Abstract
We investigated whether dual bronchodilator therapy (glycopyrrolate/formoterol fumarate; GFF; Bevespi Aerosphere) would increase exercise tolerance during a high-intensity constant work rate exercise test (CWRET) and the relative contributions of dead space ventilation (VD/VT) and dynamic hyperinflation (change in inspiratory capacity) to exercise limitation in chronic obstructive pulmonary disease (COPD). In all, 48 patients with COPD (62.9 ± 7.6 yrs; 33 male; GOLD spirometry stage 1/2/3/4, n = 2/35/11/0) performed a randomized, double blind, placebo (PL) controlled, two-period crossover, single-center trial. Gas exchange and inspiratory capacity (IC) were assessed during cycle ergometry at 80% incremental exercise peak work rate. Transcutaneous (Tc) measurement was used for VD/VT estimation. Baseline postalbuterol forced expiratory volume in 1 s (FEV1) was 1.86 ± 0.58 L (63.6% ± 13.9 predicted). GFF increased FEV1 by 0.18 ± 0.21 L relative to placebo (PL; P < 0.001). CWRET endurance time was greater after GFF vs. PL (383 ± 184 s vs. 328 ± 115 s; difference 55 ± 125 s; P = 0.013; confidence interval: 20–90 s), a 17% increase. IC on GFF was above placebo IC at all time points and fell less with GFF vs. PL (P ≤ 0.0001). Isotime tidal volume (1.54 ± 0.50 vs. 1.47 ± 0.45 L; P = 0.022) and ventilation (52.9 ± 19.9 vs. 51.0 ± 18.9 L/min; P = 0.011) were greater, and respiratory rate was unchanged (34.9 ± 9.2 vs. 35.1 ± 8.0 br/min, P = 0.865). Isotime VD/VT did not differ between groups (GFF 0.28 ± 0.08 vs. PL 0.27 ± 0.09; P = 0.926). GFF increased exercise tolerance in patients with COPD, and the increase was accompanied by attenuated dynamic hyperinflation without altering VD/VT.
NEW & NOTEWORTHY This study was a randomized clinical trial (NCT03081156) that collected detailed physiology data to investigate the effect of dual bronchodilator therapy on exercise tolerance in COPD, and additionally to determine the relative contributions of changes in dead space ventilation (VD/VT) and dynamic hyperinflation to alterations in exercise limitation. We utilized a unique noninvasive method to assess VD/VT (transcutaneous carbon dioxide, Tc) and found that dual bronchodilators yielded a moderate improvement in exercise tolerance. Importantly, attenuation of dynamic hyperinflation rather than change in dead space ventilation was the most important contributor to exercise tolerance improvement.
Keywords: COPD, CPET, exercise intolerance, hyperinflation, VD/VT
INTRODUCTION
Patients with chronic obstructive pulmonary disease (COPD) experience expiratory flow limitation, breathlessness, dyspnea, and reduced exercise tolerance relative to age and sex matched controls (1–4). A prominent mechanism responsible for exercise intolerance in COPD is thought to be dynamic hyperinflation (DH) during exercise due to end expiratory lung volume increase, usually assessed by a commensurate decrease in inspiratory capacity (IC) (5, 6). The effects of dynamic hyperinflation on dyspnea and exercise intolerance are amplified in COPD by an increased ventilatory requirement for exercise, related to increased dead space to tidal volume ratio (VD/VT) (7, 8). Treatment with bronchodilators (BD) are partially effective at reducing expiratory flow limitation, and the optimal regimen in COPD appears to be achieved with a fixed dose, long-acting inhaled combination medications (LABA/LAMA, long-acting beta agonists and long-acting muscarinic antagonists) (1, 2, 9–11). In patients with COPD, LABA/LAMA bronchodilator therapy reduces DH and increases exercise tolerance (12). However, the effect of LABA/LAMA bronchodilator therapy on VD/VT during exercise in COPD is controversial (13). Although short-acting bronchodilator therapy in COPD does not influence estimated VD/VT, long-acting bronchodilation, which increases ventilation to both well- and poorly perfused lung units, has the potential to worsen VD/VT and increase ventilatory requirement. LABA/LAMA bronchodilators also increase pulmonary blood flow, as demonstrated using gadolinium-enhanced magnetic resonance imaging at rest (14). Therefore, should the negative effects of COPD on alveolar ventilation to pulmonary blood flow ratio (V̇A/Q̇) distribution during exercise be ameliorated by LABA/LAMA bronchodilators, then VD/VT may be reduced and, thus, contribute to the mechanism by which LABA/LAMA bronchodilators increase exercise tolerance.
We hypothesized that glycopyrrolate/formoterol fumarate (GFF, Bevespi Aerosphere) would increase exercise tolerance during high intensity, constant work rate, cycle ergometer exercise in stable COPD patients by simultaneously reducing DH and VD/VT during exercise. To assess the relative contributions of these two variables, we aimed to determine DH using serial IC measurements and breath-by-breath VD/VT using transcutaneous (Tc) measurements and compare these measurements at isotime during constant work rate exercise to intolerance while receiving GFF or placebo (PL).
METHODS
The study was approved by the Institutional Review Board of The Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center (Study No. 21752-01). Written informed consent was obtained from each subject before participation.
Study Design
This was a single center, randomized, double blind, PL controlled, two-period, crossover clinical trial conducted between March 2017 and February 2019 (NCT03081156).
Participants
Male or females were enrolled, who were between 40 and 80 yr with a clinical diagnosis of COPD (postalbuterol FEV1/FVC ratio <0.70) and stable, without change in medications or exacerbation within the prior 4 wk. Participants were current or ex-smokers with >10 pack-years smoking history. Exclusion criteria included: significant diseases other than COPD that affect exercise tolerance: e.g., a history of heart failure or arthritis; a documented history of childhood or family asthma, treatment with oral corticosteroid medications above 10 mg/day, daytime oxygen use >6 hours/day, desaturation (from pulse oximetry) during exercise to <80%; a physical contraindication for exercise, e.g., marked exercise induced hypertension, serious arrhythmia; and other unstable conditions as evaluated by the principal investigator. Participants were categorized into Global Initiative for Chronic Obstructive Lung Disease (GOLD) spirometry groups: GOLD 1 (FEV1 ≥ 80% predicted), GOLD 2 (50% ≤ FEV1 predicted >80%), GOLD 3 (30% ≤ FEV1 predicted >50%), GOLD 4 (FEV1 < 30% predicted).
Procedures
Pulmonary function testing.
Participants continued their usual medications for screening. Prebronchodilator spirometry, body plethysmography, and single-breath diffusing capacity of the lung for carbon monoxide (DLCO) were measured according to recommended American Thoracic Society/European Respiratory Society (ATS/ERS) standards (15–18). Postbronchodilator spirometry was measured 20 min after inhalation of 4 puffs of albuterol (400 mg). Predicted values for spirometry and vital capacity (VC) were from National Health and Nutrition Examination Survey (NHANES) III (19); predicted lung volumes were from ERS (20); and predicted DLCO was from Cotes et al. (21).
