CPP with ACP. It exhibits anti-caries effect by suppressing demineralization and enhancing remineralization.[3] Tooth mousse plus (CPP-ACP fluoride [ACP-F]) is CPP-ACP to which 900 ppm of fluoride ions have been incorporated. The fluoride ions are released in addition to calcium and phosphate ions resulting in the formation of acid-resistant fluorapatite.[4,5]
NovaMin™ is a particulate bioactive glass composed of calcium, phosphorous, sodium, and silica (45% Sio2, 24.5% Na2O, 24.5%CaO, and 6%P2O5) which takes the chemical form of sodium phospho silicate. When the NovaMin™ particles come in contact with saliva and water, it reacts and releases calcium and phosphate ions, which binds to the tooth surface.[6,7]
With the advent of new remineralizing therapies and new conservative approach to restoring carious lesions, interest in detecting and monitoring subclinical precavitated lesions has increased. Some of the noninvasive methods available are Diagnodent and scanning electron microscope (SEM) DIAGNOdent® (KaVo), a noninvasive method, uses laser fluorescence (LF) to measure early demineralization.[8]
Although remineralization has been a major area of investigation, it is still difficult to exactly define the efficacy of various remineralization methods. Hence, the aim of this study was to compare the efficacy of two newer dentifrices, namely NovaMin™ and CPP-ACPF on enamel surface that has been exposed to an artificial caries challenge in a simulated oral environment using DIAGNOdent® and SEM The null hypothesis is that there is no difference in remineralization potential between NovaMin™ and CPP-ACPF.
Methodology
Eighty-six natural permanent maxillary first premolars extracted for therapeutic purposes were collected and stored in normal saline after thorough washing and removal of soft tissue from the crown and root surface. All the teeth were examined, and the teeth with caries, white spot lesions, erosion, or cracks were discarded. The teeth were then thoroughly cleaned of its debris, calculus, and soft tissues. All the teeth were sliced mesiodistally into buccal and lingual halves using a diamond disk bur.
The buccal halves of the sliced teeth were used for the study, taking into consideration the technique sensitivity of DIAGNOdent® (KaVo) and the ease of mounting the sample on a SEM. A white stick on paper of 3 mm × 3 mm dimension was stuck on each tooth surface to limit the area of the study. Water-resistant clear nail varnish was applied to the tooth surface to limit the area of study [Figure 1].
Figure 1.
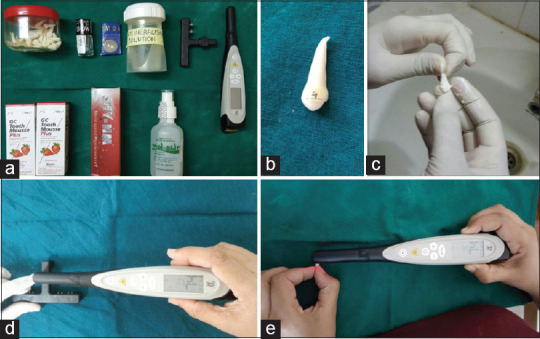
Study procedures (a: Armamentarium b: Study specimen c: Application of Remineralizing agent d: DIAGNOdent® Calibration e: DIAGNOdent® measurement)
All the samples were examined using DIAGNOdent® (KaVo) to assess for any surface changes.[4] As recommended by the manufacturer, before every measurement session, the instrument was calibrated against its own ceramic standard.
Type B probe of DIAGNOdent® for measuring the smooth surface lesions was used. Moment values between 3 and 7 on the digital display, indicating intact enamel surface, were selected for the study.[7]
The teeth were randomly divided into two groups containing 43 samples each and color-coded based on the remineralizing agents used. Group A (green) allotted to Novamine™ (SHY-NM) and Group B (Red) allotted to CPP-ACPF (GC tooth mousse plus)
The baseline values of the two groups were recorded by DIAGNOdent® using type B probe by holding the tip in close contact with the tooth surface and tilting the tip around the measuring site to measure the fluorescence in all directions. The teeth were immersed individually into separate plastic containers which were color coded based on the remineralizing agents used and numbered from 1 to 86, each containing 4 ml of demineralizing solution (buffer solution of pH (4.0 ± 0.05) which was prepared by dissolving each buffer capsule in 100 ml of deionized water followed by stirring until the powder got completely dissolved. A litmus paper was used to check the color change indicating a pH of 4. The teeth were kept in the demineralizing solution for a period of 10 h until the enamel surface was completely demineralized, thus producing a consistent subsurface lesion. After 10 h in the demineralizing solution the teeth were taken out, washed with deionized water, dried, and placed back in their respective clean containers.
