Abstract
Background
The COVID-19 pandemic, induced by the worldwide spreading of the SARS-CoV-2, is well known for its clinical picture consistent with respiratory symptoms. If pulmonary complications are the most common manifestation of the disease, neurological problems are also significantly present, with complications including acute cerebrovascular events, encephalitis, Guillain-Barré and Miller Fisher syndromes, acute necrotizing hemorrhagic encephalopathy and hemophagocytic lymphohistiocytosis. These medical signs can be considered direct effects of the virus on the nervous system, para-infectious or post-infectious immune-mediated diseases, and neurological complications of the systemic effects of the SARS-CoV-2.
Case
In the present article, the encephalitis case in a 5-year-old girl positive for COVID-19 admitted to the emergency department complaining of fever and swelling in the neck is described. At this time, her neurological examination was unremarkable. Over the next few days, the fever went down and she experienced acute behavioral changes, mild confusion, and drowsiness. The brain MRI and electroencephalography (EEG) showed CNS involvement, suggestive of encephalitis.
Conclusion
The dramatic improvement of the symptoms after immunotherapy with corticosteroids reinforced the hypothesis of an immune-related mechanism.
Keywords: COVID-19, SARS-CoV-2, Neurological manifestations, Pediatric patient, Encephalitis
Introduction
Occasional viral encephalitis in children and young adults include a large and heterogeneous group of inflammatory brain diseases, characterized by different symptoms and variable onset. The most frequent etiological agents are herpes simplex, varicella zoster, Epstein-Barr viruses, cytomegalovirus, human immunodeficiency virus (HIV), and enteroviruses [1]. The ways of access and propagation of the viruses are mainly the respiratory tract, the oral cavity, and also the peripheral nerves, and among them, the olfactory bulb represents a preferential route [2]. The differential diagnosis between the various viral forms is complex, based on laboratory data, confirmed infection, and examination of the liquor. The neuroimaging also can highlight the presence of lesions in the brain parenchyma, confined in typical locations, prevalent prerogative of a specific viral agent [3].
MRI of the brain is the main examination, essential for the study of a patient suspected of suffering from encephalitis. The test allows performing morphological and functional study of the brain, with early identification of any lesion of the cerebral parenchyma, even in the hyperacute phase [3]. After injection of the paramagnetic contrast agent, the image sequence can resolve any alteration of the blood-brain barrier and associated meningeal modification [4].
SARS-CoV-2 is a large single-stranded RNA virus. The S protein, protruding from the viral envelop, is the main antigenic component, constituted by the S1 and S2 subunits [5]. The cell invasion by SARS-CoV-2 begins with the binding of the virus to the angiotensin-converting enzyme 2 (ACE2) receptors, highly expressed in neurons and glial cells of the CNS and epithelial cells of respiratory system [6] and, to a lesser extent, in the kidneys, heart, adipose tissue, and reproductive organs of both sexes [7]. In the following step, the host transmembrane serine protease 2 cleaves the S protein at the junction between the two domains, causing the insertion of the S2 domain into the cell membrane and allowing the penetration of the viral genome into the host cell cytoplasm [7, 8]. The new viruses are assembled in the endoplasmic reticulum and Golgi apparatus and ready to infect any other cell expressing the ACE2 receptors.
The infection induced by the new SARS-CoV-2 occurs in various forms, from asymptomatic to severe, and usually they are strongly linked to the age and health conditions of the patients. In the symptomatic form, common manifestations include loss of smell and taste, fever, cough, sore throat, fatigue, and general feeling of malaise. Some gastrointestinal symptoms are also frequent, such as diarrhea, nausea, and anorexia [9–11].
Although the SARS-CoV-2 infection mainly affects the respiratory system causing, in the severe form, lung injuries and failure [12], the occurrence of neurological symptoms in COVID-19 patients is more and more evident and constantly growing. As experienced by numerous patients, also at the onset of the infection, clinical evidence has highlighted the marked neurotropism of the new SARS-CoV-2.
