Abstract
Background
Point-of-care rapid tests to identify SARS-CoV-2 can have clinical benefits.
Methods
A cross-sectional study in adults visiting emergency services or screening sites of referral hospitals for COVID-19 to validate the diagnostic performance of a rapid antigen test for SARS-CoV-2 (Abbott's Panbio) compared with reverse transcription-polymerase chain reaction (RT-PCR) testing. Tests were performed by health personnel in a routine situation during a COVID-19 outbreak.
Results
A total of 1060 participants (mean age 47, 46% with a self-reported comorbidity) were recruited from 8 hospitals in Mexico. Participants provided 1060 valid Panbio rapid test-RT-PCR test pairs with 45% testing positive in the RT-PCR. Overall sensitivity of the Panbio test was 54.2% (95% CI 51%–57%), and 69.1% (95% CI 66%–73%) for patients during the first week of symptoms. Sensitivity depended on viral load (cycle threshold (Ct) of RT-PCR) and days of symptoms. With a Ct ≤25, sensitivity was 82% (95% CI, 76%–87%). Specificity of the Panbio test was >97.8% in all groups.
Conclusions
The Panbio rapid antigen test for SARS-CoV-2 had good specificity but low sensitivity. A negative test requires confirmation with RT-PCR, especially for testing after the first week of symptoms.
Keywords: COVID-19, SARS-CoV-2, point of care test, rapid antigen test, Panbio, Abbott
Introduction
Rapid tests to identify infectious agents are beneficial since they allow decisions to be made at the point-of-care (POC) for treatment selection or separation of cohorts to avoid cross-infection. Rapid tests are especially useful in emergency situations, such as those experienced in the COVID-19 pandemic. For influenza and other respiratory viruses, rapid tests are readily available and have shown clinical benefits (Benirschke et al., 2019; Rahamat‐Langendoen et al., 2019; Shengchen et al., 2019; Wabe et al., 2019), although not in all evaluations (Schechter-Perkins et al., 2019). Rapid tests at POC can be employed to screen asymptomatic people, diagnose people with symptoms suggestive of disease, or for contact tracing and epidemiological purposes in persons with contact with suspected or confirmed cases. These situations, in which the pre-test probabilities of SARS-CoV-2 infection are very different, have different demands (Watson et al., 2020). A recent Cochrane review showed the urgent need for prospective and comparative evaluation of rapid COVID-19 tests (Dinnes et al., 2020). Having a reliable rapid test is desirable, especially in places with poor infrastructure or without access to standard laboratory tests and at reference sites, especially those with potential for patients with similar clinical manifestations but with infection with a different virus (Kubina and Dziedzic, 2020). Rapid tests could also be performed on the same subject on several occasions and at a low cost to detect and isolate positive cases and for epidemiological surveillance, even at a lower sensitivity than reverse transcription-polymerase chain reaction (RT-PCR) tests (Mina et al., 2020).
Several international regulatory bodies have authorized the emergency use of rapid tests for the presumptive diagnosis of SARS-CoV-2 infection based on the identification of antigens. While the overall recommendation is to confirm results with tests considered the gold standard, such as PCR, a readily available result obtained with a rapid antigen test can aid in making several important decisions in the clinical care workflow.
Here, we assessed the performance of the Panbio rapid antigen test for SARS-CoV-2 (Abbott Diagnostics Korea, Inc. Ref. 41FK10) administered by health personnel as a diagnostic tool in symptomatic patients who arrived at the emergency rooms (ER) and outpatient clinics of referral hospitals for COVID-19 and in symptomatic or asymptomatic contacts of patients diagnosed with SARS-CoV-2.
Methods
We conducted an observational, cross-sectional study across 8 tertiary care referral hospitals for COVID-19 in Mexico, comparing the performance of the Panbio rapid antigen test with the gold standard RT-PCR test. The participating institutions were part of the Mexican National Institutes of Health (NIH) network, including the National Institute of Respiratory Diseases (INER), National Institute of Cancer (INCAN), National Institute of Cardiology (INCIC), High Specialty Regional Hospital Ciudad Salud in Tapachula Chiapas, High Specialty Regional Hospital of Mérida Yucatán (HRAE Mérida), High Specialty Regional Hospital in Ixtapaluca State of Mexico (HRAE Ixtapaluca), National Institute of Medical Sciences and Nutrition (INCMNSZ), and the National Institute of Perinatology (INPER). The protocol was revised and approved by a single Institutional Review Board designated by the NIH authority for this study.
Inclusion criteria
Patients aged ≥18 presenting with respiratory symptoms consistent with COVID-19 or influenza syndrome at the emergency rooms or screening sites of the participating hospitals and who provided written informed consent to participate were included in the study regardless of hospitalization status. Contacts of confirmed COVID-19 cases presenting to the same sites for evaluation (mostly with respiratory symptoms but some asymptomatic) were also enrolled, all providing a written informed consent to participate.. Participants were recruited on weekdays, during the morning-afternoon shift, from the 8 participating institutions.
Gold standard
For this study, the “Berlin” SARS-CoV-2 RT-PCR methodology recommended by the World Health Organization (WHO) (Corman et al., 2020) was considered the gold standard. All participating institutions implemented this test in situ and were accredited by the corresponding national authority (National Epidemiological Reference Institute) based on the detection of 4 SARS-CoV-2 markers: the N, E, ORF and RdRp genes. The RT-PCR test for SARS-CoV-2 was performed according to the Berlin protocol on nasopharyngeal swab samples taken with a synthetic fiber swab and flexible shaft and sent to the laboratory in a transport tube. In all cases, the cycle threshold (Ct) for the different gene targets was requested. Laboratory personnel were blinded to results for the rapid tests.