All the teeth were evaluated using DIAGNOdent® and the samples showing a moment value of 9 and above on the digital display were taken indicating subsurface demineralization of the tooth surface.[4] The process was repeated until the samples were indicative of demineralization. All the readings suggestive of demineralization > 9 were recorded.
After demineralization and drying, all the teeth were kept back into their respective containers, which were filled with 6 ml of artificial saliva. Each sample in Group A was rubbed with NovaMin™ (SHY-NM) paste twice a day for 4 min using a gloved finger over the demineralized surface. The remineralizing paste was then removed from the tooth surface by thoroughly washing with deionized water. The samples were then dried and placed back into their respective containers containing artificial saliva at room temperature (37°C).
Each sample in Group B was rubbed with tooth mousse plus (CPP-ACPF) paste twice a day for 4 min using a gloved finger over the demineralized surface. The remineralizing paste was then removed from the tooth surface by thoroughly washing with deionized water. The samples were then dried and placed back into their respective containers containing artificial saliva at room temperature (37°C). This remineralization process was repeated for 1 week to induce remineralization of the enamel surface.
All the teeth surfaces were assessed using DIAGNOdent® to record the values after the remineralization procedure. Postremineralization surface changes on the enamel were assessed using an SEM (Quanta2000). To compare the enamel surface remineralization 2 samples from each group were randomly selected and compared with each other using an SEM, and pictures were taken at × 1000 and × 2000.
Statistical analysis
Statistical analysis was performed using SPSS Statistics for Windows, version 10.5 (SPSS Inc., Chicago, Ill., USA) statistical software. Descriptive statistics were expressed as mean and standard deviation. For comparing the remineralizing capacity between the groups, the Student's t-test was employed to test the statistical significance. A value of P < 0.05 was considered to be statistically significant.
Results
The results showed that before demineralization sample readings showed DIAGNOdent® values between 3 and 7, indicating normal tooth structure. After demineralization, the samples reading showed scores > 9 and after application of the remineralizing agents, reading showed scores < 9. A significant difference was seen after the application of remineralizing agents [Table 1].
Table 1.
Descriptive statistics of demineralization, remineralization, and demineralization to remineralization using DIAGNOdent® values
| Mineralization | Product used | n | Mean | SD | SE | 95% CI for mean |
Minimum | Maximum | |
|---|---|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||||||
| Baseline | CPP-ACPF NovaMin | 86 | 4.13 | 1.381 | 0.149 | 3.30 | 5.03 | 3 | 7 |
| Demineralization | CPP-ACPF | 43 | 13.09 | 3.138 | 0.479 | 12.13 | 14.06 | 9 | 22 |
| NovaMin | 43 | 12.93 | 2.806 | 0.428 | 12.07 | 13.79 | 10 | 22 | |
| Remineralization | CPP-ACPF | 43 | 6.02 | 0.938 | 0.143 | 5.73 | 6.31 | 4 | 8 |
| NovaMin | 43 | 6.56 | 1.098 | 0.167 | 6.22 | 6.90 | 4 | 8 | |
| Demineralization-remineralization | CPP-ACPF | 43 | 7.02 | 3.028 | 0.462 | 6.09 | 7.96 | 3 | 16 |
| NovaMin | 43 | 6.42 | 2.217 | 0.338 | 5.74 | 7.10 | 3 | 14 | |
CPP: Casein phosphopeptide; ACPF: Amorphous calcium phosphate fluoride; SD: Standard deviation; SE: Standard error; CI: Confidence interval
The mean value at demineralization was highest for Group B CPP-ACPF (13.09 ± 3.13) and then Group A NovaMin™ (12.93 ± 2.08). There was no statistical difference between the groups. This shows that the demineralization solution that was used for the study produced uniform artificial carious lesions. The mean value at remineralization was highest for Group B, CPP-ACPF (6.02 ± 0.93), followed by Group A NovaMin™ (6.56 ± 1.09). The maximum remineralization was seen in Group B, CPP-ACPF followed by Group A NovaMin™. The mean values at demineralization to remineralization for Group B CPP-ACPF was higher than in Group A NovaMin™. However, there was no statistically significant result in the mean value between the two groups [Table 2].
