Abstract
Purpose
To evaluate differences by sex in the neuroretina of rats with chronic glaucoma over 24 weeks of follow-up, and to assess by sex the influence on neurodegeneration of different methods of inducing ocular hypertension.
Methods
Forty-six Long–Evans rats—18 males and 28 females—with induced chronic glaucoma were analyzed. Glaucoma was achieved via 2 models: repeatedly sclerosing the episcleral veins (9 male/14 female) or by injecting poly(lactic-co-glycolic acid) microspheres measuring 20 to 10 µm (Ms20/10) into the anterior chamber (9 male/14 female). The IOP was measured weekly by tonometer; neuroretinal function was recorded by dark/light-adapted electroretinography at baseline and weeks 12 and 24; and structure was analyzed by optical coherence tomography using the retina posterior pole, retinal nerve fiber layer and ganglion cell layer protocols at baseline and weeks 8, 12, 18, and 24.
Results
Males showed statistically significant (P < 0.05) higher IOP in both chronic glaucoma models, and greater differences were found in the episcleral model at earlier stages. Males with episclerally induced glaucoma showed a statistically higher increase in retinal thickness in optical coherence tomography recordings than females and also when comparing Ms20/10 at 12 weeks. Males showed a higher percentage of retinal nerve fiber layer thickness loss in both models. Ganglion cell layer thickness loss was only detected in the Ms20/10 model. Males exhibited worse dark/light-adapted functionality in chronic glaucoma models, which worsened in the episcleral sclerosis model at 12 weeks, than females.
Conclusions
Female rats with chronic glaucoma experienced lower IOP and structural loss and better neuroretinal functionality than males. Sex and the ocular hypertension–inducing method influenced neuroretinal degeneration.
Keywords: sex, glaucoma, retina, neurodegeneration, PLGA microspheres, animal model
Traditionally, the eye was considered a sex-neutral organ. Recent publications, however, have evidenced male/female sex divergence. There are sex steroid receptors in nearly every part of the eye1,2 and even the retina synthetizes neuroestrogens via aromatase enzymes.3 Differences in ocular blood flow were found between sexes,4 and variations in the optic nerve head were described according to phase of menstrual cycle and were correlated to hormonal levels.5,6 Premenopausal females showed better recordings by perimetry7 and electroretinography (ERG).8 Physiological ophthalmologic changes caused by hyperestrogenism are also described in pregnancy.9–11 Moreover, a clear tendency to gender difference is found in ophthalmologic,12,13 neuropsychiatric, and neurodevelopmental pathologies with immune system implications14,15 and modulation of aging-linked genes influenced by sex.16,17
Glaucoma is the second biggest cause of blindness worldwide and will affect 111.8 million people by 2040.18 Glaucoma is considered an aging-linked pathology caused by progressive death of retinal ganglion cells (RGCs). The IOP is the major modifiable risk factor, with other well-known nonmodifiable factors being age and race.19 However, there are discrepancies regarding gender in the literature.20 There is evidence of higher incidence of POAG in men (adjusted by age),18 although the higher prevalence and risk of blindness are found in elderly women, possibly owing to their increased life-expectancy.21 Nonetheless, ophthalmic research data on sex differences in glaucoma remain scarce.
The protective effect of estrogens on cardiovascular areas is widely known and it has been suggested that they have a similar effect on the eye, delaying the start of glaucomatous pathology.13 In fact, the risk of POAG increases in conditions of low estrogenic exposure, such as late menarche onset, early menopause, or polycystic ovarian syndrome13,22 and better ophthalmologic parameters were found in studies with hormone replacement therapy, although its use is not recommended.20,23–25 Estrogens, and especially 17β estradiol, activate endothelial nitric oxide (NO) synthase, which diminishes vascular resistance, increases blood flow in the optic nerve26 and decreases the IOP. All of these effects of estrogens could explain the increase in IOP occurring after the onset of menopause,27 when estrogenic hormone levels decrease abruptly. Furthermore, estrogens exert a protective effect on the extracellular matrix of the trabecular meshwork and lamina cribosa.28 In contrast, estrogens act as an endogen neuroprotector owing to their chemical properties and phenolic ring,29,30 helping to maintain RGC viability31,32 and intraretinal synapsis.12,33 Neuroprotection exerted by estrogens has been demonstrated by topical, intravitreal and systemic administration in animal studies,34–36 even under conditions of induced ocular hypertension (OHT).
Sex equity in animal studies has been recently recommended on ethical grounds. Moreover, there is growing interest in increasing knowledge about the influence of sex on aging and aging-linked pathologies such as glaucoma.37 Most animal glaucoma studies are performed by inducing OHT, but usually only 1 sex is used. Moreover, even when both sexes are used, differences between them are not analyzed.
To our knowledge, this study is the first to analyze differences by sex in IOP and neuroretinal structure and function under conditions of chronic glaucoma in 2 different OHT models. We demonstrate that females showed physiological protection because they suffered from lower OHT and less structural and functional neuroretinal damage than age-matched males at an early stage of chronic glaucoma. We also showed that the induced model used to increase IOP influenced sex-dependent neuroretinal degeneration. We therefore suggest our outcomes be considered in future studies into neurodegeneration and protection.
Methods
Animals
All work with animals was approved by the Ethics Committee for Animal Research (PI34/17) and was carried out in strict accordance with the Association for Research in Vision and Ophthalmology's Statement on the Use of Animals at the Biomedical Research Center of Aragon (CIBA: Centro de Investigaciones Biomédicas de Aragón). Long–Evans rats at 4 weeks of age and weighing 50 to 100 g at the beginning of the study were housed in standard cages. To keep the same animals together throughout the study, and to preserve the animals’ welfare, a maximum of either 3 males or 4 females were placed in each cage and given environmental enrichment and water and food ad libitum. They were kept under animal welfare conditions (e.g., a 12-hour dark–light cycled room, temperature of 22 °C, and relative humidity of 55%). Body weight was measured periodically to calculate anesthetic dosage.
A total of 46 animals were used: 18 males and 28 females. Chronic glaucoma models were induced using 2 OHT methods. OHT was induced by biweekly sclerosing injections of the episcleral veins (EPI model), as described by Morrison et al38 in 23 rats (9 male/14 female). In the other 23 rats (9 male/14 female), OHT was induced by injecting a suspension of Ms20/10 (10% w/v) into the anterior chamber of the eye (Ms20/10 model) at baseline, doing so biweekly for the first month and then once monthly until week 20, as described by Rodrigo et al.39 The Ms were made of poly(lactic-co-glycolic acid) (PLGA) according to the oil in water emulsion solvent extraction–evaporation technique previously described by Garcia-Herranz et al.40 and were prepared by the Innovation, Therapy and Pharmaceutical Development in Ophthalmology (InnOftal) Research Group (Faculty of Pharmacy, Complutense University of Madrid, Spain). All OHT injections were performed in the right eye under surgical conditions: controlled temperature, topical tetracaine (1 mg/mL + oxibuprocaine 4 mg/mL) eye drops (Anestesico doble Colirccusi, Alcon Cusí SA, Barcelona, Spain) and intraperitoneal (60 mg/kg of ketamine + 0.25 mg/kg of dexmedetomidine) anesthetic. Afterward, the rats were left to recover in an enriched 2.5% oxygen atmosphere and were treated with antibiotic ointment (erythromycin 5 mg/g [Oftalmolosa Cusí eritromicina, Alcon Cusí SA]). The Ms20/10 were injected at baseline and at weeks 2, 4, 8, 12, 16, and 20. Episcleral injections were administered biweekly if the IOP was less than 20 mm Hg. Animal weight during the study was similar to that reported by the supplier (Janvier-labs, Le Genest-Saint-Isle, France). Glaucoma induction did not alter the morphometric weight parameter versus healthy animals. No correlation studies were carried out between body weight and retinal loss since these parameters have been previously demonstrated—by means of data standardization—to be independent.41
Ophthalmologic Clinical Evaluation
IOP measurements were recorded weekly, using a rodent-specific rebound tonometer (Tonolab, TiolatOy, Helsinki, Finland), under a sedative mixture of 3% sevoflurane gas and 1.5% oxygen for less than 3 minutes as recommended.42 The IOP value was obtained by averaging three consecutive measurements, taken from the average of 6 rebounds.
