Abstract
Bile acids (BAs) are a family of hydroxylated steroids secreted by the liver that aid in the breakdown and absorption of dietary fats. BAs also function as nutrient and inflammatory signaling molecules, acting through cognate receptors, to coordinate host metabolism. Commensal bacteria in the gastrointestinal tract are functional modifiers of the BA pool, affecting composition and abundance. Deconjugation of host BAs creates a molecular network that inextricably links gut microtia with their host. In this review we highlight the roles of BAs in mediating this mutualistic relationship with a focus on those events that impact host physiology and metabolism.
Keywords: BA receptors, bile acids, farnesoid X receptor, FXR, gut microbiota
Introduction
Bile acids (BAs) are cholesterol-derived, amphiphilic molecules that are synthesized by hepatocytes. BAs in most species are stored in the gallbladder and in response to the cholecystokinin elicited by a meal are released into the small intestine where they emulsify dietary fat and enhance lipid, sterol, and vitamin absorption before being reabsorbed in the terminal ileum and returned back to the liver via portal circulation (1, 2). BA uptake in the ileum is facilitated by apical bile salt transporter (ASBT) in the liver and by organic anion transporter polypeptide (OATPA/B) and sodium taurocholate cotransporting polypeptide (NTCP) transporter in the ileum. This process, termed enterohepatic circulation, is highly efficient, occurs 8–10 times per day, and is an important component of circadian timing (3, 4). Small amounts of BAs (<5%) that escape transporter-mediated uptake in the ileum then enter the colon. During their transit through the intestine and colon commensal gut bacteria oxidize, deconjugate, dehydroxylate, and epimerize BAs into secondary BAs with differing hydrophobicity (5). Recently, BA amide conjugations with phenylalanine, tyrosine, and leucine associated with the microbiome were also shown to exist (6).
BA Synthesis, Composition, and Interspecies Differences
The structures of BAs contribute to their functionality. BAs are composed of a sterol core consisting of three six-member carbon rings and one five-member carbon ring, typically having a 5β-hydrogen and a cis-configuration along the plane of the first two fused rings. BAs species are determined by the number and position of hydroxy, carboxy, sulfate, and amino acid groups conjugated to them (FIGURE 1). The hydroxy and carbonyl group face the same side of the sterol core, while the methyl groups face the opposite side. This renders BAs with amphiphilic properties as one side is hydrophobic while the other is hydrophilic (8) (FIGURE 1).
FIGURE 1.
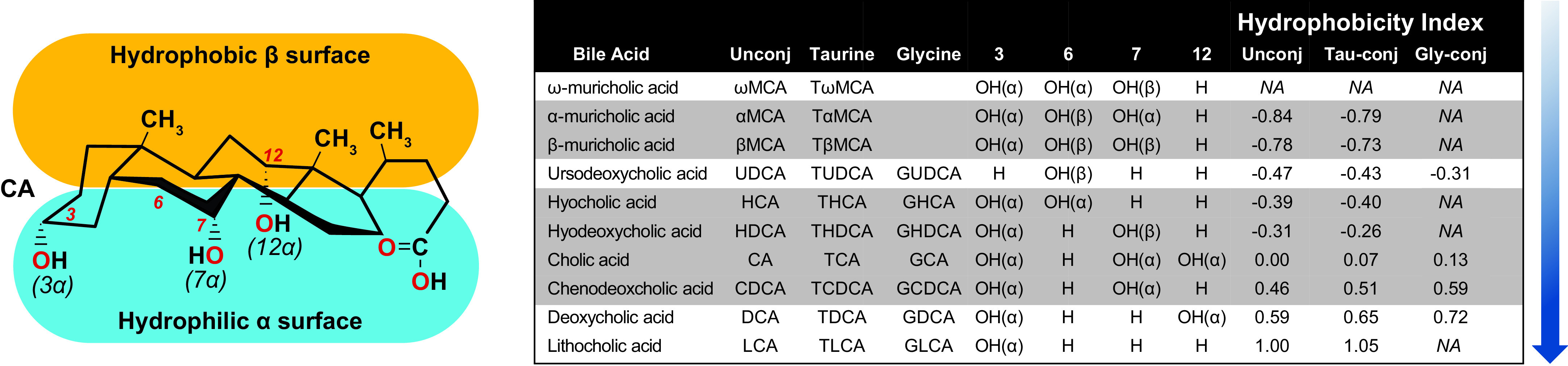
Bile acid (BA) hydrophobicity is determined by the number and position of hydroxyl and sulfate groups to the sterol ring as well whether the BA is conjugated to an amino acid, which in mice is predominantly taurine and in humans is mostly glycine. Hydroxylation at carbons 3, 6, 7, and 12 on the sterol core of primary (highlighted gray) and secondary (white) BAs increases the BA hydrophobicity index (blue shaded arrow). Adapted from Heuman et al. (7).
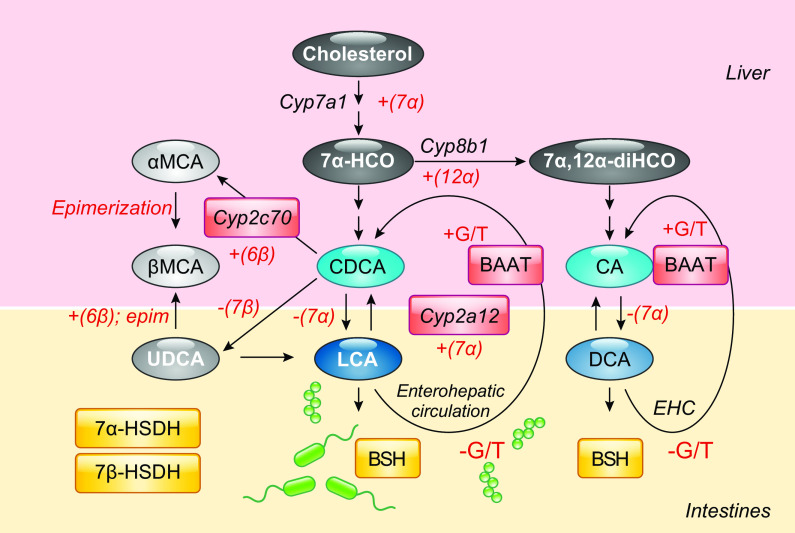
There are major differences in BA composition between humans and mice. Cholic acid (CA) and chenodeoxycholic acid (CDCA) are the two primary BAs synthesized in human liver. There are two pathways for BA biosynthesis: the classical pathway (also referred to as the neutral pathway) and the alternative pathway (also referred to as the acidic pathway, due to the production of acidic intermediates). The classical BA biosynthetic pathway accounts for >90% of total BA production under normal physiologic conditions and is considered the major BA biosynthesis pathway. The first and rate-limiting step of CDCA synthesis via this pathway is catalyzed by the microsomal protein cholesterol 7α-hydroxylase (encoded by CYP7A1), which converts cholesterol to 7α-hydroxycholesterol (7α-HCO) (FIGURE 2). Other enzymes can prevent or stimulate BA synthesis by inhibiting or activating this enzyme. Subsequent 12α hydroxylation of 7α-HCO by 3β-hydroxy-Δ5-C27-steroid hydroxylase (encoded by CYP8B1) generates 7α,12α-dihydroxy-4-cholesten-3-one (7α,12α-diHCO), a precursor to the more hydrophilic BA, CA. Whereas CYP7A1 regulates the overall rate of BA production, CYP8B1 regulates the ratio of CA/CDCA. Chenodeoxycholic acid (CDCA) is a BA end product in humans. In humans, the gut microbiota converts the primary BAs, cholic acid (CA) and CDCA, into deoxycholic acid (DCA) and lithocholic acid (LCA), respectively.
FIGURE 2.
Bile acid (BA) biosynthesis and metabolism in the liver and intestine. The primary BAs, chenodeoxycholic acid (CDCA) and cholic acid (CA) are synthesized from cholesterol and other precursors via Cyp7a1 in the liver yielding the precursors 7α-hydroxycholesterol (7α -HCO) and (7,12-dihydroxycholesterol (7α,12α-diHCO), respectively, the latter generated via the 12α-hydroxylase activity of Cyp8b1. In mice, Cyp2c70 6-hydroxylates CDCA yielding α-muricholic acid (αMCA), which is further epimerized into βMCA and ursodeoxycholic acid (UDCA) via the same enzyme. CA and CDCA are primary BAs in humans while CA, CDCA, MCAs, and UDCA, are primary BAs in mice. After conjugation with glycine (G) or taurine (T) via hepatic BA amino transferase (BAAT), these BAs are secreted into bile which is then released into the intestines. Bacterial 7α-hydroxysteroid dehydrogenase (7α-HSDH) generates deoxycholic acid (DCA), and lithocholic acid (LCA) and 7β-hydroxysteroid dehydrogenase (7β-HSDH) render UDCA. Hepatic Cyp2a12 rehydroxylates DCA and LCA into CA and CDCA, respectively. In humans, CA, CDCA, and DCA are conjugated derivatives are predominant. In mice, CA, αMCA, and βMCA and conjugated derivates are predominant. Cyp2c70-/- mice have increased CDCA and are void of MCAs. Cyp2a12-/- mice have increased DCA, CDCA, and LCA. Cyp2c70-/- and Cyp2a12-/- [double knockout (DKO)] mice have increased DCA, CDCA, and LCA.
BAs are frequently modified by conjugation with taurine (rodents) or glycine (humans), increasing solubility and reducing lipophilicity (9). BAs can also be sulfated, which increases critical micellar concentrations and diminishes bile cytotoxicity as sulfated BAs do not solubilize cholesterol. Sulfated bile acids are shown to accumulate in patients with hepatobiliary diseases (10) and in children with constipation (11) and with cholestasis (12).
