Abstract
Plasmacytoid dendritic cells (pDCs) are specialized producers of Type I interferon (IFN-I) that promote anti-viral and anti-tumor immunity. However, chronic infections and cancer inhibit pDC-derived IFN-I. While the mechanisms of this inhibition are multifarious they can be classified broadly into two categories: i) reduction or ablation of pDC IFN-I-production capacity (functional exhaustion) and/or ii) decrease in pDC numbers (altered population dynamics). Recent work has identified many processes that contribute to suppression of pDC-derived IFN-I during chronic infections and cancer, including sustained stimulation through Toll Like Receptors (TLRs), inhibitory microenvironments, inhibitory receptor ligation, and reduced development from bone marrow progenitors and apoptosis. Emerging success leveraging pDCs in treatment of disease through TLR activation illustrates the therapeutic potential of targeting pDCs. Deeper understanding of the systems that limit pDC-derived IFN-I has the potential to improve these emerging therapies as well as help devising new approaches that harness the outstanding IFN-I-production capacity of pDCs.
Introduction
Type I interferons (IFN-I) are potent immune activating, anti-viral, and anti-neoplastic cytokines (reviewed in Refs. [1,2]). They encompass a family of genes including 13 IFNα subtypes in humans (14 in mice), one IFNβ and several other less well-defined gene products (reviewed in Ref. [2]). While most cells can produce some IFN-I, the existence of a highly specialized subset of IFN-I producing cells distinct from T cells, B cells, NK Cells, and Monocytes was established in the 1980s. These interferon producing cells (IPCs), were later shown to be equivalent to a cell type originally identified for its plasmacytoid morphology providing an identity to IPCs as plasmacytoid DCs (pDCs, reviewed in Refs. [3–5]).
pDCs produce IFNsat exceptional levels, including 13 subtypes of IFN-α, IFN-β, 3 subtypes of IFN-λ and IFN-τ (reviewed in Refs. [3–5] and [6]). Indeed, within a few hours of viral activation, 60% of the new pDC transcripts encode IFN-I sequences [6]. In line with their prolific IFN-production capacity, pDCs are known to promote control of multiple types of viral infection through mechanisms that include suppression of viral replication in infected tissues, induction of apoptosis in infected cells, and enhancement of anti-viral NK and CD8 T cell responses (reviewed in Ref. [4]). In contrast, a large body of literature indicates that pDC-derived IFN-I is limited during chronic infections and cancer. This may take place either through reducing pDC numbers by modifying pDC development, survival and/or differentiation (hereafter referred to as changes in pDC population dynamics, Figure 1 top), and/or through promoting a hypo-functional state whereby pDCs produce significantly less IFN-I in response to stimulation (hereafter referred to as pDC exhaustion, Figure 1 bottom). Altered pDC population dynamics and/or pDC exhaustion have been observed in chronic viral infections such as HIV [7–13], HCV [14–21], and HBV [22–24] in humans, as well as in SIV-infected Macaques [25–28], and in chronic LCMV-infected mice [29,30,31••]. Additionally, exhausted pDCs have been observed in many different types of human cancers including head and neck stromal cell carcinoma (HNSCC) [32], breast cancer [33], chronic lymphocytic leukemia [34] and ovarian cancer [35]. Here we will discuss pDC dynamics and exhaustion, including the current understanding of the mechanisms that drive these phenomena, their outcomes, and the potential therapeutic benefit of reversing these processes. It is important to note that while this review focuses on chronic disease settings, altered pDC population dynamics and pDC exhaustion have also been observed, albeit for shorter-term, during acute infection with LCMV, HSV-1, VSV, or MCMV [29,31••,36]. Its hould also be noted that pDCs have been described to play pathogenic roles in the context of certain infections and tumors, including the induction of tolerogenic and/or immunosuppressive responses, which are beyond the scope of this review. For this and for subjects such as steady-state pDC development and function, we direct the readers to other recent reviews [5,37,38].
Figure 1.
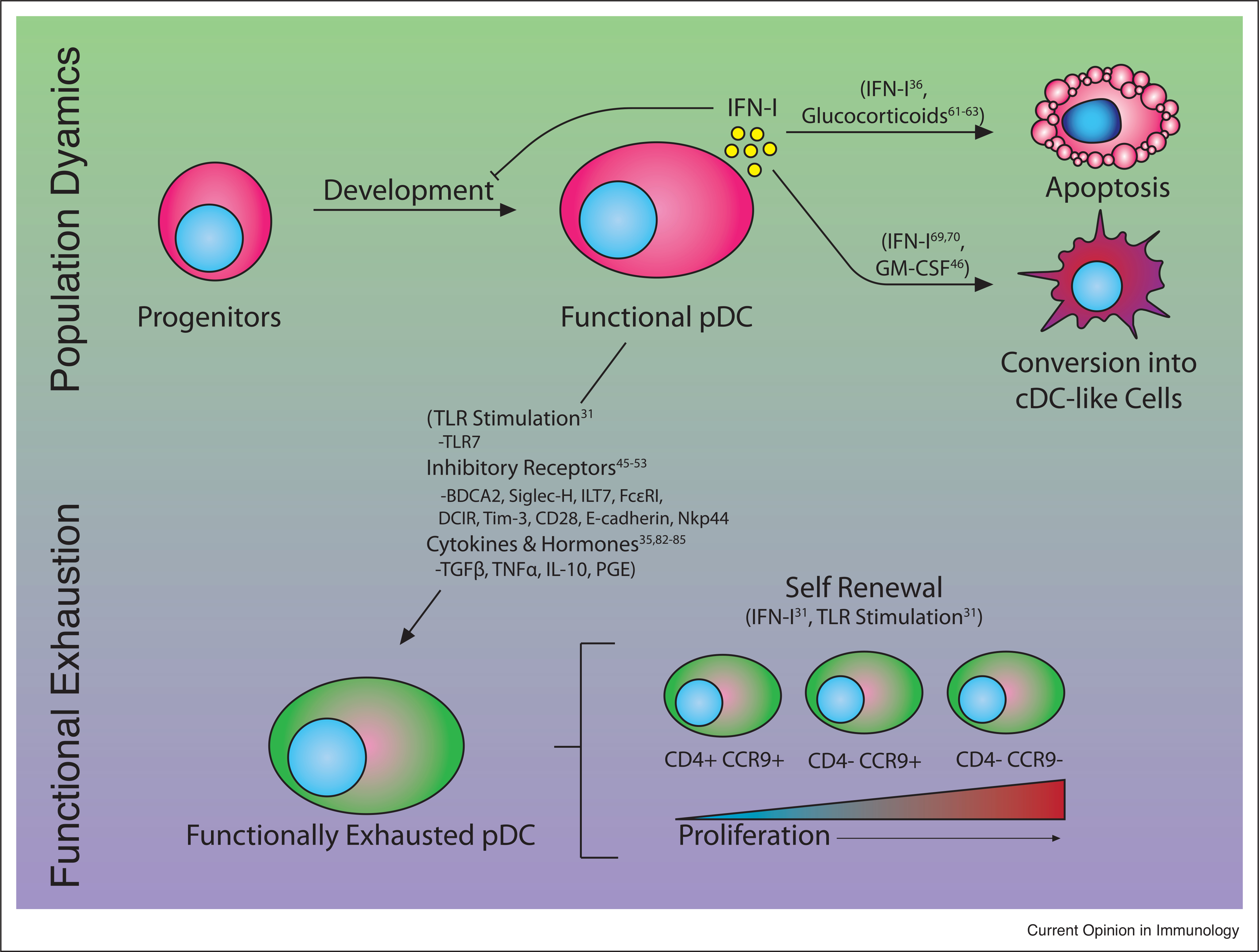
Mechanisms inhibiting pDC IFN-I production. Production of IFN-I in pDCs can be inhibited both by reducing available number of functional pDCs (Population Dynamics, top) and the amount of IFN-I produced by pDCs (Functional Exhaustion, bottom). Reduced numbers of functional pDCs may result from reduced developmental capacity from progenitors, apoptosis and/or conversion into cDC-like fates, all of which can be driven by IFN-I signaling, though this IFN-I may not be derived from pDCs. pDC functional exhaustion can be driven by a number of factors including stimulation by TLR ligands, and inhibitory receptor ligation, inhibitory cytokines and hormones. In addition, functionally exhausted pDCs encompass subsets with varying proliferative potential. Factors known to mediate the regulatory processes are in parentheses.