Optical coherence tomography (OCT) (OCT Spectralis, Heidelberg Engineering, Germany) was used to quantify the neuroretinal structure in vivo, using a contact lens adapted to the rat's cornea in order to obtain higher quality recordings. The retina posterior pole, retinal nerve fiber layer (RNFL), and ganglion cell layer (GCL) protocols were used.39–41,43,44 All of them measured a 3-mm-diameter area centered on the optic disc using 61 b-scans, and subsequent follow-up examinations were performed at this same location using eye-tracking software and a follow-up application. The retina posterior pole protocol analyzed the thickness from the inner limiting membrane to the retinal pigment epithelium divided into the 9 Early Treatment Diabetic Retinopathy areas,45 which included a central 1-mm circle centered on the optic disc (no fovea exists in rats), and inner (inferior, superior, nasal, temporal) and outer (inferior, superior, nasal, temporal) rings measuring 2 and 3 mm in diameter, respectively, as well as total volume; the RNFL protocol analyzed the thickness from the inner limiting membrane to the GCL boundaries divided into 6 sectors (inferotemporal, inferonasal, superotemporal, superonasal, nasal, and temporal); and the GCL protocol analyzed from the RNFL to the inner nuclear layer boundaries within the Early Treatment Diabetic Retinopathy areas.
ERG (Roland consult RETIanimal ERG, Germany) was used to quantify neuroretinal functionality using the scotopic full-field ERG and the light-adapted photopic negative response protocols. The scotopic ERG test was performed after 12 hours of dark adaptation and the eyes were prepared to full dilatation using topical eye drops containing tetracaine 1 mg/mL + oxibuprocaine 4 mg/mL (Anestesico doble Colirccusi, Alcon Cusí SA) and mydriatics (tropicamide 10 mg/mL, phenylephrine 100 mg/mL [Alcon Cusí SA]). Eyes were lubricated with hypromellose 2% (Methocel OmniVision, Puchheim, Germany). Active electrodes were placed on the cornea, references were placed at both sides under the skin, and the ground electrode was placed near the tail. Electrode impedance with a difference of less than 2 kΩ between electrodes was accepted.
Both eyes were tested simultaneously using a Ganzfeld Q450 SC sphere with white LED flashes for stimuli, and 7 steps with increasing luminance intensity and intervals were performed (rod response: step 1: –40 dB, 0.0003 cds/m2, 0.2 Hz [20 recordings averaged]; step 2: –30 dB, 0.003 cds/m2, 0.125 Hz [18 recordings averaged]; step 3: –20 dB, 0.03 cds/m2, 8.929 Hz [14 recordings averaged]; step 4: –20 dB, 0.03 cds/m2, 0.111 Hz [15 recordings averaged]; step 5: –10 dB, 0.3 cds/m2, 0.077 Hz [15 recordings averaged]; mixed rod–cone response: step 6: 0 dB, 3.0 cds/m2, 0.067 Hz [12 recordings averaged]; and oscillatory potentials: step 7: 0 dB, 3.0 cds/m2, 29.412 Hz [10 recordings averaged]). The photopic negative response protocol was performed after light adaptation to a blue background (470 nm, 25 cds/m2), and a red LED flash (625 nm, –10 dB, 0.30 cds/m2, 1.199 Hz [20 recordings averaged]) was used as stimulus. Latency (in milliseconds) and amplitude (in microvolts) were studied in a, b and photopic negative response waves.
OCT tests were performed at baseline, 8 weeks, 12 weeks, 18 weeks, and 24 weeks; ERG tests were performed at baseline, 12 weeks, and 24 weeks, both under intraperitoneally administered anesthesia (60 mg/kg of ketamine + 0.25 mg/kg of dexmedetomidine, as mentioned above for ocular injections). A trained, masked researcher rejected or manually corrected the recordings if the algorithm had clearly failed.
Statistical Analysis
Data from this longitudinal study were recorded in an Excel database and statistical analysis was performed using SPSS software version 20.0 (SPSS Inc., Chicago, IL). To assess sample distribution, the Kolmogorov–Smirnov test was used; however, given the nonparametric distribution of most of the data, the Mann–Whitney U test was performed to evaluate the differences between both cohorts, and a paired Wilcoxon test was used to compare the changes recorded in each eye over the 24-week study period. All values were expressed as means ± standard deviations. Values of P < 0.05 (expressed as *) were considered to indicate statistical significance, and the Bonferroni correction for multiple comparisons was calculated to avoid a high false-positive rate. The level of significance for each variable was established based on Bonferroni calculations (expressed as #).
Results
IOP
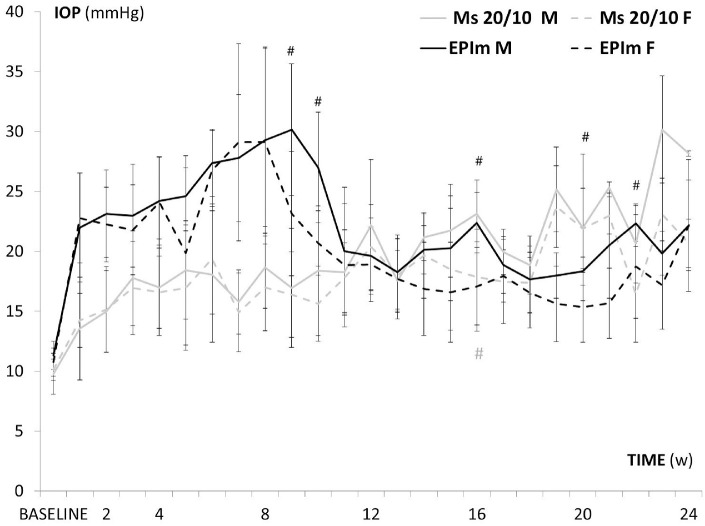
Both OHT models showed an increase in IOP over follow-up, which occurred earlier and more abruptly in the episcleral sclerosis model. Males in both OHT models, but especially the episcleral model, showed statistically significant higher IOP measurements than females (Fig. 1 and Supplementary Table S1).
Figure 1.
IOP curve in both sexes and chronic glaucoma models over follow-up. EPIm, episcleral sclerosis model; Ms 20/10, microsphere 20/10 model; M, male; F, female. #P < 0.020, for Bonferroni correction for multiple comparisons.
Structural Neuroretinal Analysis by in VIVO OCT
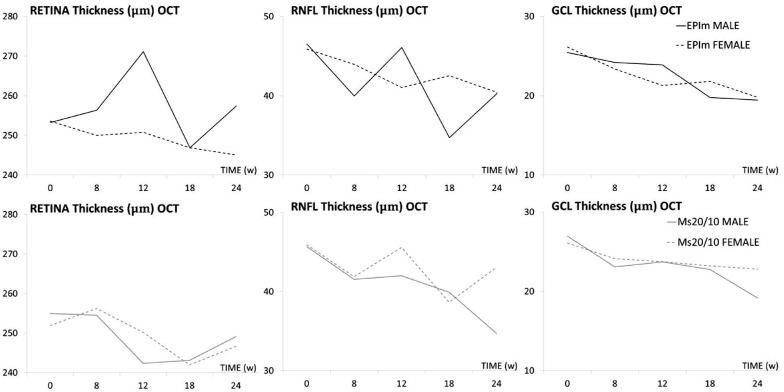
Neuroretinal thickness in males and females was quantified over the study in both chronic glaucoma models (see Supplementary Tables S2 and S3). Males in the episcleral sclerosis model had greater retinal thickness (but similar RNFL and GCL) than females at the end of the study. Moreover, males showed greater fluctuations in retina posterior pole and RNFL thickness than females, which exhibited a progressive and sustained decrease in all three OCT parameters. However, these features were not observed in the Ms20/10 model, in which males had lesser thicknesses and females exhibited greater fluctuations in RNFL (Fig. 2).
Figure 2.
Structural analysis of the neuroretina using OCT in both sexes and chronic glaucoma models. EPIm, episcleral sclerosis model; Ms 20/10, microsphere 20/10 model; OCT, optical coherence tomography; RNFL, retinal nerve fiber layer; GCL, ganglion cell layer complex; average thickness in microns (μm).