Procedures in the emergency rooms, outpatient clinics and with contacts
After participants provided their written informed consent, the Panbio rapid test and RT-PCR were performed in the ER, triage, or at the usual reception site for probable patients with COVID-19. Samples were obtained within minutes of each other with different swabs, following instructions for Panbio and RT-PCR. The Panbio test swab with the sample was inserted into the extraction tube with 300 uL of fluid buffer and processed immediately at the same place where it was taken. For RT-PCR, the specimens were inserted inside the universal viral transport tube with 2 mL of media (Becton, Dickinson, NJ, USA) and sent to the laboratory.
Rapid SARS-CoV-2 antigen test
The Panbio COVID-19 Ag Rapid Test Device (nasopharyngeal) (Abbott Diagnostics Korea, Inc. Ref. 41FK10) was evaluated. This test does not require additional equipment and is approved by the corresponding regulatory agencies in the United States, Europe and Mexico. The manufacturer reports a sensitivity of 93.3% (95% CI of 83%–98%) and a specificity of 99.4% (95% CI, 95%–99.3%) with a detection limit of 1.5 × 101.8 median tissue culture infectious dose (TCID50)/mL (Abbot 2020).
Healthcare personnel at the ER or the triage area carried out the tests following the manufacturer's instructions. A nasopharyngeal swab with a flexible shaft included with the kit was utilized to obtain the specimen with a gentle rub and roll movement. The swab was then inserted into the extraction tube and processed immediately where taken.
All participating healthcare personnel were trained in obtaining nasopharyngeal swabs and in the use of the rapid test. In all cases, a stopwatch was utilized to record the exact time of the reading. The result and a photograph of the cassette were incorporated into the database for control and later verification if necessary. Clinical information was retrieved using a REDCap database (REDCap 10.31-2021), including the WHO COVID-19 severity classification (World Health Organization 2020), the use of respiratory support (oxygen by nasal prongs or high flow), mechanical ventilation, and the presence and duration of respiratory symptoms. For hospitalized patients, the results of routine laboratory tests and clinical data were subsequently collected, including a complete blood count, serum electrolytes, glucose, urea, creatinine, electrocardiogram, liver function tests, urinalysis, and chest computed tomography.
A sample number of 600 subjects, with at least 300 symptomatic patients, and 300 contacts were considered in the protocol (Hajian-Tilaki 2014, Bujang 2016), allowing for a study power of 0.8 even given an assumed prevalence of COVID-19 positivity of 20%, and rapid test sensitivity and specificity of 80%.
Blinding: The laboratory processing the RT-PCR test and the administrator of the Panbio rapid antigen test did not know the result of the other test.
Statistical analysis
The initial comparison was performed in a 2 × 2 table between the positive and negative tests by RT-PCR and the positive and negative tests by the Panbio rapid test, with sensitivity, specificity, and positive and negative predictive value. We also evaluated concordance (kappa statistic) between the RT-PCR and the Panbio test.
Patients, contacts and participating hospitals were considered as subgroups. The impact of duration of symptoms on the positivity of the rapid test was assessed, with positivity as a dependent variable as a function of the duration of symptoms, taking into account the Ct of the RT-PCR test.
Age, sex, days from the onset of symptoms (if they occurred), symptoms, disease severity (based primarily on the type of support needed), time of arrival at the ER, time of the test, time of the result, day and the time of obtaining the RT-PCR result were taken as covariates on order to observe modifying effects on sensitivity and specificity.
Analysis was performed with STATA v13.0 statistical software, with summary statistics for diagnostic tests performed by DISGT and DIAGTEST procedures. The MIDAS procedure was utilized to analyze diagnostic performance across recruiting sites.
Results
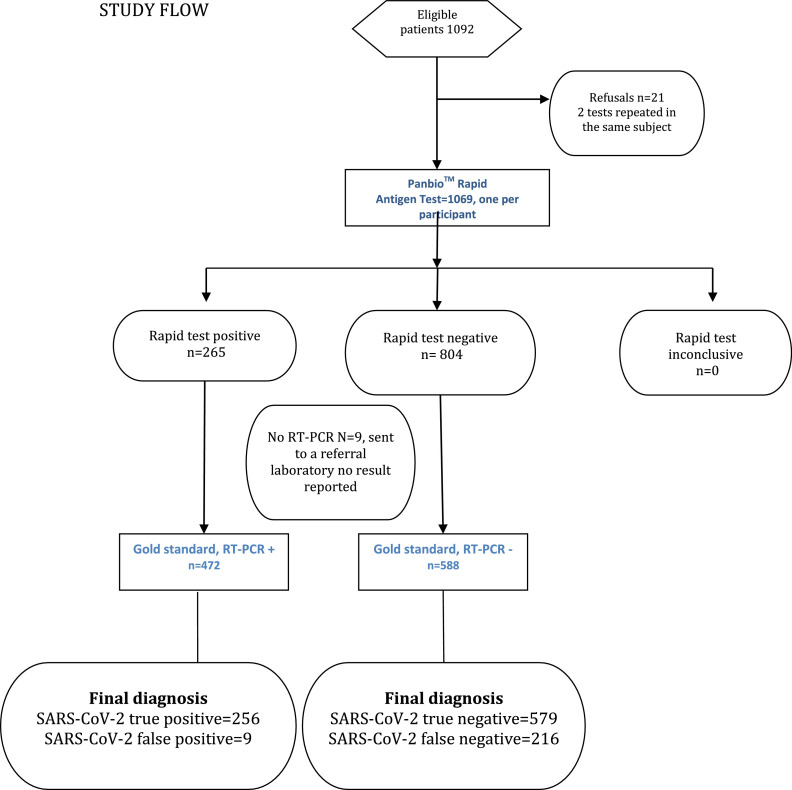
We obtained 1069 rapid test results (from 1069 patients) and 1060 rapid and RT-PCR test pairs from 1060 patients (597 women and 463 men; mean age 46.6±16 years) were further analyzed (see flow diagram). We recruited 378 study participants at INER, 246 at INCAN, 32 at INCIC, 41 at Ciudad Salud Tapachula Chiapas, 40 at HRAE Mérida, 80 at HRAE Ixtapaluca, 150 at INCMNSZ, and 93 at INPER. Ct values were reported for 386 of 472 positive RT-PCR tests (81.8%). Ct values were not available for some participating hospitals (mainly outside of Mexico City) that sent RT-PCR samples to a state laboratory for processing. In the analysis, we utilized the lowest Ct of those reported for different genes.
