ABSTRACT
Tomato is an important crop for its high nutritional and medicinal properties. The role of salicylic acid (SA) in 1-aminocyclopropane-1-carboxylate synthase (ACS), sodium-hydrogen exchanger (NHX1), salt overly sensitive 1 (sos1) and high-affinity K+ transporter (HKT1;2) transcripts, and ACS enzyme activity and ethylene (ET) production, and growth and physiological attributes was evaluated in tomato cv. Pusa Ruby under salinity stress. Thirty days-old seedlings treated with 0 mM NaCl, 250 mM NaCl, 250 mM NaCl plus 100 µM SA were assessed for different growth and physiological parameters at 45 DAS. Results showed ACS, NHX1, sos1 and HKT1;2 transcripts were significantly changed in SA treated plants. The ACS enzyme activity and ET content were considerably decreased in SA treated plants. Shoot length (SL), root length (RL), number of leaves (NL), leaf area per plant (LA), shoot fresh weight (SFW) and root fresh weight (RFW) were also improved under SA treatment. Conversely, the electrolyte leakage and sodium ion (Na+) content were significantly reduced in SA treated plants. In addition, the endogenous proline and potassium ion (K+) content, and K+/Na+ ratio were considerably increased under SA treatment. Likewise, antioxidant enzymes (SOD, CAT, APX and GR) profile were better in SA treated plant. The present findings suggest that SA reverse the negative effects of salinity stress and stress induced ET production by modulating ACS, NHX, sos1 and HKT1;2 transcript level, and improving various growth and physiological parameters, and antioxidants enzymes profile. This will contribute to a better understanding of salinity stress tolerance mechanisms of tomato plants involving SA and ET cross talk and ions homeostasis to develop more tolerant plant.
KEYWORDS: ACS, ethylene, NHX, HKT1, 2, salicylic acid, salinity stress, sos1, tomato productivity
1. Introduction
Tomato has been extensively investigated plant due to the higher content of molecules with antioxidants property such as carotenoids, lycopene, phenols, and flavonoids.1,2 Interestingly, lycopene has a potential role in maintaining cardiovascular function and health.3 Despite such an important crop, quality and productivity of tomatoes are badly affected by various abiotic stresses, particularly, salinity stress4 saline soil severely affects various growth and physiological parameters such as seed germination, plant growth, and productivity. Over and above 800 million hectares of land (c. 6% of the world’s total land area) is badly hit by salinity stress.5 Salinity stress negatively affects different morphological and physiological characteristics by modulating the endogenous content ions (Na+ and K+), and the level of biomolecules such as proline, antioxidants enzymes and plant hormones.6 Abiotic stresses (for example salinity stress) induced ethylene synthesis in plants is termed as stress ethylene.7 A potential role of salicylic acid (SA) in improving salinity stress tolerance in plants is well documented.8 Interestingly, SA functions as an ET inhibitor, and the various adverse response of excess ethylene under salinity stress can be regulated by SA,9 which might be crucial in plant salinity stress tolerance.
ET negatively regulates several plant growth and developmental processes and various stress response. ET production is induced by salinity stress, and the leaf epinasty resulted due to salinity stress or salinity stress induced ET overproduction has been considered biomarker to screen tomato cultivars under high salt.10,11 Consequently, numerous harmful effects of ET such as epinasty, restricted growth, affected leaf morphology, senescence, necrosis and cellular damage are likely to be similar to the symptoms appeared due to high sanity.7,11 In ET biosynthetic pathways, ACC (1-aminocyclo propnae 1-carboxylic acid) synthase (ACS) is a key enzyme that plays a crucial role in ET signaling and salinity stress response.12 Hence, salinity stress induced ethylene production is associated with improved ACS expression and enhanced ACS activity.6 Sato et al. (1891)13 have cloned the first ACS gene from Cucurbita pepo. ACS gene expressions have correspondingly been examined in numerous plant species.6 The phosphorylation of ACS2/ACS6 by MPK3 and MPK6 improves ACS protein that leads to higher cellular ACS enzyme activity and thereby enhanced ethylene production.14 SA, a natural phenolic compound, has been considered an important restorer of affected plant growth and development under salinity and/or ethylene. The interaction between SA and ET supports adaptive responses of plants by regulating programmed cell death (PCD) ROS generation under salinity stress.9 There is a relationship between SA and ET with respect to leaf senescence, which can be activated either coordinately or antagonistically with subject to the biological processes.14 Under salinity stress conditions, the various negative effects of salinity stress induced ET production and salinity stress alone can be nullified by regulating transcript level of ACS, NHX, sos 1 and HKT1;2 through SA.5,8,15 In the present study, we have used SA treatment to compare various morphological and physiological studies in tomato plants under salinity stress. We investigated ACS, NHX, sos 1 and HKT1;2 expression level and the endogenous ACS activity, ET content, electrolyte leakage, proline content, sodium and potassium ions accumulation, and antioxidants enzyme profile under high salt. Our study attempts to improve insights into stress tolerance mechanism involving ACS, NHX, sos 1 and HKT1;2, and SA and ET to cross-talk with growth and developmental processes to develop salinity tolerant tomato.
2. Material and methods
2.1. Plant materials and growth conditions
Tomato (Lycopersicon esculentum L.) cv. Pusa Ruby from Indian Agricultural Research Institute, New Delhi, India seeds were surface sterilized with 0.01% HgCl2. It was followed by repeated washings with deionized water. The surface sterilized seeds were sown, and then seedlings were transplanted to earthen pots filled with soil and farmyard manure (8:2). Irrigation was done by using tap water as and when required. The thirty days-old seedlings were treated with 0 mM NaCl, 250 mM NaCl and 250 mM NaCl plus 100 µM SA for three days. Electrical conductivity (EC) of 250 NaCl solution was 24 ds/m. Subsequently, plants were irrigated with tap water whenever required. Tomato plants were allowed to grow, and were assessed at 45 DAS for different growth and physiological parameters. The young leaves of earlier reproductive stages of plants were taken.
