Abstract
Background
Myocardial infarction (MI) is one of the most common ischemic heart diseases. It is very essential to explore new types of cardioprotective drugs delivery systems in this area.
Objective
The aim of the present study was to investigate the protective effect of baicalin (BA) and puerarin (PU) against acute MI rat models. BA and PU co-loaded nanoparticulate system were developed to improve bioavailability of the drugs, to prolong retention time in vivo and to enhance the protective effect.
Methods
In the present study, ANP and TPP contained ligands were synthesized. ANP/TPP-BN-LPNs were prepared and its physico-chemical properties were evaluated. The MI therapy efficiency of ANP/TPP-BN-LPNs was assessed in rats after intravenous injection. Single ligand contained LPNs, no ligand contained LPNs, and BN solution formulations were also prepared and used for the comparison.
Results
ANP/TPP-BN-LPNs were uniform and spheroidal particles. The size of ANP/TPP-BN-LPNs was 98.5 ± 2.9 nm, with a zeta potential of –19.5 ± 1.9 mV. The dual ligands modified LPNs exhibited significantly improved therapeutic efficiency compared with the single ligand modified LPNs and other systems. In vivo infarct therapy studies in rats proved that ANP/TPP-BN-LPNs were a promising system for efficient delivery of cardiovascular drugs for the treatment of cardiovascular diseases.
Conclusions
ANP/TPP-BN-LPNs could be used as a long-circulating and heart-targeting drug delivery system.
Keywords: Acute myocardial infarction, atrial natriuretic peptide, triphenylphosphonium, dual ligands modified, baicalin, lipid–polymer hybrid nanoparticles
Introduction
Myocardial infarction (MI) is a leading cause of morbidity and mortality worldwide (Higuchi et al., 2017). It is caused by the formation of plaques on the interior walls of the arteries resulting in reduced blood flow to the heart and injuring heart muscles because of lack of oxygen supply (Lu et al., 2015). It was reported that in patients suffering MI, minimizing ischemic time by reperfusion therapy was a successful strategy in reducing morbidity and mortality (Valle Raleigh et al., 2017). However, reperfusion may induce myocardial ischemia–reperfusion (IR) injury, one of the most effective methods to deal with this injury is direct delivery of cardioprotective drugs to IR myocardium (Nakano et al., 2016).
MI is known to cause the enhanced permeability and retention (EPR) effect (Sun et al., 2012), thus passive targeting via the EPR effect using nano-sized drug delivery systems such as liposomes, micelles, and nanoparticles were used (Lukyanov et al., 2004; Formiga et al., 2013; Izadifar et al., 2016). In our previous study, baicalin (BN)-loaded PEGylated lipid nanoparticles were prepared and the protective effects on acute myocardial ischemia were evaluated in rats (Zhang et al., 2016). BN is one of the major bioactive flavone glucuronides isolated from the dried roots of Scutellaria baicalensis Georgi, which was reported to attenuate acute MI by inhibiting mitochondrial damage-mediated apoptosis and mediating the mitogen-activated protein kinase pathway (Wang et al., 2013). Among various kinds of nano-sized drug delivery systems, lipid–polymer hybrid nanoparticles (LPNs) combine the advantages of liposomes and polymeric nanoparticles, which was applied for the drug delivery to achieve increased efficiency and selectivity (Wang et al., 2019). In the present research, LPNs were designed to load BN in order to avoid the low bioavailability, short half-life, and poor water solubility in vivo for MI therapy.
Surface-modification of nanocarriers such as conjugating specific ligands to nanocarrier surface can enhance the target efficacy of these nanocarriers and improve the drug efficiency (Ruckenstein & Li, 2005). Examples in MI therapy include that Yu et al. introduced an RGD modified alginate microspheres to repair the MI in the rat (Yu et al., 2010). Our previous study constructed a triphenylphosphonium (TPP) modified tanshinone-loaded nanocarriers for the target therapy of MI (Zhang et al., 2018), due to the ability of cationic TPP to pass through lipid bilayers and accumulate within mitochondria (Ong et al., 2017). Atrial natriuretic peptide (ANP) is a member of the natriuretic peptide family, which has been reported to inhibit IR injury, and reduces infarct size (Gaudin et al., 2014). In the present study, ANP and TPP dual ligands modified, BN loaded LPNs (ANP/TPP-BN-LPNs) were established, which has not been reported by other researchers.
ANP and TPP contained ligands were synthesized. ANP/TPP-BN-LPNs were prepared and its physico-chemical properties were evaluated. The MI therapy efficiency of ANP/TPP-BN-LPNs was assessed in rats after intravenous injection. Single ligand contained LPNs, no ligand contained LPNs, and BN solution formulations were also prepared and used for the comparison.