Table 2.
Comparison of means of demineralization, remineralization, and demineralization to remineralization between the groups using DIAGNOdent® values
| Mineralization | Groups compared | n | Mean | SD | Mean difference | SE difference | P |
|---|---|---|---|---|---|---|---|
| Demineralization | CPP-ACPF | 43 | 13.09 | 3.138 | 0.163 | 0.642 | 0.800 |
| Novamin | 43 | 12.93 | 2.806 | ||||
| Remineralization | CPP-ACPF | 43 | 6.65 | 1.131 | 0.093 | 0.240 | 0.700 |
| Novamin | 43 | 6.56 | 1.098 | ||||
| Demineralization-remineralization | CPP-ACPF | 43 | 7.02 | 3.028 | 0.605 | 0.572 | 0.294 |
| Novamine | 43 | 6.42 | 2.217 |
CPP: Casein phosphopeptide; ACPF: Amorphous calcium phosphate fluoride; SD: Standard deviation; SE: Standard error
Scanning electron microscope evaluation
SEM photographs taken before demineralization showed normal enamel surface (consistently smooth) in both the groups. SEM photographs taken after 10 h of demineralization showed irregular surface with the loss of some surface enamel in the study groups. Remineralization of demineralized enamel surface is seen after treatment with the remineralizing agents for 7 days in both the groups by randomly selecting samples from each group.
In Group A (NovaMin™): The interprismatic substance is evident with porosities, and areas of remineralization are also evident at × 1000 [Figure 2]. At × 2000 of the same group thick and more frequent lines of remineralization are seen along the prismatic borders. Areas of calcification can also be seen along the porosities [Figure 3].
Figure 2.

Group A (Novamin™) at ×1000
Figure 3.
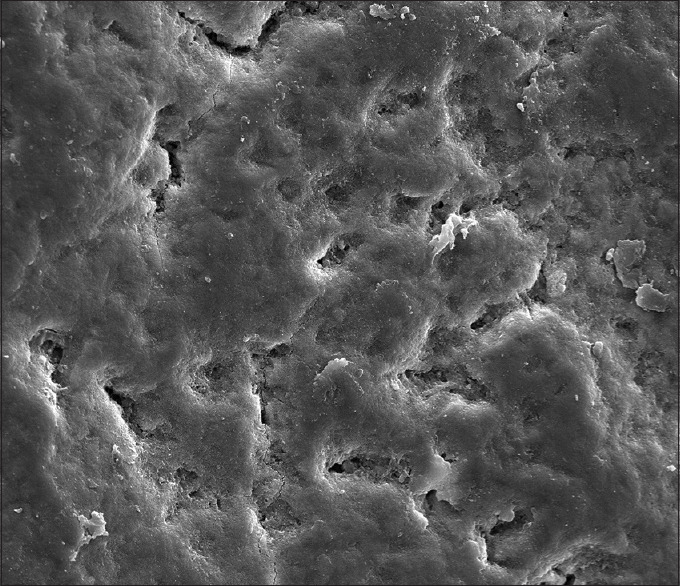
Group A (Novamin™) at ×2000
In Group B (CPP-ACPF): The enamel rods and prismatic substances are not discernible at × 1000, but the areas of calcified deposits are more evident and are concentrated along the porous defects [Figure 4]. At × 2000, areas of calcified deposits are evident and seen scattered along the porous defects. The enamel surface in this group had a maximum remineralization [Figure 5].
