Abstract
Inflammation of the kidney is a key contributor to proliferative glomerulonephritis, and kidney damage during glomerulonephritis can lead to renal failure. The immune response associated with glomerulonephritis episodes is a major determinant of patient outcomes, and understanding this response is paramount for effective therapeutic treatment. Neutrophils are the first responders to sites of infection or tissue injury and are a significant cellular infiltrate during proliferative glomerulonephritis. This immune cell was initially recognized as a “blunt” nonspecific effector cell that was recruited to kill pathogens and then die quickly. However, recent studies have shown that the behavior and function of neutrophils are substantially more complex. Neutrophil recruitment to inflammatory sites must be carefully regulated so that these potent cells accurately arrive at tissue sites and perform their functions without nonspecific injury to other locations. As the kidney contains unique microvasculature befitting their specialized role in blood filtration, the recruitment of neutrophils in the renal environment differs from other organs. This Mini-Review will describe how advances in live-animal (intravital) imaging led to the discovery of novel recruitment pathways in the kidney, particularly in the glomeruli, and highlight these differences to canonical neutrophil recruitment. In addition, molecular engagement of surface molecules that lead to intracellular signaling, which is followed by neutrophil capture in the glomeruli, is also briefly discussed. Finally, the contribution of other immune cells in renal neutrophil recruitment, the fate of the emigrated neutrophils after inflammation, and the relevance of mouse models compared with human glomerulonephritides will also be explored.
Keywords: glomerulonephritis, intravital imaging, neutrophils, neutrophil recruitment, sterile inflammation
INTRODUCTION
The kidneys are essential organs in the urinary system and are responsible for the reabsorption of water, amino acids, and glucose and the excretion of urea and ammonium through urine formation. Concurrently, the kidneys also have important roles in maintaining homeostasis and the secretion of various hormones. As such, kidney failure is associated with high mortality and morbidity. The kidney processes a unique vascular system not seen in other organs due to its filtration functions (1). The heart of the kidney filtration ability is the glomerulus, which is a high-pressure, high-flow capillary bed surrounded by arterioles. This filtration unit creates an ultrafiltrate of plasma and is drained through the proximal tubules, loop of Henle, and distal tubules, which span the cortex and medulla. As the ultrafiltrate passes through these segments, secretion and reabsorption of various substances from the filtrate occur to ultimately result in the formation of urine (1). To achieve this function, distinct capillary networks are observed in the kidney: the first capillary network exists in the glomeruli followed by peritubular capillaries in the cortex and a final capillary network following the vasa recta in the medulla (1). As a result, the unique vasculature of the kidney has culminated in distinctive mechanisms of immune cell recruitment during renal disease. This Mini-Review will present an immunological perspective of kidney injury by focusing on the recruitment of neutrophils to renal vasculature in the context of sterile inflammation such as ischemia-reperfusion and glomerulonephritis, two forms of kidney injuries that have strong immunological components (2).
Neutrophils are innate immune cells and are the first responders to the tissues during acute inflammation. Traditionally, these cells were recognized as blunt tools of the immune system whose sole purpose was to arrive at the site of infection, release robust oxidants and proteases to kill pathogens, and then die at the stricken site (3). Indeed, an inability to recruit neutrophils during infection in leukocyte adhesion deficiency (LAD) disorders results in recurrent severe, life-threatening infections (4). In contrast, aberrant recruitment of neutrophils causes unwanted inflammation and tissue and organ damage. Therefore, the recruitment of neutrophils toward a site of inflammation needs to be tightly regulated such that this effector cell can arrive at the site of injury or infection, perform its functions, and then leave or undergo apoptosis without resulting in further damage (5). Of particular importance, since neutrophil recruitment uses different molecules depending on the stimulus, for example, infection versus sterile injury and tissue or organ involved, it is essential to understand the molecular mechanisms that drive tissue-specific recruitment.
Neutrophils were initially recognized as important cells contributing to glomerular injury, but the predominant perspective of neutrophils being short-lived, nonspecific effectors at that time (6) resulted in the research community focusing on the role of other immune cells such as dendritic cells, monocytes/macrophages, and B and T cells during renal inflammation (2). With a resurgence of interest in neutrophils due to discoveries of novel roles and functions as well as new state-of-the-art microscopy techniques that can visualize neutrophil recruitment in vivo (3, 7), the importance of neutrophils in glomerulonephritis is explored in this Mini-Review. This article will focus on recent studies that describe how the unique renal vasculature causes differences in neutrophil recruitment, how this recruitment contributes to kidney injury, and the fate of the extravasated neutrophil. The limitations of using mouse models to mimic human glomerulonephritides diseases will also be briefly discussed.
A BRIEF PRIMER ON INTRAVITAL IMAGING
Time-lapse intravital microscopy provides a distinct glimpse into the lives and dynamic behaviors of diverse immune cell populations in tissues and organs under experimental conditions that closely mirror the natural environment. As an imaging technique, intravital imaging is not new as it has been used since the 19th century to visualize leukocyte trafficking in translucent tissues (8). Bright-field imaging of these tissues has led to novel findings in how leukocytes and endothelial cells interact during inflammation. However, the main limitation of this elementary technique that relies on visible light is the requirement for uniformly colorless cells to be sufficiently slowed by adhesion and, therefore, differentiated from fast-flowing cells in the blood (9). The introduction of fluorescent probes and modern optical imaging equipment has unlocked thrilling possibilities for real-time biological observations. For example, the bulk of intravital imaging is now fluorescence based as immune cells are visualized through the use of labeled fluorescent probes and/or genetically modified animals expressing specific fluorescent proteins (10). This strategy now allows real-time visualization and tracking of multiple different immune cells in vivo, under both basal and inflammatory conditions, which overcomes the initial intravital constraint of solely observing adhered colorless cells through visible light.
The increasing variety of advanced microscopes also provide important advancements to intravital imaging. Laser-scanning single-photon confocal microscopes that exclude out-of-focus light via point illumination and pinhole apertures can be used for intravital imaging at the expense of deep tissue imaging and also acquisition speed. To overcome the limitation on acquisition rates, spinning-disk confocal microscopes (with single-photon lasers) have rapid image acquisition and are ideal for capturing dynamic cell-cell interactions (11). These spinning-disk microscopes use an opaque disk dotted with multiple pinholes which, when rotated at high speeds such as 5,000 rpm, allow the pinholes to scan the sample in tandem and rapidly build up an image. This process significantly improves acquisition speed but reduces resolution and is still limited by single-photon laser penetration depth. Finally, in tissue samples that require significant depth penetration, multiphoton microscopes are used (12). These microscopes exploit different principles of fluorescence microscopy through the use of pulsed infrared laser systems that generate a dense flux of photons in a single pulse. As a result, two (or more) photons arrive at a single fluorescent molecule within attosecond (10−18s) of each other to excite the molecule. Multiphoton systems remove the need for pinhole apertures allowing greater light capture and have greater penetration due to the near-infrared laser, supporting tissue imaging up to a depth of 500 µm. A significant downside to this system is the nonlinear excitation of two-photon microscopy, and consequently, the excitation spectra of fluorescent proteins or antibodies are significantly different from single-photon systems. In the context of kidney intravital imaging, recent use of the multiphoton system has greatly contributed to the understanding of renal neutrophil recruitment. This Mini-Review focuses on neutrophil recruitment in the kidney during glomerulonephritis, but the readers are encouraged to peruse recent reviews that discuss how technical advances in intravital microscopy have yielded insights into renal cells and structure-function relationships in the kidney (13) and compared the use of different microscopes and fluorescent probes versus genetic lineage tracing technologies to determine regeneration after kidney injury (14).