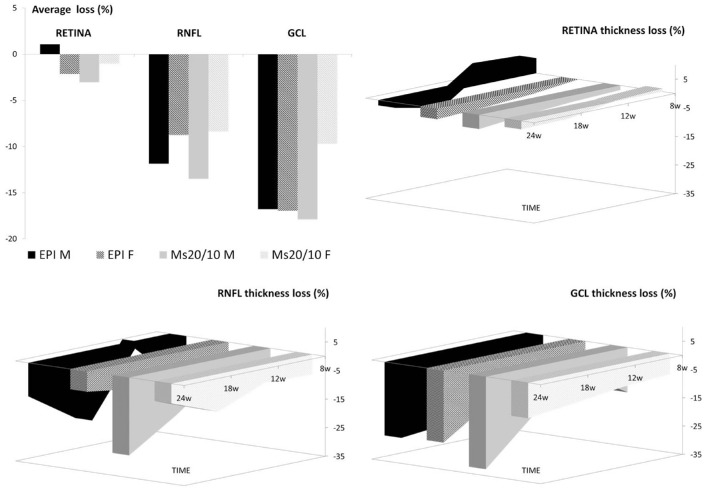
The percentages of thickness loss in both sexes and both chronic glaucoma models were then analyzed and compared over the study. As can be seen in Figure 3, the percentage of RNFL thickness loss was greater in males in both OHT models. It was also greater in the GCL and retina posterior pole in the Ms20/10 model, reaching statistical significance at an early stage (week 8). Interestingly, increased retina posterior pole thickness was found in males in the episcleral sclerosis model.
Figure 3.
Percentage neuroretinal structure loss measured using OCT in both sexes and chronic glaucoma models over 24 weeks of follow-up. EPI, episcleral sclerosis model; Ms20/10, microsphere 20/10 model; RNFL, retinal nerve fiber layer; GCL, ganglion cell layer complex; M, male; F, female.
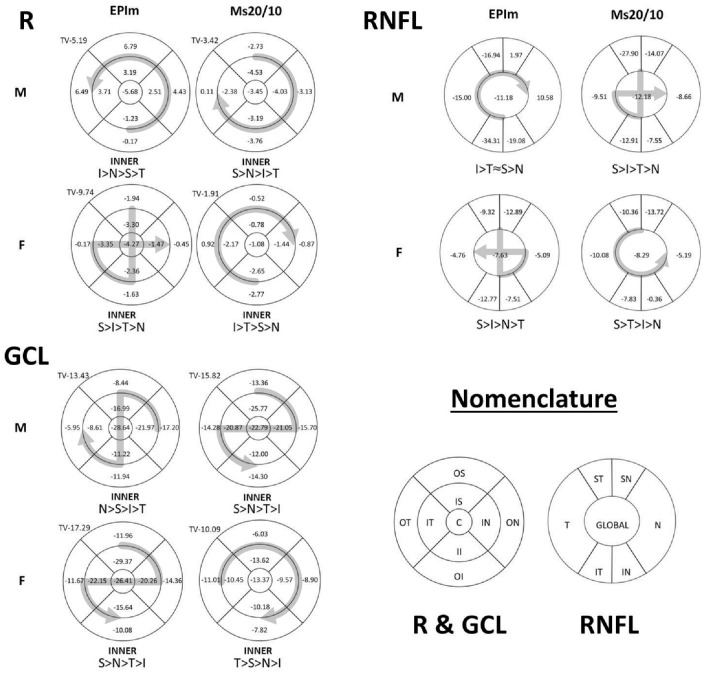
Although perceptual loss of OCT thickness in each sector was also analyzed to identify a sex-dependent neuroretinal degeneration pattern, no obvious pattern was found. Both chronic glaucoma models and sexes lost more (on average) in the inner retina posterior pole and GCL sectors and in the superior-inferior axis sectors in the RNFL. Furthermore, losses in contiguous sectors were found in females in the Ms20/10 model. The patterns of degeneration found are shown in Figure 4.
Figure 4.
Neuroretinal percentage loss in OCT sectors and loss trend averaged over 24 weeks of follow-up. R, retina; C, central, II, inner inferior; OI, outer inferior; IS, inner superior; OS, outer superior; IN, inner nasal; ON, outer nasal; IT, inner temporal; OT, outer temporal; IT, inferior temporal; IN, inferior nasal; ST, superior temporal; SN, superior nasal; N, nasal; T, temporal; EPIm, episcleral sclerosis model; Ms20/10, microsphere 20/10 model; TV, total volume; I, inferior; S, superior; N, nasal; T, temporal; >, greater loss than; ≈, similar; M, male; F, female.
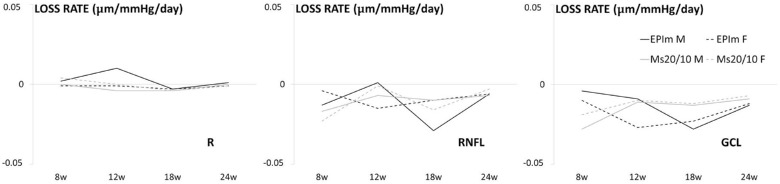
To standardize the neuroretinal loss, the right eye loss rate per day and the millimeters of mercury of increased IOP each week were calculated from the average of all OCT sectors and expressed in micrometers per millimeters of mercury per day. The parameter most affected in both OHT models per millimeter of mercury increase was GCL followed by RNFL and, finally, retina posterior pole. In the Ms20/10 model, both sexes showed a similar rate of GCL loss over time; in the episcleral sclerosis model, a similar loss rate was only observed in the later stages. This feature was also observed in retina posterior pole, although males in the episcleral sclerosis model experienced an inverted loss rate in the early stages. RNFL was the parameter that exhibited widest variations between sexes and models. At the end of the study (week 24), the loss rate in the episcleral sclerosis model was similar between sexes; however, in the Ms20/10 model females showed a slightly lower loss rate than males (Fig. 5).
Figure 5.
Neuroretinal loss rate measured using OCT in both sexes and chronic glaucoma models. EPIm, episcleral sclerosis model; Ms20/10, microsphere 20/10 model; M, male; F, female.
Functional Neuroretinal Analysis by in VIVO ERG
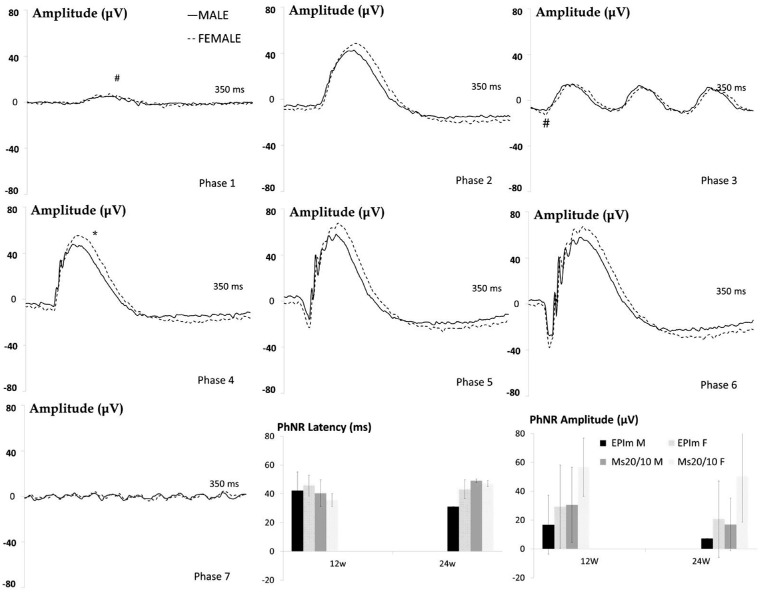
In the episcleral sclerosis model, males exhibited statistically significant lower scotopic ERG signals at week 12; in the Ms20/10 model, no differences by sex were found. In the light-adapted photopic negative response test exploring RGC functionality the males showed a clear tendency to produce worse measurements in both chronic glaucoma models up to week 24. Lower recordings were measured in both models over time, with the episcleral sclerosis model producing the lowest values (see Fig. 6).
Figure 6.
Neuroretinal functionality using dark- and light-adapted ERG in both chronic glaucoma models and sexes. PhNR, photopic negative response; EPIm, episcleral sclerosis model; Ms20/10, microsphere 20/10 model; M, male; F, female; Phases 1 to 7 of dark-adapted ERG in the EPIm at week 12.