In mice, the majority of CDCA is converted to α-muricholic acid (MCA), β-MCA, and UDCA in part by 6β-hydroxylase (Cyp2c70) (13). Therefore, CDCA, CA, α-MCA, β-MCA, and UDCA are major primary BAs in mice. These are the dominant BA species in mice, yielding a BA pool that is more hydrophilic. In the alternative pathway of BA biosynthesis, cholesterol is converted to 27-hydroxycholesterol via actions of sterol 27-hydroxylase (CYP27A1). 27-Hydroxycholesterol and other oxysterols serve as substrates for a nonspecific 7α-hydroxylase (CYP7B1) rendering CDCA (14). In the acidic environment of the duodenum, the pKa of unconjugated BAs is between 5 and 6.5, whereas at physiologic pH conjugated BAs are almost fully ionized and are termed bile salts (15).
In mice, an additional reaction occurs wherein Cyp2a12 reverts this action and converts these secondary BAs back to primary BAs. Novel mouse models deficient in Cyp2c70 (16–18), Cyp2a12 (17) or both genes [double knockout (DKO)] exhibit humanized BA compositions and varied BA pool sizes that differ in their capacity to activate BA receptors and that inhibit cytokine-mediated hepatic Cyp7a1 repression. Cyp2c70-/- mice are deficient in MCAs, Cyp2a12-/- cannot convert secondary BAs back to primary BAs, and DKO (lacking both Cyp2c70-/- and Cyp2a12-/-) mice possess both these features. These Cyp-deficient mice exhibit profoundly different BA profiles that more closely resemble humans. In addition, Cyp2c70 KO and DKO mice have a lower BA pool size (up to 50%) resulting from repressed hepatic Cyp7a1 expression. Findings with respect to the complement of enzymes catalyzing MCA formation are not entirely clear as there is evidence that some MCAs can still be produced in the absence of Cyp2c70 (16) and not all Cyp2c70-deficient lines exhibit reduced Cyp7a1 mRNA. Remarkably, none of the aforementioned mice strains exhibit altered expression of ileal Fgf15 nor do they show evidence of FXR activation. Instead, Cyp2c70 and DKO mice exhibited FXR/SHP-independent and BA/cytokine-mediated inhibition of Cyp7a1-mediated BA synthesis. Future mechanistic studies using these mice will help shed new light on how humanized BA profiles regulate metabolism.
Bacterial Transformation of Bile Salts
Bacteria colonize all surfaces of the body, but nowhere are they more abundant than the gastrointestinal tract. In mammals, the gut microbiome is made up of four major bacterial phyla: Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria. In the human gastrointestinal tract, there are more than 100 trillion (1014) microorganisms (19), including over 100,000 (104) bacterial species (19). This variety is established early in neonatal life with BAs serving as potent drivers of microbial diversity (20). Bacterial enzymes modify BAs in the small intestine, changing the structure and hydrophobicity and increasing BA diversity in enterohepatic circulation. Bacterial deconjugation of BAs via bile salt hydrolase (BSH) hydrolyzes and deconjugates taurine (rodents) or glycine (humans) from the BA sterol core, preventing active reuptake from the small intestine via ASBT (21). Metagenomic analyses demonstrated that functional BSH is present in all major bacterial divisions and archaeal species in the human gut including members of Lactobacilli, Bifidobacteria, Clostridium, and Bacteroides (21, 22). It is thought that BSH is associated with increased resistance to bile toxicity (22, 23). Novel mechanisms have been proposed for how bacteria may exploit BSH activity. For example, the enzyme 7α-HSDH converts CDCA to 7-oxo-LCA. This metabolite acts as a competitive inhibitor for 11β-HSDH-1, an enzyme responsible for the conversion of cortisone to cortisol. It is believed that the cortisol/cortisone ratio may alter the environment into one more favorable for bacteria that produce 7α-HSDH (24). A growing body of literature emerged implicating gut microbes and the abundance of bile-salt hydrolase (BSH) genes as pathologic determinants of intestinal inflammation (25, 26) and consequently metabolic (e.g., obesity and type 2 diabetes) and cardiovascular disease (27, 28). We and others have demonstrated that the gut microbiota-mediated biotransformation of the BA pool also regulates host BA signaling (by primary and secondary BAs) by affecting the activation of intestinal FXR. Recent studies showed the antioxidant Tempol alters the gut microbiome by preferentially reducing the genus Lactobacillus and its BSH activity leading to the accumulation of intestinal TβMCA with weight-lowering effects in mice (29).
Unconjugated BAs are subject to bacterial biotransformations including dehydroxylation, dehydrogenation, and epimerization leading to the formation of secondary BAs. BA oxidation and epimerization are mediated by 3α-, 7α-, 7β-, and 12α-hydroxysteroid dehydrogenase (HDSH) activities of several gut bacteria (5, 30, 31). These reactions serve to dramatically diversify the BA pool and render BA species that may serve as signaling molecules among bacteria or between bacteria and host (5). The 7α-dehydroxylation reaction is, quantitatively speaking, the most important reaction performed by gut bacteria. Deoxycholic acid (DCA) and lithocholic acid (LCA) comprise the majority of secondary bile acids and are formed from CA and CDCA, respectively, via 7α-dehydroxylation (32). This dehydroxylation, carried out mainly by Clostridium cluster XIV bacteria (5), leads to an increase in the BA hydrophobicity index, which impacts intestinal permeability (33), pathogen resistance, bacterial antibiotic secretion (34), and importantly, FXR activation (35, 36). The genes facilitating 7α-dehydroxylation are encoded by elements of the bile acid inducible (bai) operon, best described in high-activity strains of Clostridia such as Clostridium hiranonis and C. scindens. The bai operon encodes proteins needed to uptake unconjugated BAs (baiG), with additional transformation facilitated by enzymes with CoA-ligase (baiB), 3α-dehydroxylase (baiA), 7α-dehydroxylase (baiCD and baiE with different stereospecificities), 7β-dehydroxylase (baiH and baiI), and presumed BA-CoA hydrolase (baiF) activity (5). Ketohydroxyl BAs, formed by oxidoreduction, are also the result of bacterial dehydrogenation that can be produced in certain physiologic scenarios, the formation of which is influenced by oxygen and cofactor availability as well as pH.
BA Receptors
Along with being physiological detergents that aid in lipophilic molecule metabolism, BAs regulate a wide array of bioenergetic and cell signaling pathways through both nuclear and G protein-coupled receptors with different potencies (37).
The farnesoid X receptor (FXR) is a nuclear receptor that acts as a BA sensor critical in regulating BA synthesis and transport. FXR is highly expressed in the liver, intestine, and kidneys and the adrenal gland (38, 39). In the intestine, FXR controls BA availability by regulating expression of apical bile salt transporter (ASBT) and basolateral organic solute transporters-α and -β (OSTA/OSTB) (40). FXR activation also regulates intestinal secretion of FGF 15/19 (41, 42), a circulating enterokine that inhibits expression of hepatic CYP7A1. Whole body FXR KO mice exhibit worsened oral glucose tolerance (OGT) and lower glucose disposal than the wild type (WT) (43–45). Surprisingly, both agonism and antagonism of intestinal FXR in mice show beneficial effects. The gut-restricted FXR agonist fexaramine improved glycemia and reduced weight gain in mice (46, 47) while TβMCA and glycine-β-muricholate (Gly-MCA) antagonize FXR activity to improve insulin tolerance, and reduce OGT and fasting insulin levels (48). Furthermore, the antioxidant Tempol (4-hydroxy-2, 2, 6, 6-tetramethylpiperidine 1-oxyl) altered gut microbiota and BA composition, increased levels of TβMCA, and decreased FXR signaling (29). Fexaramine treatment increases LCA-producing Acetatifactor and Bacteroides, while Tempol decreases Lactobacillus and BSH activity (49). In liver, FXR activation stimulates expression of the canalicular bile salt export pump (BSEP, ACBC11). Hepatic FXR activation results in stimulation of glycogen synthesis (50) and triglyceride clearance (51–53) and inhibition of gluconeogenesis (44, 54, 55) and lipogenesis (45). This FXR signaling spans several pathways that include enhanced transintestinal cholesterol excretion via enhanced increased expression of ATP binding cassette subfamily G member 5/8 (ABCG5/8) (56); released inhibition of glucagon-like peptide-1 (GLP-1; encoded by proglucagon; Gcg) expression in enteroendocrine cells (57); and decreased hepatic glucose production resulting from the muricholic acid-induced depletions of ceramides (58). CDCA is the most efficacious endogenous FXR ligand (CDCA > DCA > LCA > CA) (59), whereas hydrophilic bile acids, such as UDCA and MCAs, do not activate FXR (35).
FXR function is modulated by phosphorylation (60), acetylation (61), sumoylation (62), O-GlcNAcylation (63), and alternative splicing (64, 65). In humans and rodents, the FXR gene encodes four isoforms (FXRα1-α4) (64–66) that are differentially expressed in tissues. Isoform pairs Fxrα1/α2 are transcribed from a common promoter but differ in that Fxrα1 contains an extra 12 nucleotides (encoding MYTG, orange) resulting from splicing events at exon 5. Splicing events skipping exon 3 encoding the longer activation function (AF-1) domain (teal) generate Fxrα3 and Fxrα4. Human livers primarily express Fxrα1/α2 isoforms, whereas intestines express Fxrα3/α4 (64). Expression of these isoforms is somehow altered in pathophysiologic states. For example, the Fxrα2/Fxrα1 ratio is decreased in human livers and tumors of hepatocellular carcinoma patients (67), while it is upregulated upon fasting and exercise in mice (68). More importantly, FXR isoforms regulate transcription of target genes in an isoform-specific manner (65, 69). Stable expression of Fxrα2 and Fxrα4 by liver-specific adeno-associated viral vectors revealed Fxrα2-specific reductions in high-density lipoprotein, increased neutral sterol expression, and trans-repressed Cyp8b1, which greatly decreased thehydrophobicity of the BA pool (70). Future studies are needed to examine the relationships between FXR isoform expression, host physiology, and the gut microbiome.
