Abstract
Background
Prenatal alcohol exposure (PAE) is associated with smaller regional and global brain volumes. In rats, gestational choline supplementation mitigates adverse developmental effects of ethanol exposure. Our recent randomized, double-blind, placebo-controlled maternal choline supplementation trial showed improved somatic and functional outcomes in infants at 6.5 and 12 months postpartum. Here we examined whether maternal choline supplementation protected the newborn brain from PAE-related volume reductions and, if so, whether these volume changes were associated with improved infant recognition memory.
Methods
52 infants born to heavy-drinking women, who had participated in our choline supplementation trial during pregnancy, received structural magnetic resonance imaging with a multiecho FLASH protocol on a 3T Siemens Allegra MRI (median age=2.8 weeks postpartum). Subcortical regions were manually segmented. Recognition memory was assessed at 12 months on the Fagan Test of Infant Intelligence (FTII). We examined the effects of choline on regional brain volumes, whether choline-related volume increases were associated with higher FTII scores, and the degree to which the regional volume increases mediated the effects of choline on the FTII.
Results
Usable MRI data were acquired in 50 infants (choline: n=27; placebo: n=23). Normalised volumes were larger in 6 of 12 regions in the choline than placebo arm (t≥2.05, p≤0.05) and were correlated with degree of maternal choline adherence (β≥0.28, p≤0.04). Larger right putamen and corpus callosum were related to higher FTII scores (r=0.36, p=0.02) and showed a trend towards partial mediation of the choline effect on recognition memory.
Conclusions
High-dose choline supplementation during pregnancy mitigated PAE-related regional volume reductions, with larger volumes associated with improved 12-month recognition memory. These results provide the first evidence that choline may be neuroprotective against PAE-related brain structural deficits in humans.
Keywords: Prenatal alcohol exposure, maternal choline supplementation, neonates, magnetic resonance imaging, brain volumes, Fagan Test of Infant Intelligence
INTRODUCTION
Fetal alcohol spectrum disorders (FASD) are the most common preventable form of neurocognitive disabilities and have been estimated at a global prevalence of 0.5–1.2% of children and youth (Lange et al., 2017). Prenatal alcohol exposure (PAE) has severe, long-lasting effects on the developing fetus, including craniofacial abnormalities (Hoyme et al., 2016; Suttie et al., 2018), cognitive disabilities (Green et al., 2009; Lewis et al., 2015; Mattson et al., 2019), and psychosocial problems (Burd et al., 2003; Panczakiewicz et al., 2016). More recently, quantitative neuroimaging has shown that PAE is associated with a number of neurostructural abnormalities, which include cortical changes (Gross et al., 2018; Hendrickson et al., 2017; Infante et al., 2015; Lebel et al., 2012; Sowell et al., 2008) as well as white matter microstructural alterations (Fan et al., 2016; Taylor et al., 2015). Perhaps most consistent are findings of PAE-associated reductions in total and regional brain volumes (Archibald et al., 2001; Biffen et al., 2017; Coles et al., 2011; Jacobson et al., 2017; Meintjes et al., 2014; Nardelli et al., 2011; Roussotte et al., 2012).
Although a range of psychosocial interventions have attempted to reduce the incidence of maternal drinking during pregnancy, it continues to be highly prevalent (Lange et al., 2017). There is, thus, an urgent need for effective pharmacological or nutritional interventions to ameliorate the effects of PAE. Choline is an essential nutrient, derived from dietary sources, including eggs, milk, and liver, and from endogenous synthesis (Resseguie et al., 2007). It is a constituent of the neurotransmitter acetylcholine and, as a precursor to the cell membrane components phosphatidylcholine and sphingomyelin, plays an important role in membrane integrity and signalling (Zeisel and Niculescu, 2006). It additionally functions as a methyl group donor in homocysteine metabolism and DNA methylation, a mechanism in epigenetic processes that may be involved in the teratogenic effects of alcohol (Zeisel, 2011a).
Laboratory studies have shown that, when administered to alcohol-exposed rats during the equivalent of the third trimester of human pregnancy, choline treatment significantly improved behavioural outcomes including memory and learning (Ryan et al., 2008; Thomas et al., 2000; Waddell and Mooney, 2017), eyeblink conditioning (Thomas and Tran, 2012), and ethanol-associated hyperactivity (Thomas et al., 2004). Administration earlier in pregnancy mitigated effects of alcohol on brain and body weight, somatic development, and reflexes and motor coordination in exposed pups (Thomas et al., 2009), as well as prenatal alcohol-related effects on spatial working memory (Thomas et al., 2010).
A small number of cohort studies have investigated whether choline supplementation is effective in mitigating the effects of PAE. Whereas supplementation in preschool-age children with PAE was associated with neurodevelopmental benefits (Wozniak et al., 2020), a randomised double-blind choline supplementation study in school-age children with FASD showed no effect of choline treatment on any of the cognitive domains tested (Nguyen et al., 2016). Prenatal supplementation in a Ukraine cohort showed no effect on infant cognition as assessed on the more global Bayley Scales of Infant Development (Coles et al., 2015) but demonstrated improvement on narrow-band information processing tasks (Kable et al., 2015) at 6 months.
