Abstract
Calponin 2 is an actin cytoskeleton-associated protein and plays a role in regulating cell motility-related functions such as phagocytosis, migration, and division. We previously reported that overexpression of calponin 2 inhibits the rate of cell proliferation. To investigate the underlying mechanism, our present study found that the levels of endogenous calponin 2 in NIH3T3 and HEK293 cells rapidly decreased before cell division characterized by an absence at the actin contractile ring. In cells lacking endogenous calponin 2, transfective expression of GFP-fusion calponin 2 inhibited cell proliferation similar to that of nonfusion calponin 2. Fluorescent imaging studies of mitotic cells indicated that a proper level of calponin 2 expression and effective degradation during cytokinesis are necessary for normal cell division. Computer-assisted dynamic image analysis of dividing cells revealed that overexpression of calponin 2 significantly affects motility and shape behaviors of cells only on the interval from the start of anaphase to the start of cytokinesis, i.e., the pre-cytokinesis phase, but not on the interval from the start of cytokinesis to 50% completion of cytokinesis. The pre-cytokinesis degradation of calponin 2 was attenuated by MG132 inhibition of the ubiquitin proteasome and inhibitor of protein kinase C (PKC), suggesting that PKC phosphorylation-triggered degradation of calponin 2 could determine the rate of cytokinesis. The novel role of calponin 2 in regulating the rate of cytokinesis may be targeted for therapeutic applications such as in an inhibition of malignant tumor growth.
Keywords: calponin 2, cell cycle, cytokinesis, PKC, proteasome
INTRODUCTION
First identified in smooth muscle (1), calponin is an actin filament-associated regulatory protein (2). Encoded by homologous genes, three calponin isoforms are present in vertebrates (3, 4). The calponin isoforms have conserved amino acid sequences in the N-terminal and middle regions but diverged structure in the C-terminal region, corresponding to their different sizes (292–330 amino acids) and overall charge with isoelectric points of 9.3 for calponin 1, 7.5 for calponin 2, and 5.3 for calponin 3 (2, 5, 6).
Calponin 1 is specifically expressed in differentiated smooth muscle cells (2) as a troponin-like inhibitory regulator of contractility (7). Calponin 1 has been shown to inhibit actin-activated myosin ATPase leading to an attenuation of smooth muscle contraction (8–11). Phosphorylation at Ser175 by protein kinase C (PKC) or Ca2+/calmodulin-dependent kinase II alleviates the inhibitory effect of calponin 1 on actomyosin ATPase and smooth muscle contraction (9–12).
Calponin 2 and calponin 3 are expressed in smooth muscle and many nonmuscle cell types with functions in regulating cell motility-based activities (2). Calponin 2 is found at abundance in multiple cell types including smooth muscle cells (4, 13), epidermal keratinocytes (14, 15), lung alveolar cells (16), vascular endothelial cells (17), fibroblasts (3, 15), myofibroblasts (18), and myeloid blood cells (19). Calponin 2 has been shown with functions in stabilizing actin cytoskeleton (15, 20), inhibiting cell migration (19) and proliferation (13), and regulating the motility, phagocytosis, and inflammatory activation of macrophages (19, 21, 22).
The actin cytoskeleton has essential functions in multiple cellular processes, such as contraction (23), migration (24), and division (25). In addition to the motor function of microtubule-based central spindles that separates the replicated chromosomes (26), actin-activated myosin motors play critical roles in powering cell division. A contractile ring formed by bundles of actin filaments and myosin motors at the cleavage furrow is an essential structure governing cytokinesis and the separation of daughter cells (27, 28). Since cytokinesis is a rate- and fate-determining step in mammalian cell cycle, the mechanisms underlying the movement and reorganization of actin cytoskeleton during cytokinesis is a foundation for the understanding of cell cycle regulation.
Although many cell types such as differentiated cardiac and skeletal muscles do not express calponin 2 and survive well (13), forced overexpression of calponin 2 slows down the rate of cell proliferation (13). On the other hand, our previous study showed that prostate cancer cells having decreased level of calponin 2 proliferated faster. Lower calponin 2 corresponds to higher rate of cell proliferation (29). Therefore, calponin 2 functions as a brake of actin-activated myosin motors for the regulation of cell motility-based activities. The broad range of calponin 2 level (from abundant to null) in various cell types may reflect the specific physiological functions of the cell or pathological changes.
To investigate the mechanism underlying the calponin 2 regulation of cytokinesis, the present study found that the levels of endogenous calponin 2 in NIH3T3 and HEK293 cells rapidly decrease before cell division characterized with an absence at the actin contractile ring. Transfective overexpression of calponin 2 inhibits cell proliferation by affecting cell motility and shape behaviors during the pre-cytokinesis period. Proper level of calponin 2 expression and its effective degradation during cytokinesis are necessary for normal cytokinesis. The pre-cytokinesis degradation of calponin 2 is attenuated by the inhibition of ubiquitin proteasome and PKC inhibitor. The results demonstrate that PKC phosphorylation-triggered rapid degradation of calponin 2 determines the rate of cytokinesis.
MATERIALS AND METHODS
Reagents
Phorbol ester phorbol 12,13-dibutyrate (PDBu, PKC activator, P1269), nocodazole (SML1665), colchicine (C9754), and MG132 (proteosome inhibitor, M7449) were purchased from Sigma-Adrich. GO6976 (PKC inhibitor, ab141413) was purchased from Abcam. Dulbecco’s modified Eagle medium (DMEM) and penicillin-streptomycin-glutamine (PSG, 100×, contains 10,000 U/mL penicillin, 10 mg/mL streptomycin, and 29.2 mg/mL l-glutamine) were purchased from Gibco.
Cell Cultures
NIH3T3, a cell line derived from mouse embryonic fibroblasts (ATCC CRL-1658), and HEK293, a cell line derived from human embryonic kidney cells (ATCC CRL-1573), were cultured in DMEM containing 10% fetal bovine serum (FBS) supplemented with PSG in a humidified incubator maintained with 5% CO2 at 37°C.
To investigate the expression of calponin 2 during cell division, rounded-up dividing NIH3T3 cells and HEK293 cells were collected using a mechanical shaking-off method to dislodge newly divided cells from the adherent monolayer cultures (30). Briefly, subconfluent NIH3T3 or HEK293 cell were cultured in 100-mm dishes to reach a log phase of growth. The culture dishes were then placed on a vibrating platform (Bel-Art Products, Pequannock, NJ) driven by a magnetic stirrer at 80 rpm/min in cell culture incubator with 5% CO2 at 37°C. The culture media were replaced every hour and the shaken-off cells in the media were collected by centrifugation at 300 g for 5 min and reseeded onto a new dish to culture in DMEM containing 10% FBS and sampled after 0, 6, 24, 48, and 72 h. After washing twice with warm phosphate-buffered saline (PBS), the cell samples were solubilized in sodium dodecyl sulfate (SDS) sample buffer (50 mM Tris-HCl, pH 8.8, 2% SDS, 140 mM β-mercaptoethanol, 0.1% bromophenol blue, 10% glycerol) to extract total proteins for SDS-polyacrylamide gel electrophoresis (PAGE) and Western blotting as previously described (13, 15).
SDS-PAGE and Western Blotting
SDS-PAGE and Western blotting were performed as described previously (19). Briefly, the cell lysates in SDS-gel sample buffer were heated at 80°C for 5 min, clarified by centrifugation at top speed in a microcentrifuge, and passed through a 25-G needle for 20 or more times to shear the cellular DNA and reduce viscosity. The protein samples were analyzed on 12% SDS-gel with an acrylamide:bis-acrylamide ratio of 29:1 in Laemmli discontinuous buffer system. Resolved gels were stained with Coomassie Blue R-250 to reveal the protein bands.