2.2. RNA isolation and quantitative real-time PCR (qRT-PCR)
The gene sequence of 1-aminocyclopropane-1-carboxylate synthase 2 (ACS2) (Accession number: NM_001247249), Na+/H+ antiporter (NHX1) (Accession number: AJ306630), Na+/H+ antiporter (sos1) and sodium transporter (HKT1;2) (Accession number: NM_001302904) from tomato were obtained by nucleotide database search of the National Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/nuccore/NM_001247249.3;https://www.ncbi.nlm.nih.gov/nuccore/AJ306630.1;https://www.ncbi.nlm.nih.gov/nuccore/NM_001247769.3 and https://www.ncbi.nlm.nih.gov/nuccore/NM_001302904.1). Leaf samples of 0 mM NaCl, 250 mM NaCl and 250 mM NaCl plus 100 µM SA treated tomato cv. Pusa Ruby plants were used for RT‐PCR. Total RNA was isolated using TRIzolH Reagent (Invitrogen, http://www.invitrogen.com) as per manufacturer’s instructions, and poly(A)-RNA was isolated.16,17 It was used to prepare cDNA using the RevertAid H minus first-strand cDNA synthesis kit (Fermentas, http://www.thermoscientificbio.c om/fermentas) as described previously.6 Expression analysis of the ACS2 gene in NaCl treated plant was performed by qRT-PCR by adopting the method of Jayaraman et al. (2008),18 and the relative levels of the transcript accumulated for the ACS2, NHX1, sos1 and HKT1;2 genes (Primers forward: 5′ – AAGAGCATGGCGAAAACTC – 3′, 5ʹ–TGCGGAGATTTTCATTTTCC–3ʹ, 5ʹ–CCACCGGAAGCTTGTTACAT–3ʹ and 5ʹ–CCGTCTTTTCGTCCTCAAAA–3ʹ; and primers reverse: 5′ – GCAATGGCCTTGAATGATTT – 3′, 5ʹ–TGTCATGCTCAGATCGCTTC–3ʹ, 5ʹ–CCTTCTTCCTCGCTTTCCTT–3ʹ and 5ʹ–TAAAAGCTTCCCCACCAAGA–3ʹ) were normalized to a–tubulin (primers: forward 5′–GTGGAGGTGATGATGCTTT–3′ and reverse 5′–ACCACGGGCAAAGTTGTTAG–3′), and ACS2, NHX1, sos1 and HKT1;2 genes expression in the control plant19 using the 2–ΔΔCt method from three independent experiments.20
2.3. Determination of ACS activity and quantification of ethylene
The leaf tissues (0.2 g) of 0 mM NaCl, 250 mM NaCl and 250 mM NaCl plus 100 µM SA treated tomato plants were ground using mortar and pestle in 3 mL of extraction buffer (0.1 M EPPS-KOH buffer, pH 8.5, 10 mM 2-mercaptoethanol, and 10 µM pyridoxal phosphate) at 2°C. ACS (EC 4.4.1.14) was extracted using the methods of Kato et al. (2000)21 with slight modification. ACC formed in the reaction was assayed by the method of Lizada and Yang (1979).22 Proteins were estimated by using the method of Bradford (1976).23 Enzyme activity was expressed as the amount of ACC (pmol) produced per mg protein per hour. Ethylene content in leaf tissues of 0 mM NaCl, 250 mM NaCl and 250 mM NaCl plus 100 µM SA treated tomato plants was quantified by following the method of Nakatsuka et al. (1997)24 using the same set of experimental conditions and gas chromatography as described by Ansari et al. (2019).6
2.4. Vegetative growth assessment
Shoot and Root length of control and treated plants were measured using a meter ruler. The number of leaves per plant was calculated in treated and untreated plants.25 Leaf area per plant was calculated.26 The change in shoot fresh weight and root fresh weight were recorded in treated and untreated plants.25
2.5. Measurement of electrolyte leakage
The harvested treated and untreated tomato plants were quickly washed with distilled water to remove adhering ions leached during the salt treatment, followed by making small incisions. Electrolyte leakage was measured by adopting the method of Bajji et al. (2004).27
2.6. Estimation of proline accumulation
Proline was extracted and estimated using the standard protocol.28 Around 100 mg leaf tissues of both salt stressed, SA treated and unstressed controls were taken. The substance was extracted from sulfosalicylic acid for which an equal volume of glacial acetic acid and ninhydrin solutions were added. The sample was heated at 100°C, to which 5 mL of toluene was added after cooling. The absorbance of the toluene layer was read at 528 nm on a spectrophotometer. Proline content was calculated from the standard graph, prepared by using pure proline.
2.7. Determination of Na+ and K+ content
For determination of endogenous Na+ and K+ contents, 100 mg of leaf tissue (treated or untreated) of tomato plants was taken and digested in 0.1% HNO3. Ions were extracted from distilled H2O by boiling for 30 min twice. The filtered extract thus obtained was used to measure specific ions with a flame photometer.29
2.8. Antioxidant enzyme assays
A crude enzyme extract was prepared by homogenizing 0.5 g of frozen leaf tissues of treated and untreated tomato plants in an extraction buffer containing 1 mM EDTA, 0.05% Triton X-100 and 2% polyvinyl pyrrolidone (PVP), 1 mM ascorbate in 50 mM potassium phosphate buffer (pH 7.8) using a chilled mortar and pestle. The homogenate was centrifuged at 10,000 g for 20 min and the supernatant was stored at −20°C for further used for the antioxidant enzymes assays.30
2.9. Statistical analysis
The experiment followed a randomized block design and the number of replicates for each treatment was 3 (n = 3). Here the ‘mean of three replicates’ represents the ‘mean of three independent plants’. Data were analyzed statistically and standard errors were calculated. Least significant differences (LSDs) between the mean values (n = 3) of control and treated tomato plants were calculated by one-way analysis of variance (ANOVA) using SPSS 10.0 (SPSS, Inc., now IBM, http:// www-01.ibm.com/software/analytics/spss). Bars showing the same letter are not significantly different from the LSD test at p < .05.
3. Results
3.1. Salicylic acid affects endogenous ACS, NHX1, sos1 and HKT1;2 transcripts, ACS activity and ethylene level in tomato plants under salinity stress
SA treatment improves the performance of tomato plant cv. Pusa Ruby under salinity at 45 DAS and 90 DAS (Figure 1a, b). The level of ACS transcript was significantly decreased in SA treated tomato plants (2.16 folds) than control plants (4.4 folds) and 250 mM NaCl treated plants (8.6 folds) (Figure 1c). However, the NHX1, sos1 and HKT1;2 transcripts (7.1, 5.9 and 11.3 folds) were significantly improved in SA treated plants as compared to 250 mM NaCl treated (209, 2.5 and 5.5 folds) and control plants (0.7, 0.9 and 1.7 folds) (Figure 1d-f). Additionally, the ACS activity (pmol ACC mg protein−1 h−1) was significantly lower in SA treated plants (101.5) as compared to 250 mM NaCl treated (398.5) and control plants (192.7) (Figure 1g). Likewise, the endogenous level ET (pmol g−1 FW min-2) of SA treated plant (77.9) was significantly declined as compared to control (149.3) and NaCl treated plants (313.6) (Figure 1h).