We conducted a randomised, double-blind placebo-controlled trial to examine the feasibility and efficacy of high-dose choline supplementation initiated in mid-pregnancy to mitigate adverse effects of PAE on birth outcomes (Jacobson et al., 2018a, 2018b). The trial was conducted from 2012 to 2016 in a high-risk socioeconomically disadvantaged community in Cape Town, South Africa (Jacobson et al., 2018a) where the incidence of FASD, estimated at 13.6–20.9%, is among the highest in the world (May et al., 2013). Maternal alcohol consumption was determined using a “gold standard” timeline follow-back interview (Jacobson et al., 2002). Heavy drinking during pregnancy was defined as an average consumption of at least two standard drinks (1.0 oz absolute alcohol [AA])/day or two or more binge-drinking episodes (4 or more drinks/occasion). A cohort of 69 heavy-drinking women initiating antenatal care before 23 weeks gestation were randomised to receive either 2g of choline or placebo daily (1 packet in the morning, 1 in the evening) from enrolment until delivery. Treatment adherence was assessed as the percentage of doses taken. The primary trial outcome was performance at 6.5 months on eyeblink conditioning, a learning paradigm affected by PAE in animals and humans (Jacobson et al., 2011). Secondary outcomes were growth at birth, 6.5 and 12 months, and recognition memory and processing speed on the Fagan Test of Infant Intelligence (FTII) at 6.5 and 12 months. Narrow-band infant tests, such as the FTII, enable the detection of alcohol-related deficits in attention or reaction time that generally could only be detected in school-age children. Performance on the FTII has been shown to predict later cognitive function, including IQ (Kavšek, 2004), and to have discriminant validity for alcohol compared to other prenatal exposures (Jacobson et al., 1996, 1993, 1992, 1985). Although the study was not powered a priori to detect treatment effects on infant outcomes due to the pilot nature of the aims, significant effects were, in fact, seen on both growth and neurobehavior (Jacobson et al., 2018b). Infants born to mothers in both the choline and placebo treatment groups were small at birth, but those in the choline-treated arm displayed considerable catch-up in weight and head circumference by age 6.5 months which persisted through the first postnatal year (Jacobson et al., 2018b). At 6.5 months choline-treated infants were more likely to meet criterion for eyeblink conditioning (Jacobson et al., 2018b), and at 12 months they demonstrated improved recognition memory on the FTII compared to placebo-treated infants.
This paper reports findings from a neonatal neuroimaging study conducted on the infants who participated in the original choline trial. It was designed to examine whether maternal choline supplementation mitigated effects of PAE on regional brain volumes. We hypothesised that brain regions reported to be affected by PAE in previous studies would be larger in infants whose mothers were in the choline-treated arm compared to the placebo-treated controls. We also investigated whether the effects of the choline treatment on these regional brain volumes partially mediated the choline-related improvement in recognition memory.
METHODS
Study Sample
This was a prospective, observational study of infants who had participated in our choline supplementation trial (Jacobson et al., 2018a, 2018b). Of 67 women who completed treatment through delivery, 62 delivered infants were enrolled and followed through trial completion (Figure 1). Mothers of 52 infants (28 choline treated; 24 placebo) agreed to participate in this neuroimaging sub-study. All investigators and research staff were blind to treatment arm. The supplementation trial was only unblinded after all infants had completed their 12-month assessment.
