Abstract
Background:
Animal models are critical to improve our understanding of the neuronal mechanisms underlying nicotine withdrawal. Nicotine dependence in rodents can be established by repeated nicotine injections, chronic nicotine infusion via osmotic minipumps, oral nicotine intake, tobacco smoke exposure, nicotine vapor exposure, and e-cigarette aerosol exposure. The time course of nicotine withdrawal symptoms associated with these methods has not been reviewed in the literature.
Aim:
The goal of this review is to discuss nicotine withdrawal symptoms associated with the cessation of nicotine, tobacco smoke, nicotine vapor, and e-cigarette aerosol exposure in rats and mice. Furthermore, age and sex differences in nicotine withdrawal symptoms are reviewed.
Results:
Cessation of nicotine, tobacco smoke, nicotine vapor, and e-cigarette aerosol exposure leads to nicotine withdrawal symptoms such as somatic withdrawal signs, changes in locomotor activity, anxiety- and depressive-like behavior, learning and memory deficits, attention deficits, hyperalgesia, and dysphoria. These withdrawal symptoms are most pronounced within the first week after cessation of nicotine exposure. Anxiety- and depressive-like behavior, and deficits in learning and memory may persist for several months. Adolescent (4–6 weeks old) rats and mice display fewer nicotine withdrawal symptoms than adults (> 8 weeks old). In adult rats and mice, females show fewer nicotine withdrawal symptoms than males. The smoking cessation drugs bupropion and varenicline reduce nicotine withdrawal symptoms in rodents.
Conclusion:
The nicotine withdrawal symptoms that are observed in rodents are similar to those observed in humans. Tobacco smoke and e-cigarette aerosol contain chemicals and added flavors that enhance the reinforcing properties of nicotine. Therefore, more valid animal models of tobacco and e-cigarette use need to be developed by using tobacco smoke and e-cigarette aerosol exposure methods to induce dependence.
Keywords: Nicotine, Mecamylamine, Spontaneous, Precipitated, Withdrawal, Dependence, Tobacco, Vapor, Smoke, e-cigarettes, Smoking cessation
Introduction
Cigarette smoking is the leading preventable cause of disease and premature death in the world. (-)-Nicotine is a natural tobacco alkaloid and the main addictive chemical in conventional and electronic cigarettes (e-cigs). When tobacco smoke is inhaled, nicotine is absorbed in the lungs and then rapidly enters the brain and binds to nicotinic acetylcholine receptors (nAChRs). The stimulation of nAChRs causes the release of dopamine in the mesolimbic system and produces a mild stimulant effect (Benowitz, 2009; Prochaska and Benowitz, 2016). Nicotine also stimulates the release of other neurotransmitters such as acetylcholine, noradrenaline, serotonin, glutamate, gamma-aminobutyric acid, and endorphins (Benowitz, 2010; Benowitz, 2009). In addition to mild euphoria, nicotine reduces stress, anxiety, and improves mood and overall wellbeing (Picciotto and Mineur, 2014; Benowitz, 2008; Xi et al., 2009). The positive reinforcing effects of nicotine play a role in the initiation of smoking. Long-term nicotine exposure causes neuroadaptations and tolerance, which leads to compulsive smoking to maintain normal brain function (Prochaska and Benowitz, 2016). Discontinuation of smoking in nicotine-dependent individuals alters brain neurotransmitter release and produces negative affective withdrawal symptoms (Benowitz, 2010).
Nicotine withdrawal is characterized by somatic symptoms, affective symptoms, attention deficits, and cognitive deficits. Somatic nicotine withdrawal symptoms include bradycardia, gastrointestinal discomfort, increased appetite, and tremors. Affective nicotine withdrawal symptoms include depression, anxiety, anhedonia, dysphoria, hyperalgesia, and irritability (Kenny and Markou, 2001; Markou, 2008; McLaughlin et al., 2015). Nicotine withdrawal symptoms peak during the first week of smoking abstinence and may last up to 4 weeks (McLaughlin et al., 2015; Paolini and De Biasi, 2011). The highly aversive nicotine withdrawal symptoms make it challenging to maintain abstinence (Hughes, 2007; Hughes et al., 1991). Negative affective nicotine withdrawal symptoms contribute more to relapse than somatic withdrawal signs (Bruijnzeel, 2017).
Nicotine replacement therapy, bupropion, and varenicline are US Food and Drug Administration (FDA) approved smoking cessation medications (Casella et al., 2010; Crooks et al., 2014; Patel et al., 2010). Bupropion is a noradrenaline-dopamine reuptake inhibitor and a neuronal nicotinic acetylcholine receptor (α3β2, α4β2, and α7 nAChRs) antagonist (Crooks et al., 2014; Slemmer et al., 2000). Varenicline is a potent partial agonist at α4β2 nAChRs and a full agonist at α7 nAChRs (Mihalak et al., 2006). Clinical studies suggest that bupropion and varenicline diminish nicotine withdrawal symptoms such as irritability, increased appetite, depressed mood, anxiety, and difficulty concentrating (Hurt et al., 1997; Mooney and Sofuoglu, 2006; Shiffman et al., 2000; Jorenby et al., 2006; Gonzales et al., 2006).
The positive and negative (withdrawal symptoms) reinforcing effects of nicotine contribute to the development of habitual smoking and the maintenance of smoking. Nicotine addiction emerges from complex neurobiological processes, and the precise mechanisms that mediate nicotine addiction are not fully understood. Animal models of nicotine addiction play a vital role in understanding and elucidating the neurobiological mechanisms underlying nicotine addiction. They are also a valuable tool for the development of new smoking cessation medications. The rewarding effects of nicotine in rodents are studied in self-administration (intravenous and oral), conditioned place preference, and intracranial self-stimulation paradigms. Nicotine withdrawal symptoms are assessed in nicotine-dependent animals. Rats and mice can be made nicotine-dependent by various exposure methods such as repeated subcutaneous and intraperitoneal injections, continuous nicotine infusion via osmotic minipumps, oral nicotine intake (drinking), tobacco smoke exposure, nicotine vapor exposure, and e-cig aerosol exposure. Spontaneous (termination of nicotine exposure) or precipitated (administration of nAChRs antagonist, e.g., mecamylamine) withdrawal methods are then used to study withdrawal symptoms. The somatic signs of nicotine withdrawal in rodents are evaluated and scored by visual observation. Behavioral tests are used to measure the affective symptoms, attention, and cognitive deficits associated with nicotine withdrawal in rodents. Somatic signs of nicotine withdrawal are mediated by central and peripheral nAChRs, whereas the affective symptoms are mediated only through the central nAChRs (Watkins et al., 2000; Jackson et al., 2008) [for detailed nAChRs mechanisms of nicotine withdrawal symptoms see the following review articles (Jackson et al., 2015b; McLaughlin et al., 2015; Cohen and George, 2013; Paolini and De Biasi, 2011; Markou, 2008)]. L-type calcium channels and calcium/calmodulin-dependent protein kinases play an important role in nicotine withdrawal symptoms (Jackson et al., 2015b). In addition to this, gamma-aminobutyric acid (GABA)-ergic, glutamatergic, dopaminergic, serotonergic, opioidergic, endocannabinoid, and neuropeptide systems play a role in nicotine withdrawal [for prior reviews see (Bruijnzeel, 2017; Jackson et al., 2015b; McLaughlin et al., 2015; Cohen and George, 2013; Markou, 2008; Kenny and Markou, 2001; Malin, 2001)].
Previous review articles describe different nicotine exposure methods and nicotine withdrawal symptoms. However, there is little information about the time course of spontaneous nicotine withdrawal symptoms in rodents. Therefore, in this review, we briefly describe different nicotine exposure methods and behavioral tests to assess nicotine withdrawal symptoms. The time course of nicotine withdrawal symptoms in mice and rats is discussed in detail. In addition, we discuss sex and age differences in nicotine withdrawal symptoms in rodents. We also review the effects of bupropion and varenicline on nicotine withdrawal symptoms in rodents. Finally, we assess the validity of the currently available rodent models of nicotine withdrawal symptoms.
Inclusion Criteria
Studies that met the following eligibility criteria are included in this review.
Subjects: mice and rats.
Age: Adolescent mice and rats (4–6 weeks old), adult mice (> 8 weeks old or > 20 g body weight), and adult rats (> 8 weeks old or >200 g body weight).
Studies investigated (-)-nicotine (salt and free base forms).
Nicotine exposure methods: intraperitoneal and subcutaneous nicotine injections, nicotine infusion through osmotic minipumps, oral nicotine exposure (drinking, single- and two-bottle methods), tobacco smoke exposure, nicotine vapor exposure, and e-cig aerosol exposure.
Number of animals per group (n ≥ 4).
Spontaneous or mecamylamine-precipitated nicotine withdrawal symptoms.
Effects of clinically approved smoking cessation drugs (bupropion and varenicline) and the effects of other compounds on nicotine withdrawal symptoms.
Animal models of nicotine withdrawal symptoms
Behavioral tests to evaluate nicotine withdrawal symptoms in mice and rats
Commonly used behavioral observations and tests to study nicotine withdrawal symptoms, such as somatic withdrawal signs, affective symptoms, attention, and cognitive deficits in mice and rats are shown in Table S1. Somatic withdrawal signs in mice and rats are scored as the occurrence of individual signs in a quiet environment by an experienced observer who is blind to the treatment conditions. Somatic nicotine withdrawal signs in mice include tremors (forepaw, body, facial), shakes (head, body, forelimb, wet dog), jumping, backing, head nodding, chewing, rearing, scratching, grooming, circling, digging, abdominal constriction, licking (paw, genital), piloerection, and ptosis. Cessation of nicotine exposure in rats leads to somatic withdrawal signs such as eye blinks, gasps, writhes, head shake, ptosis, teeth chattering, and yawns. In order to prevent a high level of activity, the mice and rats need to be habituated to the observation chambers before testing for somatic withdrawal signs (Tan et al., 2019; Stoker et al., 2008; Ponzoni et al., 2015). Environmental conditions such as light intensity affect the number of somatic withdrawal signs (Hamilton et al., 2009). Nicotine withdrawal leads to an increase in somatic signs in rodents (Damaj et al., 2003; Malin et al., 1992). Cessation of nicotine exposure in rodents also affects locomotor activity (Kota et al., 2007; Kota et al., 2008; Mannucci et al., 2006; Hamilton et al., 2010). Withdrawal-induced changes in locomotor activity are measured in the open field test and small activity chambers as locomotor counts (interruptions of photocell beams), squares crossed, and distance traveled. The affective symptoms of nicotine withdrawal in mice and rats are assessed in different behavioral tests. Depressive-like behavior in rodents is measured in the forced swim test. Nicotine withdrawal increases immobility (behavioral despair) in the forced swim test, which indicates an increase in depressive-like behavior (Zaniewska et al., 2010; Roni and Rahman, 2014). Anhedonia is one of the symptoms of depression. The intracranial self-stimulation test is an important tool to study the brain reward system quantitatively by measuring brain reward thresholds. Cessation of nicotine exposure in rodents produces an elevation of brain reward thresholds (Cryan et al., 2003). This indicates impaired reward function (anhedonia). Also, anhedonia can be measured in the sucrose preference test. Sucrose preference is decreased during nicotine withdrawal in mice, which indicates anhedonia-like behavior (Alkhlaif et al., 2017). Dysphoria (aversion) is evaluated in the conditioned place aversion test. Rodents spent less time in a compartment where they experienced negative affective withdrawal symptoms (mecamylamine-paired compartment) (Jackson et al., 2009; Malin et al., 2006). Anxiety-like behavior is measured in the elevated plus-maze, light-dark transition, open field, and marble-burying test (de la Pena et al., 2016; Zhao-Shea et al., 2013; Torres et al., 2015; Alkhlaif et al., 2017; Damaj et al., 2003). Cessation of nicotine administration in rodents leads to a decrease in the time spent in open arms and reduces the number of open arm entries in the elevated plus-maze test, reduces the duration in the light compartment in the light-dark transition test, decreases time spent in the center zone of the open field, and increases marble-burying behavior. All these behaviors are indicative of increased anxiety-like behavior during withdrawal. Hyperalgesia is studied in the hotplate, tail-flick, and thermal plantar stimulation tests. In these tests, nicotine withdrawal produces a decrease in the response latency to thermal and mechanical stimuli, which is indicative of hyperalgesia (Damaj et al., 2003; Grabus et al., 2005; Liu et al., 2014).
Learning and memory deficits during withdrawal are measured in different paradigms such as the modified elevated plus-maze test, contextual fear conditioning test, passive avoidance test, and object recognition test. The elevated plus-maze test was originally designed to study anxiety-like behavior in rodents. However, researchers have used a modified version to evaluate learning and memory deficits. In this test, the animal is placed on one end of the open arms and the transfer latency to reach the closed arms is recorded. Nicotine withdrawal leads to an increase in transfer latency time, which is indicative of a deficit in learning and memory (Biala et al., 2014). The contextual fear conditioning and object recognition tests are used to access hippocampal-dependent and -independent related learning and memory. The contextual fear conditioning test is one of the most widely used tests to study learning and memory deficits during nicotine withdrawal. Cessation of nicotine exposure leads to a decrease in contextual freezing behavior in contextual fear conditioning test and a deficit in spatial object recognition in the object recognition test (Davis et al., 2005; Kenney et al., 2011). In both these tests, nicotine withdrawal affects hippocampal-dependent learning and memory tasks. The passive avoidance test is another test that is used to evaluate learning and memory deficits. In this test, the animal receives unavoidable shocks in a dark compartment during training sessions. A decrease in latency time to enter a dark compartment on the test day is indicative of impaired learning and memory. Cessation of smoke exposure in rats does not affect the learning and memory in the passive avoidance test (de la Pena et al., 2016). The 5-choice serial reaction time task is used to measure visual attention and motor impulsivity in rodents. In this test, nicotine withdrawal decreases the correct responses after a stimulus light presentation and increases the omission responses. These behaviors indicate attention deficits (Semenova et al., 2007). See supplemental Tables S2–S6 for the detailed outcome of these behavioral tests during nicotine withdrawal in mice and rats.
Role of cotinine in the development of nicotine dependence
Nicotine reaches the brain within 10–20 secs after tobacco smoke inhalation (Hukkanen et al., 2005). Nicotine is a nonselective agonist at neuronal and muscle nAChRs. The rewarding effects of nicotine are primarily mediated via the activation of α4β2*, α6β2*, and α7 nAChRs (* denotes the possible presence of other subunits in the nAChR complex)(Brunzell et al., 2015). In most mammalian species, cotinine is the major metabolite of nicotine. In humans, about 70–80% of nicotine is metabolized to cotinine (Benowitz et al., 2009). The plasma half-life of nicotine in mice is 6–7 min, in rats 1 h, and in humans 2 h (Pekonen et al., 1993; Benowitz et al., 2009; Jarvis et al., 1988). The plasma half-life of cotinine in mice is 40 min, in rats 7 h, and in humans 16–20 h (Petersen et al., 1984; Abobo et al., 2012; Benowitz et al., 1983). The plasma half-life of nicotine and cotinine in mice and rats are shorter than in humans. In smokers, plasma cotinine levels are 13-fold higher than plasma nicotine levels (Lawson et al., 1998). Similarly, plasma cotinine levels in rats exposed to nicotine and tobacco smoke are about 5-fold higher than plasma nicotine levels (Small et al., 2010; O’Dell et al., 2006). Similar to nicotine, cotinine penetrates the blood-brain barrier in rats (Lockman et al., 2005). The half-life of cotinine in the brain (t1/2: 347 min) is much longer than the half-life of nicotine (t1/2: 97 min) in adult rats. Furthermore, brain cotinine levels are about two times higher than brain nicotine levels after 8 h of nicotine administration in adult rats (Craig et al., 2014). Cotinine is a weak α4β2 nAChR agonist and is 100 times less potent than nicotine (Alijevic et al., 2020). Dopamine is the primary neurochemical involved in rewarding effects of additive drugs. It has been reported that cotinine stimulates α4β2* nAChR and induces the release of dopamine from adult rat striatal slice preparation. This cotinine-mediated dopamine release is blocked by the nicotine receptor antagonists mecamylamine and dihydro-beta-erythroidine (Dwoskin et al., 1999). However, nicotine, but not cotinine, stimulates the release of dopamine in the nucleus accumbens in adolescent and adult rats (Marusich et al., 2017; Benwell and Balfour, 1992). Studies with rodents have shown that nicotine has stimulatory and rewarding effects. Low doses of nicotine increase locomotor activity and cause locomotor sensitization in mice and rats (Schochet et al., 2004; Marusich et al., 2017; Ulusu et al., 2005; Benwell and Balfour, 1992). Also, nicotine induces place preference and enhances brain reward function in the intracranial self-stimulation test in mice and rats (Grabus et al., 2006; Le Foll and Goldberg, 2005; Xue et al., 2018). Conversely, cotinine does not increase locomotor activity and does not induce locomotor sensitization in rats (Wiley et al., 2015; Marusich et al., 2017). Furthermore, cotinine does not produce place preference or aversion (1 – 50 mg/kg, subcutaneous (s.c.)) and does not affect brain reward function in the intracranial self-stimulation procedure (1 – 100 mg/kg, sc) in rats (Harris et al., 2015; Fudala and Iwamoto, 1986; Marusich et al., 2017). Overall, these results indicate that nicotine is primarily responsible for the rewarding effects of smoking and cotinine has no effect on reward function. Furthermore, there is no strong evidence that cotinine plays a role in the development of nicotine dependence.