Duplicate gels were electrically blotted on nitrocellulose membranes using a Bio-Rad semidry transfer apparatus for Western blot analysis. The blotted membranes were blocked with 1% bovine serum albumin (BSA) in Tris-buffered saline (TBS, 150 mM NaCl and 50 mM Tris-HCl, pH 7.5) at room temperature for 30 min and incubated with a polyclonal rabbit anti-calponin 2 antibody RAH2 raised against mouse calponin 2 with weak cross reaction to calponin 1 (31), or a rabbit anti-phospho-histone H3 (Ser10) antibody (Cell Signaling, Product No. 9701S) in TBS containing 0.1% BSA at 4°C overnight. After three 7-min washes with TBS containing 0.05% Tween-20 and two 3-min washes with TBS, the membranes were incubated with alkaline phosphatase-labeled anti-rabbit IgG secondary antibody (Sigma, A2306) at room temperature for 1 h. Washed again as aforementioned, the membranes were developed in 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium chromogenic substrate solution to reveal the protein bands recognized by the primary antibody (13).
Immunofluorescence Microscopy
NIH3T3 cells and HEK293 cells were cultured on glass cover slips coated with 0.1% gelatin to reach 70% confluence. After washing three times with TBS, the adherent cells were fixed with 4% paraformaldehyde in TBS at room temperature for 15 min. After permeabilization with 1% Triton X-100 in TBS for 10 min, the coverslips were blocked with 1% BSA in PBS for 30 min and incubated with RAH2 antibody in PBS containing 0.1% BSA at room temperature for 1 h. After washing three times with TBS containing 0.05% Tween-20, the cells were stained with fluorescein isothiocyanate (FITC)-conjugated (32) anti-rabbit IgG secondary antibody (Sigma, F9887) and/or tetramethyl rhodamine isothiocyanate (TRITC)-phalloidin (Invitrogen) at room temperature for 1 h. After three washes with TBS containing 0.05% Tween-20, the cover slips were mounted on microscopic slides with ProLong Gold antifade reagent containing DAPI nuclear dye (Invitrogen) (13).
Confocal microscopy was performed using a Leica SD6000 spinning disk confocal microscope. Representative cells were imaged for seven sections along the z-axis. The FITC and TRITC fluorescence images representing the localization of calponin 2 and F-actin, respectively, were selectively viewed with corresponding filter settings. Photographs were taken using a Leica EMCCD camera and the images acquired with LAS AF software.
Construction of Calponin 2 Expression Plasmids
Sense and antisense mouse calponin 2 cDNAs were cloned into pcDNA3.1 plasmid for transfective expression of fusion and nonfusion calponin 2 in cultured cells as described previously (13).
Full-length cDNA encoding mouse calponin 2 was isolated from a recombinant pAED4 plasmid via restriction enzyme digestion and inserted between the BsrGI and NotI sites of pEGFP-N1 plasmid vector (Clontech, Mountain View, CA). JM109 Escherichia coli colonies transformed with the recombinant plasmid were identified by PCR and verified by restriction enzyme mapping and sequencing. The recombinant plasmid was used for transfective expression of calponin 2 with GFP fused to the C-terminus.
Culture and Transfection of SM3 Cells
SM3 is a cell line derived from rabbit vascular smooth muscle and lacks endogenous expression of calponin 2 corresponding to rapid growth in culture (13, 33). We used this cell line of calponin 2-null background to compare the function of transfectively expressed GFP-calponin 2 and free calponin 2 for the inhibitory effect on the rate of cell proliferation without interference from the endogenous calponin. SM3 cells were cultured in DMEM containing 10% FBS, 100 μg/mL penicillin, and 100 μg/mL streptomycin in 5% CO2 at 37°C.
Log-phase growing cells were trypsinized and reseeded in 24-well culture plates at 2 × 104 cells per well and cultured overnight. Transfection with recombinant calponin 2 pcDNA3.1 plasmids (13) or calponin 2-pEGFPN1 plasmids was carried out using Turbofect liposomal transfection reagent (Thermo Scientific) according to the manufacturer’s instruction. Briefly, the plasmid DNA in DMEM was mixed with Turbofect and incubated at room temperature for 20 min. The Turbofect-DNA complex was then gently mixed with 0.5 mL DMEM containing 10% FBS and added to the culture dish after removing the previous media. The cells were incubated with the Turbofect-DNA media in 5% CO2 at 37°C for 6 h before changing to fresh DMEM containing 10% FBS. The transfected cells were sampled at a series of time points for analysis.
Quantification of Proliferation Rate of Cultured Cells
The proliferation rate of cultured cells was determined using crystal violet staining as described previously (13, 34). Briefly, log-phase culture of cells were collected, counted, reseeded in 96-well plates and examined at 0, 24, 48, and 72 h of culture using crystal violet staining. Proliferation curves were constructed from OD595nm measured using a microtiter plate reader (Benchmark; Bio-Rad Labs).
Microscopic Imaging of Mitotic Cells
To examine the expression and distribution of calponin 2 in mitotic cells, GFP-calponin 2 pEGFPN1 plasmid-transfected SM3 cells were seeded and grown in 6-well plates. After 12 h, the cells were passed to a 35-mm dish and cultured for another 12 h before moving to a 37°C temperature-controlled and humidified microscope stage incubation chamber (Warner Instruments, Model HMW-1) on a Zeiss Axiovert-100 inverted fluorescence microscope continuously perfused with 95% O2 and 5% CO2. The expression of GFP-calponin 2 in mitotic cells was monitored and time-lapse images were recorded at series of time points using ProgRes@ Mac Capture Pro 2.6 software (JENOPTIK Optical Systems, Jupiter, FL). The chromatin condensation in prophase, chromosome alignment along the equatorial plane in metaphase, splitting of chromatids in anaphase, formation of cleavage furrow in telophase, and contractile ring during cytokinesis were used to identify the mitotic phases of cells. The relative intensity of calponin 2 in each mitotic phase was calculated for comparison.
Dynamic Image Analysis of Dividing Cells
The morphological dynamics of dividing cells were analyzed using two-dimensional (2D) dynamic image analysis software (2D-DIAS) program (35, 36) as previously described (37–39). Briefly, a coverslip with an 80% confluent cell monolayer was assembled into a Dvorak-Stotler perfusion chamber (Lucas-Highland Co., Burnsville, VA) mounted on a temperature-controlled (35°C) Zeiss inverted microscope equipped with a ×63 DIC objective lens and a CCD camera (Optronics Inc., Galito, CA). Metaphase cells with condensed chromosomes aligned along the cell equator were identified and recorded at 5 s intervals from metaphase to cytokinesis/telophase. The recorded images were digitized and stored in a computer equipped with a frame grabber (Data Translation, Marlboro, MA) and analyzed using 2D-DIAS to generate both cell centroid and cell perimeter tracks. The instantaneous velocity and directional change were automatically compiled from the centroid paths. The maximum length, area, perimeter, radial length, convexity, and concavity were computed from the cell perimeters. Detailed description of these parameters has been presented previously (35, 36, 39). These parameters were averaged over the period of analysis for each cell and the mean of the average computed for a number (n) of cells.
Synchronization of Cell Cycle with Nocodazole
NIH3T3 cells were cultured with DMEM containing 10% FBS to reach 50%–60% confluence and then switched to medium containing 200 ng/mL of nocodazole to culture at 37°C in 5% CO2 for 28 h. After washing with warm DMEM twice to remove nocodazole, the cells were cultured in fresh DMEM containing 10% FBS and imaged using a light microscope at 0, 20, 40, 60, 80, 100, and 120 min after released from nocodazole block to count the percentage of mitotic cells. Interphase cells were identified by discrete multilobed nucleus. When cells enter mitotic phase, they became rounded up with condensed chromatin. Late mitotic phase cells were identified with spherical shape with larger mean diameter than that of newly divided cells (21.9 ± 3.5 and 8.1 ± 2.6 µm, respectively) as calculated using a phase contrast microscope with imaging software (Keyence, BZ-X810). Samples from parallel cultures were collected and processed for SDS-PAGE and Western blot analysis.
Treatments with Proteasomal Inhibitor and PKC Activator and Inhibitor
NIH3T3 cells were cultured in DMEM containing 10% FBS on 12-well plates to reach confluency and treated with 20 µM MG132, 20 µM PDBu, 20 µM PDBu plus 20 µM MG132, 500 nM GO6976, 500 nM GO6976 plus 20 µM MG132, or solvent control for 40 min. After washing three times with PBS to remove the drugs and media, the cells were lysed in SDS-gel sample buffer and stored at −20°C for SDS-PAGE and Western blot analysis.
