Summary
Background
The city of Manaus, Brazil, has seen two collapses of the health system due to the COVID-19 pandemic. We report anti-SARS-CoV-2 nucleocapsid IgG antibody seroconversion rates and associated risk factors in Manaus residents before the second wave of the epidemic in Brazil.
Methods
A convenience sample of adult (aged ≥18 years) residents of Manaus was recruited through online and university website advertising into the DETECTCoV-19 study cohort. The current analysis of seroconversion included a subgroup of DETECTCoV-19 participants who had at least two serum sample collections separated by at least 4 weeks between Aug 19 and Oct 2, 2020 (visit 1), and Oct 19 and Nov 27, 2020 (visit 2). Those who reported (or had no data on) having a COVID-19 diagnosis before visit 1, and who were positive for anti-SARS-CoV-2 nucleocapsid IgG antibodies at visit 1 were excluded. Using an in-house ELISA, the reactivity index (RI; calculated as the optical density ratio of the sample to the negative control) for serum anti-SARS-CoV-2 nucleocapsid IgG antibodies was measured at both visits. We calculated the incidence of seroconversion (defined as RI values ≤1·5 at visit 1 and ≥1·5 at visit 2, and a ratio >2 between the visit 2 and visit 1 RI values) during the study period, as well as incidence rate ratios (IRRs) through cluster-corrected and adjusted Poisson regression models to analyse associations between seroconversion and variables related to sociodemographic characteristics, health access, comorbidities, COVID-19 exposure, protective behaviours, and symptoms.
Findings
2496 DETECTCoV-19 cohort participants returned for a follow-up visit between Oct 19 and Nov 27, 2020, of whom 204 reported having COVID-19 before the first visit and 24 had no data regarding previous disease status. 559 participants were seropositive for anti-SARS-CoV-2 nucleocapsid IgG antibodies at baseline. Of the remaining 1709 participants who were seronegative at baseline, 71 did not meet the criteria for seroconversion and were excluded from the analyses. Among the remaining 1638 participants who were seronegative at baseline, 214 showed seroconversion at visit 2. The seroconversion incidence was 13·06% (95% CI 11·52–14·79) overall and 6·78% (5·61–8·10) for symptomatic seroconversion, over a median follow-up period of 57 days (IQR 54–61). 48·1% of seroconversion events were estimated to be asymptomatic. The sample had higher proportions of affluent and higher-educated people than those reported for the Manaus city population. In the fully adjusted and corrected model, risk factors for seroconversion before visit 2 were having a COVID-19 case in the household (IRR 1·49 [95% CI 1·21–1·83]), not wearing a mask during contact with a person with COVID-19 (1·25 [1·09–1·45]), relaxation of physical distancing (1·31 [1·05–1·64]), and having flu-like symptoms (1·79 [1·23–2·59]) or a COVID-19 diagnosis (3·57 [2·27–5·63]) between the first and second visits, whereas working remotely was associated with lower incidence (0·74 [0·56–0·97]).
Interpretation
An intense infection transmission period preceded the second wave of COVID-19 in Manaus. Several modifiable behaviours increased the risk of seroconversion, including non-compliance with non-pharmaceutical interventions measures such as not wearing a mask during contact, relaxation of protective measures, and non-remote working. Increased testing in high-transmission areas is needed to provide timely information about ongoing transmission and aid appropriate implementation of transmission mitigation measures.
Funding
Ministry of Education, Brazil; Fundação de Amparo à Pesquisa do Estado do Amazonas; Pan American Health Organization (PAHO)/WHO.
Research in context.
Evidence before this study
We searched published and preprint literature with the following combination of keywords: “incidence”, “risk factors”, “SARS-CoV-2”, “COVID-19”, “cohort”, “Amazon”, and “Brazil”. We found few cross-sectional studies that reported seroprevalence, and very few longitudinal studies that examined the incidence of seroconversion in the adult population. We also found no studies that examined the effect of risk factors or non-pharmaceutical interventions on SARS-CoV-2 incidence in the adult population. Previously, from our baseline DETECTCoV-19 cohort analysis, we observed that low socioeconomic status and household case clustering increased the risk of acquiring SARS-CoV-2 infection during the first COVID-19 pandemic wave in Manaus. Intrafamilial transmission might have fuelled the spread of disease among the population when voluntary isolation and protective measures were not appropriately adopted.
Added value of this study
The DETECTCoV-19 prospective cohort study allowed us to investigate the incidence of seroconversion, a proxy for disease attack rate, in Manaus between August and November, 2020 (before the second COVID-19 pandemic wave in Brazil). From our initial recruitment cohort, 2496 participants returned for a second follow-up visit. The cohort had a higher proportion of high-earning, professionally employed individuals than the Manaus population, because it was recruited through a university website. Among the participants, the crude anti-SARS-CoV-2 nucleocapsid IgG seropositivity at baseline was 27·72%, which increased to 34·33% at the second visit. When we adjusted for test sensitivity and specificity, antibody prevalence increased from 28·70% at baseline to 36·40% after follow-up. Among 1638 seronegative and uninfected participants at baseline, the overall incidence of seroconversion was 13·06% (and 6·78% for symptomatic seroconversion) indicating that 1% of the sample seroconverted every 4·5 days. We observed that having a COVID-19 case in the household and behavioural non-compliance with non-pharmaceutical intervention measures—including not wearing a mask during contact, relaxation of protective measures, and non-remote working—were associated with an increased risk of seroconversion. In addition, we estimated that around 50% of seroconversion events were asymptomatic during the study period.
Implications of all the available evidence
The seropositivity at the second visit and the seroconversion rate estimate suggest that a large proportion of this convenience sample was still susceptible to the SARS-CoV-2 after the first pandemic wave. Even after accounting for reported antibody decay rates, at least 50% of the population represented by our sample would not have developed antibodies for SARS-CoV-2. Therefore, it is probable that an intense infection transmission period preceded the second pandemic wave in Manaus. We emphasise that non-pharmaceutical interventions and protective behavioural measures are crucial to reduce the risk of acquiring the disease. Serosurvey-informed public health interventions need to be rapidly implemented, maintained, and scaled in areas of high transmission, such as Manaus. This study provides timely evidence to inform public health policies and to formulate effective communication strategies to enforce public health measures aimed at reducing the risk of acquiring SARS-CoV-2 infection.