Figure 1.
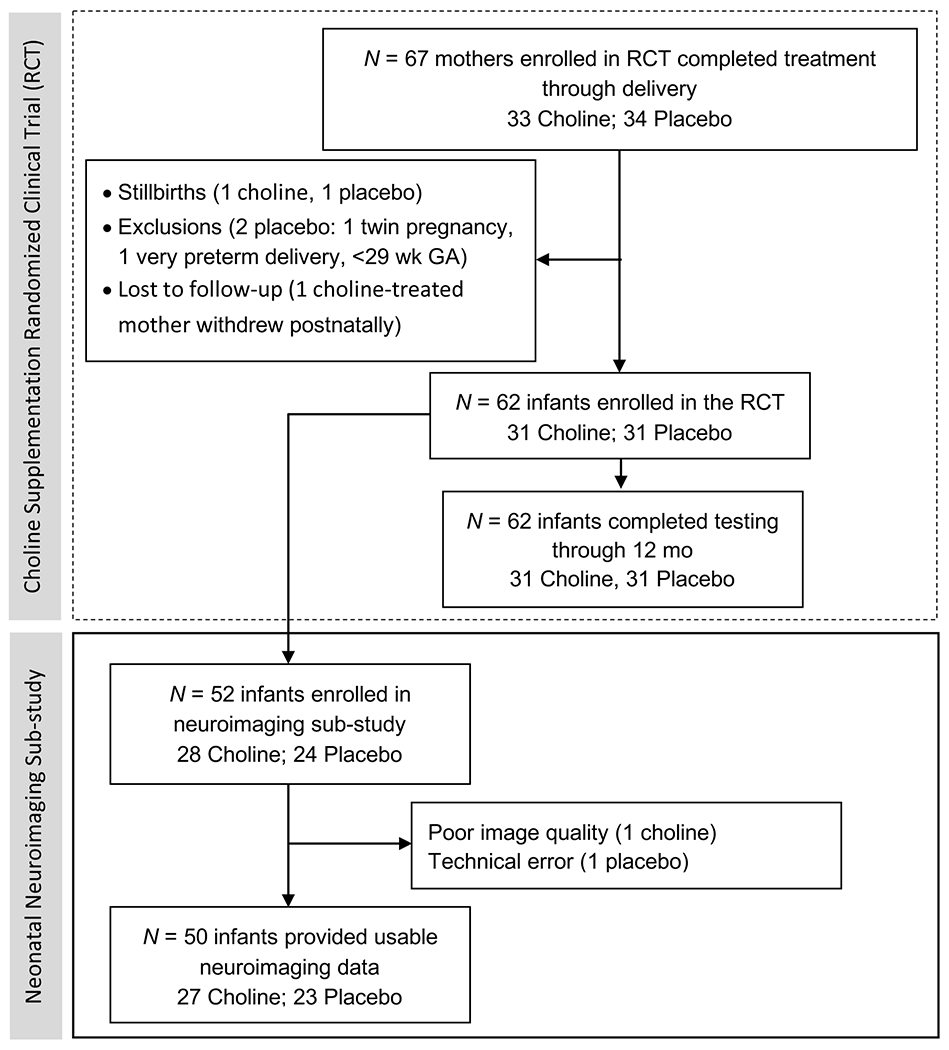
Flow diagram showing participant numbers at different stages of the choline supplementation trial and number of infants recruited into the neuroimaging sub-study.
The outcomes examined in this paper were not part of the aims of the original choline study (R21AA020332). Given the pilot nature of the parent trial, the trial was registered with the Pan African Clinical Trial Registry (PACTR202005864845358) after completion of data collection, when exploratory analyses revealed evidence of efficacy. Results regarding efficacy in the originally planned outcomes must be interpreted accordingly.
Approval for the study was obtained from the human research ethics committees at Wayne State University, the Faculty of Health Sciences at the University of Cape Town, and Columbia University Medical Center, and from the South African Medicines Control Council. Written informed consent was obtained from each mother and from the father, if available.
MRI data acquisition
Newborns were scanned asleep without sedation (Laswad et al., 2009) between 1–7 weeks postpartum (median 2.8 weeks) at the Cape Universities Brain Imaging Centre (CUBIC; see Jacobson et al., 2017; Warton et al., 2018 for details). Scanning was performed on a 3T Allegra MRI (Siemens, Erlangen, Germany) using a circularly-polarised birdcage coil designed for use in MR imaging of neonates, which was custom-built for the study at the HST/MGH Athinoula A. Martinos Center for Biomedical Imaging (HST/MGH Martinos Center). A multi-echo FLASH (MEF) sequence (van der Kouwe et al., 2008) was acquired twice with protocol parameters: 144x144 matrix, 128 sagittal slices, TR 20 ms, TE’s 1.46/3.14/4.82/6.5/8.18/9.86/11.54/13.22 ms, 1x1x1 mm3 resolution, and flip angles 5° and 20°, respectively. Of the 52 infants scanned, 50 (27 choline, 23 placebo) provided usable data (Figure 1).
Image Pre-Processing
Individual echoes from the two MEF acquisitions were split using mri_ms_fitparms in FreeSurfer (Fischl et al., 2004) and T1 and proton density estimated. A composite image volume was synthesised using mri_synthesize. A flip angle of 24° and zero echo time were selected as they provided optimal contrast for manual segmentation.
Segmentation
Manual segmentation was performed by FLW using Freeview software (FreeSurfer image analysis suite) on a Lenovo ThinkPad x220 tablet. Tracing protocols were conducted in accordance with the Infant Brain Segmentation Manual developed by the HST/MGH Martinos Center (de Macedo Rodrigues et al., 2015). All acquired structural scans were visually assessed by FLW for excessive motion and other artifacts which might render them unsuitable for manual tracing. The following regions were traced bilaterally: caudate nucleus, putamen, pallidum, nucleus accumbens, thalamus, hippocampus, amygdala and cerebellar hemispheres, and the cerebellar vermis in the midline. Segmentations were performed chiefly in the coronal view, with guidance from the sagittal and axial planes where the structure was indistinct in the coronal view. Regional volumes were then calculated using FreeSurfer. The corpus callosum was traced in two contiguous midline slices on AC-PC aligned images, from which the area was averaged to obtain a single representative value (see Jacobson et al., 2017). Median interrater reliabilities (Dice coefficients calculated for nine brains), conducted by FLW and NML, were ≥0.80 for the caudate nucleus, putamen, thalamus, hippocampus, cerebellar hemispheres, vermis and corpus callosum (Jacobson et al., 2017; Warton et al., 2018). The remaining ROIs had Dice coefficients below 0.70, and were, therefore, discarded from further analyses. Total intracranial volume (TIV) was obtained using an in-house script that extracted the brain from the T1-weighted image.