Data Analysis
Quantification of relative fluorescence intensity was carried out using ImageJ software. 2-D polygons were drawn to select the entire cell area for analysis. The integrated optical density (IOD) of each polygon was measured using ImageJ software with subtraction of per pixel background fluorescence. The total intensity value was divided by cell area to obtain mean fluorescence intensity. At least five cells in each mitotic phase were quantitatively analyzed and the experiments were performed three times.
Densitometry analysis of Western blots were carried out on 600 dpi images and normalized to the actin band in parallel SDS-gel.
Quantitative data are presented as means ± SD. Statistical significance of differences between the mean values was determined with two-tailed assays Student’s t test using Origin software. P < 0.05 was considered significant in all cases.
RESULTS
The Level of Calponin 2 is Regulated during Mitotic Cell Cycle with a Rapid Decrease Prior to Cytokinesis
To investigate the mechanism for calponin 2 overexpression to inhibit the rate of cell proliferation (13), we examined the level of calponin 2 in HEK293 cells that normally express significant levels of calponin 2 in interphase cells. The results showed that newly divided HEK293 cells contained a low level of calponin 2, which increased shortly during adherent culture (Fig. 1A). Quantification of the Western blots further detected a dip of calponin 2 at 30 h after seeding, corresponding to the completion of the first round of cell division as indicated by the doubling time on the growth curve (Fig. 1A). The significant decrease of calponin 2 during cell division was further demonstrated in synchronized cultures released from nocodazole inhibition of mitosis. The results showed that the lowest level of calponin 2 occurred at 20 min after the cells reentered cell cycle (Fig. 1B). This is a rapid and effective degradation since HEK293 cells blocked at G2/M phase with nocodazole (Fig. 1B) or at M-phase with colchicine (Fig. 1C) had high levels of calponin 2 compared with that in confluent cultures of untreated cells.
Figure 1.
The level of calponin 2 changes during cell cycle. A, top: newly divided HEK293 cells were collected from shake-off culture and reseeded for adherent preconfluent culture of 72 h. The levels of calponin 2 were examined at 0, 6, 24, 48, and 72 h. The sodium dodecyl sulfate (SDS)-gel and Western blot showed a low level of calponin 2 in rounded-up dividing cells, which was subsequently increased during culture. Middle: densitometry quantification of the Western blot normalized to actin in SDS-gel confirmed the low level of calponin 2 in newly divided cells which increased during culture. There was a trend of a second dip of calponin 2 after 30 h of culture, corresponding roughly to the completion of the first round of cell cycle indicated by the cell growth curve in the bottom that shows the number of cells per unit area doubling at 24, 48, and 72 h. B: NIH3T3 cells were treated with nocodazole for 28 h and released for synchronized growth. Western blot and densitometry analysis showed that nocodazole blocked cells expressed slightly higher level of calponin 2 as compared with untreated cells. From 0 to 20 min after releasing from nocodazole block, the level of calponin 2 decreased significantly, demonstrating a rapid degradation in dividing cells indicated by the high level of Ser10-phosphorrylated histone H3. C: SDS-gel and Western blot further showed that HEK293 cells blocked at metaphase with colchicine also maintained a high level of calponin 2. Data were summarized from 3 or more parallel cell cultures. Values are presented as means ± SD. *P value: 0.001 in Student’s t test.
Cellular Distribution of Calponin 2 in HEK293 and NIH3T3 Cells during Cell Cycle
Consistent with the Western blot results in Fig. 1 showing that newly divided cells have low levels of calponin 2, the immunofluorescence images in Fig. 2A demonstrate that cells undergoing cytokinesis contained lower levels of calponin 2 in comparison with that at other mitotic phases. Quantification of calponin 2 fluorescence confirmed the statistically significant lower mean intensity of calponin 2 during cytokinesis than that at metaphase in NIH3T3 and HEK293 cells (Fig. 2B).
Figure 2.
Regulated expression of calponin 2 during cell cycle. Proliferating HEK293 cells and NIH3T3 cells growing on gelatin-coated coverslips were examined with immunofluorescence microscopy using rabbit anti-calponin 2 antibody RAH2 and fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG secondary antibody. A: the representative phase-contrast and fluorescence images show the expression of calponin 2 in cells at different mitotic phases. B: quantitative analysis of calponin 2 stain showed that the per unit area intensity of calponin 2 decreased significantly in pre-cytokinesis cells at the state of 0% to 50% completion of cytokinesis. Values are presented as means ± SD. *P < 0.05 and ***P < 0.001, cytokinesis vs. metaphase; #P < 0.05, cytokinesis vs. anaphase and telophase in Student’s t test.
The immunofluorescence images in Fig. 3A showed the colocalization of calponin 2 with actin cytoskeleton and its absence from the contractile ring (Fig. 3B). The contractile ring is a key mitotic structure of animal cells, which consists of actin filaments and powered by myosin motors (28) to drive cell division and complete cytokinesis (27). With the established function of calponin in inhibiting actomyosin ATPase (11) and stabilizing the actin cytoskeleton (15), the absence of calponin 2 at the contractile ring indicates a novel regulatory mechanism that locally removes calponin inhibition to facilitate cytokinesis.
Figure 3.
Calponin 2 is associated with actin cytoskeleton and absent at the contractile ring during cytokinesis. A: HEK293 and NIH3T3 cells were cultured on gelatin-coated coverslips and double stained with anti-calponin 2 antibody RAH2 followed by fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG second antibody and tetramethyl rhodamine isothiocyanate (TRITC)-phalloidin for F-actin. DAPI was used to counter stain nuclei or chromosomes in these images. The phase contrast and fluorescence cell images of representative mitotic phases demonstrate colocalization of calponin 2 and actin cytoskeleton at all mitotic phases in both cell types, except at the contractile ring formed during cytokinesis (indicated by arrowheads). B: confocal microscopic images further demonstrated that calponin 2 was specifically absent at the contractile ring of HEK293 and NIH3T3 cells despite with the abundant presence of F-actin.
Nonfusion and GFP-Fusion Calponin 2 Exhibited Similar Effects on Cell Proliferation and the Architecture of Actin Cytoskeleton
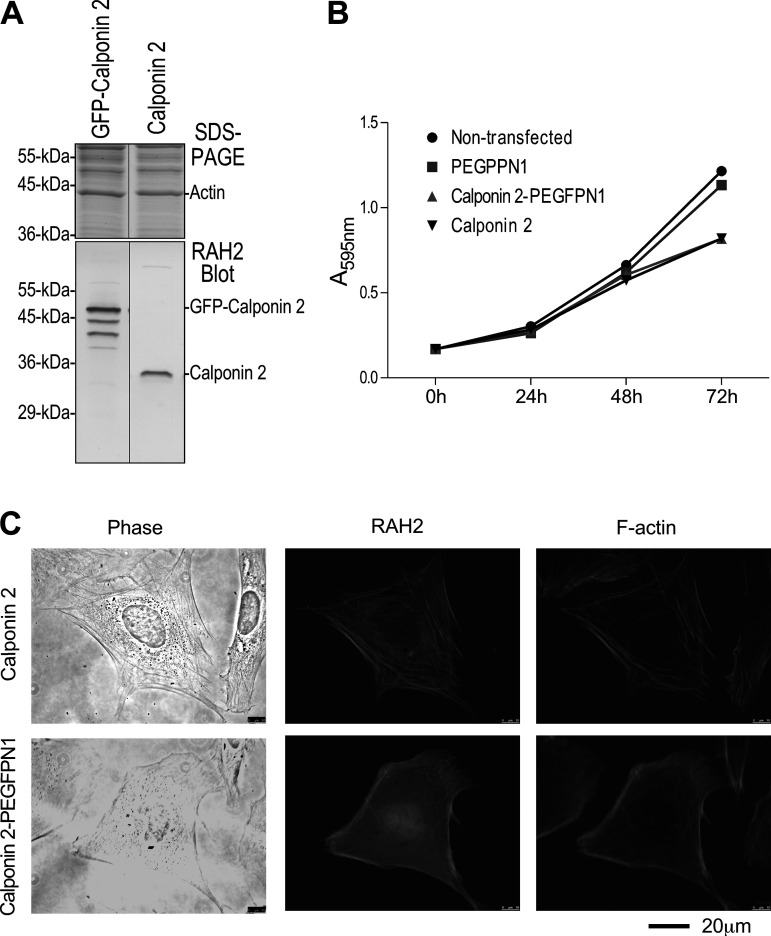
To visualize dynamic changes of calponin 2 in live cells during mitosis, we constructed a vector to express GFP-calponin 2 fusion protein. We first evaluated whether there is any significant difference between nonfusion calponin 2 and GFP-fusion calponin 2 in cytokinesis-related functions. Figure 4A shows that GFP-fusion and nonfusion calponin 2 proteins were similarly recognized in Western blot by anti-calponin 2 antibody RAH2. The similar levels of exogenous nonfusion and GFP-fusion calponin 2 expressed in SM3 cells also indicate their similarity in transfective expression.