Discussion
Several models have been developed for the study of glaucoma. However, the results regarding neurodegeneration differ among different groups, and it has been proposed that this may be due to the use of different animals, animal strains, amounts of injected solution, injection times, and even particle sizes when using microbeads.46,47 However, until now, no study considered sex as an influential factor in glaucomatous degeneration (Table). In fact, most studies employed only one sex and, when males and females were used together, differences between sexes were not analyzed.48–82 Glaucoma is a neurodegenerative disease in which associations with another neurodegenerative diseases that are influenced by sex, such as Alzheimer's disease, have been found.83,84
Table.
Sex in Rodent Glaucoma Models
| Model | Sex | References |
|---|---|---|
| Episcleral vein sclerosis model | Male | 48, 49, 50, 56, 58, 62, 63, 66, 67, 68, 69, 71, 73, 74, 76 |
| Female | – | |
| Sex not mentioned | 59, 60, 64, 65, 70, 75, 77 | |
| Both sexes | 61, 72 (sex differences not analyzed in either) | |
| Microsphere model | Male | 51, 53, 57, 82 |
| Female | 52, 54, 55, 78, 81 | |
| Sex not mentioned | 80 | |
| Both sexes | 79 (sex differences not analyzed) |
Given the lack of knowledge about the influence of sex in glaucoma, and given current needs,85 the purpose of our study was to investigate the existence of differences between males and females in 2 animal models of glaucoma (sclerosis of episcleral veins and injection of PLGA microspheres in the anterior chamber of the eye).
As stated elsewhere in this article, IOP is the main risk factor for development and worsening of glaucomatous pathology. Several glaucoma models have been developed to induce increased IOP. The episcleral sclerosis model produces very high pressures at early stages, leading to faster neuroretinal degeneration. However, the microsphere model, in which it is possible to modify injection frequency, allows for modulation of the hypertensive curve, thereby achieving a slower and more progressive IOP increase,86 as is consistent with our results. Our results also showed that females exhibit lower IOP levels than males in both models. A possible explanation for this finding is the presence of estrogenic receptors in the trabecular meshwork and endothelial cells that regulate NO synthase, these being responsible for increased vasodilation and a lower IOP.4 Therefore, females may also benefit from a protective effect in OHT. However, both sexes suffered from higher IOP in the episcleral model. This finding may be due to the induction method itself, because hypersaline solution is injected into the episcleral veins and, via a retrograde pathway, reaches the trabecular meshwork and may even opacify the lens as evidence of the solution entering the eye.47 The hyperosmotic effect could damage the endothelial cells, altering the effect of the NO synthase and eliminating, at least partially, the protective effect exerted by estrogen on the vasodilation-mediating enzyme (NO synthase) and increasing IOP in both sexes. In contrast, mineralocorticoid receptors have been found in the iris, ciliary body, endothelial cells, and even in the neuroretina and pigmentary epithelium.87 In addition to an NaCl imbalance (as with 1.8 M NaCl solution), aldosterone is upregulated and binds to the mineralocorticoid receptors, causing endothelial dysfunction and vasculopathy.88 However, because this damage would not occur so abruptly with the injection of microspheres, it seems that the OHT-inducing method could influence the results differently by sex.
As the increase in IOP produces neuroretinal degeneration, an OCT-based study was designed to evaluate the changes in the neuroretina over time. This technology allows consecutive re-explorations while decreasing the number of euthanized animals. In addition, several studies have shown an adequate correlation between OCT and immunocytochemistry and ERG.89–91 The OCT recordings were performed from baseline with first re-exploration at 8 weeks of follow-up (corresponding to 12 weeks of rat life). At this age, development and growth of the retina end and the retina reaches maturity.92
In a previous article, we demonstrated that the episcleral sclerosis model induced a more aggressive retinal glial reaction than the microsphere model.39 However, differences between the sexes were not evaluated. This article now shows that these differences are mainly found in males. It seems that the glaucoma-inducing method could also influence sex-dependent neurodegeneration. Our results showed a significant increase in retinal thickness in males at week 12 (which decreased at week 18 and increased again at week 24) in the episcleral sclerosis model; it did not occur in females and did not occur in the Ms20/10 model. These fluctuations39 may be due to greater immune infiltration93 or increased glia sensitivity to hydroelectrolytic or vascular changes, especially in males. A potential transfer of hyperosmotic solution to the posterior pole could have upregulated aldosterone. It has been demonstrated that the systemic administration of aldosterone decreases RNFL thickness under ocular normotensive conditions, and that it was counteracted by the antagonist, spironolactone.94 In this sense, Takasago et al.95 found a significant negative correlation between the aldosterone levels in plasma and the number of RGCs, which decreased earlier on the periphery and later in central sectors. However, these authors only analyzed males in their study. The mineralocorticoid receptors of the neuroretina and pigment epithelium act in retinal/choroidal homeostasis by balancing sodium and reabsorbing the fluid,96 but mineralocorticoid receptor overactivation could induce damage that has been linked to central serous chorioretinopathy.97 Central serous chorioretinopathy courses with increased capillary permeability and pressure in choroidal vessels98 that increase retinal thickness and cause edema. Moreover, this pathological entity is oddly more prevalent in males. Testosterone increases the risk of central serous chorioretinopathy with increased retinal thickness, and its improvement with finasteride has been demonstrated.99 According to the facts mentioned elsewhere in this article, bimonthly injections with hyperosmolar solution for 6 months could overactivate mineralocorticoid receptors and increase retinal thickness, although no evident edema was detected by OCT. In contrast, it is known that glial cell edema (which is also present in glaucoma) is produced by intracellular Na overload (which could be aggravated by the hyperosmotic solution). In both models, a similar pattern of RNFL fluctuation was detected, with increases in thickness at 12 and 24 weeks after induction of the increased IOP. Increases in thickness owing to immune infiltration39 or edema have been detected before cell death.100 In our episcleral sclerosis model results, fluctuations in RNFL were observed in males, but it seems that premenopausal females counteracted it. In this regard, Neuman et al.101 showed that sex steroids such as progesterone inhibit the swelling of glial cells. The suggestion therefore is that the episcleral sclerosis model would affect males more. In contrast, in the Ms20/10 model a greater fluctuation occurred in females. This suggests that sex influences axonal damage (measured as RNFL thickness change) depending on whether the hypertensive Noxa is pretrabecular (Ms 20/10 model) or post-trabecular (episcleral sclerosis model), although other unknown factors may also exert an influence.
Furthermore, O'Steen et al.102 demonstrated in rats that, in a situation of chronic stress, retinal thickness (especially in the outer layers) decreased significantly in both sexes, although males lost more than females. The degeneration began on the periphery and later affected the inner sectors. Previous OCT studies by our group found damage at an earlier stage in the outer retinal sectors and affectation of the inner sectors over the follow-up.39,40 In this study, the degeneration pattern showed greater loss (on average) in the inner sectors, which could be explained by greater damage or could be a consequence of the averaged result already including the inner sectors.
Estrogens have demonstrated a neuroretinal protective effect,12,31–36 as has progesterone in attenuating microglial-driven neurodegeneration,103 and our results showed that females experienced less neurodegeneration in all parameters in the Ms20/10 model as well as in the RNFL in the episcleral sclerosis model. O'Steen104 proposed that 17β-estradiol was to protect photoreceptors from light damage and Chaychi et al.8 showed greater functionality in premenopausal female rats than in males. We found similar results even with OHT Noxa in the episcleral sclerosis model, as the females had greater amplitudes in the scotopic ERG. Interestingly, even when the percentage loss of GCL in the episcleral sclerosis model was similar between sexes (or even slightly higher in females), ERG, and photopic negative response functionality were better preserved in females than in males, indicating better synaptic function in females. In this regard, neurosteroids (such as estrogens) synthesized in the retina have been reported to modulate the gamma-aminobutyric acid and glutamate receptors intervening in the synaptic function,85 and estrogens have shown an improvement in synaptic functionality in neuroretinal models. Nevertheless, our results partially contradict the results of other authors who claim that RGC dysfunction occurs earlier and progressively preceding cell death. It is worth mentioning that these studies were carried out with monkeys (sex was not mentioned), and with male rats.105 In our study, the same outcomes were found in males, but not in females. Thus, there could be a lag, or maintenance of the function and/or nonidentical sequence in females.