Table 1 describes the clinical characteristics of the study participants.
Table 1.
Principal characteristics of participants contributing to Panbio rapid antigen test-reverse transcription-polymerase chain reaction (RT-PCR) test pairs (Means and SD or percentage and N)
| Total (1060) | Female (597) | Male (463) | |
|---|---|---|---|
| Age | 47 (16) | 47 (16) | 46 (17) |
| Days of symptoms | 6 (8) | 6 (10) | 6 (5) |
| Asymptomatic subjects | 14% (145) | 14% (84) | 13% (61) |
| Fever | 33% (350) | 28% (169) | 39% (181) |
| Dry cough | 37% (391) | 38% (225) | 36% (166) |
| Cough and phlegm | 11% (114) | 10% (58) | 12% (56) |
| Phlegm | 9% (98) | 10% (58) | 9% (40) |
| Dyspnea | 24% (261) | 22% (132) | 28% (129) |
| Any comorbidity | 46% (485) | 46% (272) | 46% (213) |
| Diabetes | 14% (148) | 14% (81) | 14% (67) |
| Hypertension | 18% (195) | 19% (115) | 17% (80) |
| Obesity | 10% (102) | 10% (58) | 10% (44) |
| Any cancer | 10% (101) | 10% (62) | 8% (39) |
| Previous smoking | 27% (284) | 20% (121) | 35% (163) |
| Current tobacco smoker | 10% (107) | 7% (43) | 14% (64) |
| Former smoker | 18% (178) | 14% (79) | 25% (99) |
| Cigarettes per day in smokers | 4(4) | 3(3) | 5(5) |
| Influenza vaccine 2020-2021 | 49% (515) | 51% (304) | 46% (211) |
| COVID vaccine | 5% (49) | 5% (28) | 5% (21) |
| SpO2% | 90(10) | 91(10) | 89(10) |
| Breathing frequency | 21(6) | 21(5) | 22 (7) |
| Oxygen use in the moment of the test | 18% (190) | 13% (79) | 24% (111) |
| Site of recruitment | |||
| Emergency room visit | 57% (601) | 59% (350) | 54% (251) |
| Outpatient clinic | 35% (366) | 34% (202) | 35% (164) |
| Hospitalized | 9% (93) | 8% (45) | 10% (48) |
| Follow-up | |||
| Remained ambulatory | 79% (847) | 84% (449) | 75% (348) |
| Re-admission to hospital | 1% (15) | 2% (9) | 1% (6) |
| Positive RT-PCR test | 45% (472) | 41% (247) | 49% (225) |
Percentage (%) or mean (standard deviation) are shown.
From 1060 valid test pairs, 1 per participant, 915 were obtained from participants with any respiratory symptom (86.3%) and 145 (13.7%) from asymptomatic participants (usually contacts of a positive relative or coworker). Among participants reporting any symptom, 72% had symptoms lasting for <1 week.
Approximately half (57%) of participants requested attention in the ER or triage system, and 35% at an outpatient service. After initial screening, 79% of all participants were managed as outpatients. At the time of recruitment, only 49 participants (5%) had received 1 dose of the SARS-CoV-2 vaccine (none had complete vaccination) and 46% of participants presented at least 1 comorbidity, as follows: 18% had hypertension, 14% diabetes, 10% obese, and 10% current smokers.