Materials and methods
Materials
APN-NH2 was provided by ChemeGen (Los Angeles, CA). Distearoylphosphatidylethanolamine–polyethylene glycol (MW∼5 kDa)-COOH (DSPE-PEG5000-COOH, DSPE-PEG) was purchased from Ponsure Biotech (Shanghai, China). Soyabean lecithin (SL) in injection grade was supplied by Shanghai Taiwei Pharmaceutical Co., Ltd. (Shanghai, China). Baicalin, TPP, EDC·HCl, and NHS were obtained from Sigma-Aldrich (St. Louis, MO).
Cells and animals
Human cardiac myocytes (HCMs) and human umbilical vein endothelial cells (HUVECs) were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Male Sprague-Dawley (SD) rats weighing between 240 and 260 g were obtained from the Center of Experimental Animals of Shandong Province (China). All the animal experiments were approved by the Medical Ethics Committee of Linyi People’s Hospital and were performed according to the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978).
Synthesis of ANP and TPP contained ligands
ANP contained ligand was synthesized by conjugating APN-NH2 to DSPE-PEG by amide bond to achieve ANP-PEG-DSPE (Yu et al., 2018). Briefly, EDC·HCl (1.2 equivalents) and NHS (1.2 equivalents) were added to DSPE-PEG (1 equivalent), stirred for 2 h to activate the carboxyl groups (solution A). ANP was dissolved in PBS (pH 7.0) added to solution A under stirring (400 rpm at room temperature) for 24 h, then dialyzed against water for 24 h to obtain ANP-PEG-DSPE. TPP contained ligand (TPP-Lys-TPGS) was synthesized by the method in our previous study (Zhang et al., 2018). The chemical structure of ANP-PEG-DSPE and TPP-Lys-TPGS was determined by using 1H nuclear magnetic resonance (1H NMR) analysis by using DMSO-d6 as solvent.
Preparation of ANP/TPP-BN-LPNs
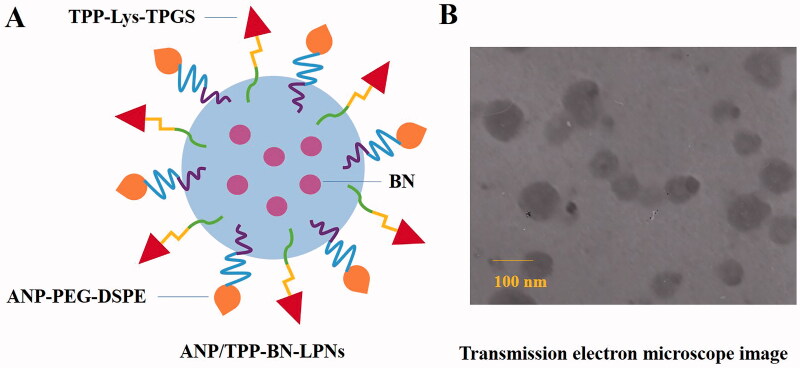
ANP/TPP-BN-LPNs were prepared by nanoprecipitation method (Qiu et al., 2017). ANP-PEG-DSPE, TPP-Lys-TPGS, and SL were dispersed in distilled water (phase A). PLGA and BN were dispersed in acetone (phase B). Phase B was added dropwise into the phase A under stirring (400 rpm) at room temperature for 4 h until complete evaporation of the organic solvent to get ANP/TPP-BN-LPNs (Figure 1(A)).
Figure 1.
A sketch (A) and TEM image (B) of ANP/TPP-BN-LPNs.
Blank ANP/TPP-LPNs were prepared by the same method without adding BN.
Single ligand modified LPNs were prepared by the same method using ANP-PEG-DSPE (ANP-BN-LPNs) or TPP-Lys-TPGS (TPP-BN-LPNs) only along with SL in phase A.
Unmodified LPNs (BN-LPNs) were prepared by the same method without adding ANP-PEG-DSPE or TPP-Lys-TPGS.
Characterization of ANP/TPP-BN-LPNs
A transmission electron microscope (JEOL, Tokyo, Japan) was used to visualize the surface morphology of ANP/TPP-BN-LPNs (Jiang et al., 2021). The particle size, polydispersity index (PDI), and zeta potential of LPNs were evaluated using dynamic light scattering (DLS) technique of NLC at room temperature on Malvern ZetaSizer Nano ZS90 (Malvern Instruments Ltd., Malvern, UK). The entrapment efficiency (EE) and drug loading (DL) were determined by HPLC using the Intertsil® ODS-3V (25 cm × 4.6 mm, 5 μm). Flow rate was kept at 0.5 mL/min and system was maintained at 35 °C, the detection was carried out at λ = 278 nm. EE and DL were calculated according to the following formulas (Jin et al., 2020): EE (%)=(weight of loaded drug/weight of feeding drug)×100; DL (%)=(weight of loaded drug/weight of drugs loaded LPNs)×100.
Stability of LPNs was evaluated by observing the appearance and diameter of the systems during the storage time (until 90 days) at the temperature of 2–8 °C (Jin et al., 2020).
