Abstract
Background
Norovirus is the most common cause of acute gastroenteritis in Canada. The illness causes great morbidity and high societal costs. The objective of this article is to describe the epidemiology of norovirus in the province of Ontario, Canada from 2009 to 2014.
Methods
To assess activity of norovirus and viral gastroenteritis (VGE) in Ontario, three datasets were acquired from the provincial government: two traditional surveillance datasets (outbreak and laboratory) and syndromic surveillance data (telehealth), all spanning 2009–2014. All outbreaks, laboratory submissions and telehealth calls were first assessed for total VGE. Norovirus and norovirus-like illness totals were calculated as a proportion of VGE to estimate agent-specific activity levels. Affected institution types, sexes and age groups were also analyzed.
Results
Between 2009 and 2014, 41.5% of VGE outbreaks, 63.4% of VGE laboratory submissions and 36.6% of all acute gastroenteritis-related (not restricted to viral causes) telehealth calls were attributed to norovirus and norovirus-like illness in Ontario. The most commonly affected institution type was long-term care homes and the most commonly affected age groups were younger (younger than five years) and older (older than 65 years) individuals. Females were slightly more frequently affected than males.
Conclusion
Norovirus and norovirus-like illnesses were the leading cause of VGE in Ontario between 2009 and 2014. They comprised the greatest percentage of VGE when compared with all other VGE-associated viruses. Additional work is needed to determine all component costs and necessary public health actions to reduce the burden of disease.
Keywords: norovirus, viral gastroenteritis, acute gastroenteritis, surveillance, Ontario, Canada
Introduction
Norovirus is the most common cause of infectious gastroenteritis in Ontario, Canada (1–3). It comprises roughly 50% of acute gastroenteritis (AGE) (all aetiologies) (4). Its high morbidity rate is due to its low infectious dose (approximately 18–1,000 viral particles), various transmission routes, extended viral shedding, short-lasting immunity and persistence in the environment (5,6). Its efficient transmission allows it to thrive in areas of concentrated populations, such as cruise ships and nursing homes (1,7). The burden of disease is high, with an estimated 3.4 million cases and hospital expenditures of 21 million CAD per year in Canada (2,8). It has also been estimated that, in the United States, the average person will experience five episodes of norovirus during their lifetime (9).
The disease is characterized by the sudden onset of nausea, vomiting, diarrhea, abdominal cramps and malaise and is transmitted via the fecal-oral route and aerosolized vomitus (1,5). The incubation period is short (approximately 10–48 h) and symptoms typically clear in 1–3 days; however, this is often longer in high-risk individuals, such as the very young and elderly (1,5). The illness is typically treated with outpatient care (10), although sequelae and serious side-effects, such as irritable bowel syndrome, necrotizing enterocolitis or death can occur (1,11).
Children are particularly susceptible to the disease, requiring medical attention more frequently than any other age group (10,12). This underscores the need for surveillance to inform public health, plan appropriate intervention measures and develop vaccines (13). A lack of formal reporting mechanisms for norovirus (and AGE in general) leads to knowledge gaps. Approximately 15% of individuals suffering from AGE seek medical care and, of those, diagnostic samples are requested from only 13% (14).
In this study, we describe the epidemiology of norovirus in the province of Ontario, Canada using confirmed outbreak data, laboratory testing data, and telemedicine calls with vomiting calls as a proxy.
Methods
This study was conducted using data from the Canadian province of Ontario, which had a population of approximately 14.3 million residents at the time of this study (15).
Datasets
All data acquired and used in this study were anonymized (no personal identifiers). For further information, see Appendix.
Outbreaks: The integrated Public Health Information System (iPHIS) dataset represents confirmed outbreaks of viral gastroenteritis (VGE) in institutions in Ontario reported to local public health units.
Laboratory reports: The Public Health Ontario Laboratories (PHOL) dataset represents all samples submitted to Public Health Ontario (PHO) with suspected VGE for confirmatory testing; more specifically, this dataset contains all samples sent to PHO with suspected norovirus or rotavirus infection.
Telehealth calls: The Telehealth Ontario (THO) dataset represents all calls made to the provincial telehealth service with gastrointestinal chief complaints. These gastrointestinal calls represent a collection of AGE symptoms, encompassing a broader scope than just VGE calls, captured by the nurses at THO. Callers may be ill with these gastrointestinal symptoms for a range of reasons including norovirus. Therefore, telehealth calls with the selected chief complaints ”vomiting” and “vomiting with diarrhea” were selected as the “vomiting chief complaints” and used as a proxy for norovirus activity in this study. The vomiting chief complaint was chosen due to its compliance with the main presenting symptoms of norovirus illness and evidence from prior studies demonstrating its role as an indicator of the disease (1,5,16).