Chest computed tomography.
Subjects underwent a volumetric computed tomography (CT) scan of the chest at full inspiration and relaxed expiration. For this study, inspiratory CT data were used. Emphysema was defined as percentage of lung attenuation areas below −950 Hounsfield Units. Airway wall thickening was measured using mean segmental wall thickness in mm of a theoretical airway of 10-mm luminal perimeter (22, 23).
Cardiopulmonary exercise testing.
Symptom-limited exercise tests were performed using cardiopulmonary measurements of gas exchange and ventilatory variables (Vmax Encore, Vyaire, Yorba Linda, CA, or CPX Ultima, Medical Graphics Corporation, St. Paul, MN) with electromagnetically braked cycle ergometry (Excalibur Sport PFM, Lode, Groningen, NL). Each subject started and finished all exercise tests (incremental and constant work rate) using the same cardiopulmonary system and cycle ergometer. Daily calibration as well as monthly biological calibrations were performed according to vendor recommendations and guidelines (24).
Incremental exercise testing.
Participants were screened with a ramp-incremental exercise test at 10 Watts (W)/min. A 3-min rest period was followed by 3 min of unloaded cycling, then increasing work rate (10 W/min) until intolerance, determined as the inability to maintain pedaling cadence >50 rpm despite encouragement. The test was concluded with at least 3 min of unloaded cycling. Subjects breathed through a mouthpiece with a nose clip in place. Heart rate was derived from a 12-lead electrocardiogram. Inspiratory capacity (IC) was measured every 2 min according to ATS standards (25, 26). Blood pressure (sphygmomanometer) and ratings of breathlessness and leg effort (modified Borg CR-10 scale) were also assessed every 2 min. Maximal voluntary ventilation (MVV) was estimated as FEV1 × 40, where FEV1 was measured seated on the cycle ergometer immediately before exercise testing (26). Peak work rate was determined as the value during the last 10 s of exercise. For variables measured breath-by-breath, peak values were defined as the average of the last 30 s of exercise averaged over three 10-s time bins. For variables assessed every 2 min, end-exercise values were taken as the last value during exercise before or during that 30-s window. The estimated lactate threshold (LAT) was assessed using the V-slope method and corroborated with ventilatory equivalent and end-tidal partial pressure responses (and ) (27). For VE/ slope, data calculated as 30-s averages were fit by linear regression. The lower point for the regression was at a work rate above 20 watts and the upper point was at the respiratory compensation point. Predicted values for ramp exercise variables were those of Hansen et al. (26).
Constant work rate exercise testing.
The work rate for constant work rate exercise testing (CWRET) was 80% of the peak work rate measured during incremental exercise. A 3-min rest period was followed by 3 min unloaded cycling, then the work rate was increased as a step change to 80% peak incremental work rate. Exercise tolerance during CWRET was determined at the time between the start of loaded exercise (work > 0 watts) and when the subject could no longer maintain pedaling cadence >50 rpm despite encouragement (specifically, the time between the 10-s bin in which loaded cycling commenced and the 10-s window in which the work rate decreased). The test was concluded with at least 3 min of unloaded cycling. CWRET was initially performed at screening. If exercise duration was outside the 3–8 min guideline duration (4), a second screening CWRET was performed at an ∼5 W higher or lower work rate—whichever was appropriate to obtain a 3–8-min CWRET duration (28). In analysis of these data, for variables measured breath-by-breath, end-exercise values were defined as the average of the last 30 s of exercise averaged over three 10-s time bins. For variables assessed every 2 min, end-exercise values were taken as the last value during exercise before or during that 30-s window. For physiologic variables, isotime responses were taken as the time of the last 10-s time bin in the shorter of the study drug and placebo tests. For variables assessed every 2 min, isotime values were taken as the value recorded at or immediately preceding isotime.
VD/VT estimation.
Transcutaneous CO2 (Tc) and oxygen saturation by pulse oximetry (; Tosca 500, Radiometer America, Brea, CA) was recorded breath-by-breath during incremental and constant work rate exercise. The sensor was attached to the left earlobe ∼10 min before exercise testing to allow the Tc measurement to stabilize. The device was set to a probe temperature of 44°C. Tc was substituted for in Eq. 1 for dead space estimation, and the fraction of the dead space of resulting from the mouthpiece breathing apparatus (VDS = 110 mL) was subtracted from the total.
| (1) |
Protocol
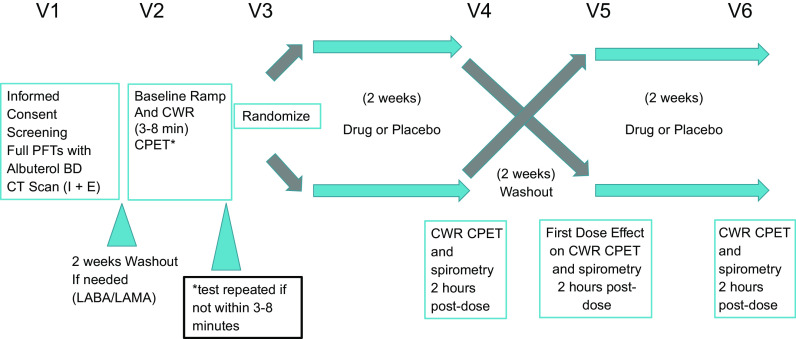
The study protocol diagram is provided in Fig. 1. Visit 1 included obtaining informed consent, vital signs including ; a focused physical examination; full pulmonary function testing (PFT) on usual respiratory medications; 6-min walk test; BODE (Body Mass index, Airflow Obstruction, Dyspnea, Exercise Capacity) index (29); modified Medical Research Council (mMRC) dyspnea score (30); inspiratory and expiratory (I + E) CT scan (31); and assessment of exacerbations in the past year (defined as new antibiotic prescription, new or increased oral corticosteroids, or a visit to the emergency room or hospital).
Figure 1.
Protocol and visit designation for this single-center, randomized, double-blind, placebo-controlled, two-period, crossover clinical trial. CPET, cardiopulmonary exercise testing; CT, computed tomography; CWR, constant work rate; LABA, long-acting beta agonists; LAMA, long-acting muscarinic antagonists; PFT, pulmonary function testing; V, visit.
Eligible participants underwent a 2-wk washout period where all LABA and LAMA bronchodilators were withheld. Short-acting beta agonists (SABA) and/or short-acting muscarinic antagonists (SAMA) were allowed during washout. Participants who were currently using inhaled corticosteroids before enrollment (either individual or as a component of a combined inhaler) were continued on a single inhaled corticosteroid agent at appropriate dosages (beclomethasone dipropionate; QVAR). Participants who were not using LABA or LAMA therapy did not require washout.