From a clinical point of view, the involvement of the CNS is manifested through the appearance of more or less serious symptoms, from headache, disturbances of smell, and anosmia, to impaired consciousness, acute cerebrovascular disease, ataxia, and seizure [2, 10, 11]. Pavone and coworkers [13] reported two cases of pediatric patients showing a temporal correlation between the insurgence of COVID-19 infection and the onset of pediatric acute neuropsychiatric syndrome, an obsessive-compulsive disorder associated with other cognitive, behavioral, and/or neurological symptoms [13].
The route for the invasion of the CNS typically starts in the peripheral tissues, and then the virus spreads to the peripheral nerves through which it reaches the CNS [10, 11]. Furthermore, the crossing of the blood-brain barrier directly from the bloodstream has been observed.
The virus can cause neurological disorders through the action of different mechanisms, that is, (i) hypoxic injury, (ii) hypercoagulability, and (iii) inflammatory response [14]. In the first one, patients with severe COVID-19 may develop serious shortness of breath and hypoxia [5]. During hypoxia, the excessive accumulation of anaerobic metabolites in the mitochondria and acid metabolites in the brain leads to edema of the brain cells and obstruction of the cerebral blood flow [15].
The hypercoagulability mechanism, frequently observed in patients with severe COVID-19 infection, could predispose to stroke [15]. A retrospective analysis revealed that abnormal coagulation, including markedly elevated levels of the D-dimer breakdown products and fibrin, longer prothrombin time, and activated partial thromboplastin time, is associated with a poor prognosis. Furthermore, disseminated intravascular coagulation is commonly associated with lethal COVID-19 form [16].
Several studies have indicated that the Sars-CoV-2 induces proinflammatory cytokine signals releasing a large amount of inflammatory factors such as IL-6, IL-12, IL-15, IL-1β, and TNF-α [17, 18]. This is one of the pathophysiological processes responsible for neurological and brain damages caused by the inflammatory mechanism in SARS-Co-2 disease [18]. Cytokine storm can stimulate an immune attack in the body, causing multiple organ failure. These mechanisms can also act, simultaneously, in combination [19].
How SARS-CoV-2 can cause neurological effects in pediatric patients is still unclear [13]. The direct viral injury to neural cells through the olfactory nerves, the endothelial vascular damage due to the interaction with angiotensin-converting enzyme 2 receptors, and the inflammatory response are the hypothesized mechanisms [13].
Although the clinical picture of the acute phase of Covid-19 is, in itself, very complex and affects many organs, recent studies have identified a new consequence of SARS-CoV-2 infection with medium- and long-term effects, involving the nervous system, called Post-COVID-19 Neurological Syndrome. Although it mainly occurs in patients with severe COVID-19 forms, it can even affect patients with mild-COVID-19 infection and it concerns one-third of the SARS-CoV-2-affected people [11]. The pathology originates from molecular mechanisms that give rise to neuroinflammation, compromises the social skills and quality of life of the affected patients and it will represent the new challenge in the fight against coronavirus in the next future [11, 20–22].
Case report
In October 2020, a 5-year-old previously healthy girl started experiencing some mild, self-limiting cough symptoms associated with a fever episode. Family and patient histories did not indicate anything significant and no medication was taken by the girl. A couple of days later, she was admitted to the emergency department complaining of a painful swelling in her neck. She especially presented a painful tumefaction in the right latero-cervical area, with a large erythematous patch of the overlying skin. The patient showed negativity to SARS-CoV-2 infection, as indicated by rapid swab test.
Therefore, due to the clinical suspect of lymph node abscess, an ultrasound of the neck was immediately requested and performed (Fig. 1). The study highlighted the presence, in correspondence with the clinical finding, of multiple lymph nodes with oval morphology and a slightly inhomogeneous and hypoechoic structure (size of about 1.6 cm), while normal morphology in the lymph nodes on the left side was observed.
Fig. 1.