Data analyses
All three datasets in this study were analyzed for total VGE outbreaks and the proportion attributed to norovirus (or in the case of THO data, gastroenteritis illness due to the inability to confirm presence of norovirus). These percentages were used to assess norovirus activity levels in Ontario. Ontario census data, as well as the total number of institution type (child care centre, long-term care home, retirement home, correctional facility) were used as denominator data to standardize select analyses (17–20).
Descriptive analyses were performed on using SAS v.9.4 (Cary, North Carolina, United States) and Microsoft Office (Excel) 2010 (Redmond, Washington, United States).
Results
Outbreaks: There were 3,100 VGE outbreaks in Ontario during the years 2009–2014; 41.6% were caused by norovirus, either by case definition and/or laboratory confirmation. The remaining 58.4% were attributed to adenoviruses, astroviruses, enteroviruses/echoviruses, rotavirus, other caliciviruses and gastroenteritis unspecified/other.
During 2009–2014, 45.1% of VGE outbreaks were in long-term care homes, 30.9% in child care facilities, 22.6% in retirement homes, 0.3% in correctional facilities and 1.2% in other settings. This distribution remained relatively consistent when the analysis was restricted to norovirus outbreaks, in which case retirement homes replaced child care facilities as the second most frequently affected institution type. Of those VGE outbreaks in long-term care homes, more than half (57.2%) were attributed to norovirus. An institutional breakdown for norovirus outbreaks is shown in Figure 1.
Figure 1.
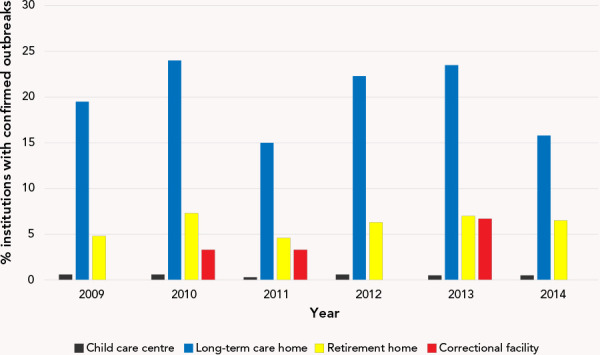
Percent of institutions affected by norovirus outbreaks in Ontario, 2009–2014
The number of norovirus outbreaks per year was relatively stable across the six years; 211 in 2009, 265 in 2010, 178 in 2011, 247 in 2012, 215 in 2013 and 175 in 2014. This is comparable to the stability of VGE outbreaks across the same period.
The seasonal distribution of norovirus outbreaks by month and year is shown in Figure 2. The average duration of VGE outbreaks was 12.6 days (range 1–78 days), and for norovirus outbreaks was 14.1 days (range 1–52 days).
Figure 2.
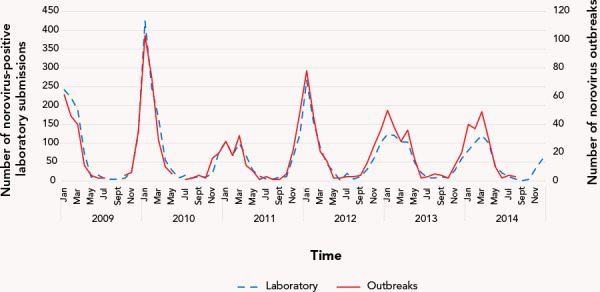
Seasonality of norovirus laboratory submissionsa and outbreaksb in Ontario by month and year, 2009–2014
a Laboratory submissions were confirmed using Public Health Ontario Laboratories (PHOL) data
b Outbreaks were confirmed using integrated Public Health Information System (iPHIS) data
Laboratory reports: There were 29,459 submitted samples for rotavirus and norovirus-like VGE to PHO between 2009 and 2014, inclusive. The majority (n=22,147; 75.2%) were negative. Among positive samples (n=7,312), 63.4% were attributed to norovirus, with the remaining 36.6% composed of various other VGE aetiologies including adenovirus (16.5%), astrovirus (1.1%), other caliciviruses (0.3%), picornaviruses (0.3%), rotavirus (81.0%) and/or sapovirus (0.5%) (Figure 3).