Suppression of pDC IFN-I production in chronic viral infection
pDC functional exhaustion
Decreased IFN-I production by blood pDCs was first observed in HIV patients in the early 2000s [7], and was subsequently confirmed in other HIV studies [8,9,11] as well as other human chronic viral infections [14–16,19,21–24]. The description of this phenomenon by our group and others in LCMV infection of mice [29,30,31••] and SIV infection of macaques [25,26] has permitted a deeper understanding of this phenomenon. We reported that, initially, both acute and chronic LCMV infections induce IFN-I and pDC exhaustion. Importantly, this phenotype is only sustained in the latter persistent infection setting and associated with dramatic reduction of systemic IFN-I (in the face of increasing viral titers in blood and multiple tissues) as well as enhanced susceptibility to unrelated secondary infections [29,31••]. Consistently, systemic IFN-I is attenuated, despite high viremia, early after HIV infection [39], and suppression of IFN-I production capacity by peripheral blood mononuclear cells has been correlated with opportunistic infections in HIV-infected individuals [10,40–42], even independently of CD4 T cell numbers [42]. More recently we showed that, analogous to the role of persistent TCR stimulation in T cell exhaustion [43], pDC stimulation through TLR7 was required for their functional exhaustion during chronic LCMV infection in mice [31••]. The mechanistic underpinnings of this observation are still not fully understood, but our group has established that TLR7 stimulation in the context of infection drives reduction in the levels of the transcription factor E2–2 [31••], which is critical for pDC development and function [44].
A relationship between TLR ligation and pDC function also likely exists in human HIV patients, as pDC exhaustion emerges in patients with high viral load [7,8] rapidly recovers during the administration of successful antiretroviral therapy [11] but subsequently becomes suppressed again upon interruption of treatment [8]. Similar to the LCMV murine infection findings, E2–2 is also reduced in human primary pDCs from HIV-viremic patients [31••] as well as in a human pDC cell line that was stimulated for two days with TLR ligands [44]. Furthermore, in human pDCs, stimulation through TLR has been shown to induce expression of the inhibitory ligand Tim-3 [9], which opposes pDC activation [45]. Coordinately, HIV patients have higher proportions of low IFN-I producing Tim-3+ pDCs [9]. While correlative, these data together suggest that persistent TLR stimulation may contribute to IFN-I exhaustion in both murine and human pDCs.
Engagement of pDC inhibitory receptors
Like many immune cells pDCs express a large variety of receptors on their surface that can temper their function such as BDCA2 [46], Siglec-H [47], ILT7 [48], FcεRI [49], DCIR [50], Tim-3 [45], CD28 [51], E-cadherin [52], and Nkp44 [53]. These systems likely represent mechanisms to tune down IFN-I production to avoid immunopathology, although they could also facilitate viral propagation. This is exemplified by the increased numbers of Tim-3+ pDCs in HIV-infected patients who exhibit reduced IFN-I production, as discussed above [9]. Additionally, mice deficient in CD28 show enhanced serum IFN-I and increased IFN-I transcript levels in pDCs after either LCMV or MCMV infection [51], and mice deficient in Siglec-H show increased systemic IFN-I after MCMV infection [54]. In contrast, it has been directly shown that the HIV protein Vpu can subvert antiviral pDC function through redistribution of BST2 on infected cells in order to allow viral exit while still engaging the inhibitory receptor ILT7 [55]. Thus, manipulation of pDC inhibitory receptors may be an effective strategy employed by hosts and pathogens to attenuate pDC-derived IFN-I in the context of infections.
Altered pDC population dynamics
Compromised pDC development from progenitors
Another consideration in the long-term suppression of pDC IFN-I production during chronic infection is that pDCs are typically short-lived [56,57], meaning that in the course of a chronic infection pDC populations could be entirely replaced, yet IFN-I levels are still suppressed with respect to peak responses. Our studies on pDC dynamics in chronic LCMV infection lend some insight into this phenomenon. We showed that pDC generation from bone marrow (BM) is decreased in chronically infected mice. This associates with a numerical reduction in progenitors with the potential to generate pDCs, their capacity to generate pDCs and their expression of E2–2 [31••]. In addition, we observed that BM progenitors from LCMV-infected mice develop into hypofunctional, E2–2-low, pDCs when cultured ex-vivo in the presence of Flt3L [31••], raising the possibility that transcriptional regulation inherited from BM progenitors contributes to IFN-I exhaustion in the pDC progeny. A caveat that should be considered in these studies is, however, the presence of virus in the cell culture, which could have caused persistent TLR stimulation to the newly generated pDCs. Remarkably, dysregulation of E2–2 and its target genes in both BM pDC progenitors and persistently stimulated splenic pDCs during chronic infection suggests down-regulation of this transcriptional pathway as a convergent mechanism imposing reductions in IFN-I production at both the levels of pDC development and function. Finally, it should be noted that some of the progenitor populations analyzed in this study are heterogeneous [58•], and so more studies are needed to discern whether the observed reduction in E2–2 in these populations represents a decrease in pDC-committed progenitor subpopulations.