Figure 1.
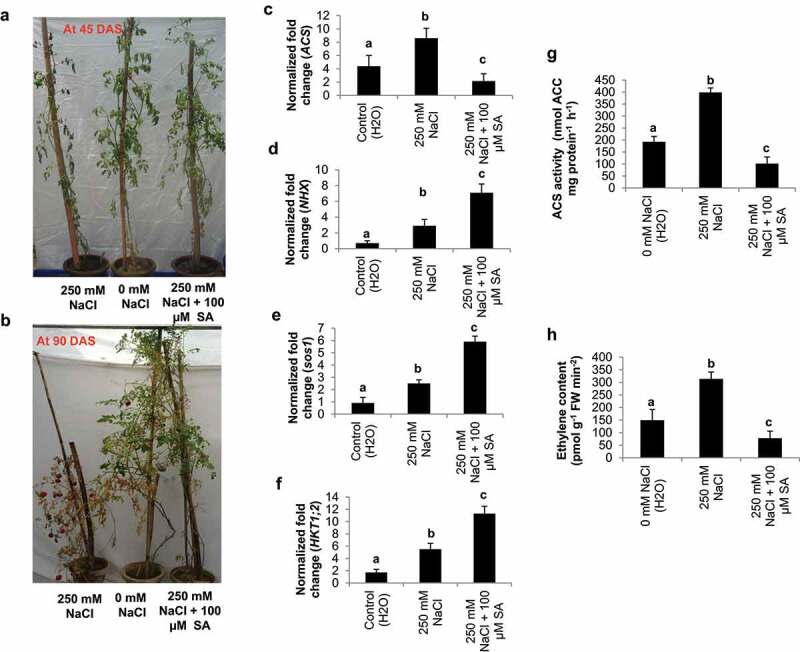
Response of tomato cv, Pusa Ruby plants to salinity stress (250 mM NaCl) and salicylic acid (100 µM SA) treatments as compared to control (H2O) at 45 and 90 DAS (a-b). The endogenous transcript levels of ACS2, NHX1, sos1 and HKT1;2 (c-f), ACC synthase enzyme activity (pmol ACC mg protein−1 h−1) (g) and ethylene content (pmol g−1 FW min−2) (h) in treated and untreated tomato cv, Pusa Ruby plants at 45 DAS as compared to control (H2O)
3.2. Salicylic acid recovers various morphological parameters in tomato plants under salinity stress
Plant growth parameters such shoot length (SL), root length (RL), number of leaves (LN), leaf area per plant (LA), shoot fresh weight (SFW) and root fresh weight (RFW) affected under salinity stress were recovered by SA treatments (Figure 2). As compared to control plants (115.6 and 25 cm), NaCl treated plants exhibited considerably reduced SL and RL (20.9 and 4.5 cm), which were significantly recovered by SA treatment (48.9 and 9.9 cm) (Figure 2a, b). SA treatment also significantly increases LN and LA (15 numbers and 57.9 cm2) than NaCl (7 numbers and 20 cm2). It was highest in control plants (26 numbers and 109 cm2) (Figure 2c, d). Similar trend was also reflected in SFW and RFW. It was significantly reduced NaCl treated plants (2.5 and 0.5 g) as compared to control plants (9 and 4.7 g), and was then significantly improved in SA treated plants (5 and 1.7 g) (Figure 2e,f).
Figure 2.
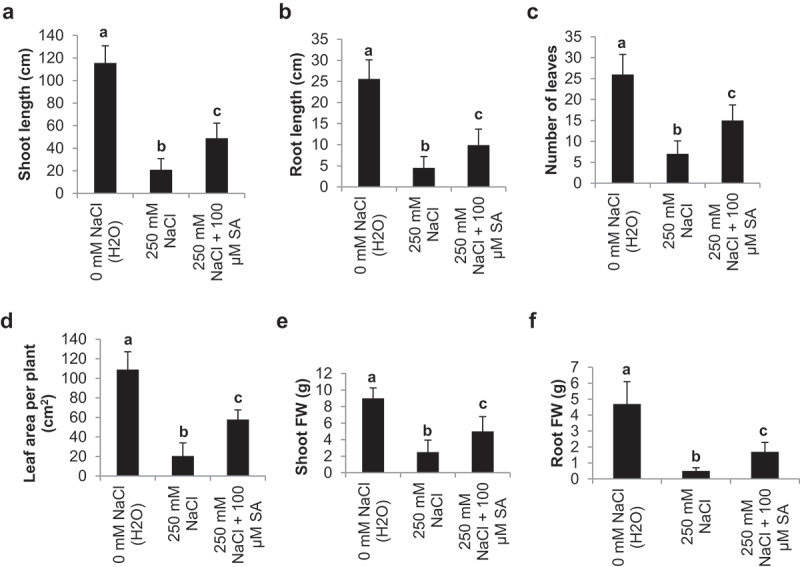
Effect of salinity stress (250 mM NaCl) and salicylic acid (100 µM SA) on shoot length (SL) (a), root length (RL) (b), number of leaves (LN) (c), leaf area per plant (LA) (d), shoot fresh weight (SFW) (e) and root fresh weight (RFW) (g) in tomato cv, Pusa Ruby at 45 DAS as compared to control (H2O)
3.3. Salicylic acid: electrolyte leakage, proline content, Na+ and K+ level, and K+/Na+ ratio in tomato plants under salinity stress
We observed cell injury in respect of electrolyte leakage in tomato plants under salinity stress and stability of cell membrane (as expressed by electrolyte leakage) was different under NaCl stress over the control (no stress). The application of SA significantly decreases electrolyte leakage (23.1%) as compared NaCl treated (92.6%) and control (30.4%) (Figure 3a). Proline (µmol g−1 FW) accumulation improved significantly with application of SA (42.8) as well as with NaCl stress treatment (22.6) than control (10) (Figure 3b). The endogenous sodium (Na+) content (µmol gDW−1) was significantly declined in SA treated plant (292.5) with respect to NaCl treated (598.5) and control plants (19.6) (Figure 3c). However, endogenous potassium (K+) content (µmol gDW−1) was considerably higher in plants treated with SA (310.9) than NaCl treated (110.6) and unstressed plants (635.6) (Figure 3d). Additionally, control (Without NaCl) plants maintain high K+/Na+ ratio (32.4), and it was declined in plants treated with NaCl (0.18). SA application significantly improved K+/Na+ ratio (0.94) as compared to NaCl treated plants (0.18) (Figure 3e).