A study in humans with POAG showed that photopic negative response was decreased in the early stages of the disease. Patients showed lower photopic negative response amplitude with the decrease in visual field sensitivity, which was correlated with the vertical cupping/disc ratio. However, no differences were found between the sexes.106 Nevertheless, the results from our study showed that females exhibited greater photopic negative response amplitudes than males in both models.
In a previous study41 analyzing differences in neurodegeneration in healthy Long–Evans rats by age and sex, we found that males had higher IOP by the end of the study (7 months of age), exhibited greater neuroretinal thickness but higher structural percentage loss, and had worse dark- and light-adapted function than females. These facts served as hypotheses for this article analyzing whether sex could also influence the findings in the OHT models studied. Moreover, as mentioned elsewhere in this article, the episcleral sclerosis model and Ms 20/10 models induce differing extents of damage at different stages of the study. episcleral sclerosis model showed earlier damage than the Ms20/10 model.39 This study constitutes a step forward; indeed, analyzing data by sex has been essential to detecting changes that would have otherwise gone unnoticed. If sex had not been considered, the average of both the male and female curves would have been taken, masking differences that would not be statistically significant in the structural and functional tests owing to an underestimation or overestimation of results (in males and females, respectively) that would counteract each other. Similarly, if the OHT model had not been considered separately, we would be facing the same dilemma owing to underestimation or overestimation (episcleral sclerosis model and Ms 20/10 model, respectively) of the thicknesses and electrical responses, ignoring differences existing since intermediate stages of the study. This argument would also apply to the analysis of the response to potential neuroprotective and hypotensive drugs. Therefore, we demonstrate that Noxa type and sex are important factors in glaucoma onset and progression.
Our group is aware of the main limitation of this study, which is the absence of histologic analysis, even when OCT is a reliable method, so it would be beneficial to support our results with histologic studies. Furthermore, hormone levels were not measured, and our animals did not reach senescence, which is when the prevalence of glaucoma rises, especially in women. It would therefore be advantageous to study more advanced ages in future research.
In conclusion, to our knowledge this study is the first to analyze the influence of sex in 2 different animal POAG models. Our results showed different patterns of degeneration and different loss rates between males and females in the same hypertensive model and also between different models. Premenopausal females exhibited lower IOP and less neuroretinal damage in glaucoma than age-matched males. Differences or discrepancies in the results of previous studies could be due in part to a sex bias, as well as to the OHT-inducing model used. It would be beneficial to consider both sex and the OHT-inducing model in future studies when evaluating both hypotensive and neuroprotective therapies, since our results suggest that premenopausal females maintained a protective effect in relation to OHT and the therapeutic outcomes could be overestimated or underestimated.
Supplementary Material
Acknowledgments
The authors thank the use of the General Research Support Service (SAI), University of Zaragoza, and to thank Zoco Estudio (zocoestudio.com) for the infographics designed for this article.
Supported by Rio Hortega Research Grants M17/00213, PI17/01726 and PI17/01946 (Carlos III Health Institute), and by MAT2017-83858-C2-2 and MAT2017-83858-C2-1 MINECO/AEI/ERDF, EU, Research Group UCM 920415, ISCIII-FEDER “Una manera de hacer Europa” RETICS Oftared, RD16/0008/0004, RD16/0008/0009, and RD16/0008/029. D.G.H. acknowledges a UCM-Santander fellowship (CT17/17-CT17-18) and AAN thanks Spain's Ministry of Science and Innovation for the fellowship granted (PRE2018-083951).
Disclosure: M.J. Rodrigo, None; T. Martinez-Rincon, None; M. Subias, None; S. Mendez-Martinez, None; L.E. Pablo, None; V. Polo, None; A. Aragon-Navas, None; D. Garcia-Herranz, None; J.G. Feijoo, None; I.B. Osuna, None; R. Herrero-Vanrell, None; E. Garcia-Martin, None
References
- 1. Wickham LA, Gao J, Toda I, Rocha EM, Ono M, Sullivan DA.. Identification of androgen, estrogen and progesterone receptor mRNAs in the eye. Acta Ophthalmol Scand. 2000; 78(2): 146–153, doi: 10.1034/j.1600-0420.2000.078002146.x. [DOI] [PubMed] [Google Scholar]
- 2. Kobayashi K, Kobayashi H, Ueda M, Honda Y.. Estrogen receptor expression in bovine and rat retinas. Investig Ophthalmol Vis Sci. 1998; 39(11): 2105–2110. [PubMed] [Google Scholar]
- 3. Salyer DL, Lund TD, Fleming DE, Lephart ED, Horvath TL.. Sexual dimorphism and aromatase in the rat retina. Dev Brain Res. 2001; 126(1): 131–136, doi: 10.1016/S0165-3806(00)00147-4. [DOI] [PubMed] [Google Scholar]
- 4. Schmidl D, Schmetterer L, Garhöfer G, Popa-Cherecheanu A.. Gender differences in ocular blood flow. Curr Eye Res. 2015; 40(2): 201–212, doi: 10.3109/02713683.2014.906625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Akar ME, Taskin O, Yucel I, Akar Y.. The effect of the menstrual cycle on optic nerve head analysis in healthy women. Acta Ophthalmol Scand. 2004; 82(6): 741–745, doi: 10.1111/j.1600-0420.2004.00351.x. [DOI] [PubMed] [Google Scholar]
- 6. Dewundara S, Wiggs J, Sullivan DA, Pasquale LR.. Is estrogen a therapeutic target for glaucoma? HHS Public Access. Semin Ophthalmol. 2016; 31(2): 140–146, doi: 10.3109/08820538.2015.1114845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Akara Y, Yucela I, Akarb ME, Taskinb O, Özera HO, Akar Y.. Menstrual cycle-dependent changes in visual field analysis of healthy women. Ophthalmologica. 2005; 219(1): 30–35, doi: 10.1159/000081780. [DOI] [PubMed] [Google Scholar]
- 8. Chaychi S, Polosa A, Lachapelle P.. Differences in retinal structure and function between aging male and female Sprague-Dawley rats are strongly influenced by the estrus cycle. PLoS One. 2015; 10(8): e0136056, doi: 10.1371/journal.pone.0136056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Phillips CI, Gore SM.. Ocular hypotensive effect of late pregnancy with and without high blood pressure. Br J Ophthalmol. 1985; 69(2): 117–119, doi: 10.1136/bjo.69.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weinreb RN, Lu A, Beeson C.. Maternal corneal thickness during pregnancy. Am J Ophthalmol. 1988; 105(3): 258–260, doi: 10.1016/0002-9394(88)90006-2. [DOI] [PubMed] [Google Scholar]
- 11. Wilke K. Episcleral venous pressure and pregnancy [proceedings]. Acta Ophthalmol Suppl. 1975;(125): 40–41, doi: 10.1111/j.1755-3768.1975.tb01228.x. [DOI] [PubMed] [Google Scholar]
- 12. Nuzzi R, Scalabrin S, Becco A, Panzica G.. Gonadal hormones and retinal disorders: A review. Front Endocrinol (Lausanne). 2018; 9: 66, doi: 10.3389/fendo.2018.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nuzzi R, Scalabrin S, Becco A, Panzica G.. Sex hormones and optic nerve disorders: a review. Front Neurosci. 2019; 13: 57, doi: 10.3389/fnins.2019.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hanamsagar R, Bilbo SD.. Sex differences in neurodevelopmental and neurodegenerative disorders: Focus on microglial function and neuroinflammation during development. J Steroid Biochem Mol Biol. 2016; 160: 127–133, doi: 10.1016/j.jsbmb.2015.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mangold CA, Wronowski B, Du M, et al.. Sexually divergent induction of microglial-associated neuroinflammation with hippocampal aging. J Neuroinflammation. 2017; 14(1): 1–19, doi: 10.1186/s12974-017-0920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Du M, Mangold CA, Bixler G V., et al.. Retinal gene expression responses to aging are sexually divergent. Mol Vis. 2017; 23: 707–717. [PMC free article] [PubMed] [Google Scholar]