Figure 3.
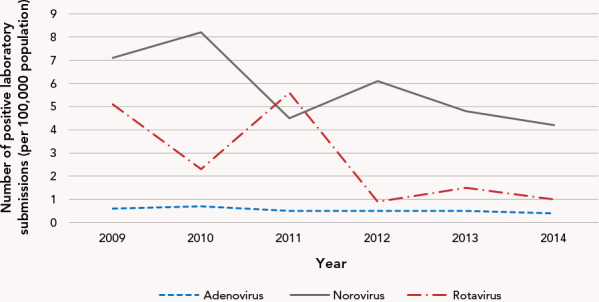
Number of positive specimens submitted to Public Health Ontario Laboratories by virus type and year, 2009–2014 (per 100,000 population)a
a Additional virus types (astrovirus, sapovirus, other Picornaviridae, and other Caliciviridae) removed due to rates less than 0.10 positive specimens per 100,000 population
Note: The rotavirus vaccine (Rotarix®) was added to the Ontario routine vaccination schedule in 2011
Female patients (40.0%) accounted for more positive VGE submissions (n=7,312) than males (32.4%); however, a large percentage of samples had incomplete sex information (27.6%). When restricting this analysis to norovirus-positive samples (n=4,633), this pattern was repeated.
The 65+ years age group had the highest number of VGE-positive submissions, followed by the 0–4 years age group; 93.5% and 15.3% of these VGE-positive submissions were positive for norovirus, respectively (Figure 4). Of all VGE positive submissions, 19% had missing age information
Figure 4.
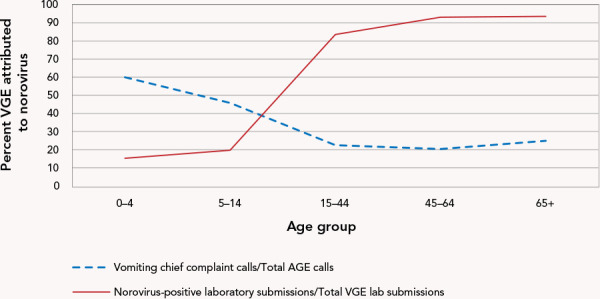
Viral gastroenteritis activity attributed to norovirus by age group 2019–2014a
Abbreviations: AGE, acute gastroenteritis; VGE, viral gastroenteritis
a Norovirus-positive laboratory submissions presented as a percent of VGE-positive specimens submitted to Public Health Ontario; vomiting chief complaint (vomiting, diarrhea, vomiting+diarrhea) calls to Telehealth Ontario as a percent of all AGE-related calls
A total of 62.3% (n=4,559/7,312) of the VGE-positive samples were linked to outbreaks. Of the outbreak samples, long-term care homes were the most commonly affected location type, followed by hospitals, retirement homes, day cares and restaurants. The seasonality of norovirus-positive laboratory submissions broken down by month and year was closely associated with the seasonality of norovirus outbreaks (Figure 2).
Telehealth calls: A total of 320,834 telehealth calls was recorded for AGE illness in the period 2009–2014. Of these calls, 36.6% were due to vomiting as the chief complaint. The percentage of AGE calls attributed to the vomiting chief complaints fluctuated between 31% and 41% during 2009–2014 (Figure 5).
Figure 5.
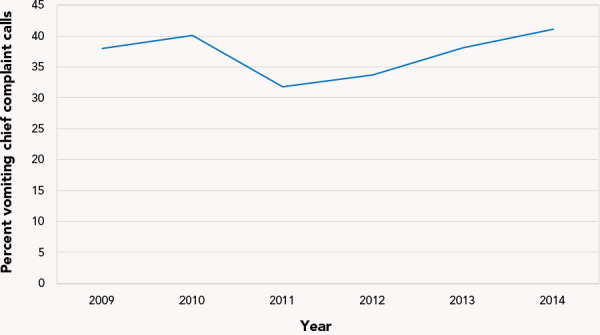
Percentage of acute gastroenteritis calls to Telehealth Ontario attributed to vomiting chief complaintsa by year, 2009–2014
a Complains involved vomiting, diarrhea and vomiting+diarrhea calls
Telehealth calls were more frequently made by females (62.6% of AGE calls); with male calls comprising 35.9% and 1.5% from unknown/blank. When analyzing the vomiting chief complaints, this pattern was repeated.