Introduction
As of February, 2021, Brazil ranks second in the reported number of COVID-19 deaths since the start of the pandemic.1 The health-care system in Manaus, capital of the Brazilian state of Amazonas, has collapsed twice in less than 8 months (April 5, 2020, and Jan 8, 2021),2, 3 and the numbers of deaths and cases in the second epidemic wave (which began at the end of December, 2020) surpassed those from the first wave.4 The high toll in morbidity and mortality has aggravated the precarious state in which the first wave left the Amazon state of Brazil, with important consequences for the families and communities affected.5 In September, 2020, Brazilian health authorities were alerted to a possible second wave of infections if physical distancing and mobility measures were not appropriately enforced.3 Additionally, non-pharmaceutical interventions were further relaxed in July, 2020, with consequences on behaviour, mobility, and adherence to protective measures, thus hindering previous public health policies’ achievements.6 Concurrently, a sequential serological survey of anti-SARS-CoV-2 antibodies among several Amazonian cities showed an increasing trend in seropositivity,7 and a blood-donor-based report showed a high attack rate in the region,8, 9 indicating high levels of transmission. These findings implied that natural immunity and the effects of, or adherence to, public health measures and non-pharmaceutical interventions were insufficient to control the rising cases. To better understand the disease dynamics in the adult population, we established a prospective observational cohort based on convenience sampling in Manaus. In our baseline cross-sectional assessment during August, 2020, we observed that seropositivity was associated with unfavourable socioeconomic status and household clustering, as previously reported.7, 10 In this study, we estimated the incidence and risk factors associated with SARS-CoV-2 seroconversion from the DETECTCoV-19 cohort before the start of the second wave of infections in Manaus.
Methods
Study design and participants
Using a convenience sampling strategy based on online and university website advertising, men and women aged 18 years and older who were residents of Manaus were recruited into the DETECTCoV-19 longitudinal study. Participants were followed up for up to 6 months, with a sample collection every 8–12 weeks, as previously described.10 Visit 1 occurred between Aug 19 and Oct 2, 2020, and visit 2 between Oct 19 and Nov 27, 2020. For the baseline study, a sample size of 2399 individuals was calculated, such that the binomial 95% CI around the point estimate of antibody test positivity was less than ±2%.10
For the current analysis of SARS-CoV-2 antibody seroconversion, we included participants in the DETECTCoV-19 study who had at least two study visits with serum sample collections separated by at least 4 weeks. We excluded individuals who reported having a COVID-19 diagnosis before the first study visit (either by PCR, serology, radiology, or clinical diagnosis); who had no data or did not answer questions regarding COVID-19 diagnosis before the first visit; and who were positive for anti-SARS-CoV-2 nucleocapsid IgG antibodies (based on the ELISA described below) at visit 1.
The research ethics committee of the Federal University of Amazonas approved this study (CAAE 34906920.4.0000.5020) in accordance with Brazilian law and the Helsinki declaration. All participants gave oral and written consent before enrolment. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guideline for cohort studies (appendix pp 14–15).
Data and sample collection
All participants filled out an electronic questionnaire and donated a blood sample for SARS-CoV-2 serological testing (appendix p 2). At both visits we collected information related to physical distancing and protective practices, as well as information related to COVID-19, symptoms since the start of the pandemic, previous diagnoses, and self-medication and prescribed medication used for the treatment of symptoms. If available, study participants provided their results from oropharyngeal SARS-CoV-2 RT-PCR or antigen tests done by local government or private laboratories. We also recorded information about COVID-19 cases diagnosed in the family and in the participant's residence. An independent form was used to record the SARS-CoV-2 serological assay results. Trained interviewers collected participant data using REDCap, an online application with integrated quality checks (eg, range and valid values, date formats, and skip logic).
An online appointment system was used to reduce unnecessary gathering of participants, and the study followed all state and federal COVID-19-related regulations throughout its duration. The collection centre was equipped with state-compliant measures for appropriate physical distancing and all members of the DETECTCoV-19 team used appropriate personal protective equipment. All test results were communicated by email or phone messaging to the study participants.
Serological testing
An indirect ELISA-based serological assay was used to measure anti-SARS-CoV-2 nucleocapsid IgG antibody titres in serum samples, using recombinant full-length SARS-CoV-2 nucleocapsid protein (residues 1–419, GenBank QHD43423.2) expressed in Escherichia coli cells and purified by affinity and size exclusion chromatography, as previously described.10 The sensitivity and specificity of the assay were determined using serum samples from patients who were SARS-CoV-2 RT-PCR-positive (n=293, 87 outpatients and 113 inpatients) and from pre-pandemic controls (n=229). The assay had a sensitivity of 89·07% (95% CI 84·79–92·30) for patients at least 7 days after onset and 94·28% (89·44–97·07) for patients at least 14 days after onset of COVID-19 symptoms. A specificity of 97·03% (95% CI 93·72–98·69) was estimated using the pre-pandemic serum samples. An anti-SARS-CoV-2 nucleocapsid IgG antibody reactivity index (RI) was calculated as the optical density ratio between the sample and the negative control for each assay plate. A cutoff value of 1·5 was obtained using a receiver operating characteristic curve analysis done on the pre-pandemic controls and SARS-CoV-2 RT-PCR-positive patients. All samples with an RI value above 1·5 (the assay cutoff) were considered positive for anti-SARS-CoV-2 nucleocapsid IgG antibodies.10
Participants were considered to show seroconversion if they had an IgG ELISA RI of 1·5 or lower at the first visit, an RI greater than 1·5 at the second visit, and a ratio greater than 2 between the second visit and first visit RI values.
Statistical analysis
Participants who had an indeterminate seroconversion status were not included in the statistical analysis (appendix p 2). We estimated seropositivity as the proportion of participating individuals with a positive antibody test result. We adjusted the crude seropositivity estimates for test sensitivity (0·89) and specificity (0·97) with the Rogan-Gladen estimator11 and computed 95% CIs with Blaker's method.12 The numbers of people in each category were listed in the first table column (appendix pp 5–9, 21–24) to show incidence denominators and account for variations due to missing data. Missing values were excluded from all calculations and, therefore, were not part of the incidence denominators, or were listed as a separate category for each variable. Percentages of missing values for each variable were calculated.