Figure 3.
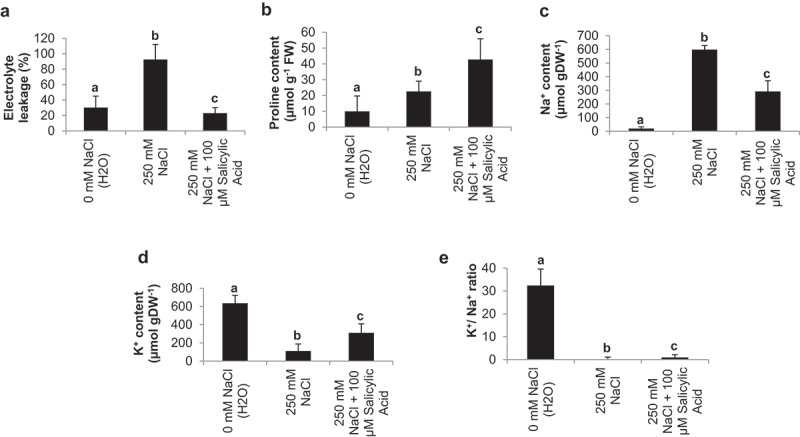
Effect of salinity stress (250 mM NaCl) and salicylic acid (100 µM SA) on electrolyte leakage (a), endogenous proline content (b), endogenous Na+ content (c), endogenous K+ content (d) and Ration of K+/Na+ (e) in tomato cv, Pusa Ruby at 45 DAS as compared to control (H2O)
3.4. Salicylic acid improves antioxidants enzymes in tomato plants under salinity stress
The activity (Unit mg-1 protein) of antioxidant enzymes such as superoxide dismutase (SOD) catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR) was deceased in NaCl treatment plants (1.9, 18.6, 7.0 and 2.9) than control plants (6.9, 70.5, 25.9 and 8.9). SA applications significantly recover the activity of SOD, CAT, APX and GR (5.2, 58.9, 18.8 and 8.2) I tomato plants with compared NaCl treated plants (1.9, 18.6, 7.0 and 2.9) (Figure 4a-d).
Figure 4.
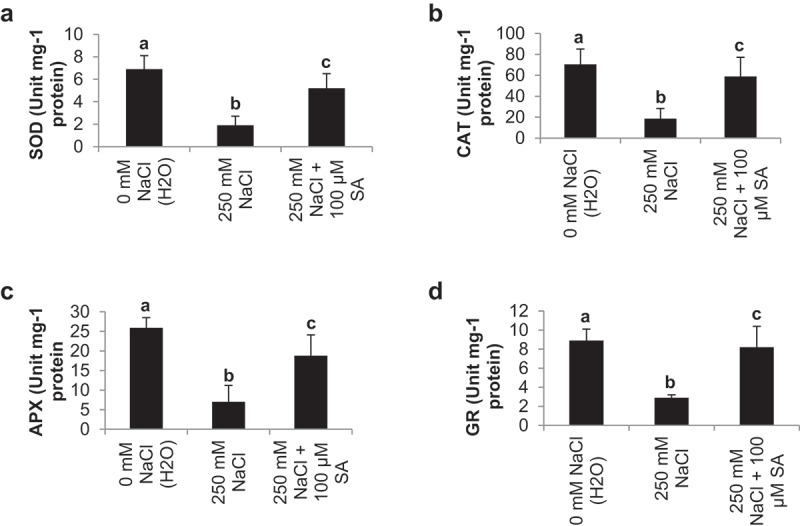
Effect of salinity stress (250 mM NaCl) and salicylic acid (100 µM SA) on antioxidants enzymes- SOD (a), CAT (b), APX (c) and GR (d) in tomato cv, Pusa Ruby at 45 DAS as compared to control (H2O)
4. Discussion
Salinity stress has become paramount environmental factor affecting agricultural productivity at large scale. It triggers many cellular events, causing morphological and physiological alterations, inducing water deficiency, osmotic stress, ion toxicity and oxidative damage which lead to reduced leaf surface expansion, yellowing and browning, epinasty, reduced growth, senescence and necrosis, and altered phenology and eventually plant death.11,31 Stress induced ethylene (ET) production is a common phenomenon in several plant response to abiotic stresses particularly salinity stress.32 The over production of ET in response to salinity stress has showed various adverse effects on plant productivity.6,32 There are indications that salicylic acid (SA) potentially regulates various responses to abiotic stresses by inhibiting ET formation under salinity stress.8,33 In the present study, it was observed that the overproduction of ET and over-accumulation Na+ ions in response to salinity stress might be associated with reduced crop productivity. Here, SA acts as a strong inhibitor of ET and potential player of K+ and Na+ ions homeostasis which is used to nullify the negative effects of stress ET and salinity stress itself.