The plasma cotinine level is a reliable measure of nicotine exposure. In smokers, the arterial nicotine level is higher (100 ng/ml) than the venous nicotine levels (10–50 ng/ml) (Matta et al., 2007; Benowitz et al., 2009). To accurately model human smoking, the blood nicotine levels in rodents must be similar to those in smokers (Matta et al., 2007). The results from previous studies suggest that experimentally administered nicotine in rodents leads to similar venous blood (plasma) nicotine and cotinine levels as in smokers (Table 1). Therefore, it is likely that the pharmacological effects of nicotine are the same in humans and rodents. In rodents, blood nicotine and cotinine levels are not detected or below detection levels 24 h after cessation of nicotine exposure. This infers that the absence of nicotine in nicotine-dependent animals leads to the expression of withdrawal symptoms. Blood nicotine and cotinine levels in mice and rats during nicotine, smoke, and e-cig aerosol exposure and withdrawal are shown in Table 1. Also, plasma nicotine and cotinine levels in light, moderate, and heavy smokers are shown in Table 1. In the sections below, plasma nicotine and cotinine levels in animal models and smokers are discussed (experimental methods 1–4).
Table 1:
Blood nicotine and cotinine levels in humans and rodents.
| Species | Nicotine exposure | N | Blood nicotine levels | Blood cotinine levels | Reference |
|---|---|---|---|---|---|
| Humans/ 18–65 years of age | light smokers: 10–15 cigarettes/day | 23 | Serum (GC/MS) *: 13.4 ± 8.4 ng/ml | Plasma (GC/MS) *: 179 ± 67 ng/ml | (Lawson et al., 1998) |
| light smokers: 16–30 cigarettes/day | 24 | Serum (GC/MS) *: 20.6 ± 7.2 ng/ml | Plasma (GC/MS) *: 266 ± 99 ng/ml | ||
| heavy smokers: >30 cigarettes/day | 24 | Serum (GC/MS) *: 23.7 ± 10.3 ng/ml | Plasma (GC/MS) *: 304 ± 148 ng/ml | ||
| NMRI mice/male/5 weeks (adolescent) | Nicotine base:1 mg/kg, s.c, pH adjusted to 7 to 7.4 | 6–8 | Plasma (GC/MS)#: 2685±876 ng/ml at 5 min and 81 ±56 ng/ml at 60 min | Plasma (GC/MS)#: 300–400 ng/ml at 30 min and 60 min | (Pekonen et al., 1993) |
| NMRI mice/male/12 weeks | Nicotine base:1 mg/kg, s.c, pH adjusted to 7 to 7.4 | 6–13 | Plasma (GC/MS)#: 1130±485 ng/ml at 5 min and 8 ±7 ng/ml at 60 min | Plasma (GC/MS)#: 250 –300 ng/ml at 30 min and 187±23 ng/ml at 60 min | |
| NMRI mice/male/12 weeks | Nicotine base: 3 mg/kg, s.c, pH adjusted to 7 to 7.4 | 8–13 | Plasma (GC/MS)#: 100 ± 29 ng/ml at 60 min | Plasma (GC/MS)#: 905±74 ng/ml at 60 min | |
| C57Bl6 mice/male/8–12 week | Nicotine base: 0.09 mg/kg, i.p | 7–9 | Plasma (after 10 min of injection; LC/MS) *: 13.40±3.73 ng/ml | Plasma (after 10 min of injection; LC/MS) *: 3.90±2.29 ng/ml | (Davis et al., 2005) |
| ICR mice/male/25–35 g | Nicotine base: 0.5 mg/kg, s.c, pH adjusted to 7 | 8 | Plasma (after 10 min of injection; HPLC/MS/MS): male and female, 20–30 ng/ml | Plasma (after 10 min of injection; HPLC/MS/MS): male and female- 5–10 ng/ml | (Lefever et al., 2017) |
| Nicotine base: 1 mg/kg, s.c, pH adjusted to 7 | 8 | Plasma (after 10 min of injection; HPLC/MS/MS): male and female, 50–60 ng/ml | Plasma (after 10 min of injection; HPLC/MS/MS): male, 5–10 ng/ml; female, 15–20 ng/ml | ||
| Nicotine base: 1.5 mg/kg, s.c, pH adjusted to 7 | 8 | Plasma (after 10 min of injection; HPLC/MS/MS): male, 70–80 ng/ml; female, 80–90 ng/ml | Plasma (after 10 min of injection; HPLC/MS/MS): male, 15–20 ng/ml; female, 20–30 ng/ml | ||
| Wistar rats/ male/ 4–5 weeks (adolescent) | Nicotine (base) minipump: 1.6 mg/kg/day × 7 days | 8 | Plasma (LC/MS)#: 26.6±5.2 ng/ml | Plasma (LC/MS)#: 150.4±18.8 ng/ml | (O’Dell et al., 2006) |
| Nicotine (base) minipump: 3.2 mg/kg/day × 7 days | 6 | Plasma (LC/MS)#: 40.5±2.0 ng/ml | Plasma (LC/MS)#: 265.5±50.1 ng/ml | ||
| Nicotine (base) minipump: 4.7 mg/kg/day × 7 days | 8 | Plasma (LC/MS)#: 76.2±7.6 ng/ml | Plasma (LC/MS)#: 460.5±74.2 ng/ml | ||
| Wistar rats/ male/10 weeks | Nicotine (base) minipump: 1 mg/kg/day × 7 days | 8 | Plasma (LC/MS)#: 22.9±3.2 ng/ml | Plasma (LC/MS)#: 143.0±42.2 ng/ml | (O’Dell et al., 2006) |
| Nicotine (base) minipump: 2.1 mg/kg/day × 7 days | 8 | Plasma (LC/MS)#: 33.8±6.4 ng/ml | Plasma (LC/MS)#: 179.3±38.1 ng/ml | ||
| Nicotine (base) minipump: 3.2 mg/kg/day × 7 days | 8 | Plasma (LC/MS)#: 65.4±9.5 ng/ml | Plasma (LC/MS)#: 297.8±53.6 ng/ml | ||
| Wistar rats/ male/ 4 weeks (adolescent) | Nicotine (base) minipump: 3 mg/kg/day × 8 days | 8 | Plasma (HPLC)#: 34.1 ±4.2 ng/ml | - | (Shram et al., 2008) |
| Nicotine (base) minipump: 4.5 mg/kg/day × 8 days | 8 | Plasma (HPLC)#: 42.1 ±7.4 ng/ml | - | ||
| Nicotine (base) minipump: 6 mg/kg/day × 8 days | 8 | Plasma (HPLC)#: 62.6 ±7.5 ng/ml | - | ||
| Nicotine (base) minipump: 9 mg/kg/day × 8 days | 8 | Plasma (HPLC)#: 92.7 ±12 ng/ml | - | ||
| Wistar rats/ male/8–9 weeks | Nicotine (base) minipump: 3 mg/kg/day × 8 days | 8 | Plasma (HPLC)#: 72.4 ±3.4 ng/ml | - | |
| Nicotine (base) minipump: 6 mg/kg/day × 8 days | 8 | Plasma (HPLC)#: 136.8±5.4 ng/ml | - | ||
| Wistar rats/male and female/8–9 weeks | Nicotine (base) minipump: 3.2 mg/kg/day × 14 days | 26 | - | Plasma (ELISA), during nicotine exposure: 250–350 ng/ml (day 6 and 10); 200–250 ng/ml (day 14). During nicotine withdrawal: 20–40 ng/ml (6 h), 1–20 ng/ml (12 and 24 h) |
(Torres et al., 2013) |
| Sprague Dawley (SD) rats/ male/ adolescent (PND 28)/ adult (PND 60) | Nicotine (base) minipump: 3.2 mg/kg/day × 7 days; pH adjusted to 7.5 | 4 | Serum (HPLC): adolescent and adult, 70–90 ng/ml | - | (de la Peña et al., 2014) |
| C57Bl6 mice/male/8–12 weeks | Nicotine (base) minipump: 6.3 mg/kg/day, 0.25 μL/h flow rate × 13 days | 7–9 | Plasma (LC/MS) *: 13±5.85 ng/ml 24 h after withdrawal: below the detection limit |
Plasma (LC/MS) *: 39.80±10.98 ng/ml 24 h after withdrawal: below the detection limit |
(Davis et al., 2005) |
| C57Bl6 mice/male/8–9 weeks |
Nicotine (base) minipump: 18 mg/kg/day, 0.11 μL/h flow rate × 28 days | 3–5 | - | Serum cotinine (ELISA)#: 131.35±1.47 ng/ml |
(Cole et al., 2015) |
| C57BL6 mice/ male/ 6– 7 weeks/ 3–4 mice/cage | Nicotine (base) oral exposure: 10–200 μg/ml × 40 days | 33 | - | Serum (day 35, during 200 μg/ml nicotine exposure; ELISA)#: 159. 53 ± 13. 55 ng/ml | (Chellian et al., 2018) |
| C57BL6 mice/ male/ 10–12 weeks/ individual housing | Nicotine (base) oral exposure: 200 μg/ml in 2 % saccharine solution × 21 days | - | - | Serum (day 14, ELISA)#: 142.86±3.7 ng/ml During withdrawal: below detection limit < 8ng/ml |
(Roni and Rahman, 2014) |
| ICR mice/male/ 8–10 weeks/3 mice per cage | Nicotine (base) oral exposure: 200 μg/ml in 2 % saccharine sodium × 28 days | 6 | Plasma (HPLC)#: 15.85 ±10.54 ng/ml 24 h after withdrawal: below detection limit < 1.0 ng/ml |
Plasma (HPLC)#: 538±153.28 ng/ml 24 h after withdrawal: below detection limit < 2.0 ng/ml |
(Grabus et al., 2005) |
| Wistar rats/ male/250–300 g | Tobacco smoke exposure (Research cigarettes, 3R4F): 4 h, 12 cigarettes/ day× 28 days | 8 | Plasma (HPLC/MS/MS)#: day 12, 111.2±5.9 ng/ml; day 20, 120.5±5.2 ng/ml; day 28, 121.3±8.1 ng/ml | Plasma (HPLC/MS/MS)#: day 12, 566.1±35.2 ng/ml; day 20, 572.0±19.8 ng/ml; day 28, 539.9±50.3 ng/ml | (Small et al., 2010) |
| Wistar rats/ male/250–300 g | Tobacco smoke exposure (Research cigarettes, 3R4F): 4 h, 12 cigarettes/ day× 6 days | 6 | Plasma (HPLC/MS/MS)#:0 h: 46.6±3.7ng/ml; not detected after 24 h | Plasma (HPLC/MS/MS)#:0h: 248.0±17.9 ng/ml; 24 h: 110.2±6.0 ng/ml; 48 h: 24.9±1.7 ng/ml; 72 h: 16.0±0.2 ng/ml | (Small et al., 2010) |
| SD rats/ male/ adolescent (PND 28)/ adult (PND 60) | Tobacco smoke exposure (Research cigarettes, 3R4F): 2 h, 12 cigarettes; twice daily × 7 days | 4 | Serum (HPLC): adolescent and adult, 60–80 ng/ml | - | (de la Peña et al., 2014) |
| ICR mice/male and female/25–35 g | e-cigarette aerosol (nicotine base in propylene glycol and glycerin mixture) exposure: 12 mg/ml, 5 min session | 8 | Plasma (after 10 min of exposure; HPLC/MS/MS): male, 10–15 ng/ml; female, 15–20 ng/ml | Plasma (after 10 min of exposure; HPLC/MS/MS): male, 20–30 ng/ml; female, 20–35 ng/ml | (Lefever et al., 2017) |
| e-cigarette aerosol exposure: 24 mg/ml, 5 min session | 8 | Plasma (after 10 min of exposure; HPLC/MS/MS): male, 20–25 ng/ml; female, 40–60 ng/ml | Plasma (after 10 min of exposure; HPLC/MS/MS): male, 20–30 ng/ml; female, 50–70 ng/ml | ||
| e-cigarette aerosol exposure: 34 mg/ml, 5 min session | 8 | Plasma (after 10 min of exposure; HPLC/MS/MS): male, 30–40 ng/ml; female, 50–70 ng/ml | Plasma (after 10 min of exposure; HPLC/MS/MS): male, 30–40 ng/ml; female, 50–70 ng/ml | ||
| Wistar rats/ male/250 g | e-cigarette aerosol (nicotine in propylene glycol and vegetable glycerin mixture) exposure: nicotine base 20 mg/ml, 60 min session / day × 10 days | 6 | Plasma (LCMS): day 1, 20 ng/ml; day 10, 20 ng/ml | Plasma (LCMS): day 1, 30 ng/ml; day 10, 30 ng/ml | (Montanari et al., 2020) |
| e-cigarette aerosol exposure: nicotine base 40 mg/ml, 60 min session/ day × 10 days | 6 | Plasma (LCMS): day 1, 50–60 ng/ml; day 10, 50–60 ng/ml | Plasma (LCMS): day 1, 50–60 ng/ml; day 10, 60 ng/ml | ||
| e-cigarette aerosol exposure: nicotine base 80 mg/ml, 60 min session /day × 10 days | 6 | Plasma (LCMS): day 1, 60–70 ng/ml; day 10, 60–70 ng/ml | Plasma (LCMS): day 1, 50–60 ng/ml; day 10, 100–110 ng/ml |
Nicotine doses are expressed as free base. Analytical method used to measure serum/plasma nicotine and cotinine levels: GC/MS, LC/MS, HPLC, HPLC/MS/MS, ELISA. PND: postnatal day; s.c.: subcutaneous; i.p.: intraperitoneal; GC/MS: gas chromatography-mass spectroscopy; LC/MS: liquid chromatography-mass spectroscopy; HPLC: high-performance liquid chromatography; HPLC/MS/MS: high performance liquid chromatography-mass spectroscopy; ELISA: enzyme-linked immunosorbent assay;
mean ± SD;
mean ± SEM.
Experimental method 1: Repeated subcutaneous or intraperitoneal nicotine injection
Nicotine withdrawal symptoms associated with the discontinuation of repeated subcutaneous or intraperitoneal nicotine injections in mice and rats are summarized in Table S2. Nicotine has a short plasma half-life in mice (t1/2: 6–7 min) and rats (t1/2: 1 h) (Pekonen et al., 1993). Therefore, to induce nicotine dependence, nicotine is injected at 4–6 h intervals in mice and 3.5–8 h intervals in rats. When using injections, at least 4 days of repeated subcutaneous or intraperitoneal nicotine injections are required to induce dependence in mice and rats (Table S2). The advantage of this method is that the dose and time of nicotine administration are well controlled and produce fluctuating plasma nicotine levels. This imitates the behavior of a smoker who smokes cigarettes at certain intervals which leads to fluctuating plasma nicotine levels. The plasma nicotine and cotinine levels after a single subcutaneous nicotine injection in mice are comparable to those in smokers (Table 1). However, in humans, nicotine from tobacco smoke reaches the brain within 10–20 secs after inhalation (80–90% of nicotine from inhaled smoke is absorbed) and activates the dopaminergic reward system quickly (Hukkanen et al., 2005). This is one of the factors that contribute to the reinforcing effects of nicotine. The rate of nicotine absorption from subcutaneous or intraperitoneal injections is slower compared to when nicotine is inhaled. This delays the nicotine delivery to the brain and therefore nicotine injections might be less rewarding than the inhalation of nicotine. Repeated daily nicotine injections may also be stressful for animals (Matta et al., 2007). In addition, repeated injections with high doses of nicotine leads to the accumulation of nicotine in the system and produce toxicity. Isola et al. reported that 25 % of mice died following repeated nicotine injections for 14 days (base, 2.5 mg/kg, sc; 4 injections per day) but no mortality was observed with the 1 and 2 mg/kg, sc dose (base) (Isola et al., 1999). Also, in rats, repeated injections with high doses of nicotine (6 mg/kg, sc; 3 injections per day × 7 days; salt or base form not specified) leads to 17 % mortality but lower doses do not leads to mortality (1 and 3 mg/kg, sc ; salt or base form not specified) (Liu et al., 2014). Similarly in other studies, repeated nicotine injections at lower doses (≤ 2 mg/kg, sc; base) did not cause toxicity in mice or rats (Kota et al., 2007; Kota et al., 2008; Akimoto et al., 2018). The median lethal dose (LD50) of subcutaneous (sc) nicotine (base) is 16 mg/kg in mice and 25 mg/kg in rats (Table 2). This shows that mice are more sensitive to the toxic effects of nicotine than rats. These studies indicate that repeated injections with nicotine at doses ≥ 10 mg/kg per day (base) in mice leads to toxicity. Therefore, a safe dose (base) for mice is 0.7 – 2 mg/kg (sc) and a safe dose (base) for rats is 0.36–0.75 mg/kg (sc). When using a between injection interval of at least 3 h, these doses can be administered up to 4 times per day (Table S2).