Frequency distribution, cumulative incidence, and incidence density (considering follow-up time) for seroconversion and for symptomatic seroconversion were calculated for the whole group and according to the study variables. χ2 or Fisher's exact tests were used to evaluate associations between seroconversion incidence and independent variables. Poisson regression models with robust variance corrected according to cluster (administrative area) were used to estimate crude relative risks (RRs) and crude incidence rate ratios (IRRs) that considered the follow-up time for each variable. A multivariate model estimating adjusted IRRs was constructed including age, sex, and all variables that showed significant associations in the crude models. p values at or below 0·05 were considered to indicate statistical significance.
Because the sample was not random, stratified, or done by clusters, a sensitivity analysis was done to test whether the results would change if the sample had a sociodemographic distribution similar to that of Manaus. Two adjustment characteristics were chosen, administrative zone and family income (0–3, 4–6, or >6 times minimum wage), considering that geographical location would probably balance other variables such as health access, housing, and social behaviour. Using data from the Manaus 2010 census obtained from the Instituto Brasileiro de Geografia e Estatística, a weight factor was calculated for each family income in each zone, totalling 18 clusters. The weight factor was the probability of belonging to the Manaus cluster divided by the probability of having been sampled in the first visit. The descriptive analysis, bivariate comparisons, and regression models were repeated using the 18 clusters and weighting factors. χ2 test with Rao-Scott adjustment and Poisson regression models with Taylor linearisation and robust variances according to cluster were used. A 5% variation in the descriptive percentages and variations higher than one SE in seroconversion incidence rates were considered pronounced. To compare the multivariate model before and after weighting, we used a Wald test to compare regression coefficients and 95% CIs for each variable of the model.
Statistical analysis was done with Stata version 15.0. Data plots were generated with GraphPad Prism for MacOS (version 9.1.0) and RStudio (version 1.4.1106; R version 4.0.4) using the package ggplot2 (version 3.3.3).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
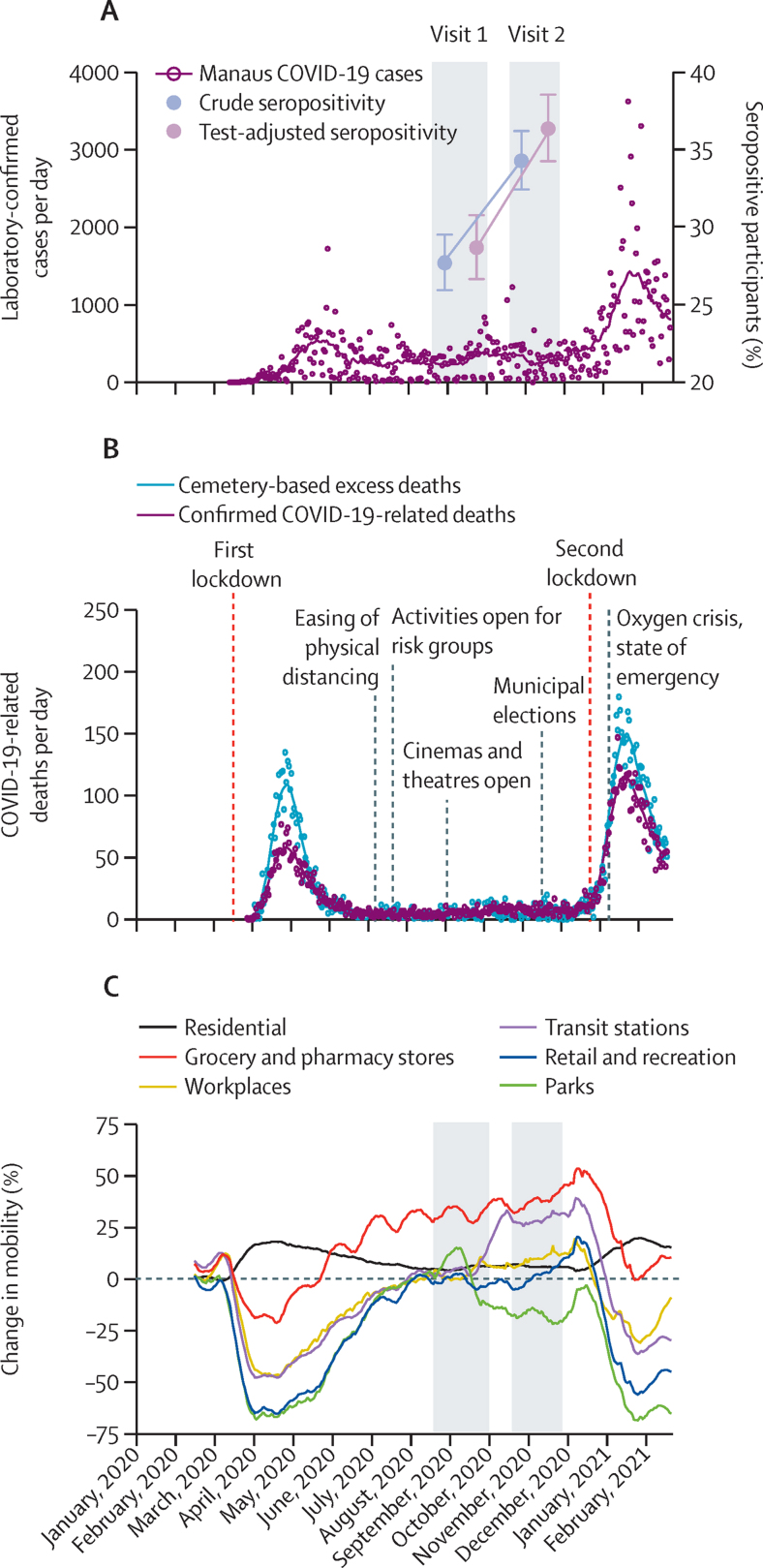
2496 of 3057 DETECTCoV-19 cohort participants returned for a second follow-up visit between Oct 19 and Nov 27, 2020 (appendix p 2). This cohort had higher proportions of high-earning, highly educated, and professionally employed people than Manaus city as a whole (appendix p 5) because a third of the participants lived in the region close to the university study site. Among these individuals, the crude anti-SARS-CoV-2 nucleocapsid IgG antibody seropositivity at baseline was 27·72% (95% CI 25·98–29·53), which increased to 34·33% (32·47–36·24) at the second visit. When adjusted for test sensitivity and specificity, antibody prevalence was 28·70% (26·70–30·80) at baseline and increased to 36·40% (34·30–38·60) after follow-up (figure 1). These findings indicate that, among the returning participants, the number of seropositive individuals and their IgG anti-nucleocapsid antibody RI values were higher at follow-up. Considering an antibody waning rate of 27·2% for symptomatic patients and double for asymptomatic patients over a 180-day follow-up period,16 at least 50% of the population represented by our sample would not have developed antibodies for SARS-CoV-2 by the second visit.