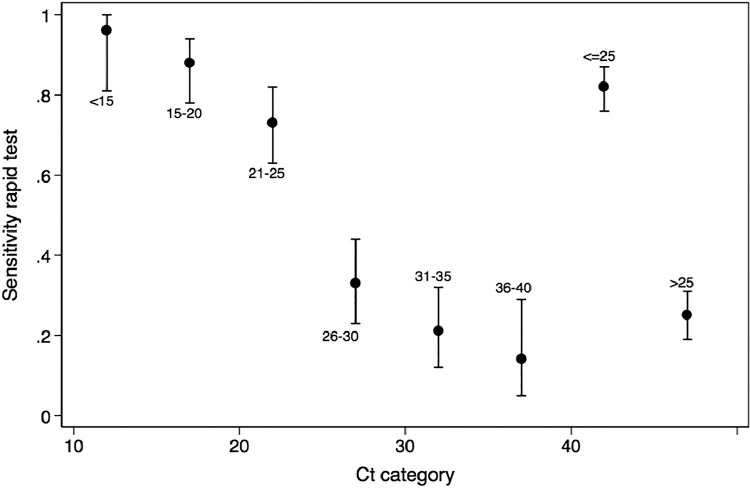
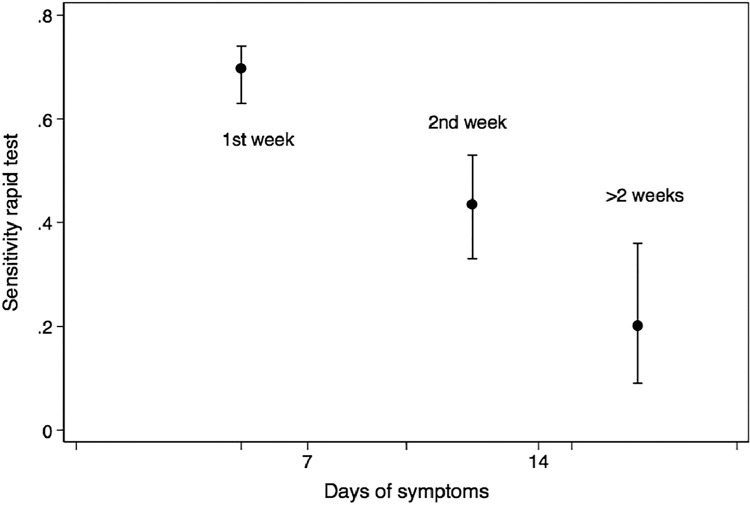
From all the test pairs analyzed, 44.5% were positive in the RT-PCR test and 25% in the Panbio rapid test. Positivity of the rapid test was strongly associated with Ct (a surrogate for viral load) (Figure 1 ), (Table S1) with a sensitivity of 82% (95% CI, 76%–87%) in the presence of a Ct ≤25 (Figure 2 ). Positivity also depended on the days since the onset of symptoms (Figure 3 ) (Table S2), with an initial sensitivity of 69.1% (95% CI 63%–74%) in the rapid test during the first week of symptoms thereafter exhibiting a progressive decline.
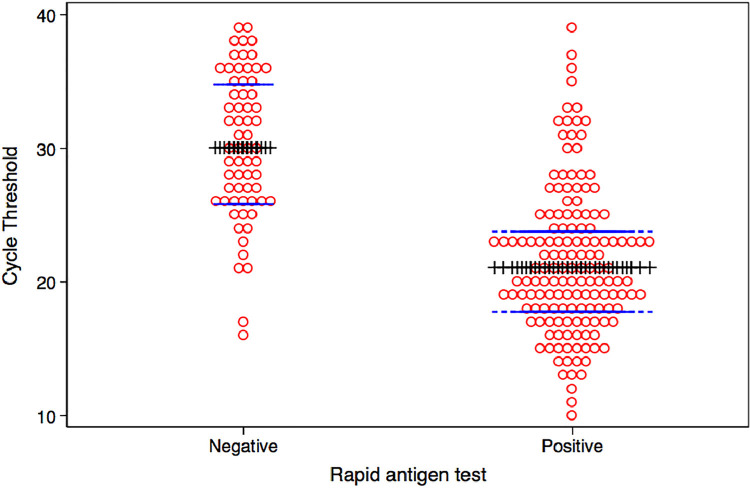
Figure 1.
Dot graph of cycle threshold (Ct) (red circles) with median value and 25% and 75% percentiles (blue lines) in individuals with negative (left side) or positive tests (right side). Those with positive rapid tests have lower Ct values.
Figure 2.
Mean sensitivity of the Panbio rapid test as a function of cycle thresholds (Ct) of the reverse transcription-polymerase chain reaction and 95% CI error bars. Sensitivity of the rapid test decreases with Ct values, indicating lower viral loads.
Figure 3.
Mean sensitivity of the Panbio rapid test as a function of the duration of respiratory symptoms in participants positive for the reverse transcription-polymerase chain reaction, and 95% CI error bars. Sensitivity drops significantly with duration of symptoms.
Table 2 shows the diagnostic performance of the rapid test for the whole group, stratified by time since the onset of symptoms. Overall sensitivity of the Panbio test was 54.2% (95% CI, 51%–57%) with a positive likelihood ratio of 35.7, a negative likelihood ratio of 0.46, and a receiver operating characteristic curve area of 0.77. For participants presenting during the first week of symptoms, sensitivity was 69.1% (95% CI, 66%–73%), decreasing considerably with a longer duration of symptoms. The presence of symptoms was a predictor of positivity for the rapid test; sensitivity of 58.1% in participants with symptoms, compared with 26.3% in persons with no symptoms but with positive RT-PCR. On the other hand, specificity of the Panbio rapid test was >97.8% in all groups.
Table 2.
Diagnostic performance of the Panbio rapid antigen test
| Group | Test pairs | Prevalence of COVID-19 positivity (%) (RT-PCR) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|---|
| All | 1060 | 44.6 | 54.2 (51.2–57.2) | 98.5 (97.7–99.2) | 96.6 (95.5–97.7) | 72.8 (70.2–75.5) |
| First week of symptoms | 710 | 38.7 | 69.1 (65.7–72.5) | 98.4 (97.5–99.3) | 96.5 (95.1–97.8) | 83.4 (80.7–86.2) |
| 2nd week of symptoms | 147 | 68.7 | 43.6 (35.3–51.5) | 97.8 (95.4–100) | 97.8 (95.4–100) | 44.1 (36.1–52.1) |
| More than 1 week of symptoms | 203 | 68.5 | 36.7 (30.1–43.3) | 98.4 (96.7–100) | 98.1 (96.2–100) | 41.7 (34.9–48.5) |
| No symptoms | 146 | 39.0 | 26.3 (19.1–33.4) | 98.9 (97.2–100) | 93.8 (89.8–97.7) | 67.7 (60.1–75.3) |
| Symptoms* | 908 | 45.5 | 58.1 (54.9–61.3) | 98.4 (97.6–99.2) | 96.8 (95.6–97.9) | 73.8 (70.9–76.7) |
PPV = positive predictive value, NPV = negative predictive value, RT-PCR = reverse transcription-polymerase chain reaction. Test pairs include a Panbio rapid antigen test and RT-PCR with minutes of each other, obtained from 1060 patients, one per patient.