In vitro drug release
In vitro BN release from LPNs was analyzed by dialysis method (Dong et al., 2017). BN loaded LPNs and BN solution (5 mL) were sealed into dialysis bags separately and immersed in 100 mL stirring release medium (100 rpm). Periodically, samples (0.5 mL) were taken out from the release media and analyzed by the HPLC method as described in the above section.
In vitro cytotoxicity
In vitro cytotoxicity of BN loaded LPNs and BN solution was evaluated by MTT assay on HCM and HUVEC (Shao et al., 2017). Briefly, cells were seeded in 96-well plates (104 cells per well) and incubated for 24 h. ANP/TPP-BN-LPNs, blank ANP/TPP-LPNs, ANP-BN-LPNs, TPP-BN-LPNs, BN-LPNs, and BN solution at various concentrations (1, 5, 10, 50, and 100 µM) were added to the cells and incubated for 48 h. Then, the cells were treated with MTT solution (5 mg/mL) and maintained for 4 h. The media containing MTT was removed, 200 μL of DMSO was added to the wells and observed by a microplate reader at 570 nm.
Acute MI model preparation
Acute MI model was established by the permanent ligation of left coronary artery method as described in our previous study (Zhang et al., 2016; Shi et al., 2017). Briefly, rats were intraperitoneal injected with chloraldurate (10%, v/v, 3.5 mL/kg) to anesthetize them. The heart was exteriorized, and ligated from the pulmonary conus to the left atrial appendage (2–3 mm). Then, the heart was returned to its normal position, and the left thorax was sutured immediately. Sham-operated rats were subjected to the same surgical procedure without ligating the coronary artery.
In vivo tissue distribution
Rats were randomly divided into eight groups (10 mice each group), each group received different treatment as follows (10 mg BN per kg of body weight via intravenous injection) (Guo et al., 2019): (1) ANP/TPP-BN-LPNs; (2) blank ANP/TPP-LPNs; (3) ANP-BN-LPNs; (4) TPP-BN-LPNs; (5) BN-LPNs; (6) BN solution; (7) physiological saline (MI group); (8) sham-operated group (sham group). Rats of groups 1, 3, 4, 5, and 6 were sacrificed at 1 and 48 h after administration. The rat heart, liver, spleen, lung, kidney, and brain were taken, washed, weighed, and homogenized. Then, the homogenate was decomposed on heating in nitric acid, evaporated to dryness, and redissolved in chloroform:methanol (1:1, v/v) solution. PUE and TAN were determined by the methods described in section ‘Preparation of ANP/TPP-BN-LPNs’.
In vivo MI therapy
The infarcted area was determined by triphenyltetrazolium chloride (TTC) staining in rats (Yao et al., 2015). Briefly, the left ventricle of heart was harvested and frozen (–20 °C) for 30 min. Then, the heart was sectioned from apex to base into slices (2 mm), incubated in a solution of TTC (1%, v/v) in phosphate buffered saline (pH 7.4, 37 °C) for 15 min, followed by fixing in formaldehyde (10%, v/v). The infarct areas were unstained while normal myocardium areas were stained brick red. The infarct size could be calculated by the following equation: percentage of infarct size (%)=infarct size/the size of the whole left ventricle × 100.
Statistical analysis
Statistical analysis was performed using an unpaired t-test between two groups with software SPSS version 21.0 (SPSS Inc., Chicago, IL). Results were expressed as a mean ± standard deviation (SD). *p<.05 and **p<.01 were considered statistically significant.
Results
Characterization of ligands and LPNs
ANP-PEG-DSPE and TPP-Lys-TPGS were determined by using 1H NMR. For ANP-PEG-DSPE: δ 2.51 (–CO–O–CH–); 2.82 (–P–O–CH2–); 3.61 (−CH2−N − C=O); 6.21 (h, –CO–NH–); 6.49–8.21 (chemical shifts belong to ANP). For TPP-Lys-TPGS: δ 2.11 (−CH2−C = O–N); 2.36 (Ph − CH3); 2.81 (−CH2−C = O–O); 3.32 (−CH2−N − C=O); 4.51 (−CH − N−C = O); 7.16 (−Ph3−P); 8.02 (−NH − C=O).
Figure 1(B) shows that ANP/TPP-BN-LPNs were uniform and spheroidal particles with a size around 100 nm. Table 1 summarizes the particle size, PDI, zeta potential, and DL of LPNs. The size of ANP/TPP-BN-LPNs was 98.5 ± 2.9 nm, with a zeta potential of –19.5 ± 1.9 mV. The EE and DL were 91.3 ± 2.8 and 5.9 ± 0.6%, respectively. During 90 days of storage time at the temperature of 2–8 °C, no obvious change appeared in the appearance and diameter of NLCs, proving the stability of the systems.
Table 1.