Fagan Test of Infant Intelligence (FTII)
As part of the supplementation trial (Jacobson et al., 2018b), visual recognition memory was assessed at 12 months on the FTII (Fagan and Singer, 1983). The infant was shown a set of 10 problems each consisting of two identical photographs, followed by a novel one paired with the familiar one. The normative response, preference for the novel stimulus, indicates ability to encode and recall the familiar stimulus and to discriminate it from the novel one. Novelty preference was determined by dividing duration of time spent looking at the novel image by total time looking at the novel and familiar images, for the 10 sets of problems. Higher recognition memory scores predict better intellectual function in childhood (Fagan and McGrath, 1981). Of the 50 infants who provided usable MRI data, FTII data were available for 41.
Statistical Analysis
Statistical analysis was performed using SPSS (v25; IBM, Armonk, NY). Analyses only included participants for whom all the data were available. The smoking variable (cigarettes/day) contained one outlier (>3 SD above the mean), which was recoded to one unit greater than the next highest observed value (Winer, 1971). Two alcohol use variables (AA/day at conception and across pregnancy) and marijuana use were skewed and, therefore, log-transformed. T tests or chi-square tests for categorical variables were used to compare sample characteristics between treatment groups.
Choline treatment effects on regional size, normalised for TIV (regional volume/TIV), were evaluated using t tests and Pearson correlations. Since low choline levels resulting from poor adherence would likely reduce our ability to detect choline effects on brain volumes, we conducted per protocol analyses in which three infants born to mothers in the choline arm with treatment adherence <20% were excluded from group comparisons, as was the single child in the placebo group whose mother was poorly adherent. The sample size for group comparisons of regional volumes was, therefore, N = 46. Dose dependent effects were examined using intent-to-treat analyses (i.e., including all the infants (N = 50)). For infants in the choline group we used maternal treatment adherence as a proxy for choline dosage, while choline dosage was coded as zero for all placebo group subjects (Jacobson et al., 2018b).
We evaluated potential confounding of observed group differences and associations by infant sex, birthweight, GA at scan, and maternal marijuana use and smoking. Any variable even weakly associated at p≤0.10 with an outcome variable was controlled for in subsequent analyses of that outcome using either ANCOVA or multiple regression.
The effect of choline treatment on FTII scores was evaluated using a t-test in a per protocol analysis excluding the four infants for whom maternal adherence was <20% (N = 37). The same procedure was used to control for potential confounding. Since low adherence to treatment regimen could be an indication of poorer maternal functioning, we additionally examined whether low adherence influenced the effect of choline on FTII by controlling for treatment adherence. As this analysis controlled for poor maternal adherence, the four infants excluded from the group comparison were now included (N = 41; intent-to-treat).
Associations between FTII scores and size in regions that showed an effect of choline treatment were similarly tested using Pearson correlations and multiple regression.
Mediation of the effects of choline treatment on FTII score by regional volume was examined in infants with treatment adherence >20% using multiple regression for each region showing an association with FTII score. Treatment group and FTII score were entered in Step 1, and regional volume in Step 2. The Clogg test (Clogg, 1995) was used to determine if the association of treatment group and FTII score was significantly reduced with the addition of volume, in which case mediation by that region’s volume was inferred.
RESULTS
Sample characteristics
Of the 52 infants scanned, 50 infants (27 choline treated; 23 placebo) provided MR images of adequate quality for manual tracing. Data from 1 choline- and 1 placebo-treated infant were excluded due to poor image quality and a technical error, respectively.
Demographic characteristics and substance use of the mothers and infants are summarised in Table 1. The sample came from an economically disadvantaged, poorly educated community. There was a higher percentage of male infants in the placebo group. Notably, TIV did not differ between males and females in the whole sample (mean [95% CI]: males 504,462 [477,523-531,402]; females 505,781 [485,429-526,133]; t = 0.077; p = 0.94), nor in separate treatment groups (p’s > 0.12). There was no between-group difference in alcohol consumption. Although more mothers in the choline group reported smoking (96% vs 70%), number of cigarettes smoked/day was the same. Marijuana use was more frequent and more prevalent (41% vs 9%) in the choline-treated than placebo-treated group.
Table 1.
