Abstract
Objective:
Extralaryngeal branching of recurrent laryngeal nerve (RLN) is frequent. In various studies, detection rate of extralaryngeal nerve branching was increased by intraoperative neuromonitorization (IONM). Our aim was evaluation of the relationship between the features of extralaryngeal branching of RLN and other anatomic variations in thyroidectomy patients under the guidance of IONM.
Methods:
Patients underwent thyroidectomy using IONM between January 2016 and December 2019 and whose RLNs were fully explored till the nerve’s entry point to the larynx, were enrolled to the study. Extralaryngeal branching of RLN was accepted as branching of the nerve at a ≥5 mm distance from its laryngeal entry point and having its all branches entering the larynx. Entrapment of RLN at the region of ligament of Berry (BL) by a vascular structure or posterior BL and relationship between RLN and inferior thyroid artery (ITA) was evaluated.
Results:
Out of 696 patients meeting the inclusion criteria, 1127 neck sides (536F and 160M) were evaluated. Mean age was 49.1±13.4 (range; 18–89). Nerve branching ratio was 35.3% and was higher in females than males (38.2%vs.25.8%, p<0.0001, respectively). Extralaryngeal branching of RLN was detected in 398 (35.3%) out of 1127 nerves. A total of 368 (92.5%) RLNs had two, 27 (6.8%) nerves had three, and 3 (0.7%) had multiple branches. RLN crossed anterior to and between branches of ITA more frequently in branching nerves than non-branching nerves (47.7 vs. 44.4% and 12.8% vs. 7.6%, respectively) but crossed posterior to ITA less frequently in branching nerves (38.5% vs. 48%, respectively, p=0.001). Entrapment of RLN at the region of BL was higher in branched nerves (25.9% vs. 17.5%, respectively, p=0.001). Entrapment of RLN wasmore frequent at the right side than left side both in branching (31.5% vs.19.4%, respectively, p=0.008) and non-branching nerves (20.6% vs. 14.4%, respectively).
Conclusion:
Extralaryngeal branching of RLN is not rare and mostly divided into two branches. Branching ratio is higher in females than males. In branching nerves, rate of crossing anterior to and between branches of ITA was higher, in non-branching nerves, rate of crossing posterior to ITA was higher. In branching nerves, possibility of entrapment of RLN at the region of BL was higher. Both in branching and non-branching nerves, entrapment of RLN at the region of BL was higher at the right side. Extralaryngeal branching, relationship between RLN and ITA, and entrapment of RLN at the region of BL are frequently seen and variable anatomic variations and cannot be foreseen preoperatively. Most of the extralaryngeal branches and their relationship with other variations can be detected by finding RLN at the level of ITA and following RLN until its entry point to the larynx.
Keywords: Intraoperative monitoring, larynx, recurrent laryngeal nerve, thyroidectomy
Introduction
Thyroidectomy is the most frequently performed operation in endocrine surgery and recurrent laryngeal nerve (RLN) is one of the main anatomical structures under risk of injury during thyroidectomy. Innervation of the larynx is supplied by motor, sensory, and parasympathetic fibers of RLN which is a branch of vagus nerve. In an embryological aspect, bilateral RLNs inclined together with heart and aortic arches. At the end of the development, the left RLN turns around ligamentum arteriosum, right RLN turns around right subclavian artery, and both return to the neck to innervate the larynx. This anatomical difference between two nerves causes a slight diversity about their angles while approaching to the neck. In their paratracheal position, right RLN has a route to the larynx in a more anterior and oblique way and approaches to the larynx from lateral aspect of the neck when compared to the left RLN which has a more perpendicular route and runs closer to the left tracheoesophageal groove. During its course, RLN may cross above or under the inferior thyroid artery (ITA) or run between the artery’s branches.[1,2]
RLNs are the motor nerves of vocal cords’ both abductor and adductor intrinsic laryngeal muscles. Cricothyroid muscle which stretches the vocal cord is innervated by the external branch of the superior laryngeal nerve.[3]
In general, RLNs follow their defined regular pathway, however, there may be acquired or embryological abnormalities on their courses.[4] As a rare embryological variation of RLN, non-RLN (NRLN) is a branch of vagus nerve that does not descend to the thorax. NRLN is rarely seen on the left side, however, mostly identified on the right side and associated with the vascular abnormalities of cardiovascular system.[5] The relationship between RLN and ITA is variable regardless of the neck side and RLN may cross the artery from its anterior side, posterior side, or between its branches.[6]
Extralaryngeal branching of the RLN is a common anatomical variation (Figs. 1 and 2). During thyroidectomy, the part of RLN which is superior to the ITA is the most common site of injury. This part of RLN often shows extralaryngeal branching and has a closer association with the tubercle of Zuckerkandl ligament of Berry (BL).[7] Anatomical variations such as distortion of RLN more medially, laterally, or anteriorly, extralaryngeal branching, and NRLN are the potential factors for RLN injury. In addition, traction injury risk will be increased if there is an anterior motor branch of RLN bifurcating close to the BL.[8]
Figure 1.