Figure 1.
Anti-SARS-CoV-2 nucleocapsid IgG antibody seropositivity, COVID-19 cases and deaths, and population mobility over time
(A) Number of laboratory-confirmed COVID-19 cases according to the Fundação de Vigilância em Saúde do Amazonas (FVS-AM) and crude and test-adjusted anti-SARS-CoV-2 nucleocapsid antibody seropositivity prevalence in the study sample (n=2496) at visits 1 and 2 (grey areas), with error bars showing 95% CIs. SARS-CoV-2 testing rates were low at the beginning of the epidemic and cases were, therefore, substantially subnotified. (B) Confirmed COVID-19-related deaths according to FVS-AM and cemetery-based excess deaths published according to the municipality of Manaus. The number of excess deaths exceeded the number of confirmed deaths during the epidemic because it includes all subnotified COVID-19 deaths as well as non-COVID-19-related deaths due to health system collapse (data extracted by Oliveira from ARPEN civil registry).13, 14 Non-pharmaceutical intervention-related and public health measures and pandemic-related events (marked with vertical lines on the graph) during the SARS-CoV-2 pandemic were collated from the Amazonas state government website. (C) Google mobility data for Manaus city15 plotted as percentage mobility change.
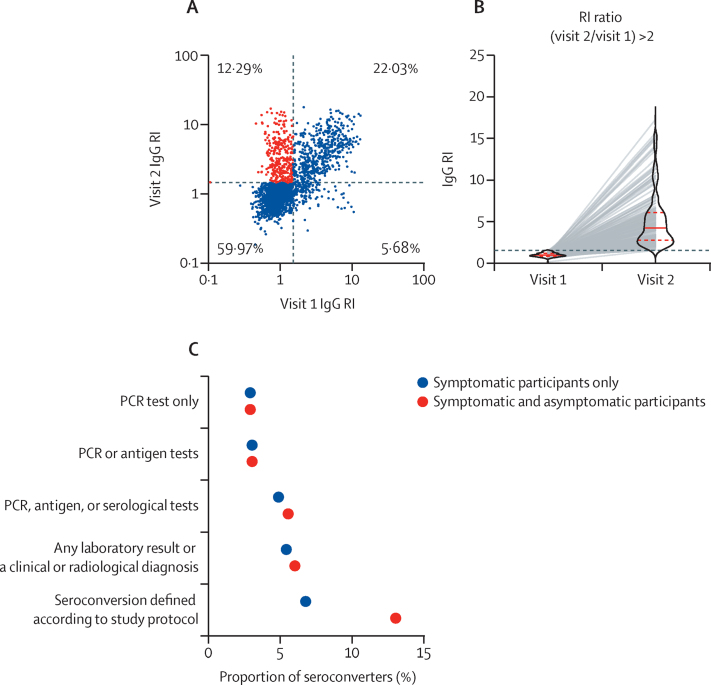
Figure 2A depicts the serological results of the 2496 participants at both visits. From the total participants evaluated at follow-up, 204 reported having COVID-19 before the first visit and 24 had no data regarding previous disease status. 559 participants were seropositive at baseline. From the remaining 2268, 1709 (75·4%) were IgG seronegative at the first visit. Among those who were seronegative at the first visit, 1424 (83·3%) had an RI value of 1·5 or lower at the second visit and were deemed to be still seronegative. 71 (4·2%) participants tested positive for IgG at second visit, but did not meet the criteria of a doubling in RI, and were thus considered to have indeterminate seroconversion status and were not included in the statistical analyses. 1638 participants were included in the analyses (appendix p 2). The percentages of missing values for each variable are shown in the appendix (p 12). Except for antigen test results, each variable was missing data for no more than 2·1% of participants, and no further adjustment for missing data was deemed necessary.
Subsequently, we calculated the incidence of SARS- CoV-2 seroconversion. 214 participants had an RI value greater than 1·5 at the second visit and an RI ratio between the second and first visit greater than 2, and (according to the protocol definition) were thus considered to show seroconversion (figure 2B; appendix p 2). The incidence of seroconversion was 13·06% (95% CI 11·52–14·79; 214 of 1638) with a median follow-up duration of 57 days (IQR 54–61); ie, 1% of the sample seroconverted every 4·5 days. Of those participants, 48·1% were asymptomatic; therefore, the incidence of symptomatic seroconversion was 6·78% (95% CI 5·61–8·10). Between the first and second visits, 2·9% of the sample had a positive RT-PCR test and 5·4% developed symptoms and were diagnosed with COVID-19 by the local government or private laboratories (figure 2C; appendix p 4).
Figure 2.
Anti-SARS-CoV-2 nucleocapsid IgG antibody seroconversion between the first and second study visits
(A) Anti-SARS-CoV-2 nucleocapsid IgG antibody RI results from study visits 1 and 2 (n=2496). Each dot represents one patient. The dotted lines denote the assay cutoff value. Red dots represent seroconverters (individuals who were negative for anti-SARS-CoV-2 nucleocapsid IgG antibodies at visit 1 and positive at visit 2 and with an RI ratio [visit 2 to visit 1] >2). (B) Paired anti-SARS-CoV-2 nucleocapsid IgG antibody RI values of seroconverters (defined according to the study protocol; n=214). The solid red lines depicts median RI values and dotted red lines indicate the upper and lower limits of the IQRs. (C) Proportion of seroconverters according to case definitions for symptomatic and asymptomatic (red) or asymptomatic-only individuals (blue); n=1638 (appendix p 4). RI=reactivity index.