Sample characteristics (N = 50)
| Choline arm (n = 27) |
Placebo arm (n = 23) |
|||||
|---|---|---|---|---|---|---|
| Mean | 95%CI | Mean | 95%CI | t or χ | p | |
| Mother | ||||||
| Parity | 1.7 | 1.2–2.3 | 1.5 | 1.2–19 | −0.602 | 0.55 |
| Maternal age (years) | 26.6 | 24.7–28.5 | 27.9 | 25.8–29.9 | 0.865 | 0.39 |
| Education (highest grade) | 8.9 | 8.2–9.6 | 9.5 | 8.8–10.2 | 1.122 | 0.27 |
| Socioeconomic status a | 20.5 | 17.4–23.6 | 19.5 | 17.8–21.2 | −0.520 | 0.61 |
| Number married (%) | 8 (30) | 10 (44) | 1.034 | 0.31 | ||
| Infant | ||||||
| Number male (%) | 8 (30) | 16 (70) | 7.936 | 0.005 | ||
| Gestational age at birth (weeks) | 38.7 | 38.1–39.3 | 39.3 | 38.7–39.8 | 1.297 | 0.20 |
| Age at scan (days) | 22.1 | 18.8–25.4 | 20.1 | 16.2–24.1 | −0.744 | 0.46 |
| Gestational age at scan (weeks) | 41.9 | 41.2–42.5 | 42.2 | 41.3–43.0 | 0.546 | 0.59 |
| Total intracranial volume (mm3) | 497,167 | 476,575–517,758 | 514,517 | 487,922–541,113 | 1.026 | 0.31 |
| Birthweight (g) | 2,833 | 2,669–2,998 | 2,896 | 2,659–3,132 | 0.435 | 0.67 |
| Exposure | ||||||
| oz AA/day at conception b,c | 1.5 | 1.0–2.2 | 1.5 | 1.1–2.1 | −0.783 | 0.44 |
| oz AA/drinking day at conception b,c | 4.6 | 2.8–6.5 | 4.2 | 3.5–7.7 | 0.427 | 0.67 |
| Drinking frequency at conception c (days/week) | 2.7 | 2.3–3.0 | 2.3 | 0.7–3.9 | −1.471 | 0.15 |
| oz AA/day across pregnancy | 0.9 | 0.5–1.2 | 0.7 | 0.5–1.2 | −0.670 | 0.51 |
| oz AA/drinking day across pregnancy | 4.1 | 3.3–4.9 | 4.3 | 3.3–5.2 | 0.278 | 0.78 |
| Drinking frequency across pregnancy (days/week) | 1.3 | 1.0–1.7 | 1.1 | 0.9–1.4 | −0.849 | 0.40 |
| Smoking (cigarettes/day) d | 6.3 | 4.8–7.9 | 6.3 | 4.6–8.0 | −0.012 | 0.99 |
| Marijuana (days/month) b,e | 8.9 | 0.5–16.0 | 5.9 | 5.0–6.8 (range) | −2.018 | 0.05 |
Median and Interquartile range;
n = 26 choline mothers who drank alcohol around conception;
n = 26 choline and n = 16 placebo mothers who smoked cigarettes during pregnancy;
n = 11 choline and n = 2 placebo mothers who used marijuana during pregnancy
Of the potential confounding variables considered, GA at scan showed associations with the volumes of the left caudate (r = −0.24, p = 0.10) and right putamen (r = −0.35, p = 0.02), and with corpus callosal area (r = −0.46, p = 0.001); infant sex was associated only with caudate volumes (left caudate r = 0.32, p = 0.03; right caudate r = 0.29, p = 0.05; all other r’s < 0.12, p’s > 0.44), and maternal marijuana use was weakly associated with right caudate volume (r = 0.26, p = 0.08). Birthweight was associated with FTII score (r = −0.31, p = 0.05).
Effect of choline supplementation on regional volumes
After excluding the four infants (3 choline, 1 placebo) with maternal adherence <20%, TIV-normalized sizes were larger in the choline-treated than placebo group for 6 of 12 regions (Figure 2 and Table 2). Group differences in all 6 regions remained significant after control for potential confounders. Size of the same six regions showed associations with choline dose, all of which remained significant after control for confounders (Table 2). Since our correlation analyses examined the dose effect of choline on regional volumes, the four infants excluded from the group comparisons were included in the analyses (N = 50). Due to the differences in sex distribution between groups, all analyses were re-run controlling for sex. Results remained essentially unchanged, with group differences and associations evident in the same regions and no new differences emerging. The scatterplots in Figure 2 show group distributions differentiated by infant sex.
Figure 2.