ACS, an important enzyme of the ET biosynthetic pathways, is regulating stress ET formation. ACSs, encoded by a multigene family, are expressed in response to environmental stresses (e.g., salinity stress). Till now, fourteen putative ACS in addition to six ACO genes were reported in the tomato genome.6,34,35 ACS 1 and ACS 12 genes were upregulation under differential treatment of salinity stress.36 The abundance of ACS transcript under abiotic stresses resulted in increased ACS activity and ethylene accumulation.6 A potential role of SA in regulating ET biosynthesis at the level of ACS transcript is well defined.37,38 Our data showed a comparatively lower accumulation of ACS transcript in SA treated plants, which substantiates the reduced ACS activity and decreases ethylene levels (Figure 1c, g-h). The individual negative effect of salinity stress on plant productivity can be reduced by maintaining the appropriate concentration of ions in the cytosol, which is mediated through functioning of antiporters (NHX and sos1) and transporter (HKT1;2). Interestingly, Na+ /H+ antiporters (NHX1 and SOS1) are a set of genes that function in ion homeostasis in plants by regulating Na+ ions in the cytosol. NHX1 are present in tonoplasts and decrease the accumulation of Na+ ions in the cytosol by pumping Na+ in the vacuole. SOS1 is located at the plasma membrane and is responsible for Na+ ions exclusion from cytosol to apoplasts.39 The increased transcript level of NHX1 and SOS1 in different plants under salinity stress confers plant tolerance to high salt.39,40 Here, our finding revealed a significantly higher level of NHX1 and SOS1 transcript in SA treated plants (Figure 1d, e). HKT transporters cause Na+ exclusion from the xylem sap of leaves and prevent Na+ from reaching the shoots and damaging photosynthetic cells.41,42 Under salinity stress, a different level of HKT transcript was studied in root and shoot of in diverse plant species.41 In the present study, SA treatment showed an improved accumulation of HKT transcript under salinity stress (Figure 1f). Further, our studied shown SA reduces salinity stress induced ACS activity and ET accumulation (Figure 1g, h). Thus, excess ET negatively effects on roots and shoots growth and leaf development.43 The BARI T1- BARI T5 tomato variety of Bangladesh is susceptible and exhibited reduced leaf number, length and width, and flowering, under high salinity.44 SA controls the overproduction of ET in plants as it suppresses ACS transcript11,45 and promotes the biosynthesis of putrescine from S-adenosyl methionine.46 Consequently, SA recovers plant productivity by improving ET mediated restricted growth of root and shoots and morphology of leaf.6,11,47 Therefore, a significantly higher shoot length, root length, number of leaves, leaf area per plant, shoot fresh weight and root fresh weight in our study might be due to reduced ET synthesis by SA (Figure 2a-f).
SA application enhanced barley plant growth by inducing protective responses and maintaining membrane integrity.48 The increased electrolyte leakage because of changes in membrane permeability is associated with enhanced ET production.49 Salinity stress essentially increased ET biosynthesis in plants,50 causing extensive electrolyte leakage and necrosis,51 which can be reduced by SA even in the presence of high salt.33 Our study showed SA treated tomato plants significantly lower electrolyte leakage than salinity stressed tomato plants (Figure 3a). Proline accumulation has been positively correlated with salt tolerance.52 It is an important multifunctional cytosolic osmolyte facilitating osmotic regulation and protecting the subcellular components in salinity stressed plants by stabilizing cellular macromolecules, such as DNA, protein, membranes.25,53 ET negatively or positively regulates the accumulation of proline under salinity stress. Under condition of no salt, a higher endogenous ethylene but lower proline was reported.54 SA limits the ET production by modulating ACS transcript and ACS activity. This will result in a relatively higher proline level.55 In the present study, we have observed comparatively higher proline in SA treated tomato plants (Figure 3b). Typically, high concentration of Na+ and low concentration of K+ status (high Na+/ K+ or low K+/Na+ ratio ratio) is most harmful for plant cells.50 Excess Na+ is particularly deleterious to plants because it competes with K+ for metabolic processes required for K+, causing a change in K+/ Na+ ratio. All of these effects lead to enzyme inactivation, nutritional imbalance, protein denaturation and oxidative stress and inhibited plant growth and developmental processes.55,56 Salinity stress induced ET is involved in cellular damage by changing in K+/ Na+ ratio under abiotic stresses together with salinity stress.6 SA application decreases the Na+ content and increases in the K+ concentration57 and normalizes various effects effects of salinity stress induced ET and excess Na+.33 Further, the NaCl-induced K+ movement from the roots was inhibited by SA treatment that resulted high K+ concentration and improved plant productivity.33,58 Here, a comparatively high K+ and low Na+ concentrations, and the high K+/ Na+ ratio were observed in the presence of SA under salinity stress (Figure 3c-e), which might be due to SA mediated over expression of NHX, sos 1 and HKT1;2 in tomato plants under salinity stress. High salinity stimulates ROS production within plant cells, causing oxidative damage of membrane lipids, proteins and nucleic acids, resulting impaired growth and developmental process.59 ROS are detoxified by many nonenzymatic and enzymatic antioxidants. Salinity stress alone and salinity stress stimulated accumulation of ethylene and/or cyanide reduce the activity of enzymatic antioxidants such as superoxide dismutase (SOD), catalase (CAT), peroxidase (POX), as well as the enzymes of the ascorbate glutathione reductase (GR).6,33,59 SA application improves the activity of enzymatic antioxidants and antioxidant enzymes inculding SOD, CAT, APX and GR in many plant species.33,60 Our results showed an improved activity of SOD, CAT, APX and GR in SA treated tomato plants (Figure 4a-d) that might be substantiated ACS, NHX, sos 1 and HKT1;2 gene expressions. As shown in (Figure 5), our study explains that salinity stress affects plant productivity by causing Na+ cytotoxicity and inducing ET production. SA application reduces ACS transcript level and improves NHX1, sos1 and HKT1;2 transcript abundance in tomato plants under salinity stress. Further, SA maintains low ACS enzyme activity and ET accumulation. This will lead to improved shoot length (SL), root length (RL), number of leaves (NL), leaf area per plant (LA), shoot fresh weight (SFW) and root fresh weight (RFW) in SA treated plants under salinity stress. On the other hand, in response to salinity stress the increased Na+ ions concentration in xylem parenchyma is regulated through the action of HKT 1;2, NHX and sos1. In this case, SA upregulates HKT 1;2, NHX and sos1 expression in tomato plants. The primary mechanism of salinity stress tolerance is the exclusion of Na+ from xylem parenchyma to cytosol, which could be due to high HKT 1;2 transcript in SA treated tomato plants. The increased HKT1:2 transcripts cause enhanced Na+ exclusion from xylem parenchyma to cytosol. Likewise, SA induces transcript accumulation of Na+ /H+ antiporters (NHX1 and SOS1). The improved NHX (found in tonoplast) transcript lowers the Na+ ions accumulation in the cytosol by driving Na+ ions in the vacuole. Conversely, the higher level of sos1 (located plasma membrane) transcripts in SA treated tomato plants promotes Na+ ions exclusion from cytosol to apoplasts. This will promote leads to reduced Na+ ions toxicity and electrolyte leakage in SA treated plants, and improved proline level and K+ ions content, and K+/Na+ ratio in SA treatment. Likewise, antioxidants enzymes (SOD, CAT, APX and GR) profile were superior in SA treated plant. Here, we suggest that SA positively mediates various negative effects of high ET, Na+ ions and salinity stress by controlling the expression of ACS, NHX, sos1 and HKT1;2 that in turn regulates different growth and developmental processes to improve tolerance to high salinity. In conclusion, the present finding provides insight into salinity stress tolerance mechanism of tomato plants cv. Pusa Ruby in which SA and ET are the key players that function in cell signaling network of plant involving various growth and developmental and physiological process in response to salinity stress.