Table 2:
Acute toxicity of nicotine in mice and rats.
| Route of administration | Mouse: LD50 nicotine base dose | Rat: LD50 nicotine base dose | LD50 rat/LD50 mouse | Reference |
|---|---|---|---|---|
| Intravenous | 0.3 mg/kg | 2.8 mg/kg | 9.3 | (Karaconji, 2005) (see PubChem CID: 89594-toxicity section) |
| Intraperitoneal | 5.9 mg/kg | 14.6 mg/kg | 2.5 | |
| Subcutaneous | 16 mg/kg | 25 mg/kg | 1.6 | |
| Oral | 3.3 mg/kg | 50 mg/kg | 15.2 |
LD50, median lethal dose.
Adult male outbred mice and rats are mostly used to study nicotine withdrawal symptoms using repeated nicotine injection methods of dependence (Table S2). The termination of nicotine injections in mice leads to spontaneous withdrawal symptoms such as somatic withdrawal signs, decreased locomotor activity, depressive- and anxiety-like behavior, hyperalgesia, and learning and memory deficits (Kotagale et al., 2015; Biala et al., 2014; Mannucci et al., 2006; Biala and Weglinska, 2005; Isola et al., 1999; Bagosi et al., 2016) (Table S2). Cessation of nicotine injections in mice does not produce anhedonia (Johnson et al., 2008) (Table S2). In contrast, the administration of mecamylamine to nicotine-exposed mice causes precipitated withdrawal symptoms. This includes somatic withdrawal signs, increased locomotor activity, anxiety-like behavior, and hyperalgesia (Rehni et al., 2012; Biala and Weglinska, 2005; Isola et al., 1999; Kota et al., 2007) (Table S2). Mecamylamine precipitated nicotine withdrawal symptoms are less severe in adult female mice than male mice. Cessation of nicotine injections in adult female mice leads to somatic withdrawal signs but not anxiety-like behavior, hyperalgesia, or changes in locomotor activity (Kota et al., 2007; Kota et al., 2008). In rats, cessation of nicotine injections produces decreased locomotor activity, anxiety, and depressive-like behavior (Zaniewska et al., 2010; Akimoto et al., 2018; Pandey et al., 2001). One study reported that during the first week of nicotine withdrawal, rats do not show changes in anxiety-like behavior (Morud et al., 2018). However, 2 and 3 months after cessation of the nicotine injections, the rats showed decreased anxiety-like behavior (Morud et al., 2018). The administration of mecamylamine to nicotine-exposed rats leads to precipitated withdrawal symptoms including somatic withdrawal signs and hyperalgesia (Liu et al., 2014) (Table S2).
Repeated subcutaneous nicotine injections decreased body weight gain in mice and rats. After nicotine exposure, the body weights returned to baseline levels (Biala and Weglinska, 2005; Liu et al., 2014). This pattern of results is similar to body weight changes observed in smokers. The body weights of smokers are lower than those of nonsmokers and smoking cessation leads to an increase in body weights (Audrain-McGovern and Benowitz, 2011). Only a few studies have evaluated the effects of age and sex on precipitated nicotine withdrawal symptoms in mice that received nicotine via injections. The administration of mecamylamine to nicotine-exposed adolescent mice produced fewer nicotine withdrawal symptoms than the administration of mecamylamine to nicotine-exposed adult mice (Kota et al., 2007; Kota et al., 2008). Mecamylamine precipitated nicotine withdrawal symptoms are more severe in adolescent female mice than male mice. Somatic withdrawal signs, anxiety-like behavior, and hyperalgesia are observed in adolescent female mice, but only somatic withdrawal signs are observed in male mice (Kota et al., 2007; Kota et al., 2008) (Table S2). The following factors contribute to the severity of withdrawal symptoms associated with the cessation of nicotine injections in rodents: route of nicotine administration (subcutaneous and intraperitoneal route), nicotine dose, dosing interval, and duration of nicotine exposure (Table S2). Cessation of nicotine injections leads to a long-lasting spontaneous withdrawal state in rodents (Figure 1). Somatic withdrawal signs and decreased locomotor activity last up to 1 or 2 days after cessation of nicotine administration. Hyperalgesia, anxiety-like behavior, and learning and memory deficits can last up to 14 days after cessation of nicotine administration. Interestingly, depressive-like behavior can last up to 60 days. The effects of bupropion and varenicline on withdrawal symptoms have not been studied using this model to induce nicotine dependence. The contextual fear conditioning test is the best validated and most reliable test for evaluating learning and memory deficits during nicotine withdrawal (Davis et al., 2005; Portugal and Gould, 2007; Raybuck et al., 2008). Therefore, learning and memory deficits induced by the cessation of repeated nicotine injection need to be studied with this test. It is not known if the cessation of nicotine injections leads to a dysphoria-like state in mice and rats. It is also not known if age and sex affect the severity of the spontaneous withdrawal symptoms after the cessation of nicotine injections.
Figure 1.
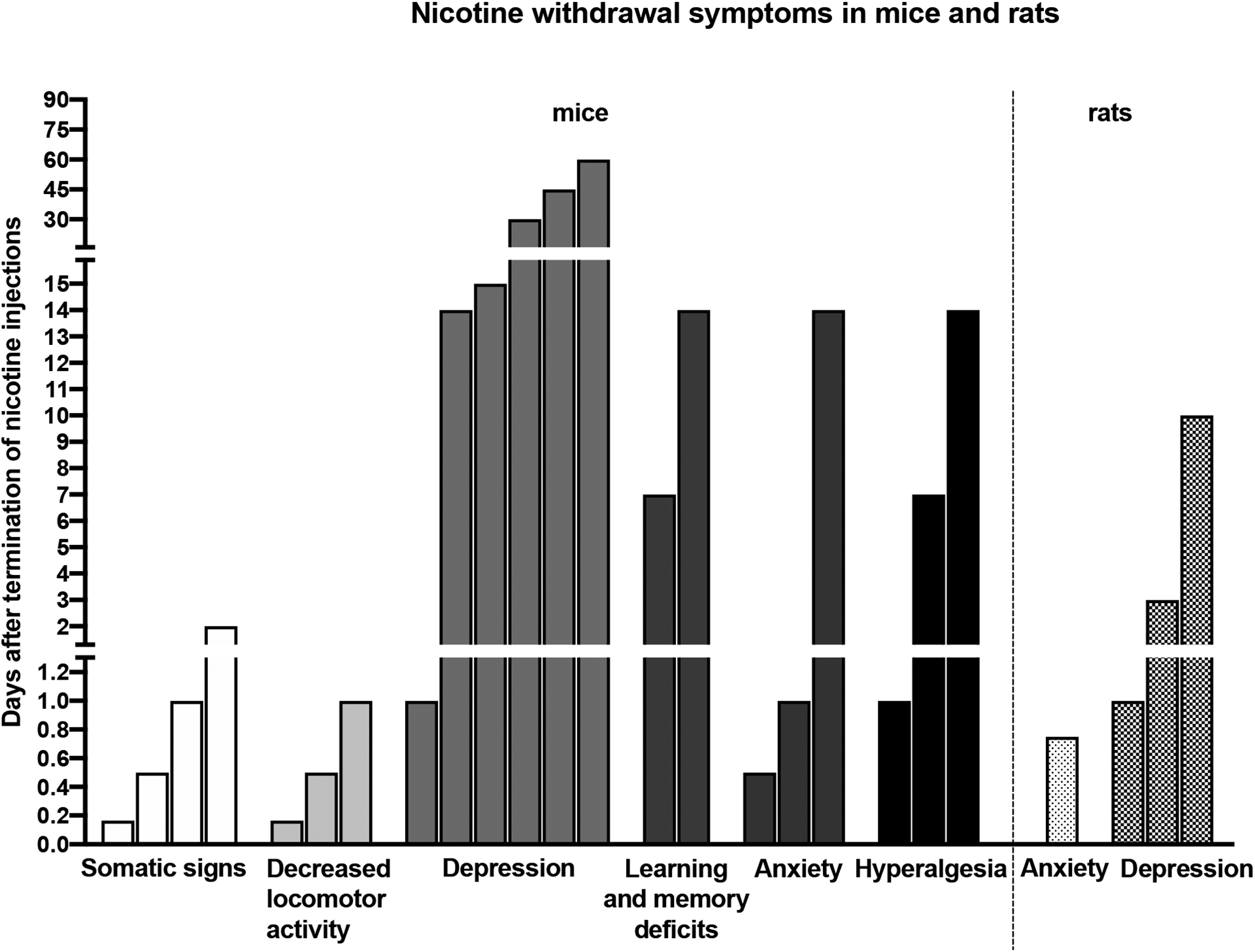
Time course of nicotine withdrawal symptoms after the cessation of repeated nicotine injections in adult mice and rats.
Experimental method 2: Subcutaneous nicotine infusion through osmotic minipumps
One of the most widely used methods to induce nicotine dependence in rodents is the continuous subcutaneous infusion of nicotine with osmotic minipumps. Minor surgery is required to implant and remove the minipumps. The removal of minipumps is required for the assessment of spontaneous withdrawal but not for precipitated withdrawal. This method of nicotine exposure is less stressful than daily nicotine injections (Matta et al., 2007; Hall et al., 2015). Nicotine is slowly and continuously released from the osmotic minipumps and that results in a steady-state plasma nicotine level in animals. The flow-rate of minipumps depends on the nicotine exposure period (rats 1–10 μL/h and mice 0.25–1 μL/h). The nicotine dose and exposure period play an important role in the development of dependence in rodents. In rats, a typical nicotine dose is 3.16 mg/kg/day (free base) delivered via minipumps for one to two weeks to produce nicotine dependence (Table S3). In mice, a wide range of nicotine doses, 6.3–48 mg/kg/day (free base), are infused for one or more weeks to induce dependence (Table S4). The main advantage of this method is that the blood nicotine or cotinine levels in nicotine-infused male and female adolescent or adult rats and mice are similar to the plasma nicotine and cotinine levels in smokers (Table 1). The accuracy of nicotine delivery can be determined by measuring the blood nicotine levels and by measuring the remaining solution in the minipump at the end of the study.
The use of minipumps to induced dependence has three limitations. First, the continuous release of nicotine from the minipump into the systemic circulation results in a steady-state blood nicotine level. This does not correlate well with variable blood levels of nicotine in smokers who smoke intermittently during the day and do not smoke during the night. Secondly, the nicotine concentration in minipumps is adjusted based on the initial body weights of mice and rats and the animals may gain or lose weight during the experiment. Therefore, the nicotine dose delivered via minipump might not remain exactly the same during the study. Finally, nicotine is relatively unstable at a neutral pH and approximately 50% of a neutralized nicotine solution in minipumps degrades over 10 days. Nicotine tartrate or bitartrate salt (as opposed to free base) dissolved in saline or water is acidic (approximately pH 4) and minipumps filled with this acidic solution degrade more slowly, with less than 10 % loss after 10 days (Matta et al., 2007). The serum cotinine levels in adult male and female rats on days 7, 10, and 14 of the nicotine infusions (3.2 mg/kg/day, free base) are therefore similar (Table 1) (Torres et al., 2013). This supports that nicotine at acidic pH does not degrade quickly in the minipumps, and therefore produces stable blood nicotine levels concentration for weeks.
Nicotine infusion at lower doses for one week does not lead to mecamylamine precipitated somatic withdrawal signs in adolescent and adult rats (O’Dell et al., 2006). Similarly, in mice, cessation of the infusion of low doses of nicotine does not produce spontaneous withdrawal symptoms such as somatic withdrawal signs, anxiety-like behavior, hyperalgesia, and learning and memory deficits (Damaj et al., 2003; Portugal et al., 2012). These findings indicate that the level of dependence in rodents depends on the nicotine dose and exposure duration. The administration of high doses of nicotine via minipumps leads to more severe withdrawal signs than the administration of low doses of nicotine (Table S3 and S4). Nicotine withdrawal symptoms using this method of nicotine dependence are extensively studied in adult male rats and mice (Table S3 and S4).
Removal of nicotine pumps in rats induces withdrawal symptoms such as somatic withdrawal signs, attention deficits, and anxiety- and depressive-like behavior (Malin et al., 1992; Torres et al., 2015; Semenova et al., 2007; Shoaib and Bizarro, 2005; Igari et al., 2014) (Table S3). The administration of mecamylamine to nicotine-infused rats leads to precipitated withdrawal symptoms such as somatic withdrawal signs, dysphoria, and depressive-like behavior (Malin et al., 1994; Bruijnzeel et al., 2006; Watkins et al., 2000; Suzuki et al., 1996; Suzuki et al., 1999; O’Dell et al., 2006) (Table S3). In mice, cessation of nicotine infusion after one or more weeks induces spontaneous withdrawal symptoms including somatic withdrawal signs, increased locomotor activity, anxiety-like behavior, hyperalgesia, learning and memory deficits, and depressive-like behavior (anhedonia) (Davis et al., 2005; Kenney et al., 2011; Damaj et al., 2003; Stoker et al., 2008; Alkhlaif et al., 2017; Kota et al., 2007; Johnson et al., 2008; Gould et al., 2012) (Table S4). The administration of mecamylamine to mice that have received nicotine via minipumps leads to somatic withdrawal signs, increased locomotor activity, hyperalgesia, dysphoria, anxiety-like behavior, and depressive-like behavior (anhedonia) (Hilario et al., 2012; Jackson et al., 2009; Alajaji et al., 2013; Jackson et al., 2008; Kota et al., 2008; Damaj et al., 2003) (Table S4). Nicotine withdrawal signs vary by mouse strain. ICR and C57Bl6 mice mostly show clear nicotine withdrawal symptoms but many other strains do not show withdrawal symptoms (Jackson et al., 2009; Varani et al., 2015; Stoker et al., 2008; Jonkman et al., 2005; Damaj et al., 2003; Portugal et al., 2012) (Table S4). During the nicotine withdrawal period, two or more nicotine withdrawal symptoms can be measured in the same animals at different time points (Table S4).