EARLY STUDIES ON NEUTROPHIL RECRUITMENT IN THE KIDNEY
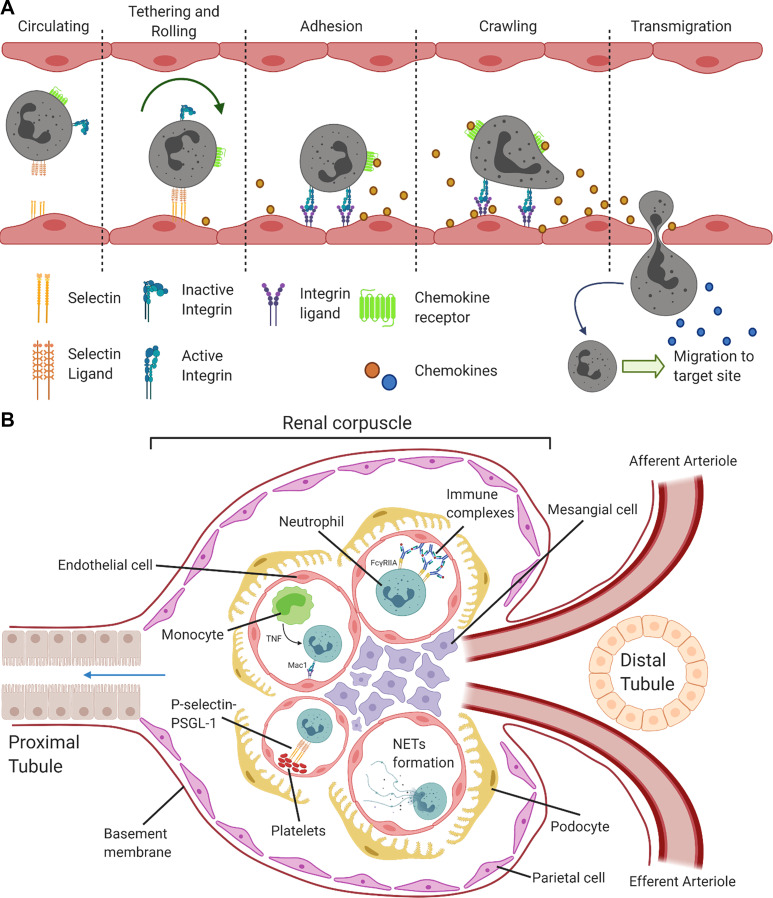
Intravital imaging of translucent tissues as well as the skin revealed that neutrophil recruitment occurs in postcapillary venules and follows a canonical sequence of tethering and rolling, adhesion, crawling, and eventual transmigration (Fig. 1A). At the end of this process, neutrophils are then guided by chemokines toward the infection or site of injury. It is important to note that this form of neutrophil recruitment was also observed during superficial imaging of the kidney cortex, but later studies using multiphoton imaging revealed significantly different recruitment mechanisms in the renal glomeruli. Due to space limitations, the classic pathway of neutrophil recruitment will not be discussed in this Mini-Review and the readers are directed to a recent comprehensive review (15).
Figure 1.
Neutrophil recruitment in vivo. A: canonical neutrophil recruitment sequence. Neutrophil recruitment from the vasculature into inflamed tissue is depicted in this illustration. Neutrophil tethering and rolling is dependent on endothelial cell selectins binding to selectin ligands on neutrophils, which slows down neutrophils flowing in the blood stream. Encountering chemokines and cytokines, neutrophils then upregulate integrins on activation and adhere to the endothelium. Neutrophils also begin to crawl along the endothelial cells in a direction along a chemokine gradient. Reaching a preferred site on the endothelium, neutrophils begin to transmigrate across ECs and release proteases to transverse the basement membrane. Emigrated neutrophils then follow another chemokine gradient to the inflamed site. A brief description of adhesion molecules and chemokines involved in neutrophil recruitment are selectins and ligands: P-, E-, and L-selectin with P-selectin glycoprotein ligand 1 (PSGL-1); integrins and ligands: lymphocyte function-associated antigen (LFA-1, CD11a complexed with CD18, αLβ2), macrophage-1 antigen (Mac-1, CD11b-CD18, αMβ2) with intercellular adhesion molecules 1 and 2; and chemokines: chemokines with a glutamate-leucine-arginine motif before the amino-terminal CXC motif (ELR-CXC) such as CXCL8 (known as IL-8 in humans), its analog in mice: CXCL1 [or keratinocyte-derived chemokine (KC)], CXCL2 [or macrophage-inflammatory protein 2 (MIP-2)], and CXCL5 (or LPS-induced CXC chemokine (LIX)). These chemokines signal through CXCR2 on neutrophils. Created with BioRender.com. B: neutrophil recruitment in kidney glomeruli. During glomerulonephritis, neutrophils were not observed to roll in glomerular capillaries. Instead, neutrophils were captured by various molecules and arrested in the capillaries. In the first method of neutrophil arrest, neutrophils bind directly to immune complexes deposited on the endothelium via Fcγ receptors. Alternatively, monocytes can assist in neutrophil capture by the production of TNFα, which upregulates integrin expression on neutrophils and slow their crawling. Finally, platelets also exist as an “secondary” capture method by serving as a bridge between endothelial cells and neutrophils. This interaction occurs through selectin to selectin-ligand interaction. In addition to the release of cytotoxic molecules such as ROS production, neutrophils are also able to release NETs causing damage to surrounding tissues and present autoantigen. ECs, endothelial cells; NETs, neutrophil extracellular traps; ROS, reactive oxygen species; TNFα, tumor necrosis factor alpha. Created with BioRender.com.