Figure 4.
SM3 cells transfectively expressing nonfusion or green fluorescent protein (GFP)-fusion calponin 2 had similar growth curves and structure of actin cytoskeleton. A: Western blot analysis showed similar levels of fusion and nonfusion calponin 2 expressed in SM3 cells 48 h after transfection. B: cell proliferation rates were examined by quantifying cell numbers at designated time points using crystal violet staining. Consistent with our previous observations, transfective expression of calponin 2 inhibited the rate of cell proliferation. Nonfusion and GFP-fusion calponin 2 had nearly identical effects. C: immunofluorescence microscopic images showed that actin cytoskeleton architecture in SM3 cells transfected with nonfusion or GFP-fusion calponin 2 was also similar. Data were summarized from 3 or more parallel cell cultures.
SM3 cells transfectively expressing GFP-fusion or nonfusion calponin 2 were examined for the rate of proliferation and the association of calponin 2 with actin filaments. The results showed that nonfusion and GFP-fusion calponin 2 had similar effects on inhibiting the rate of cell proliferation (Fig. 4B). The actin cytoskeleton structures in SM3 cells transfected with nonfusion or GFP-fusion calponin 2 were also similar (Fig. 4C). This observation is consistent with previous studies showing that a GFP-fusion actin-binding protein, coronin, had similar functions in regulating cytokinesis (40), and GFP-kinesin Kif12 could restore normal mitotic phenotype of Kif12-deleted cells (41). Therefore, the results justify that the C-terminal fusion of GFP has no significant effect on cellular functions of calponin 2 and can be used for investigation of living cells for the function of calponin 2 in cytokinesis.
Appropriate Expression Level and Effective Post-Metaphase Degradation of Calponin 2 Are Required for Normal Cytokinesis
To study the regulatory effect of calponin 2 on cytokinesis in live cells, we monitored the changes of calponin 2 in dividing SM3 cells transfectively expressing GFP-fusion calponin 2. Representative cells were imaged using fluorescent microscopy at a series of time points during the course of cell division for correlation between the expression level and fate of GFP-calponin 2 and the outcome of cytokinesis. The results showed that the GFP-calponin 2 expressing cells can be qualitatively placed into three groups. Group I cells expressed a proper level of GFP-fusion calponin 2 with a timely decrease during cytokinesis and successfully completed mitosis within the normal time frame of 1 h. The relative mean intensity of calponin 2 in Group I cells decreased by ∼36% during cytokinesis from that in metaphase (Fig. 5A). Group II cells expressed higher levels of calponin 2 in metaphase (∼2.7 folds of that in Group I cells), which were effectively decreased by ∼28% during cytokinesis. Although at a slower rate, Group II cells could complete cytokinesis in ∼2.5 h (Fig. 5B). Group III cells also expressed high levels of calponin 2 in the metaphase (∼1.8 folds of that in Group I cells), which, however, was not decreased during cytokinesis. The ineffective degradation of high levels of calponin 2 in Group III cells corresponds to the failure of completing cytokinesis followed by cell death (Fig. 5C).
Figure 5.
Effects of calponin 2 on the rate and fate of cytokinesis. Green fluorescent protein (GFP)-fusion calponin 2 was expressed in SM3 cells via transient transfection for 24 h. Individual cells were monitored with fluorescence microscopy using a stage culture chamber. GFP fluorescent images were recorded throughout the course of mitosis. The representative recordings show the correlation between calponin 2 level and the velocity and fate of cytokinesis. A: Group I cells expressed appropriate levels of calponin 2 with an effective decrease after metaphase and showed normal completion of cytokinesis within 1 h. B: Group II cells expressed higher levels of calponin 2 with an effective decrease after metaphase could also complete cytokinesis although with a significantly longer time. C: in contrast, Group III cells expressed high levels of calponin 2 without notable decrease after metaphase failed to complete cytokinesis and ended up with cell death after a few hours. The lower level of GFP-fusion calponin 2 seen in the cleavage furrow of successfully dividing cell is indicated by arrows. D: quantification of GFP-calponin 2 fluorescence intensity shows the level and change of calponin 2 in metaphase and cytokinesis. The pattern illustrates that cells expressing normal or high level of calponin 2 can complete cytokinesis by having an effective degradation after metaphase. The longer duration of cytokinesis for cells that need to remove high level of calponin 2 suggest that calponin 2 degradation is a determinant of the rate of cytokinesis, by which the failure of post metaphase degradation of calponin 2 results in failure of cytokinesis followed by cell death. Values are presented as means ± SD.
The relationship between the expression level and pre-mitotic degradation of calponin 2 and the rate and fate of cytokinesis are summarized in Fig. 5D. The results demonstrate that cells expressing normal or high level of calponin 2 can complete cytokinesis with effective post-metaphase degradation. Cells would need a longer time to remove higher level of calponin 2 before cytokinesis whereas ineffective decrease of calponin 2 halted cell division, rendering calponin 2 degradation as a determining regulator of the rate and fate of cytokinesis.
Effects of Calponin 2 on the Dynamics of Mitosis
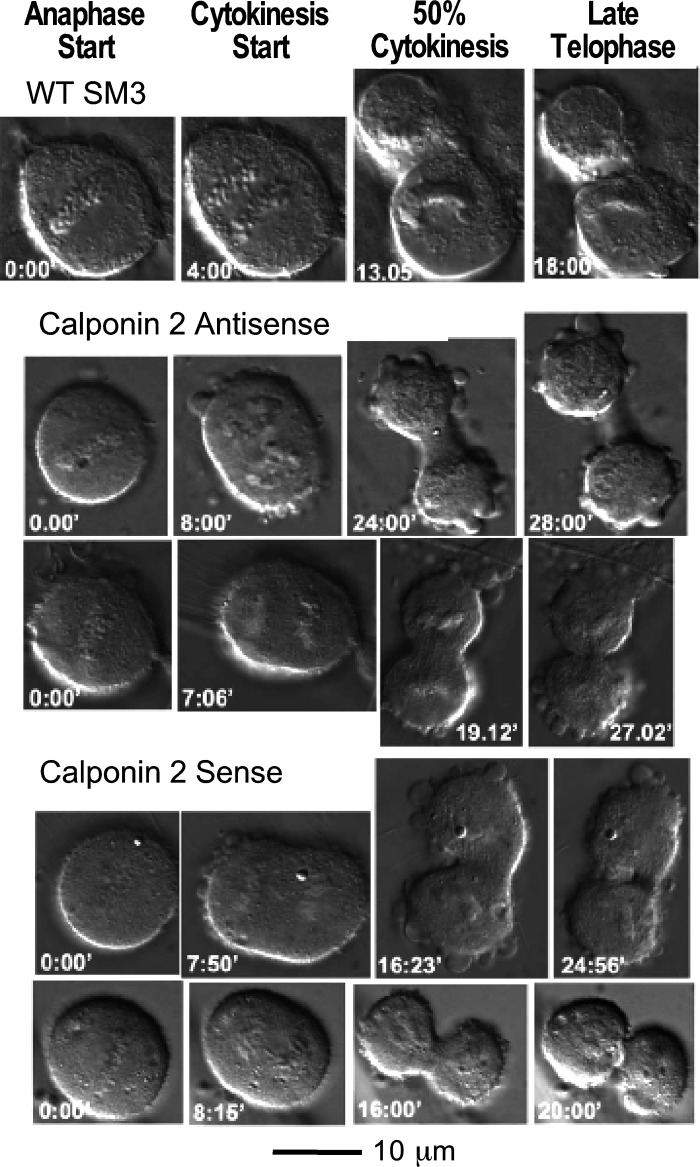
To assess the effects of calponin 2 on the dynamics of mitosis, dividing cells from wild-type (WT) SM3 that lacks endogenous calponin 2, calponin 2 antisense cDNA transfected control SM3, and calponin 2 sense cDNA-expressing SM3 cells were motion-analyzed using the 2D-DIAS program (Fig. 6). The motility and morphology parameters of mitotic cells undergoing Interval 1 (from the start of anaphase to the start of cytokinesis) and Interval 2 (from the start of cytokinesis to 50% cytokinesis) are summarized in Table 1.