Interestingly, few studies have compared spontaneous and precipitated nicotine withdrawal symptoms in adolescent rats and mice (Table S3 and S4). Spontaneous and mecamylamine precipitated somatic withdrawal signs and decreased locomotor activity have been observed in adolescent rats that were exposed to nicotine (Hamilton et al., 2010; Shram et al., 2008; O’Dell et al., 2006). In contrast, other studies reported that nicotine-infused adolescent rats do not display mecamylamine precipitated somatic withdrawal signs (Shram et al., 2008; Torres et al., 2013). Also, mecamylamine does not induce dysphoria and anhedonia-like behaviors in adolescent rats that received nicotine via minipumps (O’Dell et al., 2007; Shram et al., 2008; O’Dell et al., 2006) (Table S3). In adolescent mice, both spontaneous and mecamylamine-precipitated nicotine withdrawal lead to somatic withdrawal signs, increased locomotor activity, or hyperalgesia (Table S4). However, nicotine withdrawal in adolescent mice did not lead to anxiety- and depressive-like behaviors (Jackson et al., 2009; Kota et al., 2007; Kota et al., 2008). These studies suggest that nicotine withdrawal in adolescent rodents causes somatic withdrawal signs and hyperalgesia but not affective withdrawal symptoms. In contrast, cessation of nicotine administration causes somatic withdrawal signs, affective symptoms, and attention and cognitive deficits in adult rodents. Therefore, this implies that nicotine withdrawal symptoms are less severe in adolescent than in adult rodents
Sex differences in nicotine withdrawal have been reported in adolescent and adult mice and rats (Table S3 and S4). Both adolescent male and female Sprague Dawley (SD) rats showed nicotine withdrawal symptoms such as decreased locomotor activity and somatic withdrawal signs, but these signs were not observed in adolescent male and female Wistar rats (Hamilton et al., 2010; Torres et al., 2013). Sex differences in affective, attention, and learning and memory deficits associated with nicotine withdrawal in adolescent rats are unknown. In adolescent male and female mice, spontaneous and mecamylamine-precipitated nicotine withdrawal leads to somatic withdrawal signs, increased locomotor activity, and hyperalgesia but not anxiety and dysphoria (Jackson et al., 2009; Kota et al., 2007; Kota et al., 2008). Nicotine withdrawal in adult male and female SD and Wistar rats leads to somatic withdrawal signs (Hamilton et al., 2009; Torres et al., 2013). In our recent study, removal of minipumps led to greater anhedonia in male rats (6–96h after withdrawal) than female rats (only at 6h after withdrawal) (Tan et al., 2019). In addition, low doses of mecamylamine produced greater anhedonia in males than females and precipitated somatic withdrawal signs were detected in males but not in females (Tan et al., 2019). In contrast, mecamylamine precipitated somatic withdrawal signs are detected in male and female rats exposed to nicotine via minipumps. Furthermore, mecamylamine precipitated anxiety-like behavior is only observed in female rats but not in male rats (Correa et al., 2019). However, anxiety-like behavior was not observed in male and female rats after removal of the minipumps (spontaneous withdrawal) (Tan et al., 2019). On the other hand, in adult mice, spontaneous and mecamylamine precipitated nicotine withdrawal leads to anxiety-like behavior, hyperalgesia, and increased locomotor activity in males but not in females (Kota et al., 2007; Kota et al., 2008). These studies suggest that nicotine withdrawal symptoms might be more severe in male than female mice or rats.
Spontaneous nicotine withdrawal symptoms such as changes in locomotor activity and attention deficits are only observed at 12 h after the removal of nicotine pumps (Figure 2). Other symptoms such as somatic withdrawal signs, anxiety-like behavior, learning and memory deficits, and anhedonia can last for 3–5 days after the removal of the minipumps (Figure 2). Also, hyperalgesia lasts up to 1 day after the cessation of the nicotine infusions (Figure 2). However, spontaneous withdrawal symptoms due to the cessation of nicotine infusion in rodents did not last longer than 1–2 weeks. The smoking cessation drugs bupropion and varenicline decrease nicotine withdrawal symptoms in mice and rats. Bupropion treatment suppresses nicotine withdrawal symptoms including somatic signs, anxiety-like behavior, hyperalgesia, dysphoria, learning and memory deficits, and depressive-like behavior (anhedonia) in mice and rats (Cryan et al., 2003; Malin et al., 2006; Wing and Shoaib, 2007; Damaj et al., 2010; Portugal and Gould, 2007) (Table S3 and S4). Varenicline also diminishes nicotine withdrawal symptoms such as somatic withdrawal signs, learning and memory deficits, dysphoria, hyperalgesia, and depressive-like behavior (anhedonia) in mice and rats (Igari et al., 2014; Raybuck and Gould, 2009; Bagdas et al., 2018) (Table S3 and S4).
Figure 2.
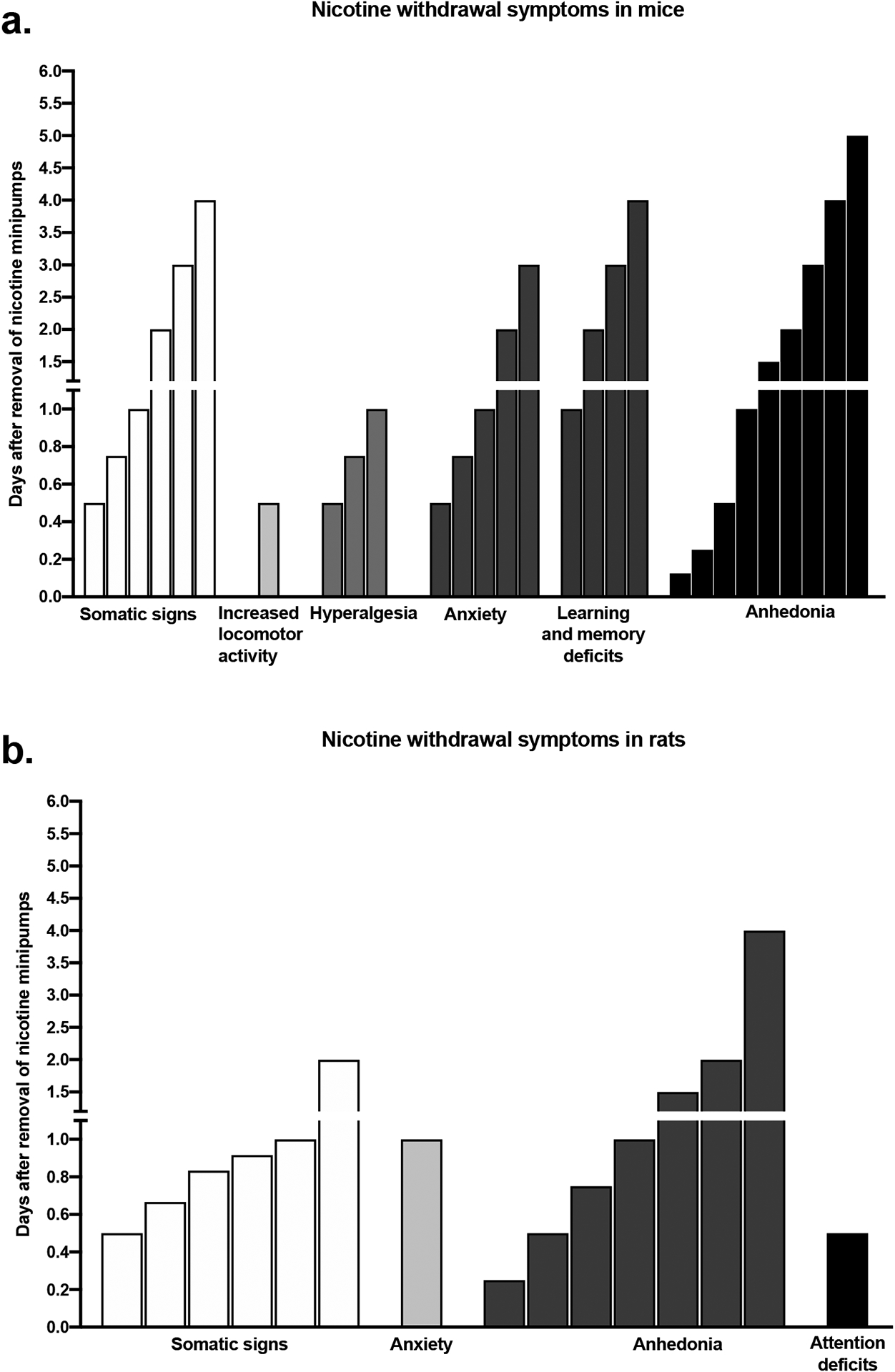
Time course of spontaneous nicotine withdrawal symptoms after removal of nicotine minipumps in adult mice (a) and rats (b).
Experimental method 3: Oral nicotine exposure
Nicotine administration in drinking water is a simple method to induce nicotine dependence in rodents. Nicotine is dissolved in drinking water and available as the only source of fluid in the home cage (single bottle administration). Nicotine can be administered chronically via drinking water to induce dependence. One of the main characteristic features of smoking is fluctuating blood nicotine levels that arise from smoking cigarettes at regular or irregular intervals throughout the day. In addition, the blood nicotine levels of smokers decline during the night. These fluctuating blood nicotine levels in smokers can be replicated in rodents by adding nicotine to drinking water. Mostly rodents drink the nicotine solution during the active period (i.e. during the night). Nicotine drinking behavior in rodents is episodic and this can lead to numerous peaks of blood nicotine levels (Pekonen et al., 1993; Sparks and Pauly, 1999; Pietila and Ahtee, 2000). However, nicotine exposure via drinking water has several disadvantages. This method forces animals to drink nicotine solution as they do not have access to another source of water. Although this might be somewhat stressful for the animals, it is less stressful than the repeated nicotine injections method (Matta et al., 2007; Hall et al., 2015). To overcome some of the disadvantages of using nicotine-containing water as the sole source of fluid, in a recent study a two-bottle choice paradigm was used for nicotine exposure in mice (Bagdas et al., 2019). Daily fluid intake, plasma nicotine levels (~16 ng/ml), and withdrawal symptoms (somatic withdrawal signs and anxiety) of mice exposed to nicotine using two-bottle choice paradigm (nicotine base, 60 μg/ml) are comparable to those of mice exposed to nicotine (nicotine base, 200 μg/ml) via a single bottle method (Grabus et al., 2005; Bagdas et al., 2019; Zhao-Shea et al., 2013). Therefore, the two-bottle choice model can be used to induce nicotine dependence in rodents. Nicotine is not completely absorbed in the oral mucosa and the oral bioavailability of nicotine is low. Oral nicotine has a high degree of first-pass metabolism. Seventy percent of nicotine is primarily metabolized to cotinine before reaching the systemic circulation (Matta et al., 2007). Therefore, a longer period of nicotine exposure is needed to induce dependence in rodents using this method.
Chronic oral nicotine exposure (200 μg/mL × 30 days) leads to an upregulation of nAChRs expressions in the brain in mice In addition, chronic oral nicotine intake causes an increase in extracellular dopamine levels in the nucleus accumbens in mice (Gäddnäs et al., 2002). This increase in dopamine levels can be reversed with mecamylamine treatment (Gäddnäs et al., 2002). In a recent study, varenicline decreased oral nicotine intake in mice. Also, oral nicotine intake is diminished in β2, α6, and α7 nAChR knockout mice compared to wild-type mice (Bagdas et al., 2019). These studies suggest that oral nicotine intake mediates an increase in brain dopamine release via the activation of nAChRs, which may model mesolimbic dopamine release in smokers (Sharma and Brody, 2009). However, chronic oral nicotine exposure in rodents cannot model changes in the brain reward system caused by the rapid increase in nicotine levels by smoking cigarettes. There are three important experimental considerations researchers should make before selecting an oral nicotine exposure method for the induction of nicotine dependence. Firstly, nicotine has a bitter taste and therefore rodents might drink less fluid than normal. This can be overcome by adding a sweetening agent (saccharin sodium) to the nicotine solution or choosing a mouse strain with a high level of nicotine intake. Recent studies show that the oral nicotine intake in C57Bl6 mice is higher than in other inbred mouse strains. Interestingly, adding saccharin sodium to the nicotine solution does not increase oral nicotine consumption (10–200 μg/mL) in C57Bl6 mice (Robinson et al., 1996; Wilking et al., 2012; Chellian et al., 2018; Roni and Rahman, 2014). Based on these studies, it is suggested that the C57Bl6 mouse strain is an outstanding mouse strain for chronic oral nicotine studies and that it is not necessary to add sweetening agents. Secondly, it has been shown that a neutralized nicotine solution is unstable (Pekonen et al., 1993). A neutralized (pH adjusted: 6.8–7.0) nicotine solution at relatively low concentrations (50 and 100 μg/mL) is stable for up to 2 days. However, a 13% loss has been observed in a 500 μg/mL nicotine solution (pH adjusted: 6.8–7.0) after 1 day of preparation and a 25% loss is observed in 300 and 500 μg/mL nicotine solutions (pH adjusted: 6.8–7.0) after 2 days of preparation (Pekonen et al., 1993). Therefore, it is not necessary to adjust the pH of oral nicotine solutions. However, a fresh nicotine solution should be prepared every 2 days. In recent studies, nicotine dependence was successfully established in mice that were exposed to an oral nicotine solution without pH adjustment (Chellian et al., 2018; Roni and Rahman, 2014; Grabus et al., 2005; Bagdas et al., 2019). Finally, single housing for chronic nicotine exposure period as the only source of fluid might be stressful for animals. In recent studies, it was found that there are no differences in daily nicotine consumption and withdrawal symptoms between single and group-housed animals (Table S5). General behavioral differences are observed between individually and socially housed C57Bl6 mice (Voikar et al., 2005). Individual housing affects anxiety- and depressive-like behavior and impairs learning and memory in C57Bl6 mice (Voikar et al., 2005). Therefore, group housing (3–4 mice/cage) might minimize the stress to animals during the chronic oral nicotine exposure period.
Chronic oral nicotine (≥ 200 μg/mL) intake decreased body weight gain and fluid intake in mice. However, body weight gain normalized within one week of nicotine abstinence (Pietila et al., 1998; Grabus et al., 2005; Chellian et al., 2018). This animal model may be used to study decreased body weight gain in smokers and weight gain during smoking cessation (Audrain-McGovern and Benowitz, 2011). Therefore, body weights, fluid consumption, food intake, and nicotine intake should be recorded during the nicotine exposure period in mice. Chronic oral nicotine intake in mice elicits an increase in blood nicotine and cotinine levels comparable to human smokers (Table 1). Blood cotinine levels correlate with oral nicotine intake and therefore, cotinine levels can be used to assess the variation in nicotine intake between mice. Blood nicotine and cotinine levels in mice are undetectable 2 h after cessation of oral nicotine intake (Roni and Rahman, 2014; Grabus et al., 2005; Pekonen et al., 1993). These studies show that chronic oral nicotine intake in mice leads to nicotine dependence. The nicotine concentration and the duration of nicotine exposure play an important role in the development of nicotine dependence in mice (Table S5).
Spontaneous nicotine withdrawal in mice can be induced by replacing the nicotine solution with drinking water. Termination of chronic oral nicotine exposure in mice induces withdrawal symptoms including somatic withdrawal signs, decreased locomotor activity, hyperalgesia, anxiety- and depressive-like behaviors (Table S5). Other nicotine withdrawal symptoms such as anhedonia, dysphoria, attention, and cognitive deficits have not yet been studied. In addition, the administration of mecamylamine to nicotine-exposed mice leads to withdrawal symptoms such as somatic signs and anxiety-like behavior (Zhao-Shea et al., 2015) (Table S5). Cessation of oral nicotine exposure in adolescent mice leads to anxiety-like behavior (Manhães et al., 2008). Two or more nicotine withdrawal symptoms can be measured in the same mouse exposed to oral nicotine solution (Table S5). Bupropion decreases depressive-like behavior after the cessation of oral nicotine intake in mice (Chellian and Pandy, 2018; Roni and Rahman, 2014). Cessation of oral nicotine exposure in mice leads to a state of withdrawal that lasts 8 days (Figure 3). Somatic withdrawal signs, hyperalgesia, and depressive-like behavior last from 6 h to 8 days after the cessation of nicotine intake (Figure 3). The decrease in locomotor activity is only observed 12 –14 h after the cessation of nicotine intake (Figure 3). Cessation of oral nicotine intake leads to anxiety-like behavior at the 6 and 24 h time points (Bagdas et al., 2019). Nicotine withdrawal symptoms associated with the cessation of oral nicotine intake are mostly studied in adult C57Bl6 male mice. Oral nicotine intake is higher in female than male mice (Bagdas et al., 2019). However, it is not known if there are sex differences in nicotine withdrawal symptoms in adult mice or rats. Cessation of oral nicotine intake in adolescent male and female C57Bl6 mice leads to anxiety-like behavior (Table S5). Other nicotine withdrawal symptoms in adolescent rodents still need to be explored.
Figure 3.