After the appropriate washout period, participants performed screening ramp-incremental and CWRET exercise tests (visit 2) separated by at least 1 h of rest. Participants and study personnel were blinded to the statistician created randomization that the research pharmacist followed to provide either PL first or GFF for visits 3 and 5. A coded, indistinguishable inhaler (either GFF or PL) was dispensed at visit 3 and used for 2 wk. At visits 4 and 6 spirometry was followed by CWRET. Visit 5 was preceded by another 2-wk washout period, after which participants received their crossed-over inhaler (either PL or GFF) and took the first dose in the clinic. The acute effect of either PL or GFF on spirometry was determined 2 h postdose on visit 5. Participants then resumed their prestudy medications, and a follow-up visit (visit 7) was performed 1 wk later to assess patient safety.
Statistics
Data are presented as mean ± SD or 95% confidence interval (CI). Differences in physiologic and perceptual responses between study drug and placebo were analyzed using paired Student’s t tests or repeated measures analysis of variance (rmANOVA). Statistical significance was accepted at P < 0.05. Multivariable linear regression analysis was used to determine whether differences between study drug and placebo for a variety of physiologic variables were predictive of increases in endurance time (Sigmaplot v.14, Systat Software, San Jose, CA). The sample size was estimated assuming an effect size on endurance time of 60 s [lower bound of the minimum clinically important difference (MCID) estimated for CWRET (32)] and a standard deviation of change in endurance time of 150 s (4). A desired power (1 − β) of 0.8 and α of 0.05 resulted in a sample size of 50 participants.
RESULTS
Participants
Sixty participants were consented. Eight subjects failed screening procedures related to failure to meet pulmonary function inclusion criteria (5), recent hospitalization for heart failure (1), chest mass found on the study CT scan (1), and inability to tolerate bronchodilator washout (1). The four subjects who entered, but did not complete the study, all withdrew consent in the prerandomization period (before visit 3), related to family emergency (1), knee pain (1), and unhappiness with the possibility of being in the placebo group (1); one subject declined to give a reason for withdrawal. Forty-eight subjects completed the study.
Participant demographics were: age 62.9 ± 7.6 yr; height 172.5 ± 8.6 cm; weight 86.7 ± 18.4 kg; body mass index 29.1 ± 5.9 kg/m2; gender: 33 male (69%); race: 26 Caucasian (54%); 21 African American (43%); 1 Asian (2%). GOLD spirometry stage 1/2/3/4 was n = 2/35/11/0. Thirty were past smokers, 18 were current smokers. The average pack-years of cigarette smoking was 38 ± 21. All participants were stable for at least 4 weeks before screening, but 11 (23%) had experienced a COPD exacerbation within the prior year and one subject had experienced two exacerbations. At screening, the mean 6-min walk distance was 393 ± 86 m, BODE (8) index was 1.83 ± 1.36 and mMRC score was 1.75 ± 0.5. Five (11%) subjects had been receiving LABA, four (8%) had been receiving LAMA therapy; nine (19%) had been receiving dual LABA/LAMA therapy. Nineteen participants (40%) had been receiving inhaled corticosteroids, which were continued during the study using inhaled beclomethasone at a dosage of either 40 or 80 µg depending on the previous dosage. None of the subjects had been receiving PDE4 inhibitors or daily azithromycin. Average hemoglobin concentration was within the normal range (14.4 ± 1.6 g/dL) and the average eosinophil count was 209 ± 165 cells/uL or 2.66 ± 1.79%. CT scans (n = 46, 2 subjects had recent CT scans, which were not repeated for the study) demonstrated emphysema 9.09 ± 7.71% and mean segmental wall thickness 3.61 ± 0.47 mm.
Pulmonary Function
Screening pulmonary function with usual medications and before washout are presented in Table 1. The majority of participants had mild-moderate (71%) or severe (23%) COPD. The FEV1/FVC ratio was 51.8 ± 11.6%. After 4 puffs of albuterol, the average FEV1 increase (0.14 ± 0.14 L and 9.1 ± 9.4%) and FVC increase (0.21 ± 0.30 L and 6.2 ± 8.8%) did not fulfill ATS/ERS criteria for a positive bronchodilator response (17). The subjects on average were not hyperinflated with respect to TLC (101 ± 17% predicted); however, the RV (118 ± 45%) and FRC (110.5 ± 39.9%) were elevated. The average gas transfer/DLCO was reduced at 59.3 ± 16.2% of predicted with DLCO ranging from 23 to 88% of predicted.
Table 1.
Baseline pre- and postbronchodilator pulmonary function (n = 48)
| Prebronchodilator | Postbronchodilator (Albuterol) | Bronchodilator Effect (Albuterol) | % Increase | |
|---|---|---|---|---|
| FEV1, L | 1.72 ± 0.56 | 1.86 ± 0.58 | 0.14 ± 0.14 | 9.1 ± 9.4% |
| FEV1, % Predicted (19) | 58.6 ± 13.8 | 63.6 ± 13.9 | - | - |
| FVC, L | 3.35 ± 0.89 | 3.56 ± 0.98 | 0.21 ± 0.30 | 6.2 ± 8.8% |
| FVC, % Predicted (19) | 87.1 ± 14.4 | 92.2 ± 15.3 | - | - |
| FEV1/FVC, % (19) | 51.8 ± 11.6 | 53.2 ± 11.4 | - | - |
| TLC, L | 6.15 ± 1.72 | - | - | - |
| TLC, % Predicted (20) | 101 ± 17 | - | - | - |
| FRC, L | 3.55 ± 1.57 | - | - | - |
| FRC, % Predicted | 110.5 ± 39.9 | - | - | - |
| RV, L | 2.68 ± 1.19 | - | - | - |
| RV, % Predicted | 118 ± 45 | - | - | - |
| RV/TLC, % | 42.8 ± 8.9 | - | - | - |
| IC | 2.60 ± 0.66 | - | - | - |
| IC, % Predicted | 98 ± 22 | - | - | - |
| DLCO, mL/min/mmHg | 15.2 ± 4.9 | - | - | - |
| DLCO, % Predicted (21) | 59.3 ± 16.1 | - | - | - |
| CT emphysema, % < LA 950* | 9.09 ± 7.71 | - | - | - |
| CT mean segmental wall thickness, mm* | 3.61 ± 0.47 | - | - | - |
All values, mean ± SD. FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; TLC, total lung capacity; RV, residual volume; DLCO, diffusing capacity of the lung for carbon monoxide. *CT, computed tomography, n = 46 (2 subjects had a recent CT and did not repeat a quantitative CT for the study).
At visit 5, 23 subjects received GFF and 25 subjects received PL per their randomization sequence. Spirometry obtained 2-h after GFF or placebo inhalation at visit 5 following a 2-wk wash out from long-acting bronchodilators (LABA/LAMA) is displayed in Table 2. After administration of GFF, FEV1 increased by 0.18 ± 0.20 L (11.8 ± 15.6%, P < 0.001 versus predose). There was no increase in FEV1 following PL (0.01 ± 0.10 L; P > 0.050 vs. predose).