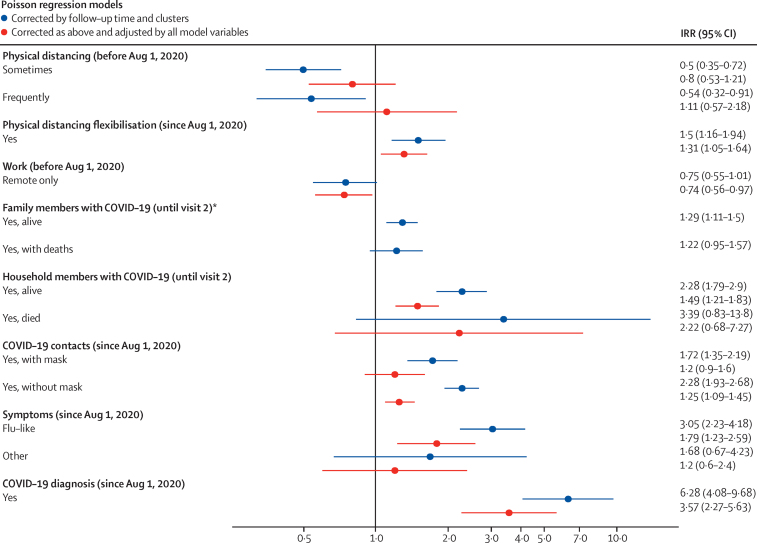
We then analysed the effects of sociodemographic, health-related, behavioural, case clustering, and COVID-19 testing variables on seroconversion (appendix pp 5–9). Statistically significant variables were then tested in a multivariate regression model for SARS-CoV-2 seroconversion (appendix p 10). Figure 3 shows IRRs obtained via Poisson regression, considering follow-up time with robust variance corrected by clusters. IRRs are shown unadjusted and adjusted according to all model variables (appendix pp 5–10). We observed that working remotely was associated with lower incidence (IRR 0·74 [95% CI 0·56–0·97]), whereas relaxation or flexibility of physical distancing measures (1·31 [1·05–1·64]), having a household member with COVID-19 (1·49 [1·21–1·83]), and direct contact with people with COVID-19 without a mask (1·25 [1·09–1·45]) were independently associated with an increase in incidence. Additionally, having flu-like symptoms (1·79 [1·23–2·59]) and a COVID-19 diagnosis between the first and second visits (3·57 [2·27–5·63]) were associated with increased IRRs (figure 3; appendix p 4).
Figure 3.
Multivariate regression model for SARS-CoV-2 seroconversion (n=1618)
Forest plot showing IRRs obtained via Poisson Regression considering follow-up time with robust variance corrected by clusters. IRRs are shown unadjusted and adjusted according to all model variables (appendix p 4). IRR=incidence rate ratio. *Not included in the multivariate model due to collinearity with the household contact variable.
In the sensitivity analysis, we observed changes in frequencies of seroconversion after applying the weights in the descriptive sensitivity analysis tables (appendix pp 16–26). The frequency decreased from 35·47% to 11·48% among people living in the Centre-South area (where the study collection centre is located), but increased among people living in areas with lower socioeconomic levels (from 9·95% to 18·83% in the East zone and from 16·73% to 26·29% in the North zone). Similarly, the frequency decreased from 45·57% to 26·63% among people with family income more than 6 times minimum wage, but increased from 30·11% to 45·70% among people with income 0–3 times minimum wage.
Seroconversion incidence rates increased after weighting for male sex, White ethnicity, east Asian ethnicity, people who self-medicated with over-the-counter drugs, people who had COVID-19 contact with mask use, negative SARS-CoV-2 PCR test results, and negative serology results. Incidence dropped for African Brazilians and for people with a COVID-19 diagnosis (appendix p 21). The multivariate model showed no significant changes in the regression coefficients of any variable, and the calculated IRRs maintained similar values to those in the adjusted model. However, the variables relating to physical distancing flexibility, remote working, and having a COVID-19 contact lost significance, compared with those in the adjusted model, due to the widening of 95% CIs (appendix p 4).
Discussion
In this study, we found that the anti-SARS-CoV-2 seropositivity rate increased from 27·72% (95% CI 25·98–29·53) to 34·33% (32·47–36·24) in the DETECTCoV-19 cohort before the second wave of COVID-19 in Manaus. We observed a high incidence of seroconversion at 13·06% (11·52–14·79), with a median follow-up duration of 57 days (IQR 54–61 days). Our regression models showed that seroconversion was associated with modifiable behaviours and case clustering in households. The risk factors for seroconversion outlined in this study provide evidence to support that adherence to physical distancing and non-pharmaceutical interventions decreases the risk of infection with SARS-CoV-2. Because this cohort oversampled wealthier and more educated people, we explored the effect of adjusting the sample to Manaus city demographics and found a slight increase in the proportion of participants who showed seroconversion (13·39% [10·47–16·97]) compared with the main analysis. Seroconversion ranged from 10·22% to 19·53% for the different regions of the city, which reflects that the least affluent areas of the city had higher frequency of seroconversion. This highlights the need to have a differentiated health surveillance and response systems for areas of the city with marked disparities, especially in the context of the emergence of the P.1 (gamma) variant17, 18 and the second epidemic wave.
To our knowledge, this is the first study to report SARS-CoV-2 seroconversion rates prospectively in Brazil. Between the start of the first round of sample collection and the end of the second round (Aug 19 to Nov 27, 2020), Manaus city Fundação de Vigilância em Saúde do Amazonas reported 28 550 adult COVID-19 cases confirmed by laboratory testing, which correspond to a case reporting rate of 1·97% (95% CI 1·95–1·99) in the study period. The difference between the seroconversion rate in our convenience sample and the official reporting rate highlights the variations obtained when using different case definition criteria, whether it is seroconversion, symptomatic seroconversion, clinical diagnosis, diagnosis by PCR, or reporting to health agencies. Overall, it is important to rapidly identify these divergent incidences and their dynamics during the pandemic to adequately plan resource allocation and mitigation strategies. Crucially, we observed that a considerable proportion of COVID-19 transmission during the study period consisted of asymptomatic cases. This finding underlines the importance of continuous disease monitoring to inform appropriate and timely disease control measures, especially when incidence rates are driven by a relatively high proportion of undocumented infections.19, 20, 21, 22
By contrast with our previous report,10 which evaluated the prevalence of seropositivity during August, 2020, this study examined factors that affected incidence over a median 2-month period between August and November, 2020. In our first report, we found that prevalence was strongly associated with sociodemographic characteristics (male sex, older age, lower income, occupation, and number of household members), whereas these factors were no longer associated with the emergence of new cases during this study period. By contrast, we found that the main risk factors were related to the social behaviour of the participants, such as not maintaining physical distancing before August, 2020, relaxing physical distancing after August, on-site working, and having contact with people with COVID-19 without a mask. These results show that, between the first and second epidemic waves in Manaus, behavioural risk factors that increased exposure to SARS-CoV-2 were more important than biological or structural characteristics such as sex or poverty. This finding could be due to changes in epidemic dynamics; some studies have shown that patients were younger and healthier during the second wave, thus reducing the effects of age and comorbidities on infection.23, 24 Conversely, compliance with protective measures increased after the first wave, as shown in some other countries,25, 26 acquiring a more prominent role in the control of the epidemic. Having a person with a COVID-19 diagnosis in the household affected both the prevalence in August 2020, and the incidence in the following 2–3 months, with similar magnitude. Importantly, independently of the time period within the pandemic, our findings confirm that having a COVID-19 contact in the household remains a robust predictor for acquiring the disease.27 Having symptoms or having been diagnosed during the observation period were also strongly associated with seroconversion, more so than in the cross-sectional study,10 highlighting the inherent correlation between these events.