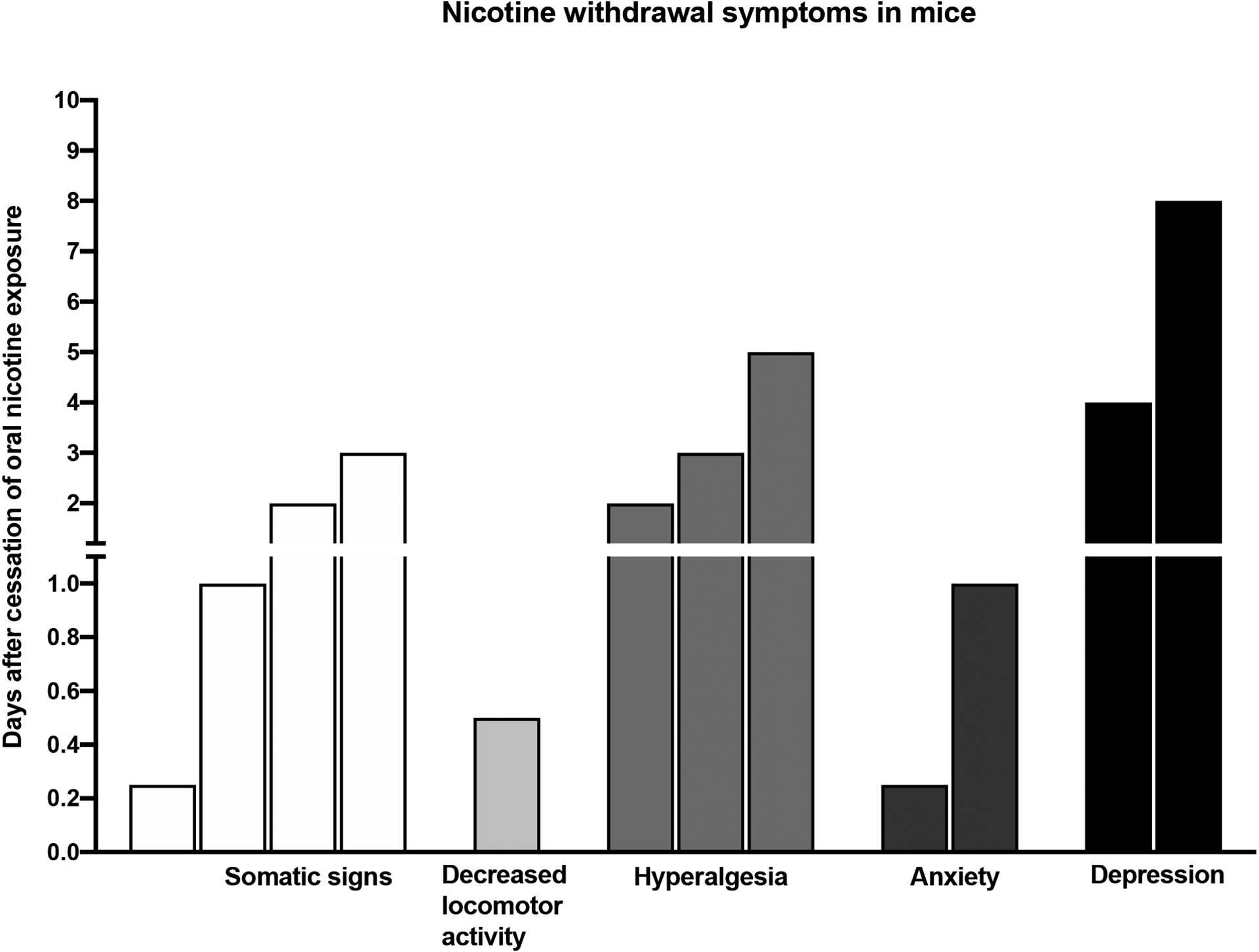
Time course of spontaneous nicotine withdrawal symptoms after cessation of oral nicotine exposure in mice.
Experimental method 4: Tobacco smoke, nicotine vapor, and e-cigarette aerosol exposure
Nicotine from smoke, vapor, or aerosol quickly enters the brain. The advantage of these inhalation methods is that it is non-invasive and less stressful compared to repeated nicotine injections and less invasive than the implantation of osmotic pumps. However, the animals are exposed to smoke, vapor, or aerosol continuously for a prolonged period of time (e.g., 4 h session/day). Smokers titrate the level of nicotine in their system by adjusting the number of puffs, puff duration, puff interval, and/or the number of cigarettes smoked (Griffiths et al., 1982). Therefore, nicotine delivery in rodents via smoke, vapor, or aerosol might not model active smoking. However, blood nicotine and cotinine levels of rats exposed to tobacco smoke are similar to those of heavy smokers (Table 1). Importantly, exposure to cigarette smoke leads to an increase in the expression of α7 nAChRs or non- α7 nAChRs in the CA2/3 region of the hippocampus, stratum oriens, dentate gyrus, and thalamus in rats (Small et al., 2010). On a similar note, tobacco smoke, and e-cig aerosol exposure increases the expressions of α4 and β2 nAChRs and increases [3H]-epibatidine (non- α7 nAChRs) binding in the cortex, hippocampus, nucleus accumbens, and caudate putamen in mice (Ponzoni et al., 2015). Tobacco smoke exposure decreases body weight gain compared to rats that were not exposed to tobacco smoke (Small et al., 2010). Also, food intake and body weight gain were decreased in mice exposed to tobacco smoke but not in mice exposed to e-cig aerosols. Body weights and food intake returned to normal after the cessation of smoke exposure (Ponzoni et al., 2015). These results suggest that tobacco smoke exposure in mice and rats resembles the activation of nAChRs and reduced body weight gain in smokers (Audrain-McGovern and Benowitz, 2011). Exposure to lower and intermediate levels of e-cig aerosol in mice and rats led to plasma nicotine levels similar to those of smokers. However, exposure to high levels of e-cig aerosol in mice and rats led to higher plasma nicotine levels than in heavy smokers (Table 1) (Montanari et al., 2020; Lefever et al., 2017; Chen et al., 2007).
Chronic cigarette smoke, nicotine vapor, or e-cig aerosol exposure in mice and rats induces nicotine dependence and cessation of exposure produces withdrawal symptoms (Table S6). The administration of mecamylamine to mice exposed to tobacco smoke or e-cig aerosol leads to somatic withdrawal signs and decreases locomotor activity. The mecamylamine-precipitated withdrawal symptoms are more severe in mice exposed to tobacco smoke than in mice exposed to e-cig aerosol (Ponzoni et al., 2015). Also, tobacco smoke, nicotine vapor, or e-cig aerosol exposed rats that were treated with mecamylamine showed precipitated nicotine withdrawal symptoms, including somatic withdrawal signs and depressive-like behavior (anhedonia) (Montanari et al., 2020; Gilpin et al., 2014; Small et al., 2010; Chellian et al., 2020). Cessation of tobacco smoke and e-cig aerosol after 7 weeks of exposure in mice leads to somatic withdrawal signs, learning and memory deficits, and anxiety- and depressive-like behavior (Ponzoni et al., 2015; Ponzoni et al., 2020). In rats, cessation of a brief period of nicotine vapor exposure (1 and 10 days) produces somatic withdrawal signs (Montanari et al., 2020). However, cessation of 2–5 weeks of cigarette smoke exposure did not lead to anxiety- and depressive-like behaviors or cognitive deficits in rats (de la Pena et al., 2016; Chellian et al., 2020). These results suggest that a prolonged period of tobacco smoke, nicotine vapor, or e-cig aerosol exposure is necessary to induce spontaneous affective nicotine withdrawal symptoms in rodents. Interestingly, discontinuation of chronic cigarette smoke exposure in adolescent rats leads to anxiety-like behavior (days 1, 3, and 7) and increased locomotor activity (days 3 and 7) (de la Pena et al., 2016). Abstinence from tobacco smoke exposure in adolescent and adult rats does not produce cognitive deficits (de la Pena et al., 2016). Cessation of tobacco smoke and e-cig aerosol exposure in mice produces long-lasting withdrawal symptoms. Learning and memory deficits, anxiety- and depressive-like behaviors last from day 1 to 90 days after cessation of smoke or e-cig aerosol exposure in mice (Ponzoni et al., 2015; Ponzoni et al., 2020) (Figure 4). In a recent study by our group, mecamylamine precipitated somatic withdrawal signs were observed in male but not in female rats that had been exposed to tobacco smoke for 35 days (Chellian et al., 2020). Additional studies are needed to study the withdrawal symptoms associated with the cessation of tobacco smoke and e-cig aerosol exposure in rats. Also, little is known about the role of age and sex differences in nicotine withdrawal symptoms associated with the cessation of tobacco smoke and e-cig aerosol exposure.
Figure 4.

Time course of spontaneous nicotine withdrawal symptoms after cessation of tobacco smoke and e-cigarette aerosol exposure in mice.
Summary
(-)-Nicotine is a natural tobacco alkaloid that is widely used by humans. To induce nicotine dependence in rodents, the majority of studies used (-)-nicotine hydrogen tartrate salt (molecular weight: 462.4 g/mol; PubChem CID: 6174). Most studies reported free base doses and some studies reported salt doses. However, e-cig aerosol and nicotine vapor exposure studies used pure (-)-nicotine (liquid form, molecular weight: 162.23 g/mol; PubChem CID: 89594). Therefore, it has been recommended to report the doses of nicotine as base forms (1 mg/kg nicotine hydrogen tartrate salt equals 0.3508 mg/kg nicotine free base) (Varani et al., 2015; Matta et al., 2007). The LD50 doses of nicotine in mice and rats are reported in Table 2. These data show that mice are more sensitive to the toxic effects of nicotine than rats (see PubChem CID: 89594 toxicity section for more information on the toxicity studies of nicotine in rodents). Nicotine dependence can be induced in mice and rats with different nicotine exposure methods, such as repeated subcutaneous and intraperitoneal injections, implantation of osmotic minipumps, oral nicotine intake, and inhalation methods. However, the oral nicotine intake method of dependence has only been studied in mice and not in rats. To induce dependence, mice are treated with a higher dose of nicotine than rats. For example, rats are made dependent with 3.16 mg/kg/day of nicotine (free base) delivered via minipumps. In mice, 6.3–48 mg/kg/day of nicotine (free base) is administered via minipumps to induce dependence. Chronic exposure to nicotine and tobacco smoke causes reduced body weight gain and food intake in rodents. This is mainly due to the anorexic effect of nicotine (Dandekar et al., 2011). During nicotine withdrawal, body weight and food intake returns to baseline levels. Cessation of nicotine exposure or administration of a nAChR antagonist to nicotine-dependent animals leads to withdrawal symptoms in mice and rats. The nicotine withdrawal symptoms in rodents include somatic signs, changes in locomotor activity, anxiety-like behavior, depressive-like behaviors (anhedonia), learning and memory deficits, attention deficits, dysphoria, and hyperalgesia. Nicotine withdrawal disrupts hippocampal-dependent tasks (learning and memory) but does not affect hippocampal-independent tasks (contextual fear conditioning and object recognition tests) in mice (Kenney et al., 2011; Davis et al., 2005). Learning and memory deficit and hyperalgesia associated with nicotine withdrawal have only been studied in mice. Nicotine withdrawal induced attention deficits have only been studied in rats. Nicotine withdrawal symptoms are more evident in adult than adolescent rodents. Nicotine exposure and its withdrawal symptoms are mainly studied in male rodents. Therefore, sex differences in nicotine withdrawal symptoms are not yet completely understood. However, nicotine withdrawal symptoms might be somewhat more severe in male than female rats and mice. Nicotine withdrawal symptoms are mostly studied in outbred rodents (ICR mice, Wistar, and SD rats). However, learning and memory deficits during withdrawal can be most clearly observed in C57bl6 mice (inbred strain), and nicotine dependence induced by oral nicotine intake is mostly studied in C57bl6 mice. Spontaneous nicotine withdrawal symptoms can be observed from 6 h to several months after nicotine exposure irrespective of the nicotine exposure method used. The time course of somatic withdrawal signs, depressive-like behaviors in mice is comparable to rats. However, cessation of nicotine or smoke produces more prolonged anxiety-like behavior in mice than in rats. The clinically approved smoking cessation drugs, bupropion, and varenicline are highly effective against the nicotine withdrawal symptoms in mice and rats.
The validity of rodent models of nicotine withdrawal symptoms
The validity of animal models can be accessed with three criteria, namely construct validity, face validity, and predictive validity (van der Staay et al., 2009; Willner and Mitchell, 2002). Animal studies can be used to model human tobacco and e-cig use. The validity of the animal models of nicotine withdrawal depends on the species, method of nicotine administration, duration of nicotine exposure, and the methods used to determine nicotine withdrawal.
Construct validity: Do the rodent models accurately represent the nicotine dependence and the withdrawal symptoms associated with smoking cessation (similarity in mechanisms to induce nicotine dependence and withdrawal)?
Nicotine is a nonselective agonist at neuronal and muscle nAChRs. The nAChRs are pentameric ligand-gated cation channels, which are assembled as homomeric and heteromeric complexes. Several subunits of neuronal (12 subunits: α2- α10; β2- β4) and muscle (5 subunits: α1, β1, γ, δ, and ε) nAChRs have been identified. The α4, α6, β2, and α7 nAChRs subunits are predominantly expressed in the brain. In humans, the genes encoded for the α4, α6, β2, and α7 nAChRs subunits are CHRNA4, CHRNA6, CHRNB2, and CHRNA7, respectively. Human genetic studies suggest that these genes play a role in the initiation of smoking and withdrawal (for detailed genetic studies of smoking see review articles authored by (Brunzell et al., 2015; Gold and Lerman, 2012; Portugal and Gould, 2008; McLaughlin et al., 2015). The rewarding effects of nicotine are primarily mediated via the activation of α4β2*, α6β2*, and α7 nAChRs (* denotes the possible presence of other subunits in the nAChR complex). The rewarding effects of nicotine are not observed in mice lacking α4, α6, β2, and α7 nAChRs subunits (Brunzell et al., 2015). In humans, smoking leads to an upregulation of α4β2* nAChRs in the brain (Benwell et al., 1988; Staley et al., 2006). Similarly in rodents, long-term smoke or nicotine exposure increases the α4β2* and α7 nAChR levels in the brain (Small et al., 2010; Ponzoni et al., 2015; Perez et al., 2013). The neurobiology of nicotine withdrawal has been extensively studied in rodent models. For example, in both smokers and rodents, nicotine produces a rewarding effect via the activation of neuronal nAChRs and increasing dopamine signaling in the mesocorticolimbic system. Long-term nicotine exposure in humans and rodents leads to increased nAChRs expressions in the brain. The increase in brain nAChRs and decrease in dopaminergic transmissions in the mesocorticolimbic brain regions of smokers and rodents during withdrawal causes aversive withdrawal symptoms [for detailed mechanisms of nicotine withdrawal symptoms see the review articles authored by (Jackson et al., 2015b; Paolini and De Biasi, 2011; McLaughlin et al., 2015)]. Neuronal nAChRs play an important role in the expression of somatic withdrawal signs, affective symptoms, cognitive deficits, and attention deficits. Somatic withdrawal signs are absent or diminished in mice lacking α2, α3, α5, α7, and β4 subunits. Mice lacking α6 and β2 nAChR subunits display normal somatic nicotine withdrawal signs but do not display nicotine withdrawal-induced anxiety-like behavior, dysphoria, or cognitive deficits. However, α7 nAChR knockout does not affect nicotine withdrawal-induced anxiety and cognitive deficits (Paolini and De Biasi, 2011; Brunzell et al., 2015; Portugal and Gould, 2008). These studies indicate that α7 nAChRs are mainly involved in somatic withdrawal signs whereas α6 and β2 nAChR subunits are primarily involved in affective symptoms and cognitive deficits. Therefore, it has been suggested that the rodent models of nicotine and tobacco smoke exposure can be used to study the neurobiological mechanisms underlying smoking in humans.
Long-term exposure to nicotine and tobacco smoke affects body weight gain in humans and rodents. Smokers gain less weight over time than nonsmokers and smoking cessation can cause excessive weight gain (Audrain-McGovern and Benowitz, 2011). On a similar note, chronic exposure to nicotine and tobacco smoke causes reduced body weight gain in rodents. During nicotine withdrawal, body weight returns to baseline levels (Biala and Weglinska, 2005; Chellian et al., 2018; Small et al., 2010; Grabus et al., 2005; Chellian et al., 2020; Ponzoni et al., 2015). This pattern of results suggests that exposure to nicotine or smoke and withdrawal has a similar effect on body weights in rodents and human tobacco users. Smoking cessation in nicotine-dependent individuals leads to nicotine withdrawal symptoms. Repeated nicotine injections, nicotine infusion via osmotic minipumps, oral nicotine intake, and nicotine vapor exposure methods are used to induce nicotine dependence in rodents. These methods have been used to examine the positive and negative reinforcing effects of nicotine in animals. However, tobacco smoke and e-cigs contain other hazardous or biologically active chemical compounds (Kaur et al., 2018; Kenny and Markou, 2001). For example, tobacco smoke contains chemicals that enhance the reinforcing effect of nicotine in mice or rats (O’Dell and Khroyan, 2009). The rodent models using different routes and methods of nicotine or smoke exposure and all these methods differ from active smoking. In addition, the duration of nicotine exposure to induce nicotine dependence is different in rodents than in humans. Tobacco smoke and e-cig aerosol exposure methods in rodents might be similar to second-hand smoke exposure. However, refinement of tobacco smoke and e-cig aerosol exposure models in rodents is necessary to model human smoking more closely. For example, multiple smoke exposure sessions per day during the dark cycle can model smoking in humans. The behavioral effects of passive exposure to tobacco smoke and e-cig aerosol might not be the same as the effects of active smoking in humans. However, exposure to nicotine and tobacco smoke in rodents leads to similar plasma nicotine and cotinine levels in active smokers (Table 1). Overall, the nicotine exposure methods that are used to induce dependence and withdrawal in rodents have good construct validity. However, it should be noted that animal models that use tobacco smoke exposure or e-cig aerosol exposure to induce dependence have greater construct validity than other nicotine exposure methods.