- 17. Tower J. Sex-specific gene expression and life span regulation HHS Public Access. Trends Endocrinol Metab. 2017; 28(10): 735–747, doi: 10.1016/j.tem.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY.. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014; 121(11): 2081–2090, doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 19. Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S.. Glaucoma. Lancet. 2017; 390(10108): 2183–2193, doi: 10.1016/S0140-6736(17)31469-1. [DOI] [PubMed] [Google Scholar]
- 20. Tehrani S. Gender difference in the pathophysiology and treatment of glaucoma. Curr Eye Res. 2015; 40(2): 191–200, doi: 10.3109/02713683.2014.968935. [DOI] [PubMed] [Google Scholar]
- 21. Vajaranant TS, Nayak S, Wilensky JT, Joslin CE.. Gender and glaucoma: what we know and what we need to know. Curr Opin Ophthalmol. 2010; 21(2): 91–99, doi: 10.1097/ICU.0b013e3283360b7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yu S, Eh H, Mh K, H C, M S. The association between female reproductive factors and open-angle glaucoma in Korean Women: the Korean National Health and Nutrition Examination Survey V. J Ophthalmol. 2018; 2018: 27507863, doi: 10.1155/2018/2750786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deschênes MC, Descovich D, Moreau M, et al.. Postmenopausal hormone therapy increases retinal blood flow and protects the retinal nerve fiber layer. Investig Ophthalmol Vis Sci. 2010; 51(5): 2587–2600, doi: 10.1167/iovs.09-3710. [DOI] [PubMed] [Google Scholar]
- 24. Rossouw JE, Anderson GL, Prentice RL, et al.. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the women's health initiative randomized controlled trial. JAMA. 2002; 288(3): 321–333, doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 25. Hulley S, Grady D, Bush T, et al.. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998; 280(7): 605–613, doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 26. Tobe LA, Harris A, Trinidad J, et al.. Should men and women be managed differently in glaucoma? Ophthalmol Ther. 2012; 1(1): 1, doi: 10.1007/s40123-012-0001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Altintaş Ö, Çaǧlar Y, Yüksel N, Demirci A, Karabaş L.. The effects of menopause and hormone replacement therapy on quality and quantity of tear, intraocular pressure and ocular blood flow. Ophthalmologica. 2004; 218(2): 120–129, doi: 10.1159/000076148. [DOI] [PubMed] [Google Scholar]
- 28. Zalewski A, Cecchini EL, Deroo BJ.. Expression of extracellular matrix components is disrupted in the immature and adult estrogen receptor β-null mouse ovary. PLoS One. 2012; 7(1): e29927, doi: 10.1371/journal.pone.0029937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pietrucha-Dutczak M, Amadio M, Govoni S, Lewin-Kowalik J, Smedowski A.. The role of endogenous neuroprotective mechanisms in the prevention of retinal ganglion cells degeneration. Front Neurosci. 2018; 12: 834, doi: 10.3389/fnins.2018.00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moosmann B, Behl C.. The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proc Natl Acad Sci U S A. 1999; 96(16): 8867–8872, doi: 10.1073/pnas.96.16.8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nakazawa T, Takahashi H, Shimura M.. Estrogen has a neuroprotective effect on axotomized RGCs through ERK signal transduction pathway. Brain Res. 2006; 1093(1): 141–149, doi: 10.1016/j.brainres.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 32. Chen X, Liu Y, Zhang Y, Kam WR, Pasquale LR, Sullivan DA.. Impact of aromatase absence on murine intraocular pressure and retinal ganglion cells. Sci Rep. 2018; 8(1), doi: 10.1038/s41598-018-21475-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaja S, Yang SH, Wei J, et al.. Estrogen protects the inner retina from apoptosis and ischemia-induced loss of Vesl-1L/Homer 1c immunoreactive synaptic connections. Investig Ophthalmol Vis Sci. 2003; 44(7): 3155–3162, doi: 10.1167/iovs.02-1204. [DOI] [PubMed] [Google Scholar]
- 34. Prokai-Tatrai K, Xin H, Nguyen V, et al.. 17β-estradiol eye drops protect the retinal ganglion cell layer and preserve visual function in an in vivo model of glaucoma. Mol Pharm. 2013; 10(8): 3253–3261, doi: 10.1021/mp400313u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Russo R, Cavaliere F, Watanabe C, et al.. 17β-Estradiol prevents retinal ganglion cell loss induced by acute rise of intraocular pressure in rat. Prog Brain Res. 2008; 173: 583–590, doi: 10.1016/S0079-6123(08)01144-8. [DOI] [PubMed] [Google Scholar]
- 36. Zhou X, Li F, Ge J, et al.. Retinal ganglion cell protection by 17-β-estradiol in a mouse model of inherited glaucoma. Dev Neurobiol. 2007; 67(5): 603–616, doi: 10.1002/dneu.20373. [DOI] [PubMed] [Google Scholar]
- 37. Cascio C, Deidda I, Russo D, Guarneri P.. The estrogenic retina: The potential contribution to healthy aging and age-related neurodegenerative diseases of the retina. Steroids. 2015; 103: 31–41, doi: 10.1016/j.steroids.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 38. Morrison JC, Moore CG, Deppmeier LMH, Gold BG, Meshul CK, Johnson EC.. A rat model of chronic pressure-induced optic nerve damage. Exp Eye Res. 1997; 64(1): 85–96, doi: 10.1006/exer.1996.0184. [DOI] [PubMed] [Google Scholar]
- 39. Rodrigo MJ, Garcia-Herranz D, Subias M, et al.. Chronic glaucoma using biodegradable microspheres to induce intraocular pressure elevation. six-month follow-up. Biomedicines. 2021; 9(6): 682, doi: 10.3390/biomedicines9060682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Garcia-Herranz D, Rodrigo MJ, Subias M, et al.. Novel use of PLGA microspheres to create an animal model of glaucoma with progressive neuroretinal degeneration. Pharmaceutics. 2021; 13(2): 237, doi: 10.3390/pharmaceutics13020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rodrigo MJ, Martinez-Rincon T, Subias M, et al.. Effect of age and sex on neurodevelopment and neurodegeneration in the healthy eye: longitudinal functional and structural study in the Long–Evans rat. Exp Eye Res. August 2020; 200: 108208, doi: 10.1016/j.exer.2020.108208. [DOI] [PubMed] [Google Scholar]
- 42. Ding C, Wang P, Tian N.. Effect of general anesthetics on IOP in elevated IOP mouse model. Exp Eye Res. 2011; 92(6): 512–520, doi: 10.1016/j.exer.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rodrigo MJ, Cardiel MJ, Fraile JM, et al.. Brimonidine-LAPONITE® intravitreal formulation has an ocular hypotensive and neuroprotective effect throughout 6 months of follow-up in a glaucoma animal model. Biomater Sci. 2020; 8(22): 6246–6260, doi: 10.1039/d0bm01013h. [DOI] [PubMed] [Google Scholar]
- 44. Rodrigo MJ, Del Palomar AP, Montolío A, et al.. Monitoring new long-lasting intravitreal formulation for glaucoma with vitreous images using optical coherence tomography. Pharmaceutics. 2021; 13(2): 217, doi: 10.3390/pharmaceutics13020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Photocoagulation for diabetic macular edema. Arch Ophthalmol. 1985; 103(12): 1796, doi: 10.1001/archopht.1985.01050120030015. [DOI] [PubMed] [Google Scholar]