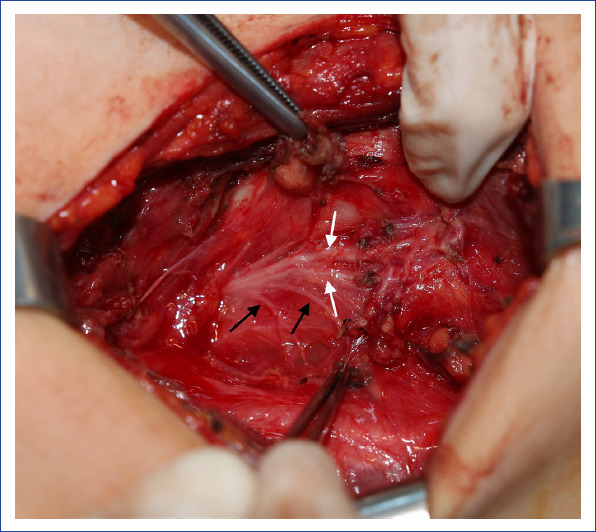
An extralaryngeal branching recurrent laryngeal nerve (black arrows show esophageal branches, white arrows show laryngeal branches).
Figure 2.
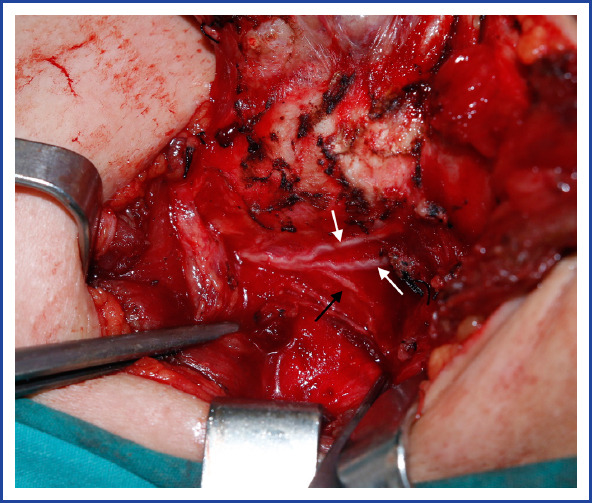
An extralaryngeal branching recurrent laryngeal nerve (black arrow shows esophageal branches, white arrows show laryngeal branches).
The main purpose of this study was to evaluate of the prevalence and features of extralaryngeal branching of RLN and its relationship with other anatomical variations.
Materials and Methods
The data of the patients who underwent primary or secondary thyroidectomy with or without parathyroidectomy between January 2016 and December 2019 were evaluated retrospectively. The patients, whose RLNs were fully explored till the nerve’s entry point to the larynx, were enrolled to the study. The present study was approved by the local ethics committee (no: 1876, approval date: April 27, 2021). Informed consents of the patients were obtained. The patients underwent thyroidectomy (+/-parathyroidectomy) using intraoperative neuromonitorization (IONM) and intraoperative data were recorded. Data collection process was compatible with the instructions of the Helsinki Declaration.
The data of each neck side were recorded and evaluated separately. The patients with recorded data of RLNs branching pattern and other variations were included to this study.
Neck side in which RLNs course was not adequately explored as in parathyroidectomy or subtotal thyroidectomy, RLNs with unidentified branching pattern, patients with locally advanced thyroid cancer with invasion to RLN, and patients with missing intraoperative records have been excluded from the study.
Thyroidectomies have been performed under general anesthesia using low-dose muscle relaxants and under the guidance of IONM. Thyroidectomy technique has been detailed in the previous studies.[9–12]
The branching distance was defined as the distance between the division point of the RLN and its laryngeal entry point which was measured with a digital Vernier caliper and recorded in millimeters with a resolution of 0.1 mm. Extralaryngeal branching of RLN was accepted as branching of the nerve at a ≥5 mm distance from its laryngeal entry point and having all its branches entering the larynx.[10]
According to the International RLN Anatomic Classification system, entrapment of RLN at the region of BL has been described as trapping of RLN by a vascular structure or posterior BL.[4]
One hundred and four RLNs without the records regarding the entrapment of the nerve by posterior BL were not evaluated. The relationship between RLN and ITA was not able to be evaluated in 10 neck sides due to NRLN, secondary intervention, or lacking ITA, anatomical relationship between ITA and RLN was defined and grouped as RLN crossing the ITA anteriorly, posteriorly, or between the branches of ITA.
Statistical Analysis
In statistical analysis, SPSS version 27.0 (IBM, Armonk, NY) was used. Categorical values were evaluated with Chi-square test or Fisher’s exact test. Parametric continuous variables were evaluated with Student’s t-test and non-parametric continuous variables were evaluated with Mann–Whitney U-test. p<0.05 was accepted as statistically significant. Continuous values are presented as mean±standard deviation.
Results
Out of 696 patients meeting the inclusion criteria, 1127 neck sides (536F and 160M) were evaluated and the mean age was 49.1±13.4 (range; 18–89). Post-operative diagnosis was benign for 672, malignant for 343, and Graves’ disease for 112 neck sides.
Extralaryngeal branching of RLN was detected in 398 (35.3%) out of 1127 nerves. A total of 368 (92.5%) RLNs had two, 27 (6.8%) nerves had three, and 3 (0.7%) had multiple branches.
Extralaryngeal branching of RLN was more frequent in female patients rather than male patients (38.2% vs. 25.8%, p<0.00001, respectively). Relationship between RLN and ITA was evaluated in 1117 nerves. In extralaryngeal branching nerves, the rates of RLNs anterior to ITA and between the branches of ITA were significantly higher than the rate of RLNs posterior to ITA compared to the non-branching nerves (p=0.001) (Table 1). In branching nerves, BL entrapment was detected significantly higher (25.9% vs. 17.5%; p=0.001). Five out of 1127 nerves (0.4%) were identified as NRLN and 2 of them (40%) were branching. Extralaryngeal branching rates were found similar regarding the right and left sides (37.4% vs. 33.2%) (p=0.135).
Table 1.