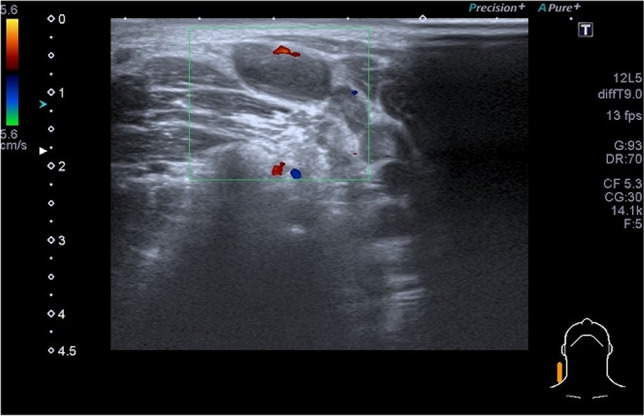
Ultrasound of the neck indicating the lymph node swelling
On the next day, the little patient underwent a standard chest X-ray examination and ultrasound test of the complete abdomen. They were both normal.
In the following days, a sudden worsening of the clinical conditions was observed with the appearance of neurological symptoms such as altered mental status, increased irritability, sleepiness, lack of energy, and lethargy.
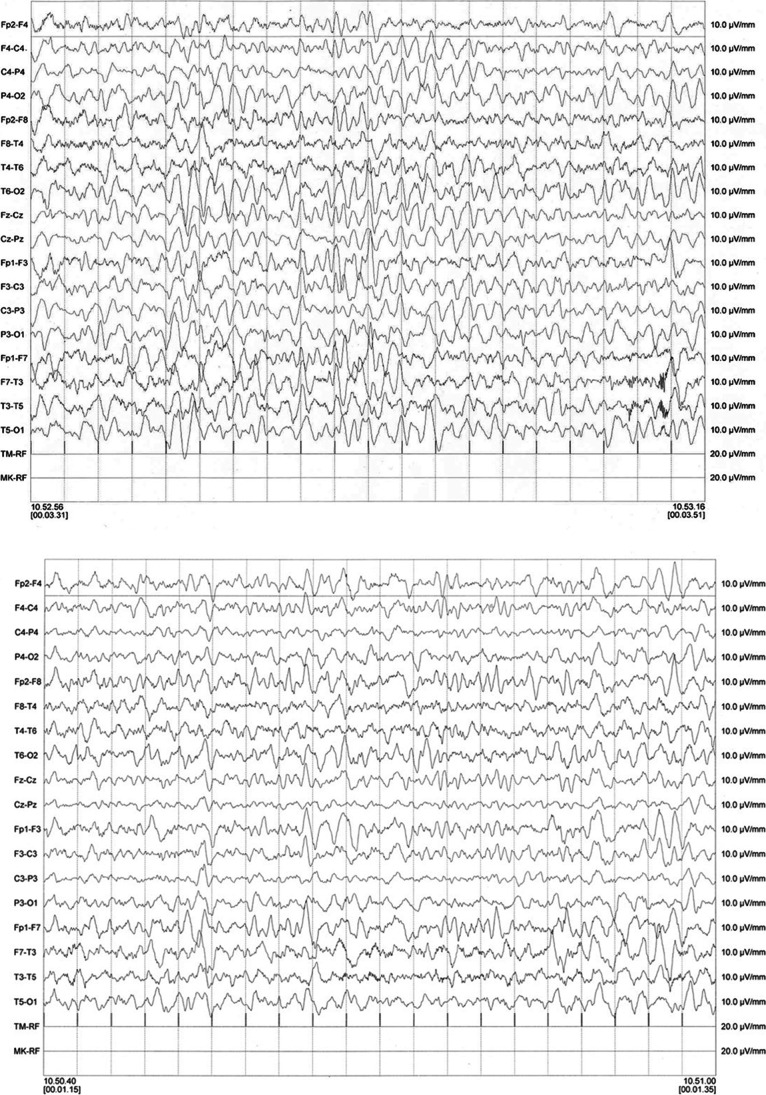
Serological investigations for HSV1, varicella-zoster, Epstein-Barr, and CMV were negative. The RT-PCR test for SARS-CoV-2 was performed using a nasopharyngeal swab which was positive. Arterial blood gas analysis indicated a PO2/FiO2 ratio of 240 mmHg. Electroencephalogram did not display any significant epileptic discharges, but a widespread slowing of the underlying rhythm. The EEG (Fig. 2) showed a slow base rhythm (theta-delta) together with synchronous bilateral potentials formed by slow waves with predominance on the right side.