FXR plays a critical role in intestinal bacterial growth (40). WT and FXR-deficient mice raised in conventional or germ-free conditions showed differential accumulation of BAs and an abolishment of FXR signaling in the ileum (71). Fecal microbial transfer (FMT) of human or mouse feces into germ-free mice showed that mice with human bacteria produced relatively less TβMCA in both the liver and gall bladder, as well as the portal and caval veins. In addition, fewer secondary BAs were observed from MCA than mice who received mouse feces (72). In mice, FXR deficiency enriched Desulfovibrionaceae, Deferribacteraceae, and Helicobacteraceae and increased hepatic taurine-conjugated CA and β-MCA as well as hepatic and serum lipids (73). Treatment of mice with VSL#3, a commercial mixture of Lactobacilli (L. casei, L. plantarum, L. acidophilus, and L. delbrueckii), Bifidobacteria (B. longum, B. breve, and B. infantis), and Streptococcus (S. salivarius), promoted BA deconjugation and enhanced fecal BA excretion (74). In mice with mucosal injury caused by bile duct ligation FXR deficiency, intestinal FXR activation inhibited bacterial overgrowth and blocked bacteria-induced mucosal injury (75). In humans, administration of obeticholic acid, a BA analog and potent FXR agonist, led to a reversible induction of gram-positive bacteria and suppression of endogenous BA synthesis (76).
The vitamin D receptor (VDR) is involved in immunity, cellular growth, insulin secretion, and detoxification of secondary bile acids (77). VDR has an affinity for the following BAs: dehydro-LCA > LCA > CDCA > DCA (59). VDR is more sensitive than other receptors to LCA and 3-oxo-LCA and upon activation induces expression CYP3A genes, cytochrome P450 enzymes responsible for detoxifying LCA in the liver and intestine (78). Genome-wide association studies of gut microbiota from 1,812 individuals identified differences in beta-diversity that associated with the VDR gene (79). In the same study, VDR-deficient mice exhibit shifts in microbial flora that associated with changes in serum BAs, fatty acids. These studies supported earlier findings where Lactobacillus were depleted and Clostridium and Bacteroidetes species were enriched in Vdr-/- mice (80). Schmidt et al. (81) reported that Vdr-/- mice have an enlarged (∼30%) BA pool at 3 mo old and more than twice the amount at 6 mo of age. In addition, increased expression of FGF15 was linked with VDR activation. Highlighting its role in tumorigenesis, intestinal deletion of VDR resulted in shifts in bacterial taxa abundance that correlated with increased intestinal tumor burden and secondary BAs and activated JAK/STAT signaling (82).
Pregnane X receptor (PXR) is a well-known orphan nuclear receptor that regulates gene expression in response to xenobiotic and BA exposure (83, 84). PXR also plays important roles in counteracting inflammation (85). PXR has an affinity for the following BAs: LCA > DCA > CA (86). As FXR is unresponsive to LCA, LCA signaling through PXR provides an important sensory role in catabolism of BAs, particularly in a setting of LCA accumulation (87) or cholestasis (88). In clinical studies, it was revealed that patients with inflammatory bowel disease (IBD) had downregulated PXR in inflamed tissue (59). In germ-free mice versus conventionally housed mice, PXR was among those genes most differentially expressed, being lower in GF mice then conventionally housed mice (89). When adequately expressed, PXR acts as a sensor for both BA dysregulation and bacterial derived metabolites such indole 3-propionic acid (90), thereby potentiating immune responses in the host.
Takeda G-protein receptor 5 (TGR5), also known as G protein-coupled bile acid receptor (Gpbar1) is cell surface BA receptor that imparts the nongenomic actions of BAs. TGR5 has an affinity for the following BAs: DCA > LCA > CDCA > CA (86). TGR5 is expressed in monocytes, enteroendocrine cells, adipose tissue, smooth and skeletal muscle, pancreas, and the central nervous system (91–99). Through kinase signaling pathways, TGR5 activation stimulates gallbladder filling (100), modulates energy expenditure (99), stimulates GLP-1 release from intestinal L cells (91, 98, 101), suppresses hepatic glycogenolysis (102), and reduces inflammation and macrophage activation (92, 103–106). TGR5-/- mice have mildly reduced BA pools (107, 108), impaired glucose tolerance (98), and exacerbated inflammatory responses (109). TGR5 has an anti-inflammatory function in the intestine by inhibiting proinflammatory cytokine production in macrophages and modifying innate immune responses (110).
Bile Salts as Modulators of Microbiome-Host Interactions
Alterations in gut microbiota and bile acids are associated with diet, age, antibiotic exposure, as well as the onset and remission of disease. Below are vignettes highlighting the interactions among BAs, microbiota, and host metabolism.
Diet
High-fat diet determines the composition of the murine gut microbiome independently of obesity and is an important determinant of gut microbiota composition (111). In turn, the gut microbiota drives the impact of dietary fat and bile acids on metabolism (112). Western diets composed of highly refined carbohydrate (113) and fat (114) have been attributed to the prevalence of obesity and metabolic inflammation (115). Certain dietary fats present in Western diets have a greater capacity to precipitate intestinal inflammation through their actions on the enteric microbiota of genetically susceptible hosts (115). In particular, studies in mice have shown that milk fat promotes colonic inflammation by promoting taurine conjugation with bile acids and increases the availability of organic sulfur used by the bacteria. This form of fat, when coadministered with high sugar, particularly highly refined sugars such as fructose and glucose (116), represents a Westernized diet that is a potent mediator of metabolic derangement (117). Transplantation of gut communities from obese mice fed a Western diet into germ-free mice induced adiposity in recipient mice within 2 wk (118). These conventionalization (i.e., microbiota transfer by feeding fecal material) experiments have shown that the pathogenesis of obesity can be transferred from obese mice or humans to healthy mice (119, 120).
Nonalcoholic Fatty Liver Disease
Nonalcoholic fatty liver disease (NAFLD) is one of the most prevalent forms of chronic liver disease in the United States (121). The spectrum of NAFLD ranges from simple steatosis alone to nonalcoholic steatohepatitis (NASH) to cirrhosis and occurs frequently in the setting of obesity, dyslipidemia, hyperinsulinemia, and insulin resistance (122–125). Gut dysbiosis has been ascribed often to NAFLD (126, 127) and may afford predictive power in identifying NAFLD-cirrhosis (128). Altered BA profiles including increased total primary BAs and decreased secondary BAs characterize NASH; a higher ratio of total secondary to primary BA ratio decreased the odds of fibrosis (129). Similarly, BA derivatives and compounds that influence BA-related signaling have emerged as potentially useful therapeutic agents for NAFLD and NASH (130, 131). For more information on the role of gut microbiota in liver disease we refer readers to an excellent recent review (132).
Diabetes and Diabetes Resolution after Bariatric Surgery
Gut microbiota and BAs exert considerable influence over insulin sensitivity (57, 133, 134) and enteroendocrine cell hormone release (135). We (135–137) and others have shown that many of the metabolic improvements after bariatric surgery in humans (136, 138–141) and rodents (135, 137, 142, 143) can be attributed to the prevailing bile acid (BA) profile. Surgeries including ileal transposition (144), Roux-en Y gastric bypass (RYGB) (145, 146), vertical sleeve gastrectomy (VSG) (147, 148), and biliary diversion (135, 137) markedly change the gut microbiome. We recently employed fecal metagenomics and metabolomics to understand the impact of bariatric surgery on gastrointestinal microbiota (149). In 21 RYGB and VSG patients assessed at 1 wk, 1 mo, and/or months postoperative, the alpha-diversity and abundance of bacteria with aero-tolerant, probiotic, or inflammatory/anti-diabetic properties (e.g., A. muciniphila) were increased, while Bacteroidetes were decreased. Beta-diversity and fecal metabolites, including indoles, butyrate, and secondary BAs, were also changed. These findings support our earlier work reporting longitudinal increases in secondary BAs in humans after RYGB (136). The postoperative increases in A. muciniphila are also consistent with those we observed in mice where TβMCA was increased after surgical diversion of gallbladder bile to the terminal ileum (135, 137) and in humans where A. muciniphila and GUDCA were increased after treatment with the insulin-sensitizer metformin (150).
Inflammation
Importantly, bacterial BA metabolism also plays a key role in modulating T-cell innate immunity by controlling the balance of proinflammatory T-helper cells and anti-inflammatory Treg cells in both the intestine (151, 152) and periphery (153, 154). Derivatives of lithocholic acid (LCA), 3-oxoLCA and isoalloLCA, induce peripheral Tregs by increasing FoxP3 expression (155). They also inhibit the differentiation of T-helper cells that express IL-17a (TH17) cells by directly binding to the retinoid-related orphan receptor-γt (RORγt) transcription factor (156). IsoalloLCA also increased the differentiation of Treg cells by increasing expression of FoxP3 and binding at an intronic enhancer, CNS3. It has also been reported that another secondary bile acid, 3β-hydroxydeoxycholic acid, increased FoxP3 induction by acting on dendritic cells, probably via interaction with FXR (155).
Inflammatory Bowel Disease
IBD-associated dysbiosis was characterized by a decrease in the ratio of Faecalibacterium prausntizii and Escherichia coli. BA deconjugation, transformation, and desulfation are reportedly impaired in fecal bacteria such that conjugated BAs levels are significantly higher and secondary BAs are lower in active IBD patients (157). These BA differences were recently demonstrated to impact PXR and FXR activation consequently reducing CYP3A4 activity and reducing FGF19 expression (158). Differences in the ratio of primary to secondary BAs have also been observed in subjects with ulcerative colitis versus non-IBD controls (159). Likewise, patients with Crohn’s disease exhibited significantly higher bile UDCA and decreased BA pool sizes (160).