pDC apoptosis
In addition to reduced development it has been observed that pDCs show higher rates of apoptosis in HIV and SIV infection compared to uninfected controls [26,27,59]. The precise mechanisms that drive increased pDC death in these infections is still not fully understood, but IFN-I signaling has been shown to drive pDC death in response to multiple other types of infections in mice [36], and thus it is tempting to speculate that IFN-I driven apoptosis is part of a negative feedback loop limiting pDC IFN-I production after infection. Alternatively, it is established that glucocorticoids (GCs), which are elevated in HIV-infected patients [60], can induce apoptosis in pDCs [61–63]. TLR stimulation, however, opposes GC-induced apoptosis [61–63], and it is therefore unclear whether these elevated levels of GC would induce apoptosis of pDCs in the context of infection.
pDC proliferation
Despite the aforementioned impaired pDC development from progenitors and pDC apoptosis, the numbers of pDCs in peripheral lymphoid tissues are not always dramatically reduced and they are even enhanced at day 10 after chronic LCMV infection [31••]. This was explained by the discovery that exhausted splenic and BM pDCs undergo significant self-renewal early after acute infection and throughout chronic infection with LCMV in mice. Such pDC expansion coincides with increased proportions of CD4-CCR9− and CD4-CCR9+ pDC subsets which exhibit the highest proliferative capacity [31••]. This was a striking finding as almost all splenic pDCs are CCR9+ and do not proliferate under steady state conditions [56,64,65]. In contrast, CD4-CCR9− and CD4−CCR9+ pDCs have been described as minor BM populations with proliferative potential and as the immediate precursors of CD4+CCR9+ pDCs [56,64,65]. Notably, the gain of pDC proliferation after LCMV infection depends on both IFN-I and TLR signaling, while functional exhaustion only requires the latter [31••]. Similarly, an increase in pDCs expressing the cell cycle marker Ki67 has been reported during chronic SIV infection in macaques [26–28]. Given this, it is tempting to speculate that the suppression of de-novo pDC development together with the gain in self-renewal capacity of exhausted pDCs may perpetuate a pool of hypofunctional cells that compromise IFN-I production throughout chronic infections.
pDC conversion
It is well established that after stimulation with IL-3/CD40L or TLR ligands in vitro pDCs increase their antigen presenting capacity and reduce IFN-I production potential [66–68]. We also demonstrated that viral infections (acute and chronic) and administration of IFN-I primes pDCs to differentiate into CD11b+ cDC like cells, exhibiting phenotypic and functional features of bonafide cDCs, both in vitro [69] and in vivo [70]. Similar conversion into CD11b+ cDC-like cells was observed by other groups using Ly49Q- pDCs from PolyI:C injected mice [71] or CCR9− pDCs stimulated with intestinal epithelial cell supernatant or GM-CSF [65] or cell transfer under steady-state conditions [64]. The conversion of pDCs into cDC-like cells is consistent with murine and human studies that reported intermediary DCs exhibiting features of both pDCs and cDCs [72–77,78•,79•]. These non-canonical DCs [5] have been defined as Axl+ in humans [72,78•], Cx3cr1+ CD8+ cDC in mice [75,76] and in recent high dimensional mapping and cross-species analysis studies they have been grouped in with the newly described population of transitional (t)DCs [72,79•]. The population defined as tDCs specifically show variable E2–2 expression and have reduced IFN-I production when compared to pDCs. Indeed, as E2–2 is necessary to maintain pDC fate [80] and is downregulated in pDCs and their progenitors during infection [31••], pDC conversion into cDC like cells may be, at least partially, driven via the suppression of E2–2. Further work will be necessary to determine whether the aforementioned intermediary DCs [72–77,78•,79•] are transitional stages along the pDC conversion into CD11b+ cDC-like cells that we first reported after LCMV infection [69,70]. Nonetheless, these observations suggest that enhanced pDC conversion into cDC-like cells, which exhibit diminished IFN-I production capacity, may be another mechanism by which infections suppress overall pDC-mediated IFN-I production.
pDC IFN-I exhaustion in cancer
Tumor microenvironments are complex, highly immunosuppressive, and diverse. Thus, disentangling the exact mechanisms by which tumors suppress pDC function is complicated, and it is likely that, as with immunosuppression of other cell types [81] many overlapping mechanisms suppress tumor-associated (TA)pDC function in a given malignancy. Below we provide some examples of mechanisms that have been associated to pDC exhaustion in specific tumor settings.
Immunosuppressive cytokines and hormones
Tumor microenvironments are rife with immunosuppressive cytokines and hormones such as Transforming Growth Factor Beta (TGFβ), Interleukin (IL)-10, and prostaglandin E2 all of which have been shown to inhibit pDC production of IFN-I [35,82–85]. Reducing the levels of PGE2 in HNSCC supernatants slightly relieves pDC IFN-I suppression [83], while blockade of TGFβ partially rescues IFN-I production in pDCs exposed to supernatants from ovarian cancer [35]. The typically pro-inflammatory cytokine Tumor Necrosis Factor Alpha (TNFα), can also inhibit IFN-I production by pDCs [82]. This may represent an autocrine negative feedback loop as pDCs can also produce TNFα upon TLR [5] or FCεRl engagement [49,86]. Indeed, in the above-mentioned ovarian cancer model TNFα also contributed significantly to the inhibition of IFN-I production by pDC in the presence of tumor supernatant [35].
Tumor metabolic environment as a challenge to pDC function
In addition to active evasion mechanisms employed by cancers, the tumor microenvironment can be naturally hostile for immune function as a result of the high metabolic activity of cancerous cells, which puts them in direct competition for nutrients with infiltrating immune populations [87]. As pDC production of IFN is a highly metabolically demanding activity requiring de novo synthesis of a large quantity of mRNA and protein, pDC function has been intrinsically linked to metabolism relying on both glycolysis and OxPhos [88,89]. It is thus plausible that some tumor microenvironments may lack the necessary nutrients to support pDC IFN-I production. Furthermore, many tumors have been established to produce and secrete high levels of lactate [90] which has recently been shown to inhibit pDC function both through nutrient sensing and direct metabolic pathways [91•]. Importantly, if this is the case, typical experiments where pDC are taken ex vivo from tumors and cultured in nutrient rich media for stimulation without lactate may underrepresent the amount of in vivo pDC functional suppression in the tumor microenvironment.