Table 2.
The effect of first dose of LABA/LAMA bronchodilator (GFF) or placebo (PL) on spirometry at visit 5
| Subjects Receiving Placebo (n = 25) |
Subjects Receiving GFF (n = 23) |
|||
|---|---|---|---|---|
| Predose | Postdose Change | Predose | Postdose Change | |
| FEV1, L | 1.79 ± 0.64 | 0.01 ± 0.10 | 1.64 ± 0.55 | 0.18 ± 0.21# |
| FVC, L | 3.62 ± 1.14 | 0.03 ± 0.26 | 3.21 ± 0.82 | 0.17 ± 0.23* |
Postdose change is the difference in the variables (FEV1 and FVC) between predose and 120 min postdose of either PL or GFF at visit 5. FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; GFF, glycopyrrolate/formoterol fumarate; LABA, long-acting beta agonists; LAMA, long-acting muscarinic antagonists.
*P ≤ 0.05, #P ≤ 0.001, Student’s t test.
Incremental Exercise Test
Table 3 presents the baseline characteristics during screening ramp-incremental exercise. Participants had impaired aerobic function: reduced V̇o2 peak [1.36 L/min, 67 ± 19% predicted (26), 15.8 ± 4.9 mL/kg/min], low LAT (0.88 ± 0.20 L/min, 92% ± 21 of predicted lower limit of normal). They had a peak work rate of 91 ± 33 W, abnormal indices of ventilatory efficiency and gas exchange, including a high V̇E/MVV (0.71 ± 0.16), increased V̇E/V̇co2 at the LAT (34.5 ± 5.1), and V̇E versus V̇co2 slope (29.5 ± 6.3). Twenty-two of the 48 subjects (46%) had a peak V̇E/MVV above 0.75, and subjects showed signs of dynamic hyperinflation, as evidenced by a fall in IC, with inspiratory reserve volume (IRV, IC-VT) averaging 0.60 ± 0.40L at end exercise. The average VD/VT for the group at the LAT was 0.29 ± 0.08.
Table 3.
Baseline ramp incremental exercise testing results (n = 48)
| Incremental Exercise Test | Means ± SD |
|---|---|
| Peak V̇o2, L/min | 1.36 ± 0.41 |
| Peak V̇o2, mL/kg/min | 15.8 ± 4.9 |
| Peak V̇o2, % Predicted | 67 ± 19 |
| Peak Work Rate, W | 91 ± 33 |
| V̇o2 at LAT, L/min | 0.88 ± 0.20 |
| V̇o2 at LAT, % Predicted | 92 ± 21 |
| Work rate at LAT, W | 44.3 ± 15.8 |
| Peak HR, beat/min | 123 ± 17 |
| O2 saturation at peak exercise, % | 97 ± 3 |
| Peak O2 pulse, mL/beat | 11.0 ± 2.8 |
| Peak V̇E, L/min | 52.3 ± 20.2 |
| Peak V̇E/V̇co2 | 37.0 ± 7.0 |
| V̇E/V̇co2 at LAT | 34.5 ± 5.1 |
| V̇E vs. V̇co2 slope | 29.5 ± 6.3 |
| VD/VT at the LAT | 0.29 ± 0.08 |
| Peak V̇E/MVV | 0.71 ± 0.16 |
| Peak V̇E/MVV above 0.75 [n (%)] | 22 (18) |
| ΔIC (peak-rest), L | −0.41 ± 0.46 |
| Peak IRV, L | 0.60 ± 0.40 |
V̇o2, oxygen uptake; LAT, estimated lactic acidosis threshold; for V̇o2 at LAT the % predicted is 40% of peak V̇o2; V̇E, minute ventilation; V̇co2, carbon dioxide output; V̇E vs. V̇co2 slope, the slope of the relationship between V̇E and V̇co2 measured between 20 W and the respiratory compensation point; WR, work rate (watts); MVV, maximum voluntary ventilation estimated from forced expiratory volume in 1 s × 40; ΔIC (peak-rest), the difference in inspiratory capacity between resting and peak exercise; IRV, inspiratory reserve volume. Peak HR, maximum HR recorded during the cardiopulmonary exercise testing. O2 saturation at peak exercise. Peak O2 pulse: peak V̇o2/peak HR. All predicted values from Wasserman et al. (26).
Constant Work Rate Exercise Test
Figures 2 and 3 present the time course of a number of physiologic and perceptual responses to CWR exercise in response to GFF and placebo. The average CWRET work rate was 73 ± 27 W. The endurance time (primary outcome) was 383 ± 184 s on GFF versus 328 ± 115 s on PL, with a mean individual difference of 55 ± 125 s (17% increase; P = 0.013, CI 20–90 s). Figure 4 displays the individual CWRET responses in seconds for 48 subjects on placebo and GFF. There were large increases in exercise tolerance in certain individuals, with the average increasing by 55 ± 125 s. The MCID for endurance time for CWRET has been postulated to be 100 s or 33% (34); by both of these metrics, 18 of 48 subjects, demonstrated clinically important increases in exercise tolerance. Isotime occurred 4.53 ± 1.45 min after the onset of loaded CWRET exercise.
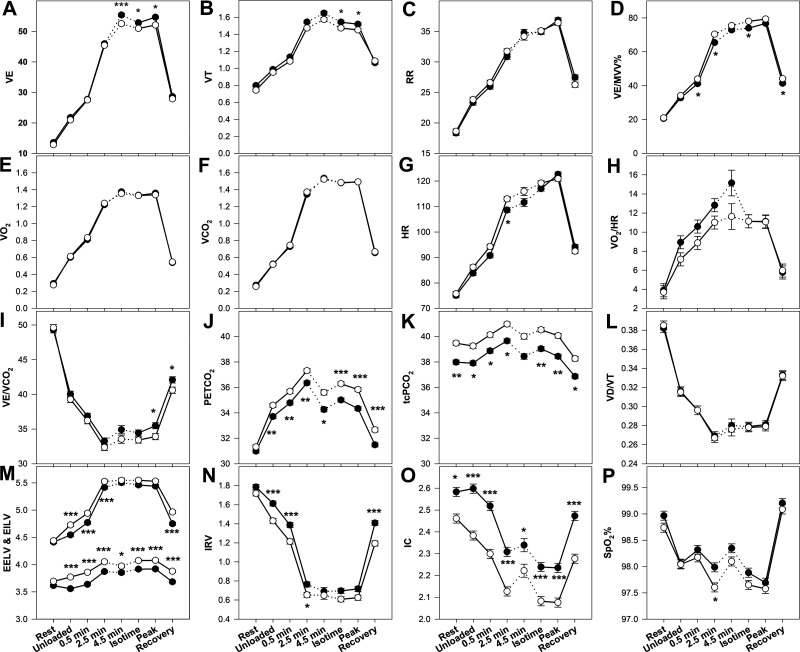
Figure 2.