Concordance (kappa statistic) between the Panbio rapid test and RT-PCR (Table S3) during the first week of symptoms was 0.72 (SE 0.04), but across all participants, it was 0.53 (SE 0.03). For participants presenting during the second week of symptoms, sensitivity was 0.31 (SE 0.06) and 0.13 (SE 0.07) for participants with >2 weeks of symptoms.
In a logistic regression model, a positive result on the RT-PCR was predicted by a positive result on the Panbio rapid test (odds ratio (OR) 98; 95% CI, 47–203), by days of symptoms (OR 1.08; 95% CI, 1.05–1.11), male sex (OR 1.5; 95% CI, 1.03–2.08), and age (OR 1.01; 95% CI, 1.00–1.03), and these same variables were associated with the Ct (Pseudo R2 0.38).
In multivariate logistic regression, modeling positivity for the Panbio rapid test, the most important predictor was the RT-PCR test and especially the Ct (adjusted odds ratio (aOR) 0.79; 95% CI, 0.75–0.84), but also the days of symptoms (aOR 0.88; 95% CI, 0.83–0.94) adjusting for age, sex and comorbidities (Pseudo R2 0.34).
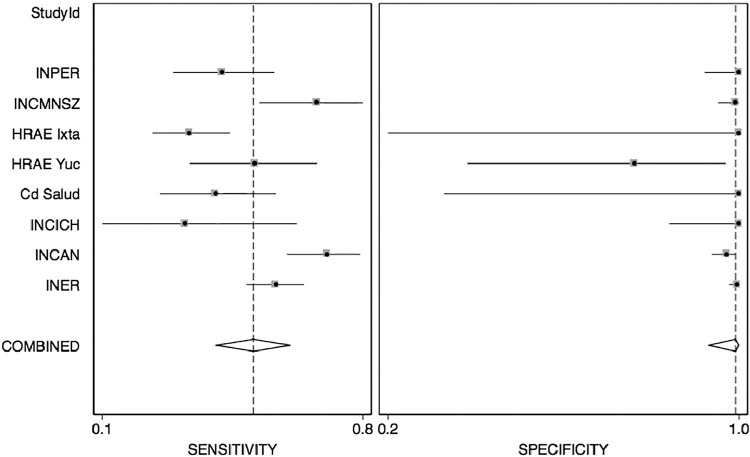
The diagnostic characteristics of the Panbio rapid test varied across sites, with substantial heterogeneity in sensitivity (I2 80.9; 95% CI, 68–93) and specificity (I2 73.5; 95% CI, 54–92), with a relevant influence of centers with fewer study participants (Figure 4 ).
Figure 4.
Forest plot depicting combined sensitivity and specificity as a function of participant hospitals. Combined sensitivity was 0.53 (range 0.42–0.63) with I2 of 80.2 (range 67–93), whereas specificity was consistent with an overall value of 0.99 (0.93–1.0) and I2 of 73 (range 55–92)
Discussion
We present the results of a validation experiment of a commercial POC rapid antigen rapid test for SARS-CoV-2 infection (Panbio), revealing high variability in sensitivity with respect to time from symptoms onset and viral load. In our study, during the first week of symptoms, the sensitivity of the Panbio rapid antigen test was 69.1%, although the overall sensitivity was 54.2%, much lower than that reported by the manufacturer (Abbott, 2020). The test was performed in patients with a longer duration of symptoms or with no respiratory symptoms (predominantly contacts of symptomatic cases); such patients were common in ERs or at screening sites during outbreak peaks when access to hospitals was difficult. On the other hand, the specificity was consistently high in all subgroups.
In studies conducted elsewhere with the Panbio test, lower sensitivities have also been found, ranging from 45% in pediatric patients with <5 days of symptoms compatible with COVID-19 (Villaverde et al., 2021), 48% in household contacts of positive cases, 37% in non-domestic contacts (Torres et al., 2021), and up to 73% in primary-care patients (Linares et al., 2020). In a meta-analysis, days of symptoms were strongly associated with the Panbio rapid test result, with sensitivity of 86.5% if symptoms were present for <7 days and 54% if symptoms were present for >7 days (Linares et al., 2020). In a study conducted under normal working conditions, the sensitivity of the Panbio test was 73% in the Netherlands, 81% in Aruba, and >95% in Aruba if the Ct was <32 (Gremmels et al., 2021). In another study in symptomatic patients in primary care, sensitivity was 80% and specificity 100% (Albert et al., 2021). In all studies, the specificity has been 100% or close to 100%.
The Canadian authorities issued recommendations that took into account sensitivities incorporated into the annex to the Panbio test (Interim guidance on the use of the Abbott PanbioTM COVID-19 Antigen Rapid Test, 2021) before independent assessments of the test... and sensitivities in the abbott inserts were much higher than those found later.
An update of a Cochrane review (Dinnes et al., 2021), including 78 studies (20 pre-prints) and 24 087 samples (approximately one-third positive for SARS-CoV-2), confirmed a substantial variation in sensitivity according to the presence or absence of symptoms (72% vs 58%), first versus second week of symptoms onset (78% vs 51%), and a Ct of ≤25 versus >25 (94.5% vs 40.7%) (Dinnes et al., 2021)() available rapid antigen tests (several) but with a section on Panbio test. Sensitivities reported for rapid antigen tests for SARS-CoV-2 from different manufacturers ranged from 34% to 88%, but specificities were in general >99% (Dinnes et al., 2021)(). The pooled sensitivity of the Panbio test (11 studies) was 75.1% in symptomatic patients during the first week of symptoms but dropped to 58% in asymptomatic individuals (Dinnes et al., 2021)(). Specificity was 99.5%. In the first week of symptoms, the sensitivity in our study was 69.1%, and specificity was 98.5%. It is relevant that only 1 study included in the Cochrane analysis (Alemany et al., 2021) had a test sensitivity with a lower limit of confidence >80%, the recommended cut-off point by the WHO for a rapid antigen test.