- 46. Dey A, Manthey AL, Chiu K, Do CW.. Methods to induce chronic ocular hypertension: reliable rodent models as a platform for cell transplantation and other therapies. Cell Transplant. 2018; 27(2): 213–229, doi: 10.1177/0963689717724793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Morrison JC, Cepurna WO, Johnson EC.. Modeling glaucoma in rats by sclerosing aqueous outflow pathways to elevate intraocular pressure. Exp Eye Res. 2015; 141: 23–32, doi: 10.1016/j.exer.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Johnson EC, Morrison JC, Farrell S, Deppmeier L, Moore CG, Mcginty MR.. The effect of chronically elevated intraocular pressure on the rat optic nerve head extracellular matrix. Exp Eye Res. 1996; 62(6): 663–674, doi: 10.1006/exer.1996.0077. [DOI] [PubMed] [Google Scholar]
- 49. Fortune B, Bui B V., Morrison JC, et al.. Selective ganglion cell functional loss in rats with experimental glaucoma. Investig Ophthalmol Vis Sci. 2004; 45(6): 1854–1862, doi: 10.1167/iovs.03-1411. [DOI] [PubMed] [Google Scholar]
- 50. Almasieh M, MacIntyre JN, Pouliot M, et al.. Acetylcholinesterase inhibition promotes retinal vasoprotection and increases ocular blood flow in experimental glaucoma. Investig Ophthalmol Vis Sci. 2013; 54(5): 3171–3183, doi: 10.1167/iovs.12-11481. [DOI] [PubMed] [Google Scholar]
- 51. Almasieh M, Zhou Y, Kelly ME, Casanova C, Di Polo A.. Structural and functional neuroprotection in glaucoma: role of galantamine-mediated activation of muscarinic acetylcholine receptors. Cell Death Dis. 2010; 1(2): e27, doi: 10.1038/cddis.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Johnson EC, Cepurna WO, Choi D, Choe TE, Morrison JC.. Radiation pretreatment does not protect the rat optic nerve from elevated intraocular pressure–induced injury. Investig Ophthalmol Vis Sci. 2014; 56(1): 412–419, doi: 10.1167/iovs.14-15094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pang IH, Johnson EC, Jia L, et al.. Evaluation of inducible nitric oxide synthase in glaucomatous optic neuropathy and pressure-induced optic nerve damage. Investig Ophthalmol Vis Sci. 2005; 46(4): 1313–1321, doi: 10.1167/iovs.04-0829. [DOI] [PubMed] [Google Scholar]
- 54. Nissirios N, Chanis R, Johnson E, et al.. Comparison of anterior segment structures in two rat glaucoma models: an ultrasound biomicroscopic study. Investig Ophthalmol Vis Sci. 2008; 49(6): 2478–2482, doi: 10.1167/iovs.07-0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Walsh MM, Yi H, Friedman J, et al.. Gene and protein expression pilot profiling and biomarkers in an experimental mouse model of hypertensive glaucoma. Exp Biol Med. 2009; 234(8): 918–930, doi: 10.3181/0811-RM-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Colafrancesco, V, Coassin, M, Rossi, S, Aloe, L. Effect of eye NGF administration on two animal models of retinal ganglion cells degeneration. Ann Ist Super Sanita. 2011; 47(3): 284–289, doi: 10.4415/ANN_11_03_08. [DOI] [PubMed] [Google Scholar]
- 57. Huang W, Fileta JB, Filippopoulos T, Ray A, Dobberfuhl A, Grosskreutz CL.. Hsp27 phosphorylation in experimental glaucoma. Investig Ophthalmol Vis Sci. 2007; 48(9): 4129–4135, doi: 10.1167/iovs.06-0606. [DOI] [PubMed] [Google Scholar]
- 58. Taylor S, Calder CJ, Albon J, Erichsen JT, Boulton ME, Morgan JE.. Involvement of the CD200 receptor complex in microglia activation in experimental glaucoma. Exp Eye Res. 2011; 92(5): 338–343, doi: 10.1016/j.exer.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yang MH, Dibas A, Tyan YC.. Changes in retinal aquaporin-9 (AQP9) expression in glaucoma. Biosci Rep. 2013; 33(2): 379–385, doi: 10.1042/BSR20130005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chauhan BC, Pan J, Archibald ML, LeVatte TL, Kelly ME TF. Effect of intraocular pressure on optic disc topography, electroretinography, and axonal loss in a chronic pressure-induced rat model of optic nerve damage. Invest Ophthalmol Vis Sci. 2002; 43(9): 2969–2976. [PubMed] [Google Scholar]
- 61. Guo Y, Cepurna WO, Dyck JA, Doser TA, Johnson EC, Morrison JC.. Retinal cell responses to elevated intraocular pressure: a gene array comparison between the whole retina and retinal ganglion cell layer. Investig Ophthalmol Vis Sci. 2010; 51(6): 3003–3018, doi: 10.1167/iovs.09-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Johnson EC, Doser TA, Cepurna WO, et al.. Cell proliferation and interleukin-6-type cytokine signaling are implicated by gene expression responses in early optic nerve head injury in rat glaucoma. Investig Ophthalmol Vis Sci. 2011; 52(1): 504–518, doi: 10.1167/iovs.10-5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Johnson EC, Jia L, Cepurna WO, Doser TA, Morrison JC.. Global changes in optic nerve head gene expression after exposure to elevated intraocular pressure in a rat glaucoma model. Investig Ophthalmol Vis Sci. 2007; 48(7): 3161–3177, doi: 10.1167/iovs.06-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sappington RM, Carlson BJ, Crish SD, Calkins DJ.. The microbead occlusion model: a paradigm for induced ocular hypertension in rats and mice. Investig Ophthalmol Vis Sci. 2010; 51(1): 207–216, doi: 10.1167/iovs.09-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cone FE, Gelman SE, Son JL, Pease ME, Quigley HA.. Differential susceptibility to experimental glaucoma among 3 mouse strains using bead and viscoelastic injection. Exp Eye Res. 2010; 91(3): 415–424, doi: 10.1016/j.exer.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kalesnykas G, Oglesby EN, Zack DJ, et al.. Retinal ganglion cell morphology after optic nerve crush and experimental glaucoma. Investig Ophthalmol Vis Sci. 2012; 53(7): 3847–3857, doi: 10.1167/iovs.12-9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cone FE, Steinhart MR, Oglesby EN, Kalesnykas G, Pease ME, Quigley HA.. The effects of anesthesia, mouse strain and age on intraocular pressure and an improved murine model of experimental glaucoma. Exp Eye Res. 2012; 99(1): 27–35, doi: 10.1016/j.exer.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Frankfort BJ, Kareem Khan A, Tse DY, et al.. Elevated intraocular pressure causes inner retinal dysfunction before cell loss in a mouse model of experimental glaucoma. Investig Ophthalmol Vis Sci. 2013; 54(1): 762–770, doi: 10.1167/iovs.12-10581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Smedowski A, Pietrucha-Dutczak M, Kaarniranta K, Lewin-Kowalik J.. A rat experimental model of glaucoma incorporating rapid-onset elevation of intraocular pressure. Sci Rep. 2014; 4: 5910, doi: 10.1038/srep05910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Khan Kareem A, Tse DY, Van Der Heijden ME, et al.. Prolonged elevation of intraocular pressure results in retinal ganglion cell loss and abnormal retinal function in mice. Exp Eye Res. 2015; 130: 29–37, doi: 10.1016/j.exer.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tehrani S, Johnson EC, Cepurna WO, Morrison JC.. Astrocyte processes label for filamentous actin and reorient early within the optic nerve head in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2014; 55(10): 6945–6952, doi: 10.1167/iovs.14-14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Samsel PA, Kisiswa L, Erichsen JT, Cross SD, Morgan JE.. A novel method for the induction of experimental glaucoma using magnetic microspheres. Investig Ophthalmol Vis Sci. 2011; 52(3): 1671–1675, doi: 10.1167/iovs.09-3921. [DOI] [PubMed] [Google Scholar]
- 73. Urcola JH, Hernández M, Vecino E.. Three experimental glaucoma models in rats: comparison of the effects of intraocular pressure elevation on retinal ganglion cell size and death. Exp Eye Res. 2006; 83(2): 429–437, doi: 10.1016/j.exer.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 74. Dai C, Khaw PT, Yin ZQ, Li D, Raisman G, Li Y.. Structural basis of glaucoma: the fortified astrocytes of the optic nerve head are the target of raised intraocular pressure. Glia. 2012; 60(1): 13–28, doi: 10.1002/glia.21242. [DOI] [PubMed] [Google Scholar]