Fig. 2.
EEG showing a widespread slowing of the underlying rhythm
A MRI examination of the brain was immediately requested, with and without contrast medium.
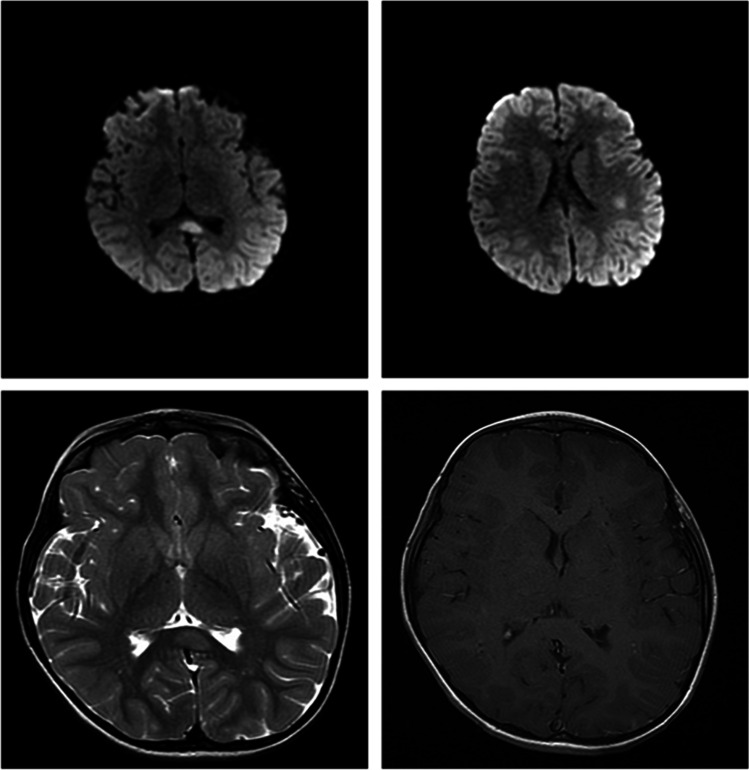
The MRI studies were promptly performed at the Radiology Unit of the hospital on 1.5-T MR scanner. The sequences included fast spin echo (FSE), diffusion-weighted imaging (DWI), T1WI, T2-weighted imaging (T2WI), and T2-fluid-attenuated inversion recovery (T2-FLAIR) in the axial plane, T2WI or T2-FLAIR in the sagittal plane, and CE T1WI in the axial, coronal, and sagittal planes after injection of 0.1 mmol/kg of GBCAs (Fig. 3).
Fig. 3.
Magnetic resonance imaging (MRI) of brain. Axial DWI images show an hyperintense focal lesion in the splenium of corpus callosum and an additional area in the left parietal subcortical site (A and B). Axial T2 image of the same lesion of splenium of corpus callosum that appears subtly hyperintense (C). Axial MRI CE does not evidence any pathological enhancement of the lesion (D)
Axial DWI demonstrated the presence of a hyperintense focal lesion in the splenium of the corpus callosum, which appeared slightly swollen, and an additional area with the same signal characteristics in the right parietal subcortical area. In the long TR sequences, the lesions appeared hyperintense, but did not show any contrast enhancement.
The remaining regions were within limits.
These lesions were compatible with parenchymal cerebral edema due to brain inflammation in accordance with the diagnosis of encephalitis in patient with SARS-CoV-2.