Figure 5.
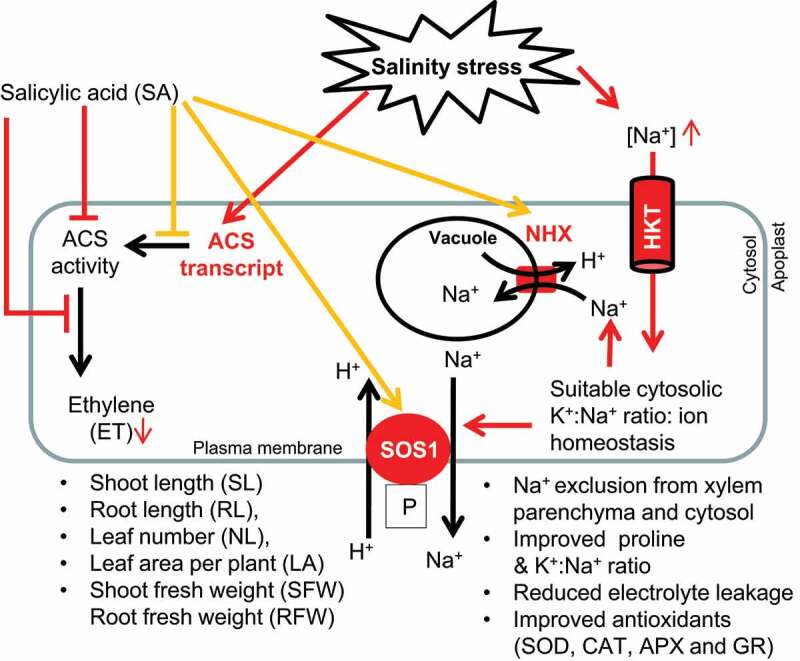
A tentative model of novel insight into plant salinity stress tolerance mechanisms mediated through cross talk between SA and ET to regulate ACS, NHX, sos1 and HKT1;2 genes expression, and thereby improved proline and K+ ions content, and K+/Na+ and antioxidants enzymes, and improved productivity
Acknowledgments
The work was supported by Department of Science and Technology (DST), Government of India under Fast Track Scheme of young scientist.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Periago MJ, García-Alonso J, Jacob K, Belén Olivares A, José Bernal M, Dolores Iniesta M, Martínez C, Ros G.. Bioactive compounds, folates and antioxidant properties of tomatoes (Lycopersicum esculentum) during vine ripening. Intr J Food Sci Nutr. 2009;60(8):1–9. doi: 10.3109/09637480701833457. [DOI] [PubMed] [Google Scholar]
- 2.Gerszberg A, Hnatuszko‑Konka K.. Tomato tolerance to abiotic stress: a review of most often engineered target sequence. Plant Growth Regul. 2017;83(2):175–198. doi: 10.1007/s10725-017-0251-x. [DOI] [Google Scholar]
- 3.Costa-Rodrigues J, Pinho O, Monteiro PRR. Can lycopene be considered an effective protection against cardiovascular disease? Food Chem. 2018;245:1148–1153. doi: 10.1016/j.foodchem.2017.11.055. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z, Hong Y, Li Y, Shi H, Yao J, Liu X, Wang F, Huang S, Zhu G, Zhu JK. Natural variations in SlSOS1 contribute to the loss of salt tolerance during tomato domestication. Plant Biotechnol J. 2021;19(1):20–22. doi: 10.1111/pbi.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59(1):651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 6.Ansari MW, Kaushik S, Bains G, Tula S, Joshi B, Rani V, Wattal RK, Rakwal R, Shukla A, Pant RC, et al. Cyanide produced with ethylene by ACS and its incomplete detoxification by β-CAS in mango inflorescence leads to malformation. Sci Rep. 2019;9(1):18361. doi: 10.1038/s41598-019-54787-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ansari MW, Rani V, Shukla A, Bains G, Pant RC, Tuteja N. Mango (Mangifera indica L.) malformation: a malady of stress ethylene origin. Physiol Mol Biol Plants. 2015;21(1):1–8. doi: 10.1007/s12298-014-0258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens J, Senaratna T, Sivasithamparam K. Salicylic acid induces salinity tolerance in tomato (Lycopersico nesculentum cv. Roma): associated changes in gas exchange, water relations and membrane stabilization. Plant Growth Regul. 2006;49:77–83. [Google Scholar]
- 9.Poór P, Borbély PG, Bódi N, Bagyánszki M, Görgényi-Miklósné-Tari IE. Effects of salicylic acid on photosynthetic activity and chloroplast morphology under light and prolonged darkness. Photosynthetica. 2019;57(2):367–376. doi: 10.32615/ps.2019.040. [DOI] [Google Scholar]
- 10.Jones RA, El-Beltagy AS. Epinasty promoted by salinity or ethylene is an indicator of salt-sensitivity in tomatoes. Plant Cell Environ. 1989;12(8):813–817. doi: 10.1111/j.1365-3040.1989.tb01643.x. [DOI] [Google Scholar]
- 11.Rao YR, Ansari MW, Singh AK, Bharti N, Rani V, Verma A, Gupta R, Siddiqui ZH, Abbas ZK, Bains G, et al. Ethylene mediated physiological response for in vitro development of salinity tolerant tomato. J Plant Interact. 2020;15(1):303–313. doi: 10.1080/17429145.2020.1820591. [DOI] [Google Scholar]
- 12.Win KT, Fukuyo T, Keiki O, Ohwaki Y. The ACC deaminase expressing endophyte Pseudomonas spp. enhances NaCl stress tolerance by reducing stress-related ethylene production, resulting in improved growth, photosynthetic performance, and ionic balance in tomato plants. Plant Physiol Biochem. 2018;127:599–607. doi: 10.1016/j.plaphy.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 13.Sato T, Theologis A. Cloning the mRNA encoding 1-aminocyclopropane-1-carboxylate synthase, the key enzyme for ethylene biosynthesis in plants. Proc Natl Acad Sci. 1989;86(17):6621–6625. doi: 10.1073/pnas.86.17.6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu X, Xu Y, Yan S. Salicylic acid and ethylene coordinately promote leaf senescence. J Integr Plant Biol. 2021;63(5):823–827. doi: 10.1111/jipb.13074. [DOI] [PubMed] [Google Scholar]
- 15.Eun HD, Ali S, Jung H, Kim K, Kim WC. Profiling of ACC synthase gene (ACS11) expression in Arabidopsis induced by abiotic stresses. Appl Biol Chem. 2019;62(1):42. doi: 10.1186/s13765-019-0450-4. [DOI] [Google Scholar]
- 16.Deng W, Yang Y, Ren Z, Audran-Delalande C, Mila I, Wang X, Song H, Hu Y, Bouzayen M, Li Z. The tomato SlIAA15 is involved in trichome formation and axillary shoot development. New Phytol. 2012;194(2):379–390. doi: 10.1111/j.1469-8137.2012.04053.x. [DOI] [PubMed] [Google Scholar]
- 17.Tuteja N, Sahoo RK, Garg B, Tuteja R. OsSUV3 dual helicase functions in salinity stress tolerance by maintaining photosynthesis and antioxidant machinery in rice (Oryza sativa L. cv. IR64). Plant J. 2013;76:115–127. [DOI] [PubMed] [Google Scholar]
- 18.Jayaraman A, Puranik S, Rai NK, Vidapu S, Sahu PP, Lata C, Prasad M. cDNA-AFLP analysis reveals differential gene expression in response to salt stress in foxtail millet (Setaria italica L.). Mol Biotechnol. 2008;40(3):241–251. doi: 10.1007/s12033-008-9081-4. [DOI] [PubMed] [Google Scholar]