Figure 6.
Differential interference contrast micrographs of mitotic cells. Images of wild-type (WT), calponin 2 antisense-expressing, and calponin 2 sense-expressing SM3 cells show that at the start of anaphase, condensed chromosomes aligned along the metaphase plate begin to separate. During anaphase, the cells elongate and at the start of cytokinesis, the membranes at the cell equators begin to invaginate. 50% cytokinesis was defined as the time when the cleavage furrow was half the width of the cell prior to invagination. The numbers (in min) on the bottom left of each image represent their relative times to the start of anaphase.
Table 1.
Computer-assisted measurements of motility and morphology parameters of dividing cells
|
Interval 1 (from the start of anaphase to the start of cytokinesis)
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cell Line | Numbers of Cells | Total Elapsed Time, min | Mean Instantaneous Velocity, μm/min | Mean Maximal Length, μm | Mean Area, μm2 | Mean Perimeter, μm | Mean Radial Length, μm | Mean Convexity, ° | Mean Concavity, ° |
| WT SM3a | 10 | 9.4 ± 1.43 | 0.14 ± 0.01 | 10.91 ± 0.38 | 60.47 ± 2.35 | 29.99 ± 0.74 | 4.47 ± 0.09 | 691 ± 26 | 331 ± 26 |
| AS#I-5b | 10 | 9.95 ± 1.15 | 0.16 ± 0.01 | 11.9 ± 1.32 | 65.86 ± 7.67 | 31.72 ± 2.59 | 4.7 ± 0.32 | 725 ± 41 | 365 ± 41 |
| S#105c | 10 | 8.45 ± 2.02 | 0.23 ± 0.04 | 16.05 ± 2.16 | 82.0 ± 8.1 | 40.01 ± 4.26 | 5.63 ± 0.46 | 813 ± 67 | 453 ± 67 |
| P value | a vs. b | 0.77 | 0.21 | 0.47 | 0.62 | 0.79 | 0.85 | 0.49 | 0.49 |
| a vs. c | 0.34 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.24 | 0.24 | |
| b vs. c | 0.14 | 0.10 | 0.08 | 0.17 | 0.09 | 0.11 | 0.28 | 0.28 | |
|
Interval 2 (from the start of cytokinesis to 50% cytokinesis)
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cell Line | Numbers of Cells Analyzed | Total Elapsed Time, min | Mean Instantaneous Velocity, μm/min | Mean Maximal Length, μm | Mean Area, μm2 | Mean Perimeter, μm | Mean Radial Length, μm | Mean Convexity, ° | Mean Concavity, ° |
| WT SM3a | 10 | 8.1 ± 0.68 | 0.21 ± 0.02 | 12.49 ± 0.49 | 63.48 ± 2.7 | 33.77 ± 1.07 | 4.65 ± 0.11 | 867 ± 49 | 510 ± 50 |
| AS#I-5b | 10 | 6.6 ± 0.56 | 0.27 ± 0.04 | 14.15 ± 1.01 | 74.27 ± 7.15 | 37.97 ± 2.25 | 5.05 ± 0.27 | 1013 ± 56 | 650 ± 55 |
| S#105c | 10 | 6.4 ± 0.73 | 0.25 ± 0.03 | 16.85 ± 2.18 | 86.6 ± 9.06 | 43.76 ± 4.11 | 5.75 ± 0.47 | 1046 ± 74 | 686 ± 74 |
| P value | a vs. b | 0.11 | 0.39 | 0.16 | 0.43 | 0.11 | 0.19 | 0.07 | 0.08 |
| a vs. c | 0.10 | 0.23 | 0.12 | 0.09 | 0.08 | 0.09 | 0.06 | 0.06 | |
| b vs. c | 0.79 | 0.65 | 0.57 | 0.30 | 0.23 | 0.21 | 0.72 | 0.70 | |
WT SM3, antisense calponin 2 cDNA transfected (AS#I-5) and sense calponin 2 cDNA transfected (S#105) cells were studied using 2D-DIAS. The values are presented as means ± SD and statistical analysis was performed using Student’s t test after testing normality and equal variance. Mann–Whitney rank sum test was used where normality or equal variance failed in Student’s t test. P < 0.05 is considered significant (indicated with bold font).
No statistically significant differences were detected in all parameters between WT and antisense calponin 2 cDNA transfected SM3 cells, indicating that both groups may serve as control for comparison with calponin 2 sense cDNA expressing SM3 cells. Consistent with the results of GFP-fusion calponin 2 studies, WT, antisense, and sense cDNA expressing cells that successfully completed mitosis as described earlier for Group I cells showed no significant difference in the total time of Intervals 1 and 2 (Table 1). However, during Interval 1, the mean instantaneous velocity for calponin 2 sense expression cells was significantly faster than that for WT and antisense expression cells. This finding suggests that more active reorganization of cytoskeleton (including microfilaments and microtubules) is needed when calponin 2 is present. Other motility parameters such as mean total path and mean net path of cell centroid movement during Interval 1 were also greater for sense expression cells than that for WT and antisense expression cells although statistical significance was not reached (data not shown).
The size parameters such as mean maximal length, mean area, mean perimeter, and mean radial length were significantly greater for sense expression cells during Interval 1. A trend of bigger size parameters was also observed with sense expression cells as compared with antisense expression cells during Interval 1. Cell complexity parameters such as mean convexity and mean concavity showed no statistically significant difference among the three groups. No difference in motility, size, and cell complexity was found during Interval 2, consistent with the hypothesis that a rapid degradation of calponin 2 occurs before the start of cytokinesis.
Calponin 2 is Degraded Prior to Cytokinesis via Ubiquitin Proteasomal Pathway
Nocodazole treatment of NIH3T3 cells effectively arrested 56.7% of cells at G2/M phase as compared with 8.9% in untreated cells. Western blot analysis showed nocodazole arrested cells expressed a higher level of calponin 2 than that in untreated cells. A rapid decrease in calponin 2 occurred 20 min after releasing from nocodazole arrest, which restored at 40 min (Fig. 7A). The treatment with ubiquitin proteasomal inhibitor MG132 significantly attenuated the pre-cytokinetic decrease of calponin 2 (Fig. 7B). The results indicate that the downregulation of calponin 2 before cytokinesis is by degradation via the ubiquitin proteasomal pathway.
Figure 7.
Calponin 2 degradation via ubiquitin proteasomal pathway. A: NIH3T3 cells were treated with nocodazole for 28 h and released for synchronized culture and treated with 20 µM MG132 or dimethyl sulfoxide (DMSO) solvent control. Western blot analysis showed that the nocodazole blocked cells contained a higher level of calponin 2 as compared with untreated cells. The level of calponin 2 decreased significantly at 20 min after releasing from nocodazole block and then restored at 40 min. MG132 treatment resulted in a higher level of calponin 2 as compared with the solvent (DMSO) control, especially at 20 min as shown by the densitometry analysis (B). Values are presented as means ± SD. *P < 0.05 in Student’s t test.
PKC Regulates Pre-Cytokinetic Degradation of Calponin 2
The PKC phosphorylation site Ser175 identified in calponin 1 (10, 11, 42, 43) and flanking sequences are conserved in calponin 2 (2). Therefore, we hypothesized that PKC phosphorylation may regulate calponin 2 functions during cytokinesis. NIH3T3 cells were treated with PKC activator PDBu or inhibitor GO6976 with or without the presence of proteasomal inhibitor MG132. The results showed that PKC activator decreased the level of calponin 2 (Fig. 8A) whereas PKC inhibitor increased calponin 2 as compared with that in untreated cells (Fig. 8B), suggesting a role of PKC in the regulation of pre-cytokinetic degradation of calponin 2.
Figure 8.