Figure 4.
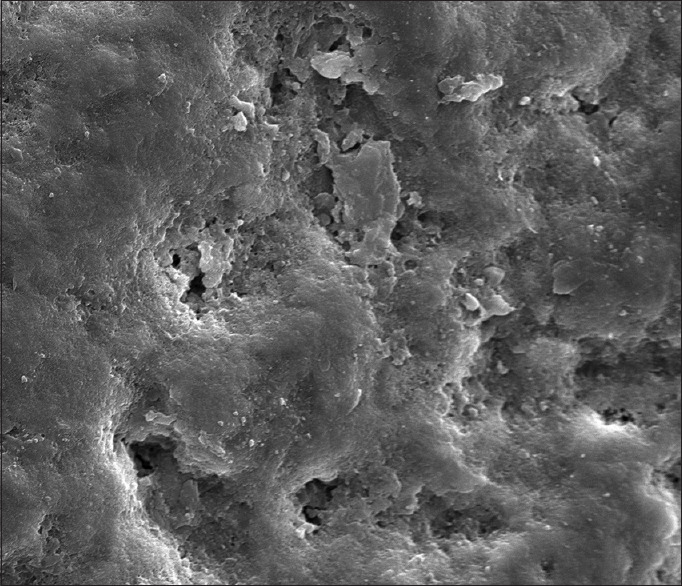
Group B casein phosphopeptide-amorphous calcium phosphate fluoride at ×1000
Figure 5.

Group B casein phosphopeptide-amorphous calcium phosphate fluoride at ×2000
Discussion
Caries is a chronic, slowly progressing disease, with symptoms not detected at the onset of the disease but generally much later. Its initiation is associated with the demineralization of calcium and phosphate loss from the subsurface tooth enamel, resulting in the formation of a subsurface lesion.[9]
Demineralization results from a complex chemistry between the bacteria, diet and salivary components. A drop in the pH in the oral cavity results in demineralization. In the demineralization process, the bacteria produce organic acids that diffuse into the tooth through the water among the hydroxyapatite crystals, which are the major composition of tooth enamel and dentin. When the acid reaches a susceptible site on a crystal surface, where impurities and inclusions of other ions (especially carbonate ion) incorporated in the crystal lattice producing defects and calcium deficient regions, calcium and phosphate are dissolved and transferred into the surrounding aqueous phase between the crystals. If the diffusion of calcium, phosphate, and carbonate out of the tooth can continue without proper remineralization, cavitation will eventually take place.[1]
Remineralization is the body's natural repair process for subsurface noncavitated lesions. This occurs when the pH rises and there is the deposition of calcium, phosphate, and fluoride ions in the form of fluorapatite, which is more resistant to crystal dissolution by organic acids. During remineralization, the growth of newly formed crystals (fluorapatite) takes place, and with advancing growth, the crystals fuse with each other to form large crystals with hexagonal outlines.[10]
Different preventive therapies have been studied to enhance the remineralization, decrease, and arrest carious lesions. Much of the recent studies have focused on the concentration of calcium and phosphate present in the tooth. Since both ions are major components of the tooth and ultimately related to the demineralization of tooth structure, most of the efforts have been directed toward their deposition in the dental structure.[11]
Demineralization can be detected by two methods: (1) invasive and (2) noninvasive. The noninvasive diagnostic methods available are quantitative light fluorescence, electrical resistance monitoring device, fiberoptic transillumination, optical coherence tomography, LF (DIAGNOdent®) and SEM.