The particle size, PDI, zeta potential, EE, and DL of LPNs.
| LPNs | Particle size (nm) | PDI | Zeta potential (mV) | EE (%) | DL (%) |
|---|---|---|---|---|---|
| ANP/TPP-BN-LPNs | 98.5 ± 2.9 | 0.12 ± 0.01 | –19.5 ± 1.9 | 91.3 ± 2.8 | 5.9 ± 0.6 |
| Blank ANP/TPP-LPNs | 96.9 ± 3.2 | 0.13 ± 0.02 | –18.1 ± 2.2 | – | – |
| ANP-BN-LPNs | 97.3 ± 3.4 | 0.14 ± 0.02 | –26.7 ± 2.1 | 88.9 ± 2.6 | 7.3 ± 0.7 |
| TPP-BN-LPNs | 95.1 ± 3.5 | 0.14 ± 0.02 | –13.2 ± 1.5 | 90.4 ± 3.1 | 8.1 ± 0.9 |
| BN-LPNs | 75.3 ± 2.6 | 0.13 ± 0.01 | –31.2 ± 2.8 | 92.3 ± 2.7 | 10.1 ± 0.8 |
In vitro drug release
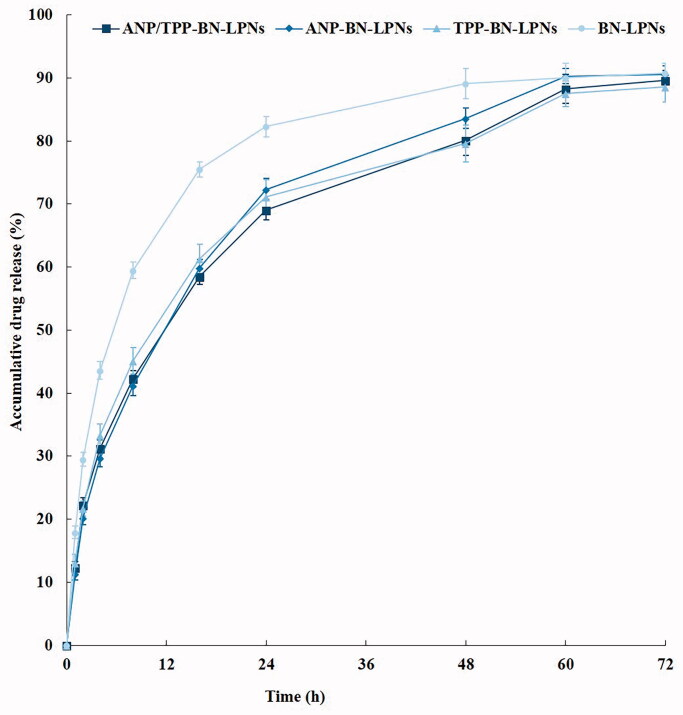
In vitro BN release behaviors from LPNs are illustrated in Figure 2. ANP/TPP-BN-LPNs, ANP-BN-LPNs, and TPP-BN-LPNs showed similar sustained release pattern, while BN-LPNs without modification exhibited relatively faster release profile. Nearly, all of drugs were released from BN-LPNs after 48 h, which is 60 h for ANP/TPP-BN-LPNs.
Figure 2.
In vitro BN release from LPNs analyzed by dialysis method.
In vitro cytotoxicity
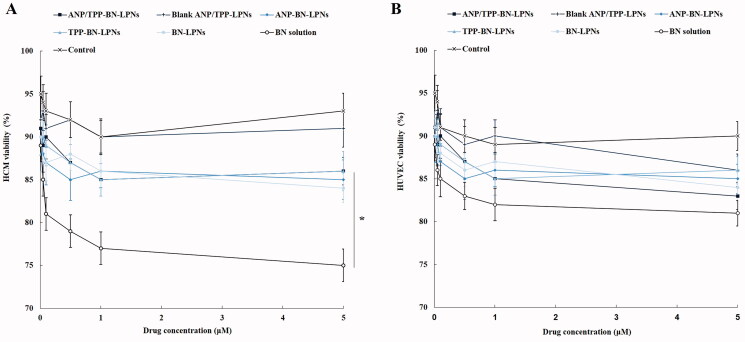
HCM viability was calculated to determine the cytotoxicity of drug loaded LPNs and drug solution in cardiac myocytes. Figure 3(A) shows that various kinds of LPNs including ANP/TPP-BN-LPNs did not exhibit significant cytotoxicity at the designed drug concentrations, by whom over 85% of HCM viabilities were achieved. However, BN solution reduced the cell viability at the high concentrations (higher than 1 μM). Lower cytotoxicity compared with BN solution (p<.05) could prove the fine protective effects of LPNs and well cytocompatibility of the nanoparticle formulations, which may benefit the use of them in vivo. The cytocompatibility of formulation was checked in normal cell line: HUVEC. Figure 3(B) shows that both LPNs and drug solutions did not introduce significant cytotoxicity on HUVEC, proving the good compatibility of these systems.