A–P: physiologic responses to constant work rate exercise testing in patients with COPD receiving LAMA/LAMA therapy (closed circles) vs. Placebo (open circles). Description of visits is given in text. All data points are for 48 subjects, except the 4.5 minute time point, in which only 22 subjects completed this duration in both tests. *P < 0.05, **P = 0.01, ***P ≤ 0.001 by rmANOVA. V̇E (L/min), pulmonary ventilation; VT (L), tidal volume; RR (breaths/min), respiratory rate; V̇E/MVV (%), pulmonary ventilation divided by maximum voluntary ventilation; V̇o2 (L/min), oxygen uptake; V̇co2 (L/min), carbon dioxide output; HR (beats/min), heart rate; V̇o2/HR (mL/beat), oxygen pulse; V̇E/V̇co2, pulmonary ventilation divided by carbon dioxide output; (mmHg), end-tidal carbon dioxide partial pressure; Tc (mmHg), transcutaneous carbon dioxide partial pressure; VD/VT, dead space to tidal volume ratio; IRV (L), inspiratory reserve volume; IC (L), inspiratory capacity; (%), oxygen saturation by pulse oximetry. COPD, chronic obstructive pulmonary disease; LAMA, long-acting muscarinic antagonists.
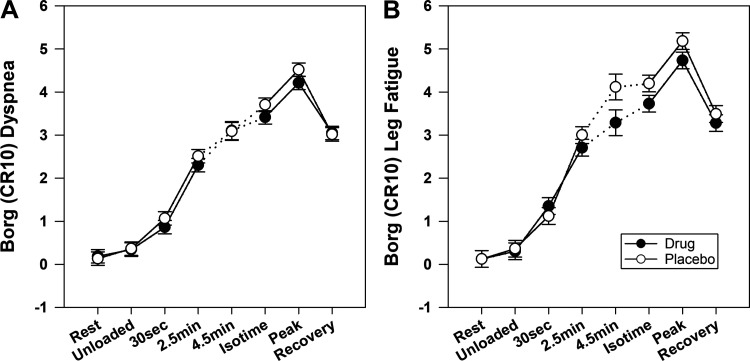
Figure 3.
Perceptual responses to constant work rate exercise in patients with COPD receiving LAMA/LAMA therapy (closed circles) vs. Placebo (open circles). All data points are for 48 subjects except the 4.5-min time point, in which only 22 subjects completed this duration in both tests. There were no statistical differences in the Borg scores for either dyspnea (A) or leg fatigue (B). COPD, chronic obstructive pulmonary disease; CR10, Category and Ratio Scale of 10; LAMA, long-acting muscarinic antagonists.
Figure 4.
Constant work rate exercise duration while receiving dual bronchodilator vs. placebo for 48 individual subjects with COPD. COPD, chronic obstructive pulmonary disease; CWRET, constant work rate exercise time; placebo versus GFF, dual LABA/LAMA bronchodilator; GFF, glycopyrrolate/formoterol fumarate; LABA, long-acting beta agonists; LAMA, long-acting muscarinic antagonists.
In Fig. 2A, V̇E increased progressively during CWRET in both GFF (solid circles) and Placebo (open circles) studies, but were little changed from 2.5 min onward. V̇E was statistically significantly higher with GFF versus Placebo at 4.5 min, isotime, and peak exercise time points. In Fig. 2B, tidal volume (VT) peaked at approximately ∼ 1.5–1.6 L and decreased thereafter; however, VT in GFF remained statistically significantly higher than placebo at isotime and peak exercise. In Fig. 2C, respiratory rate increased progressively throughout exercise with an acceleration at peak exercise, but the time course was essentially identical in GGF and placebo studies. In Fig. 2D, V̇E/MVV was slightly lower throughout exercise in the GFF studies; this achieved statistical significant at 30 s, 2.5 min, isotime, and recovery.
In Fig. 2, E and F, both V̇o2 and V̇co2 were unchanging from 4.5 min through isotime and peak exercise at ∼1.35 and 1.50 L/min, respectively, with no significant differences between the GFF versusplacebo studies. In Fig. 2G, HR progressively increased throughout exercise, with values slightly lower in GFF than placebo studies during exercise, but only the 2.5 min difference achieved statistical significance. In Fig. 2H, O2 pulse (V̇o2/HR) rose during exercise, but no differences between GFF and placebo achieved statistical significance.
In Fig. 2I, V̇E/V̇co2 was higher in the GFF group relative to placebo throughout exercise; however, this difference achieved statistical significance at peak exercise and recovery. In Fig. 2J, the end tidal CO2 values were statistically significantly lower in GFF versus placebo (by ∼1 mmHg) throughout exercise and recovery. In Fig. 2K, transcutaneous (Tc) was statistically significantly lower at rest, during all levels of exercise, and recovery relative to placebo also by ∼ 1 mmHg. In Fig. 2L, VD/VT was essentially identical at all points of the study with no statistically significant differences.
In Fig. 2M, end-inspiratory lung volume was lower with GFF at 30 s, 2.5 min, and 4.5 min relative to placebo, but was not statistically different at isotime or peak exercise. For end-expiratory lung volume, GFF was statistically significantly lower at all times during exercise as well as in recovery compared to placebo. In Fig. 2N, IRV with GFF remained slightly higher than placebo throughout exercise, and was statistically different at unloaded cycling, 30 s, 2.5 min, and recovery. In Fig. 2O, IC remained statistically significantly higher in GFF as compared to placebo studies throughout all measured points of rest, exercise, and recovery. In Fig. 2P, the oxygen saturation was not statistically different between GFF and placebo except at the 2.5 min time point where saturation was lower in the placebo group.
Figure 3A presents the Borg Category and Ratio Scale of 10 (CR10) scores for dyspnea during rest, exercise, and recovery. GFF trended lower versusplacebo, but no significant differences were observed. In Fig. 3B, the Borg CR 10 scores for leg fatigue demonstrated trends for lower scores on GFF relative to placebo, but no significant differences emerged.
The increase in isotime IC was not significantly correlated with the endurance time improvement with BFF. For the 46 subjects who had quantitative CT scans, percent emphysema and segmental wall thickness were not significantly correlated with constant work rate endurance time improvement (R2 = 0.0043 and 0.066, respectively). There was also no correlation of VD/VT change with these CT parameters. DLCO as % predicted also showed no significant correlation with endurance time improvement (R2 = 0.0086).