Face validity: Is there any similarity between nicotine withdrawal symptoms in humans and rodents?
Smoking cessation leads to nicotine withdrawal symptoms such as somatic withdrawal signs, depressed mood, anxiety, anhedonia, dysphoria, hyperalgesia, irritability, attention, and cognitive deficits (McLaughlin et al., 2015). Similarly, termination of nicotine exposure in rodents leads to all the withdrawal symptoms observed in humans. Importantly, rodents and humans tend to express emotional states through different behaviors. In humans, the nicotine withdrawal syndrome is not expressed as a specific behavioral change but as a set of changes in basic physiological states (irritability, cognitive deficits, anxiety, depression, attention deficits, and appetite) (Malin and Goyarzu, 2009). The somatic manifestation of nicotine withdrawal symptoms in humans includes bradycardia, gastrointestinal discomfort, increased appetite, and tremors. In rodents, somatic withdrawal signs that can be observed are tremors, shakes, gasps writhes, teeth chattering, and yawns, and ptosis. Nicotine withdrawal symptoms in rodents and humans are somewhat different and therefore animal models do not completely model the nicotine withdrawal symptoms in humans. The important feature of smoking cessation is the nature of nicotine withdrawal symptoms. Smoking cessation leads to spontaneous nicotine withdrawal symptoms in humans. However, in rodents, both spontaneous and precipitated withdrawal symptoms are studied. Spontaneous and precipitated withdrawal symptoms can be studied in rodents by exposing them to repeated nicotine injections, osmotic minipumps, nicotine in drinking water, tobacco smoke, and e-cig aerosol. However, so far only precipitated withdrawal has been investigated in rodents exposed to nicotine vapor. One of the key features of smoking cessation is that the nicotine withdrawal symptoms start a few hours after quitting smoking, peak in the first few weeks after quitting and may last up to 4 weeks(McLaughlin et al., 2015; Paolini and De Biasi, 2011). Withdrawal symptoms play a role in relapse. In rodents, somatic withdrawal signs, changes in locomotor activity, anxiety-like behavior, hyperalgesia, learning and memory deficits, depressive-like behavior, and attention deficits have been observed during the first week of nicotine abstinence. Furthermore, anxiety-like behavior, learning and memory deficits, and depressive like-behavior can last up to 90 days and hyperalgesia lasts up to 14 days after cessation of nicotine administration in rodents. This suggests that rodent models of spontaneous nicotine withdrawal symptoms can mimic important aspects of smoking cessation. Therefore, it is suggested that rodent models of spontaneous nicotine withdrawal have good face validity.
Predictive validity: Are the animal models of nicotine withdrawal useful in predicting the efficacy of new treatments for smoking cessation?
Many compounds have been tested in animal models of nicotine dependence. In mice that had received nicotine injections, the calcium channel antagonists nimodipine, flunarizine, verapamil diminished nicotine-withdrawal induced learning and memory deficits, hyperalgesia, and anxiety - and depressive-like behavior. (Biala et al., 2014). In addition, all these calcium channel antagonists are effective in reducing mecamylamine precipitated somatic withdrawal signs in mice that received nicotine injections (Biala and Weglinska, 2005). A selective Src kinase inhibitor, SU-6656 decreased mecamylamine-induced somatic signs and anxiety in mice that received nicotine injections (Rehni et al., 2012). In rats, imipramine (tricyclic antidepressant drug), M100,907 (5-HT2A receptor antagonist), Ro 60–0175 (5-HT2B and 5-HT2C receptor agonist), SB 242,084 (5-HT2C receptor antagonist), and WAY 163,909 (5-HT2C receptor agonist) decrease the depressive-like behavior after cessation of nicotine injections (Zaniewska et al., 2010).
In the great majority of the studies, rodents are made nicotine-dependent using minipumps and then potential new treatments for nicotine withdrawal are tested. Mice that are treated with nicotine or nornicotine during the withdrawal phase display fewer somatic withdrawal signs, less anxiety-like behavior, and reduced hyperalgesia. However, cotinine (a major nicotine metabolite) does not diminish these withdrawal symptoms in mice (Elhassan et al., 2017). These results support the effectiveness of nicotine replacement therapy for withdrawal symptoms during smoking cessation (Jorenby et al., 1999). Cytisine and varenicline (a synthetic analog of cytisine) are partial agonists at α4β2 nAChRs and full agonists at α7 nAChRs. Varenicline diminishes learning and memory deficits during nicotine withdrawal in mice (Raybuck et al., 2008). Also, varenicline reduces mecamylamine precipitated somatic withdrawal signs, hyperalgesia, and dysphoria in mice (Bagdas et al., 2018). Varenicline and cytisine decrease depressive-like behavior (anhedonia) in rats during nicotine withdrawal (Igari et al., 2014). Other nAChR ligands such as PNU282987 (a selective α7 nAChRs agonist) and desformylflustrabromide (a positive allosteric modulator at α4β2 nAChRs) also decreased somatic withdrawal signs, anxiety-like behavior, and hyperalgesia in mice (Jackson et al., 2018; Hamouda et al., 2018). The antidepressant drug bupropion (a noradrenaline-dopamine reuptake inhibitor and an α3β2, α4β2, and α7 nAChRs antagonist) is found to be effective in reducing somatic withdrawal signs, anxiety-like behavior, hyperalgesia, learning and memory deficits, dysphoria and depressive-like behavior (anhedonia) associated with nicotine withdrawal in mice and rats (Portugal and Gould, 2007; Damaj et al., 2010; Cryan et al., 2003; Malin et al., 2006). Bupropion is also effective in reducing mecamylamine precipitated somatic withdrawal signs in rats (Wing and Shoaib, 2007; Malin et al., 2006). In addition, mice treated with bupropion or its hydroxy metabolites showed less nicotine withdrawal symptoms such as somatic withdrawal signs, anxiety-like behavior, and hyperalgesia (Damaj et al., 2010). Another antidepressant drug (tricyclic class), desipramine is effective in diminishing the somatic withdrawal signs and depressive-like behavior (anhedonia) associated with nicotine withdrawal in rats (Paterson et al., 2008). Nortriptyline, a tricyclic antidepressant is effective in reducing mecamylamine precipitated somatic withdrawal signs in rats (Wing and Shoaib, 2007). The monoaminoxidase-B inhibitor selegiline decreases somatic withdrawal signs in nicotine-dependent rats, while the monoaminoxidase-A inhibitor, clorgyline potentiates the somatic signs of nicotine withdrawal in rats (Malin et al., 2013). The acetylcholine esterase inhibitors donepezil and galantamine (also a positive allosteric modulator of nAChRs) and a norepinephrine-reuptake inhibitor, atomoxetine reversed the learning and memory deficits associated with nicotine withdrawal in mice (Davis and Gould, 2007; Poole et al., 2014; Wilkinson and Gould, 2011). The beta-lactam antibiotic ceftriaxone diminished mecamylamine precipitated somatic withdrawal signs, anxiety-like behavior, and hyperalgesia (Alajaji et al., 2013). The alpha-1 adrenergic receptor antagonist prazosin and alpha-2 adrenergic receptor antagonist idazoxan are effective in reversing mecamylamine precipitated depressive-like behavior (anhedonia) but not somatic withdrawal signs in rats (Bruijnzeel et al., 2010). However, clonidine (alpha-2 adrenergic/imidazoline-1 receptor agonist), and propranolol (non-selective beta-adrenergic antagonist) do not diminish depressive-like behavior (anhedonia) in rats but decrease the somatic withdrawal signs (Bruijnzeel et al., 2010). The alpha-2 adrenergic receptor antagonist idazoxan is effective in reversing depressive-like behavior (anhedonia) but not somatic withdrawal signs in rats (Semenova and Markou, 2010). In contrast, the 5-HT2A receptor antagonist M100907 potentiates the somatic signs and anhedonia in rats (Semenova and Markou, 2010). Treatment with the selective kappa-opioid receptor antagonist LY2456302reduces anxiety-like behavior, somatic signs, hyperalgesia, and dysphoria associated with nicotine withdrawal in mice (Jackson et al., 2015a). Bupropion and lobeline (α3β2 and α4β2 nAChRs antagonist) diminished depressive-like behavior after cessation of oral nicotine intake in mice (Chellian et al., 2018; Roni and Rahman, 2014). Overall, not only bupropion and varenicline, but also other drugs with different mechanism of actions (e.g., acetylcholine esterase inhibitors, monoaminoxidase-B inhibitor, and alpha-2 adrenergic receptor antagonist), have been shown the diminish nicotine withdrawal. This indicates that a wide range of pharmacotreatments that counteract some of the neurochemical changes associated with the cessation of nicotine administration can diminish nicotine withdrawal (McRobbie et al., 2005; Moerke et al., 2020).
Cytisine has been approved as a smoking cessation drug in Eastern Europe but not in the US (Crooks et al., 2014). Nicotine replacement therapy, varenicline, and bupropion are US FDA-approved medications for the treatment of smoking cessation (Crooks et al., 2014). All these FDA approved smoking cessation drugs diminish the withdrawal symptoms in rodents exposed to nicotine via minipumps. Many different pharmacological classes of compounds have been studied in rodents that were made dependent on using minipumps. Several compounds that diminish nicotine withdrawal in rodents are clinically approved for other psychiatric disorders (e.g. tricyclic antidepressants, acetylcholinesterase inhibitors, etc., see above). However, these compounds are not approved for the treatment of smoking cessation in humans. Importantly, the minipump model might be better than some other nicotine dependence models to evaluate the effectiveness of drugs for smoking cessation. For example, idazoxan diminished the depressive-like behavior, but not somatic signs of nicotine withdrawal. Conversely, clonidine reduced the somatic signs of withdrawal, but not the depressive-like behavior associated with nicotine withdrawal. Overall, treatments that help people quit smoking diminish withdrawal symptoms in rodents that received nicotine via osmotic minipumps. Therefore, this model has a high predictive validity. It is currently not well known if FDA approved smoking cessation drugs diminish withdrawal in rats that were made dependent using other methods such as nicotine injections, oral nicotine intake, tobacco smoke exposure, or e-cig aerosol exposure.
Future directions and implications
Animal models of nicotine withdrawal reproduce most features of the withdrawal syndrome that have been observed in smokers. Only a few studies have investigated sex and age (adolescence and adult) differences in nicotine withdrawal symptoms. Clinical studies have reported that women experience more severe smoking abstinence symptoms and are less successful in quitting smoking than men (Pang and Leventhal, 2013; Leventhal et al., 2007; Bohadana et al., 2003). Adolescents are more vulnerable to the initiation of smoking (conventional and e-cigarettes). Individuals who smoke at a young age are more likely to transition to regular smoking in adulthood (Barrington-Trimis et al., 2016; Kandel et al., 2007). Therefore, it is important to gain a better understanding of the role of sex and age in the severity of nicotine withdrawal symptoms.
A major challenge is to develop animal models of nicotine dependence and withdrawal with higher validity. Cigarette smoke is a complex mixture of more than 7000 chemical compounds and some of these chemicals (e.g., acetaldehyde) enhance the reinforcing effects of nicotine in adolescent and adult rodents (Myers et al., 1982; Smith et al., 1984; Belluzzi et al., 2005; Cao et al., 2007). Tobacco smoke also contains two beta-carboline alkaloids, namely norharman and harman. These alkaloids are potent reversible inhibitors of human recombinant monoaminoxidase-A and monoaminoxidase-B isoenzymes (Herraiz and Chaparro, 2005). Positron emission tomography imaging indicated that brain monoaminoxidase-A and -B activities are lower in smokers than non-smokers (Fowler et al., 1996b; Fowler et al., 1996a; Joanna S. Fowler et al., 2000). Furthermore, some commercial cigarettes and e-cig liquids contain added flavors (e.g. menthol). In rodents, menthol enhances the reinforcing effects of nicotine (Biswas et al., 2016; Cooper et al., 2020). These studies indicate that tobacco smoke and e-cig aerosol exposure in rodents can closely model human smoking. Therefore, tobacco smoke and e-cig aerosol exposure methods in rodents will be a valuable tool to gain knowledge about the neural mechanisms that underlie nicotine withdrawal. Also, it is important to develop animal models of spontaneous nicotine withdrawal by using tobacco smoke and e-cig aerosol exposure methods with improved construct, face, and predictive validity. For example, an animal model in which animals self-administer e-cig aerosol and display withdrawal signs upon the cessation of nicotine use would have high construct and face validity. Currently, the predictive validity of tobacco smoke and e-cig aerosol models of nicotine withdrawal symptoms is low. In almost all the animal studies only adult male rodents were used to evaluate the effects of drugs on nicotine withdrawal. In clinical and animal studies, robust sex differences in nicotine withdrawal symptoms have been reported. Therefore, to improve the predictive validity of tobacco smoke and e-cig aerosol models, it is pivotal to study the effects of clinically approved smoking cessation drugs on spontaneous withdrawal symptoms in adolescent and adult rats of both sexes.
Overall, this review provides an overview of the animal models that are being used to study withdrawal symptoms associated with smoking cessation in humans. Animals models for smoking cessation and withdrawal with more validity will have greater translation significance to humans. This may lead to the development of novel pharmacotherapies to aid smoking cessation. In addition, studying the sex and age differences in the severity of nicotine withdrawal symptoms in more valid animal models can help with the development of improved treatment strategies for smoking cessation.