We observed a strong association between the Panbio rapid test result and viral load (estimated with the Ct) with no positive results in samples with a Ct of >39. It is also relevant that in our study, the Panbio test was performed in patients with <5 days of symptoms but also with >5 days from the onset of symptoms, a group in which positivity decreases significantly due to an expected lower viral load. Nevertheless, even in this group in which sensitivity is considerably lower, a positive test would be highly informative.
It is noteworthy that with symptoms of longer duration, the cost-effectiveness of applying POC rapid antigen tests would drop progressively. Thus, given that specificity is high, the greatest clinical advantage of utilizing a rapid antigen test would present when the result is positive, in which case, a confirmatory RT-PCR would not be needed.
In the presence of symptoms compatible with COVID-19, or a period of high incidence of infection in the community, a negative RT-PCR test result would be unreliable, even during the first week of symptoms. In these cases, the RT-PCR test is usually repeated a second or even third time (Ramdas et al., 2020). As the infection progresses, the viral and nucleic acid load tends to decrease, and the RT-PCR test tends to be negative, while antibody titers against SARS-CoV-2 begin to appear (Ghaffari et al., 2020; Kilic et al., 2020; Laureano and Riboldi 2020; Shyu et al., 2020). Thus, confirming a SARS-CoV-2 infection ideally involves both the positivity of an RT-PCR test (not necessarily the first test) and the consideration of a combination of epidemiological variables, including the rate of community transmission, the presence of compatible symptoms and the presence of antibodies.
False-positive RT-PCR test results have rarely been reported and are attributed to contamination with viral genetic material in any of the steps between sampling and processing. On the other hand, in the case of a negative antigen test, confirmation with RT-PCR will be required, especially in the presence of compatible symptoms, a high rate of community infections, or in the case of persons with direct contact with confirmed COVID-19 patients. In any case, according to our observations, if a Panbio rapid antigen test were employed, 69.1% of the RT-PCR tests would be avoided during the first week of symptoms, which represents a considerable saving. The latter represents an enormous advantage in settings where decision-making is needed and where the lack of infrastructure and high costs render it difficult to implement molecular testing.
Our study was observational, subject to all possible biases of cross-sectional studies; it was performed predominately during the daytime, the shift with more available staff and in-training personnel. Testing was carried out with the Mexican health system and personnel under stress, a very different situation from that of rapid tests performed under controlled circumstances in a laboratory by the same expert personnel, which could explain, at least in part, the reduced sensitivity found in our study. On the other hand, our results are expected to be closer to what can be observed during outbreaks that saturate or overwhelm screening sites and ERs, that is, more demanding circumstances than those found under strictly controlled laboratory testing. Furthermore, overall RT-PCR positivity in participating hospitals was relatively high (44.6%, ranging from 27% to 93% in different hospitals), allowing for a proper evaluation of sensitivity and specificity. Actual conditions of use may be even more demanding than those present in our study, for example, if testing includes primary care, community hospitals, during all shifts including weekends and nights, characterized by the presence of fewer personnel, and especially if the overall positivity of the tests or community transmission is low.
Conclusions
We have shown that the sensitivity of the Panbio SARS-CoV-2 rapid antigen test is limited, with an overall estimated value of 69.1% in the present study. As expected, sensitivity was highly associated with time from the onset of symptoms and with viral load. The low sensitivity and high specificity observed in the Panbio test make it necessary to confirm all negative results, especially in the presence of symptoms compatible with COVID-19 and in settings with a high community infection rate. Nevertheless, the Panbio rapid antigen test could be beneficial to screen for positive tests and to reduce the number of cases to be confirmed, enabling rapid clinical decision-making.
Figure 5.
STUDY FLOW.RT-PCR=real time polymerase chain reaction. Description of the participants, a total of 1060, each providing one pair of PanbioTM-RT-PCR tests, and their results.
Acknowledgments
Acknowledgments
The Panbio rapid antigen test kits were donated by the World Health Organization/Pan American Health Organization, and the Mexican Health Secretariat purchased additional tests of the same type for the hospital care of the patients.
Competing financial interests or personal relationships that could have appeared to influence the work reported
The authors declare no known competing interests.
Funding source
The antigen test kits were donated by the Pan American Health Organization, and the Mexican Health Secretariat purchased additional tests of the same type for the hospital care of the patients. No other additional support was available, and the expenses incurred were covered by the participating hospitals. Care of patients with COVID-19 was complimentary in all participating hospitals.
Ethical approval
The protocol was revised and approved (approval code C93-20) by the Investigation, Ethics in Investigation and Biosecurity Unique Committee from the Coordination of the National Institutes of Health and High Specialty Hospitals, and a single Institutional Review Board appointed by the Mexican Institutes of Health authority for this multicentric study.
All hospitals included in the protocol belong to the same organization, share the same administrative coordination and adhered to the same protocol and consent form (adding only the principal investigator of the participating institutions).