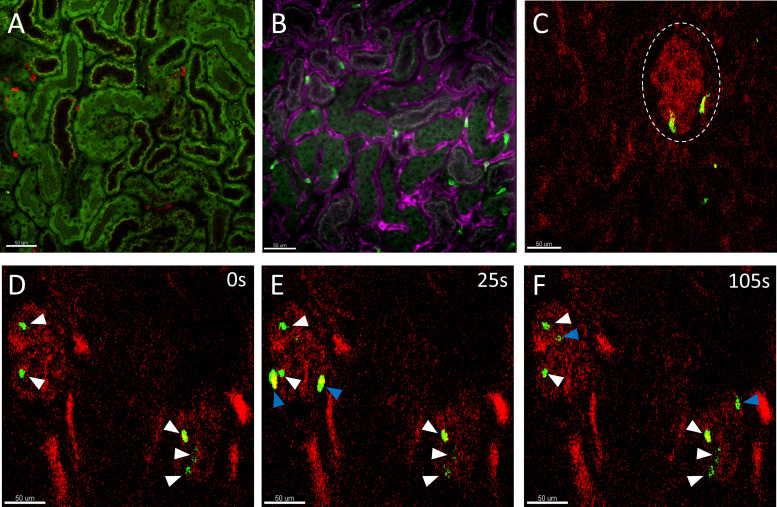
Before the advent of multiphoton intravital microscopy, neutrophil recruitment during noninfectious inflammation could only be investigated using mouse models (such as ischemia-reperfusion) that caused indiscriminate injury to multiple regions of the kidney. These studies focused on visualizing neutrophil recruitment in the peritubular capillaries in the kidney cortex. For example, using spinning-disk laser confocal microscopes to visualize the cortex of the kidney, neutrophils (bright green) were observed to be present in the capillaries (magenta) surrounding renal tubules (dull green) (Fig. 2, A and B). During renal ischemia-reperfusion, E-selectin was found to be important for neutrophil infiltration and aggravation of kidney injury (16, 17). In contrast, P-selectin on platelets instead of endothelial cells (ECs) were more important for neutrophil infiltration into the parenchyma (18, 19). Other molecules have also been discovered to be important in neutrophil recruitment. Binding of neutrophil hyaluronic acid to endothelial CD44 regulates neutrophil infiltration after ischemia-reperfusion (20). In contrast, adenosine 2A receptor on neutrophils serves as a negative regulator of neutrophil recruitment as its activation reduces neutrophil transmigration and vascular permeability (21). Neutrophil adhesion led to the margination of neutrophils intravascularly before transendothelial migration of neutrophils into the kidney interstitium occurred (21). Later, these pathways were examined in a renal ischemia-reperfusion study using intravital microscopy with Lysozyme M (LysM)-GFP+ mice, which were expressed in neutrophils (and to a lesser extent, monocytes). Rolling neutrophils in the kidney cortex were observed to be dependent on E-selectin, which was consistent with earlier studies (16). Further dissection of this pathway revealed that E-selectin engagement signaled through SLP-76 (SH2 domain containing leukocyte phosphoprotein of 76 kDa) and ADAP (adhesion- and degranulation-promoting adaptor protein), which then mediated inside-out activation of lymphocyte function-associated antigen 1 (LFA-1) resulting in firm adhesion of neutrophils and recruitment into inflamed tissue (16).
Figure 2.
Intravital snapshots of the murine kidney cortex. A: neutrophils (red) are observed among spaces located between renal tubules (dull green, autofluorescence) in the kidney cortex. Some S1 and S2 renal tubules that can be differentiated as S1 proximal tubules have coarse green-red (yellow) autofluorescence vs. S2 with only fine green autofluorescence. During disease, these neutrophils would transmigrate from the capillaries into the kidney interstitium contributing to renal injury. Scale bar 50 μm. B: neutrophils (green) that are between renal tubules are located in the peritubular capillaries (magenta). Labeling with additional fluorescent antibodies against capillaries diminished the autofluorescence contrast between S1 and S2 renal tubules. Scale bar 50 μm. A and B were visualized with a Leica DMI6000 and Nikon Ti2 inverted spinning-disk confocal microscope, respectively, equipped with a ×20 Plan Apo objective lens (NA0.7 and NA0.8) on a C57Bl/6 mouse. Alexa Fluor (AF)594-conjugated and AF488-conjugated Anti-Ly6G (clone 1A8) were used to label neutrophils in A and B correspondingly. AF647 Isolectin B4 and Brilliant Violet 421 Anti-CD31 (clone 390) were used to label the endothelial cells in B, the acquired signals were combined into a single color (magenta) to highlight the endothelium. C: neutrophils (green) transiently present in the ball-like glomerulus (red, dotted white circle outline) labeled with AF594-conjugated 10 kDa dextran and visualized by a custom-made multiphoton microscope equipped with coherent tunable femtosecond Ti-Sapphire laser tuned to 780 nm (×20 water objective, NA0.95) on a Lysozyme M (LysM)-GFP mouse. Scale bar 50 μm. D–F: timelapse snapshots of neutrophils (green) in glomeruli (red) under 2 min, 2 glomeruli could be seen in this field of view. Some neutrophils persisted in the glomeruli during the imaging period (white arrows), whereas others were observed to rapidly flow through the glomeruli (blue arrows). During anti-GBM glomerulonephritis, these neutrophils significantly increase their dwell time within the glomeruli through the upregulation of adhesion factors and interaction with other immune cells and cause injury through release of reactive oxygen species, proteases, and NETs (Fig. 1B). Scale bar 50 μm. GBM, glomerular basement membrane; NETs, neutrophil extracellular traps.
ADVANCES IN INTRAVITAL MICROSCOPY LEADING TO NOVEL NEUTROPHIL RECRUITMENT PATHWAYS
Neutrophil recruitment in the kidney cortex also occur through the capillaries of the renal glomeruli. Glomerulonephritides, as a whole, are the third leading cause of end-stage renal disease and a leading cause for renal transplantation in the United States (https://adr.usrds.org/). Glomerulonephritis is categorized into proliferative glomerulonephritis, which is identified with increased glomerular cellularity due to immune cell recruitment and proliferation of intrinsic cells, and nonproliferative glomerulonephritis (22). Clinically, manifestations of glomerulonephritis are hematuria, proteinuria, hypertension, edema, and decreased urine output (23). In most cases of proliferative glomerulonephritis that are not due to intrinsic immunological complications (such as postsurgery or critical illnesses requiring intensive care), immune complexes play a significant role. Under physiological conditions, circulating immune complexes (ICs) are rapidly cleared from blood by phagocytes and are of little pathological significance. However, during glomerulonephritis caused by autoimmune diseases such as lupus nephritis, paraproteinemia (the presence of excessive amounts of monoclonal γ-globulin in blood), or certain postinfections, circulating immune complexes lodge within the vasculature and are subsequently deposited on the endothelium where they eventually accumulate in the extravascular space (2).