Extralaryngeal branching features of RLN and its relationship with other anatomical variations
| Total | Non-branching n=729 (64.7%) | Branching n=398 (35.3%) | P-values | |
|---|---|---|---|---|
| Age (mean±SD) (year) | 49.2±13.4 | 49.1±13.5 | 49.3±13.4 | 0.792 |
| BMI (kg/m2) | 29.3±5.9 | 28.9±5.8 | 30±6.1 | 0.948 |
| Gender | 0.152 | |||
| Female | 867 (66.9%) | 536 (61.8%) | 331 (38.2%) | <0.000 |
| Male | 260 (23.1%) | 193 (74.2%) | 67 (25.8%) | |
| Number of branches | ||||
| 1 | 729 (64.6%) | 729 (64.6%) | ||
| 2 | 368 (32.7%) | 368 (92.5%) | ||
| 3 | 27 (2.4%) | 27 (6.8%) | ||
| >4 | 3 (0.3%) | 3 (0,7%) | ||
| Neck side | 0.135 | |||
| Right | 572 (50.8%) | 358 (62.6%) | 214 (37.4%) | |
| Left | 555 (49.2%) | 371 (66.8%) | 184 (33.2%) | |
| Relationship between RLN and ITA (n=1117) | 0.001 | |||
| Anterior1 (n=511) | 511 (45.7%) | 321 (44.4%) | 190(47.7%) | |
| Posterior2 (n=500) | 500 (44.8%) | 347 (48%) | 153 (38.5%) | |
| Between branches3 (n=106) | 106 (9.5%) | 55 (7.6%) | 51 (12.8%) | |
| Presence of non-recurrent laryngeal nerve | 1 | |||
| (+) | 5 (0.4%) | 3 (60%) | 2 (40%) | |
| (−) | 1122 (99.6%) | 726 (64.7%) | 396 (35%) | |
| Ligament of Berry entrapment (n=1023) | 0.001 | |||
| (+) | 210 (20.5%) | 114 (17.5%) | 96 (25.9%) | |
| (−) | 813 (79.5%) | 539 (82.5%) | 274 (74.1%) | |
| Branching distance (cm) Mean±SD (min-max) | 1.91±0.9 (0.5-5.0) |
SD: Standard deviation; BMI: Body mass index; RLN: Recurrent laryngeal nerve; ITA: Inferior thyroid artery.
Branching Distances
Mean branching distance for all RLNs was 1.91±0.9 cm (range, 0.5–5 cm). Mean branching distance in female and male patients was 1.91±0.91 cm (range, 0.5–5 cm) and 1.77±0.89 cm (range, 0.5–4 cm), respectively, and there was no statistically significant difference between the two genders (p=0.128). Mean branching distance was 1.82±0.86cm (range, 0.5–4 cm) at the right side, and it was 1.97±0.95cm (range, 0.5–5 cm) at the left side and no difference was detected between the two groups (p=0.128).
Features of Branching in Female Patients
By virtue of high ratio of extralaryngeal branching in female patients, branching features of both genders were analyzed separately. In female patients, of the 331 extralaryngeal branching nerve; 308 (93.1%) had 2, 21 nerves (6.3%) had 3, and 2 nerves (0.6%) had multiple branches. Branching ratio was 41% at the right side, 35.2% at the left side, and there was no difference between the two groups (p=0.077). In branching nerves, RLNs that crossing anterior to ITA and between the branches were detected with a significantly higher rate compared to the non-branching nerves (p=0.007). BL entrapment was more common in extralaryngeal branching nerves compared to non-branched nerves (25.7% vs. 16.4%, respectively; p=0.002) (Table 2).
Table 2.
Extralaryngeal branching features of RLN and its relationship with other anatomical variations in female patients
| Female | Total | Non-branching n=536 | Branching n=331 | p-values |
|---|---|---|---|---|
| Number of branches | ||||
| 1 | 536 (61.8%) | 536 | ||
| 2 | 308 (35.5%) | 308 (93.1%) | ||
| 3 | 21 (2.4%) | 21 (6.3%) | ||
| >4 | 2 (0.3%) | 2 (0.6%) | ||
| Neck side | 0.077 | |||
| Right | 441 (50.9%) | 260 (59%) | 181 (41%) | |
| Left | 426 (49.1%) | 276 (64.8%) | 150 (35.2%) | |
| Relationship between RLN and ITA (n=858) | 0.007 | |||
| Anterior | 394 (45.9%) | 238 (44.8%) | 156(47.7%) | |
| Posterior | 385 (44.9%) | 255 (48%) | 130 (39.8%) | |
| Between branches | 79 (9.2%) | 38 (7.2%) | 41 (12.5%) | |
| Presence of non-recurrent Nerve (n=4) | 0.639 | |||
| (+) | 4 (0.4%) | 2(50%) | 2(50%) | |
| (−) | 863 (99.6%) | 534(61.9%) | 329(38.1%) | |
| Ligament of Berry entrapment (n=794) | 0.002 | |||
| (+) | 159 (20%) | 80(16.4%) | 79(25.7%) | |
| (−) | 635 (80%) | 407(83.6%) | 228(74.3%) | |
| Branching distance (cm) | 1.91±0.91 | |||
| Mean+SD (min-max) (cm) | (0.5–5.0) |
SD: Standard deviation; RLN: Recurrent laryngeal nerve; ITA: Inferior thyroid artery.
Features of Branching in Male Patients
In male patients, of the 67 extralaryngeal branching nerves, 60 nerves (89.6%) had 2, 6 nerves (8.9%) had 3, and 1 nerve (1.5%) had multiple branches. Branching ratio was 25.2% at the right side, 26.4% at the left side, and there was no difference between the two groups (p=0.830). In branching nerves compared to non-branching nerves, the rates of RLNs crossing anterior to ITA and between the branches of ITA were significantly higher than the rate of RLNs crossing posterior to ITA (P =0.108). BL entrapment was similar both in extralaryngeal branching nerves and unbranched nerves (25.8% vs. 21.1%, respectively; p=0.446) (Table 3).