The 15–44 age group comprised the highest number of AGE telehealth calls with 131,271 (40.9%) calls between 2009 and 2014. However, for vomiting chief complaint calls the highest call volumes (n=60,058 calls) were recorded for the 0–4 years age group. The 65+ years group consistently had the lowest number of calls. The youngest age groups (0–4, 5–14 years) displayed higher percentages of AGE calls attributed to the vomiting chief complaints in comparison to the older age groups (15–44, 45–64, 65+ years) (Figure 4). Less than 1% of AGE and vomiting chief complaint calls had missing age information.
Discussion
Norovirus was the most common cause of VGE cases and outbreaks in Ontario during the years 2009–2014. This work confirms previous research that has identified norovirus as the most common cause of VGE and intestinal infections in the community (1,14,21,22). The outbreak dataset showed that norovirus comprised 41.6% of all VGE outbreaks during the study period, the laboratory dataset showed that norovirus comprised 63.4% of all VGE submissions, and the telehealth dataset showed 36.6% of all AGE calls had vomiting as the chief complaint.
The 2009–2014 outbreak data demonstrated that long-term care homes were the most commonly implicated institution type for both VGE and norovirus (Figure 1). This was not unexpected due to the higher incidence of VGE in older adults; the virus disproportionately causes more severe illness in vulnerable populations, such the elderly, young children and those with compromised immune systems (1). The number of VGE outbreaks per year in child care facilities decreased after 2011 (Figure 1). This finding is likely due to the introduction of the Rotarix® vaccine administered at the ages of two and four months in the Ontario childhood vaccination schedule in August 2011; this primarily impacted outbreaks occurring in child care centres (23). Rotavirus is a common illness of children younger than five years of age, its primary symptom being diarrhea. Therefore, the presence of rotavirus infection in these data likely impacts the number of VGE outbreaks in young age groups. Other studies, both in Ontario and in countries worldwide, have also reported using surveillance to identify decreases in in rotavirus activity following the introduction of the vaccine (23–25).
Both the outbreak and laboratory data illustrated a rise in norovirus activity above normal seasonal activity during the winter of 2009/2010 (Figure 2). A rise above normal seasonal levels of norovirus activity typically occurs with the introduction of a new strain, mostly due to the quick mutation rate of the virus and lack of herd immunity in the population (22,26). The introduction of new strains can cause shifts in seasonality and/or increases in the number of outbreaks (27). This is likely a result of the emergence of two novel strains: the GII.4 New Orleans strain which affected countries globally; and a GII.12 strain (28,29). The GII.4 New Orleans strain caused many outbreaks and was so widespread that it was still detected in high numbers up until 2013; this may also explain the higher peak seen in Figure 2 for the 2011/2012 season (30).
The two most commonly reported pathogens were norovirus and rotavirus. Norovirus tends to disproportionately affect very young (younger than five years) and older (65+ years) individuals when compared with middle-aged healthy people. Outbreaks are very common in high-density areas, such as daycares, retirement homes and long-term care homes (4,31). Rotavirus has a similar outcome in that it disproportionately affects young children (younger than five years), also resulting in outbreaks in daycares, preschools, etc. (1,23). Older individuals (65+ years) and those living in long-term care homes may be privy to more acute medical care and a higher likelihood of samples being collected and submitted to public health authorities, which might explain these findings. It should be noted that the difference in norovirus and rotavirus-positive samples was present even before the introduction of Rotarix into the Ontario childhood vaccination schedule. Further, our analyses clearly demonstrated the acute decrease in rotavirus cases following the introduction of Rotarix in 2011. From 2012 onwards, the laboratory-positive specimens were almost entirely norovirus.
The difference in telehealth call volumes for AGE and vomiting chief complaints for the 15–44 and 0–4-years age groups, respectively, is likely influenced by rotavirus. Because the illness disproportionately affects young populations, it would lead to a higher call volume for the 0–4 age group. It is likely that many callers phoning telehealth are parents concerned about their children. The 15–44 age group likely had the highest number of callers for AGE because of the various of illnesses affecting this population and their preference for virtual care.
While the datasets provided insight into activity of VGE and norovirus in Ontario, there was one clear disadvantage: a lack of community data (i.e. data from people suffering from illness at-home). Both the laboratory and outbreak datasets are biased towards institutional settings primarily because, outside of institutional settings, it is not mandatory for norovirus and other VGE to be reported to Ontario public health authorities. In addition, many VGE cases (norovirus specifically) suffer from underreporting (32). The inclusion of telehealth data in this study helps to bridge this gap in that it primarily collects community-based data that is not well-represented by the outbreak or laboratory data. Syndromic data are known for their ability to reduce underreporting and represent a higher percentage of the population (33). Therefore, including telehealth data provides a greater understanding of VGE activity in Ontario and reduces bias.