Cancer
Colorectal cancer (CRC) is the third most common cancer diagnosis and the fourth leading cause of cancer-related death worldwide (161). Obesity, and in particular the increased intake of dietary fat (162) and the decreased intake of fiber (163), is associated with increased risk of CRC. Gut dysbiosis and secondary BAs appear to drive proinflammatory responses and epithelial cell transformation, leading to cancer or may be permissive for engraftment of opportunistic allogenic species that are keystone pathogens. In support of this, genetically predisposed mice developed fewer tumors under germ-free conditions than conventionally raised mice (164). Alterations in both fecal microbiota (165, 166) and fecal BAs (167, 168) are associated with CRC. While enhanced DCA and LCA as well as increased levels of Fusobacterium and Campylobacter species are elevated in CRC (169, 170) and in some studies with resistance to CRC chemotherapy (171), it remains unclear if such associations are causal or of consequence. Recent evidence showing that intestinal FXR knockout (KO) mice have increased susceptibility to intestinal cancer (172), particularly those cancers associated with inflammation (173), and that BAs promote protumorigenic phenotypes (174) provides fertile ground for further examining the contributions of BAs to CRC.
Neurologic Disorders
The emergence of gut bacteria and bacterial metabolites as functional modifiers of the central nervous system suggest an equally important role for endosymbiotic bacteria and bile acids in host regulation at a higher level. Gut dysbiosis in a 5xFAD mouse model of Alzheimer’s disease increased C/EBPβ/AEP signaling in the brain concomitant with age-related progression of disease severity and antibiotic treatment diminished this signaling, attenuated amyloidogenic processes, and improved cognitive functions (175). These effects were reversed with antibiotic administration. Autistic individuals exhibit maldigestion and malabsorption disorders with symptoms suggesting impaired carbohydrate digestion. Here, restoring the relative deficiency of Prevotella (176) or conjugated BAs (177) may afford therapeutic relief. Finally, dysregulated bile acid metabolism is also a component of multiple sclerosis (MS) and supplementation with tauroursodeoxycholic acid prevents astrocyte and microglia polarization and ameliorated neuropathology in animal models of MS.
Conclusions and Perspectives
The evidence reviewed here indicates that BA composition and gut bacteria are tightly coupled and together play key etiologic roles in regulating host metabolism. Experiments in rodent model systems offer important mechanistic clues about the metabolic interplay between BAs and receptors; however, major differences in enzyme activity and therefore BA species between humans and mice often complicate clinical translations. The recent development of mice with humanized BA metabolism provides new opportunities to reexamine earlier findings where observations in rats and mice occurred in tandem with changes in rodent-specific BAs, such as MCAs, complicating clinical translation. Underscoring this are recent findings in mice with a humanized BA profile showing that despite very different BA compositions, the build of the commensal microbiome was largely the same (16). While additional functional studies are needed to better understand such differences, these observations suggest that BA composition and gut bacteria may not be inextricably linked.
Acknowledgments
The National Institute of Diabetes and Digestive and Kidney Diseases Grants DK105847 and DK020593 (Vanderbilt Diabetes Research and Training Center) supported this work .
No conflicts of interest, financial or otherwise, are declared by the authors.
C.R.F. conceived and designed research; C.R.F. performed experiments; C.R.F. analyzed data; C.R.F. interpreted results of experiments; C.R.F. prepared figures; J.C.P. and C.R.F. drafted manuscript; J.C.P. and C.R.F. edited and revised manuscript; J.C.P. and C.R.F. approved final version of manuscript.
References
- 1.Hofmann AF. Biliary secretion and excretion in health and disease: current concepts. Ann Hepatol 6: 15–27, 2007. doi: 10.1016/S1665-2681(19)31949-0. [DOI] [PubMed] [Google Scholar]
- 2.Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev 83: 633–671, 2003. doi: 10.1152/physrev.00027.2002. [DOI] [PubMed] [Google Scholar]
- 3.Le Martelot G, Claudel T, Gatfield D, Schaad O, Kornmann B, Lo Sasso G, Moschetta A, Schibler U. REV-ERBalpha participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol 7: e1000181, 2009. doi: 10.1371/journal.pbio.1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma K, Xiao R, Tseng HT, Shan L, Fu L, Moore DD. Circadian dysregulation disrupts bile acid homeostasis. PLoS One 4: e6843, 2009. doi: 10.1371/journal.pone.0006843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res 47: 241–259, 2006. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Quinn RA, Melnik AV, Vrbanac A, Fu T, Patras KA, Christy MP, et al. Global chemical effects of the microbiome include new bile-acid conjugations. Nature 579: 123–129, 2020. doi: 10.1038/s41586-020-2047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heuman DM. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J Lipid Res 30: 719–730, 1989. doi: 10.1016/S0022-2275(20)38331-0. [DOI] [PubMed] [Google Scholar]
- 8.Li T, Chiang JY. Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev 66: 948–983, 2014. doi: 10.1124/pr.113.008201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roda A, Minutello A, Angellotti MA, Fini A. Bile acid structure-activity relationship: evaluation of bile acid lipophilicity using 1-octanol/water partition coefficient and reverse phase HPLC. J Lipid Res 31: 1433–1443, 1990. doi: 10.1016/S0022-2275(20)42614-8. [DOI] [PubMed] [Google Scholar]
- 10.Makino I, Hashimoto H, Shinozaki K, Yoshino K, Nakagawa S. Sulfated and nonsulfated bile acids in urine, serum, and bile of patients with hepatobiliary diseases. Gastroenterology 68: 545–553, 1975. doi: 10.1016/S0016-5085(75)80094-1. [DOI] [PubMed] [Google Scholar]
- 11.Hofmann AF, Loening-Baucke V, Lavine JE, Hagey LR, Steinbach JH, Packard CA, Griffin TL, Chatfield DA. Altered bile acid metabolism in childhood functional constipation: inactivation of secretory bile acids by sulfation in a subset of patients. J Pediatr Gastroenterol Nutr 47: 598–606, 2008. doi: 10.1097/MPG.0b013e31816920a6. [DOI] [PubMed] [Google Scholar]
- 12.van Berge Henegouwen GP, Brandt KH, Eyssen H, Parmentier G. Sulphated and unsulphated bile acids in serum, bile, and urine of patients with cholestasis. Gut 17: 861–869, 1976. doi: 10.1136/gut.17.11.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi S, Fukami T, Masuo Y, Brocker CN, Xie C, Krausz KW, Wolf CR, Henderson CJ, Gonzalez FJ. Cyp2c70 is responsible for the species difference in bile acid metabolism between mice and humans. J Lipid Res 57: 2130–2137, 2016. doi: 10.1194/jlr.M071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem 72: 137–174, 2003. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 15.Vlahcevic X, Heuman D, Pb H. Physiology and Pathophysiology of Enterohepatic Circulation of Bile Acids (3rd ed.). Philadelphia, PA: Saunders, vol. 1, 1996. [Google Scholar]
- 16.de Boer JF, Verkade E, Mulder NL, de Vries HD, Huijkman N, Koehorst M, Boer T, Wolters JC, Bloks VW, van de Sluis B, Kuipers F. A human-like bile acid pool induced by deletion of hepatic Cyp2c70 modulates effects of FXR activation in mice. J Lipid Res 61: 291–305, 2020. doi: 10.1194/jlr.RA119000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honda A, Miyazaki T, Iwamoto J, Hirayama T, Morishita Y, Monma T, Ueda H, Mizuno S, Sugiyama F, Takahashi S, Ikegami T. Regulation of bile acid metabolism in mouse models with hydrophobic bile acid composition. J Lipid Res 61: 54–69, 2020. doi: 10.1194/jlr.RA119000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Straniero S, Laskar A, Savva C, Hardfeldt J, Angelin B, Rudling M. Of mice and men: murine bile acids explain species differences in the regulation of bile acid and cholesterol metabolism. J Lipid Res 61: 480–491, 2020. doi: 10.1194/jlr.RA119000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minemura M, Shimizu Y. Gut microbiota and liver diseases. World J Gastroenterol 21: 1691–1702, 2015. doi: 10.3748/wjg.v21.i6.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Best N, Rolle-Kampczyk U, Schaap FG, Basic M, Olde Damink SW, Bleich A, Savelkoul PH, von Bergen M, Penders J, Hornef MW. Bile acids drive the newborn's gut microbiota maturation. Nat Commun 11: 3692, 2020. doi: 10.1038/s41467-020-17183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab 24: 41–50, 2016. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Moser SA, Savage DC. Bile salt hydrolase activity and resistance to toxicity of conjugated bile salts are unrelated properties in Lactobacilli. Appl Environ Microbiol 67: 3476–3480, 2001. doi: 10.1128/AEM.67.8.3476-3480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones BV, Begley M, Hill C, Gahan CG, Marchesi JR. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci U S A 105: 13580–13585, 2008. doi: 10.1073/pnas.0804437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odermatt A, Da Cunha T, Penno CA, Chandsawangbhuwana C, Reichert C, Wolf A, Dong M, Baker ME. Hepatic reduction of the secondary bile acid 7-oxolithocholic acid is mediated by 11beta-hydroxysteroid dehydrogenase 1. Biochem J 436: 621–629, 2011. doi: 10.1042/BJ20110022. [DOI] [PubMed] [Google Scholar]
- 25.Chavez-Talavera O, Tailleux A, Lefebvre P, Staels B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology 152: 1679–1694, 2017. doi: 10.1053/j.gastro.2017.01.055. [DOI] [PubMed] [Google Scholar]