Table 3.
Extralaryngeal branching features of RLN and its relationship with other anatomical variations in male patients
| Male | Total | Non-branching n=193 | Branching n=67 | P-values |
|---|---|---|---|---|
| Number of branches | ||||
| 1 | 193 (74.2%) | 193 | ||
| 2 | 60 (23.1%) | 60 (89.6%) | ||
| 3 | 6 (2.3%) | 6 (8.9%) | ||
| >4 | 1 (0.4%) | 1 (1.5%) | ||
| Neck side | ||||
| Right | 131 (50.4%) | 98(74.8%) | 33(25.2%) | 0.829 |
| Left | 129 (49.6%) | 95(73.6%) | 34(26.4%) | |
| Relationship between RLN and ITA (n=259) | 0.108 | |||
| Anterior | 117 (45.2%) | 83(43.2%) | 34(50.7%) | |
| Posterior | 115 (44.4%) | 92(47.9%) | 23(34.3%) | |
| Between branches | 27 (10.4%) | 17(8.9%) | 10(14.9%) | |
| Presence of non-recurrent nerve | 1 | |||
| Yes | 1 (0.4%) | 1 (100%) | 0(00.0%) | |
| No | 259 (99.6%) | 192(74.1%) | 67(25.9%) | |
| Ligament of Berry entrapment (n=229) | 0.446 | |||
| (+) | 51 (22.3%) | 34(21.1%) | 17(25.8%) | |
| (−) | 178 (87.7%) | 132(78.9%) | 46(74.2%) | |
| Branching distance, Mean±SD (min-max) (cm) | 1.77±0.89 (0.5-4.0) |
SD: Standard deviation; RLN: Recurrent laryngeal nerve; ITA: Inferior thyroid artery.
Features of Branching on the Right and Left Sides
Number of extralaryngeal branching was different on either side of the neck and nerves with two branches were more frequently detected on the right side (35.8% vs. 29.4%, respectively), non-branching nerves (62.6% vs. 66.8%, respectively), and nerves with three branches (1% vs. 3.8%, respectively) were detected more commonly on the left side (p=0.001). Branching rates of the RLNs were 37.4% on the right side, 33.2% on the left side, and there was no difference between the two groups (p=0.077) (Table 4). BL entrapment was more frequent on the right side compared to the left side both in the branching (31.5% vs. 19.4%, respectively, p=0.008) and non-branching nerves (20.6% vs. 14.4%, respectively; p=0.037).
Table 4.
Extralaryngeal branching features of RLN and its relationship with other anatomical variations on both sides of the neck
| Total | Right (n=572) | Left (n=555) | p-values | |
|---|---|---|---|---|
| Number of branches (n=1127) | 0.001 | |||
| 1 | 729 (64.7%) | 358 (62.6%) | 371 (66.8%) | |
| 2 | 368 (32.7) | 205 (35.8%) | 163 (29.4%) | |
| 3 | 27 (2.4%) | 6 (1%) | 21 (3.8%) | |
| 4 | 3 (0.3%) | 3 (0.5%) | 0 | |
| Number of branching nerves | 398 (35.4%) | 214 (37.4%) | 184 (33.2%) | 0.077 |
| Branching distance Mean+SD (min-max) (cm) | 1.91±0.91 (0.5–5.0) | 1.82±0.86 (0.5–4.0) | 1.97±0.95 (0.5–5.0) | 0.128 |
| Ligament of Berry entrapment in branching nerves (n=229) | 0.008 | |||
| (+) | 96 (25.9%) | 63(31.5%) | 33(19.4%) | |
| (−) | 274 (74.1%) | 137(68.5%) | 137(80.6%) | |
| Ligament of Berry entrapment in non-branching nerves(n=653) | 0.037 | |||
| (+) | 114 (17.5%) | 66 (20.6%) | 48 (14.4%) | |
| (−) | 539 (82.5%) | 254 (79.4%) | 285 (85.6%) | |
| Relationship between RLN and ITA in branching nerves(n=394) | 0.002 | |||
| Anterior | 190 (48.2%) | 114 (54%) | 76 (41.5%) | |
| Posterior | 153(38.8%) | 65 (30.8%) | 88 (48.1%) | |
| Between branches | 51 (12.9%) | 32 (15.2%) | 19 (10.4%) | |
| Relationship between RLN and ITA in non-branching nerves(n=723) | ||||
| Anterior | 321(44.4%) | 185(52.0%) | 136(37.0%) | <0.000 |
| Posterior | 347(48.0%) | 135(37.9%) | 212(57.8%) | |
| Between branches | 55(7.6%) | 36(10.1%) | 19(5.2%) |
SD: Standard deviation; RLN: Recurrent laryngeal nerve; ITA: Inferior thyroid artery.
The rates of RLNs crossing anterior and posterior to ITA were similar regardless the neck side, however, on the right side when compared to the left side, RLN crossed anterior to ITA (52.9% vs. 38.4%, respectively) or between branches of ITA (12% vs. 6.9%, respectively) more frequently, but crossed posterior to ITA less frequently (38.4% vs. 48.1%, respectively).
On the right side when compared to the left side, both in branching nerves and non-branching nerves, the rates of RLNs anterior to ITA and between the branches of ITA were significantly higher than the rate of the nerves posterior to ITA (P =0.002 vs. P <0.0001, respectively).