Figure 3.
In vitro cytotoxicity of BN loaded LPNs and BN solution evaluated on HCM (A) and HUVEC (B) by MTT assay.
In vivo tissue distribution
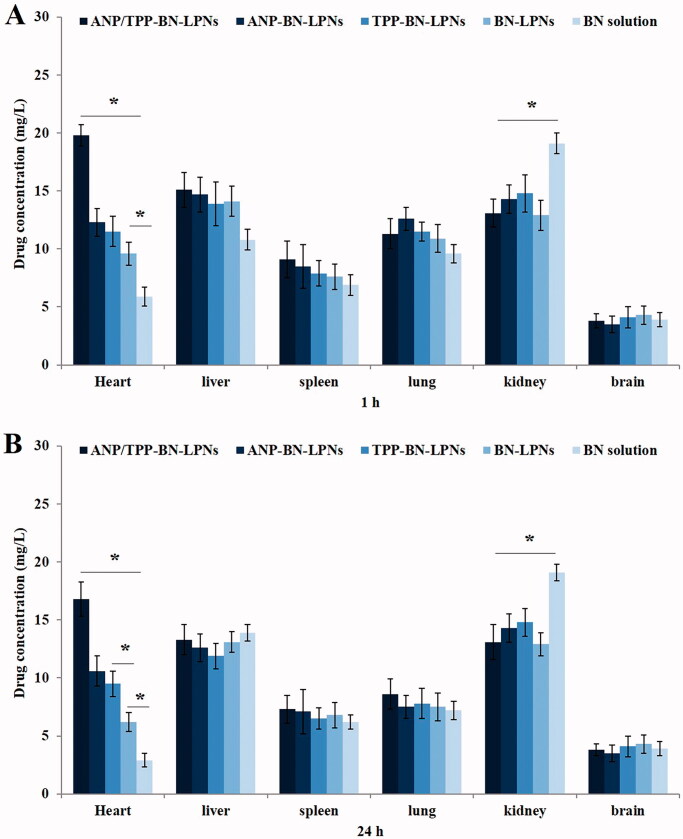
In vivo tissue distribution behaviors of drug contained LPNs and drug solution were evaluated on acute MI rats (Figure 4). Drug distribution of ANP/TPP-BN-LPNs in the heart was the highest, higher than other LPNs and drug solution at both 1 and 24 h (p<.05). BN-LPNs showed higher heart accumulation compared with BN solution at 1 and 24 h (p<.05). More ANP-BN-LPNs and TPP-BN-LPNs were accumulated in the heart than BN-LPNs at 24 h (p<.05). BN solution exhibited higher distribution in the kidney compared with LPNs formulations (p<.05).
Figure 4.
In vivo tissue distribution in rats received different treatment at 1 (A) and 24 h (B) after administration.
In vivo MI therapy
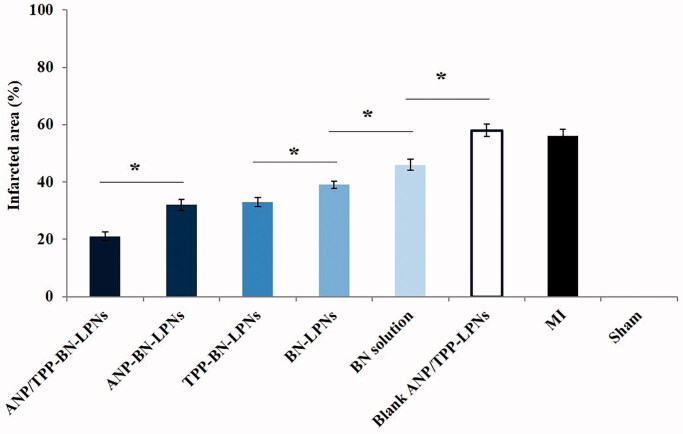
In vivo anti MI effects of drug contained LPNs and drug solution were determined by measuring the infarct size (Figure 5). ANP/TPP-BN-LPNs exhibited the most significant infract size reduction, which was 21 ± 1.5% of infract area compared with control. The reduction of infract size carried by ANP/TPP-BN-LPNs was remarkably higher than that of ANP-BN-LPNs (32 ± 1.9%) and TPP-BN-LPNs (33 ± 1.5%) (p<.05). BN-LPNs showed better efficiency (39 ± 1.3%) than that of BN solution (46 ± 1.9%) (p<.05). The infarct size of blank ANP/TPP-LPNs and MI groups was 58 ± 2.1%, and 56 ± 2.3%, respectively.
Figure 5.
In vivo MI therapy by measuring the infarcted area in rats.