Immune complexes can also be generated in situ (such as antiglomerular basement membrane antibodies, antineutrophil cytoplasmic antibodies, and antibody-mediated transplant rejection). Kidney glomeruli capillaries are recurrent sites where ICs deposit (24). As a consequence of IC deposition, Fcγ receptors (FcγRs) on neutrophils are cross linked, inducing the aggregation of receptors, which is followed by activation and results in glomerular neutrophil recruitment (25); a situation that might be exacerbated in patients with lupus expressing the nonsynonymous Macrophage-1 antigen (Mac-1, CD11b-CD18, αMβ2) functional variant rs1143679 (26). This variant, rs1143679, is a result of an Arg to His substitution at position 77 of the extracellular β-propeller unit of CD11b (27, 28). The adhesion molecule Mac-1 is typically considered to be proinflammatory. For example, absence of CD18 integrins in patients with LAD leads to recurrent infections (4). However, Mac-1 also exhibits an inhibitory role through allostery interactions with FcγRs (28). By reversing Mac-1 inhibition of FcγRIIA, the rs1143679 variant results in increased FcγRIIA-dependent recruitment to IgG-coated endothelium and may be a contributing factor to neutrophil influx during lupus nephritis (26). Furthermore, the presence of ICs on the endothelium promotes tumor necrosis factor (TNF) production, which further drives neutrophil influx (29). Development of proliferative glomerulonephritis might also occur due to a dysfunction in the alternative activation pathway of complement where complement proteins accumulate along glomeruli walls resulting in neutrophil recruitment during complement-dependent glomerulonephritis. The fact that neutrophils are recruited to glomerular capillaries is unusual as canonical neutrophil recruitment occurs in postcapillary venules. As a result, it is of compelling interest to understand neutrophil behavior in glomeruli through systematic imaging of the immune response.
Mechanisms of Neutrophil Recruitment in the Kidney Glomeruli
Before the advent of multiphoton microscopy, the glomeruli were extremely difficult to visualize as the kidney is a solid organ and even superficial glomeruli are located at least 100 µm beneath the kidney surface. Use of multiphoton microscopy has overcome several of these restrictions and have been pivotal in investigating the recruitment and function of neutrophils in the glomeruli (Fig. 1B). The use of anti-GBM (glomerular basement membrane) antibodies is the commonly accepted approach to study glomerulonephritis in mice (2, 12). Using different monoclonal antibodies [anti-Gr-1 and anti-Ly6G (clone 1A8)] in separate experiments to label neutrophils for multiphoton microscopy, Devi et al. (12) observed that neutrophils would persist in glomerular vasculature for several minutes. A subset of these neutrophils (∼50%) would also crawl within the glomeruli, and this crawling was independent of blood flow. Induction of glomerulonephritis significantly increased the dwell time of neutrophils (defined as the number of minutes neutrophils were present in a field-of-view from a 60-min recording) to more than 20 min. Both the dwell times of static and crawling neutrophils were observed to increase after glomerulonephritis. Importantly, when these two sets of neutrophils were adhered, they were responsible for the generation of reactive oxygen species (ROS) that caused tissue injury and resulted in glomerular dysfunction. Intravital imaging revealed that Mac-1 was required for neutrophil retention in the glomerulus during inflammation (12). In Fig. 2C, multiphoton microscopy was used to demonstrate the presence of neutrophils (green) located within ball-like glomeruli (red). Under basal conditions, most neutrophils flowed through glomeruli, whereas some dwelled in the glomeruli for a few minutes (Fig. 2, D–F).
To study the role of immune complexes in mediating neutrophil recruitment in glomeruli, humanized mice expressing FcγRIIA but not murine Fc common γ-chain (FcγRIIA+/−γ–/–) was used in a separate study of glomerulonephritis (24). In this study, bone marrow-derived mature neutrophils from FcγRIIA+/−γ–/– mice or γ–/– mice were differentially labeled with two different fluorescent dyes and injected into anti-GBM-treated recipient mice. This dual labeling allowed concurrent tracking of two different neutrophil populations. Intravital imaging of glomeruli revealed that FcγRIIA+/−γ–/– neutrophils persisted significantly longer in recipient mice as compared with neutrophils isolated from γ–/– mice. In vitro binding assays of immobilized IgG to FcγRIIA showed that the lifetime bonds of these two molecules was increased in an F-actin-dependent manner and regulated through Abl/Src kinases. Abl/Src kinases are part of an intracellular signaling pathway of cell surface molecules, such as integrins, that is induced by extracellular stimuli. During capture of neutrophils under physiological flow conditions, signaling via Abl/Src kinases regulates F-actin polymerization, which then strengthens IgG-FcγR interactions for neutrophils experiencing mechanical forces (as in the case as they transit through glomeruli) (24). Bosutinib, which inhibits the Abl/Src kinases, reduced the capture of FcγRIIA+/−γ–/– neutrophils in the glomeruli. By treating anti-GBM mice with bosutinib over a 21-day period, neutrophil accumulation as well as kidney injury was reduced as measured by albuminuria, crescent formation, and tubulointerstitial injury (24).
Contribution of other immune cells to renal neutrophil recruitment.
A plethora of immune cells are present in the kidney, and these immune cells can either be distributed in the renal interstitium (30) or transiting through the renal vasculature at any given time. This section shall discuss studies that examined immune cells in the vasculature (monocytes and platelets), which mediated neutrophil capture during glomerulonephritis (Fig. 1B). Generally, monocytes exist in two populations—the classical monocyte [C-C Motif Chemokine Receptor (CCR) 2hiC-X3-C Motif Chemokine Receptor 1 (CX3CR1)lo] that is recruited into inflamed tissues in a CCR2-dependent manner and produces inflammatory factors and the regulatory nonclassical monocyte (CCR2loCX3CR1hi) that patrols vasculatures under resting states and performs an immune homeostatic role. Using intravital microscopy of CX3CR1GFP+ mice, patrolling monocytes were observed in the glomeruli under baseline and inflamed conditions (31). This patrolling behavior was dependent on β2 and α4 integrins. During immune complex-mediated glomerulonephritis, depletion of monocytes via clodronate treatment reduced ∼60% of neutrophil recruitment to glomerular capillaries. Imaging the glomeruli under these conditions, monocytes were observed to interact with reactive oxygen species (ROS)-generating neutrophils, which increased the glomerular retention of neutrophils leading to neutrophil activation, increased ROS production, and renal injury. Monocytes were observed to be responsible for producing TNFα, and the depletion of monocytes or inhibition of TNFα reduced neutrophil retention, ROS generation, and renal injury (31).