Discussion
In thyroid surgery, having the knowledge of RLNs anatomy and its possible variations are critical for visual identification of the RLN which is the gold standard technique to preserve the RLN. Extralaryngeal branching is the most common variation of RLN. To be able to maintain RLN function, all the branches must be preserved. RLN injury may occur in case of an undetected motor branch of RLN being divided unintentionally leading to an unexpected vocal cord dysfunction. Although many studies have been conducted on RLN anatomy and its variations, characteristics of extralaryngeal branching of RLN and its relationship with other anatomical variations have not been fully evaluated in the literature.[7]
In this study, 1127 nerves were assessed for branching features and its relations with other anatomical variations (relationship between RLN and ITA, BL entrapment, and NRLN) between the genders and the two sides of the neck. The prevalence of extralaryngeal branching of RLN was detected higher in female patients. In branching and non-branching nerves, relationship of RLN and ITA was also different both in the female and total patient groups. When branching and non-branching nerves were analyzed separately, the difference was statistically significant regarding the RLN and ITA relation between the right and left neck sides. In branching nerves compared to the non-branching nerves, the rates of RLN entrapment at BL region were detected significantly higher both in the female and total patient groups. Both in branching and non-branching nerves, entrapment of BL was detected with a higher rate on the right side compared to the left side of the neck.
In the current literature, the prevalence of extralaryngeal branching of RLN has been debatable and reported in a large scale of 5–100%. Various results have been reported from cadaveric or intraoperative studies and subgroup analyses.[7]
This study is a clinical investigation that was conducted under the guidance of IONM and extralaryngeal branching of RLN was 35.3% in all patients, furthermore, seen more frequently in female patients than male patients (38.2% vs. 25.8%, P <0.0001, respectively).
There was no difference of extralaryngeal branching of RLN between the right and left sides of the neck in entire patient group (37.4% vs. 33.2%, p=0.135, respectively), in female patients (41% vs. 35.2%, p=0.077, respectively), and in male patients (25.2% vs. 26.4%, p=0.829, respectively). In addition, extralaryngeal branching rate was 40% in NRLNs.
In a recent meta-analysis, extralaryngeal branching of RLN was estimated as 39.2% (95% CI:29.0–49.9) in clinical series and 73.3% (95% CI: 61.0–84.0) in cadaveric studies. It is speculated that in clinical studies, some of the thin branches may not be recognized and lesser amount of extralaryngeal branching of RLNs than cadaveric studies are detected. In addition, inadequate exploration of RLN and rejecting small accessory branches as an extralaryngeal branch may cause difference in numbers between studies.[7]
Similar to the present study, Wu et al. reported a higher rate of extralaryngeal branching of RLN in female patients, however, many studies in the literature have reported similar rates of branching between the male and female patients.[11,13–17]
In the present study, the rates of extralaryngeal branching of RLNs were a like between the right and left sides of the neck. Nevertheless, several studies reported higher number of branching nerves on the right side than the left side of the neck.[11,15,16,18,19]
On the other hand, Asgharpour et al. found higher rate of extralaryngeal branching of RLN on the left side compared to the right side in the cadaveric studies. A total of 142 cadavers have been investigated and the rate of extralaryngeal branching was 54.6% and branching was more common on the left side.[20]
In another study, a relationship was found between extralaryngeal branching and the ethnicity. Higher prevalence of extralaryngeal branching was detected in African-Americans than Caucasians (42.1% vs. 33.2%, respectively).[14]
In addition, complete exploration of RLN rather than only exposing the distal part of the RLN close to the BL, using surgical loops and IONM, has been reported to increase the detection rate of the extralaryngeal branches of RLN.[11,21–24]
Extralaryngeal branching of RLN has been mostly asymmetrical and unilateral in clinical series and percentage of bilaterality has been reported to beranging between 3.7 and 33.3%.[9,11,16–19,25]
In the current study, 64.7% of the nerves were non-branching, 32.7% had two, 2.4% had three, and 0.3% had multiple branches. In a meta-analysis that was reported by Henry et al., type of branching has been evaluated both in clinical series and cadaveric studies and results were found different between the two groups. The rates of branching in cadaveric series and clinical series were 61.1% (95% CI: 33.8–78.4) versus 37.6% (95% CI: 26.2–49.4) for two branches, 9.0% (95% CI: 0.3–20.9) versus1.0% (95% CI: 0–4.1) for three branches, and 6.5% (95% CI: 0–15.5) versus 0.1% (95% CI: 0–1.7) for multiple branches, respectively.[7]
In the present study, branching rates between the two sides of the neck were similar, but branching type was different between the two groups. On the right side, the rate of bifurcation was higher than that of the left side (35.8% vs. 29.4%, respectively), but the rates of non-branching (62.6% vs. 66.8%, respectively) and trifurcation(1% vs. 3.8%, respectively) were less than those of the left side (P =0.001). Between the two sides of the neck similar bifurcation and trifurcation rates have been reported before, however, Beneragama et al. and Sormaz et al., together with Serpell et al., detected higher rates of bifurcation and trifurcation on the right side of the neck.[11,17,19]
Another feature of branching that has been evaluated in the present study was the branching distance of RLN. Mean branching distance was 1.91±0.9 cm (range, 0.5–5.0) and there was no difference detected regarding the genders and the two sides of the neck. Branching distance has been generally found similar between the right and left sides of the neck in the previous studies.[11,19] The branching distance was found longer in female population in a study conducted by Fontenot et al.[14] Sormaz et al. were found that branching distances were higher in female patients than male patients on the left side (11.57±3.72 mm vs. 9.78±2.89 mm, respectively; p=0.031), however, branching distances were similar on the right side (11.42±3.60 mm vs. 10.89±2.84 mm; p>0.05, respectively).[17]
In another study, branching distance was detected longer in the nerves with motor function both in the anterior and posterior branches compared to the nerves with motor function only in the anterior branch.[10] In a meta-analysis by Henry et al., RLNs were found to branch at the last 2 cm part to the entry point to the larynx in approximately 90% of cases.[7]
The surgeon must be aware of variable relationship between ITA and RLN in thyroidectomy, because of the critical role of ITA for the identification of RLN.[6] The rates of RLN crossing ITA anteriorly and posteriorly were found similar between the right and left sides of the neck(crossing anteriorly: 45.7%, posteriorly: 44.8%, and between branches: 9.5%). However, on the right side of the neck compared to the left side RLN passed more frequently from anterior of ITA (52.9% vs. 38.4%, respectively), between branches of ITA (12% vs. 6.9%, respectively) and less frequently from posterior of ITA (38.4% vs. 48.1%).