An intravenous treatment with ceftriaxone (50 mg/kg/day), vancomycin (15 mg/kg/day), acyclovir (0.8 mg/kg every 8 h), and steroids (dexamethasone 0.6 mg/kg/day) was immediately started. Lumbar puncture was performed and the cerebrospinal fluid (CSF) was clear and colorless. CSF laboratory tests showed WBC 0.5×107/L (normal value <4), protein 0.27 g/L (normal 0.15–0.45), and normal glucose. The CSF specimen was negative for SARS-CoV-2 test. The patient did not evidence any bacterial or tuberculous infection of the CNS. Anti-HSV 1, varicella-zoster, Epstein-Barr, and CMV IgM antibodies were not detected in serum samples. After a cycle of high-dose intravenous methylprednisolone, a quick and complete remission of symptoms was observed. Two weeks later, the patient was discharged from hospital after two consecutive and negative throat swab tests for SARS-CoV-2. No consequences of the infection can be recorded at the present time.
Discussion
Paterson and colleagues [23] described in 2020 the emerging spectrum of COVID-19 neurological syndromes. Guillain-Barré and Miller Fisher syndromes, acute necrotizing hemorrhagic encephalopathy, and acute disseminated encephalomyelitis have been described in COVID-19 patients suggesting a key role of the host-immune response mechanism rather than a direct neuro-invasion of the SARS-CoV-2 [24–27]. In fact, a subsidiary effect of the virus invasion is the reaction of the innate immune system leading to uncontrolled inflammatory response and the so-called cytokine storm. This, in turn, causes apoptosis of cells and vascular leakage and, in many cases, death [28].
The early suspicion of COVID-19 encephalitis and the appropriate CSF studies were the key to establish the correct diagnosis and timely management of the pathology. Despite the absence of CSF pleocytosis, the suspicion of CNS encephalitis should still be considered. Although the conclusive diagnosis of viral encephalitis largely depends on virus isolation, this can be difficult for COVID-19, because SARS-CoV-2 dissemination is transient and its CSF titer may be extremely low [29]. Consistently, anti-SARS-CoV-2 IgM and IgG were not detectable in the patient’s CSF sample [29]. Therefore, as mentioned before, a physical evaluation of neurological symptoms and noninvasive tests such as brain RMN and EEG are important to lead a presumptive diagnosis [30]. From this case, it can be assumed that encephalitis in children is a complication of COVID-19 infection or, indeed, that it represents the first manifestation of the infection [29]. After multiplication in the primary (subcutaneous, respiratory mucosa, lymph nodes) and secondary (endothelium, muscle, marrow) sites, the SARS-CoV-2 would trigger an abnormal immune and/or inflammatory response, through still not completely understood mechanisms, causing damage to the vascular endothelium of the brain, arteriolar angiopathy, and in some cases direct, onconeural damage as described in literature in many dysimmune encephalitis [31]. The precise pathogenesis of encephalitis with SARS-CoV-2 infection is still unclear as well as its histopathological features. Viral epitopes resembling myelin antigens have the ability to activate myelin-reactive T-cell clones via molecular mimicry [32], and may thus elicit a CNS-specific autoimmune response, as in acute disseminated encephalomyelitis. Taken together, our findings support the hypothesis that corticosteroids therapy can be effective in the treatment of severe COVID-19-related encephalitis. Although rare, pediatric encephalitis cases related to SARS-CoV-2 para-infection and post-infection have already been reported in literature. Some results, highlighting symptom similarities and differences with the case here reported, are summarized in Table 1. Once more, it is important to highlight that the early establishment of the diagnosis and the immediate commencement of a management plan may contribute to a better outcome.
Table 1.