Potential commonalities in pDC exhaustion in chronic infection and cancer
In addition to the above-described work, it is tempting to speculate that, like chronic infection, persistent stimulation of TApDCs through TLRs may also contribute to their exhaustion in cancer. Indeed, nucleic acid release by dying cells, which would be sensed by TApDCs, has been described in tumor microenvironments [92]. In contrast, regarding the dynamics of pDCs in cancer, it is known that pDC can be recruited to tumors that produce Stromal Derived Factor-1 (SDF-1 a.k.a. CXCL12) [93] and that CCL2 is involved in pDC recruitment upon TLR7 ligand administration in tumor settings [94]. However, whether TApDCs are repopulated by BM progenitors or derived from local proliferative pDC populations in the tumor or draining lymph nodes, as observed during chronic infections [26–28,31••], remains unknown. It would also be interesting to explore whether TApDCs or pDCs in tumor draining lymph nodes may differentiate into tDC or cDC-like populations, as observed in the context of infection [69,70]. Answering these questions will be paramount to understanding which populations to target with therapeutics aimed at restoring pDC IFN-I production in the context of cancer.
Regarding pDC inhibitory receptors that have been involved in pDC suppression during chronic infections, Tim-3 is known to suppress pDC function in a tumor microenvironment [45]. In addition, it has been posited that BST2 expression by tumors may suppress pDCs through ILT7, though in multiple myeloma it was found that suppression of pDC function was mediated by E-cadherin and not BST2 [52]. As many cancers express, produce, or present the ligands of pDC inhibitory receptors (e.g. NKp44-L, BST2, phosphatidylserine, Galectin-9, HMGB1) [95–100], and expression of these ligands is a strong predictor of poor outcome or metastasis [96–99], it is conceivable that ligands derived from cancer cells may contribute to the suppression of TApDCs. However, as these ligands have been studied mostly for their impact on other immune cells, which express the same receptors, and their intrinsic impacts on cancer fitness, future work will be needed to elucidate their impact on pDC function in specific tumor microenvironments.
Concluding remarks
Despite the role of pDCs as specialized producers of IFN-I, there are diverse mechanisms, which temper or entirely shut down their IFN-I production. Given the consequences of excessive and/or persistent IFN-I signaling exemplified by both genetic interferonopathies such as Aicardi-Goutieres Syndrome, Chilblain Lupus, Systemic Lupus Erthematosus and others (reviewed by Ref. [101]) as well as IFN-I induced immunopathology that has been observed in some infections (reviewed by Ref. [2]), this may represent an evolved abundance of caution around the power of pDCs to produce such large amounts of IFN-I. Indeed, as mentioned above, altered pDC population dynamics and functional exhaustion still emerges (albeit temporarily) in response to multiple types of acute viral infection, suggesting that pDC suppression may be the result of a general default program that is initiated after pDC activation and is sustained in the context of a chronic insult like a persistent infection or a tumor [31••]. Thus, like PD1 expression in CD8 T cells, which prevents autoimmunity and immunopathology, but favors chronic infections and tumors [81], pDC IFN-I suppression may represent a similar natural mechanism of immune-regulation. Promising studies activating pDCs for treatment of cancer [102–105] suggest the utility of leveraging pDC IFN-I production. However, pDC IFN-I exhaustion and altered pDC population dynamics may be significant stumbling blocks in revealing the full therapeutic potential of pDCs in chronic infections and tumor settings. Further understanding of the mechanisms underlying pDC suppression will not only provide opportunities to restore IFN-I production to fight infections and cancer but may also allow harnessing of these natural braking systems to inhibit pDC function in the context of autoimmunity and other chronic inflammatory diseases.
Acknowledgements
We thank members of the Zuniga lab for fruitful discussions. Research in the Zuniga lab is supported by N.I.H. grants AI145314, AI081923, AI113923 and AI132122. As citations were limited we were unable to cite all available studies, and so apologize to any authors who feel their studies were not adequately represented in our accounting.
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G: Type I interferons in anticancer immunity. Nat Rev Immunol 2015, 15:405–414. [DOI] [PubMed] [Google Scholar]
- 2.McNab F, Mayer-Barber K, Sher A, Wack A, O’Garra A: Type I interferons in infectious disease. Nat Rev Immunol 2015, 15:87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzgerald-Bocarsly P, Feng D: The role of type I interferon production by dendritic cells in host defense. Biochimie 2007, 89:843–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swiecki M, Colonna M: The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol 2015, 15:471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reizis B: Plasmacytoid dendritic cells: development, regulation, and function. Immunity 2019, 50:37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito T, Kanzler H, Duramad O, Cao W, Liu Y-J: Specialization, kinetics, and repertoire of type 1 interferon responses by human plasmacytoid predendritic cells. Blood 2006, 107:2423–2431. [DOI] [PubMed] [Google Scholar]
- 7.Feldman S, Stein D, Amrute S, Denny T, Garcia Z, Kloser P, Sun Y, Megjugorac N, Fitzgerald-Bocarsly P: Decreased interferon-α production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin Immunol 2001, 101:201–210. [DOI] [PubMed] [Google Scholar]
- 8.Tilton JC, Manion MM, Luskin MR, Johnson AJ, Patamawenu AA, Hallahan CW, Cogliano-Shutta NA, Mican JM, Davey RT, Kottilil S et al. : Human immunodeficiency virus viremia induces plasmacytoid dendritic cell activation in vivo and diminished alpha interferon production in vitro. J Virol 2008, 82:3997–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz JA, Clayton KL, Mujib S, Zhang H, Rahman AKMN, Liu J, Yue FY, Benko E, Kovacs C, Ostrowski MA: Tim-3 is a marker of plasmacytoid dendritic cell dysfunction during HIV infection and is associated with the recruitment of IRF7 and p85 into lysosomes and with the submembrane displacement of TLR9. J Immunol 2017, 198:3181–3194. [DOI] [PubMed] [Google Scholar]