- 19.Jain M, Nijhawan A, Tyagi AK, Khurana JP. Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem Biophys Res Commun. 2006;345(2):646–651. doi: 10.1016/j.bbrc.2006.04.140. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 200(25):402–408. [DOI] [PubMed] [Google Scholar]
- 21.Kato M, Hayakawa Y, Hyodo H, Ikom Y, Yano M. Wound-induced ethylene synthesis and expression and formation of 1-aminocyclopropane-1-carboxylate (ACC) synthase, ACC oxidase, phenylalanine ammonia-lyase, and peroxidase in wounded mesocarp tissue of Cucurbita maxima. Plant Cell Physiol. 2000;41(4):440–447. doi: 10.1093/pcp/41.4.440. [DOI] [PubMed] [Google Scholar]
- 22.Lizada MC, Yang SF, Yang SF. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979;100(1):140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- 23.Bradfor MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 24.Nakatsuka A, Shiomi S, Kubo Y, Inaba A. Expression and internal feedback regulation of ACC synthase and ACC oxidase genes in ripening tomato fruit. Plant Cell Physiol. 1997;38(10):1103–1110. doi: 10.1093/oxfordjournals.pcp.a029094. [DOI] [PubMed] [Google Scholar]
- 25.Basu S, Giri RK, Benazir I, Kumar S, Rajwanshi R, Dwivedi SK, Kumar G. Comprehensive physiological analyses and reactive oxygen species profiling in drought-tolerant rice genotypes under salinity stress. Physiol Mol Biol Plants. 2017;23(4):837–850. doi: 10.1007/s12298-017-0477-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan MN. Growth and physiological attributes of tomato (Lycopersicon esculentum Mill.) Genotypes as affected by NaCl stress. American J Plant Sci. 2016;7(3):453–460. doi: 10.4236/ajps.2016.73039. [DOI] [Google Scholar]
- 27.Bajji M, Bertin P, Lutts S, Kinet JM. Evaluation of drought resistance-related traits in durum wheat somaclonal lines selected in vitro. Aust J Exp Agric. 2004;44(1):27–35. doi: 10.1071/EA02199. [DOI] [Google Scholar]
- 28.Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39(1):205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- 29.Kumar G, Purty RS, Sharma MP, Singla-Pareek SL, Pareek A. Physiological responses among Brassica species under salinity stress show strong correlation with transcript abundance for SOS pathway-related genes. J Plant Physiol. 2009;166(5):507–520. doi: 10.1016/j.jplph.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Garg B, Jaiswal JP, Misra S, Tripathi BN, Prasad MA. A comprehensive study on dehydration-induced antioxidative responses during germination of Indian bread wheat (Triticum aestivum L. em Thell) cultivars collected from different agroclimatic zones. Physiol Mol Biol Plants. 2012;18(3):217–228. doi: 10.1007/s12298-012-0117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasouli F, Kiani-Pouya A, Shabala L, Li L, Tahir A, Yu M, Hedrich R, Chen Z, Wilson R, Zhang H, et al. Salinity effects on guard cell proteome in Chenopodium quinoa. Int J Mol Sci. 2021;22(1):428. doi: 10.3390/ijms22010428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dubois M, Van-den-Broeck L, Inzé D. The pivotal role of ethylene in plant growth. Trends Plant Sci. 2018;23(4):311–323. doi: 10.1016/j.tplants.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed W, Imran M, Yaseen M, Haq TU, Jamshaid MU, Rukh S, Ikram RM, Ali M, Ali A, Maqbool M, et al. Role of salicylic acid in regulating ethylene and physiological characteristics for alleviating salinity stress on germination, growth and yield of sweet pepper. Peer J. 2020;8:e8475. doi: 10.7717/peerj.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mata CI, Fabre B, Parsons HT, Hertog MLATM, Van Raemdonck G, Baggerman G, Van de Poel B, Lilley KS, Nicolaï BM. Ethylene receptors, ctrs and ein2 target protein identification and quantification through parallel reaction monitoring during tomato fruit ripening. Front Plant Sci. 2018;9:1626. doi: 10.3389/fpls.2018.01626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quinet M, Angosto T, Yuste-Lisbona FJ, Blanchard-Gros R, Bigot S, Martinez JP, Lutts S. Tomato fruit development and metabolism. Front Plant Sci. 2019;10:1554. doi: 10.3389/fpls.2019.01554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng Z, He S, Gong W, Sun J, Pan Z, Xu F, Lu Y, Du X. Comprehensive analysis of differentially expressed genes and transcriptional regulation induced by salt stress in two contrasting cotton genotypes. BMC Genom. 2014;15(1):760. doi: 10.1186/1471-2164-15-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li N, Parsons BL, Liu DR, Mattoo AK. Accumulation of wound-inducible ACC synthase transcript in tomato fruit is inhibited by salicylic acid and polyamines. Plant Mol Biol. 1992;18(3):477–487. doi: 10.1007/BF00040664. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Wang W, Wang M, Zhang HY, Liu JH. The miR396b of Poncirus trifoliata functions in cold tolerance by regulating ACC oxidase gene expression and modulating ethylene–polyamine homeostasis. Plant Cell Physiol. 2016;57(9):1865–1878. doi: 10.1093/pcp/pcw108. [DOI] [PubMed] [Google Scholar]