Since the start of the pandemic, disease control strategies that include effective surveillance, availability and ease of access to testing, well implemented non-pharmaceutical interventions, and public health response measures linked to testing (ie, contact tracing, quarantine, and isolation) have been fundamental in controlling disease transmission.28, 29, 30 Our findings reveal that COVID-19 index cases were likely to have driven seroconversion—a representative proxy for infection burden—within the household, as observed in other high-transmission settings.31 Our data suggest that household close contacts of COVID-19 patients (survivors or deceased) should be tested regardless of symptoms, and advised to undertake voluntary isolation;32 strict follow-up of cases and contacts is essential to reduce virus transmission.28, 33 Non-pharmaceutical interventions including physical distancing, mask use, and hygiene have been essential to the reduction of community SARS-CoV-2 transmission worldwide; therefore, abruptly lifting non-pharmaceutical interventions diminishes the gains accumulated by previously implemented policies.29, 34 In our study, individuals who relaxed physical distancing measures or had contact with people with COVID-19 without a mask35, 36 had the highest risk of being infected with SARS-CoV-2.
Our work has some limitations. The convenience sampling strategy based on online and university website advertising potentially excluded individuals who did not have access to this information, and might not completely represent the adult population of Manaus. To test whether this limitation had any effect on our results, we did a thorough sensitivity analysis using weighting to make the sample more similar to the actual population of Manaus. Marked differences in socioeconomic and geographical distributions were found between the unweighted and weighted samples: our sample had a disproportionate number of people with higher income, higher education, and residence around the university campus. After correcting for those factors, some seroconversion incidence rates changed, but without significantly modifying the crude IRRs; therefore, no new variable became significant, nor did any lose significance compared with the previous analysis. This finding is reflected in the final multivariate model, in which the regressions coefficients remained remarkably similar after weighting. The variables of physical distance flexibility, remote work, and having a COVID-19 contact lost significance due to widening of 95% CIs, but we attribute this finding to the expanded number of clusters from 6 to 18, and not to changes in the coefficients themselves. Therefore, although it is true that there were marked differences in geographical and socioeconomic distribution between our sample and the actual Manaus population, these differences did not alter our final risk factors model, nor our study conclusions. Additionally, although our in-house serological assay highly correlates with commercial SARS-CoV-2 tests, it is possible that performance variation among asymptomatic patients might affect our seropositivity estimates.
We also had shortcomings in active surveillance to identify symptomatic infections and positive RT-PCR test results. Additionally, most symptomatic participants had a mild or moderate infection, but our surveillance method could not exclude the possibility that cases of severe illness or death occurred among the non-returning participants, which would lead to underestimation of total seroconversion events. Although anti-SARS-CoV-2 IgG antibody seropositivity, specifically against spike and nucleocapsid proteins, is a robust indicator of previous infection,37, 38 antibody response kinetics and their variability among populations limit our interpretations.39 Nevertheless, our longitudinal serosurvey approach to assess exposure and burden, and the size of our cohort, made possible an in-depth statistical analysis to identify the risk factors associated with seroconversion in a setting of high transmission and low non-pharmaceutical intervention containment measures. Unfortunately, we could not evaluate the roles of different SARS-CoV-2 strains. The study period between August and November, 2020, theoretically predates the surge and dominance of the gamma variant in the region; however, we cannot rule out that the high rate of seroconversion in our cohort could have been influenced partially by the emergence of a more infectious strain, such as gamma, in Manaus.17, 18 We hypothesise that the role of the gamma variant could be ascribed to accelerating the transmission rate observed after November, but we consider that the high seroconversion incidence found in our cohort during the study period might be explained by case clustering and host-related behavioural factors, as observed in high-transmission settings.27, 31, 40, 41
Rampant spread of SARS-CoV-2 infection in settings with low compliance with behavioural and non-pharmaceutical intervention containment measures is a cause of concern because of its high human costs, elevated burden imposed on health-care systems, and a possible effect on the emergence of new variants—favoured in high transmission settings—which can be detrimental to the effectiveness of available and future countermeasures, including diagnostics, vaccines, and therapeutics. Our study shows that increased testing can provide timely information about ongoing transmission levels and can contribute to mitigation of the pandemic by informing adequate public health measures and non-pharmaceutical interventions in areas of high transmission such as Manaus and elsewhere.
Data sharing
De-identified participant data can be made available to researchers after approval from the research ethics committee. Requests should be directed to the corresponding authors.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
JDBL was supported by funds from the Ministry of Education (MEC), Brazil. PL received funding from the Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM). JDBL and PL were supported by WHO Unity Studies, a global seroepidemiological standardisation initiative, with funding to WHO by the COVID-19 Solidarity Response Fund and the German Federal Ministry of Health (BMG) COVID-19 Research and Development. BBS, IVPF, ARCB, and WBSS received scholarship from CAPES. DSSS, TBNM, and MFJ received scholarship from FAPEAM. We would like to thank Comitê de Enfrentamento de Coronavírus/UFAM and Escola de Enfermagem de Manaus (EEM/UFAM) for the logistical support. We are also grateful to Laboratório Central de Saúde Pública do Amazonas (LACEN/AM) for providing SARS-CoV-2 RT-PCR testing. We sincerely thank Bernardo Horta for suggestions and discussions.