In a meta-analysis, Henry et al. analyzed 14269 nerves out of 79 cadaveric or clinical studies and detected that RLN passed from anterior of ITA in 27.6% (95% CI:23.2–30.6), from posterior in 50,7% (95% CI:45.2–53.5), and between branches of ITA in 21.7% (95% CI: 17.8–24.6). In subgroup analysis, there was no significant difference between study types (clinical or cadaveric), gender, or geographical area. Consistent with our results, they found that on the left side of the neck 62.6% of RLNs crossed posterior to ITA (95% CI: 56.3–65.7) compared to the right side on which only 37% crossed posterior to ITA (95% CI: 45.2–53.5) and the difference was statistically significant.[6]
As far as our knowledge, there has been no study that evaluated the relationship between RLN and ITA in extralaryngeal branching and non-branching RLNs. According to our results, the relationship between RLN and ITA was statistically different in branching nerves compared to the non-branching nerves (p=0.001) and RLN passed from anterior of ITA (47.7% vs. 45.7%, respectively), from posterior of ITA (38.5% vs. 48%, respectively) and between the branches of ITA (12.8% vs. 7.6%, respectively). About 61.5% of branching nerves were found to cross anterior to ITA or between the branches of ITA. It has been claimed by Skandalakis et al. that incautious dividing of the artery branches might lead to RLN injury crossing anterior to or between the branches of ITA.[26]
Low number of branching in clinical studies compared to cadaveric studies in which branching ratio is close to 90%, high frequency of crossing from posterior of ITA in non-branching nerves, and low frequency of crossing from posterior of ITA in branching nerves may be associated with undetected or unidentified branches which pass from posterior of ITA. In addition, Thomas et al. found the ratio of extralaryngeal branching of RLN 89.1% on the right side and 74.6% on the left side and these results showed that there is a lower percentage of extralaryngeal branching of RLN on the left side.[27]
In female and male patients, branching and non-branching nerves were compared separately and the rates regarding the relationship between RLN and ITA were similar to those of the entire group in both genders. However, the difference was found significant in females, but not statistically significant in male patients between the branching and non-branching nerves due to the low number of nerves (p=0.007 in females and p=0.108 in males). On the right and left sides of the neck, branching and non-branching nerves were analyzed for their relationship with ITA, and in both nerve groups, the rates of RLNs crossing anterior to ITA and between the branches of ITA were higher on the right side and the rate of RLNs crossing posterior to ITA was higher on the left side (p=0.002 on branching nerve and p<0.0001 on non-branching nerve).
BL is a reliable anatomical landmark, nevertheless, the anatomical relationship between RLN and BL has been still controversial. According to many cadaveric studies, 100% of RLNs pass through lateral side of the BL, on the other hand, various studies mention that few of the RLNs penetrate through the branches of BL or go deeper to the BL.[28,29]
In a meta-analysis, 16 cadaveric studies and 2500 RLNs were investigated and 78.2% of RLNs were passing on the surface of BL (95% CI: 51.5–90.8), 14.8% of RLNs were going deeper to the BL (95% CI: 0–33.0), and 7.0% of RLNs were passing between the branches of BL (95% CI: 0–19.6).[29]
The RLN might be entrapped by the vascular structure or the BL running posteriorly to the nerve in the region of Berry leading to traction trauma in up to 75% of nerve injuries. Thus, this feature has been included to the International RLN Classification System. It has been estimated that RLN entrapment exists in 10% of all cases at this region.[4]
In the previous studies, increased risk of RLN injury in branching nerves has been reported.[18,30] Along with that in branching nerves, especially the anterior motor branch of RLN is under the increased risk of injury due to the entrapment by the BL.[8]
In the current study, rate of BL entrapment was 20.5% and it was significantly high in branching nerves (25.9% in branching vs. 17.5% in non-branching nerves, p=0.001). In female patients, BL entrapment was higher in branching nerves compared to non-branching nerves (25.7% vs. 16.4%, respectively, P =0.002). Furthermore, in male patients, BL entrapment was higher in branching nerves compared to non-branching nerves, but the difference was not statistically significant (25.8% vs. 21.1%, respectively, p=0.446). On the right side of the neck compared to the left side, BL entrapment was more frequent both in branching (31.5% vs. 19.4%, respectively, p=0.008) and non-branching nerves (20.6% vs. 14.4%, respectively, p=0.037). Anatomical findings in the present study were in compliance with the previous studies in the literature. On the right side of the neck, the higher rates of RLNs anterior to ITA pattern and also BL entrapment both in branching and non-branching nerves support the idea that the rightside harbors anatomical variations more frequently compared to the left side.