- 10.Soumelis V, Scott I, Gheyas F, Bouhour D, Cozon G, Cotte L, Huang L, Levy JA, Liu Y-J: Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood 2001, 98:906–912. [DOI] [PubMed] [Google Scholar]
- 11.Chehimi J, Campbell DE, Azzoni L, Bacheller D, Papasavvas E, Jerandi G, Mounzer K, Kostman J, Trinchieri G, Montaner LJ: Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J Immunol 2002, 168:4796–4801. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Fu J, Zhao Q, He Y, Jin L, Zhang H, Yao J, Zhang L, Wang F-S: Differential restoration of myeloid and plasmacytoid dendritic cells in HIV-1-infected children after treatment with highly active antiretroviral therapy. J Immunol 2006, 176:5644–5651. [DOI] [PubMed] [Google Scholar]
- 13.Meyers JH, Justement JS, Hallahan CW, Blair ET, Sun YA, O’Shea MA, Roby G, Kottilil S, Moir S, Kovacs CM et al. : Impact of HIV on cell survival and antiviral activity of plasmacytoid dendritic cells. PLoS One 2007, 2:e458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanto T, Inoue M, Miyatake H, Sato A, Sakakibara M, Yakushijin T, Oki C, Itose I, Hiramatsu N, Takehara T et al. : Reduced numbers and impaired ability of myeloid and plasmacytoid dendritic cells to polarize T helper cells in chronic hepatitis C virus infection. J Infect Dis 2004, 190:1919–1926. [DOI] [PubMed] [Google Scholar]
- 15.Ulsenheimer A, Gerlach JT, Jung M-C, Gruener N, Wächtler M, Backmund M, Santantonio T, Schraut W, Heeg MHJ, Schirren CA et al. : Plasmacytoid dendritic cells in acute and chronic hepatitis C virus infection. Hepatology 2005, 41:643–651. [DOI] [PubMed] [Google Scholar]
- 16.Goutagny N, Vieux C, Decullier E, Ligeoix B, Epstein A, Trépo C, Couzigou P, Inchauspe G, Bain C: Quantification and functional analysis of plasmacytoid dendritic cells in patients with chronic hepatitis C virus infection. J Infect Dis 2004, 189:1646–1655. [DOI] [PubMed] [Google Scholar]
- 17.Anthony DD, Yonkers NL, Post AB, Asaad R, Heinzel FP, Lederman MM, Lehmann PV, Valdez H: Selective impairments in dendritic cell-associated function distinguish hepatitis C virus and HIV infection. J Immunol 2004, 172:4907–4916. [DOI] [PubMed] [Google Scholar]
- 18.Wertheimer AM, Bakke A, Rosen HR: Direct enumeration and functional assessment of circulating dendritic cells in patients with liver disease. Hepatology 2004, 40:335–345. [DOI] [PubMed] [Google Scholar]
- 19.Lai WK, Curbishley SM, Goddard S, Alabraba E, Shaw J, Youster J, McKeating J, Adams DH: Hepatitis C is associated with perturbation of intrahepatic myeloid and plasmacytoid dendritic cell function. J Hepatol 2007, 47:338–347. [DOI] [PubMed] [Google Scholar]
- 20.Szabo G, Dolganiuc A: Subversion of plasmacytoid and myeloid dendritic cell functions in chronic HCV infection. Immunobiology 2005, 210:237–247. [DOI] [PubMed] [Google Scholar]
- 21.Dolganiuc A, Chang S, Kodys K, Mandrekar P, Bakis G, Cormier M, Szabo G: Hepatitis C virus (HCV) core protein-induced, monocyte-mediated mechanisms of reduced IFN-α and plasmacytoid dendritic cell loss in chronic HCV infection. J Immunol 2006, 177:6758–6768. [DOI] [PubMed] [Google Scholar]
- 22.van der Molen RG, Sprengers D, Binda RS, de Jong EC, Niesters HGM, Kusters JG, Kwekkeboom J, Janssen HLA: Functional impairment of myeloid and plasmacytoid dendritic cells of patients with chronic hepatitis B. Hepatology 2004, 40:738–746. [DOI] [PubMed] [Google Scholar]
- 23.Xie Q, Shen H-C, Jia N-N, Wang H, Lin L-Y, An B-Y, Gui H-L, Guo S-M, Cai W, Yu H et al. : Patients with chronic hepatitis B infection display deficiency of plasmacytoid dendritic cells with reduced expression of TLR9. Microbes Infect 2009,11:515–523. [DOI] [PubMed] [Google Scholar]
- 24.Duan X-Z, Wang M, Li H-W, Zhuang H, Xu D, Wang F-S: Decreased frequency and function of circulating plasmocytoid dendritic cells (pDC) in hepatitis B virus infected humans. J Clin Immunol 2004, 24:637–646. [DOI] [PubMed] [Google Scholar]
- 25.Malleret B, Manéglier B, Karlsson I, Lebon P, Nascimbeni M, Perié L, Brochard P, Delache B, Calvo J, Andrieu T et al. : Primary infection with simian immunodeficiency virus: plasmacytoid dendritic cell homing to lymph nodes, type I interferon, and immune suppression. Blood 2008, 112:4598–4608. [DOI] [PubMed] [Google Scholar]
- 26.Bruel T, Dupuy S, Démoulins T, Rogez-Kreuz C, Dutrieux J, Corneau A, Cosma A, Cheynier R, Dereuddre-Bosquet N, Le Grand R et al. : Plasmacytoid dendritic cell dynamics tune interferon-alfa production in SIV-infected cynomolgus macaques. PLoS Pathog 2014, 10:e1003915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown KN, Wijewardana V, Liu X, Barratt-Boyes SM: Rapid influx and death of plasmacytoid dendritic cells in lymph nodes mediate depletion in acute simian immunodeficiency virus infection. PLoS Pathog 2009, 5:e1000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H, Evans TI, Gillis J, Connole M, Reeves RK: Bone marrow–imprinted gut-homing of plasmacytoid dendritic cells (pDCs) in acute simian immunodeficiency virus infection results in massive accumulation of hyperfunctional CD4 + pDCs in the mucosae. J Infect Dis 2015, 211:1717–1725. [DOI] [PubMed] [Google Scholar]
- 29.Zuniga EI, Liou L-Y, Mack L, Mendoza M, Oldstone MBA: Persistent virus infection inhibits type I interferon production by plasmacytoid dendritic cells to facilitate opportunistic infections. Cell Host Microbe 2008, 4:374–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee LN, Burke S, Montoya M, Borrow P: Multiple mechanisms contribute to impairment of Type 1 interferon production during chronic lymphocytic choriomeningitis virus infection of mice. J Immunol 2009, 182:7178–7189. [DOI] [PubMed] [Google Scholar]
- 31. Macal M, Jo Y, Dallari S, Chang AY, Dai J, Swaminathan S, Wehrens EJ, Fitzgerald-Bocarsly P, Zúñiga EI: Self-Renewal and toll-like receptor signaling sustain exhausted plasmacytoid dendritic cells during chronic viral infection. Immunity 2018, 48:730–744.e5 •• Using LCMV as a model system the authors demonstrate that sensing through TLR7 drives functional exhaustion of pDCs, that pDCs in the periphery gain proliferative capacity, and that development of pDCs from progenitors is compromised both early in acute viral infection as well as late in chronic viral infection.