Encephalitis pediatric cases developed as SARS-CoV-2 para-infection or post-infection in previously healthy patients. The sex of patients is indicated as F for female and M for male
| Reference | Age (years) | Condition | COVID-19 symptoms | Encephalitis symptoms |
|---|---|---|---|---|
| Present case | 5 F | Para-infection | Cough, fever | Neck swelling, right latero-cervical and painful lymphadenopathy, altered mental status, irritability, sleepiness, lethargy, lack of energy |
| [13] | 12 M | Para-infection | Absent | Severe emotional lability, facial tics, obsessive-compulsive disorder |
| [13] | 13 M | Para-infection | Cough, fever, skin rush | Obsessive-compulsive disorder, facial tic, hyperactivity, aggressiveness, irritability, inattentiveness |
| [33] | 16 F | Para-infection | Sore throat, fever | Insomnia, anorexia, paranoia, hallucinations, severe encephalopathy |
| [34] | 10 F | Para-infection | Ageusia, headache, malaise, urinary incontinence | Stop speaking, mobilizing, and using right arm, hypertonia, brisk reflexes, right-sided Babinski, and sluggish pupils |
| [35] | 13 F | Para-infection | Fever | Headache, non-explosive vomiting, sudden-onset sensory disorder |
| [36] | 11 M | Para-infection | Weakness | Epilepsy |
| [37] | 7 M | Para-infection | Absent | Unsteady gait, ataxia, somnolence, seizures, not elicited deep tendon reflexes |
| [38] | 5 F | Para-infection | Fever, painful abdomen, diarrhea | Neck swelling, right latero-cervical and painful lymphadenopathy |
| [39] | 0.75 Unknown | Para-infection | Fever, breath shortness | Seizures, enlargement of lateral ventricles, hydrocephalus |
Conclusions
From this clinical case, it emerges that encephalitis in children can be a complication of COVID-19 infection, or it can be even considered that the encephalitis itself could be the initial manifestation of a not yet overt infection. Furthermore, the case suggests that neurological manifestations might be expected in COVID-19 infection, despite the absence of significant respiratory symptoms.
The neurological mechanism of viral agents, in general, and of COVID-19 in particular, could occur in different steps. Initially, we observe a multiplication in the primary (subcutaneous, respiratory mucous membranes, lymph nodes) and secondary (endothelium, muscle, marrow) sites. Then, the subsequent viremia would cause capillary infiltration, an abnormal immune and/or inflammatory response, which, with a still unknown mechanism, could damage the vascular endothelium of the brain, and cause an arteriolar angiopathy and development of lesions of the cerebral parenchyma of the relative affected regions. In conclusion, it appears quite evident that COVID-19 is a complex pathology made more complex by the presence of comorbidities, such as obesity and diabetes, and age of the patients.
Declarations
Ethical approval
Ethical approval for this study was obtained from the Institutional Review Board Statement (May 19, 2021). All procedures performed were in accordance with the ethical standards of the institutional and national research committees and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
The patient parents gave their written consent to analyze retrospectively her clinical data in order to publish the present case report.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lidia Urso, Email: lidiaurso76@gmail.com.
Maria Grazia Distefano, Email: mary.distefano@live.it.
Gaetano Cambula, Email: gaetanocambula@libero.it.
Angela Irene Colomba, Email: angecolomba@gmail.com.
Domenico Nuzzo, Email: domenico.nuzzo@cnr.it.
Pasquale Picone, Email: pasquale.picone@cnr.it.
Daniela Giacomazza, Email: daniela.giacomazza@cnr.it.
Luigi Sicurella, Email: luigi.sicurella@asptrapani.it.