Major limitation of this study is its retrospective nature. Along with that, adequate number of RLNs enrolled to the study increased the significance of the obtained data regarding the relationship between RLN and ITA as well as BL entrapment.
Conclusion
RLNs extralaryngeal branching is not uncommon, and it usually has two branches. Branching rate in females is higher than in men. Extralaryngeal branching is associated with the other anatomical variations. In branching nerves, the rates of RLNs crossing anterior to ITA and crossing between the branches were higher, whereas the rate of RLNs crossing posterior to ITA was higher in non-branching nerves.
In branching nerves, nerve entrapment was more likely in the Berry region. Entrapment of the RLN by the posterior BL was detected more frequently on the right side in both branching and non-branching nerves. Extralaryngeal branching, RLN-ITA relationship, and entrapment in the Berry region are common anatomical variations that can be variable and cannot be predicted preoperatively. By determining the RLN at the level of the ITA and exposing it completely till its laryngeal entry, it may be possible to detect most of the extralaryngeal branches and their relationship with the other anatomical variations.
Disclosures
Ethics Committee Approval: This study was approved by the local ethics committee (no: 1876, approval date: April 27, 2021).
Peer-review: Externally peer-reviewed.
Conflict of Interest: None declared.
Authorship Contributions: Concept – M.K., M.U.; Design – M.K., M.U.; Supervision – M.U.; Materials – M.K., O.C., C.Y., Y.C., M.U.; Data collection &/ or processing – M.K., O.C., C.Y., Y.C., M.U.; Analysis and/or interpretation – M.K., O.C., C.Y., Y.C., M.U.; Literature search – M.K., O.C., C.Y., Y.C., M.U.; Writing – M.K., O.C., C.Y., Y.C., M.U.; Critical review – M.K., O.C., C.Y., Y.C., M.U.
References
- 1.Fundakowski CE, Hales NW, Agrawal N, Barczyński M, Camacho PM, Hartl DM, et al. Surgical management of the recurrent laryngeal nerve in thyroidectomy:American Head and Neck Society Consensus Statement. Head Neck. 2018;40:663–75. doi: 10.1002/hed.24928. [DOI] [PubMed] [Google Scholar]
- 2.Lombardi CP, Raffaelli M, De Crea C, Carnassale G, Bellantone R. RLN nerve and inferior thyroid crossing. In: Randolph GW, editor. The recurrent and superior laryngeal nerves. 1st ed. Switzerland: Springer International Publishing; 2016. pp. 79–82. [Google Scholar]
- 3.Zealear DL, Billante CR. Neurophysiology of vocal fold paralysis. Otolaryngol Clin North Am. 2004;37:1–23. doi: 10.1016/S0030-6665(03)00165-8. [DOI] [PubMed] [Google Scholar]
- 4.Randolph GW, Wu CW, Dionigi G, Kamani D, Modi RR, Chiang FY, et al. The international RLN anatomic classification system. In: Randolph GW, editor. The recurrent and superior laryngeal nerves. 1st ed. Switzerland: Springer International Publishing; 2016. pp. 125–38. [Google Scholar]
- 5.Henry BM, Sanna S, Graves MJ, Vikse J, Sanna B, Tomaszewska IM, et al. The Non-Recurrent Laryngeal Nerve:a meta-analysis and clinical considerations. PeerJ. 2017;5:e3012. doi: 10.7717/peerj.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henry BM, Vikse J, Graves MJ, Sanna S, Sanna B, Tomaszewska IM, et al. Variable relationship of the recurrent laryngeal nerve to the inferior thyroid artery:A meta-analysis and surgical implications. Head Neck. 2017;39:177–86. doi: 10.1002/hed.24582. [DOI] [PubMed] [Google Scholar]
- 7.Henry BM, Vikse J, Graves MJ, Sanna S, Sanna B, Tomaszewska IM, et al. Extralaryngeal branching of the recurrent laryngeal nerve:a meta-analysis of 28,387 nerves. Langenbecks Arch Surg. 2016;401:913–23. doi: 10.1007/s00423-016-1455-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snyder SK, Lairmore TC, Hendricks JC, Roberts JW. Elucidating mechanisms of recurrent laryngeal nerve injury during thyroidectomy and parathyroidectomy. J Am Coll Surg. 2008;206:123–30. doi: 10.1016/j.jamcollsurg.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Uludağ M, Yetkin G, Oran EŞ, Aygün N, Celayir F, İşgör A. Extralaryngeal division of the recurrent laryngeal nerve:A common and asymmetric anatomical variant. Turk J Surg. 2017;33:164–8. doi: 10.5152/UCD.2016.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uludag M, Aygun N, Isgor A. Motor function of the recurrent laryngeal nerve:Sometimes motor fibers are also located in the posterior branch. Surgery. 2016;160:153–60. doi: 10.1016/j.surg.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Uludag M, Yazici P, Aygun N, Citgez B, Yetkin G, Mihmanli M, et al. A closer look at the recurrent laryngeal nerve focusing on branches &diameters:A prospective cohort study. J Invest Surg. 2016;29:383–8. doi: 10.1080/08941939.2016.1176279. [DOI] [PubMed] [Google Scholar]