Protein kinase C (PKC) regulation of pre-cytokinetic calponin 2 degradation. A: PKC activator phorbol 12,13-dibutyrate (PDBu) induced calponin 2 degradation. NIH3T3 cells were treated with 20 µM PDBu, 20 µM PDBu plus 20 µM MG132, or 20 µM MG132 for 40 min. The treated and untreated control cells were collected for SDS-PAGE and Western blot analysis. Densitometry quantitation of Western blots showed that the level of calponin 2 was significantly decreased after PDBu treatment whereas cells treated with MG132 with or without PDBu both showed higher level of calponin 2 when compared with that in untreated cells. B: PKC inhibitor GO6976 attenuated calponin 2 degradation. NIH3T3 cells were treated with 500 nM GO6976, GO6976 plus MG132 or MG132 only. Western blot analysis showed that calponin 2 levels were higher in GO6976, GO6976 plus MG132, and MG132 treated cells in comparison with that in untreated control cells. Values are presented as means ± SD. *P < 0.05 vs. untreated control in Student’s t test.
Although the phosphorylation regulation of calponin has been shown in in vitro studies, phosphorylated calponin is undetectable in living cells under physiologic conditions (44). It is known that PKC phosphorylation of calponin weakens the binding affinity for F-actin (43). Therefore, our hypothesis is that phosphorylated calponin 2 will dissociate from the actin cytoskeleton and be rapidly degraded. Our finding in the present study that PKC activation decreased calponin 2, which was minimized by cotreatment of proteosome inhibitor whereas PKC inhibitors increased the level of calponin 2 supports this hypothesis. Future studies to identify the PKC phosphorylation site(s) in calponin 2 in correlation with the binding affinity for F-actin would further establish this regulatory mechanism.
DISCUSSION
Calponin 2 is a regulatory protein that inhibits myosin ATPase and myosin motor-powered contractile and motility functions (2), including cell divisions during proliferation (13, 45). Cytokinesis is the final step of cell division that requires myosin motor functions (46–48). Our present study investigated the role of calponin 2 in the inhibitory regulation of the rate of cytokinesis. The results provide several new findings.
The Role of Calponin 2 in Regulating the Rate of Cell Proliferation via Inhibiting Cytokinesis
Stringent regulation of the proliferation rate is a fundamental requirement for maintaining physiological structure and function of multi-cell organisms. Most previous studies of this regulation have focused on the control of chromosomal DNA replication and the overall gene expression and protein synthesis (49). Cell division is a motility function that involves complex and rapid reorganization of the cytoskeleton. The critical role of microtubules-based motility in the segregation of sister chromosomes during mitosis have been extensively described (50). Actin cytoskeleton and actomyosin-based motility play essential roles in the division of cellular contents into daughter cells (51) and the final step of cytokinesis (46–48). Biochemical studies have demonstrated the function of calponin 2 in regulating the actin-activated myosin ATPase (11) and stabilizing the actin filaments (15, 16, 42, 52). The role of calponin in regulating actin-myosin interaction, myosin ATPase activity, and actin’s interactions with other structural and regulatory proteins (2, 5, 6) forms a foundation for its function in the regulation of cytokinesis.
Our present study demonstrated that the level of calponin 2 decreases during cytokinesis thus newly divided cells had significantly lower levels of calponin 2 as compared with that in confluent interphase cells or mixed-phase cultures (Figs. 1 and 2). Although a high level of calponin 2 was maintained when the cell cycle was blocked at M phase by colchicine (Fig. 1), a post-metaphase degradation of calponin 2 during the cell cycle appears essential for cytokinesis. Supporting the role of calponin 2 as an inhibitory regulator of the rate and fate of cytokinesis, our results are consistent with the previous observations that overexpression of calponin 2 resulted in slower rate of cell proliferation (13, 45).
Calponin 2 Regulation May Act on the Function of Actin Contractile Ring of Mitotic Cells
Cytokinesis involves a series of linked processes, and inhibition of one or more of these steps may block the process (48). Abnormality of actin filaments organization (47) and perturbation of myosin localization or its activity (46) have been suggested to cause failure of cytokinesis. Experimental evidence has indicated that calponin plays a role in stabilizing the actin cytoskeleton (15, 42, 52). Together with the decrease in total calponin 2 in dividing and newly divided cells, a striking finding in our present study was the selective exclusion of calponin 2 from the contractile ring during cytokinesis (Fig. 3B).
The contractile ring is made of actin filaments and its function requires the interaction of F-actin and myosin (27). It is a structure critical to cytokinesis by providing mechanical force for the final division of the daughter cells. Regulated polymerization-depolymerization of actin filaments contributes to the assembly, maintenance, and closure of the contractile ring (51). Although the high level of calponin 2 in metaphase (Fig. 2) corresponding to stable actin filaments may be critical to a proper partition of cellular contents before cell division, the rapid remodeling of actin filaments at the contractile ring is an essential event during cytokinesis and would require increased dynamics in the movements of local actin filaments. The rapid exclusion of calponin 2 specifically in the contractile ring during cytokinesis indicates a novel function of calponin 2 in controlling the rate of cell division.
Regulated Expression and Degradation of Calponin 2 Are Required for Normal Cytokinesis
We used GFP-fusion calponin 2 to directly monitor the changes of calponin 2 during mitosis of live cells and investigate the effects of calponin 2 on inhibiting cell proliferation. To preclude the effect of GFP, we first compared nonfusion calponin 2 (no GFP) and GFP-fusion calponin 2 to show that they have similar effects on cell structure and growth (Fig. 4). The effects of calponin 2 level in metaphase and its degradation before cytokinesis on the rate and fate of cytokinesis showed an inverted correlation between the level of GFP-fusion calponin 2 in metaphase cells and the duration of cytokinesis, confirming the inhibitory effect of calponin 2 on the rate of cell proliferation.
The results show that cells transfectively expressing high levels of calponin 2 could still divide although at a slower rate, as long as effective calponin 2 degradation occurred before cytokinesis (Fig. 5B). In contrast, cells that expressed high levels of calponin 2 without effective degradation failed to complete cytokinesis (Fig. 5C). The fact that those cells eventually died in culture suggests a powerful consequence of calponin 2 inhibition in the fate of cytokinesis. These findings conclude that not only the level of calponin 2 gene expression but also its cell cycle-specific degradation contributes to the regulation of cell division.
PKC Phosphorylation Leads to the Degradation of Calponin 2 in Cytokinesis
Previous studies have found that the biochemical function of calponin 1 is regulated by phosphorylation and dephosphorylation (53–55). The mechanism that controls the pre-cytokinetic calponin 2 degradation is worth investigating. Calponin is associated with actin cytoskeleton which may prevent it from rapid degradation. We previously showed that PKC phosphorylation of calponin 1 decreases the binding affinity to F-actin (43). Therefore, activation of PKC may promote the pre-cytokinetic degradation of calponin 2 by phosphorylation-induced dissociation from the actin cytoskeleton and entering the ubiquitin proteasomal pathway.
Rho-kinase phosphorylates calponin 1 stoichiometrically in vitro and inhibits the binding of calponin to F-actin (56). Insufficient activation of Rho A is correlated to the failure of cytokinesis (57). The potential role of Rho-kinase in the regulation of calponin 2 degradation during cytokinesis is also worth investigating.
Calponin 2 expresses at high levels in epithelial, endothelial, fibroblast, and myeloid blood cells but absent in many other cell types (2). Cells that do not express calponin can proliferate as that seen in SM3 cells. When calponin 2 is present, however, it slows down the rate of cell proliferation (13). The rapid degradation of calponin 2 during cytokinesis in calponin-expressing cells demonstrated in the present study suggests a potential target to control cell proliferation. Metastatic cancer cells have decreased expression of calponin 2 (29). Therefore, an increased expression and/or decreased PKC-induced degradation of calponin 2 may be targeted as therapeutic approach.
In conclusion, results of the present study demonstrate that a rapid degradation of calponin 2 is necessary for normal cytokinesis, and PKC phosphorylation is implicated as one of the possible mechanisms that initiates the degradation of calponin 2 before cytokinesis. The function of calponin 2 in regulating cytokinesis through modifying the activity of actin cytoskeleton especially the dynamic reorganization of the contractile ring suggests a novel mechanism for the control of the rate of cell proliferation during development, tissue regeneration, pathophysiological remodeling, wound healing, and malignant growth.