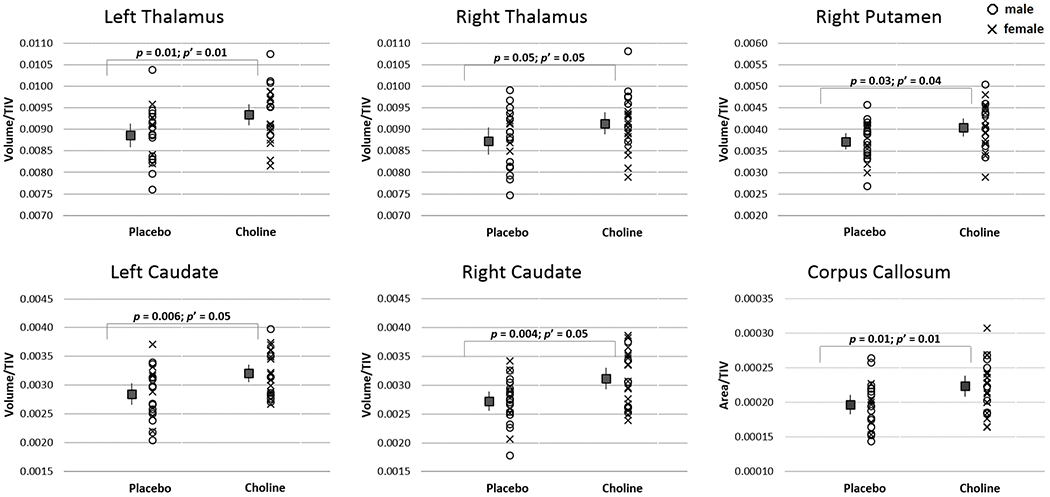
Mean ± 95% confidence intervals of volumes (or areas), normalised to TIV, for regions showing a difference (p ≤ 0.05) between choline- and placebo-treated infants (choline: n = 24; placebo: n = 22). p: from t test; p’: from ANCOVA after adjustment for confounders. Scatterplots show the distribution of sex in the treatment groups.
Table 2.
Choline treatment effects on regional sizes
| Choline (n = 24)a | Placebo (n = 22)a | Comparison by group of TIV-normalizedb sizes (N = 46)a | Associations of TIV-normalizedb sizes with choline dosec (N = 50) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Region | Mean | 95%CI | Mean | 95%CI | ||||||||
|
| ||||||||||||
| Regional Volume (mm3) | t | p | F | p | r | p | β | p | ||||
| Left cerebellum | 11059 | 10451–11667 | 11107 | 10325–11890 | −0.90 | 0.37 | - | - | 0.16 | 0.27 | - | - |
| Right cerebellum | 10927 | 10356–11497 | 11145 | 10409–11881 | −0.32 | 0.75 | - | - | 0.11 | 0.46 | - | - |
| Left thalamus | 4650 | 4406–4894 | 4500 | 4311–4691 | −2.63 | 0.01 | 6.94 | 0.01 | 0.38 | 0.007 | 0.38 | 0.007 |
| Right thalamus | 4541 | 4318–4764 | 4439 | 4199–4679 | −2.05 | 0.05 | 4.22 | 0.05 | 0.33 | 0.02 | 0.33 | 0.02 |
| Left caudate d,e | 1584 | 1501–1667 | 1444 | 1338–1549 | −2.90 | 0.006 | 4.15 | 0.05 | 0.39 | 0.006 | 0.32 | 0.03 |
| Right caudate e,f | 1551 | 1437–1664 | 1385 | 1295–1476 | −3.03 | 0.004 | 4.06 | 0.05 | 0.39 | 0.005 | 0.34 | 0.02 |
| Left putamen | 1957 | 1825–2090 | 1901 | 1804–1999 | −1.38 | 0.17 | - | - | 0.20 | 0.16 | - | - |
| Right putamen d | 2006 | 1887–2125 | 1890 | 1790–1990 | −2.19 | 0.03 | 4.47 | 0.04 | 0.30 | 0.03 | 0.28 | 0.04 |
| Left hippocampus | 1343 | 1261–1425 | 1319 | 1236–1401 | −1.05 | 0.30 | - | - | 0.14 | 0.33 | - | - |
| Right hippocampus | 1309 | 1230–1387 | 1280 | 1203–1357 | −1.18 | 0.24 | - | - | 0.16 | 0.26 | - | - |
| Vermis | 2373 | 2180–2566 | 2397 | 2170–2624 | −0.36 | 0.73 | - | - | 0.08 | 0.56 | - | - |
|
|
||||||||||||
| Regional Area (mm2) | ||||||||||||
|
| ||||||||||||
| Corpus callosum d | 110 | 103–117 | 99 | 94–104 | −2.56 | 0.01 | 6.64 | 0.01 | 0.35 | 0.01 | 0.32 | 0.01 |
BOLD denotes significance at p ≤ 0.05.
Group means and group comparisons exclude infants (3 choline treated, 1 placebo) whose mothers had treatment adherence < 20%
Regional sizes were normalized relative to total intracranial volume (TIV) (i.e., regional size/TIV)
In choline-treated infants, treatment adherence was used as a proxy for choline dose; in the placebo group, choline dose = 0
t unpaired student’s t test; F ANCOVA controlling for potential confounders
r Pearson correlation coefficient; β Standardised regression coefficient after adjustment for potential confounders
Confounders: GA at scan;
Infant sex;
Marijuana
Effect of choline supplementation on FTII
Usable data for the FTII were available for 41 of the infants who were scanned (21 choline treated, 20 placebo). Of these, the four infants whose mothers had poor adherence were excluded from the group comparison (N = 37). Although the larger sample of the choline supplementation trial demonstrated significantly better visual recognition memory on the FTII in the choline than placebo arm, as indicated by preferential looking at the novel stimulus (Jacobson et al., 2018b), the effect of choline treatment in the present smaller sub-sample fell short of significance (mean [95% CI]: choline 63.2 [60.8–65.7]; placebo 60.2 [57.4–62.9]; t(1,35) = −1.64, p = 0.11). When including all 41 infants, but controlling for maternal treatment adherence, the effect of choline treatment on FTII was significant F(1,38) = 5.23, p = 0.03. This result remained significant (F(1, 37) = 4.87, p = 0.03) after control for birthweight.