Supplementary Material
Funding
This work was supported by a NIDA/NIH and FDA Center for Tobacco Products (CTP) grant (DA042530) and NIDA grant (DA046411) to AB. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
Reference
- Abobo CV, Ma J and Liang D (2012) Effect of menthol on nicotine pharmacokinetics in rats after cigarette smoke inhalation. Nicotine Tob Res 14(7): 801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimoto H, Oshima S, Michiyama Y, et al. (2018) Metabolic Profiling of the Hippocampus of Rats Experiencing Nicotine-Withdrawal Symptoms. Biol Pharm Bull 41(12): 1879–1884. [DOI] [PubMed] [Google Scholar]
- Alajaji M, Bowers MS, Knackstedt L, et al. (2013) Effects of the beta-lactam antibiotic ceftriaxone on nicotine withdrawal and nicotine-induced reinstatement of preference in mice. Psychopharmacology (Berl) 228(3): 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alijevic O, McHugh D, Rufener L, et al. (2020) An electrophysiological characterization of naturally occurring tobacco alkaloids and their action on human α4β2 and α7 nicotinic acetylcholine receptors. Phytochemistry 170: 112187. [DOI] [PubMed] [Google Scholar]
- Alkhlaif Y, Bagdas D, Jackson A, et al. (2017) Assessment of nicotine withdrawal-induced changes in sucrose preference in mice. Pharmacol Biochem Behav 161: 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J and Benowitz NL (2011) Cigarette smoking, nicotine, and body weight. Clin Pharmacol Ther 90(1): 164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D, Alkhlaif Y, Jackson A, et al. (2018) New insights on the effects of varenicline on nicotine reward, withdrawal and hyperalgesia in mice. Neuropharmacology 138: 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D, Diester CM, Riley J, et al. (2019) Assessing nicotine dependence using an oral nicotine free-choice paradigm in mice. Neuropharmacology 157: 107669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagosi Z, Palotai M, Simon B, et al. (2016) Selective CRF2 receptor agonists ameliorate the anxiety- and depression-like state developed during chronic nicotine treatment and consequent acute withdrawal in mice. Brain Res 1652: 21–29. [DOI] [PubMed] [Google Scholar]
- Barrington-Trimis JL, Urman R, Berhane K, et al. (2016) E-Cigarettes and Future Cigarette Use. Pediatrics 138(1): e20160379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi JD, Wang R and Leslie FM (2005) Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology 30(4): 705–712. [DOI] [PubMed] [Google Scholar]
- Benowitz NL (2008) Neurobiology of nicotine addiction: implications for smoking cessation treatment. Am J Med 121(4 Suppl 1): S3–10. [DOI] [PubMed] [Google Scholar]
- Benowitz NL (2009) Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol 49: 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL (2010) Nicotine addiction. The New England journal of medicine 362(24): 2295–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Hukkanen J and Jacob P 3rd (2009) Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. Epub ahead of print 2009/02/03. DOI: 10.1007/978-3-540-69248-5_2.(192): 29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Kuyt F, Jacob P 3rd, et al. (1983) Cotinine disposition and effects. Clin Pharmacol Ther 34(5): 604–611. [DOI] [PubMed] [Google Scholar]
- Benwell ME and Balfour DJ (1992) The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. British journal of pharmacology 105(4): 849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ and Anderson JM (1988) Evidence that tobacco smoking increases the density of (-)-[3H]nicotine binding sites in human brain. Journal of neurochemistry 50(4): 1243–1247. [DOI] [PubMed] [Google Scholar]
- Biala G, Polak P, Michalak A, et al. (2014) Influence of calcium channel antagonists on nonsomatic signs of nicotine and D-amphetamine withdrawal in mice. Pharmacol Rep 66(2): 212–222. [DOI] [PubMed] [Google Scholar]
- Biala G and Weglinska B (2005) Blockade of the expression of mecamylamine-precipitated nicotine withdrawal by calcium channel antagonists. Pharmacol Res 51(5): 483–488. [DOI] [PubMed] [Google Scholar]
- Biswas L, Harrison E, Gong Y, et al. (2016) Enhancing effect of menthol on nicotine self-administration in rats. Psychopharmacology (Berl) 233(18): 3417–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohadana A, Nilsson F, Rasmussen T, et al. (2003) Gender differences in quit rates following smoking cessation with combination nicotine therapy: influence of baseline smoking behavior. Nicotine Tob Res 5(1): 111–116. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW (2017) Neuropeptide systems and new treatments for nicotine addiction. Psychopharmacology (Berl) 234(9): 1419–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Bishnoi M, van Tuijl IA, et al. (2010) Effects of prazosin, clonidine, and propranolol on the elevations in brain reward thresholds and somatic signs associated with nicotine withdrawal in rats. Psychopharmacology (Berl) 212(4): 485–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Zislis G, Wilson C, et al. (2006) Antagonism of CRF Receptors Prevents the Deficit in Brain Reward Function Associated with Precipitated Nicotine Withdrawal in Rats. Neuropsychopharmacology 32(4): 955–963. [DOI] [PubMed] [Google Scholar]
- Brunzell DH, Stafford AM and Dixon CI (2015) Nicotinic receptor contributions to smoking: insights from human studies and animal models. Current addiction reports 2(1): 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Belluzzi JD, Loughlin SE, et al. (2007) Acetaldehyde, a major constituent of tobacco smoke, enhances behavioral, endocrine, and neuronal responses to nicotine in adolescent and adult rats. Neuropsychopharmacology 32(9): 2025–2035. [DOI] [PubMed] [Google Scholar]
- Casella G, Caponnetto P and Polosa R (2010) Therapeutic advances in the treatment of nicotine addiction: present and future. Therapeutic Advances in Chronic Disease 1(3): 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellian R, Behnood-Rod A, Wilson R, et al. (2020) Exposure to smoke from high- but not low-nicotine cigarettes leads to signs of dependence in male rats and potentiates the effects of nicotine in female rats. Pharmacol Biochem Behav 196: 172998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellian R and Pandy V (2018) Protective effect of alpha-asarone against nicotine-induced seizures in mice, but not by its interaction with nicotinic acetylcholine receptors. Biomed Pharmacother 108: 1591–1595. [DOI] [PubMed] [Google Scholar]
- Chellian R, Pandy V and Mohamed Z (2018) Alpha-asarone attenuates depression-like behavior in nicotine-withdrawn mice: Evidence for the modulation of hippocampal pCREB levels during nicotine-withdrawal. European Journal of Pharmacology 818: 10–16. [DOI] [PubMed] [Google Scholar]
- Chen H, Matta SG and Sharp BM (2007) Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology 32(3): 700–709. [DOI] [PubMed] [Google Scholar]
- Cohen A and George O (2013) Animal Models of Nicotine Exposure: Relevance to Second-Hand Smoking, Electronic Cigarette Use, and Compulsive Smoking. Frontiers in Psychiatry 4(41). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole RD, Poole RL, Guzman DM, et al. (2015) Contributions of β2 subunit-containing nAChRs to chronic nicotine-induced alterations in cognitive flexibility in mice. Psychopharmacology (Berl) 232(7): 1207–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SY, Akers AT and Henderson BJ (2020) Flavors Enhance Nicotine Vapor Self-administration in Male Mice. Nicotine & Tobacco Research. DOI: 10.1093/ntr/ntaa165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa VL, Flores RJ, Carcoba LM, et al. (2019) Sex differences in cholinergic systems in the interpeduncular nucleus following nicotine exposure and withdrawal. Neuropharmacology 158: 107714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig EL, Zhao B, Cui JZ, et al. (2014) Nicotine pharmacokinetics in rats is altered as a function of age, impacting the interpretation of animal model data. Drug metabolism and disposition: the biological fate of chemicals 42(9): 1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks PA, Bardo MT and Dwoskin LP (2014) Nicotinic Receptor Antagonists as Treatments for Nicotine Abuse. Advances in pharmacology (San Diego, Calif.) 69: 513–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Bruijnzeel AW, Skjei KL, et al. (2003) Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology (Berl) 168(3): 347–358. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Grabus SD, Navarro HA, et al. (2010) Effects of Hydroxymetabolites of Bupropion on Nicotine Dependence Behavior in Mice. The Journal of Pharmacology and Experimental Therapeutics 334(3): 1087–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Kao W and Martin BR (2003) Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther 307(2): 526–534. [DOI] [PubMed] [Google Scholar]
- Dandekar MP, Nakhate KT, Kokare DM, et al. (2011) Effect of nicotine on feeding and body weight in rats: involvement of cocaine- and amphetamine-regulated transcript peptide. Behav Brain Res 219(1): 31–38. [DOI] [PubMed] [Google Scholar]
- Davis JA and Gould TJ (2007) Atomoxetine reverses nicotine withdrawal-associated deficits in contextual fear conditioning. Neuropsychopharmacology 32(9): 2011–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, et al. (2005) Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci 25(38): 8708–8713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Pena JB, Ahsan HM, Botanas CJ, et al. (2016) Cigarette smoke exposure during adolescence but not adulthood induces anxiety-like behavior and locomotor stimulation in rats during withdrawal. Int J Dev Neurosci 55: 49–55. [DOI] [PubMed] [Google Scholar]
- de la Peña JB, Ahsan HM, Botanas CJ, et al. (2014) Adolescent nicotine or cigarette smoke exposure changes subsequent response to nicotine conditioned place preference and self-administration. Behavioural Brain Research 272: 156–164. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Teng L, Buxton ST, et al. (1999) (S)-(-)-Cotinine, the major brain metabolite of nicotine, stimulates nicotinic receptors to evoke [3H]dopamine release from rat striatal slices in a calcium-dependent manner. J Pharmacol Exp Ther 288(3): 905–911. [PubMed] [Google Scholar]
- Elhassan S, Bagdas D and Damaj MI (2017) Effects of Nicotine Metabolites on Nicotine Withdrawal Behaviors in Mice. Nicotine Tob Res 19(6): 763–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, et al. (1996a) Inhibition of monoamine oxidase B in the brains of smokers. Nature 379(6567): 733–736. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, et al. (1996b) Brain monoamine oxidase A inhibition in cigarette smokers. Proc Natl Acad Sci U S A 93(24): 14065–14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudala PJ and Iwamoto ET (1986) Further studies on nicotine-induced conditioned place preference in the rat. Pharmacology Biochemistry and Behavior 25(5): 1041–1049. [DOI] [PubMed] [Google Scholar]
- Gäddnäs H, Piepponen TP and Ahtee L (2002) Mecamylamine decreases accumbal dopamine output in mice treated chronically with nicotine. Neuroscience Letters 330(3): 219–222. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Whitaker AM, Baynes B, et al. (2014) Nicotine vapor inhalation escalates nicotine self-administration. Addict Biol 19(4): 587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold AB and Lerman C (2012) Pharmacogenetics of smoking cessation: role of nicotine target and metabolism genes. Human genetics. DOI: 10.1007/s00439-012-1143-9. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, et al. (2006) Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 296(1): 47–55. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Portugal GS, André JM, et al. (2012) The Duration of Nicotine Withdrawal-Associated Deficits in Contextual Fear Conditioning Parallels Changes in Hippocampal High Affinity Nicotinic Acetylcholine Receptor Upregulation. Neuropharmacology 62(5–6): 2118–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Batman AM, et al. (2005) Nicotine physical dependence and tolerance in the mouse following chronic oral administration. Psychopharmacology (Berl) 178(2–3): 183–192. [DOI] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Brown SE, et al. (2006) Nicotine place preference in the mouse: influences of prior handling, dose and strain and attenuation by nicotinic receptor antagonists. Psychopharmacology (Berl) 184(3–4): 456–463. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Henningfield JE and Bigelow GE (1982) Human cigarette smoking: manipulation of number of puffs per bout, interbout interval and nicotine dose. J Pharmacol Exp Ther 220(2): 256–265. [PubMed] [Google Scholar]
- Hall FS, Der-Avakian A, Gould TJ, et al. (2015) Negative affective states and cognitive impairments in nicotine dependence. Neuroscience & Biobehavioral Reviews 58: 168–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KR, Berger SS, Perry ME, et al. (2009) Behavioral effects of nicotine withdrawal in adult male and female rats. Pharmacology Biochemistry and Behavior 92(1): 51–59. [DOI] [PubMed] [Google Scholar]
- Hamilton KR, Perry ME, Berger SS, et al. (2010) Behavioral effects of nicotine withdrawal differ by genetic strain in male and female adolescent rats. Nicotine Tob Res 12(12): 1236–1245. [DOI] [PubMed] [Google Scholar]
- Hamouda AK, Jackson A, Bagdas D, et al. (2018) Reversal of Nicotine Withdrawal Signs Through Positive Allosteric Modulation of α4β2 Nicotinic Acetylcholine Receptors in Male Mice. Nicotine Tob Res 20(7): 903–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Tally L, Muelken P, et al. (2015) Effects of nicotine and minor tobacco alkaloids on intracranial-self-stimulation in rats. Drug Alcohol Depend 153: 330–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herraiz T and Chaparro C (2005) Human monoamine oxidase is inhibited by tobacco smoke: β-carboline alkaloids act as potent and reversible inhibitors. Biochemical and Biophysical Research Communications 326(2): 378–386. [DOI] [PubMed] [Google Scholar]
- Hilario MR, Turner JR and Blendy JA (2012) Reward sensitization: effects of repeated nicotine exposure and withdrawal in mice. Neuropsychopharmacology 37(12): 2661–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR (2007) Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res 9(3): 315–327. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Gust SW, Skoog K, et al. (1991) Symptoms of tobacco withdrawal. A replication and extension. Arch Gen Psychiatry 48(1): 52–59. [DOI] [PubMed] [Google Scholar]
- Hukkanen J, Jacob P and Benowitz NL (2005) Metabolism and Disposition Kinetics of Nicotine. Pharmacological Reviews 57(1): 79. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Sachs DP, Glover ED, et al. (1997) A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med 337(17): 1195–1202. [DOI] [PubMed] [Google Scholar]
- Igari M, Alexander JC, Ji Y, et al. (2014) Varenicline and Cytisine Diminish the Dysphoric-Like State Associated with Spontaneous Nicotine Withdrawal in Rats. Neuropsychopharmacology 39(2): 455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isola R, Vogelsberg V, Wemlinger TA, et al. (1999) Nicotine abstinence in the mouse. Brain Res 850(1–2): 189–196. [DOI] [PubMed] [Google Scholar]
- Jackson A, Papke RL and Damaj MI (2018) Pharmacological modulation of the α7 nicotinic acetylcholine receptor in a mouse model of mecamylamine-precipitated nicotine withdrawal. Psychopharmacology (Berl) 235(7): 1897–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Jackson A, Carroll FI, et al. (2015a) Effects of orally-bioavailable short-acting kappa opioid receptor-selective antagonist LY2456302 on nicotine withdrawal in mice. Neuropharmacology 97: 270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Kota DH, Martin BR, et al. (2009) The role of various nicotinic receptor subunits and factors influencing nicotine conditioned place aversion. Neuropharmacology 56(0): 10.1016/j.neuropharm.2009.1001.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, et al. (2008) Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther 325(1): 302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Muldoon PP, De Biasi M, et al. (2015b) New mechanisms and perspectives in nicotine withdrawal. Neuropharmacology 96(0 0): 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MJ, Russell MA, Benowitz NL, et al. (1988) Elimination of cotinine from body fluids: implications for noninvasive measurement of tobacco smoke exposure. American journal of public health 78(6): 696–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler, Ph.D. Joanna S. ,, Wang, M.D. Gene-Jack ,, Volkow, M.D. Nora D. ,, et al. (2000) Maintenance of Brain Monoamine Oxidase B Inhibition in Smokers After Overnight Cigarette Abstinence. American Journal of Psychiatry 157(11): 1864–1866. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Hollander JA and Kenny PJ (2008) Decreased brain reward function during nicotine withdrawal in C57BL6 mice: evidence from intracranial self-stimulation (ICSS) studies. Pharmacol Biochem Behav 90(3): 409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman S, Henry B, Semenova S, et al. (2005) Mild anxiogenic effects of nicotine withdrawal in mice. Eur J Pharmacol 516(1): 40–45. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, et al. (2006) Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 296(1): 56–63. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, et al. (1999) A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med 340(9): 685–691. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Hu M-C, Griesler PC, et al. (2007) On the development of nicotine dependence in adolescence. Drug and alcohol dependence 91(1): 26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaconji IB (2005) Facts about nicotine toxicity. Arh Hig Rada Toksikol 56(4): 363–371. [PubMed] [Google Scholar]
- Kaur G, Pinkston R, McLemore B, et al. (2018) Immunological and toxicological risk assessment of e-cigarettes. Eur Respir Rev 27(147). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Adoff MD, Wilkinson DS, et al. (2011) The effects of acute, chronic, and withdrawal from chronic nicotine on novel and spatial object recognition in male C57BL/6J mice. Psychopharmacology (Berl) 217(3): 353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ and Markou A (2001) Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav 70(4): 531–549. [DOI] [PubMed] [Google Scholar]