GRANTS
This study was supported in part by a grant from the National Institutes of Health Grant HL-086720 (to J.-P. Jin). T.-B. Hsieh was a recipient of Women’s Reproductive Health Research fellowship program from National Institute of Child Health and Human Development (HD001254 to Dr. Chau-Dong Hsu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.-P.J. conceived and designed research; A.Q., T.-B.H., M.M.H., and J.J.-C.L. performed experiments; A.Q., T.-B.H., M.M.H., and J.J.-C.L. analyzed data; A.Q., T.-B.H., M.M.H., J.J.-C.L., and J.-P.J. interpreted results of experiments; A.Q., T.-B.H., M.M.H., J.J.-C.L., and J.-P.J. prepared figures; A.Q., T.-B.H., and J.-P.J. drafted manuscript; T.-B.H., J.J.-C.L., and J.-P.J. edited and revised manuscript; A.Q., T.-B.H., M.M.H., J.J.-C.L., and J.-P.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Hui Wang for technical assistance.
REFERENCES
- 1.Takahashi K, Hiwada K, Kokubu T. Isolation and characterization of a 34,000-dalton calmodulin- and F-actin-binding protein from chicken gizzard smooth muscle. Biochem Biophys Res Commun 141: 20–26, 1986. doi: 10.1016/s0006-291x(86)80328-x. [DOI] [PubMed] [Google Scholar]
- 2.Liu R, Jin JP. Calponin isoforms CNN1, CNN2 and CNN3: regulators for actin cytoskeleton functions in smooth muscle and non-muscle cells. Gene 585: 143–153, 2016. doi: 10.1016/j.gene.2016.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strasser P, Gimona M, Moessler H, Herzog M, Small JV. Mammalian calponin. Identification and expression of genetic variants. FEBS Lett 330: 13–18, 1993. doi: 10.1016/0014-5793(93)80909-e. [DOI] [PubMed] [Google Scholar]
- 4.Applegate D, Feng W, Green RS, Taubman MB. Cloning and expression of a novel acidic calponin isoform from rat aortic vascular smooth muscle. J Biol Chem 269: 10683–10690, 1994. [PubMed] [Google Scholar]
- 5.Jin JP, Zhang Z, Bautista JA. Isoform diversity, regulation, and functional adaptation of troponin and calponin. Crit Rev Eukaryot Gene Expr 18: 93–124, 2008. doi: 10.1615/critreveukargeneexpr.v18.i2.10. [DOI] [PubMed] [Google Scholar]
- 6.Wu KC, Jin JP. Calponin in non-muscle cells. Cell Biochem Biophys 52: 139–148, 2008. doi: 10.1007/s12013-008-9031-6. [DOI] [PubMed] [Google Scholar]
- 7.Rozenblum GT, Gimona M. Calponins: adaptable modular regulators of the actin cytoskeleton. Int J Biochem Cell Biol 40: 1990–1995, 2008. doi: 10.1016/j.biocel.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Abe M, Takahashi K, Hiwada K. Effect of calponin on actin-activated myosin ATPase activity. J Biochem 108: 835–838, 1990. doi: 10.1093/oxfordjournals.jbchem.a123289. [DOI] [PubMed] [Google Scholar]
- 9.Obara K, Szymanski PT, Tao T, Paul RJ. Effects of calponin on isometric force and shortening velocity in permeabilized taenia coli smooth muscle. Am J Physiol Cell Physiol 270: C481–C487, 1996. doi: 10.1152/ajpcell.1996.270.2.C481. [DOI] [PubMed] [Google Scholar]
- 10.Tang DC, Kang HM, Jin JP, Fraser ED, Walsh MP. Structure-function relations of smooth muscle calponin. The critical role of serine 175. J Biol Chem 271: 8605–8611, 1996. doi: 10.1074/jbc.271.15.8605. [DOI] [PubMed] [Google Scholar]
- 11.Winder SJ, Walsh MP. Smooth muscle calponin. Inhibition of actomyosin MgATPase and regulation by phosphorylation. J Biol Chem 265: 10148–10155, 1990. [PubMed] [Google Scholar]
- 12.Itoh T, Suzuki S, Suzuki A, Nakamura F, Naka M, Tanaka T. Effects of exogenously applied calponin on Ca2+-regulated force in skinned smooth muscle of the rabbit mesenteric artery. Pflugers Arch 427: 301–308, 1994. doi: 10.1007/BF00374538. [DOI] [PubMed] [Google Scholar]
- 13.Hossain MM, Hwang DY, Huang QQ, Sasaki Y, Jin JP. Developmentally regulated expression of calponin isoforms and the effect of h2-calponin on cell proliferation. Am J Physiol Cell Physiol 284: C156–C167, 2003. doi: 10.1152/ajpcell.00233.2002. [DOI] [PubMed] [Google Scholar]
- 14.Fukui Y, Masuda H, Takagi M, Takahashi K, Kiyokane K. The presence of h2-calponin in human keratinocyte. J Dermatol Sci 14: 29–36, 1997. doi: 10.1016/s0923-1811(96)00545-2. [DOI] [PubMed] [Google Scholar]
- 15.Hossain MM, Crish JF, Eckert RL, Lin JJ, Jin JP. h2-calponin is regulated by mechanical tension and modifies the function of actin cytoskeleton. J Biol Chem 280: 42442–42453, 2005. doi: 10.1074/jbc.M509952200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hossain MM, Smith PG, Wu K, Jin JP. Cytoskeletal tension regulates both expression and degradation of h2-calponin in lung alveolar cells. Biochemistry 45: 15670–15683, 2006. doi: 10.1021/bi061718f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang J, Hu G, Hanai J, Yadlapalli G, Lin Y, Zhang B, Galloway J, Bahary N, Sinha S, Thisse B, Thisse C, Jin JP, Zon LI, Sukhatme VP. A critical role for calponin 2 in vascular development. J Biol Chem 281: 6664–6672, 2006. doi: 10.1074/jbc.M506991200. [DOI] [PubMed] [Google Scholar]
- 18.Plazyo O, Liu R, Moazzem Hossain M, Jin JP. Deletion of calponin 2 attenuates the development of calcific aortic valve disease in ApoE−/− mice. J Mol Cell Cardiol 121: 233–241, 2018. doi: 10.1016/j.yjmcc.2018.07.249. [DOI] [PubMed] [Google Scholar]
- 19.Huang QQ, Hossain MM, Wu K, Parai K, Pope RM, Jin JP. Role of H2-calponin in regulating macrophage motility and phagocytosis. J Biol Chem 283: 25887–25899, 2008. doi: 10.1074/jbc.M801163200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danninger C, Gimona M. Live dynamics of GFP-calponin: isoform-specific modulation of the actin cytoskeleton and autoregulation by C-terminal sequences. J Cell Sci 113: 3725–3736, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Liu R, Jin JP. Deletion of calponin 2 in macrophages alters cytoskeleton-based functions and attenuates the development of atherosclerosis. J Mol Cell Cardiol 99: 87–99, 2016. doi: 10.1016/j.yjmcc.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plazyo O, Sheng JJ, Jin JP. Downregulation of calponin 2 contributes to the quiescence of lung macrophages. Am J Physiol Cell Physiol 317: C749–C761, 2019. doi: 10.1152/ajpcell.00036.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filipe JA, Nunes JP. Functional importance of the actin cytoskeleton in contraction of bovine iris sphincter muscle. Auton Autacoid Pharmacol 22: 155–159, 2002. doi: 10.1046/j.1474-8673.2002.00255.x. [DOI] [PubMed] [Google Scholar]
- 24.Ponti A, Machacek M, Gupton SL, Waterman-Storer CM, Danuser G. Two distinct actin networks drive the protrusion of migrating cells. Science 305: 1782–1786, 2004. doi: 10.1126/science.1100533. [DOI] [PubMed] [Google Scholar]