Discussion
ANP or TPP contained drug delivery systems have been reported respectively by researchers for MI therapy (Kim et al., 2012; Mueller et al., 2019). In the present study, a novel dual ligands system was introduced for MI therapy. ANP/TPP-BN-LPNs were nano-sized and spheroidal particles, which are uniform when characterized by TEM. Ferreira et al. argued that drug-loaded multifunctional nanoparticles could target the injured heart (Ferreira et al., 2017), which was also proved by our previous study (Zhang et al., 2016). In this study, we would like to combine ANP and TPP modified system together, which has not been reported yet by other researchers. The LPNs showed sizes of about 100 nm, which could be used as promising carriers for the delivery of drugs.
In vitro BN release behaviors from LPNs are illustrated in Figure 2. To evaluate whether the modification of ligands affect the release behaviors of BN, the release profiles were expressed as a function of time (Wang et al., 2018). In vitro drug release of LPNs may be controlled by erosion, corrosion, and diffusion processes (Lu et al., 2019). BN releases from LPNs showed sustained behaviors, mechanistically may be attributed to slow degradation of PLGA and the release of BN from the lipid layer by diffusion. ANP/TPP-BN-LPNs, ANP-BN-LPNs, and TPP-BN-LPNs showed similar sustained release pattern, while BN-LPNs without modification exhibited relatively faster release profile. Nearly, all of drugs were released from BN-LPNs after 48 h, which is 60 h for ANP/TPP-BN-LPNs. Surface modification of nano-systems could bring the systems more sustained release behaviors, which have been reported by researchers (Zhang et al., 2018; Shao et al., 2019): Zhang et al. summarized that no-ligand-modified nanoparticles exhibited faster release than modified systems (Zhang et al., 2018); and Shao et al. also argue that modified nanoparticles reveal slower release than the non-modified ones, which may be caused by the ligands coating on the surface which delayed the drug release (Shao et al., 2019). Surface modified ligands were reported as molecular fences that could retain the drugs inside the particles (Fu et al., 2020). The longer release time of ANP/TPP-BN-LPNs than that of BN-LPNs could protect the drugs for a relatively longer time from being degraded in the circulation system, prevent premature drug release prior to reaching the tumor sites and thus may perform persistent therapeutic effect.
Lipid materials were reported as biocompatible and less toxic, which were introduced to reduce the cytotoxicity of the drug delivery systems (Li et al., 2020). Zheng et al. (2019) reported that the cytotoxicity of the empty nanoparticles was investigated to study the safety of the drug carriers and lipid nanoparticles exhibited no obvious impact on the cell viability because the lipids were good biocompatible materials which showed nontoxicity to cells. In this study, various kinds of LPNs including ANP/TPP-BN-LPNs did not exhibit significant cytotoxicity at the designed drug concentrations. However, BN solution reduced the cell viability at the high concentrations. Lower cytotoxicity could prove the fine protective effects of LPNs and well cytocompatibility of the nanoparticle formulations, which may benefit the use of them in vivo.
In vivo biodistribution study results exhibited similar long-circulating characteristics of LPNs. Yu et al. (2018) suggested that nanoparticles could elongate the circulation time of drugs in serum and have great potential to accumulate in the myocardial infarct area. The higher heart distribution of ANP/TPP-BN-LPNs than non-modified LPNs is in accordance with the aim of the study: to deliver more nanocarriers to the heart. This phenomenon could be due to the ligands mediated targeting of modified LPNs, which is also discussed by Dong et al. that the active targeting ability of the ligands could help with the heart accumulation (Dong et al., 2017; Hao et al., 2017). Lower BN concentration of ANP/TPP-BN-LPNs in the kidney compared with BN solution may reduce the systemic toxicity of the system. In vivo infarct therapy effect was evaluated by measuring the infarct size, which is conceived as a critical indicator to evaluate the cardiac damage (Zuurbier et al., 2020). ANP/TPP-BN-LPNs reduced the infract size to the largest content, suggesting the best cardioprotective effect of the dual ligands modified LPNs. Significant higher efficiency ANP/TPP-BN-LPNs compared with ANP-BN-LPNs and TPP-BN-LPNs could be the evidence of the better infarct therapy ability of dual ligands modified LPNs than the single ligand modified ones. These results were in accordance with the protective effect and sustained release behavior of the system, which could let the drug remain within the heart, then provide lasting MI therapy effects after in vivo administration.