DISCUSSION
The physiologic mechanisms limiting exercise in COPD include increased ventilatory requirements consequent to one or more mechanisms including, greater V̇A/Q̇ inequality, lower LAT, larger VD/VT, and abnormal ventilatory mechanics (dynamic hyperinflation; constrained VT; lower IRV; increased respiratory muscle work) (3, 7, 8, 26, 35, 36). These mechanisms are not exclusive, and may potentiate one another, exacerbating dyspnea and reducing exercise tolerance. This study aimed to determine the contributions of alterations in DH and VD/VT to the increases in exercise tolerance observed with LABA/LAMA bronchodilator therapy in COPD. We found, as anticipated, that LABA/LAMA bronchodilator therapy (GFF) significantly increased exercise tolerance in patients with mild to very severe COPD (∼17%), while reducing isotime dynamic hyperinflation (on average by ∼150 mL or ∼ 7% increase in IC) without significant isotime change in VD/VT. Together these data suggest that the primary mechanism by which LABA/LAMA bronchodilator therapy increases exercise tolerance in patients with COPD is via beneficial effects on respiratory mechanics to reduce dynamic hyperinflation, rather than increased ventilatory efficiency (reduced VD/VT).
The increase in exercise tolerance LABA/LAMA treatment in this placebo controlled, double blinded, study was modest (17%). The average increase in exercise tolerance by 55 s (CI 20–90 s) was less than the consensus MCID of 105 s or 33% increase, based on interventions including bronchodilator therapy, pulmonary rehabilitation, oxygen supplementation and noninvasive ventilation (4). Our findings were, however, approximately equal to the lower 95% confidence interval of the MCID (60 s or 22% increase) (4) and similar to previous studies of LABA/LAMA or short-acting beta agonists (SABA) and short-acting muscarinic antagonists (SAMA) therapy (4). A subanalysis of our data showed that the average increase in exercise tolerance tended to be greater in GOLD 3–4 than GOLD 1–2 participants (124 ± 143 s, or 38% increase vs. 34 ± 144 s or 10% increase; P = 0.076). Although our study was not powered to identify the effect of combination LABA/LAMA across disease severities, these observations are consistent with earlier studies that found that bronchodilator therapy is less effective in patients with COPD with mild obstruction in whom exercise limitation may be dominated by peripheral muscle function (2).
Assessment of ventilatory inefficiency during exercise is challenging. The ratio of ventilation to CO2 output (V̇E/V̇co2) at LAT (or nadir) is commonly used to infer inefficient ventilation. However, because V̇E/V̇co2 depends on both ventilatory efficiency (VD/VT) and , it is inaccurate to determine the normalcy (or not) of VD/VT in patients with intermediate V̇E/V̇co2 values (32). Further, using end-tidal values (rather than arterial ) to calculate VD/VT is highly inaccurate, in all but normal subjects (37–39). The most appropriate method to calculate VD/VT uses , utilizing the modified Bohr-Enghoff correction (40, 41). However, the blood draws during exercise that this necessitates, either from an indwelling catheter or from a single arterial puncture, is painful, time consuming and yields poor time resolution. may be estimated noninvasively using transcutaneous (33, 34, 42–45), and may be reasonably applied (i.e., have good agreement with ) to estimate VD/VT on a breath-by-breath basis during a cardiopulmonary exercise testing in patients with COPD (37).
To address the mechanism(s) of the observed improvement in exercise tolerance resulting from LABA/LAMA therapy, we assessed both dynamic hyperinflation using serial inspiratory capacity maneuvers every 2 min and the VD/VT calculated breath-by-breath using Tc. At isotime during constant work rate exercise, we observed that LABA/LAMA bronchodilator therapy yielded significantly greater tidal volume, allowing greater ventilation and V̇E/V̇co2 for an identical exercise task (Fig. 2B). End-exercise comparisons also showed greater V̇E, V̇E/V̇co2, and VT as well as higher Tc with bronchodilator therapy, consistent with relief of pulmonary mechanical constraints (Fig. 2, A, B, I, and K). Average respiratory rate was not statistically different. However, the overall effect on VD/VT both at isotime and at end-exercise for GFF versusplacebo was negligible, and did not reach statistical significance. Therefore, contrary to our hypothesis, increased exercise tolerance following LABA/LAMA therapy in COPD was not associated with reduced exercising VD/VT. Consistent with the literature, increased exercise tolerance following LABA/LAMA therapy in COPD was coincident with an attenuated fall in IC (reduced dynamic hyperinflation).
Some previous studies (but not all) suggest that VD/VT is increased following bronchodilation therapy (14, 46, 47). Elbehairy et al. studied 20 patients with COPD who performed constant work rate exercise following acute administration of LABA/LAMA bronchodilators versus placebo; no difference in isotime VD/VT was detected (7). Marvin et al. (47) studied 15 men with COPD who performed 6 min of low-intensity constant work rate exercise after receiving oral theophylline plus an oral short acting beta agonist or placebo for 10 days; a trend for a greater exercise-induced fall in VD/VT was seen with the combination oral bronchodilator (23). Vogel-Claussen et al. utilized gadolinium-enhanced MRI and phase-resolved functional lung MRI to measure pulmonary microvascular blood flow and regional ventilation, respectively, in 62 patients with COPD receiving 2 wk of LABA/LAMA versus placebo; though VD/VT was not directly measured, LABA/LAMA therapy was found to decrease both nonventilated and hypoventilated lung regions (14).
This could occur by increased ventilation of poorly perfusion lung regions following bronchodilation in the proximal airways. Nevertheless, our RCT found no difference between VD/VT at isotime in GFF and PL groups (Fig. 2L), suggesting that GFF does not have a negative effect on V̇A/Q̇ inequality during exercise in COPD, leading to an increase in VD/VT.
Although this was a rigorous physiologic study of a pharmaceutical intervention in COPD subjects, there were some weaknesses. First, the small overall effect size on endurance time was likely due to the majority of participants (75%) being mild or moderately obstructed. The effects on exercise tolerance and VD/VT may have been different if more severe patients had been included in the study. Second, VD/VT estimation relied on Tc measurements and we did not draw simultaneous arterial bloods samples for .
We conclude that combination bronchochodilator therapy (glycopyrrolate/formoterol fumarate; GFF) increases exercise tolerance in patients with COPD by attenuating dynamic hyperinflation without increasing dead space ventilation (VD/VT).
GRANTS
AstraZeneca provided funding for this project through an investigator-initiated grant provided to the Lundquist Institute for which Dr. W. W. Stringer was the principal investigator.
DISCLOSURES
AstraZeneca also provided the medications and a placebo inhaler for the study. Dr. S. Rennard and S. Siddiqui were paid employees of AstraZeneca at the time the project was designed and performed. Dr. R. Casaburi has been a consultant for AstraZeneca.