- Kota D, Martin BR and Damaj MI (2008) Age-dependent differences in nicotine reward and withdrawal in female mice. Psychopharmacology (Berl) 198(2): 201–210. [DOI] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, et al. (2007) Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther 322(1): 399–407. [DOI] [PubMed] [Google Scholar]
- Kotagale NR, Chopde CT, Umekar MJ, et al. (2015) Chronic agmatine treatment prevents behavioral manifestations of nicotine withdrawal in mice. Eur J Pharmacol 754: 190–198. [DOI] [PubMed] [Google Scholar]
- Lawson GM, Hurt RD, Dale LC, et al. (1998) Application of serum nicotine and plasma cotinine concentrations to assessment of nicotine replacement in light, moderate, and heavy smokers undergoing transdermal therapy. J Clin Pharmacol 38(6): 502–509. [DOI] [PubMed] [Google Scholar]
- Le Foll B and Goldberg SR (2005) Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology (Berl) 178(4): 481–492. [DOI] [PubMed] [Google Scholar]
- Lefever TW, Lee YO, Kovach AL, et al. (2017) Delivery of nicotine aerosol to mice via a modified electronic cigarette device. Drug Alcohol Depend 172: 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Boyd S, et al. (2007) Gender differences in acute tobacco withdrawal: effects on subjective, cognitive, and physiological measures. Exp Clin Psychopharmacol 15(1): 21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Liu XW, Lu SF, et al. (2014) Effect of nicotine withdrawal on pain sensitivity in rats to mechanical stimulation and thermal stimulation. Eur Rev Med Pharmacol Sci 18(18): 2759–2765. [PubMed] [Google Scholar]
- Lockman PR, McAfee G, Geldenhuys WJ, et al. (2005) Brain uptake kinetics of nicotine and cotinine after chronic nicotine exposure. J Pharmacol Exp Ther 314(2): 636–642. [DOI] [PubMed] [Google Scholar]
- Malin DH (2001) Nicotine dependence: studies with a laboratory model. Pharmacol Biochem Behav 70(4): 551–559. [DOI] [PubMed] [Google Scholar]
- Malin DH and Goyarzu P (2009) Rodent Models of Nicotine Withdrawal Syndrome. In: Henningfield JE, London EDand Pogun S (eds) Nicotine Psychopharmacology. Berlin, Heidelberg: Springer Berlin Heidelberg, pp.401–434. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Newlin-Maultsby P, et al. (1992) Rodent model of nicotine abstinence syndrome. Pharmacol Biochem Behav 43(3): 779–784. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Smith TD, et al. (2006) Bupropion attenuates nicotine abstinence syndrome in the rat. Psychopharmacology (Berl) 184(3): 494–503. [DOI] [PubMed] [Google Scholar]
- Malin DH, Moon WD, Goyarzu P, et al. (2013) Inhibition of monoamine oxidase isoforms modulates nicotine withdrawal syndrome in the rat. Life Sciences 93(12): 448–453. [DOI] [PubMed] [Google Scholar]
- Malin DH, Ronald Lake J, Carter VA, et al. (1994) The nicotinic antagonist mecamylamine precipitates nicotine abstinence syndrome in the rat. Psychopharmacology (Berl) 115(1): 180–184. [DOI] [PubMed] [Google Scholar]
- Manhães AC, Guthierrez MCS, Filgueiras CC, et al. (2008) Anxiety-like behavior during nicotine withdrawal predict subsequent nicotine consumption in adolescent C57BL/6 mice. Behavioural Brain Research 193(2): 216–224. [DOI] [PubMed] [Google Scholar]
- Mannucci C, Tedesco M, Bellomo M, et al. (2006) Long-term effects of nicotine on the forced swimming test in mice: an experimental model for the study of depression caused by smoke. Neurochem Int 49(5): 481–486. [DOI] [PubMed] [Google Scholar]
- Markou A (2008) Neurobiology of nicotine dependence. Philosophical Transactions of the Royal Society B: Biological Sciences 363(1507): 3159–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Darna M, Wilson AG, et al. (2017) Tobacco’s minor alkaloids: Effects on place conditioning and nucleus accumbens dopamine release in adult and adolescent rats. Eur J Pharmacol 814: 196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, et al. (2007) Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 190(3): 269–319. [DOI] [PubMed] [Google Scholar]
- McLaughlin I, Dani JA and De Biasi M (2015) Nicotine Withdrawal. Current topics in behavioral neurosciences 24: 99–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRobbie H, Lee M and Juniper Z (2005) Non-nicotine pharmacotherapies for smoking cessation. Respir Med 99(10): 1203–1212. [DOI] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI and Luetje CW (2006) Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol 70(3): 801–805. [DOI] [PubMed] [Google Scholar]
- Moerke MJ, McMahon LR and Wilkerson JL (2020) More than Smoke and Patches: The Quest for Pharmacotherapies to Treat Tobacco Use Disorder. Pharmacological reviews 72(2): 527–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanari C, Kelley LK, Kerr TM, et al. (2020) Nicotine e-cigarette vapor inhalation effects on nicotine & cotinine plasma levels and somatic withdrawal signs in adult male Wistar rats. Psychopharmacology (Berl) 237(3): 613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney ME and Sofuoglu M (2006) Bupropion for the treatment of nicotine withdrawal and craving. Expert Rev Neurother 6(7): 965–981. [DOI] [PubMed] [Google Scholar]
- Morud J, Strandberg J, Andren A, et al. (2018) Progressive modulation of accumbal neurotransmission and anxiety-like behavior following protracted nicotine withdrawal. Neuropharmacology 128: 86–95. [DOI] [PubMed] [Google Scholar]
- Myers WD, Ng KT and Singer G (1982) Intravenous self-administration of acetaldehyde in the rat as a function of schedule, food deprivation and photoperiod. Pharmacol Biochem Behav 17(4): 807–811. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Bruijnzeel AW, Smith RT, et al. (2006) Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology (Berl) 186(4): 612–619. [DOI] [PubMed] [Google Scholar]
- O’Dell LE and Khroyan TV (2009) Rodent Models of Nicotine Reward: What do they tell us about tobacco abuse in humans? Pharmacology, biochemistry, and behavior 91(4): 481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Torres OV, Natividad LA, et al. (2007) Adolescent nicotine exposure produces less affective measures of withdrawal relative to adult nicotine exposure in male rats. Neurotoxicol Teratol 29(1): 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Xu T, et al. (2001) Effects of protracted nicotine exposure and withdrawal on the expression and phosphorylation of the CREB gene transcription factor in rat brain. Journal of neurochemistry 77(3): 943–952. [DOI] [PubMed] [Google Scholar]
- Pang RD and Leventhal AM (2013) Sex differences in negative affect and lapse behavior during acute tobacco abstinence: a laboratory study. Exp Clin Psychopharmacol 21(4): 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini M and De Biasi M (2011) Mechanistic insights into nicotine withdrawal. Biochemical Pharmacology 82(8): 996–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel DR, Feucht C, Reid L, et al. (2010) Pharmacologic agents for smoking cessation: A clinical review. Clinical pharmacology : advances and applications 2: 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Semenova S and Markou A (2008) The effects of chronic versus acute desipramine on nicotine withdrawal and nicotine self-administration in the rat. Psychopharmacology (Berl) 198(3): 351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekonen K, Karlsson C, Laakso I, et al. (1993) Plasma nicotine and cotinine concentrations in mice after chronic oral nicotine administration and challenge doses. European Journal of Pharmaceutical Sciences 1(1): 13–18. [Google Scholar]
- Perez XA, McIntosh JM and Quik M (2013) Long-term nicotine treatment down-regulates α6β2* nicotinic receptor expression and function in nucleus accumbens. Journal of neurochemistry 127(6): 762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen DR, Norris KJ and Thompson JA (1984) A comparative study of the disposition of nicotine and its metabolites in three inbred strains of mice. Drug metabolism and disposition: the biological fate of chemicals 12(6): 725–731. [PubMed] [Google Scholar]
- Picciotto MR and Mineur YS (2014) Molecules and circuits involved in nicotine addiction: The many faces of smoking. Neuropharmacology 76 Pt B: 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietila K and Ahtee L (2000) Chronic nicotine administration in the drinking water affects the striatal dopamine in mice. Pharmacol Biochem Behav 66(1): 95–103. [DOI] [PubMed] [Google Scholar]
- Pietila K, Lahde T, Attila M, et al. (1998) Regulation of nicotinic receptors in the brain of mice withdrawn from chronic oral nicotine treatment. Naunyn Schmiedebergs Arch Pharmacol 357(2): 176–182. [DOI] [PubMed] [Google Scholar]
- Ponzoni L, Braida D, Carboni L, et al. (2020) Persistent cognitive and affective alterations at late withdrawal stages after long-term intermittent exposure to tobacco smoke or electronic cigarette vapour: Behavioural changes and their neurochemical correlates. Pharmacological Research 158: 104941. [DOI] [PubMed] [Google Scholar]
- Ponzoni L, Moretti M, Sala M, et al. (2015) Different physiological and behavioural effects of e-cigarette vapour and cigarette smoke in mice. European Neuropsychopharmacology 25(10): 1775–1786. [DOI] [PubMed] [Google Scholar]
- Poole RL, Connor DA and Gould TJ (2014) Donepezil reverses nicotine withdrawal-induced deficits in contextual fear conditioning in C57BL/6J mice. Behav Neurosci 128(5): 588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS and Gould TJ (2007) Bupropion Dose-Dependently Reverses Nicotine Withdrawal Deficits in Contextual Fear Conditioning. Pharmacology, biochemistry, and behavior 88(2): 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS and Gould TJ (2008) Genetic variability in nicotinic acetylcholine receptors and nicotine addiction: converging evidence from human and animal research. Behavioural Brain Research 193(1): 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Wilkinson DS, Kenney JW, et al. (2012) Strain-dependent effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning. Behav Genet 42(1): 133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JJ and Benowitz NL (2016) The Past, Present, and Future of Nicotine Addiction Therapy. Annual Review of Medicine 67(1): 467–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD and Gould TJ (2009) Nicotine Withdrawal-Induced Deficits in Trace Fear Conditioning in C57BL/6 Mice: A Role for High-Affinity β2 Subunit-Containing Nicotinic Acetylcholine Receptors. The European journal of neuroscience 29(2): 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Portugal GS, Lerman C, et al. (2008) Varenicline ameliorates nicotine withdrawal-induced learning deficits in C57BL/6 mice. Behav Neurosci 122(5): 1166–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehni AK, Singh TG and Arora S (2012) SU-6656, a selective Src kinase inhibitor, attenuates mecamylamine-precipitated nicotine withdrawal syndrome in mice. Nicotine Tob Res 14(4): 407–414. [DOI] [PubMed] [Google Scholar]
- Robinson SF, Marks MJ and Collins AC (1996) Inbred mouse strains vary in oral self-selection of nicotine. Psychopharmacology (Berl) 124(4): 332–339. [DOI] [PubMed] [Google Scholar]
- Roni MA and Rahman S (2014) The effects of lobeline on nicotine withdrawal-induced depression-like behavior in mice. Psychopharmacology (Berl) 231(15): 2989–2998. [DOI] [PubMed] [Google Scholar]
- Schochet TL, Kelley AE and Landry CF (2004) Differential behavioral effects of nicotine exposure in adolescent and adult rats. Psychopharmacology (Berl) 175(3): 265–273. [DOI] [PubMed] [Google Scholar]
- Semenova S and Markou A (2010) The alpha2 adrenergic receptor antagonist idazoxan, but not the serotonin-2A receptor antagonist M100907, partially attenuated reward deficits associated with nicotine, but not amphetamine, withdrawal in rats. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 20(10): 731–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova S, Stolerman IP and Markou A (2007) Chronic nicotine administration improves attention while nicotine withdrawal induces performance deficits in the 5-choice serial reaction time task in rats. Pharmacology, biochemistry, and behavior 87(3): 360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A and Brody AL (2009) In vivo brain imaging of human exposure to nicotine and tobacco. Handb Exp Pharmacol. Epub ahead of print 2009/02/03. DOI: 10.1007/978-3-540-69248-5_6.(192): 145–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Johnston JA, Khayrallah M, et al. (2000) The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology (Berl) 148(1): 33–40. [DOI] [PubMed] [Google Scholar]
- Shoaib M and Bizarro L (2005) Deficits in a sustained attention task following nicotine withdrawal in rats. Psychopharmacology (Berl) 178(2–3): 211–222. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Siu EC, Li Z, et al. (2008) Interactions between age and the aversive effects of nicotine withdrawal under mecamylamine-precipitated and spontaneous conditions in male Wistar rats. Psychopharmacology (Berl) 198(2): 181–190. [DOI] [PubMed] [Google Scholar]
- Slemmer JE, Martin BR and Damaj MI (2000) Bupropion is a nicotinic antagonist. J Pharmacol Exp Ther 295(1): 321–327. [PubMed] [Google Scholar]
- Small E, Shah HP, Davenport JJ, et al. (2010) Tobacco smoke exposure induces nicotine dependence in rats. Psychopharmacology (Berl) 208(1): 143–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BR, Amit Z and Splawinsky J (1984) Conditioned place preference induced by intraventricular infusions of acetaldehyde. Alcohol 1(3): 193–195. [DOI] [PubMed] [Google Scholar]
- Sparks JA and Pauly JR (1999) Effects of continuous oral nicotine administration on brain nicotinic receptors and responsiveness to nicotine in C57Bl/6 mice. Psychopharmacology (Berl) 141(2): 145–153. [DOI] [PubMed] [Google Scholar]
- Staley JK, Krishnan-Sarin S, Cosgrove KP, et al. (2006) Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. The Journal of neuroscience : the official journal of the Society for Neuroscience 26(34): 8707–8714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker AK, Semenova S and Markou A (2008) Affective and somatic aspects of spontaneous and precipitated nicotine withdrawal in C57BL/6J and BALB/cByJ mice. Neuropharmacology 54(8): 1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Ise Y, Maeda J, et al. (1999) Mecamylamine-precipitated nicotine-withdrawal aversion in Lewis and Fischer 344 inbred rat strains. European Journal of Pharmacology 369(2): 159–162. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Ise Y, Tsuda M, et al. (1996) Mecamylamine-precipitated nicotine-withdrawal aversion in rats. Eur J Pharmacol 314(3): 281–284. [DOI] [PubMed] [Google Scholar]
- Tan S, Xue S, Behnood-Rod A, et al. (2019) Sex differences in the reward deficit and somatic signs associated with precipitated nicotine withdrawal in rats. Neuropharmacology 160: 107756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Gentil LG, Natividad LA, et al. (2013) Behavioral, Biochemical, and Molecular Indices of Stress are Enhanced in Female Versus Male Rats Experiencing Nicotine Withdrawal. Front Psychiatry 4: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Pipkin JA, Ferree P, et al. (2015) Nicotine withdrawal increases stress-associated genes in the nucleus accumbens of female rats in a hormone-dependent manner. Nicotine Tob Res 17(4): 422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulusu U, Uzbay IT, Kayir H, et al. (2005) Evidence for the role of nitric oxide in nicotine-induced locomotor sensitization in mice. Psychopharmacology (Berl) 178(4): 500–504. [DOI] [PubMed] [Google Scholar]
- van der Staay FJ, Arndt SS and Nordquist RE (2009) Evaluation of animal models of neurobehavioral disorders. Behav Brain Funct 5: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani AP, Pedron VT, Machado LM, et al. (2015) Lack of GABAB receptors modifies behavioural and biochemical alterations induced by precipitated nicotine withdrawal. Neuropharmacology 90: 90–101. [DOI] [PubMed] [Google Scholar]
- Voikar V, Polus A, Vasar E, et al. (2005) Long-term individual housing in C57BL/6J and DBA/2 mice: assessment of behavioral consequences. Genes Brain Behav 4(4): 240–252. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Stinus L, Koob GF, et al. (2000) Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. J Pharmacol Exp Ther 292(3): 1053–1064. [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Thomas BF, et al. (2015) Determination of behaviorally effective tobacco constituent doses in rats. Nicotine Tob Res 17(3): 368–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilking JA, Hesterberg KG, Nguyen VH, et al. (2012) Comparison of nicotine oral consumption and baseline anxiety measures in adolescent and adult C57BL/6J and C3H/Ibg mice. Behav Brain Res 233(2): 280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DS and Gould TJ (2011) The effects of galantamine on nicotine withdrawal-induced deficits in contextual fear conditioning in C57BL/6 mice. Behavioural Brain Research 223(1): 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P and Mitchell PJ (2002) The validity of animal models of predisposition to depression. Behav Pharmacol 13(3): 169–188. [DOI] [PubMed] [Google Scholar]
- Wing VC and Shoaib M (2007) Examining the clinical efficacy of bupropion and nortriptyline as smoking cessation agents in a rodent model of nicotine withdrawal. Psychopharmacology (Berl) 195(3): 303–313. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Spiller K and Gardner EL (2009) Mechanism-based medication development for the treatment of nicotine dependence. Acta Pharmacol Sin 30(6): 723–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue S, Behnood-Rod A, Wilson R, et al. (2018) Rewarding effects of nicotine in adolescent and adult male and female rats as measured using intracranial self-stimulation. Nicotine Tob Res. Epub ahead of print 2018/11/20. DOI: 10.1093/ntr/nty249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaniewska M, McCreary AC, Wydra K, et al. (2010) Effects of serotonin (5-HT)2 receptor ligands on depression-like behavior during nicotine withdrawal. Neuropharmacology 58(7): 1140–1146. [DOI] [PubMed] [Google Scholar]
- Zhao-Shea R, DeGroot SR, Liu L, et al. (2015) Increased CRF signalling in a ventral tegmental area-interpeduncular nucleus-medial habenula circuit induces anxiety during nicotine withdrawal. Nat Commun 6: 6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao-Shea R, Liu L, Pang X, et al. (2013) Activation of GABAergic neurons in the interpeduncular nucleus triggers physical nicotine withdrawal symptoms. Curr Biol 23(23): 2327–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