Conclusions
ANP/TPP-BN-LPNs could be used as a long-circulating and heart-targeting drug delivery system owing to its fine biocompatibility and low toxicity. The dual ligands modified LPNs exhibited significantly improved therapeutic efficiency compared with the single ligand modified LPNs and other systems. In vivo infarct therapy studies in rats proved that ANP/TPP-BN-LPNs were a promising system for efficient delivery of cardiovascular drugs for the treatment of cardiovascular diseases.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Dong Z, Guo J, Xing X, et al. (2017). RGD modified and PEGylated lipid nanoparticles loaded with puerarin: formulation, characterization and protective effects on acute myocardial ischemia model. Biomed Pharmacother 89:297–304. [DOI] [PubMed] [Google Scholar]
- Ferreira MPA, Ranjan S, Kinnunen S, et al. (2017). Drug-loaded multifunctional nanoparticles targeted to the endocardial layer of the injured heart modulate hypertrophic signaling. Small 13:1701276. [DOI] [PubMed] [Google Scholar]
- Formiga FR, Garbayo E, Díaz-Herráez P, et al. (2013). Biodegradation and heart retention of polymeric microparticles in a rat model of myocardial ischemia. Eur J Pharm Biopharm 85:665–72. [DOI] [PubMed] [Google Scholar]
- Fu Q, Wang J, Liu H. (2020). Chemo-immune synergetic therapy of esophageal carcinoma: trastuzumab modified, cisplatin and fluorouracil co-delivered lipid–polymer hybrid nanoparticles. Drug Deliv 27:1535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudin A, Yemisci M, Eroglu H, et al. (2014). Squalenoyl adenosine nanoparticles provide neuroprotection after stroke and spinal cord injury. Nat Nanotechnol 9:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Xing X, Lv N, et al. (2019). Therapy for myocardial infarction: in vitro and in vivo evaluation of puerarin-prodrug and tanshinone co-loaded lipid nanoparticulate system. Biomed Pharmacother 120:109480. [DOI] [PubMed] [Google Scholar]
- Hao J, Tong T, Jin K, et al. (2017). Folic acid-functionalized drug delivery platform of resveratrol based on Pluronic 127/d-α-tocopheryl polyethylene glycol 1000 succinate mixed micelles. Int J Nanomedicine 12:2279–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi A, Ku NJ, Tseng YC, et al. (2017). Stem cell therapies for myocardial infarction in clinical trials: bioengineering and biomaterial aspects. Lab Invest 97:1167–79. [DOI] [PubMed] [Google Scholar]
- Izadifar M, Kelly ME, Chen X. (2016). Regulation of sequential release of growth factors using bilayer polymeric nanoparticles for cardiac tissue engineering. Nanomedicine 11:3237–59. [DOI] [PubMed] [Google Scholar]
- Jiang T, Ma S, Shen Y, et al. (2021). Topical anesthetic and pain relief using penetration enhancer and transcriptional transactivator peptide multi-decorated nanostructured lipid carriers. Drug Deliv 28:478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Wang Y, Liu X, et al. (2020). Synergistic combination chemotherapy of lung cancer: cisplatin and doxorubicin conjugated prodrug loaded, glutathione and pH sensitive nanocarriers. Drug Des Devel Ther 14:5205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Kim HS, Le UN, et al. (2012). Evaluation of a mitochondrial voltage sensor, (18F-fluoropentyl)triphenylphosphonium cation, in a rat myocardial infarction model. J Nucl Med 53:1779–85. [DOI] [PubMed] [Google Scholar]
- Li M, Feng S, Xing H, Sun Y. (2020). Dexmedetomidine and levobupivacaine co-loaded, transcriptional transactivator peptide modified nanostructured lipid carriers or lipid–polymer hybrid nanoparticles, which performed better for local anesthetic therapy? Drug Deliv 27:1452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G, Cao L, Zhu C, et al. (2019). Improving lung cancer treatment: hyaluronic acid‑modified and glutathione‑responsive amphiphilic TPGS‑doxorubicin prodrug‑entrapped nanoparticles. Oncol Rep 42:361–9. [DOI] [PubMed] [Google Scholar]
- Lu L, Liu M, Sun R, et al. (2015). Myocardial infarction: symptoms and treatments. Cell Biochem Biophys 72:865–7. [DOI] [PubMed] [Google Scholar]
- Lukyanov AN, Hartner WC, Torchilin VP. (2004). Increased accumulation of PEG-PE micelles in the area of experimental myocardial infarction in rabbits. J Control Release 94:187–93. [DOI] [PubMed] [Google Scholar]
- Mueller C, McDonald K, de Boer RA, et al. (2019). Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail 21:715–31. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Matoba T, Tokutome M, et al. (2016). Nanoparticle-mediated delivery of irbesartan induces cardioprotection from myocardial ischemia-reperfusion injury by antagonizing monocyte-mediated inflammation. Sci Rep 6:29601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong SB, Lu S, Katwadi K, et al. (2017). Nanoparticle delivery of mitoprotective agents to target ischemic heart disease. Future Cardiol 13:195–8. [DOI] [PubMed] [Google Scholar]