Contributors
PL and JDBL are the principal investigators of this study and acquired necessary funding. PL and JDBL conceived the study with input from CFC, PES, BCA, CAG, and RVA. Protein expression and purification were done by BBS, IBC, JNSN, ENA, and SAF. Sample collection was led by JDBL with assistance from CFC, PES, and BCA. The laboratory setup and sample processing were coordinated by PL and BBS. BBS, IVPF, DSSS, TBNM, MFJ, JVO, ARCB, and WBSS processed blood samples, did laboratory testing, collected data, and approved test results, with supervision from PL. PL, BBS, and JDBL coordinated data acquisition and data management. Data were cleaned and prepared by PL and RVA. Statistical analyses and data visualisation were done by PL and CAG. RVA led the statistical analyses. PL, JDBL, CAG, and RVA analysed the results and wrote the manuscript. All authors revised and approved the final version of this manuscript. All authors had full access to the raw data and accept responsibility to submit for publication.
DETECTCoV-19 Study Team
Aldina Iacy Paulain Holanda, Ana Lúcia Silva Gomes, Ana Paula Souza de França, André Victor Rabelo Monteiro, Andressa dos Passos Santos, Antônia de Sousa Teixeira, Antônio Vinicius Soares de Souza, Beatriz Pinheiro, Bianca Pires dos Santos, Brenda Pereira Farias, Bruno Nicolau Paulino, Caio Lúcio Andreola da Silva, Cinthya Iamile Frithz Brandão de Oliveira, Dalila de Alcântara Martins, Eline Araújo de Oliveira, Elisson Denny da Costa Carvalho, Evillyn Fernandes Da Costa, Fernanda Guilhon Simplicio, Fernanda Serrão Pereira, Gabriele Pimentel Sinimbu, Genilton de Oliveira Cardenes, Giane Alves da Silva, Iago Sampaio Fernandes da Costa, Ingrid Silva Correia, Ilia Gilmara Carvalho dos Santos, Jackeline Vieira Guimarães, Jessica Samile Batista Pinheiro, Juliana Correa Romana, Josineide de Oliveira Novo França, Kerollen Runa Pinto, Maria Fiamma Farias Freitas, Marne Carvalho de Vasconcellos, Marizete Candido Moraes, Matheus da Silva Damasceno, Michelle Araújo Ruiz, Milena Maria Cardoso de Lemos, Neila Soares Picanço, Rayara Gonzaga Maia, Regiane Carneiro Bezerra, Romeu Santos de Souza, Susy Cavalcante Harjani, Vitor Batista de Souza, Wellington Barbosa de Melo. Members are listed in alphabetical order.
Contributor Information
Pritesh Lalwani, Email: pritesh.lalwani@fiocruz.br.
Jaila Dias Borges Lalwani, Email: jaila@ufam.edu.br.
the DETECTCoV-19 Study Team:
Aldina Iacy Paulain Holanda, Ana Lúcia Silva Gomes, Ana Paula Souza de França, André Victor Rabelo Monteiro, Andressa dos Passos Santos, Antônia de Sousa Teixeira, Antônio Vinicius Soares de Souza, Beatriz Pinheiro, Bianca Pires dos Santos, Brenda Pereira Farias, Bruno Nicolau Paulino, Caio Lúcio Andreola da Silva, Cinthya Iamile Frithz Brandão de Oliveira, Dalila de Alcântara Martins, Eline Araújo de Oliveira, Elisson Denny da Costa Carvalho, Evillyn Fernandes Da Costa, Fernanda Guilhon Simplicio, Fernanda Serrão Pereira, Gabriele Pimentel Sinimbu, Genilton de Oliveira Cardenes, Giane Alves da Silva, Iago Sampaio Fernandes da Costa, Ingrid Silva Correia, Ilia Gilmara Carvalho dos Santos, Jackeline Vieira Guimarães, Jessica Samile Batista Pinheiro, Juliana Correa Romana, Josineide de Oliveira Novo França, Kerollen Runa Pinto, Maria Fiamma Farias Freitas, Marne Carvalho de Vasconcellos, Marizete Candido Moraes, Matheus da Silva Damasceno, Michelle Araújo Ruiz, Milena Maria Cardoso de Lemos, Neila Soares Picanço, Rayara Gonzaga Maia, Regiane Carneiro Bezerra, Romeu Santos de Souza, Susy Cavalcante Harjani, Vitor Batista de Souza, and Wellington Barbosa de Melo
Supplementary Material
References
- 1.WHO WHO coronavirus (COVID-19) dashboard. 2021. https://covid19.who.int
- 2.Taylor L. COVID-19: is Manaus the final nail in the coffin for natural herd immunity? BMJ. 2021;372:n394. doi: 10.1136/bmj.n394. [DOI] [PubMed] [Google Scholar]
- 3.Ferrante L, Steinmetz WA, Almeida ACL. Brazil's policies condemn Amazonia to a second wave of COVID-19. Nat Med. 2020;26:1315. doi: 10.1038/s41591-020-1026-x. [DOI] [PubMed] [Google Scholar]
- 4.FVS-AM Fundação de vigilância em Saúde do Amazonas: dados epidemiológicos e financeiros das ações de combate à COVID-19. 2021. https://www.fvs.am.gov.br/transparenciacovid19
- 5.Castro MC, Kim S, Barberia L. Spatiotemporal pattern of COVID-19 spread in Brazil. Science. 2021;372:821–826. doi: 10.1126/science.abh1558. [DOI] [PubMed] [Google Scholar]
- 6.Faria de Moura Villela E, López RVM, Sato APS. COVID-19 outbreak in Brazil: adherence to national preventive measures and impact on people's lives, an online survey. BMC Public Health. 2021;21:152. doi: 10.1186/s12889-021-10222-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallal PC, Hartwig FP, Horta BL. SARS-CoV-2 antibody prevalence in Brazil: results from two successive nationwide serological household surveys. Lancet Glob Health. 2020;8:e1390–e1398. doi: 10.1016/S2214-109X(20)30387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabino EC, Buss LF, Carvalho MPS. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. Lancet. 2021;397:452–455. doi: 10.1016/S0140-6736(21)00183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buss LF, Prete CA, Jr, Abrahim CMM. Three-quarters attack rate of SARS-CoV-2 in the Brazilian Amazon during a largely unmitigated epidemic. Science. 2020 doi: 10.1126/science.abe9728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lalwani P, Salgado BB, Pereira Filho IV. SARS-CoV-2 seroprevalence and associated factors in Manaus, Brazil: baseline results from the DETECTCoV-19 cohort study. Int J Infect Dis. 2021;110:141–150. doi: 10.1016/j.ijid.2021.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogan WJ, Gladen B. Estimating prevalence from the results of a screening test. Am J Epidemiol. 1978;107:71–76. doi: 10.1093/oxfordjournals.aje.a112510. [DOI] [PubMed] [Google Scholar]
- 12.Reiczigel J, Földi J, Ozsvári L. Exact confidence limits for prevalence of a disease with an imperfect diagnostic test. Epidemiol Infect. 2010;138:1674–1678. doi: 10.1017/S0950268810000385. [DOI] [PubMed] [Google Scholar]
- 13.Oliveira M. Brazil civil registry data. https://github.com/capyvara/brazil-civil-registry-data