Another important immune cell is the platelet because in addition to roles in thrombosis and hemostasis, platelets are important during inflammation as they can directly interact with immune cells, including neutrophils, through surface receptors (32). Furthermore, microthrombi formation has been observed in a subset of patients with lupus nephritis, although the direct clinical contribution of microthombi to the severity of renal damage is debatable (33, 34). Using fluorescently labeled platelets transferred into recipient animals, platelets were observed to be recruited to inflamed glomeruli in a integrin αIIbβ3/glycoprotein VI (GPVI)-dependent manner during anti-GBM glomerulonephritis (35). To study whether endogenous platelets interacted with neutrophils and mediated neutrophil recruitment, intravital microscopy of LysM-GFP+ mice was performed where endogenous platelets were labeled with fluorescently tagged antibody against CD49b (33). Platelets were observed to “touch-and-go” with endothelial cells and neutrophils in the glomeruli, and the glomerular inflammation increased platelet-neutrophil interaction. P-selectin was responsible for increased interaction during inflammation as well as promoting neutrophil recruitment and ROS production. Depletion of platelets or inhibition of platelet-activating factor reduced both neutrophil recruitment and activation. The Chemokine C-X-C motif ligand 7 (CXCL7) was discovered to regulate this behavior. However, release of ROS by adhered neutrophils was not affected, as this was instead regulated by adenosine disphosphate and thromboxane A2 pathways (33).
Neutrophil Extracellular Traps during Glomerulonephritis
Although neutrophils were captured in the glomeruli capillaries, intravital imaging revealed that some neutrophils that were present released neutrophil extracellular traps (NETs) (36) (Fig. 1B). NETs are web-like structures of DNA and proteins released from neutrophils that trap pathogens (37). NETs formation (NETosis) is a regulated event stimulated by activation of toll-like receptors, Fc receptors, and cytokines such as interferons, TNF and IL-8. The webs of NETs contain a variety of cytotoxic molecules such as neutrophil elastase that degrades proteins, peptidyl arginine deiminase type 4 (PAD4) that citrullinates histones, and gasdermin D that generates membranal pores (37). NETs are beneficial as host defense against pathogens by trapping and killing pathogens, but disproportionate NET formation (especially during a cytokine storm) triggers inflammatory cascades, damages surrounding tissues, and can cause permanent injury to multiple organs including the renal system (38). NETs are particularly important in lupus nephritis as factors such as multiple immune complexes and circulating apoptotic microparticles that are produced during systemic lupus erythematosus (SLE) accelerate NETosis (39–41). This is collaborated in patients with SLE who demonstrate increased NET formation, and these patients with active SLE lesions exhibit impaired degradation of NETs due to anti-NETs antibodies and DNase I inhibitors (42, 43). Furthermore, NETs are also able to present autoantigens such as myeloperoxidase and proteinase-3, which are then bound by circulating autoantibodies in patients with lupus nephritis (44). Intravital microscopy was used to visualize NETs formation in anti-GBM-induced glomerulonephritis. In this acute model, NETs formation was observed to be transient and short lived (36). This was likely due to the high-shear stress environment present in the glomeruli. Although NETs formation was reduced using DNase I or PAD4-inhibitor CI-amidine, albuminuria was not significantly reduced in this model of acute glomerulonephritis. NETs formation reduction did reduce hematuria which suggests that NETs might only provide a modest contribution of glomerular injury during acute glomerulonephritis (36).
THE FATE OF THE EMIGRATED NEUTROPHIL
Initially considered as a short-lived immune cell with a circulating half-life of 6–8 h in humans and mice (45), a newer study has proposed that the life span of neutrophils is 5.4 days in humans (18 h for mice) (46). It must be noted that orally administered deuterium-labeled water was used to track human neutrophils in this study and could have overestimated the longevity of neutrophils in circulating blood as bone marrow neutrophils could have been labeled as well (47). Nevertheless, the life span of activated neutrophils increases significantly following inflammation due to the presence of cytokines, growth factors, and other products at the inflamed tissue (48). Tracking of photo-activated neutrophils at a site of sterile injury revealed a life span as long as 48 h (5). This allows the neutrophil to persist at the site of injury to perform complex functions such as inflammatory resolution, tissue healing, or shaping adaptive immune responses.
Following the fulfillment of function, neutrophils are postulated to die and are then cleared by macrophages in situ or in the liver, spleen, and bone marrow, particularly in the context of infection (15). However, the mechanisms of neutrophil cell death and turnover after sterile inflammation remain poorly defined as intravital imaging of these organs has not revealed significant uptake of dying neutrophils and the depletion of monocytes or macrophages did not affect neutrophil disappearance during tissue healing (5). Another possibility for the dissipation of neutrophils after inflammation is a “reverse transmigration” exit strategy. In zebrafish, neutrophils were able to leave the tissue and return to the vasculature through reverse transmigration after sterile injury (49). This has also been observed after sterile injury in the liver (5), postischemic muscle (50), and exposure to UV light on the skin (51). The implications of this neutrophil behavior are significant, but the fate and function of these reverse transmigrated neutrophils remain uncertain, as these neutrophils disseminated inflammation following ischemia-reperfusion in a Cremaster injury model (50) or UV light exposure on the skin (51) but caused no injury after hepatic sterile inflammation (5). Instead, reverse transmigrated neutrophils terminated their journey in the bone marrow and underwent apoptosis, suggesting a potential mechanism that allowed the host to recycle neutrophils following sterile injury in the liver (5). Further work is needed to determine the role of reverse transmigrating neutrophils during health and in disease.
GLOMERULONEPHRITIDES IN HUMANS AND THE LIMITATIONS/RELEVANCE OF MOUSE MODELS
This Mini-Review has approached glomerulonephritis as a single kidney disease, but glomerulonephritides in humans are a disparate family of renal disorders with differing pathogeneses (Table 1). Therefore, the singular use of the anti-GBM glomerulonephritis mouse model to study the immune response represents a very limited glimpse of immunity in the wide range of glomerulonephritides etiologies. Notably, anti-GBM disease is also a rare autoimmune form of glomerulonephritis. Furthermore, although most types of glomerulonephritides are immune-mediated, not all glomerulonephritides have significant inflammatory components, as other causes such as metabolic disease (diabetes, amyloidosis) can also result in glomerular damage.
Table 1.