- 32.Hartmann E, Wollenberg B, Rothenfusser S, Wagner M, Wellisch D, Mack B, Giese T, Gires O, Endres S, Hartmann G: Identification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancer. Cancer Res 2003, 63:6478–6487. [PubMed] [Google Scholar]
- 33.Sisirak V, Faget J, Gobert M, Goutagny N, Vey N, Treilleux I, Renaudineau S, Poyet G, Labidi-Galy SI, Goddard-Leon S et al. : Impaired IFN-α production by plasmacytoid dendritic cells favors regulatory T-cell expansion that may contribute to breast cancer progression. Cancer Res 2012, 72:5188–5197. [DOI] [PubMed] [Google Scholar]
- 34.Saulep-Easton D, Vincent FB, Le Page M, Wei A, Ting SB, Croce CM, Tam C, Mackay F: Cytokine-driven loss of plasmacytoid dendritic cell function in chronic lymphocytic leukemia. Leukemia 2014, 28:2005–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Labidi-Galy SI, Sisirak V, Meeus P, Gobert M, Treilleux I, Bajard A, Combes J-D, Faget J, Mithieux F, Cassignol A et al. : Quantitative and functional alterations of plasmacytoid dendritic cells contribute to immune tolerance in ovarian cancer. Cancer Res 2011, 71:5423–5434. [DOI] [PubMed] [Google Scholar]
- 36.Swiecki M, Wang Y, Vermi W, Gilfillan S, Schreiber RD, Colonna M: Type I interferon negatively controls plasmacytoid dendritic cell numbers in vivo. J Exp Med 2011, 208:2367–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demoulin S, Herfs M, Delvenne P, Hubert P: Tumor microenvironment converts plasmacytoid dendritic cells into immunosuppressive/tolerogenic cells: insight into the molecular mechanisms. J Leukoc Biol 2013, 93:343–352. [DOI] [PubMed] [Google Scholar]
- 38.Barrat FJ, Su L: A pathogenic role of plasmacytoid dendritic cells in autoimmunity and chronic viral infection. J Exp Med 2019, 216:1974–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, Lebedeva M, DeCamp A, Li D, Grove D et al. : Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus Type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol 2009, 83:3719–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez C, Fitzgerald PA, Siegal FP: Severe acquired immune deficiency syndrome in male homosexuals: diminished capacity to make interferon-a in vitro associated with severe opportunistic infections. J Infect Dis 1983, 148:962–966. [DOI] [PubMed] [Google Scholar]
- 41.Siegal FP, Lopez C, Fitzgerald PA, Shah K, Baron P, Leiderman IZ, Imperato D, Landesman S: Opportunistic infections in acquired immune deficiency syndrome result from synergistic defects of both the natural and adaptive components of cellular immunity. J Clin Invest 1986, 78:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siegal FP, Fitzgerald-Bocarsly P, Holland BK, Shodell M: Interferon-α generation and immune reconstitution during antiretroviral therapy for human immunodeficiency virus infection. AIDS 2001, 15:1603–1612. [DOI] [PubMed] [Google Scholar]
- 43.Mueller SN, Ahmed R: High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A 2009, 106:8623–8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cisse B, Caton ML, Lehner M, Maeda T, Scheu S, Locksley R, Holmberg D, Zweier C, den Hollander NS, Kant SG et al. : Transcription factor E2–2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell 2008,135:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, Fujioka Y, Ohba Y, Gorman Jv, Colgan JD et al. : Tumor-infiltrating DCs suppress nucleic acid–mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol 2012, 13:832–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, Gunther G, Johnston I, Lanzavecchia A, Nagasaka T et al. : BDCA-2, a novel plasmacytoid dendritic cell-specific Type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon α/β induction. J Exp Med 2001, 194:1823–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blasius AL, Cella M, Maldonado J, Takai T, Colonna M: Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12. Blood 2006, 107:2474–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao W, Rosen DB, Ito T, Bover L, Bao M, Watanabe G, Yao Z, Zhang L, Lanier LL, Liu Y-J: Plasmacytoid dendritic cell-specific receptor ILT7-FcεRIγ inhibits toll-like receptor–induced interferon production. J Exp Med 2006, 203:1399–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Novak N, Allam J-P, Hagemann T, Jenneck C, Laffer S, Valenta R, Kochan J, Bieber T: Characterization of FcεRI-bearing CD123+ blood dendritic cell antigen-2+ plasmacytoid dendritic cells in atopic dermatitis. J Allergy Clin Immunol 2004, 114:364–370. [DOI] [PubMed] [Google Scholar]
- 50.Meyer-Wentrup F, Benitez-Ribas D, Tacken PJ, Punt CJA, Figdor CG, de Vries IJM, Adema GJ: Targeting DCIR on human plasmacytoid dendritic cells results in antigen presentation and inhibits IFN-α production. Blood 2008, 111:4245–4253. [DOI] [PubMed] [Google Scholar]
- 51.Macal M, Tam MA, Hesser C, Di Domizio J, Leger P, Gilliet M, Zuniga EI: CD28 deficiency enhances Type I IFN production by murine plasmacytoid dendritic cells. J Immunol 2016, 196:1900–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bi E, Li R, Bover LC, Li H, Su P, Ma X, Huang C, Wang Q, Liu L, Yang M et al. : E-cadherin expression on multiple myeloma cells activates tumor-promoting properties in plasmacytoid DCs. J Clin Invest 2018, 128:4821–4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fuchs A, Cella M, Kondo T, Colonna M: Paradoxic inhibition of human natural interferon-producing cells by the activating receptor NKp44. Blood 2005, 106:2076–2082. [DOI] [PubMed] [Google Scholar]
- 54.Puttur F, Arnold-Schrauf C, Lahl K, Solmaz G, Lindenberg M, Mayer CT, Gohmert M, Swallow M, van Helt C, Schmitt H et al. : Absence of Siglec-H in MCMV infection elevates interferon alpha production but does not enhance viral clearance. PLoS Pathog 2013, 9:e1003648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bego MG, Côté É, Aschman N, Mercier J, Weissenhorn W, Cohen ÉA: Vpu exploits the cross-talk between BST2 and the ILT7 receptor to suppress anti-HIV-1 responses by plasmacytoid dendritic cells. PLOS Pathog 2015, 11:e1005024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhan Y, Chow KV, Soo P, Xu Z, Brady JL, Lawlor KE, Masters SL, O’keeffe M, Shortman K, Zhang J-G et al. : Plasmacytoid dendritic cells are short-lived: reappraising the influence of migration, genetic factors and activation on estimation of lifespan. Sci Rep 2016, 6:25060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M: Origin of dendritic cells in peripheral lymphoid organs of mice. Nat Immunol 2007, 8:578–583. [DOI] [PubMed] [Google Scholar]
- 58. Dress RJ, Dutertre C-A, Giladi A, Schlitzer A, Low I, Shadan NB, Tay A, Lum J, Kairi MFBM, Hwang YY et al. : Plasmacytoid dendritic cells develop from Ly6D+ lymphoid progenitors distinct from the myeloid lineage. Nat Immunol 2019, 20:852–864 • Using a combination of single-cell sequencing, mass cytometry, and fate mapping the authors identify a pDC committed progenitor population (pre-pDC).