LF is a method introduced for early diagnosis of dental caries. It is useful for the early detection of hidden caries in noncavitated teeth through a noninvasive method. The organic and inorganic materials present on the tooth surface absorb the laser light and emit fluorescence in the infrared region (655 nm) of the spectrum. The presence of a demineralized area increases the fluorescence, with an audible sound indicating the fluorescence increase showing various scales between 0 and 99. The higher number of sound and higher-pitched sounds indicate more demineralization. It can reliably detect even the tiniest of the lesions without requiring X-rays.[8]
The exact mechanism of DIAGNOdent® is still not clear, but two theories exist: First, when the red light meets a change in tooth tissue, such as porosity due to demineralization or hypomineralization, it stimulates fluorescent light of a different wavelength. Second, some bacterial metabolites such as porphyrins (proto-porphyrin, meso-porphyrin, or co-porphyrin), result in the red fluorescence of carious teeth.[12]
In the present study, the buccal half of the teeth were used for DIAGNOdent® evaluation to simulate clinical conditions, in which the buccal surface of the teeth is used for caries detection. In the present study, the specimens were kept in the demineralization solution (4 ml of buffer solution having a Ph of 4.5) for 10 h at 37°C created a subsurface demineralization with an intact surface simulating an early enamel lesion.
All the samples were observed using DIAGNOdent® (KaVo) and baseline value, the amount of demineralization and the remineralization values were tabulated. An SEM (Quanta 2000) was used to assess the surface changes seen on the enamel.
The results of the present study showed that on comparing the remineralization value of Group B (CPP-ACPF; 6.02 ± 0.93) and Group A (NovaMin™; 6.56 ± 1.0) to its demineralization value Group B (CPP-ACPF; 13.09 ± 3.13) and Group A NovaMin™; 12.93 ± 2.8) it is evident that a significant amount of remineralization has occurred in both the groups. On comparing Group, A and Group B, the Group B CPP-ACPF (6.02 ± 0.93) showed a higher amount of remineralization compared to Group A NovaMin™ (6.56 ± 1.09). There was no statistically significant difference between the groups.
Although saliva has some remineralizing potential, it cannot by itself increase the levels of calcium and phosphate release. For remineralization to occur within the body of the lesion calcium and phosphate ions must first penetrate the surface calcium phosphate layer of enamel. The ability of CPP-ACPF to remineralize artificial enamel lesions in this study is due to the localization of ACP to tooth surface, which then buffers the free calcium and phosphate ions activity, thereby helping to maintain a state of supersaturation with respect to enamel depressing demineralization and promoting remineralization.[13]
SHY-NM is a “fluoride-free” toothpaste containing nanometric bioactive glass NovaMin™. Fluoride has been shown to have substantial positive effects on preventing tooth decay.[14] There are also negative effects to excessive ingestion of fluoride. Evidence mount that everyday ingestion of fluoride through normal food, beverage, and water intake supplies most or all of the fluoride necessary for good oral health.
When NovaMin particle comes in contact with the saliva and water, it reacts and release calcium and phosphate ions. Na2 ± ions in the NovaMin particle exchange the H ± ions, which allows Ca2 ± and Po4 ions to be released. A calcium phosphate layer is formed, which then crystallizes into hydroxy carbonate apatite resulting in remineralization of teeth.[15]
On comparing the change in the mean values from demineralization to remineralization between the groups, there was no statistically significant result between the groups. Group B CPP-ACPF (7.02 ± 3.02) showed more amount of demineralization to remineralization compared to Group A NovaMin™ (6.42 ± 2.21).
This can be attributed to the synergistic anti-cariogenic effects of CPP-ACP and fluoride. The fluoride ions are adsorbed onto the surface of enamel crystals, inhibiting dissolution and increasing remineralization.[16] With the use of low fluoride concentration as is present in CPP-ACPF (0.9% or 900 ppm), there is a complex localization of free calcium phosphate and fluoride ion activities, which help in maintaining a state of supersaturation by suppressing demineralization. Elsayad reported that the addition of fluoride to CPP-ACP could give a synergistic effect on enamel remineralization.[9]
Calcium sodium phosphosilicate (NovaMin™) is delivered in the form of a solid bioactive glass that must first dissolve before it can be active. CPP-ACPF, on the other hand, are in a dissolved active, noncrystalline form, which accounts for its superior remineralizing capacity.[12]
The results of the present study were similar to the studies done by Ogaard et al.[17] in 1998, Jayarajan et al.,[8] Preethee T et al.[16] in 2011. The studies reported that CPP-ACPF-containing low fluoride concentration (0.2% or 900 ppm of Na F) maintains a state of supersaturation and act as an excellent local delivery system to treat the white spot lesions. Agnihotri et al.,[18] in 2012, noted that when CPP-ACP was used in combination with fluoride, it lowered the caries score and had a better effect on inhibiting the demineralization of sound enamel. The results of the studies revealed that CPP-ACPF showed superior remineralizing ability.