Highlights of the forms of proliferative (and often crescentic) glomerulonephritis in humans
| Human Proliferative Glomerulonephritis | Features and Observations | Antibody Patterns | Effector Cells (Leukocytes)in the different forms of human glomerulonephritis |
|---|---|---|---|
| Anti-GBM glomerulonephritis | Autoantibodies develop against the α3 chain of type IV collagen (noncollagenous domain). | Linear | Neutrophils, T cells and macrophages |
| ANCA-associated glomerulonephritis | Can be subdivided into Wegener’s granulomatosis, microscopic polyangiitis, and Churg–Strauss granulomatosis. | Minimal, “Pauci-Immune” | |
| SLE nephritis | Complex multiorgan autoimmune disease triggering lupus nephritis. Highly variable histological features in glomeruli with 6 classes for histology classifications. | Granular | |
| IgA nephropathy | Most common type of glomerulonephritis in most countries and cause of mesangial proliferative glomerulonephritis (NIH GARD). Shares features with Henoch–Schonlein Purpura, a systemic form of vasculitis. | Granular | |
| Membranoproliferative (mesangiocapillary) glomerulonephritis | A group of pathological descriptions of glomerular lesions made up of three subgroups. Some secondary cases are associated with viral infections. | Mixed (fine to coarse) depending on subgroup | |
| Postinfectious glomerulonephritis | Found after bacterial infections (typically Streptococcus pyogenes) but can also occur after infection by other organisms. | Granular |
Several glomerulonephritis diseases are also further classified into subgroups depending on histological findings and proliferative changes determined by light microscopy. Therefore, although serological testing, urinalysis, blood counts, etc., are important during clinical evaluation of the patient, the kidney biopsy remains the gold standard for diagnosis. A nephrologist can also be consulted for renal biopsy indications and differential diagnosis. Although there are multiple stages in the stages of pathogenesis leading to glomerulonephritis (e.g. loss of tolerance, T helper polarization, and B cell modification/production of autoantibodies), the end stage is the recruitment of effector immune cells such as neutrophils, T cells, monocytes, and macrophages, which leads to kidney injury. ANCA, antineutrophil cytoplasmic antibody; GBM, glomerular basement membrane; NIH GARD, National Institutes of Health Genetic and Rare Diseases Information Center; SLE, systemic lupus erythematosus.
Despite the broad range of immune dysregulation that leads to glomerulonephritis, the downstream inflammatory mechanisms causing renal injury is similar. Indeed, common to glomerulonephritis is the participation of neutrophils, which accumulate at the glomeruli rapidly and is responsible for injury during inflammation (12). The crucial role of neutrophils in glomerulonephritis has been documented four decades ago (6), and they are observed to participate in a number of human glomerulonephritis diseases such as lupus nephritis, anti-GBM disease, and antineutrophil antibody-associated vasculitis (52–54). Although mice are not humans and may not accurately mimic human molecular mechanisms of disease during inflammation, the mechanisms delineated using mice models do provide a better understanding of the immunological response that occur during human disease.
Due to its consistency in evoking a strong inflammatory response and rapid time course, the anti-GBM glomerulonephritis mouse model is now commonly used to study immune effector mechanisms in regulating glomerular injury. However, the observations from the anti-GBM model might not translate well to other forms of glomerulonephritis, particularly in the nature of the induction of disease. To study the pathogenesis of other forms of glomerulonephritis, some specific models do exist: for example, 1) anti-myeloperoxidase (MPO) IgG models to study antineutrophil cytoplasm autoantibody vasculitis and 2) anti-Thy-1 IgG models to study mesangial proliferative glomerulonephritis. However, due to the considerable diversity in the different types of human glomerulonephritides, there is a current lack of mouse models to investigate all the myriad forms of human glomerulonephritides diseases.
CONCLUDING REMARKS
Neutrophils are an essential front-line immune cell necessary for the clearance of pathogens. Recently, they have also been implicated as important modulators in tissue healing as well as other biological processes (3). However, the roles of neutrophils in adaptive immunity and regular homeostatic functions require more studies. At this time, evidence points toward neutrophils as being detrimental in noninfectious renal inflammation. No treatment exists to inhibit neutrophil responses during inflammation in the kidney as current approaches rely on corticosteroids with moderate efficacy and global immunosuppression. An improved understanding of the role of neutrophils and how they are recruited to the kidney will pave the way for therapeutic strategies that can fine-tune the renal inflammatory response to provide a scalpel approach. By explicitly targeting the recruitment of neutrophils at the renal level without affecting systems elsewhere will minimize risk of infection and malignancy while modulating the immune system to attenuate renal damage and treat kidney injuries.
GRANTS
P. X. Liew is supported through Dr. Tanya Mayadas US National Institute of Health (R01AI152522).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
P.X.L. conceived and designed research; P.X.L. performed experiments; P.X.L. analyzed data; P.X.L. interpreted results of experiments; P.X.L. prepared figures; P.X.L. drafted manuscript; P.X.L. edited and revised manuscript; P.X.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The author thanks Dr. Jerrold Turner for the use of Leica microscope, the Harvard Medical School MicRoN (Microscopy Resources on the North Quad) Core facility for use of Nikon microscope, and Dr. Mark J Miller for training, education, and use of his multiphoton microscope. Due to space limitations, the author apologizes for the many manuscripts that couldn’t be cited in this review.
REFERENCES
- 1.Molema G, Aird WC. Vascular heterogeneity in the kidney. Semin Nephrol 32: 145–155, 2012. doi: 10.1016/j.semnephrol.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Ernandez T, Mayadas TN. The changing landscape of renal inflammation. Trends Mol Med 22: 151–163, 2016. doi: 10.1016/j.molmed.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ley K, Hoffman HM, Kubes P, Cassatella MA, Zychlinsky A, Hedrick CC, Catz SD. Neutrophils: new insights and open questions. Sci Immunol 3: eaat4579, 2018. doi: 10.1126/sciimmunol.aat4579. [DOI] [PubMed] [Google Scholar]