References
- 1.Kennedy PEG. Viral encephalitis: causes, differential diagnosis, and management. J Neurol Neurosurg Psychiatry. 2004;75:i10–i15. doi: 10.1136/jnnp.2003.034280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu J, Jolkkonen J, Zhao C. Neurotropism of SARS-CoV-2 and its neuropathological alterations: similarities with other coronaviruses. Neurosci Behav Rev. 2020;119:184–193. doi: 10.1016/j.neubiorev.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10:102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kihira S, Delman BN, Belani P, Stein L, Aggarwal A, Rigney B, Schefflein J, Doshi AH, Pawha PS. Imaging features of acute encephalopathy in patients with COVID-19: a case series. Am J Neuroradiol. 2020;41:1804–1808. doi: 10.3174/ajnr.A6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Y, Yang C, Xu XF, Xu W, Liu SW. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41:1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alenina N, Bader M. ACE2 in brain physiology and pathophysiology: evidence from transgenic animal models. Neurochem Res. 2019;44:1323–1329. doi: 10.1007/s11064-018-2679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murgolo N, Therien AG, Howell B, Klein K, et al. SARS- CoV-2 tropism, entry, replication, and propagation: considerations for drug discovery and development. PLoS Pathog. 2021;17:e1009225. doi: 10.1371/journal.ppat.1009225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nuzzo D, Cambula G, Bacile I, Rizzo M, Galia M, PMangiapane P, Picone P, Giacomazza D, Scalisi L. Long-term brain disorders in PostCovid-19 neurological syndrome patient. Brain Sci. 2021;10:1947. doi: 10.3390/brainsci11040454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nuzzo D, Vasto S, Scalisi L, Cottone S, Cambula G, Rizzo M, Giacomazza D, Picone P. Post-acute COVID-19 neurological syndrome: a new medical challenge. J Clin Med. 2021;11:454. doi: 10.3390/jcm10091947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung NHL (2021) Trasmissibility and transmission of respiratory viruses. Nature Rev – Microbiol. 10.1038/s41579-021-00535-6 [DOI] [PMC free article] [PubMed]
- 13.Pavone P, Ceccarelli M, Marino S, Caruso D, Falsaperla R, Berretta M, Rullo EV, Nunnari G. SARS-CoV-2 related paediatric acute-onset neuropsychiatric syndrome. Lancet Child Adolesc Health. 2021;5:e19–e21. doi: 10.1016/S2352-4642(21)00135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zirpe KG, Dixit S, Kulkarni AP, Kakkar G, Gupta R, Bansal AR, Garg A, et al. Pathophysiological mechanisms and neurological manifestations in COVID-19. Indian J Crit Care Med. 2020;24:975–980. doi: 10.5005/2Fjp-journals-10071-23592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdennour L, Zeghal C, Dème M, Puybasset L. Interaction brain-lungs. Ann Fr Anesth Reanim. 2012;2012(31):e101–e107. doi: 10.1016/j.annfar.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chowdhury MA, Hossain N, Kashem MA, Shaid A, Alam A. Immune response in COVID-19. A review. J Inf Pub Health. 2020;13:1619–16-29. doi: 10.1016/j.jiph.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Fu L, Gonzales DM, Lavi E. Coronavirus neurovirulence correlates with the ability of the virus to induce proinflammatory cytokine signals from astrocytes and microglia. J Virol. 2004;78:3398–3406. doi: 10.1128/JVI.78.7.3398-3406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paramo JA (2020) Inflammatory response in relation to COVID-19 and other prothrombotic phenotypes. Reumatologia Clinica (Eng. Ed.). 10.1016/j.reumae.2020.06.007 [DOI] [PMC free article] [PubMed]
- 20.Camargo-Martinez W, Lozada-Martinez I, Escobar-Collazos A, Navarro-Coronado A, Moscote-Salazar L, Pacheco-Hernandez A, Janjua T, Bosque-Varela P. Post-Covid 19 neurological syndrome: implications for sequelae’s treatment. J Clin Neurosci. 2021;88:219–225. doi: 10.1016/j.jocn.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frontera JA, Yang D, Lewis A, Patel P, Medicherla C, et al. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J Neurol Sci. 2021;426:17486. doi: 10.1016/j.jns.2021.117486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez-Herazo MA, Silva-Nugnoz DC, Guevara-Martinez PA, Lozada-Martinez ID (2021) Post-COVID 19 neurological syndrome: a fresh challenge in neurological management. Polish J Neurol Neurosurg. 10.5603/PJNNS.a2021.0052 [DOI] [PubMed]