The results of our study contradict to the study done by Narayana et al.[13] in 2014 and Rajan et al.[14] in 2015. The results of the studies concluded that bioactive glass (SHY-NM) showed an increase of calcium, suggesting greater remineralizing properties than fluoride toothpaste and CPP-ACPF (tooth mousse plus). Allan et al.[19] in their study has shown that fine particulate bioactive glass (NovaMin™) incorporated into nonaqueous dentifrice was able to reduce the viability of planktonic bacterial cultures of Streptococcus mutans, Fusobacterium nucleatum, Actinomyces naeslundi, and Streptococcus sanguis. This increase in the bacterial flora is attributed to the increase in the pH of bioactive glass in solution.
Based on the present results and under the current experimental conditions, CPP-ACPF showed superior remineralizing capacity. Furthermore, remineralization is facilitated by the nanometric bioactive glass– NovaMin™ (SHY-NM) and can be comparable with CPP-ACPF.
The major drawback of this study was that it is an in vitro study and did not include organic components such as oral bacteria and plaque. In this study, the application of the remineralizing agent was limited to 1 week. If the same has been extended to a scheduled routine protocol, the amount of remineralization probably could have been more approaching the baseline value.
Conclusion
The results obtained from the present study demonstrated that:
Both Group B CPP-ACPF and Group A NovaMin™ when compared with the demineralization values has shown significant remineralization potential
On comparing Group B CPP-ACPF and Group A NovaMin™, the Group A CPP-ACPF (6.02 ± 0.93) showed a higher amount of remineralization than Group A Novamin™ (6.56 ± 1.09). This is mostly due to the synergistic anticariogenic effects of CPP-ACP and fluoride
Remineralization is facilitated by nanometric bioactive glass NovaMin™ and can be comparable with CPP-ACPF
From the results obtained, it can be inferred that both the experimental groups have the potential for remineralization, and further studies with long follow-up is evident to support this finding.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Reynolds EC. Calcium phosphate-based remineralization systems: Scientific evidence? Aust Dent J. 2008;53:268–7. doi: 10.1111/j.1834-7819.2008.00061.x. [DOI] [PubMed] [Google Scholar]
- 2.Rosen S, Min DB, Harper DS, Harper WJ, Beck EX, Beck FM. Effect of cheese, with and without sucrose, on dental caries and recovery of Streptococcus mutans in rats. J Dent Res. 1984;63:894–6. doi: 10.1177/00220345840630061601. [DOI] [PubMed] [Google Scholar]
- 3.Rahiotis C, Vougiouklakis G. Effect of a CPP-ACP agent on the demineralization and remineralization of dentine in vitro. J Dent. 2007;35:695–8. doi: 10.1016/j.jdent.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds EC. Remineralization of enamel subsurface lesions by casein phosphopeptide-stabilized calcium phosphate solutions. J Dent Res. 1997;76:1587–95. doi: 10.1177/00220345970760091101. [DOI] [PubMed] [Google Scholar]
- 5.Fan Y, Sun Z, Moradian-Oldak J. Effect of fluoride on the morphology of calcium phosphate crystals grown on acid-etched human enamel. Caries Res. 2009;43:132–6. doi: 10.1159/000209346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kontonasaki E, Zorba T, Papadopoulou L, Pavlidou E, Chatzistavrou X, Paraskevopoulos K, et al. Hydroxy carbonate apatite formation on particulate bioglass in vitro as a function of time. Cryst Res Technol. 2002;37:1165–71. [Google Scholar]