- 14.Manaus_excess_deaths https://drive.google.com/drive/folders/1DYmuzzOJwHrB3LtXMrmguc1mIauJH4V_?usp=sharing
- 15.Google COVID-19 community mobility reports. https://www.google.com/covid19/mobility/
- 16.Chia WN, Zhu F, Ong SWX. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe. 2021;2:e240–e249. doi: 10.1016/S2666-5247(21)00025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naveca FG, Nascimento V, de Souza VC. COVID-19 in Amazonas, Brazil, was driven by the persistence of endemic lineages and P.1 emergence. Nat Med. 2021;27:1230–1238. doi: 10.1038/s41591-021-01378-7. [DOI] [PubMed] [Google Scholar]
- 18.Faria NR, Mellan TA, Whittaker C. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science. 2021;372:815–821. doi: 10.1126/science.abh2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansson MA, Quandelacy TM, Kada S. SARS-CoV-2 transmission from people without COVID-19 symptoms. JAMA Netw Open. 2021;4:e2035057. doi: 10.1001/jamanetworkopen.2020.35057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subramanian R, He Q, Pascual M. Quantifying asymptomatic infection and transmission of COVID-19 in New York City using observed cases, serology, and testing capacity. Proc Natl Acad Sci USA. 2021;118 doi: 10.1073/pnas.2019716118. e2019716118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li R, Pei S, Chen B. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2) Science. 2020;368:489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hao X, Cheng S, Wu D, Wu T, Lin X, Wang C. Reconstruction of the full transmission dynamics of COVID-19 in Wuhan. Nature. 2020;584:420–424. doi: 10.1038/s41586-020-2554-8. [DOI] [PubMed] [Google Scholar]
- 23.Iftimie S, López-Azcona AF, Vallverdú I. First and second waves of coronavirus disease-19: a comparative study in hospitalized patients in Reus, Spain. PLoS One. 2021;16:e0248029. doi: 10.1371/journal.pone.0248029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Domingo P, Pomar V, Mur I, Castellví I, Corominas H, de Benito N. Not all COVID-19 pandemic waves are alike. Clin Microbiol Infect. 2021;27:1040.e7–1040.e10. doi: 10.1016/j.cmi.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rader B, White LF, Burns MR. Mask-wearing and control of SARS-CoV-2 transmission in the USA: a cross-sectional study. Lancet Digit Health. 2021;3:e148–e157. doi: 10.1016/S2589-7500(20)30293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Betsch C, Korn L, Sprengholz P. Social and behavioral consequences of mask policies during the COVID-19 pandemic. Proc Natl Acad Sci USA. 2020;117:21851–21853. doi: 10.1073/pnas.2011674117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madewell ZJ, Yang Y, Longini IM, Jr, Halloran ME, Dean NE. Household transmission of SARS-CoV-2: a systematic review and meta-analysis. JAMA Netw Open. 2020;3:e2031756. doi: 10.1001/jamanetworkopen.2020.31756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lokuge K, Banks E, Davis S. Exit strategies: optimising feasible surveillance for detection, elimination, and ongoing prevention of COVID-19 community transmission. BMC Med. 2021;19:50. doi: 10.1186/s12916-021-01934-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh S, Shaikh M, Hauck K, Miraldo M. Impacts of introducing and lifting nonpharmaceutical interventions on COVID-19 daily growth rate and compliance in the United States. Proc Natl Acad Sci USA. 2021;118 doi: 10.1073/pnas.2021359118. e2021359118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chu DK, Akl EA, Duda S. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li F, Li Y-Y, Liu M-J. Household transmission of SARS-CoV-2 and risk factors for susceptibility and infectivity in Wuhan: a retrospective observational study. Lancet Infect Dis. 2021;21:617–628. doi: 10.1016/S1473-3099(20)30981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kucharski AJ, Klepac P, Conlan AJK. Effectiveness of isolation, testing, contact tracing, and physical distancing on reducing transmission of SARS-CoV-2 in different settings: a mathematical modelling study. Lancet Infect Dis. 2020;20:1151–1160. doi: 10.1016/S1473-3099(20)30457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Litvinova M, Wang W. Evolving epidemiology and transmission dynamics of coronavirus disease 2019 outside Hubei province, China: a descriptive and modelling study. Lancet Infect Dis. 2020;20:793–802. doi: 10.1016/S1473-3099(20)30230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oraby T, Tyshenko MG, Maldonado JC. Modeling the effect of lockdown timing as a COVID-19 control measure in countries with differing social contacts. Sci Rep. 2021;11:3354. doi: 10.1038/s41598-021-82873-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howard J, Huang A, Li Z. An evidence review of face masks against COVID-19. Proc Natl Acad Sci USA. 2021;118 doi: 10.1073/pnas.2014564118. e2014564118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leung NHL, Chu DKW, Shiu EYC. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26:676–680. doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kennedy-Shaffer L, Baym M, Hanage WP. Perfect as the enemy of good: tracing transmissions with low-sensitivity tests to mitigate SARS-CoV-2 outbreaks. Lancet Microbe. 2021;2:e219–e224. doi: 10.1016/S2666-5247(21)00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Long Q-X, Liu B-Z, Deng H-J. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 39.Amanat F, Stadlbauer D, Strohmeier S. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26:1033–1036. doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Del Brutto OH, Costa AF, Mera RM, Recalde BY, Bustos JA, García HH. Household clustering of SARS-CoV-2 in community settings: a study from rural Ecuador. Am J Trop Med Hyg. 2020;103:1207–1210. doi: 10.4269/ajtmh.20-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rader B, Scarpino SV, Nande A. Crowding and the shape of COVID-19 epidemics. Nat Med. 2020;26:1829–1834. doi: 10.1038/s41591-020-1104-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified participant data can be made available to researchers after approval from the research ethics committee. Requests should be directed to the corresponding authors.