Syndromic data are becoming increasingly more common and frequently utilized in public health practice due to their array of advantages. They are timely, can detect new/emerging threats, supplement data from traditional surveillance systems and are non-specific (34). Telehealth data are particularly beneficial as they represent one of the timeliest syndromic data options available; telemedicine helplines are one of the first points of medical care for symptomatic patients (34). These data are also known for their availability in real-time and ease of access. However, telehealth data are not as specific as other sources and cannot necessarily be used to detect specific or severe outbreaks (34). In this study, telehealth data do not need to be specific because non-specific gastrointestinal calls provide the early warning of illness required for the system designed, and will be the most effective at observing norovirus and gastrointestinal illness in Ontario when combined with laboratory and outbreak data.
Limitations
There are a few notable limitations to this study. In the outbreak dataset, there was no age-related data. Rather, the institution type was used as an age proxy. In addition, there were many “gastroenteritis unspecified” and “gastroenteritis other” entries in the dataset, which likely contained additional norovirus cases, but were unusable. Furthermore, there was a lack of standardized reporting for norovirus and VGE in Ontario. Only institutional cases of norovirus are required to be reported to Ontario public health authorities (which also suffers from underreporting and time lags). As a result, analyses of norovirus and VGE activity are challenging due to the data gaps, as well as biases in age group reporting across the province. It is also important to note there is likely overrepresentation in the laboratory dataset. This dataset includes both outbreak samples as well as sporadic; when an outbreak occurs, one or more samples may be submitted. This study is, therefore, unable to describe specifically the burden of sporadic norovirus in Ontario, and there is likely a heavier focus on outbreak-related data. Finally, the telehealth calls may have included non-viral causes and may have contained some duplicate callers; however, it was not possible to stratify this in the dataset. As well, it was assumed vomiting was the main symptom of norovirus for telehealth analyses, which likely excluded additional norovirus-related calls from our results. Each dataset had limitations in terms of representative population; however, when combined, an overall summary of norovirus epidemiology in Ontario during the period 2009–2014 was generated.
Conclusion
This study describes the epidemiology of VGE and, specifically, norovirus in the province of Ontario, Canada between 2009 and 2014.Our study demonstrates that norovirus is a highly prevalent illness and the most dominant cause of VGE in the province. Our findings are in line with those of similar international studies, demonstrating norovirus as the leading cause of VGE (1–3). While a vaccine has been introduced in Ontario and countries worldwide to mitigate rotavirus infection, there is no vaccine for norovirus. Introducing preventative interventions, such as a vaccine, is ideal; however, other public health actions, such as novel surveillance techniques, are also necessary to inform public health interventions A combination of traditional and novel surveillance techniques will best capture data representative of Ontarians and reduce bias in surveillance. Additional techniques to help estimate norovirus disease burden knowledge gaps, such as sporadic norovirus, should be considered (35).
Acknowledgements
We are grateful for the assistance provided by Public Health England throughout this study. In addition, many thanks are necessary to W Sears at the University of Guelph for his assistance with the analyses in this study.
Appendix.
integrated Public Health Information System
The integrated Public Health Information System (iPHIS) dataset contains information on confirmed outbreaks of viral gastroenteritis (VGE) in Ontario reported to public health between January 1, 2009 and December 13, 2014. It is mandated in Ontario that all Reportable Diseases (as of 2018, these are now called “Diseases of Public Health Significance”) be submitted to the database (1,2). Local public health units are responsible for collecting case information on all reportable diseases and entering them into iPHIS, as part of provincial and federal surveillance.
All outbreaks in the dataset are institutional (i.e. occurred at long-term care homes, retirement homes, child care facilities, correctional facilities, etc.).Additional information found in iPHIS includes the method of exposure (if determined), aetiologic agent (if determined), public health unit, disease confirmed status, date the outbreak was declared by the medical officer of health, date the outbreak was declared over by the medical officer of health, the initial onset date (time of index case) and final onset date (time of last case). The onset dates (time of index case) were used for analyses were used for this study, and the range between the onset date (time of index case) and onset date (time of last case) was used to calculate average VGE and norovirus outbreak duration.