- 7.Alaudin SS, Fontana M. Evaluation of NovaMin® As an Adjunct to Fluoride for Caries Lesion Remineralization Novamin Research Report. 2008 [Google Scholar]
- 8.Jayarajan J, Janardhanam P, Jayakumar P. Efficacy of CPP-ACP and CPP-ACPF on enamel remineralization – An in vitro study using scanning electron microscope and DIAGNOdent®. Indian J Dent Res. 2011;22:77. doi: 10.4103/0970-9290.80001. [DOI] [PubMed] [Google Scholar]
- 9.Elsayad I, Sakr A, Badr Y. Combining casein phosphopeptide-amorphous calcium phosphate with fluoride: Synergistic remineralization potential of artificially demineralized enamel or not? J Biomed Opt. 2009;14:1–7. doi: 10.1117/1.3210780. [DOI] [PubMed] [Google Scholar]
- 10.Rao A, Malhotra N. The role of remineralizing agents in dentistry: A review. Compend Contin Educ Dent. 2011;32:26–33. [PubMed] [Google Scholar]
- 11.Pulido MT, Wefel JS, Hernandez MM, Denehy GE, Guzman-Armstrong S, Chalmers JM, et al. The inhibitory effect of MI paste, fluoride and a combination of both on the progression of artificial caries-like lesions in enamel. Oper Dent. 2008;33:550–5. doi: 10.2341/07-136. [DOI] [PubMed] [Google Scholar]
- 12.Bahrololoomi Z, Musavi SA, Kabudan M. In vitro evaluation of the efficacy of laser fluorescence (DIAGNOdent) to detect demineralization and remineralization of smooth enamel lesions. J Conserv Dent. 2013;16:362–6. doi: 10.4103/0972-0707.114360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narayana SS, Deepa VK, Ahamed S, Sathish ES, Meyappan R, Satheesh Kumar KS. Remineralization efficiency of bioactive glass on artificially induced carious lesion an in vitro study. J Indian Soc Pedod Prev Dent. 2014;32:19–25. doi: 10.4103/0970-4388.127047. [DOI] [PubMed] [Google Scholar]
- 14.Rajan R, Krishnan R, Bhaskaran B, Kumar SV. A polarized light microscopic study to comparatively evaluate four remineralizing agents on enamel viz CPP-ACPF, ReminPro, SHY-NM and Colgate strong teeth. Int J Clin Pediatr Dent. 2015;8:42–7. doi: 10.5005/jp-journals-10005-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srinivasan N, Kavitha M, Loganathan SC. Comparison of the remineralization potential of CPP-ACP and CPP-ACP with 900 ppm fluoride on eroded human enamel: An in situ study. Arch Oral Biol. 2010;55:541–4. doi: 10.1016/j.archoralbio.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Preethee T, Kandaswamy D, Rosaline H, Arathi G. Comparing the remineralization potential of NovaMin and casein phosphopeptide-amorphous calcium phosphate using quantitative light induced flourescence. Amrita J Med. 2011;7:28–32. [Google Scholar]
- 17.Ogaard B, Rølla G, Arends J, ten Cate JM. Orthodontic appliances and enamel demineralization. Part 2. Prevention and treatment of lesions. Am J Orthod Dentofacial Orthop. 1988;94:123–8. doi: 10.1016/0889-5406(88)90360-5. [DOI] [PubMed] [Google Scholar]
- 18.Agnihotri Y, Pragada NL, Patri G, Thajuraj PK. The effect of CPP-ACP on remineralization of artificial caries like lesions: An in vitro study. Indian J Multidiscip Dent. 2011;2:366–9. [Google Scholar]
- 19.Allan I, Newman H, Wilson M. Antibacterial activity of particulate Bioglass® against supra-and subgingival bacteria. Biomaterials. 2001;22:1683–7. doi: 10.1016/s0142-9612(00)00330-6. [DOI] [PubMed] [Google Scholar]