- 26.Das P, Marcisauskas S, Ji B, Nielsen J. Metagenomic analysis of bile salt biotransformation in the human gut microbiome. BMC Genomics 20: 517, 2019. doi: 10.1186/s12864-019-5899-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Groot P, Scheithauer T, Bakker GJ, Prodan A, Levin E, Khan MT, Herrema H, Ackermans M, Serlie MJ, de Brauw M, Levels JH, Sales A, Gerdes VE, Stahlman M, Schimmel AW, Dallinga-Thie G, Bergman JJ, Holleman F, Hoekstra JB, Groen A, Backhed F, Nieuwdorp M. Donor metabolic characteristics drive effects of faecal microbiota transplantation on recipient insulin sensitivity, energy expenditure and intestinal transit time. Gut 69: 502–512, 2020. doi: 10.1136/gutjnl-2019-318320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labbe A, Ganopolsky JG, Martoni CJ, Prakash S, Jones ML. Bacterial bile metabolising gene abundance in Crohn's, ulcerative colitis and type 2 diabetes metagenomes. PLoS One 9: e115175, 2014. doi: 10.1371/journal.pone.0115175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li F, Jiang C, Krausz KW, Li Y, Albert I, Hao H, Fabre KM, Mitchell JB, Patterson AD, Gonzalez FJ. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun 4: 2384, 2013. doi: 10.1038/ncomms3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JY, Arai H, Nakamura Y, Fukiya S, Wada M, Yokota A. Contribution of the 7beta-hydroxysteroid dehydrogenase from Ruminococcus gnavus N53 to ursodeoxycholic acid formation in the human colon. J Lipid Res 54: 3062–3069, 2013. doi: 10.1194/jlr.M039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridlon JM, Alves JM, Hylemon PB, Bajaj JS. Cirrhosis, bile acids and gut microbiota: unraveling a complex relationship. Gut Microbes 4: 382–387, 2013. doi: 10.4161/gmic.25723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staley C, Weingarden AR, Khoruts A, Sadowsky MJ. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl Microbiol Biotechnol 101: 47–64, 2017. doi: 10.1007/s00253-016-8006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stenman LK, Holma R, Eggert A, Korpela R. A novel mechanism for gut barrier dysfunction by dietary fat: epithelial disruption by hydrophobic bile acids. Am J Physiol Gastrointest Liver Physiol 304: G227–G234, 2013. doi: 10.1152/ajpgi.00267.2012. [DOI] [PubMed] [Google Scholar]
- 34.Kang JD, Myers CJ, Harris SC, Kakiyama G, Lee IK, Yun BS, Matsuzaki K, Furukawa M, Min HK, Bajaj JS, Zhou H, Hylemon PB. Bile acid 7alpha-dehydroxylating gut bacteria secrete antibiotics that inhibit clostridium difficile: role of secondary bile acids. Cell Chem Biol 26: 27–34, 2019. doi: 10.1016/j.chembiol.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li T, Chiang JY. Nuclear receptors in bile acid metabolism. Drug Metab Rev 45: 145–155, 2013. doi: 10.3109/03602532.2012.740048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moschetta A, Bookout AL, Mangelsdorf DJ. Prevention of cholesterol gallstone disease by FXR agonists in a mouse model. Nat Med 10: 1352–1358, 2004. doi: 10.1038/nm1138. [DOI] [PubMed] [Google Scholar]
- 37.Chiang JY. Bile acids: regulation of synthesis. J Lipid Res 50: 1955–1966, 2009. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW, Evans RM, Weinberger C. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell 81: 687–693, 1995. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 39.Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM. Bile acids: natural ligands for an orphan nuclear receptor. Science 284: 1365–1368, 1999. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 40.Lee FY, Lee H, Hubbert ML, Edwards PA, Zhang Y. FXR, a multipurpose nuclear receptor. Trends Biochem Sci 31: 572–580, 2006. doi: 10.1016/j.tibs.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Jones S. Mini-review: endocrine actions of fibroblast growth factor 19. Mol Pharm 5: 42–48, 2008. doi: 10.1021/mp700105z. [DOI] [PubMed] [Google Scholar]
- 42.Kir S, Kliewer SA, Mangelsdorf DJ. Roles of FGF19 in liver metabolism. Cold Spring Harb Symp Quant Biol 76: 139–144, 2011. doi: 10.1101/sqb.2011.76.010710. [DOI] [PubMed] [Google Scholar]
- 43.Cariou B, van Harmelen K, Duran-Sandoval D, van Dijk TH, Grefhorst A, Abdelkarim M, Caron S, Torpier G, Fruchart JC, Gonzalez FJ, Kuipers F, Staels B. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J Biol Chem 281: 11039–11049, 2006. doi: 10.1074/jbc.M510258200. [DOI] [PubMed] [Google Scholar]
- 44.Ma K, Saha PK, Chan L, Moore DD. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest 116: 1102–1109, 2006. doi: 10.1172/JCI25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, Willson TM, Edwards PA. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A 103: 1006–1011, 2006. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, Yoshihara E, Perino A, Jacinto S, Lukasheva Y, Atkins AR, Khvat A, Schnabl B, Yu RT, Brenner DA, Coulter S, Liddle C, Schoonjans K, Olefsky JM, Saltiel AR, Downes M, Evans RM. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med 21: 159–165, 2015. doi: 10.1038/nm.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pathak P, Xie C, Nichols RG, Ferrell JM, Boehme S, Krausz KW, Patterson AD, Gonzalez FJ, Chiang JY. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology 68: 1574–1588, 2018. doi: 10.1002/hep.29857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang C, Xie C, Lv Y, Li J, Krausz KW, Shi J, Brocker CN, Desai D, Amin SG, Bisson WH, Liu Y, Gavrilova O, Patterson AD, Gonzalez FJ. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat Commun 6: 10166, 2015. doi: 10.1038/ncomms10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gonzalez FJ, Jiang C, Patterson AD. An intestinal microbiota-farnesoid X receptor axis modulates metabolic disease. Gastroenterology 151: 845–859, 2016. doi: 10.1053/j.gastro.2016.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K, Xu HE, Shulman GI, Kliewer SA, Mangelsdorf DJ. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science 331: 1621–1624, 2011. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prawitt J, Abdelkarim M, Stroeve JH, Popescu I, Duez H, Velagapudi VR, Dumont J, Bouchaert E, van Dijk TH, Lucas A, Dorchies E, Daoudi M, Lestavel S, Gonzalez FJ, Oresic M, Cariou B, Kuipers F, Caron S, Staels B. Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes 60: 1861–1871, 2011. doi: 10.2337/db11-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102: 731–744, 2000. doi: 10.1016/S0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 53.Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, Moore DD, Auwerx J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest 113: 1408–1418, 2004. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cipriani S, Mencarelli A, Palladino G, Fiorucci S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J Lipid Res 51: 771–784, 2010. doi: 10.1194/jlr.M001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Potthoff MJ, Boney-Montoya J, Choi M, He T, Sunny NE, Satapati S, Suino-Powell K, Xu HE, Gerard RD, Finck BN, Burgess SC, Mangelsdorf DJ, Kliewer SA. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1alpha pathway. Cell Metab 13: 729–738, 2011. doi: 10.1016/j.cmet.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Boer JF, Schonewille M, Boesjes M, Wolters H, Bloks VW, Bos T, van Dijk TH, Jurdzinski A, Boverhof R, Wolters JC, Kuivenhoven JA, van Deursen JM, Oude Elferink RP, Moschetta A, Kremoser C, Verkade HJ, Kuipers F, Groen AK. Intestinal farnesoid X receptor controls transintestinal cholesterol excretion in mice. Gastroenterology 152: 1126–1138, 2017. doi: 10.1053/j.gastro.2016.12.037. [DOI] [PubMed] [Google Scholar]
- 57.Trabelsi MS, Daoudi M, Prawitt J, Ducastel S, Touche V, Sayin SI, Perino A, Brighton CA, Sebti Y, Kluza J, Briand O, Dehondt H, Vallez E, Dorchies E, Baud G, Spinelli V, Hennuyer N, Caron S, Bantubungi K, Caiazzo R, Reimann F, Marchetti P, Lefebvre P, Bäckhed F, Gribble FM, Schoonjans K, Pattou F, Tailleux A, Staels B, Lestavel S. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nat Commun 6: 7629, 2015. doi: 10.1038/ncomms8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xie C, Jiang C, Shi J, Gao X, Sun D, Sun L, Wang T, Takahashi S, Anitha M, Krausz KW, Patterson AD, Gonzalez FJ. An intestinal farnesoid X receptor-ceramide signaling axis modulates hepatic gluconeogenesis in mice. Diabetes 66: 613–626, 2017. doi: 10.2337/db16-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dring MM, Goulding CA, Trimble VI, Keegan D, Ryan AW, Brophy KM, Smyth CM, Keeling PW, O'Donoghue D, O'Sullivan M, O'Morain C, Mahmud N, Wikstrom AC, Kelleher D, McManus R. The pregnane X receptor locus is associated with susceptibility to inflammatory bowel disease. Gastroenterology 130: 341–348, 2006. doi: 10.1053/j.gastro.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 60.Gineste R, Sirvent A, Paumelle R, Helleboid S, Aquilina A, Darteil R, Hum DW, Fruchart JC, Staels B. Phosphorylation of farnesoid X receptor by protein kinase C promotes its transcriptional activity. Mol Endocrinol 22: 2433–2447, 2008. doi: 10.1210/me.2008-0092. [DOI] [PubMed] [Google Scholar]
- 61.Kemper JK, Xiao Z, Ponugoti B, Miao J, Fang S, Kanamaluru D, Tsang S, Wu SY, Chiang CM, Veenstra TD. FXR acetylation is normally dynamically regulated by p300 and SIRT1 but constitutively elevated in metabolic disease states. Cell Metab 10: 392–404, 2009. doi: 10.1016/j.cmet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim DH, Xiao Z, Kwon S, Sun X, Ryerson D, Tkac D, Ma P, Wu SY, Chiang CM, Zhou E, Xu HE, Palvimo JJ, Chen LF, Kemper B, Kemper JK. A dysregulated acetyl/SUMO switch of FXR promotes hepatic inflammation in obesity. EMBO J 34: 184–199, 2015. doi: 10.15252/embj.201489527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berrabah W, Aumercier P, Gheeraert C, Dehondt H, Bouchaert E, Alexandre J, Ploton M, Mazuy C, Caron S, Tailleux A, Eeckhoute J, Lefebvre T, Staels B, Lefebvre P. Glucose sensing O-GlcNAcylation pathway regulates the nuclear bile acid receptor farnesoid X receptor (FXR). Hepatology 59: 2022–2033, 2014. doi: 10.1002/hep.26710. [DOI] [PubMed] [Google Scholar]