The study was registered with ClinicalTrials.gov
Footnotes
On behalf of the Rapid Antigen Tests Working Group, also formed by:
Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas: Dra. Angélica Margarita Portillo-Vásquez, Dr. Sebastián Rodríguez-Llamazares, Dr. Maribel Soto-Nava, Dr. Dulce María López-Sánchez, QFB. Daniela Tapia Trejo, Biol. Gonzalo Salgado-Montes de Oca
HOSPITAL REGIONAL DE ALTA ESPECIALIDAD DE IXTAPALUCA
Dr. Sayuri Itzel Espíndola-Chavarría, Dr. Jan Kuschick-Fehér
INSTITUTO NACIONAL DE CANCEROLOGIA
TIT Carla Vázquez-Marín, BN Giselle Pineda, Dr. Pamela Alatorre, Dr. Beda Islas, Dr. Alexandra Martin, Dr. Carolina Pérez, Dra. Diana Vilar.
Hospital Regional de Alta Especialidad Ciudad Salud Tapachula
QFB Roberto Alejandro Sánchez-González, QFB Ma Guadalupe Trujillo-Vizuet, QFB Fabiola Yeseline Zamudio-Castellanos, Dr. Diana de León-Rodríguez
INSTITUTO NACIONAL DE CARDIOLOGÍA IGNACIO CHÁVEZ
Dr. Rodrigo Gopar-Nieto
INSTITUTO NACIONAL DE CIENCIAS MÉDICAS Y NUTRICIÓN SALVADOR ZUBIRÁN
Dr. Sergio Ponce de León, Dr. Juan Sierra-Madero, QFB Patricia Bárcenas-Bautista
Instituto Nacional Perinatología
Dr. Manuel Cortés-Bonilla, Dr. Cecilia Helguera- Reppeto, Dr. Angélica Yong-Mendoza, Dr. Óscar Villavicencio-Carrisoza, Dr. Mónica Selena Fonseca-Pérez, Dr. Luis Evaristo Ruiz-Sánchez, Dr. Mildred Montoya-Narváez, Dr. Claudia Viridiana Rivera-Aguilar, Dr. Rocio Alvarado-Flores, Dr. Nancy Guadalupe Chavero-Hernández, Dr. Jorge Arturo Cardona-Pérez
Hospital Regional de Alta Especialidad de la Península de Yucatán, Unidad de Infectología y Vigilancia Epidemiológica
Dr. María Alejandra Salcedo-Parra, Dr. Nelda Judith Núñez-Caamal
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2021.10.027.
Appendix. Supplementary materials
References
- Abbot. Insert, Abbot Panbio COVID-19 Ag Rapid Test Device. 2020. https://www.globalpointofcare.abbott/es/product-details/panbio-covid-19-ag-antigen-test.html
- Albert E., Torres I., Bueno F., Huntley D., Molla E., Fernández-Fuentes M., Martínez M., Poujois S., Forqué L., Valdivia A., Solano de la Asunción C., Ferrer J., Colomina J., Navarro D. Field evaluation of a rapid antigen test (Panbio™ COVID-19 Ag Rapid Test Device) for COVID-19 diagnosis in primary healthcare centres. Clin Microbiol Infect. 2021;27(3) doi: 10.1016/j.cmi.2020.11.004. 472.e477-472.e410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemany A., Baró B., Ouchi D., Rodó P., Ubals M., Corbacho-Monné M., Vergara-Alert J., Rodon J., Segalés J., Esteban C., Fernández G., Ruiz L., Bassat Q., Clotet B., Ara J., Vall-Mayans M., G-Beiras C., Blanco I., Mitjà O. Analytical and clinical performance of the panbio COVID-19 antigen-detecting rapid diagnostic test. The Journal of Infection. 2021;82:186–230. doi: 10.1016/j.jinf.2020.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benirschke R.C., McElvania E., Thomson R.B., Kaul K.L., Das S. Clinical Impact of Rapid Point-of-Care PCR Influenza Testing in an Urgent Care Setting: a Single-Center Study. Journal of Clinical Microbiology. 2019;57(3):e01281. doi: 10.1128/JCM.01281-18. -18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujang M.A. Requirements for Minimum Sample Size for Sensitivity and Specificity Analysis. Journal of Clinical and Diagnostic Research. 2016;10(10):YE01–YE06. doi: 10.7860/JCDR/2016/18129.8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.-L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinnes J., Deeks J.J., Adriano A., Berhane S., Davenport C., Dittrich S., Emperador D., Takwoingi Y., Cunningham J., Beese S., Dretzke J., Ferrante di Ruffano L., Harris I.M., Price M.J., Taylor-Phillips S., Hooft L., Leeflang M.M., Spijker R., Van den Bruel A. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2020;8 doi: 10.1002/14651858.CD013705. Cd013705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinnes J., Deeks J.J., Berhane S., Taylor M., Adriano A., Davenport C., Dittrich S., Emperador D., Takwoingi Y., Cunningham J., Beese S., Domen J., Dretzke J., Ferrante di Ruffano L., Harris I.M., Price M.J., Taylor-Phillips S., Hooft L., Leeflang M.M., McInnes M.D., Spijker R., Van den Bruel A., Cochrane C.-D.T.A.G. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2021;3 doi: 10.1002/14651858.CD013705.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffari A, Meurant R., Ardakani A. COVID-19 Serological Tests: How Well Do They Actually Perform? Diagnostics (Basel) 2020;10(7):453. doi: 10.3390/diagnostics10070453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremmels H., Winkel B.M.F., Schuurman R., Rosingh A., Rigter N.A.M., Rodriguez O., Ubijaan J., Wensing A.M.J., Bonten M.J.M., Hofstra L.M. Real-life validation of the Panbio™ COVID-19 antigen rapid test (Abbott) in community-dwelling subjects with symptoms of potential SARS-CoV-2 infection. EClinicalMedicine. 2021;31 doi: 10.1016/j.eclinm.2020.100677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajian-Tilaki K. Sample size estimation in diagnostic test studies of biomedical informatics. Journal of Biomedical Informatics. 2014;48:193–204. doi: 10.1016/j.jbi.2014.02.013. [DOI] [PubMed] [Google Scholar]