AUTHOR CONTRIBUTIONS
W.W.S., J.P., H.B.R., S.S., S.R., and R.C. conceived and designed research; W.W.S., J.P., and M.C. performed experiments; W.W.S., J.P., M.C., and R.C. analyzed data; W.W.S., J.P., M.C., H.B.R., and R.C. interpreted results of experiments; W.W.S., J.P., and M.C. prepared figures; W.W.S. and J.P. drafted manuscript; W.W.S., J.P., H.B.R., S.R., and R.C. edited and revised manuscript; W.W.S., J.P., M.C., H.B.R., S.S., S.R., and R.C. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors express their gratitude to Michael von Plato, MD, for data analysis, graphical programming, and statistical support; Arash Khosrovi-Eghbal, MD, MSc, and Mohan Zopey, BS, for data analysis; Leticia Diaz, MA, and Robert Calmelat, MS, for subject recruitment and data collection; April Soriano, BS, for data collection; and Agustin Leyva, AA, for data collection and recruitment. The authors are grateful for their combined efforts to support and complete this study.
REFERENCES
- 1.Casaburi R. Strategies to reduce dynamic hyperinflation in chronic obstructive pulmonary disease. Pneumonol Alergol Pol 77: 192–195, 2009. [PubMed] [Google Scholar]
- 2.Casaburi R, Maltais F, Porszasz J, Albers F, Deng Q, Iqbal A, Paden HA, O'Donnell DE; 205.440 Investigators. Effects of tiotropium on hyperinflation and treadmill exercise tolerance in mild to moderate chronic obstructive pulmonary disease. Ann Am Thorac Soc 11: 1351–1361, 2014. doi: 10.1513/AnnalsATS.201404-174OC. [DOI] [PubMed] [Google Scholar]
- 3.Neder JA, Berton DC, Müller PT, Elbehairy AF, Rocha A, Palange P, O'Donnell DE; Canadian Respiratory Research Network. Ventilatory inefficiency and exertional dyspnea in early chronic obstructive pulmonary disease. Ann Am Thorac Soc 14: S22–S29, 2017. doi: 10.1513/AnnalsATS.201612-1033FR. [DOI] [PubMed] [Google Scholar]
- 4.Puente-Maestu L, Palange P, Casaburi R, Laveneziana P, Maltais F, Neder JA, O'Donnell DE, Onorati P, Porszasz J, Rabinovich R, Rossiter HB, Singh S, Troosters T, Ward S. Use of exercise testing in the evaluation of interventional efficacy: an official ERS statement. Eur Respir J 47: 429–460, 2016. doi: 10.1183/13993003.00745-2015. [DOI] [PubMed] [Google Scholar]
- 5.O'Donnell DE, Lam M, Webb KA. Measurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 158: 1557–1565, 1998. doi: 10.1164/ajrccm.158.5.9804004. [DOI] [PubMed] [Google Scholar]
- 6.Puente-Maestu L, Stringer WW. Hyperinflation and its management in COPD. Int J Chron Obstruct Pulmon Dis 1: 381–400, 2006. doi: 10.2147/copd.2006.1.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elbehairy AF, Ciavaglia CE, Webb KA, Guenette JA, Jensen D, Mourad SM, Neder JA, O'Donnell DE; Canadian Respiratory Research Network. Pulmonary gas exchange abnormalities in mild chronic obstructive pulmonary disease. Implications for dyspnea and exercise intolerance. Am J Respir Crit Care Med 191: 1384–1394, 2015. doi: 10.1164/rccm.201501-0157OC. [DOI] [PubMed] [Google Scholar]
- 8.O'Donnell DE, Neder JA, Elbehairy AF. Physiological impairment in mild COPD. Respirology 21: 211–223, 2016. doi: 10.1111/resp.12619. [DOI] [PubMed] [Google Scholar]
- 9.Bateman ED, Mahler DA, Vogelmeier CF, Wedzicha JA, Patalano F, Banerji D. Recent advances in COPD disease management with fixed-dose long-acting combination therapies. Expert Rev Respir Med 8: 357–379, 2014. doi: 10.1586/17476348.2014.910457. [DOI] [PubMed] [Google Scholar]
- 10.Cope S, Donohue JF, Jansen JP, Kraemer M, Capkun-Niggli G, Baldwin M, Buckley F, Ellis A, Jones P. Comparative efficacy of long-acting bronchodilators for COPD: a network meta-analysis. Respir Res 14: 100, 2013. doi: 10.1186/1465-9921-14-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huisman EL, Cockle SM, Ismaila AS, Karabis A, Punekar YS. Comparative efficacy of combination bronchodilator therapies in COPD: a network meta-analysis. Int J Chron Obstruct Pulmon Dis 10: 1863–1881, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di MF, Sotgiu G, Santus P, O'Donnell DE, Beeh KM, Dore S, Roggi MA, Giuliani L, Blasi F, Centanni S. Long-acting bronchodilators improve exercise capacity in COPD patients: a systematic review and meta-analysis. Respir Res 19: 18, 2018. doi: 10.1186/s12931-018-0721-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loring SH, Garcia-Jacques M, Malhotra A. Pulmonary characteristics in COPD and mechanisms of increased work of breathing. J Appl Physiol (1985) 107: 309–314, 2009. doi: 10.1152/japplphysiol.00008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogel-Claussen J, Schönfeld CO, Kaireit TF, Voskrebenzev A, Czerner CP, Renne J, Tillmann HC, Berschneider K, Hiltl S, Bauersachs J, Welte T, Hohlfeld JM. Effect of indacaterol/glycopyrronium on pulmonary perfusion and ventilation in hyperinflated patients with chronic obstructive pulmonary disease (CLAIM). A double-blind, randomized, crossover trial. Am J Respir Crit Care Med 199: 1086–1096, 2019. doi: 10.1164/rccm.201805-0995OC. [DOI] [PubMed] [Google Scholar]
- 15.Graham BL, Brusasco V, Burgos F, Cooper BG, Jensen R, Kendrick A, MacIntyre NR, Thompson BR, Wanger J. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J 49: 1600016, 2017.doi: 10.1183/13993003.00016-2016. [DOI] [PubMed] [Google Scholar]
- 16.MacIntyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, Gustafsson P, Hankinson J, Jensen R, McKay R, Miller MR, Navajas D, Pedersen OF, Pellegrino R, Wanger J. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 26: 720–735, 2005. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 17.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J; ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J 26: 319–338, 2005. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 18.Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson D, Macintyre N, McKay R, Miller MR, Navajas D, Pellegrino R, Viegi G. Standardisation of the measurement of lung volumes. Eur Respir J 26: 511–522, 2005. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 19.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 159: 179–187, 1999. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 20.Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 16: 5–40, 1993. doi: 10.1183/09041950.005s1693. [DOI] [PubMed] [Google Scholar]
- 21.Cotes J. Lung Function (5th ed.). London: Blackwell Scientific Publications, 1993, p. 225–250. [Google Scholar]