Relation of regional volumes to FTII score
Of the regions in which choline treatment effects were observed, larger right putamen and corpus callosum were associated (at p ≤ 0.05) with higher FTII scores (Table 3). The weaker association in left thalamus became significant after adjustment for potential confounding by birthweight, which acted as a suppressor.
Table 3.
Relation of TIV-normalized size with FTII scores in the 6 regions where choline treatment effects were observed (N = 41)
| Region | r | p | β | p |
|---|---|---|---|---|
| Left thalamus | 0.28 | 0.08 | 0.30 | 0.05 |
| Right thalamus | 0.13 | 0.44 | - | - |
| Left caudate | 0.04 | 0.81 | - | - |
| Right caudate | 0.14 | 0.37 | - | - |
| Right putamen | 0.36 | 0.02 | 0.44 | 0.003 |
| Corpus callosum | 0.36 | 0.02 | 0.32 | 0.04 |
Regional sizes normalized for total intracranial volume (TIV); BOLD denotes significance at p ≤ 0.05
r Pearson correlation coefficient; β Standardised regression coefficient after adjustment for birthweight
Mediation of the effect of choline treatment on FTII score by regional volumes
In our mediation analysis, we found that the effect of choline treatment on the FTII was substantially reduced when the right putamen volume (Clogg p = 0.08) or corpus callosum area (Clogg p = 0.06) was added to the regression (Figure 3). Note that α represents the relation between treatment group and the mediating variable (i.e. region size), and τ the relation between treatment group and FTII score. The Clogg test evaluates whether the association of FTII score with treatment group (i.e., τ) is significantly reduced when the mediating variable is added to the model. In that model, β is the standardised regression coefficient for the mediating variable. No mediating effect of the left thalamus volume was observed.
Figure 3.
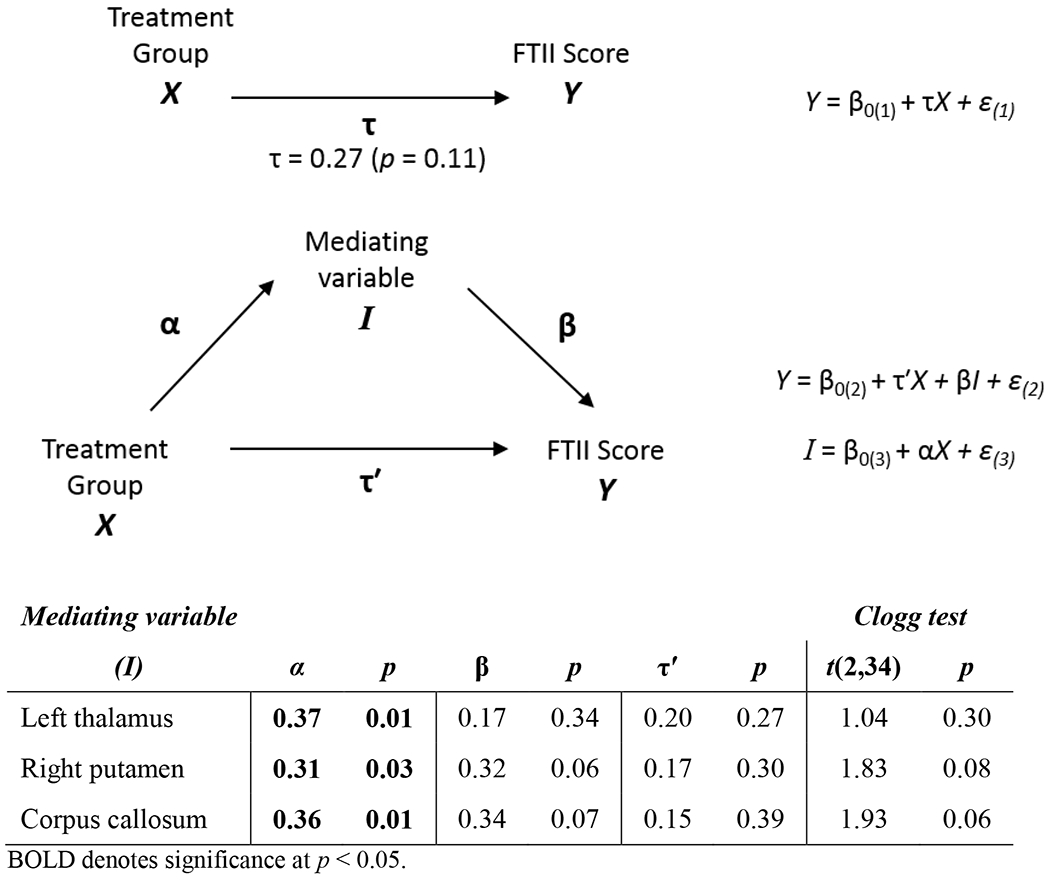
Mediation by region size of the effect of choline treatment on Fagan Test of Infant Intelligence (FTII) score (18 choline treated; 19 placebo). Infants were included if maternal treatment adherence ≥ 20%.