While iPHIS data are continuously updated, public health authorities are required to enter all outbreaks from the past year by each August; therefore, incurring time delays. It should be noted laboratory confirmation is not required for an outbreak to be entered into the dataset, and when laboratory confirmation is present not all cases associated with an outbreak are laboratory tested.
Public Health Ontario Laboratories
The Public Health Ontario Laboratories (PHOL) dataset represents all the samples submitted to Public Health Ontario (PHO) between January 1, 2009 and December 31, 2014 for testing in Ontario. The data represent samples which were submitted to PHO from patients ill with suspected norovirus and/or rotavirus (i.e. symptoms of vomiting and/or diarrhea). Samples are submitted by medical professionals in the form of stool samples and are either tested by polymerase chain reaction, electron microscopy or immunochromatographic test. The dataset provides age, gender and public health unit information, as well as the dates the samples were collected and subsequently entered into the dataset. For analyses, the sample collection dates were used (i.e. the results represent the date the samples were collected from the ill person by the healthcare practitioner for testing). It should be noted not all VGE test requisitions from institutions and physicians are tested by PHO in Ontario; samples may also be sent to other private labs in Ontario, or in duplicates to multiple labs. As a result, not all laboratory-confirmed samples of VGE from Ontario are captured in this dataset.
Telehealth Ontario
The Telehealth Ontario (THO) dataset represents all phone calls made by residents of Ontario between January 1, 2009 and December 31, 2014 with gastrointestinal symptoms as chief complaints. Telehealth Ontario is a 24-hour, 7-days-a-week, confidential telephone hotline service which has been in service since 2001 and is available to anyone (providing a health card number is optional). The service is operated by Sykes Assistance Services Corporation, who are contracted by the Ontario Ministry of Health and Long-Term Care. An individual may call the hotline for any reason, where a responding nurse provides basic medical advice and directs the caller to an appropriate next course of action (i.e. visit an emergency room immediately, see a family physician very soon or within the next day, or self-care). The hotline helps alleviate the pressure on emergency departments and doctor's offices, while simultaneously providing free medical advice to millions of Ontarians. The THO dataset provides information on the date and time of call, the caller's chief complaint (primary reason for calling), the nurse's suggested next steps, age, gender, and location (city) of call.
The calls made to THO are referred to as acute gastroenteritis (AGE) in this study rather than VGE as in the iPHIS and PHOL datasets due to the fact they are less specific and encompass all causes of AGE, not strictly viral aetiologies. In addition, the calls made to THO for norovirus-like illness has not been confirmed as norovirus. Therefore, the “vomiting chief complaints” are used as a proxy for norovirus and norovirus-like illness and the burden of AGE calls cannot be attributed strictly to norovirus, but rather norovirus and norovirus-like illness.
Competing interests: None.
Funding: This work was supported by the University of Guelph.
References
- 1.Patel MM, Hall AJ, Vinjé J, Parashar UD. Noroviruses: a comprehensive review. J Clin Virol 2009;44(1):1–8. 10.1016/j.jcv.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 2.Morton VK, Thomas MK, McEwen SA. Estimated hospitalizations attributed to norovirus and rotavirus infection in Canada, 2006-2010. Epidemiol Infect 2015;143(16):3528–37. 10.1017/S0950268815000734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yen C, Hall AJ. Challenges to estimating norovirus disease burden. J Pediatric Infect Dis Soc 2013;2(1):61–2. 10.1093/jpids/pis134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karst SM. Pathogenesis of noroviruses, emerging RNA viruses. Viruses 2010;2(3):748–81. 10.3390/v2030748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glass RI, Parashar UD, Estes MK. Norovirus gastroenteritis. N Engl J Med 2009;361(18):1776–85. 10.1056/NEJMra0804575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teunis PF, Moe CL, Liu P, Miller SE, Lindesmith L, Baric RS, Le Pendu J, Calderon RL. Norwalk virus: how infectious is it? J Med Virol 2008;80(8):1468–76. 10.1002/jmv.21237 [DOI] [PubMed] [Google Scholar]