- 59.Lehmann C, Lafferty M, Garzino-Demo A, Jung N, Hartmann P, Fätkenheuer G, Wolf JS, van Lunzen J, Romerio F: Plasmacytoid dendritic cells accumulate and secrete interferon alpha in lymph nodes of HIV-1 patients. PLoS One 2010, 5:e11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.George MM, Bhangoo A: Human immune deficiency virus (HIV) infection and the hypothalamic pituitary adrenal axis. Rev Endocr Metab Disord 2013, 14:105–112. [DOI] [PubMed] [Google Scholar]
- 61.Boor PPC, Metselaar HJ, Mancham S, Tilanus HW, Kusters JG, Kwekkeboom J: Prednisolone suppresses the function and promotes apoptosis of plasmacytoid dendritic cells. Am J Transplant 2006, 6:2332–2341. [DOI] [PubMed] [Google Scholar]
- 62.Guiducci C, Gong M, Xu Z, Gill M, Chaussabel D, Meeker T, Chan JH, Wright T, Punaro M, Bolland S et al. : TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature 2010, 465:937–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lepelletier Y, Zollinger R, Ghirelli C, Raynaud F, Hadj-Slimane R, Cappuccio A, Hermine O, Liu Y-J, Soumelis V: Toll-like receptor control of glucocorticoid-induced apoptosis in human plasmacytoid predendritic cells (pDCs). Blood 2010, 116:3389–3397. [DOI] [PubMed] [Google Scholar]
- 64.Schlitzer A, Heiseke AF, Einwächter H, Reindl W, Schiemann M, Manta C-P, See P, Niess J-H, Suter T, Ginhoux F et al. : Tissue-specific differentiation of a circulating CCR9− pDC-like common dendritic cell precursor. Blood 2012, 119:6063–6071. [DOI] [PubMed] [Google Scholar]
- 65.Schlitzer A, Loschko J, Mair K, Vogelmann R, Henkel L, Einwachter H, Schiemann M, Niess J-H, Reindl W, Krug A: Identification of CCR9–murine plasmacytoid DC precursors with plasticity to differentiate into conventional DCs. Blood 2011, 117:6562–6570. [DOI] [PubMed] [Google Scholar]
- 66.Kadowaki N, Antonenko S, Lau JY-N, Liu Y-J: Natural interferon α/β-producing cells link innate and adaptive immunity. J Exp Med 2000, 192:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siegal FP: The nature of the principal type 1 interferon-producing cells in human blood. Science (80-) 1999, 284:1835–1837. [DOI] [PubMed] [Google Scholar]
- 68.Grouard G, Rissoan M-C, Filgueira L, Durand I, Banchereau J, Liu Y-J: The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med 1997, 185:1101–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zuniga EI, McGavern DB, Pruneda-Paz JL, Teng C, Oldstone MBA: Bone marrow plasmacytoid dendritic cells can differentiate into myeloid dendritic cells upon virus infection. Nat Immunol 2004, 5:1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liou L-Y, Blasius AL, Welch MJ, Colonna M, Oldstone MBA, Zuniga EI: In vivo conversion of BM plasmacytoid DC into CD11b+ conventional DC during virus infection. Eur J Immunol 2008, 38:3388–3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Toma-Hirano M, Namiki S, Miyatake S, Arai K, Kamogawa-Schifter Y: Type I interferon regulates pDC maturation and Ly49Q expression. Eur J Immunol 2007, 37:2707–2714. [DOI] [PubMed] [Google Scholar]
- 72.Alcántara-Hernández M, Leylek R, Wagar LE, Engleman EG, Keler T, Marinkovich MP, Davis MM, Nolan GP, Idoyaga J: High-dimensional phenotypic mapping of human dendritic cells reveals interindividual variation and tissue specialization. Immunity 2017, 47:1037–1050.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.See P, Dutertre C-A, Chen J, Günther P, McGovern N, Irac SE, Gunawan M, Beyer M, Händler K, Duan K et al. : Mapping the human DC lineage through the integration of high-dimensional techniques. Science (80-) 2017, 356 eaag3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matsui T, Connolly JE, Michnevitz M, Chaussabel D, Yu C-I, Glaser C, Tindle S, Pypaert M, Freitas H, Piqueras B et al. : CD2 distinguishes two subsets of human plasmacytoid dendritic cells with distinct phenotype and functions. J Immunol 2009, 182:6815–6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bar-On L, Birnberg T, Lewis KL, Edelson BT, Bruder D, Hildner K, Buer J, Murphy KM, Reizis B, Jung S: CX3CR1+ CD8 + dendritic cells are a steady-state population related to plasmacytoid dendritic cells. Proc Natl Acad Sci U S A 2010,107:14745–14750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lau CM, Nish SA, Yogev N, Waisman A, Reiner SL, Reizis B: Leukemia-associated activating mutation of Flt3 expands dendritic cells and alters T cell responses. J Exp Med 2016, 213:415–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang H, Gregorio JD, Iwahori T, Zhang X, Choi O, Tolentino LL, Prestwood T, Carmi Y, Engleman EG: A distinct subset of plasmacytoid dendritic cells induces activation and differentiation of B and T lymphocytes. Proc Natl Acad Sci U S A 2017, 114:1988–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Villani A-C, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, Griesbeck M, Butler A, Zheng S, Lazo S et al. : Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science (80-) 2017, 356: eaah4573. • Using single cell RNA-seq the authors for the first time identified a population of non-cannonical Axl+ DCs with features of both pDCs and cDCs in human blood.
- 79. Leylek R, Alcántara-Hernández M, Lanzar Z, Lüdtke A, Perez OA, Reizis B, Idoyaga J: Integrated cross-species analysis identifies a conserved transitional dendritic cell population. Cell Rep 2019, 29:3736–3750.e8 • In a cross species analysis using mass cytometry, transcriptomics, and functional assays the authors identified a population of DCs in both humans and mice (dubbed tDCs) that are intermediate between pDCs and cDCs.