- 25.Heng YW, Koh CG. Actin cytoskeleton dynamics and the cell division cycle. Int J Biochem Cell Biol 42: 1622–1633, 2010. doi: 10.1016/j.biocel.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Glotzer M. Cytokinesis: integrating signaling, the cytoskeleton, and membranes to create new daughter cells. Semin Cell Dev Biol 21: 865, 2010. doi: 10.1016/j.semcdb.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Chang F, Burgess D. The contractile ring. Curr Biol 13: R692–R693, 2003. doi: 10.1016/j.cub.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 28.Pollard TD. Mechanics of cytokinesis in eukaryotes. Curr Opin Cell Biol 22: 50–56, 2010. doi: 10.1016/j.ceb.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moazzem Hossain M, Wang X, Bergan RC, Jin JP. Diminished expression of h2-calponin in prostate cancer cells promotes cell proliferation, migration and the dependence of cell adhesion on substrate stiffness. FEBS Open Bio 4: 627–636, 2014. doi: 10.1016/j.fob.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kasinathan P, Knott JG, Moreira PN, Burnside AS, Jerry DJ, Robl JM. Effect of fibroblast donor cell age and cell cycle on development of bovine nuclear transfer embryos in vitro. Biol Reprod 64: 1487–1493, 2001. doi: 10.1095/biolreprod64.5.1487. [DOI] [PubMed] [Google Scholar]
- 31.Nigam R, Triggle CR, Jin JP. h1- and h2-calponins are not essential for norepinephrine- or sodium fluoride-induced contraction of rat aortic smooth muscle. J Muscle Res Cell Motil 19: 695–703, 1998. doi: 10.1023/a:1005389300151. [DOI] [PubMed] [Google Scholar]
- 32.Dunn DE, Jin JP, Lancki DW, Fitch FW. An alternative pathway of induction of lymphokine production by T lymphocyte clones. J Immunol 142: 3847–3856, 1989. [PubMed] [Google Scholar]
- 33.Sasaki Y, Uchida T, Sasaki Y. A variant derived from rabbit aortic smooth muscle: phenotype modulation and restoration of smooth muscle characteristics in cells in culture. J Biochem 106: 1009–1018, 1989. doi: 10.1093/oxfordjournals.jbchem.a122956. [DOI] [PubMed] [Google Scholar]
- 34.Gillies RJ, Didier N, Denton M. Determination of cell number in monolayer cultures. Anal Biochem 159: 109–113, 1986. doi: 10.1016/0003-2697(86)90314-3. [DOI] [PubMed] [Google Scholar]
- 35.Soll DR. The use of computers in understanding how animal cells crawl. Int Rev Cytol 163: 43–104, 1995. [PubMed] [Google Scholar]
- 36.Wessels D, Voss E, Von Bergen N, Burns R, Stites J, Soll DR. A computer-assisted system for reconstructing and interpreting the dynamic three-dimensional relationships of the outer surface, nucleus and pseudopods of crawling cells. Cell Motil Cytoskeleton 41: 225–246, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 37.Eppinga RD, Li Y, Lin JL, Lin JJ. Tropomyosin and caldesmon regulate cytokinesis speed and membrane stability during cell division. Arch Biochem Biophys 456: 161–174, 2006. doi: 10.1016/j.abb.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Wessels D, Wang T, Lin JL, Soll DR, Lin JJ. Regulation of caldesmon activity by Cdc2 kinase plays an important role in maintaining membrane cortex integrity during cell division. Cell Mol Life Sci 60: 198–211, 2003. doi: 10.1007/s000180300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong K, Wessels D, Krob SL, Matveia AR, Lin JL, Soll DR, Lin JJ. Forced expression of a dominant-negative chimeric tropomyosin causes abnormal motile behavior during cell division. Cell Motil Cytoskeleton 45: 121–132, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Mishima M, Nishida E. Coronin localizes to leading edges and is involved in cell spreading and lamellipodium extension in vertebrate cells. J Cell Sci 112: 2833–2842, 1999. doi: 10.1242/jcs.112.17.2833. [DOI] [PubMed] [Google Scholar]
- 41.Lakshmikanth GS, Warrick HM, Spudich JA. A mitotic kinesin-like protein required for normal karyokinesis, myosin localization to the furrow, and cytokinesis in Dictyostelium. Proc Natl Acad Sci USA 101: 16519–16524, 2004. doi: 10.1073/pnas.0407304101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gimona M, Kaverina I, Resch GP, Vignal E, Burgstaller G. Calponin repeats regulate actin filament stability and formation of podosomes in smooth muscle cells. Mol Biol Cell 14: 2482–2491, 2003. doi: 10.1091/mbc.e02-11-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin JP, Walsh MP, Sutherland C, Chen W. A role for serine-175 in modulating the molecular conformation of calponin. Biochem J 350: 579–588, 2000. doi: 10.1042/0264-6021:3500579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gimona M, Sparrow MP, Strasser P, Herzog M, Small JV. Calponin and SM 22 isoforms in avian and mammalian smooth muscle. Absence of phosphorylation in vivo. Eur J Biochem 205: 1067–1075, 1992. doi: 10.1111/j.1432-1033.1992.tb16875.x. [DOI] [PubMed] [Google Scholar]
- 45.Jiang Z, Grange RW, Walsh MP, Kamm KE. Adenovirus-mediated transfer of the smooth muscle cell calponin gene inhibits proliferation of smooth muscle cells and fibroblasts. FEBS Lett 413: 441–445, 1997. doi: 10.1016/S0014-5793(97)00944-7. [DOI] [PubMed] [Google Scholar]
- 46.Matsumura F. Regulation of myosin II during cytokinesis in higher eukaryotes. Trends Cell Biol 15: 371–377, 2005. doi: 10.1016/j.tcb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Murthy K, Wadsworth P. Myosin-II-dependent localization and dynamics of F-actin during cytokinesis. Curr Biol 15: 724–731, 2005. [Erratum in Curr Biol 15: 883, 2005]. doi: 10.1016/j.cub.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 48.Normand G, King RW. Understanding cytokinesis failure. Adv Exp Med Biol 676: 27–55, 2010. doi: 10.1007/978-1-4419-6199-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson BJ. Developmental control of cell growth and division in Drosophila. Curr Opin Cell Biol 22: 788–794, 2010. doi: 10.1016/j.ceb.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 50.Elad N, Abramovitch S, Sabanay H, Medalia O. Microtubule organization in the final stages of cytokinesis as revealed by cryo-electron tomography. J Cell Sci 124: 207–215, 2011. doi: 10.1242/jcs.073486. [DOI] [PubMed] [Google Scholar]
- 51.Pelham RJ, Chang F. Actin dynamics in the contractile ring during cytokinesis in fission yeast. Nature 419: 82–86, 2002. doi: 10.1038/nature00999. [DOI] [PubMed] [Google Scholar]
- 52.Dykes AC, Wright GL. Down-regulation of calponin destabilizes actin cytoskeletal structure in A7r5 cells. Can J Physiol Pharmacol 85: 225–232, 2007. doi: 10.1139/y07-005. [DOI] [PubMed] [Google Scholar]
- 53.Abouzaglou J, Benistant C, Gimona M, Roustan C, Kassab R, Fattoum A. Tyrosine phosphorylation of calponins. Inhibition of the interaction with F-actin. Eur J Biochem 271: 2615–2623, 2004. doi: 10.1111/j.1432-1033.2004.04190.x. [DOI] [PubMed] [Google Scholar]
- 54.Winder SJ, Allen BG, Fraser ED, Kang HM, Kargacin GJ, Walsh MP. Calponin phosphorylation in vitro and in intact muscle. Biochem J 296: 827–836, 1993. doi: 10.1042/bj2960827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winder SJ, Pato MD, Walsh MP. Purification and characterization of calponin phosphatase from smooth muscle. Effect of dephosphorylation on calponin function. Biochem J 286: 197–203, 1992. doi: 10.1042/bj2860197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaneko T, Amano M, Maeda A, Goto H, Takahashi K, Ito M, Kaibuchi K. Identification of calponin as a novel substrate of Rho-kinase. Biochem Biophys Res Commun 273: 110–116, 2000. doi: 10.1006/bbrc.2000.2901. [DOI] [PubMed] [Google Scholar]
- 57.Kamijo K, Ohara N, Abe M, Uchimura T, Hosoya H, Lee JS, Miki T. Dissecting the role of Rho-mediated signaling in contractile ring formation. Mol Biol Cell 17: 43–55, 2006. doi: 10.1091/mbc.e05-06-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]