- 12.Uludağ M, Tanal M, İşgör A. A review of methods for the preservation of laryngeal nerves during thyroidectomy. Sisli Etfal Hastan Tip Bul. 2018;52:79–91. doi: 10.14744/SEMB.2018.37928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu KT, Chan YC, Chou FF, Wu YJ, Chi SY. Association between recurrent laryngeal nerve calibre and body figure:A preoperative tool to assess thin-diameter nerves in thyroidectomy. World J Surg. 2020;44:3036–42. doi: 10.1007/s00268-020-05549-4. [DOI] [PubMed] [Google Scholar]
- 14.Fontenot TE, Randolph GW, Friedlander PL, Masoodi H, Yola IM, Kandil E. Gender, race, and electrophysiologic characteristics of the branched recurrent laryngeal nerve. Laryngoscope. 2014;124:2433–7. doi: 10.1002/lary.24631. [DOI] [PubMed] [Google Scholar]
- 15.Barczyński M, Stopa M, Konturek A, Nowak W. The overwhelming majority but not all motor fibers of the bifid recurrent laryngeal nerve are located in the anterior extralaryngeal branch. World J Surg. 2016;40:629–35. doi: 10.1007/s00268-015-3257-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wojtczak B, Kaliszewski K, Sutkowski K, Bolanowski M, Barczyński M. A functional assessment of anatomical variants of the recurrent laryngeal nerve during thyroidectomies using neuromonitoring. Endocrine. 2018;59:82–9. doi: 10.1007/s12020-017-1466-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sormaz IC, Tunca F, Şenyürek YG. Bilateral patterns and motor function of the extralaryngeal branching of the recurrent laryngeal nerve. Surg Radiol Anat. 2018;40:1077–83. doi: 10.1007/s00276-018-1989-1. [DOI] [PubMed] [Google Scholar]
- 18.Casella C, Pata G, Nascimbeni R, Mittempergher F, Salerni B. Does extralaryngeal branching have an impact on the rate of postoperative transient or permanent recurrent laryngeal nerve palsy? World J Surg. 2009;33:261–5. doi: 10.1007/s00268-008-9832-1. [DOI] [PubMed] [Google Scholar]
- 19.Beneragama T, Serpell JW. Extralaryngeal bifurcation of the recurrent laryngeal nerve:a common variation. ANZ J Surg. 2006;76:928–31. doi: 10.1111/j.1445-2197.2006.03899.x. [DOI] [PubMed] [Google Scholar]
- 20.Asgharpour E, Maranillo E, Sañudo J, Pascual-Font A, Rodriguez-Niedenführ M, Valderrama FJ, et al. Recurrent laryngeal nerve landmarks revisited. Head Neck. 2012;34:1240–6. doi: 10.1002/hed.21882. [DOI] [PubMed] [Google Scholar]
- 21.Cernea CR, Hojaij FC, De Carlucci D, Jr, Gotoda R, Plopper C, Vanderlei F, et al. Recurrent laryngeal nerve:a plexus rather than a nerve? Arch Otolaryngol Head Neck Surg. 2009;135:1098–102. doi: 10.1001/archoto.2009.151. [DOI] [PubMed] [Google Scholar]
- 22.Chiang FY, Lu IC, Chen HC, Chen HY, Tsai CJ, Hsiao PJ, et al. Anatomical variations of recurrent laryngeal nerve during thyroid surgery:how to identify and handle the variations with intraoperative neuromonitoring. Kaohsiung J Med Sci. 2010;26:575–83. doi: 10.1016/S1607-551X(10)70089-9. [DOI] [PubMed] [Google Scholar]
- 23.Reeve T, Thompson NW. Complications of thyroid surgery:how to avoid them, how to manage them, and observations on their possible effect on the whole patient. World J Surg. 2000;24:971–5. doi: 10.1007/s002680010160. [DOI] [PubMed] [Google Scholar]
- 24.Anuwong A, Lavazza M, Kim HY, Wu CW, Rausei S, Pappalardo V, et al. Recurrent laryngeal nerve management in thyroid surgery:consequences of routine visualization, application of intermittent, standardized and continuous nerve monitoring. Updates Surg. 2016;68:331–41. doi: 10.1007/s13304-016-0393-9. [DOI] [PubMed] [Google Scholar]
- 25.Kandil E, Abdelghani S, Friedlander P, Alrasheedi S, Tufano RP, Bellows CF, et al. Motor and sensory branching of the recurrent laryngeal nerve in thyroid surgery. Surgery. 2011;150:1222–7. doi: 10.1016/j.surg.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Skandalakis JE, Droulias C, Harlaftis N, Tzinas S, Gray SW, Akin JT., Jr The recurrent laryngeal nerve. Am Surg. 1976;42:629–34. [PubMed] [Google Scholar]
- 27.Thomas AM, Fahim DK, Gemechu JM. Anatomical variations of the recurrent laryngeal nerve and implications for injury prevention during surgical procedures of the neck. Diagnostics (Basel) 2020;10:670. doi: 10.3390/diagnostics10090670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasou S, Nakamura S, Kurihara H. Suspensory ligament of Berry:its relationship to recurrent laryngeal nerve and anatomic examination of 24 autopsies. Head Neck. 1998;20:695–8. doi: 10.1002/(sici)1097-0347(199812)20:8<695::aid-hed6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 29.Henry BM, Sanna B, Graves MJ, Sanna S, Vikse J, Tomaszewska IM, et al. The reliability of the tracheoesophageal groove and the ligament of berry as landmarks for ıdentifying the recurrent laryngeal nerve:a cadaveric study and meta-analysis. Biomed Res Int. 2017;2017:4357591. doi: 10.1155/2017/4357591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sancho JJ, Pascual-Damieta M, Pereira JA, Carrera MJ, Fontané J, Sitges-Serra A. Risk factors for transient vocal cord palsy after thyroidectomy. Br J Surg. 2008;95:961–7. doi: 10.1002/bjs.6173. [DOI] [PubMed] [Google Scholar]