- 4.Hanna S, Etzioni A. Leukocyte adhesion deficiencies. Ann N Y Acad Sci 1250: 50–55, 2012. doi: 10.1111/j.1749-6632.2011.06389.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Hossain M, Thanabalasuriar A, Gunzer M, Meininger C, Kubes P. Visualizing the function and fate of neutrophils in sterile injury and repair. Science 358: 111–116, 2017. doi: 10.1126/science.aam9690. [DOI] [PubMed] [Google Scholar]
- 6.Cochrane CG, Unanue ER, Dixon FJ. A role of polymorphonuclear leukocytes and complement in nephrotoxic nephritis. J Exp Med 122: 99–116, 1965. doi: 10.1084/jem.122.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kubes P. The enigmatic neutrophil: what we do not know. Cell Tissue Res 371: 399–406, 2018. doi: 10.1007/s00441-018-2790-5. [DOI] [PubMed] [Google Scholar]
- 8.Wagner R. Erlauterungstaflen zur Physiologie und Entwicklungsgeschichte. Leipzig: Leopold Voss, 1839. [Google Scholar]
- 9.Gill V, Doig C, Knight D, Love E, Kubes P. Targeting adhesion molecules as a potential mechanism of action for intravenous immunoglobulin. Circulation 112: 2031–2039, 2005. doi: 10.1161/CIRCULATIONAHA.105.546150. [DOI] [PubMed] [Google Scholar]
- 10.Liew PX, Kim JH, Lee WY, Kubes P. Antibody-dependent fragmentation is a newly identified mechanism of cell killing in vivo. Sci Rep 7: 10515, 2017. doi: 10.1038/s41598-017-10420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liew PX, Lee WY, Kubes P. iNKT Cells orchestrate a switch from inflammation to resolution of sterile liver injury. Immunity 47: 752–765.e5, 2017. doi: 10.1016/j.immuni.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Devi S, Li A, Westhorpe CL, Lo CY, Abeynaike LD, Snelgrove SL, Hall P, Ooi JD, Sobey CG, Kitching AR, Hickey MJ. Multiphoton imaging reveals a new leukocyte recruitment paradigm in the glomerulus. Nat Med 19: 107–112, 2013. [Erratum in Nat Med 22: 446, 2016]. doi: 10.1038/nm.3024. [DOI] [PubMed] [Google Scholar]
- 13.Martins JR, Haenni D, Bugarski M, Polesel M, Schuh C, Hall AM. Intravital kidney microscopy: entering a new era. Kidney Int 2021. doi: 10.1016/j.kint.2021.02.042. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Li Z. Intravital microscopy imaging of kidney injury and regeneration. Ren Replace Ther 7: 23, 2021. doi: 10.1186/s41100-021-00342-y. [DOI] [Google Scholar]
- 15.Liew PX, Kubes P. The neutrophil’s role during health and disease. Physiol Rev 99: 1223–1248, 2019. doi: 10.1152/physrev.00012.2018. [DOI] [PubMed] [Google Scholar]
- 16.Block H, Herter JM, Rossaint J, Stadtmann A, Kliche S, Lowell CA, Zarbock A. Crucial role of SLP-76 and ADAP for neutrophil recruitment in mouse kidney ischemia-reperfusion injury. J Exp Med 209: 407–421, 2012. doi: 10.1084/jem.20111493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singbartl K, Ley K. Protection from ischemia-reperfusion induced severe acute renal failure by blocking E-selectin. Crit Care Med 28: 2507–2514, 2000. doi: 10.1097/00003246-200007000-00053. [DOI] [PubMed] [Google Scholar]
- 18.Singbartl K, Forlow SB, Ley K. Platelet, but not endothelial, P-selectin is critical for neutrophil-mediated acute postischemic renal failure. FASEB J 15: 2337–2344, 2001. doi: 10.1096/fj.01-0199com. [DOI] [PubMed] [Google Scholar]
- 19.Singbartl K, Green SA, Ley K. Blocking P-selectin protects from ischemia/reperfusion-induced acute renal failure. FASEB J 14: 48–54, 2000. doi: 10.1096/fasebj.14.1.48. [DOI] [PubMed] [Google Scholar]
- 20.Rouschop KM, Roelofs JJ, Claessen N, da Costa Martins P, Zwaginga JJ, Pals ST, Weening JJ, Florquin S. Protection against renal ischemia reperfusion injury by CD44 disruption. J Am Soc Nephrol 16: 2034–2043, 2005. doi: 10.1681/ASN.2005010054. [DOI] [PubMed] [Google Scholar]
- 21.Awad AS, Rouse M, Huang L, Vergis AL, Reutershan J, Cathro HP, Linden J, Okusa MD. Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury. Kidney Int 75: 689–698, 2009. doi: 10.1038/ki.2008.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sethi S, Fervenza FC. Standardized classification and reporting of glomerulonephritis. Nephrol Dial Transplant 34: 193–199, 2019. doi: 10.1093/ndt/gfy220. [DOI] [PubMed] [Google Scholar]
- 23.Rahman M, Shad F, Smith MC. Acute kidney injury: a guide to diagnosis and management. Am Fam Physician 86: 631–639, 2012. [PubMed] [Google Scholar]
- 24.Nishi H, Furuhashi K, Cullere X, Saggu G, Miller MJ, Chen Y, Rosetti F, Hamilton SL, Yang L, Pittman SP, Liao J, Herter JM, Berry JC, DeAngelo DJ, Zhu C, Tsokos GC, Mayadas TN. Neutrophil FcγRIIA promotes IgG-mediated glomerular neutrophil capture via Abl/Src kinases. J Clin Invest 127: 3810–3826, 2017. doi: 10.1172/JCI94039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuboi N, Asano K, Lauterbach M, Mayadas TN. Human neutrophil Fcγ receptors initiate and play specialized nonredundant roles in antibody-mediated inflammatory diseases. Immunity 28: 833–846, 2008. doi: 10.1016/j.immuni.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saggu G, Okubo K, Chen Y, Vattepu R, Tsuboi N, Rosetti F, Cullere X, Washburn N, Tahir S, Rosado AM, Holland SM, Anthony RM, Sen M, Zhu C, Mayadas TN. Cis interaction between sialylated FcγRIIA and the αI-domain of Mac-1 limits antibody-mediated neutrophil recruitment. Nat Commun 9: 5058, 2018. doi: 10.1038/s41467-018-07506-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han S, Kim-Howard X, Deshmukh H, Kamatani Y, Viswanathan P, Guthridge JM, Thomas K, Kaufman KM, Ojwang J, Rojas-Villarraga A, Baca V, Orozco L, Rhodes B, Choi CB, Gregersen PK, Merrill JT, James JA, Gaffney PM, Moser KL, Jacob CO, Kimberly RP, Harley JB, Bae SC, Anaya JM, Alarcón-Riquelme ME, Matsuda K, Vyse TJ, Nath SK. Evaluation of imputation-based association in and around the integrin-alpha-M (ITGAM) gene and replication of robust association between a non-synonymous functional variant within ITGAM and systemic lupus erythematosus (SLE). Hum Mol Genet 18: 1171–1180, 2009. doi: 10.1093/hmg/ddp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosetti F, Chen Y, Sen M, Thayer E, Azcutia V, Herter JM, Luscinskas FW, Cullere X, Zhu C, Mayadas TN. A lupus-associated Mac-1 variant has defects in integrin allostery and interaction with ligands under force. Cell Rep 10: 1655–1664, 2015. doi: 10.1016/j.celrep.2015.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venkatesh D, Ernandez T, Rosetti F, Batal I, Cullere X, Luscinskas FW, Zhang Y, Stavrakis G, García-Cardeña G, Horwitz BH, Mayadas TN. Endothelial TNF receptor 2 induces IRF1 transcription factor-dependent interferon-β autocrine signaling to promote monocyte recruitment. Immunity 38: 1025–1037, 2013. doi: 10.1016/j.immuni.