- 64.Vaquero J, Monte MJ, Dominguez M, Muntane J, Marin JJ. Differential activation of the human farnesoid X receptor depends on the pattern of expressed isoforms and the bile acid pool composition. Biochem Pharmacol 86: 926–939, 2013. doi: 10.1016/j.bcp.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y, Kast-Woelbern HR, Edwards PA. Natural structural variants of the nuclear receptor farnesoid X receptor affect transcriptional activation. J Biol Chem 278: 104–110, 2003. doi: 10.1074/jbc.M209505200. [DOI] [PubMed] [Google Scholar]
- 66.Huber RM, Murphy K, Miao B, Link JR, Cunningham MR, Rupar MJ, Gunyuzlu PL, Haws TF, Kassam A, Powell F, Hollis GF, Young PR, Mukherjee R, Burn TC. Generation of multiple farnesoid-X-receptor isoforms through the use of alternative promoters. Gene 290: 35–43, 2002. doi: 10.1016/S0378-1119(02)00557-7. [DOI] [PubMed] [Google Scholar]
- 67.Chen Y, Song X, Valanejad L, Vasilenko A, More V, Qiu X, Chen W, Lai Y, Slitt A, Stoner M, Yan B, Deng R. Bile salt export pump is dysregulated with altered farnesoid X receptor isoform expression in patients with hepatocellular carcinoma. Hepatology 57: 1530–1541, 2013. doi: 10.1002/hep.26187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Correia JC, Massart J, de Boer JF, Porsmyr-Palmertz M, Martinez-Redondo V, Agudelo LZ, Sinha I, Meierhofer D, Ribeiro V, Bjornholm M, Sauer S, Dahlman-Wright K, Zierath JR, Groen AK, Rua JL. Bioenergetic cues shift FXR splicing towards FXRalpha2 to modulate hepatic lipolysis and fatty acid metabolism. Mol Metab 4: 891–902, 2015. doi: 10.1016/j.molmet.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anisfeld AM, Kast-Woelbern HR, Meyer ME, Jones SA, Zhang Y, Williams KJ, Willson T, Edwards PA. Syndecan-1 expression is regulated in an isoform-specific manner by the farnesoid-X receptor. J Biol Chem 278: 20420–20428, 2003. doi: 10.1074/jbc.M302505200. [DOI] [PubMed] [Google Scholar]
- 70.Boesjes M, Bloks VW, Hageman J, Bos T, van Dijk TH, Havinga R, Wolters H, Jonker JW, Kuipers F, Groen AK. Hepatic farnesoid X-receptor isoforms alpha2 and alpha4 differentially modulate bile salt and lipoprotein metabolism in mice. PLoS One 9: e115028, 2014. doi: 10.1371/journal.pone.0115028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sayin SI, Wahlstrom A, Felin J, Jantti S, Marschall HU, Bamberg K, Angelin B, Hyotylainen T, Oresic M, Backhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 17: 225–235, 2013. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 72.Wahlstrom A, Kovatcheva-Datchary P, Stahlman M, Khan MT, Backhed F, Marschall HU. Induction of farnesoid X receptor signaling in germ-free mice colonized with a human microbiota. J Lipid Res 58: 412–419, 2017. doi: 10.1194/jlr.M072819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sheng L, Jena PK, Liu HX, Kalanetra KM, Gonzalez FJ, French SW, Krishnan VV, Mills DA, Wan YY. Gender differences in bile acids and microbiota in relationship with gender dissimilarity in steatosis induced by diet and FXR inactivation. Sci Rep 7: 1748, 2017. doi: 10.1038/s41598-017-01576-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Degirolamo C, Rainaldi S, Bovenga F, Murzilli S, Moschetta A. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr-Fgf15 axis in mice. Cell Rep 7: 12–18, 2014. doi: 10.1016/j.celrep.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 75.Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ, Mangelsdorf DJ, Kliewer SA. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A 103: 3920–3925, 2006. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Friedman ES, Li Y, Shen TD, Jiang J, Chau L, Adorini L, Babakhani F, Edwards J, Shapiro D, Zhao C, Carr RM, Bittinger K, Li H, Wu GD. FXR-dependent modulation of the human small intestinal microbiome by the bile acid derivative obeticholic acid. Gastroenterology 155: 1741–1752, 2018. doi: 10.1053/j.gastro.2018.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Aguiar Vallim TQ, Tarling EJ, Edwards PA. Pleiotropic roles of bile acids in metabolism. Cell Metab 17: 657–669, 2013. doi: 10.1016/j.cmet.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ. Vitamin D receptor as an intestinal bile acid sensor. Science 296: 1313–1316, 2002. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 79.Wang J, Thingholm LB, Skiecevičienė J, Rausch P, Kummen M, Hov JR, et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat Genet 48: 1396–1406, 2016. doi: 10.1038/ng.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jin D, Wu S, Zhang YG, Lu R, Xia Y, Dong H, Sun J. Lack of vitamin D receptor causes dysbiosis and changes the functions of the murine intestinal microbiome. Clin Ther 37: 996–1009, 2015. doi: 10.1016/j.clinthera.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 81.Schmidt DR, Holmstrom SR, Fon Tacer K, Bookout AL, Kliewer SA, Mangelsdorf DJ. Regulation of bile acid synthesis by fat-soluble vitamins A and D. J Biol Chem 285: 14486–14494, 2010. doi: 10.1074/jbc.M110.116004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang YG, Lu R, Wu S, Chatterjee I, Zhou D, Xia Y, Sun J. Vitamin D receptor protects against dysbiosis and tumorigenesis via the JAK/STAT pathway in intestine. Cell Mol Gastroenterol Hepatol 10: 729–746, 2020. doi: 10.1016/j.jcmgh.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jonker JW, Liddle C, Downes M. FXR and PXR: potential therapeutic targets in cholestasis. J Steroid Biochem Mol Biol 130: 147–158, 2012. doi: 10.1016/j.jsbmb.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev 23: 687–702, 2002. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- 85.Mohandas S, Vairappan B. Role of pregnane X-receptor in regulating bacterial translocation in chronic liver diseases. World J Hepatol 9: 1210–1226, 2017. doi: 10.4254/wjh.v9.i32.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol 30: 332–338, 2014. doi: 10.1097/MOG.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ishii M, Toda T, Ikarashi N, Kusunoki Y, Kon R, Ochiai W, Machida Y, Sugiyama K. Gastrectomy increases the expression of hepatic cytochrome P450 3A by increasing lithocholic acid-producing enteric bacteria in mice. Biol Pharm Bull 37: 298–305, 2014. doi: 10.1248/bpb.b13-00824. [DOI] [PubMed] [Google Scholar]
- 88.Jung D, Mangelsdorf DJ, Meyer UA. Pregnane X receptor is a target of farnesoid X receptor. J Biol Chem 281: 19081–19091, 2006. doi: 10.1074/jbc.M600116200. [DOI] [PubMed] [Google Scholar]
- 89.Bjorkholm B, Bok CM, Lundin A, Rafter J, Hibberd ML, Pettersson S. Intestinal microbiota regulate xenobiotic metabolism in the liver. PLoS One 4: e6958, 2009. doi: 10.1371/journal.pone.0006958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, Qiu Z, Maher L, Redinbo MR, Phillips RS, Fleet JC, Kortagere S, Mukherjee P, Fasano A, Le Ven J, Nicholson JK, Dumas ME, Khanna KM, Mani S. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 41: 296–310, 2014. doi: 10.1016/j.immuni.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Habib AM, Richards P, Rogers GJ, Reimann F, Gribble FM. Co-localisation and secretion of glucagon-like peptide 1 and peptide YY from primary cultured human L cells. Diabetologia 56: 1413–1416, 2013. doi: 10.1007/s00125-013-2887-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M. A G protein-coupled receptor responsive to bile acids. J Biol Chem 278: 9435–9440, 2003. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 93.Keitel V, Reinehr R, Gatsios P, Rupprecht C, Gorg B, Selbach O, Haussinger D, Kubitz R. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology 45: 695–704, 2007. doi: 10.1002/hep.21458. [DOI] [PubMed] [Google Scholar]
- 94.Kumar DP, Rajagopal S, Mahavadi S, Mirshahi F, Grider JR, Murthy KS, Sanyal AJ. Activation of transmembrane bile acid receptor TGR5 stimulates insulin secretion in pancreatic beta cells. Biochem Biophys Res Commun 427: 600–605, 2012. doi: 10.1016/j.bbrc.2012.09.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Poole DP, Godfrey C, Cattaruzza F, Cottrell GS, Kirkland JG, Pelayo JC, Bunnett NW, Corvera CU. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil 22: 814–825, 2010. doi: 10.1111/j.1365-2982.2010.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rajagopal S, Kumar DP, Mahavadi S, Bhattacharya S, Zhou R, Corvera CU, Bunnett NW, Grider JR, Murthy KS. Activation of G protein-coupled bile acid receptor, TGR5, induces smooth muscle relaxation via both Epac- and PKA-mediated inhibition of RhoA/Rho kinase pathway. Am J Physiol Gastrointest Liver Physiol 304: G527–G535, 2013. doi: 10.1152/ajpgi.00388.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Svensson PA, Olsson M, Andersson-Assarsson JC, Taube M, Pereira MJ, Froguel P, Jacobson P. The TGR5 gene is expressed in human subcutaneous adipose tissue and is associated with obesity, weight loss and resting metabolic rate. Biochem Biophys Res Commun 433: 563–566, 2013. doi: 10.1016/j.bbrc.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 10: 167–177, 2009. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439: 484–489, 2006. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 100.Li T, Holmstrom SR, Kir S, Umetani M, Schmidt DR, Kliewer SA, Mangelsdorf DJ. The G protein-coupled bile acid receptor, TGR5, stimulates gallbladder filling. Mol Endocrinol 25: 1066–1071, 2011. doi: 10.1210/me.2010-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun 329: 386–390, 2005. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- 102.Potthoff MJ, Potts A, He T, Duarte JA, Taussig R, Mangelsdorf DJ, Kliewer SA, Burgess SC. Colesevelam suppresses hepatic glycogenolysis by TGR5-mediated induction of GLP-1 action in DIO mice. Am J Physiol Gastrointest Liver Physiol 304: G371–380, 2013. doi: 10.1152/ajpgi.00400.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Keitel V, Donner M, Winandy S, Kubitz R, Haussinger D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun 372: 78–84, 2008. doi: 10.1016/j.bbrc.2008.04.171. [DOI] [PubMed] [Google Scholar]