- Kilic T., Weissleder R., Lee H. Molecular and Immunological Diagnostic Tests of COVID-19: Current Status and Challenges. iScience. 2020;23(8) doi: 10.1016/j.isci.2020.101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubina R., Dziedzic A. Molecular and Serological Tests for COVID-19 a Comparative Review of SARS-CoV-2 Coronavirus Laboratory and Point-of-Care Diagnostics. Diagnostics (Basel) 2020;10(6):434. doi: 10.3390/diagnostics10060434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureano A.F.S., Riboldi M. The different tests for the diagnosis of COVID-19 - A review in Brazil so far. JBRA Assist Reprod. 2020;24(3):340–346. doi: 10.5935/1518-0557.20200046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares M., Pérez-Tanoira R., Carrero A., Romanyk J., Pérez-García F., Gómez-Herruz P., Arroyo T., Cuadros J. Panbio antigen rapid test is reliable to diagnose SARS-CoV-2 infection in the first 7 days after the onset of symptoms. J Clin Virol. 2020;133 doi: 10.1016/j.jcv.2020.104659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mina M.J., Parker R., Larremore D.B. Rethinking Covid-19 Test Sensitivity - A Strategy for Containment. N Engl J Med. 2020;383(22):e120. doi: 10.1056/NEJMp2025631. [DOI] [PubMed] [Google Scholar]
- Rahamat-Langendoen J., Groenewoud H., Kuijpers J., Melchers W.J.G., van der Wilt G.J. Impact of molecular point-of-care testing on clinical management and in-hospital costs of patients suspected of influenza or RSV infection: a modeling study. Journal of Medical Virology. 2019;91(8):1408–1414. doi: 10.1002/jmv.25479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramdas K., Darzi A., Jain S. Test, re-test, re-test': using inaccurate tests to greatly increase the accuracy of COVID-19 testing. Nat Med. 2020;26(6):810–811. doi: 10.1038/s41591-020-0891-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter-Perkins E.M., Mitchell P.M., Nelson K.P., Liu J.H., Shannon A., Ahern J., Orr B., Miller N.S. Point-of-care influenza testing does not significantly shorten time to disposition among patients with an influenza-like illness. The American Journal of Emergency Medicine. 2019;37(5):873–878. doi: 10.1016/j.ajem.2018.08.005. [DOI] [PubMed] [Google Scholar]
- Shengchen D., Gu X., Fan G., Sun R., Wang Y., Yu D., Li H., Zhou F., Xiong Z., Lu B., Zhu G., Cao B. Evaluation of a molecular point-of-care testing for viral and atypical pathogens on intravenous antibiotic duration in hospitalized adults with lower respiratory tract infection: a randomized clinical trial. Clinical Microbiology and Infection: The Official Publication of the. European Society of Clinical Microbiology and Infectious Diseases. 2019;25(11):1415–1421. doi: 10.1016/j.cmi.2019.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu D., Dorroh J., Holtmeyer C., Ritter D., Upendran A., Kannan R., Dandachi D., Rojas-Moreno C., Whitt S.P., Regunath H. Laboratory Tests for COVID-19: A Review of Peer-Reviewed Publications and Implications for Clinical UIse. Mo Med. 2020;117(3):184–195. [PMC free article] [PubMed] [Google Scholar]
- Torres I., Poujois S., Albert E., Colomina J., Navarro D. Evaluation of a rapid antigen test (Panbio™ COVID-19 Ag rapid test device) for SARS-CoV-2 detection in asymptomatic close contacts of COVID-19 patients. Clin Microbiol Infect. 2021;27(4) doi: 10.1016/j.cmi.2020.12.022. 636.e631-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villaverde S., Domínguez-Rodríguez S., Sabrido G., Pérez-Jorge C., Plata M., Romero M.P., Grasa C.D., Jiménez A.B., Heras E., Broncano A., Núñez M.D.M., Illán M., Merino P., Soto B., Molina-Arana D., Bermejo A., Mendoza P., Gijón M., Pérez-Moneo B., Moraleda C., Tagarro A. Diagnostic Accuracy of the Panbio Severe Acute Respiratory Syndrome Coronavirus 2 Antigen Rapid Test Compared with Reverse-Transcriptase Polymerase Chain Reaction Testing of Nasopharyngeal Samples in the Pediatric Population. J Pediatr. 2021;232:287–289. doi: 10.1016/j.jpeds.2021.01.027. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wabe N., Li L., Lindeman R., Yimsung R., Dahm M.R., McLennan S., Clezy K., Westbrook J.I., Georgiou A. Impact of Rapid Molecular Diagnostic Testing of Respiratory Viruses on Outcomes of Adults Hospitalized with Respiratory Illness: a Multicenter Quasi-experimental Study. Journal of Clinical Microbiology. 2019;57(4):e01727. doi: 10.1128/JCM.01727-18. -18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Whiting P.F., Brush J.E. Interpreting a covid-19 test result. BMJ. 2020;369:m1808. doi: 10.1136/bmj.m1808. [DOI] [PubMed] [Google Scholar]
- Canadian Public Health Laboratory Network Laboratory Directors Council C, Public Health Laboratory Network Respiratory Virus Infection Working Group Interim guidance on the use of the abbott panbioTM COVID-19 antigen rapid test. Can Commun Dis Rep. 2021;47:17–22. doi: 10.14745/ccdr.v47i01a04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20(8):e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.