- 80.Ghosh HS, Cisse B, Bunin A, Lewis KL, Reizis B: Continuous expression of the transcription factor E2–2 maintains the cell fate of mature plasmacytoid dendritic cells. Immunity 2010, 33:905–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pauken KE, Wherry EJ: Overcoming T cell exhaustion in infection and cancer. Trends Immunol 2015, 36:265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gary-Gouy H, Lebon P, Dalloul AH: Type I interferon production by plasmacytoid dendritic cells and monocytes is triggered by viruses, but the level of production is controlled by distinct cytokines. J Interferon Cytokine Res 2002, 22:653–659. [DOI] [PubMed] [Google Scholar]
- 83.Bekeredjian-Ding I, Schäfer M, Hartmann E, Pries R, Parcina M, Schneider P, Giese T, Endres S, Wollenberg B, Hartmann G: Tumour-derived prostaglandin E 2 and transforming growth factor-β synergize to inhibit plasmacytoid dendritic cell-derived interferon-α. Immunology 2009, 128:439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li L, Liu S, Zhang T, Pan W, Yang X, Cao X: Splenic stromal microenvironment negatively regulates virus-activated plasmacytoid dendritic cells through TGF-β. J Immunol 2008, 180:2951–2956. [DOI] [PubMed] [Google Scholar]
- 85.Son Y, Ito T, Ozaki Y, Tanijiri T, Yokoi T, Nakamura K, Takebayashi M, Amakawa R, Fukuhara S: Prostaglandin E2 is a negative regulator on human plasmacytoid dendritic cells. Immunology 2006, 119:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schroeder JT, Bieneman AP, Xiao H, Chichester KL, Vasagar K, Saini S, Liu MC: TLR9- and FcεRI-mediated responses oppose one another in plasmacytoid dendritic cells by down-regulating receptor expression. J Immunol 2005, 175:5724–5731. [DOI] [PubMed] [Google Scholar]
- 87.Buck MD, Sowell RT, Kaech SM, Pearce EL: Metabolic instruction of immunity. Cell 2017, 169:570–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bajwa G, DeBerardinis RJ, Shao B, Hall B, Farrar JD, Gill MA: Cutting edge: critical role of glycolysis in human plasmacytoid dendritic cell antiviral responses. J Immunol 2016, 196:2004–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu D, Sanin DE, Everts B, Chen Q, Qiu J, Buck MD, Patterson A, Smith AM, Chang C-H, Liu Z et al. : Type 1 interferons induce changes in core metabolism that are critical for immune function. Immunity 2016, 44:1325–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de la Cruz-López KG, Castro-Muñoz LJ, Reyes-Hernández DO, García-Carrancá A, Manzo-Merino J: Lactate in the regulation of tumor microenvironment and therapeutic approaches. Front Oncol 2019, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Raychaudhuri D, Bhattacharya R, Sinha BP, Liu CSC, Ghosh AR, Rahaman O, Bandopadhyay P, Sarif J, D’Rozario R, Paul S et al. : Lactate induces pro-tumor reprogramming in intratumoral plasmacytoid dendritic cells. Front Immunol 2019, 10. • The authors demonstrate that pDC IFN-I production is inhibited by lactate both in vitro and in vivo and demonstrate that this relies both on sensing of extracellular lactate as well as on lactate uptake by pDCs.
- 92.lurescia S, Fioretti D, Rinaldi M: Targeting cytosolic nucleic acid-sensing pathways for cancer immunotherapies. Front Immunol 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zou W, Machelon V, Coulomb-L’Hermin A, Borvak J, Nome F, Isaeva T, Wei S, Krzysiek R, Durand-Gasselin I, Gordon A et al. : Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med 2001, 7:1339–1346. [DOI] [PubMed] [Google Scholar]
- 94.Drobits B, Holcmann M, Amberg N, Swiecki M, Grundtner R, Hammer M, Colonna M, Sibilia M: Imiquimod clears tumors in mice independent of adaptive immunity by converting pDCs into tumor-killing effector cells. J Clin Invest 2012, 122:575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Parodi M, Favoreel H, Candiano G, Gaggero S, Sivori S, Mingari MC, Moretta L, Vitale M, Cantoni C: NKp44-NKp44 ligand interactions in the regulation of natural killer cells and other innate lymphoid cells in humans. Front Immunol 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tiwari R, de la Torre JC, McGavern DB, Nayak D: Beyond tethering the viral particles: immunomodulatory functions of tetherin (BST-2). DNA Cell Biol 2019, 38:1170–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chou F-C, Chen H-Y, Kuo C-C, Sytwu H-K: Role of galectins in tumors and in clinical immunotherapy. Int J Mol Sci 2018, 19:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.He S-J, Cheng J, Feng X, Yu Y, Tian L, Huang Q: The dual role and therapeutic potential of high-mobility group box 1 in cancer. Oncotarget 2017, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dankner M, Gray-Owen SD, Huang Y-H, Blumberg RS, Beauchemin N: CEACAM1 asa multi-purpose target for cancer immunotherapy. Oncoimmunology 2017, 6:e1328336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Birge RB, Boeltz S, Kumar S, Carlson J, Wanderley J, Calianese D, Barcinski M, Brekken RA, Huang X, Hutchins JT et al. : Phosphatidylserine is a global immunosuppressive signal in efferocytosis, infectious disease, and cancer. Cell Death Differ 2016, 23:962–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee-Kirsch MA: The Type I interferonopathies. Annu Rev Med 2017, 68:297–315. [DOI] [PubMed] [Google Scholar]
- 102.Nierkens S, den Brok MH, Garcia Z, Togher S, Wagenaars J, Wassink M, Boon L, Ruers TJ, Figdor CG, Schoenberger SP et al. : Immune adjuvant efficacy of CpG oligonucleotide in cancer treatment is founded specifically upon TLR9 function in plasmacytoid dendritic cells. Cancer Res 2011, 71:6428–6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Le Mercier I, Poujol D, Sanlaville A, Sisirak V, Gobert M, Durand I, Dubois B, Treilleux I, Marvel J, Vlach J et al. : Tumor promotion by intratumoral plasmacytoid dendritic cells is reversed by TLR7 ligand treatment. Cancer Res 2013, 73:4629–4640. [DOI] [PubMed] [Google Scholar]
- 104.Tel J, Aarntzen EHJG, Baba T, Schreibelt G, Schulte BM, Benitez-Ribas D, Boerman OC, Croockewit S, Oyen WJG, van Rossum M et al. : Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res 2013, 73:1063–1075. [DOI] [PubMed] [Google Scholar]
- 105.Lou Y, Liu C, Lizée G, Peng W, Xu C, Ye Y, Rabinovich BA, Hailemichael Y, Gelbard A, Zhou D et al. : Antitumor activity mediated by CpG: the route of administration is critical. J Immunother 2011, 34:279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]