- 75. Hänninen VA, Pantcheva MB, Freeman EE, Poulin NR, Grosskreutz CL.. Activation of caspase 9 in a rat model of experimental glaucoma. Curr Eye Res. 2002; 25(6): 389–395, doi: 10.1076/ceyr.25.6.389.14233. [DOI] [PubMed] [Google Scholar]
- 76. Mukai R, Park DH, Okunuki Y, et al.. Mouse model of ocular hypertension with retinal ganglion cell degeneration. PLoS One. 2019; 14(1): e0208713, doi: 10.1371/journal.pone.0208713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schlamp CL, Johnson EC, Li Y, Morrison JC, Nickells RW.. Changes in Thy1 gene expression associated with damaged retinal ganglion cells. Mol Vis. 2001; 7: 192–201. [PubMed] [Google Scholar]
- 78. Iwamoto K, Birkholz P, Schipper A, Mata D, Linn DM, Linn CL.. A nicotinic acetylcholine receptor agonist prevents loss of retinal ganglion cells in a glaucoma model. Investig Ophthalmol Vis Sci. 2014; 55(2): 1078–1087, doi: 10.1167/iovs.13-12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jia L, Cepurna WO, Johnson EC MJ. Effect of general anesthetics on IOP in rats with experimental aqueous outflow obstruction. Invest Ophthalmol Vis Sci. 2000; 41(11): 3415–3419. [PubMed] [Google Scholar]
- 80. Jia L, Cepurna WO, Johnson EC MJ. Patterns of intraocular pressure elevation after aqueous humor outflow obstruction in rats. Invest Ophthalmol Vis Sci. 2000; 41(6): 1380–1385. [PubMed] [Google Scholar]
- 81. Cordeiro MF, Guo L, Luong V, et al.. Real-time imaging of single nerve cell apoptosis in retinal neurodegeneration. Proc Natl Acad Sci U S A. 2004; 101(36): 13352–13356, doi: 10.1073/pnas.0405479101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Guo L, Moss SE, Alexander RA, Ali RR, Fitzke FW, Cordeiro MF.. Retinal ganglion cell apoptosis in glaucoma is related to intraocular pressure and IOP-induced effects on extracellular matrix. Investig Ophthalmol Vis Sci. 2005; 46(1): 175–182, doi: 10.1167/iovs.04-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cesareo M, Martucci A, Ciuffoletti E, et al.. Association between Alzheimer's disease and glaucoma: a study based on Heidelberg retinal tomography and frequency doubling technology perimetry. Front Neurosci. 2015; 9(DEC): 1–8, doi: 10.3389/fnins.2015.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Xu XH, Zou JY, Geng W, Wang AY.. Association between glaucoma and the risk of Alzheimer's disease: a systematic review of observational studies. Acta Ophthalmol. 2019; 97(7): 665–671, doi: 10.1111/aos.14114. [DOI] [PubMed] [Google Scholar]
- 85. Ishikawa M, Yoshitomi T, Covey DF, Zorumski CF, Izumi Y.. Neurosteroids and oxysterols as potential therapeutic agents for glaucoma and Alzheimer's disease. Neuropsychiatry (London). 2018; 08(1): 344–359, doi: 10.4172/neuropsychiatry.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pang I-H, Clark AF.. Inducible rodent models of glaucoma. Prog Retin Eye Res. 2020; 75: 100799, doi: 10.1016/J.PRETEYERES.2019.100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhao M, Valamanesh F, Celerier I, et al.. The neuroretina is a novel mineralocorticoid target: aldosterone up-regulates ion and water channels in Müller glial cells. FASEB J. 2010; 24(9): 3405–3415, doi: 10.1096/fj.09-154344. [DOI] [PubMed] [Google Scholar]
- 88. Brown NJ. Aldosterone and end-organ damage. Curr Opin Nephrol Hypertens. 2005; 14(3): 235–241, doi: 10.1097/01.mnh.0000165889.60254.98. [DOI] [PubMed] [Google Scholar]
- 89. Guo L, Normando EM, Nizari S, Lara D, Francesca Cordeiro M. Tracking longitudinal retinal changes in experimental ocular hypertension using the cSLO and spectral domain-OCT. Investig Ophthalmol Vis Sci. 2010; 51(12): 6504–6513, doi: 10.1167/iovs.10-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cuenca N, Fernández-Sánchez L, Sauvé Y, et al.. Correlation between SD-OCT, immunocytochemistry and functional findings in an animal model of retinal degeneration. Front Neuroanat. 2014; 8(DEC): 1–20, doi: 10.3389/fnana.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Adachi K, Takahashi S, Yamauchi K, Mounai N, Tanabu R, Nakazawa M.. Optical coherence tomography of retinal degeneration in royal college of surgeons rats and its correlation with morphology and electroretinography. PLoS One. 2016; 11(9): e0162835, doi: 10.1371/journal.pone.0162835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Nadal-Nicolás FM, Vidal-Sanz M, Agudo-Barriuso M.. The aging rat retina: from function to anatomy. Neurobiol Aging. 2018; 61: 146–168, doi: 10.1016/j.neurobiolaging.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 93. Ramirez AI, de Hoz R, Salobrar-Garcia E, et al.. The role of microglia in retinal neurodegeneration: Alzheimer's disease, Parkinson, and glaucoma. Front Aging Neurosci. 2017; 9(JUL): 1–21, doi: 10.3389/fnagi.2017.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Nitta E, Hirooka K, Tenkumo K, et al.. Aldosterone: A mediator of retinal ganglion cell death and the potential role in the pathogenesis in normal-tension glaucoma. Cell Death Dis. 2013; 4(7): e711–6, doi: 10.1038/cddis.2013.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Takasago Y, Hirooka K, Nakano Y, Kobayashi M, Ono A.. Elevated plasma aldosterone levels are associated with a reduction in retinal ganglion cell survival. J Renin Angiotensin Aldosterone Syst. 2018; 19(3): 1470320318795001, doi: 10.1177/1470320318795001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhao M, Rodríguez-Villagra E, Kowalczuk L, et al.. Tolerance of high and low amounts of PLGA microspheres loaded with mineralocorticoid receptor antagonist in retinal target site. J Control Release. 2017; 266: 187–197, doi: 10.1016/j.jconrel.2017.09.029. [DOI] [PubMed] [Google Scholar]
- 97. Zhao M, Célérier I, Bousquet E, et al.. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest. 2012; 122(7): 2672–2679, doi: 10.1172/JCI61427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Prunte C, Flammer J.. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996; 121(1): 26–34, doi: 10.1016/S0002-9394(14)70531-8. [DOI] [PubMed] [Google Scholar]
- 99. Forooghian F, Meleth AD, Cukras C, Chew EY, Wong WT, Meyerle CB.. Finasteride for chronic central serous chorioretinopathy. Retina. 2011; 31(4): 766–771, doi: 10.1097/IAE.0b013e3181f04a35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Moore S, Thanos S.. Differential increases in rat retinal ganglion cell size with various methods of optic nerve lesion. Neurosci Lett. 1996; 207(2): 117–120, doi: 10.1016/0304-3940(96)12500-3. [DOI] [PubMed] [Google Scholar]
- 101. Neumann F, Wurm A, Linnertz R, et al.. Sex steroids inhibit osmotic swelling of retinal glial cells. Neurochem Res. 2010; 35(4): 522–530, doi: 10.1007/s11064-009-0092-8. [DOI] [PubMed] [Google Scholar]
- 102. O'steen WK, Sweatt AJ, Eldridge JC, Brodish A. Gender and chronic stress effects on the neural retina of young and mid-aged Fischer-344 rats. Neurobiol Aging. 1987; 8(5): 449–455, doi: 10.1016/0197-4580(87)90040-6. [DOI] [PubMed] [Google Scholar]
- 103. Roche SL, Wyse-Jackson AC, Gómez-Vicente V, et al.. Progesterone attenuates microglial-driven retinal degeneration and stimulates protective fractalkine-CX3CR1 signaling. PLoS One. 2016; 11(11): 1–27, doi: 10.1371/journal.pone.0165197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. O'Steen WK. Ovarian steroid effects on light-induced retinal photoreceptor damage. Exp Eye Res. 1977; 25(4): 361–369, doi: 10.1016/0014-4835(77)90103-8. [DOI] [PubMed] [Google Scholar]
- 105. Porciatti V. Electrophysiological assessment of retinal ganglion cell function. Exp Eye Res. 2014; 141: 164–170, doi: 10.1016/j.exer.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Viswanathan S, Frishman LJ, Robson JG WJ. The photopic negative response of the flash electroretinogram in primary open angle glaucoma. Invest Ophthalmol Vis Sci. 2001; 42(2): 514–522. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.