- 7.Kaplan JE, Gary GW, Baron RC, Singh N, Schonberger LB, Feldman R, Greenberg HB. Epidemiology of Norwalk gastroenteritis and the role of Norwalk virus in outbreaks of acute nonbacterial gastroenteritis. Ann Intern Med 1982;96(6 Pt 1):756–61. 10.7326/0003-4819-96-6-756 [DOI] [PubMed] [Google Scholar]
- 8.Thomas MK, Murray R, Flockhart L, Pintar K, Pollari F, Fazil A, Nesbitt A, Marshall B. Estimates of the burden of foodborne illness in Canada for 30 specified pathogens and unspecified agents, circa 2006. Foodborne Pathog Dis 2013;10(7):639–48. 10.1089/fpd.2012.1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall AJ, Lopman BA, Payne DC, Patel MM, Gastañaduy PA, Vinjé J, Parashar UD. Norovirus disease in the United States. Emerg Infect Dis 2013;19(8):1198–205. 10.3201/eid1908.130465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burke RM, Mattison C, Marsh Z, Shioda K, Donald J, Salas SB, Naleway AL, Biggs C, Schmidt MA, Hall AJ. Norovirus and other viral causes of medically attended acute gastroenteritis across the age spectrum: Results from the Medically Attended Acute Gastroenteritis Study in the United States. Clin Infect Dis. 2021;73(6):e913-20. 10.1093/cid/ciab033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cremon C, De Giorgio R, Giovanni B. Norovirus gastroenteritis. N Engl J Med 2010l362(6):577-8. 10.1056/NEJMc0911723 [DOI] [PubMed] [Google Scholar]
- 12.Tarr GA, Pang XL, Zhuo R, Lee BE, Chui L, Ali S, Vanderkooi OG, Michaels-Igbokwe C, Tarr PI, MacDonald SE, Currie G, MacDonald J, Kim K, Freedman SB. Attribution of pediatric acute gastroenteritis episodes and emergency department visits to norovirus genogroups I and II. J Infect Dis 2021;223(3):452–61. 10.1093/infdis/jiaa391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esposito S, Principi N. Norovirus vaccine: priorities for future research and development. Front Immunol 2020;11:1383. 10.3389/fimmu.2020.01383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall AJ, Rosenthal M, Gregoricus N, Greene SA, Ferguson J, Henao OL, Vinjé J, Lopman BA, Parashar UD, Widdowson MA. Incidence of acute gastroenteritis and role of norovirus, Georgia, USA, 2004-2005. Emerg Infect Dis 2011;17(8):1381–8. 10.3201/eid1708.101533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Statistics Canada. Population estimates on July 1st, by age and sex. Ottawa (ON): Government Canada; 2021 (accessed 2021-04-10). https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000501&pickMembers%5B0%5D=1.7&pickMembers%5B1%5D=2.1
- 16.Freedman SB, Xie J, Lee BE, Ali S, Pang X-L, Chui L, Zhuo R, Vankerkooi OG, Tellier R, Funk AL, Tarr PI, Alberta Provincial Pediatric EnTeric Infection Team (APPETITE). Microbial etiologies and clinical characteristics of children seeking emergency department care due to vomiting in the absence of diarrhea. Clin Infect Dis 2021:ciab451. (Epub ahead of print). 10.1093/cid/ciab451 [DOI]
- 17.Ministry of Education. Find Licensed Child Care. Toronto (ON): Government of Ontario; 2019 (accessed 2021-04-10). https://www.iaccess.gov.on.ca/LCCWWeb/childcare/search.xhtml
- 18.Ministry of the Solicitor General. Corrections: Facilities - locations and visiting hours. Toronto (ON): Government of Ontario; 2020 (accessed 2021-04-10). https://www.mcscs.jus.gov.on.ca/english/corr_serv/facilitieslocationsandvisitinghours/facilities.html
- 19.Ontario Long Term Care Association. About long-term care in Ontario: Facts and figures. Toronto (ON): OLTCA; 2019 (accessed 2021-04-10). http://www.oltca.com/oltca/OLTCA/LongTermCare/OLTCA/Public/LongTermCare/FactsFigures.aspx
- 20.Statistics Canada. Annual demographic estimates by economic region, age and sex, based on the Standard Geographical Classification (SGC) 2011, inactive. Ottawa (ON): Government of Canada; 2021 (accessed 2021-04-10). https://www150.statcan.gc.ca/t1/tbl1/en/cv.action?pid=1710008101#timeframe