- 104.Lou G, Ma X, Fu X, Meng Z, Zhang W, Wang YD, Van Ness C, Yu D, Xu R, Huang W. GPBAR1/TGR5 mediates bile acid-induced cytokine expression in murine Kupffer cells. PLoS One 9: e93567, 2014. doi: 10.1371/journal.pone.0093567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, Nakamura T, Itadani H, Tanaka K. Identification of membrane-type receptor for bile acids (M-BAR.). Biochem Biophys Res Commun 298: 714–719, 2002. doi: 10.1016/S0006-291X(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 106.Wang YD, Chen WD, Wang M, Yu D, Forman BM, Huang W. Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response. Hepatology 48: 1632–1643, 2008. doi: 10.1002/hep.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maruyama T, Tanaka K, Suzuki J, Miyoshi H, Harada N, Nakamura T, Miyamoto Y, Kanatani A, Tamai Y. Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. J Endocrinol 191: 197–205, 2006. doi: 10.1677/joe.1.06546. [DOI] [PubMed] [Google Scholar]
- 108.Vassileva G, Golovko A, Markowitz L, Abbondanzo SJ, Zeng M, Yang S, Hoos L, Tetzloff G, Levitan D, Murgolo NJ, Keane K, Davis HR Jr, Hedrick J, Gustafson EL. Targeted deletion of Gpbar1 protects mice from cholesterol gallstone formation. Biochem J 398: 423–430, 2006. doi: 10.1042/BJ20060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pean N, Doignon I, Garcin I, Besnard A, Julien B, Liu B, Branchereau S, Spraul A, Guettier C, Humbert L, Schoonjans K, Rainteau D, Tordjmann T. The receptor TGR5 protects the liver from bile acid overload during liver regeneration in mice. Hepatology 58: 1451–1460, 2013. doi: 10.1002/hep.26463. [DOI] [PubMed] [Google Scholar]
- 110.Rao J, Yang C, Yang S, Lu H, Hu Y, Lu L, Cheng F, Wang X. Deficiency of TGR5 exacerbates immune-mediated cholestatic hepatic injury by stabilizing the beta-catenin destruction complex. Int Immunol 32: 321–334, 2020. doi: 10.1093/intimm/dxaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, Knight R, Ahima RS, Bushman F, Wu GD. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 137: 1716–1724, 2009. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Just S, Mondot S, Ecker J, Wegner K, Rath E, Gau L, Streidl T, Hery-Arnaud G, Schmidt S, Lesker TR, Bieth V, Dunkel A, Strowig T, Hofmann T, Haller D, Liebisch G, Gerard P, Rohn S, Lepage P, Clavel T. The gut microbiota drives the impact of bile acids and fat source in diet on mouse metabolism. Microbiome 6: 134, 2018. doi: 10.1186/s40168-018-0510-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tappy L, Le KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev 90: 23–46, 2010. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 114.Caesar R, Tremaroli V, Kovatcheva-Datchary P, Cani PD, Backhed F. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab 22: 658–668, 2015. doi: 10.1016/j.cmet.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 487: 104–108, 2012. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Payne AN, Chassard C, Lacroix C. Gut microbial adaptation to dietary consumption of fructose, artificial sweeteners and sugar alcohols: implications for host-microbe interactions contributing to obesity. Obes Rev 13: 799–809, 2012. doi: 10.1111/j.1467-789X.2012.01009.x. [DOI] [PubMed] [Google Scholar]
- 117.Asgharpour A, Cazanave SC, Pacana T, Seneshaw M, Vincent R, Banini BA, Kumar DP, Daita K, Min HK, Mirshahi F, Bedossa P, Sun X, Hoshida Y, Koduru SV, Contaifer D Jr, Warncke UO, Wijesinghe DS, Sanyal AJ. A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J Hepatol 65: 579–588, 2016. doi: 10.1016/j.jhep.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A 102: 11070–11075, 2005. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ, Ilkayeva O, Semenkovich CF, Funai K, Hayashi DK, Lyle BJ, Martini MC, Ursell LK, Clemente JC, Van Treuren W, Walters WA, Knight R, Newgard CB, Heath AC, Gordon JI. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341: 1241214, 2013. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027–1031, 2006. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 121.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 116: 1413–1419, 1999. doi: 10.1016/S0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 122.Angulo P, Alba LM, Petrovic LM, Adams LA, Lindor KD, Jensen MD. Leptin, insulin resistance, and liver fibrosis in human nonalcoholic fatty liver disease. J Hepatol 41: 943–949, 2004. doi: 10.1016/j.jhep.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 123.Kim CH, Younossi ZM. Nonalcoholic fatty liver disease: a manifestation of the metabolic syndrome. Cleve Clin J Med 75: 721–728, 2008. doi: 10.3949/ccjm.75.10.721. [DOI] [PubMed] [Google Scholar]
- 124.Pagano G, Pacini G, Musso G, Gambino R, Mecca F, Depetris N, Cassader M, David E, Cavallo-Perin P, Rizzetto M. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic association. Hepatology 35: 367–372, 2002. doi: 10.1053/jhep.2002.30690. [DOI] [PubMed] [Google Scholar]
- 125.Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol 56: 704–713, 2012. doi: 10.1016/j.jhep.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jiao N, Baker SS, Chapa-Rodriguez A, Liu W, Nugent CA, Tsompana M, Mastrandrea L, Buck MJ, Baker RD, Genco RJ, Zhu R, Zhu L. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut 67: 1881–1891, 2018. doi: 10.1136/gutjnl-2017-314307. [DOI] [PubMed] [Google Scholar]
- 127.Mouzaki M, Wang AY, Bandsma R, Comelli EM, Arendt BM, Zhang L, Fung S, Fischer SE, McGilvray IG, Allard JP. Bile acids and dysbiosis in non-alcoholic fatty liver disease. PLoS One 11: e0151829, 2016. doi: 10.1371/journal.pone.0151829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Oh TG, Kim SM, Caussy C, Fu T, Guo J, Bassirian S, Singh S, Madamba EV, Bettencourt R, Richards L, Raffatellu M, Dorrestein PC, Yu RT, Atkins AR, Huan T, Brenner DA, Sirlin CB, Knight R, Downes M, Evans RM, Loomba R. A universal gut-microbiome-derived signature predicts cirrhosis. Cell Metab 32: 878–888.e6, 2020. doi: 10.1016/j.cmet.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Puri P, Daita K, Joyce A, Mirshahi F, Santhekadur PK, Cazanave S, Luketic VA, Siddiqui MS, Boyett S, Min HK, Kumar DP, Kohli R, Zhou H, Hylemon PB, Contos MJ, Idowu M, Sanyal AJ. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology 67: 534–548, 2018. dois: 10.1002/hep.29359], . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe E, Dillon P, Pruzanski M, Shapiro D. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 145: 574–582, 2013. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 131.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, Kowdley KV, McCullough A, Terrault N, Clark JM, Tonascia J, Brunt EM, Kleiner DE, Doo E, NASH Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 385: 956–965, 2015. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jiang L, Schnabl B. Gut microbiota in liver disease: what do we know and what do we not know? Physiology (Bethesda 35: 261–274, 2020. doi: 10.1152/physiol.00005.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kuipers F, Bloks VW, Groen AK. Beyond intestinal soap-bile acids in metabolic control. Nat Rev Endocrinol 10: 488–498, 2014. doi: 10.1038/nrendo.2014.60. [DOI] [PubMed] [Google Scholar]
- 134.Sonne DP, Hansen M, Knop FK. Bile acid sequestrants in type 2 diabetes: potential effects on GLP-1 secretion. Eur J Endocrinol 171: R47–R65, 2014. doi: 10.1530/EJE-14-0154. [DOI] [PubMed] [Google Scholar]
- 135.Albaugh VL, Banan B, Antoun J, Xiong Y, Guo Y, Ping J, Alikhan M, Clements BA, Abumrad NN, Flynn CR. Role of bile acids and GLP-1 in mediating the metabolic improvements of bariatric surgery. Gastroenterology 156: 1041–1051, 2019. doi: 10.1053/j.gastro.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Albaugh VL, Flynn CR, Cai S, Xiao Y, Tamboli RA, Abumrad NN. Early increases in bile acids post Roux-en-Y gastric bypass are driven by insulin-sensitizing, secondary bile acids. J Clin Endocrinol Metab 100: E1225–1233, 2015. doi: 10.1210/jc.2015-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Flynn CR, Albaugh VL, Cai S, Cheung-Flynn J, Williams PE, Brucker RM, Bordenstein SR, Guo Y, Wasserman DH, Abumrad NN. Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery. Nat Commun 6: 7715, 2015. doi: 10.1038/ncomms8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ahmad NN, Pfalzer A, Kaplan LM. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Intl J Obesity 37: 1553–1559, 2013. doi: 10.1038/ijo.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism 58: 1400–1407, 2009. doi: 10.1016/j.metabol.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 140.Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, Badman MK, Maratos-Flier E, Mun EC, Pihlajamaki J, Auwerx J, Goldfine AB. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring 17: 1671–1677, 2009. doi: 10.1038/oby.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pournaras DJ, Glicksman C, Vincent RP, Kuganolipava S, Alaghband-Zadeh J, Mahon D, Bekker JH, Ghatei MA, Bloom SR, Walters JR, Welbourn R, Le Roux CW. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology 153: 3613–3619, 2012. doi: 10.1210/en.2011-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kohli R, Kirby M, Setchell KD, Jha P, Klustaitis K, Woollett LA, Pfluger PT, Balistreri WF, Tso P, Jandacek RJ, Woods SC, Heubi JE, Tschoep MH, D'Alessio DA, Shroyer NF, Seeley RJ. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J Physiol Gastrointest Liver Physiol 299: G652–660, 2010. doi: 10.1152/ajpgi.00221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kohli R, Setchell KD, Kirby M, Myronovych A, Ryan KK, Ibrahim SH, Berger J, Smith K, Toure M, Woods SC, Seeley RJ. A surgical model in male obese rats uncovers protective effects of bile acids post-bariatric surgery. Endocrinology 154: 2341–2351, 2013. doi: 10.1210/en.2012-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cummings BP, Bettaieb A, Graham JL, Kim J, Ma F, Shibata N, Stanhope KL, Giulivi C, Hansen F, Jelsing J, Vrang N, Kowala M, Chouinard ML, Haj FG, Havel PJ. Bile-acid-mediated decrease in endoplasmic reticulum stress: a potential contributor to the metabolic benefits of ileal interposition surgery in UCD-T2DM rats. Dis Model Mech 6: 443–456, 2013. doi: 10.1242/dmm.010421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature 489: 242–249, 2012. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 146.Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, Parameswaran P, Crowell MD, Wing R, Rittmann BE, Krajmalnik-Brown R. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A 106: 2365–2370, 2009. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Murphy R, Tsai P, Jullig M, Liu A, Plank L, Booth M. Differential changes in gut microbiota after gastric bypass and sleeve gastrectomy bariatric surgery vary according to diabetes remission. Obes Surg 27: 917–925, 2017. doi: 10.1007/s11695-016-2399-2. [DOI] [PubMed] [Google Scholar]
- 148.Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Perez HE, Sandoval DA, Kohli R, Backhed F, Seeley RJ. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 509: 183–188, 2014. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yu D, Shu X, Howard EF, Long J, English WJ, Flynn CR. Fecal metagenomics and metabolomics reveal gut microbial changes after bariatric surgery. Surg Obes Relat Dis 16: 1772–1782, 2020.doi: 10.1016/j.soard.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, Bae JW. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 63: 727–735, 2014. dois: 10.1136/gutjnl-2012-303839, 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 151.Kim KS, Hong SW, Han D, Yi J, Jung J, Yang BG, Lee JY, Lee M, Surh CD. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science 351: 858–863, 2016. doi: 10.1126/science.aac5560. [DOI] [PubMed] [Google Scholar]
- 152.Zheng W, Nurmi H, Appak S, Sabine A, Bovay E, Korhonen EA, Orsenigo F, Lohela M, D'Amico G, Holopainen T, Leow CC, Dejana E, Petrova TV, Augustin HG, Alitalo K. Angiopoietin 2 regulates the transformation and integrity of lymphatic endothelial cell junctions. Genes Dev 28: 1592–1603, 2014. doi: 10.1101/gad.237677.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504: 451–455, 2013. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nutsch K, Chai JN, Ai TL, Russler-Germain E, Feehley T, Nagler CR, Hsieh CS. Rapid and efficient generation of regulatory t cells to commensal antigens in the periphery. Cell Rep 17: 206–220, 2016. doi: 10.1016/j.celrep.2016.08.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Campbell C, McKenney PT, Konstantinovsky D, Isaeva OI, Schizas M, Verter J, Mai C, Jin WB, Guo CJ, Violante S, Ramos RJ, Cross JR, Kadaveru K, Hambor J, Rudensky AY. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 581: 475–479, 2020. doi: 10.1038/s41586-020-2193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hang S, Paik D, Yao L, Kim E, Trinath J, Lu J, Ha S, Nelson BN, Kelly SP, Wu L, Zheng Y, Longman RS, Rastinejad F, Devlin AS, Krout MR, Fischbach MA, Littman DR, Huh JR. Bile acid metabolites control TH17 and Treg cell differentiation. Nature 576: 143–148, 2019. doi: 10.1038/s41586-019-1785-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Duboc H, Rajca S, Rainteau D, Benarous D, Maubert MA, Quervain E, Thomas G, Barbu V, Humbert L, Despras G, Bridonneau C, Dumetz F, Grill JP, Masliah J, Beaugerie L, Cosnes J, Chazouilleres O, Poupon R, Wolf C, Mallet JM, Langella P, Trugnan G, Sokol H, Seksik P. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut 62: 531–539, 2013. doi: 10.1136/gutjnl-2012-302578. [DOI] [PubMed] [Google Scholar]
- 158.Wilson A, Almousa A, Teft WA, Kim RB. Attenuation of bile acid-mediated FXR and PXR activation in patients with Crohn's disease. Sci Rep 10: 1866, 2020. doi: 10.1038/s41598-020-58644-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gnewuch C, Liebisch G, Langmann T, Dieplinger B, Mueller T, Haltmayer M, Dieplinger H, Zahn A, Stremmel W, Rogler G, Schmitz G. Serum bile acid profiling reflects enterohepatic detoxification state and intestinal barrier function in inflammatory bowel disease. World J Gastroenterol 15: 3134–3141, 2009. doi: 10.3748/wjg.15.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rutgeerts P, Ghoos Y, Vantrappen G. Bile acid studies in patients with Crohn's colitis. Gut 20: 1072–1077, 1979. doi: 10.1136/gut.20.12.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 49: 1374–1403, 2013. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 162.Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut 62: 933–947, 2013. doi: 10.1136/gutjnl-2013-304701. [DOI] [PubMed] [Google Scholar]
- 163.Aune D, Chan DS, Lau R, Vieira R, Greenwood DC, Kampman E, Norat T. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ 343: d6617, 2011. doi: 10.1136/bmj.d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Uronis JM, Muhlbauer M, Herfarth HH, Rubinas TC, Jones GS, Jobin C. Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS One 4: e6026, 2009. doi: 10.1371/journal.pone.0006026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cremonesi E, Governa V, Garzon JF, Mele V, Amicarella F, Muraro MG, Trella E, Galati-Fournier V, Oertli D, Daster SR, Droeser RA, Weixler B, Bolli M, Rosso R, Nitsche U, Khanna N, Egli A, Keck S, Slotta-Huspenina J, Terracciano LM, Zajac P, Spagnoli GC, Eppenberger-Castori S, Janssen KP, Borsig L, Iezzi G. Gut microbiota modulate T cell trafficking into human colorectal cancer. Gut 67: 1984–1994, 2018. doi: 10.1136/gutjnl-2016-313498. [DOI] [PubMed] [Google Scholar]
- 166.Wang T, Cai G, Qiu Y, Fei N, Zhang M, Pang X, Jia W, Cai S, Zhao L. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J 6: 320–329, 2012. dois: 10.1038/ismej.2011.109, 10.1038/ismej.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Imray CH, Radley S, Davis A, Barker G, Hendrickse CW, Donovan IA, Lawson AM, Baker PR, Neoptolemos JP. Faecal unconjugated bile acids in patients with colorectal cancer or polyps. Gut 33: 1239–1245, 1992. doi: 10.1136/gut.33.9.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Reddy BS, Wynder EL. Metabolic epidemiology of colon cancer. Fecal bile acids and neutral sterols in colon cancer patients and patients with adenomatous polyps. Cancer 39: 2533–2539, 1977. doi:. [DOI] [PubMed] [Google Scholar]
- 169.Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, El-Omar EM, Brenner D, Fuchs CS, Meyerson M, Garrett WS. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 14: 207–215, 2013. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Warren RL, Freeman DJ, Pleasance S, Watson P, Moore RA, Cochrane K, Allen-Vercoe E, Holt RA. Co-occurrence of anaerobic bacteria in colorectal carcinomas. Microbiome 1: 16, 2013. doi: 10.1186/2049-2618-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, Qian Y, Kryczek I, Sun D, Nagarsheth N, Chen Y, Chen H, Hong J, Zou W, Fang JY. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell 170: 548–563, 2017. doi: 10.1016/j.cell.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Modica S, Gadaleta RM, Moschetta A. Deciphering the nuclear bile acid receptor FXR paradigm. Nucl Recept Signal 8: e005, 2010. doi: 10.1621/nrs.08005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Maran RR, Thomas A, Roth M, Sheng Z, Esterly N, Pinson D, Gao X, Zhang Y, Ganapathy V, Gonzalez FJ, Guo GL. Farnesoid X receptor deficiency in mice leads to increased intestinal epithelial cell proliferation and tumor development. J Pharmacol Exp Ther 328: 469–477, 2009. doi: 10.1124/jpet.108.145409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gadaleta RM, Garcia-Irigoyen O, Moschetta A. Bile acids and colon cancer: Is FXR the solution of the conundrum? Mol Aspects Med 56: 66–74, 2017. doi: 10.1016/j.mam.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 175.Chen C, Ahn EH, Kang SS, Liu X, Alam A, Ye K. Gut dysbiosis contributes to amyloid pathology, associated with C/EBPβ/AEP signaling activation in Alzheimer’s disease mouse model. Sci Adv 6: eaba0466–15, 2020. doi: 10.1126/sciadv.aba0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Qiao Y, Wu M, Feng Y, Zhou Z, Chen L, Chen F. Alterations of oral microbiota distinguish children with autism spectrum disorders from healthy controls. Sci Rep 8: 1597, 2018. doi: 10.1038/s41598-018-19982-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Golubeva AV, Joyce SA, Moloney G, Burokas A, Sherwin E, Arboleya S, Flynn I, Khochanskiy D, Moya-Perez A, Peterson V, Rea K, Murphy K, Makarova O, Buravkov S, Hyland NP, Stanton C, Clarke G, Gahan CG, Dinan TG, Cryan JF. Microbiota-related changes in bile acid & tryptophan metabolism are associated with gastrointestinal dysfunction in a mouse model of autism. EBioMedicine 24: 166–178, 2017. doi: 10.1016/j.ebiom.2017.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]