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tadagavadi RK, Reeves WB. Renal dendritic cells ameliorate nephrotoxic acute kidney injury. J Am Soc Nephrol 21: 53–63, 2010. doi: 10.1681/ASN.2009040407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finsterbusch M, Hall P, Li A, Devi S, Westhorpe CL, Kitching AR, Hickey MJ. Patrolling monocytes promote intravascular neutrophil activation and glomerular injury in the acutely inflamed glomerulus. Proc Natl Acad Sci USA 113: E5172–E5181, 2016. doi: 10.1073/pnas.1606253113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deppermann C, Kubes P. Start a fire, kill the bug: The role of platelets in inflammation and infection. Innate Immun 24: 335–348, 2018.doi: 10.1177/1753425918789255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finsterbusch M, Norman MU, Hall P, Kitching AR, Hickey MJ. Platelet retention in inflamed glomeruli occurs via selective prolongation of interactions with immune cells. Kidney Int 95: 363–374, 2019. doi: 10.1016/j.kint.2018.08.042. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalo E, Toldos O, Martínez-Vidal MP, Ordoñez MC, Santiago B, Fernández-Nebro A, Loza E, García I, León M, Pablos JL, Galindo M. Clinicopathologic correlations of renal microthrombosis and inflammatory markers in proliferative lupus nephritis. Arthritis Res Ther 14: R126, 2012. doi: 10.1186/ar3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devi S, Kuligowski MP, Kwan RY, Westein E, Jackson SP, Kitching AR, Hickey MJ. Platelet recruitment to the inflamed glomerulus occurs via an αIIbβ3/GPVI-dependent pathway. Am J Pathol 177: 1131–1142, 2010. doi: 10.2353/ajpath.2010.091143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Westhorpe CL, Bayard JE, O'Sullivan KM, Hall P, Cheng Q, Kitching AR, Hickey MJ. In vivo imaging of inflamed glomeruli reveals dynamics of neutrophil extracellular trap formation in glomerular capillaries. Am J Pathol 187: 318–331, 2017. doi: 10.1016/j.ajpath.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Castanheira FVS, Kubes P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood 133: 2178–2185, 2019. doi: 10.1182/blood-2018-11-844530. [DOI] [PubMed] [Google Scholar]
- 38.Nakazawa D, Marschner JA, Platen L, Anders HJ. Extracellular traps in kidney disease. Kidney Int 94: 1087–1098, 2018. doi: 10.1016/j.kint.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 39.Dieker J, Tel J, Pieterse E, Thielen A, Rother N, Bakker M, Fransen J, Dijkman HB, Berden JH, de Vries JM, Hilbrands LB, van der Vlag J. Circulating apoptotic microparticles in systemic lupus erythematosus patients drive the activation of dendritic cell subsets and prime neutrophils for NETosis. Arthritis Rheumatol 68: 462–472, 2016. doi: 10.1002/art.39417. [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, Barrat FJ, Banchereau J, Pascual V. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med 3: 73ra20, 2011. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, Bassett R, Amuro H, Fukuhara S, Ito T, Liu YJ, Gilliet M. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med 3: 73ra19, 2011. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hakkim A, Fürnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, Herrmann M, Voll RE, Zychlinsky A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci USA 107: 9813–9818, 2010. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, Rubin CJ, Zhao W, Olsen SH, Klinker M, Shealy D, Denny MF, Plumas J, Chaperot L, Kretzler M, Bruce AT, Kaplan MJ. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol 187: 538–552, 2011. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruschi M, Petretto A, Santucci L, Vaglio A, Pratesi F, Migliorini P, Bertelli R, Lavarello C, Bartolucci M, Candiano G, Prunotto M, Ghiggeri GM. Neutrophil extracellular traps protein composition is specific for patients with lupus nephritis and includes methyl-oxidized αenolase (methionine sulfoxide 93). Sci Rep 9: 7934, 2019. doi: 10.1038/s41598-019-44379-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Basu S, Hodgson G, Katz M, Dunn AR. Evaluation of role of G-CSF in the production, survival, and release of neutrophils from bone marrow into circulation. Blood 100: 854–861, 2002. doi: 10.1182/blood.v100.3.854. [DOI] [PubMed] [Google Scholar]
- 46.Pillay J, den Braber I, Vrisekoop N, Kwast LM, de Boer RJ, Borghans JA, Tesselaar K, Koenderman L. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood 116: 625–627, 2010. doi: 10.1182/blood-2010-01-259028. [DOI] [PubMed] [Google Scholar]
- 47.Tofts PS, Chevassut T, Cutajar M, Dowell NG, Peters AM. Doubts concerning the recently reported human neutrophil lifespan of 5.4 days. Blood 117: 6050–6052; author reply 6053–6054, 2011. doi: 10.1182/blood-2010-10-310532. [DOI] [PubMed] [Google Scholar]
- 48.Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood 80: 2012–2020, 1992. doi: 10.1182/blood.V80.8.2012.2012. [DOI] [PubMed] [Google Scholar]
- 49.Mathias JR, Perrin BJ, Liu TX, Kanki J, Look AT, Huttenlocher A. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J Leukoc Biol 80: 1281–1288, 2006. doi: 10.1189/jlb.0506346. [DOI] [PubMed] [Google Scholar]
- 50.Colom B, Bodkin JV, Beyrau M, Woodfin A, Ody C, Rourke C, Chavakis T, Brohi K, Imhof BA, Nourshargh S. Leukotriene B4-neutrophil elastase axis drives neutrophil reverse transendothelial cell migration in vivo. Immunity 42: 1075–1086, 2015. doi: 10.1016/j.immuni.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skopelja-Gardner S, Tai J, Sun X, Tanaka L, Kuchenbecker JA, Snyder JM, Kubes P, Mustelin T, Elkon KB. Acute skin exposure to ultraviolet light triggers neutrophil-mediated kidney inflammation. Proc Natl Acad Sci USA 118: e2019097118, 2021. doi: 10.1073/pnas.2019097118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brouwer E, Huitema MG, Mulder AH, Heeringa P, van Goor H, Tervaert JW, Weening JJ, Kallenberg CG. Neutrophil activation in vitro and in vivo in Wegener’s granulomatosis. Kidney Int 45: 1120–1131, 1994. doi: 10.1038/ki.1994.149. [DOI] [PubMed] [Google Scholar]
- 53.O’Sullivan KM, Lo CY, Summers SA, Elgass KD, McMillan PJ, Longano A, Ford SL, Gan PY, Kerr PG, Kitching AR, Holdsworth SR. Renal participation of myeloperoxidase in antineutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis. Kidney Int 88: 1030–1046, 2015. doi: 10.1038/ki.2015.202. [DOI] [PubMed] [Google Scholar]
- 54.Wither JE, Prokopec SD, Noamani B, Chang NH, Bonilla D, Touma Z, Avila-Casado C, Reich HN, Scholey J, Fortin PR, Boutros PC, Landolt-Marticorena C. Identification of a neutrophil-related gene expression signature that is enriched in adult systemic lupus erythematosus patients with active nephritis: clinical/pathologic associations and etiologic mechanisms. PloS one 13: e0196117, 2018. doi: 10.1371/journal.pone.0196117. [DOI] [PMC free article] [PubMed] [Google Scholar]