- 39.Zhang WD, Wang P, Bao Z, Ma Q, Duan LJ, Bao AK, Zhang JL, Wang S-M. SOS1, HKT1;5, and NHX1 synergistically modulate Na+ homeostasis in the halophytic grass Puccinellia tenuiflora. Front Plant Sci. 2017;8:576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ali A, Raddatz N, Pardo JM, Yun DJ. HKT sodium and potassium transporters in Arabidopsis thaliana and related halophyte species. Physiol Plant. 2021;171(4):546–558. doi: 10.1111/ppl.13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horie T, Hauser F, Schroeder JI. HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants. Trends Plant Sci. 2009;14(12):660–668. doi: 10.1016/j.tplants.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Almeida P, Katschnig D, Boer AHD. HKT transporters: state of the art. Int J Mol Sci. 2013;14(10):20359–20385. doi: 10.3390/ijms141020359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van-de-Poel B, Smet D, Van-Der-Straeten D. Ethylene and hormonal cross talk in vegetative growth and development. Plant Physiol. 2015;169(1):61–72. doi: 10.1104/pp.15.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rahman MM, Hossain M, Binte-Hossain KF, Sikder MT, Shammi M, Rasheduzzaman M, Hossain MA, Alam AKMM, Uddin MK. Effects of NaCl-Salinity on tomato (Lycopersicon esculentum Mill.) plants in a pot experiment. Open Agric. 2018;3(1):578–585. doi: 10.1515/opag-2018-0061. [DOI] [Google Scholar]
- 45.Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Ann Rev Plant Physiol. 1984;35(1):155–189. doi: 10.1146/annurev.pp.35.060184.001103. [DOI] [Google Scholar]
- 46.Singh A, Ansari MW, Singh CP, Shukla A, Pant RC, Tuteja N, Bains G, Bains G. First evidence of putrescine involvement in mitigating floral malformation in mango: a scanning electron microscope study. Protoplasma. 2014;251(5):1255–1261. doi: 10.1007/s00709-014-0611-6. [DOI] [PubMed] [Google Scholar]
- 47.Souri MK, Tohidloo G. Effectiveness of different methods of salicylic acid application on growth characteristics of tomato seedlings under salinity. Chem Biol Technol Agric. 2019;6(1):26. doi: 10.1186/s40538-019-0169-9. [DOI] [Google Scholar]
- 48.El-Tayeb MA. Response of barley grains to the interactive effect of salinity and salicylic acid. Plant Growth Regul. 2005;45(3):215–224. doi: 10.1007/s10725-005-4928-1. [DOI] [Google Scholar]
- 49.Guthrie DS, Cothren JT. Enhanced ethylene evolution and electrolyte leakage from methomyl-treated cotton (Gossypium hirsutum). Field Crops Res. 1989;19(4):241–252. doi: 10.1016/0378-4290(89)90096-8. [DOI] [Google Scholar]
- 50.Tao JJ, Chen HW, Ma B, Zhang WK, Chen SY, Zhang JS. The role of ethylene in plants under salinity stress. Front Plant Sci. 2015;6:1059. doi: 10.3389/fpls.2015.01059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bailey BA, Dean JF, Anderson JD. An ethylene biosynthesis-inducing endoxylanase elicits electrolyte leakage and necrosis in nicotiana tabacum cv xanthi leaves. Plant Physiol. 1990;94(4):1849–1854. doi: 10.1104/pp.94.4.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alotaibi S, Ali E, Darwesh H, Ahmed AT, Al-Thubaiti E. Effect of proline on growth and nutrient uptake of Simmondsia chinensis (Link) Schneider under salinity stress. Pak J Biol Sci. 2019;22(9):412–418. doi: 10.3923/pjbs.2019.412.418. [DOI] [PubMed] [Google Scholar]
- 53.Kavi-Kishor PB, Sangam S, Amrutha RN, Sri Laxmi P, Naidu KR, Rao KRSS, Rao S, Reddy KJ, Theriappan P, Sreenivasulu N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr Sci. 2005;88:424–438. [Google Scholar]
- 54.Iqbal N, Umar S, Khan NA. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J Plant Physiol. 2015;178:84–91. doi: 10.1016/j.jplph.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 55.Sharma A, Shahzad B, Kumar V, Kohli SK, Sidhu GPS, Bali AS, Handa N, Kapoor D, Bhardwaj R, Zheng B. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 2019;9:285.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li L, Gu W, Zhang L, Li C, Chen X, Qian C, Wang Z, Li W, Zuo S, Wei S. Exogenous 2-(3,4-Dichlorophenoxy) triethylamine alleviates salinity stress in maize by enhancing photosynthetic capacity, improving water status and maintaining K(+)/Na(+) homeostasis. BMC Plant Biol. 2020;20(1):348. doi: 10.1186/s12870-020-02550-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kovacik J, Klejdus B, Hedbavny J, Backor M. Salicylic acid alleviates NaCl-induced changes in the metabolism of Matricaria chamomilla plants. Ecotoxicology. 2009;18(5):544–554. doi: 10.1007/s10646-009-0312-7. [DOI] [PubMed] [Google Scholar]
- 58.Jayakannan M, Bose J, Babourina O, Rengel Z, Shabala S. Salicylic acid improves salinity tolerance in Arabidopsis by restoring membrane potential and preventing salt-induced K+ loss via a GORK channel. J Exp Bot. 2013;64(8):2255–2268. doi: 10.1093/jxb/ert085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–930. [DOI] [PubMed] [Google Scholar]
- 60.Loutfy N, Sakuma Y, Gupta DK, Inouhe M. Modifications of water status, growth rate and antioxidant system in two wheat cultivars as affected by salinity stress and salicylic acid. J Plant Res. 2020;133(4):549–570. doi: 10.1007/s10265-020-01196-x. [DOI] [PubMed] [Google Scholar]