- 21.Loveridge P, Cooper D, Elliot AJ, Harris J, Gray J, Large S, Regan M, Smith GE, Lopman B. Vomiting calls to NHS Direct provide an early warning of norovirus outbreaks in hospitals. J Hosp Infect 2010;74(4):385–93. 10.1016/j.jhin.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 22.Gastañaduy PA, Hall AJ, Curns AT, Parashar UD, Lopman BA. Burden of norovirus gastroenteritis in the ambulatory setting--United States, 2001-2009. J Infect Dis 2013;207(7):1058–65. 10.1093/infdis/jis942 [DOI] [PubMed] [Google Scholar]
- 23.Wilson SE, Rosella LC, Wang J, Le Saux N, Crowcroft NS, Harris T, Bolotin S, Deeks SL. Population-level impact of Ontario’s infant rotavirus immunization program: evidence of direct and indirect effects. PLoS One 2016;11(5):e0154340. 10.1371/journal.pone.0154340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas SL, Walker JL, Fenty J, Atkins KE, Elliot AJ, Hughes HE, Stowe J, Ladhani S, Andrews NJ. Impact of the national rotavirus vaccination programme on acute gastroenteritis in England and associated costs averted. Vaccine 2017;35(4):680–6. 10.1016/j.vaccine.2016.11.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bawa Z, Elliot AJ, Morbey RA, Ladhani S, Cunliffe NA, O’Brien SJ, Regan M, Smith GE. Assessing the likely impact of a rotavirus vaccination program in England: the contribution of syndromic surveillance. Clin Infect Dis 2015;61(1):77–85. 10.1093/cid/civ264 [DOI] [PubMed] [Google Scholar]
- 26.Karst SM, Baric RS. What is the reservoir of emergent human norovirus strains? J Virol 2015;89(11):5756–9. 10.1128/JVI.03063-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allen DJ, Adams NL, Aladin F, Harris JP, Brown DW. Emergence of the GII-4 Norovirus Sydney2012 strain in England, winter 2012-2013. PLoS One 2014;9(2):e88978. 10.1371/journal.pone.0088978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vega E, Vinjé J. Novel GII.12 norovirus strain, United States, 2009-2010. Emerg Infect Dis 2011;17(8):1516–8. 10.3201/eid1708.110025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yen C, Wikswo ME, Lopman BA, Vinjé J, Parashar UD, Hall AJ. Impact of an emergent norovirus variant in 2009 on norovirus outbreak activity in the United States. Clin Infect Dis 2011;53(6):568–71. 10.1093/cid/cir478 [DOI] [PubMed] [Google Scholar]
- 30.Medici MC, Tummolo F, Grazia S, Calderaro A, Conto F, Terio V, Chironna M, Bonura F, Pucci M, Bányai K, Martella V, Giammanco GM. Epidemiological dynamics of norovirus GII.4 variant New Orleans 2009. J Gen Virol 2015;96(9):2919–27. 10.1099/vir.0.000204 [DOI] [PubMed] [Google Scholar]
- 31.Said MA, Perl TM, Sears CL. Healthcare epidemiology: gastrointestinal flu: norovirus in health care and long-term care facilities. Clin Infect Dis 2008;47(9):1202–8. 10.1086/592299 [DOI] [PubMed] [Google Scholar]
- 32.Tam CC, Rodrigues LC, Viviani L, Dodds JP, Evans MR, Hunter PR, Gray JJ, Letley LH, Rait G, Tompkins DS, O’Brien SJ; IID2 Study Executive Committee. Longitudinal study of infectious intestinal disease in the UK (IID2 study): incidence in the community and presenting to general practice. Gut 2012;61(1):69–77. 10.1136/gut.2011.238386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silva AD, Evangelista MS. Syndromic surveillance: etiologic study of acute febrile illness in dengue suspicious cases with negative serology. Brazil, Federal District, 2008. Rev Inst Med Trop São Paulo 2010;52(5):237–42. 10.1590/S0036-46652010000500003 [DOI] [PubMed] [Google Scholar]
- 34.French Institute for Public Health Surveillance. Triple-S Project (Syndromic Surveillance System in Europe). Guidelines for designing and implementing a syndromic surveillance system. InVS; 2013. https://webgate.ec.europa.eu/chafea_pdb/assets/files/pdb/20091112/20091112_d08_giss_en_ps.pdf
- 35.Vanderkooi OG, Xie J, Lee BE, Pang XL, Chui L, Payne DC, MacDonald J, Ali S, MacDonald S, Drews S, Osterreicher L, Kim K, Freedman SB; Alberta Provincial Pediatric EnTeric Infection TEam (APPETITE) and Pediatric Emergency Research Canada (PERC). A prospective comparative study of children with gastroenteritis: emergency department compared with symptomatic care at home. Eur J Clin Microbiol Infect Dis 2019;38(12):2371–9. 10.1007/s10096-019-03688-8 [DOI] [PubMed] [Google Scholar]