- 23.Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gutierrez-Ortiz C, Mendez A, Rodrigo-Rey S, San Pedro-Murillo E, Bermejo-Guerrero L, Gordo-Manas R, de Aragon-Gomez F, Benito-Leon J. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95:e601–e605. doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 25.Novi G, Rossi T, Pedemonte E, Saitta L, Rolla C, Roccatagliata L, Inglese M, Farinini D. Acute disseminated encephalomyelitis after SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7:e797. doi: 10.1212/NXI.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, Franciotta D, Baldanti F, Daturi R, Postorino P, Cavallini A, Micieli G. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382:2574–2576. doi: 10.1056/nejmc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weyhern CV, Kaufmann I, Neff F, Kremer M (2020) Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet 395:e109. 10.1016/S0140-6736(20)31282-4 [DOI] [PMC free article] [PubMed]
- 28.Tang Y, Liu L, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haider H, Siddiqa A, Ali N, Dhallu M. COVID-19 and the brain: acute encephalitis as a clinical manifestation. Cureus. 2020;12:e10784. doi: 10.7759/2Fcureus.10784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nuzzo D, Picone P. Potential neurological effects of severe COVID-19 infection. Neurosci Res. 2020;158:1–5. doi: 10.1016/j.neures.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Dault E, Lagarde S, Guedj E, Dufournet B, Rey C, Kaphan E, Tanguy G, Bregigeon M, Sagui E, Brosset C (2016) Unexplicated neuropsychiatric disorders: do not ignore dysimmune encephalitis. A case report of a dysimmune encephalitis with anti-leucine rich glioma inactivated 1 (LGI-1) antibodies. Rev Med Interne 37:127–130. 10.1016/j.revmed.2015.06.007 [DOI] [PubMed]
- 32.Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaughan M. Pediatric parainfectious encephalitis associated with COVID-19. Neurology. 2021;96:541–544. doi: 10.1212/WNL.0000000000011476. [DOI] [PubMed] [Google Scholar]
- 34.Vraka K, Ram D, West S, Chia WYE, Kurup P, Subramanian G, Tan HJ (2021) Two paediatric patients with encephalopathy and concurrent COVID-19 infection: Two sides of the same coin? Case Rep Neurol Med:6658000. 10.1155/2021/6658000 [DOI] [PMC free article] [PubMed]
- 35.Conto-Palomino NM, Cabrera-Bueno ML, Vargas-Ponce KG, Rondón-Abuhadba EA, Atamari-Anahui N. Encephalitis associated with COVID-19 in a 13-year-old girl: a case report. Medwave. 2020;20:e7985. doi: 10.5867/medwave.2020.07.7984. [DOI] [PubMed] [Google Scholar]
- 36.McAbee GN, Brosgol Y, Pavlakis S, Agha R, Gaffoor M. Encephalitis associated with COVID-19 infection in an 11-year-old child. Pediatr Neurol. 2020;109:94. doi: 10.1016/j.pediatrneurol.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarigecili E, Arslan I, Ucar HK, Celik U. Pediatric anti-NMDA receptor encephalitis associated with COVID-19. Child’s Nerv Syst. 2021;14:1–4. doi: 10.1007/s00381-021-05155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siracusa L, Cascio A, Giordano S, Medaglia AA, Restivo GA, Pirrone I, Saia GF, Collura F, Colomba C. Neurological complications in pediatric patients with SARS-CoV-2 infection: a systematic review of the literature. Ital J Pediatr. 2021;47:123. doi: 10.1186/s13052-021-01066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sofijanova A, Bojadzieva S, Duma F, Superlishka E, Murtezani A, Jordanova O. Severe encephalitis in infant with COVID-19: a case report. Maced J Med Sci. 2020;8:514–517. doi: 10.3889/oamjms.2020.5485. [DOI] [Google Scholar]
- 40.Hess DC, Eldahshan W, Rutkowski E. COVID-19-related stroke. Transl Stroke Res. 2020;11:322–325. doi: 10.1007/s12975-020-00818-9. [DOI] [PMC free article] [PubMed] [Google Scholar]