- 22.Lynch DA, Al-Qaisi MA. Quantitative computed tomography in chronic obstructive pulmonary disease. J Thorac Imaging 28: 284–290, 2013. doi: 10.1097/RTI.0b013e318298733c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lynch DA, Austin JH, Hogg JC, Grenier PA, Kauczor HU, Bankier AA, Barr RG, Colby TV, Galvin JR, Gevenois PA, Coxson HO, Hoffman EA, Newell JD, Pistolesi M, Silverman EK, Crapo JD. CT-definable subtypes of chronic obstructive pulmonary disease: a statement of the Fleischner Society. Radiology 277: 192–205, 2015. doi: 10.1148/radiol.2015141579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porszasz J, Stringer W, Casaburi R. Equipment, measurements and quality control. In: Clinical Exercise Testing (ERS Monograph), edited by Palange P, Laveneziana P, Neder JA, Ward S.. Sheffeld, UK: European Respiratory Society, 2018, p. 59–81. doi: 10.1183/2312508X.10011117. [DOI] [Google Scholar]
- 25.American Thoracic Society; American College of Chest Physicians. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 167: 211–277, 2003. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 26.Wasserman K, Hansen JE, Sue DY, Stringer WW, Sietsema K, S X-G, Whipp BJ. Principles of Exercise Testing and Interpretation: Pathophysiology and Clinical Applications. Baltimore, MD: Lippincott Williams & Wilkins, 2012. [Google Scholar]
- 27.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting the anaerobic threshold by gas exchange. J Appl Physiol (1985) 60: 2020–2027, 1986. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 28.Degani-Costa LH, O'Donnell DE, Webb K, Aranda LC, Carlstron JP, Cesar TDS, Plachi F, Berton DC, Neder JA, Nery LE. A simplified approach to select exercise endurance intensity for interventional studies in COPD. COPD 15: 139–147, 2018. doi: 10.1080/15412555.2018.1428944. [DOI] [PubMed] [Google Scholar]
- 29.Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, Pinto Plata V, Cabral HJ. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 350: 1005–1012, 2004. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 30.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 93: 580–586, 1988. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 31.Ross JC, Estépar RSJ, Díaz A, Westin C-F, Kikinis R, Silverman EK, Washko GR. Lung extraction, lobe segmentation and hierarchical region assessment for quantitative analysis on high resolution computed tomography images. Med Image Comput Comput Assist Interv 12: 690–698, 2009. doi: 10.1007/978-3-642-04271-3_84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roman MA, Casaburi JD, Porszasz J, Casaburi R. Noninvasive assessment of normality of VD/VT in clinical cardiopulmonary exercise testing utilizing incremental cycle ergometry. Eur J Appl Physiol 113: 33–40, 2013. doi: 10.1007/s00421-012-2407-8. [DOI] [PubMed] [Google Scholar]
- 33.Carter R, Banham SW. Use of transcutaneous oxygen and carbon dioxide tensions for assessing indices of gas exchange during exercise testing. Respir Med 94: 350–355, 2000. doi: 10.1053/rmed.1999.0714. [DOI] [PubMed] [Google Scholar]
- 34.Planès C, Leroy M, Foray E, Raffestin B. Arterial blood gases during exercise: validity of transcutaneous measurements. Arch Phys Med Rehabil 82: 1686–1691, 2001. doi: 10.1053/apmr.2001.26248. [DOI] [PubMed] [Google Scholar]
- 35.O'Donnell DE, Elbehairy AF, Faisal A, Webb KA, Neder JA, Mahler DA. Exertional dyspnoea in COPD: the clinical utility of cardiopulmonary exercise testing. Eur Respir Rev 25: 333–347, 2016. doi: 10.1183/16000617.0054-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varga J, Casaburi R, Ma S, Hecht A, Hsia D, Somfay A, Porszasz J. Relation of dynamic airway compression to dynamic hyperinflation during exercise in COPD. Resp Physiol Neurobiol 234: 79–84, 2016. doi: 10.1016/j.resp.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Cao M, Stringer WW, Corey S, Orogian A, Cao R, Calmelat R, Lin F, Casaburi R, Rossiter HB, Porszasz J. Transcutaneous PCO2 for exercise gas exchange efficiency in chronic obstructive pulmonary disease. COPD 18: 16–25, 2021. doi: 10.1080/15412555.2020.1858403. [DOI] [PubMed] [Google Scholar]
- 38.Jones NL, McHardy GJR, Naimark A, Cambell EJM. Physiological dead space and alveolar-arterial gas pressure differences during exercise. Clin Sci 31: 19–29, 1966. [PubMed] [Google Scholar]
- 39.Jones NL, Robertson DG, Kane JW. Difference between end-tidal and arterial PCO2 in exercise. J Appl Physiol Respir Environ Exerc Physiol 47: 954–960, 1979. doi: 10.1152/jappl.1979.47.5.954. [DOI] [PubMed] [Google Scholar]
- 40.Kreit JW. Volume capnography in the intensive care unit: physiological principles, measurements, and calculations. Ann Am Thorac Soc 16: 291–300, 2019. doi: 10.1513/AnnalsATS.201807-501CME. [DOI] [PubMed] [Google Scholar]
- 41.Verscheure S, Massion PB, Verschuren F, Damas P, Magder S. Volumetric capnography: lessons from the past and current clinical applications. Crit Care 20: 184, 2016. doi: 10.1186/s13054-016-1377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandes TM, Alotaibi M, Strozza DM, Stringer WW, Porszasz J, Faulkner GG, Castro CF, Tran DA, Morris TA. Dyspnea postpulmonary embolism from physiological dead space proportion and stroke volume defects during exercise. Chest 157: 936–944, 2020. doi: 10.1016/j.chest.2019.10.047. [DOI] [PubMed] [Google Scholar]
- 43.Lambert LL, Baldwin MB, Gonzalez CV, Lowe GR, Willis JR. Accuracy of transcutaneous CO2 values compared with arterial and capillary blood gases. Respir Care 63: 907–912, 2018. doi: 10.4187/respcare.05936. [DOI] [PubMed] [Google Scholar]
- 44.Sridhar MK, Carter R, Moran F, Banham SW. Use of a combined oxygen and carbon dioxide transcutaneous electrode in the estimation of gas exchange during exercise. Thorax 48: 643–647, 1993. doi: 10.1136/thx.48.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stege G, van den Elshout FJ, Heijdra YF, van de Ven MJ, Dekhuijzen PN, Vos PJ. Accuracy of transcutaneous carbon dioxide tension measurements during cardiopulmonary exercise testing. Respiration 78: 147–153, 2009. doi: 10.1159/000187631. [DOI] [PubMed] [Google Scholar]
- 46.Elbehairy AF, Webb KA, Laveneziana P, Domnik NJ, Neder JA, O'Donnell DE; Canadian Respiratory Research Network (CRRN). Acute bronchodilator therapy does not reduce wasted ventilation during exercise in COPD. Respir Physiol Neurobiol 252-253: 64–71, 2018. doi: 10.1016/j.resp.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 47.Marvin PM, Baker BJ, Dutt AK, Murphy ML, Bone RC. Physiologic effects of oral bronchodilators during rest and exercise in chronic obstructive pulmonary disease. Chest 84: 684–689, 1983. doi: 10.1378/chest.84.6.684. [DOI] [PubMed] [Google Scholar]