- Qiu J, Cai G, Liu X, Ma D. (2017). αvβ3 integrin receptor specific peptide modified, salvianolic acid B and panax notoginsenoside loaded nanomedicine for the combination therapy of acute myocardial ischemia. Biomed Pharmacother 96:1418–26. [DOI] [PubMed] [Google Scholar]
- Ruckenstein E, Li ZF. (2005). Surface modification and functionalization through the self-assembled monolayer and graft polymerization. Adv Colloid Interface Sci 113:43–63. [DOI] [PubMed] [Google Scholar]
- Shao M, Yang W, Han G. (2017). Protective effects on myocardial infarction model: delivery of schisandrin B using matrix metalloproteinase-sensitive peptide-modified, PEGylated lipid nanoparticles. Int J Nanomedicine 12:7121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Luo W, Guo Q, et al. (2019). In vitro and in vivo effect of hyaluronic acid modified, doxorubicin and gallic acid co-delivered lipid–polymeric hybrid nano-system for leukemia therapy. Drug Des Devel Ther 13:2043–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi ZY, Liu Y, Dong L, et al. (2017). Cortistatin improves cardiac function after acute myocardial infarction in rats by suppressing myocardial apoptosis and endoplasmic reticulum stress. J Cardiovasc Pharmacol Ther 22:83–93. [DOI] [PubMed] [Google Scholar]
- Sun G, Lin X, Hong Y, et al. (2012). PEGylation for drug delivery to ischemic myocardium: pharmacokinetics and cardiac distribution of poly(ethylene glycol)s in mice with normal and ischemic myocardium. Eur J Pharm Sci 46:545–52. [DOI] [PubMed] [Google Scholar]
- Valle Raleigh J, Mauro AG, Devarakonda T, et al. (2017). Reperfusion therapy with recombinant human relaxin-2 (Serelaxin) attenuates myocardial infarct size and NLRP3 inflammasome following ischemia/reperfusion injury via eNOS-dependent mechanism. Cardiovasc Res 113:609–19. [DOI] [PubMed] [Google Scholar]
- Wang G, Wang Z, Li C, et al. (2018). RGD peptide-modified, paclitaxel prodrug-based, dual-drugs loaded, and redox-sensitive lipid–polymer nanoparticles for the enhanced lung cancer therapy. Biomed Pharmacother 106:275–84. [DOI] [PubMed] [Google Scholar]
- Wang J, Su G, Yin X, et al. (2019). Non-small cell lung cancer-targeted, redox-sensitive lipid–polymer hybrid nanoparticles for the delivery of a second-generation irreversible epidermal growth factor inhibitor-afatinib: in vitro and in vivo evaluation. Biomed Pharmacother 120:109493. [DOI] [PubMed] [Google Scholar]
- Wang X, He F, Liao Y, et al. (2013). Baicalin pretreatment protects against myocardial ischemia/reperfusion injury by inhibiting mitochondrial damage-mediated apoptosis. Int J Cardiol 168:4343–5. [DOI] [PubMed] [Google Scholar]
- Yao C, Shi X, Lin X, et al. (2015). Increased cardiac distribution of mono-PEGylated Radix Ophiopogonis polysaccharide in both myocardial infarction and ischemia/reperfusion rats. Int J Nanomedicine 10:409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Du KT, Fang Q, et al. (2010). The use of human mesenchymal stem cells encapsulated in RGD modified alginate microspheres in the repair of myocardial infarction in the rat. Biomaterials 31:7012–20. [DOI] [PubMed] [Google Scholar]
- Yu J, Li W, Yu D. (2018). Atrial natriuretic peptide modified oleate adenosine prodrug lipid nanocarriers for the treatment of myocardial infarction: in vitro and in vivo evaluation. Drug Des Devel Ther 12:1697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Xiao X, Zhu J, et al. (2018). Lactoferrin- and RGD-comodified, temozolomide and vincristine-coloaded nanostructured lipid carriers for gliomatosis cerebri combination therapy. Int J Nanomedicine 13:3039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li J, Hu S, et al. (2018). Triphenylphosphonium and d-α-tocopheryl polyethylene glycol 1000 succinate-modified, tanshinone IIA-loaded lipid–polymeric nanocarriers for the targeted therapy of myocardial infarction. Int J Nanomedicine 13:4045–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Wang J, Pan J. (2016). Baicalin-loaded PEGylated lipid nanoparticles: characterization, pharmacokinetics, and protective effects on acute myocardial ischemia in rats. Drug Deliv 23:3696–703. [DOI] [PubMed] [Google Scholar]
- Zheng G, Zheng M, Yang B, et al. (2019). Improving breast cancer therapy using doxorubicin loaded solid lipid nanoparticles: synthesis of a novel arginine-glycine-aspartic tripeptide conjugated, pH sensitive lipid and evaluation of the nanomedicine in vitro and in vivo. Biomed Pharmacother 116:109006. [DOI] [PubMed] [Google Scholar]
- Zuurbier CJ, Bertrand L, Beauloye CR, et al. (2020). Cardiac metabolism as a driver and therapeutic target of myocardial infarction. J Cell Mol Med 24:5937–54. [DOI] [PMC free article] [PubMed] [Google Scholar]