DISCUSSION
This is the first study to show efficacy of prenatal choline supplementation in mitigating effects of PAE on brain structure in humans. High-dose choline supplementation initiated in mid-pregnancy was associated with improved structural outcomes in infants with heavy PAE. Infants in the choline-treated arm showed larger volumes in 6 of 12 regions, namely bilateral thalamus and caudate, right putamen and corpus callosum. Intervening variable analysis showed that the more optimal recognition memory observed in the original larger cohort (Jacobson et al., 2018b) was partially mediated by the larger right putamen and corpus callosum.
Findings from previous choline supplementation studies, which administered lower doses of choline, have shown inconsistent effects in ameliorating the impact of PAE. In a U.S. study choline supplementation in 5- to 10-year-olds with FASD produced no observable effect on the cognitive domains tested (Nguyen et al., 2016). By contrast, in a second study involving a four-year follow-up of U.S. children with PAE who had been given choline or placebo at 2.5–4 years of age, choline supplementation was associated with neurodevelopmental benefits (Wozniak et al., 2020). A third study in which pregnant women in Ukraine were administered a choline supplement with a multivitamin showed no effect of supplementation on infant cognition assessed on a global test of development, the Bayley Scales of Infant Development, at 6 months of age (Coles et al., 2015). However, another study of a subset of the same Ukraine cohort used a more sensitive, narrow-band test and found that infants in both choline-supplemented arms (with and without PAE) exhibited improved cardiac orienting responses to visual stimuli (Kable et al., 2015). These findings suggest an improvement in basic attentional regulation systems following maternal choline treatment and are in agreement with the findings of the current study.
Our research has shown improved infant outcomes following maternal choline supplementation during pregnancy in several modalities which are affected in PAE, including FTII recognition memory (Jacobson et al., 2018b). The FTII, which examines novelty preference and processing speed in infants, has marked predictive validity for later performance on intelligence tests (Fagan and Singer, 1983). The current study extends our previous findings by demonstrating that high-dose prenatal choline supplementation may protect against regional brain volume reductions, including the thalamus, dorsal striatum and corpus callosum, which have been consistently found in individuals with PAE (Biffen et al., 2017; Jacobson et al., 2017; Nardelli et al., 2011; Roussotte et al., 2012).
In typically-developing children aged 6-13 years of age, larger volumes of the caudate and cerebellum were observed to be associated with higher IQ, and larger putamen volume was associated with improved working memory (Pangelinan et al., 2011). Larger corpus callosum area has also been shown to be associated with higher IQ in older children and to partially mediate effects of PAE on IQ (Biffen et al., 2017). Our findings that larger putamen and corpus callosum were associated with improved performance on the FTII are consistent with those results. In addition, the mediating role of the corpus callosum in the effects of PAE on IQ lends support to the hypothesis that the effects of choline on FTII are mediated by certain ROI sizes.
In the larger infant clinical trial, infants in the choline arm were more likely to meet criterion for EBC than placebo-treated infants (Jacobson et al., 2018b). EBC performance is supported by cerebellar structure and function (Jacobson et al., 2011, 2008), and effects of choline treatment on cerebellar volume might have been expected. However, postnatal brain volume changes are regionally asynchronous. The growth rates, measured primarily by change in volume, of the telencephalon (including the caudate, putamen and corpus callosum) and the diencephalon (including the thalamus) peak earlier in postnatal life than the rhombencephalon, which includes the cerebellum (Rice and Barone, 2000). The cerebellum undergoes the most rapid postnatal growth of the regions evaluated in the current study (Holland et al., 2014). The infants in this study were scanned within the first postnatal month, while EBC was assessed at 6.5 months and the FTII was administered at 6.5 and 12 months. While volumetric effects in the cerebellum were not observed during the neonatal period, these might have been detected at the later time-points. It is possible, however, that the effect of choline supplementation on cerebellar functioning may be mediated by structural changes that are not volumetric. For example, increasing alcohol exposure was shown to be associated with lower fractional anisotropy and higher mean diffusivity in the cerebellar peduncles, and these indices were associated with poorer performance on EBC, suggesting a mediating role for these white matter deficits in the effects of PAE on EBC (Fan et al., 2015). Investigating white matter microstructure of choline-treated infants with PAE may reveal effects of supplementation on these indices that are not observed in volumetric analyses.
Two features of the choline trial intervention may explain the robust findings in our study. The treatment was initiated during the second trimester of pregnancy, much earlier in development than the childhood supplementation trials, and the maternal choline dose was markedly higher than in previous studies. These features of the treatment are consistent with laboratory animal studies in which supplementation has been observed to be more effective when administered earlier in pregnancy (Meck et al., 1989; Thomas et al., 2009) and which used much higher choline doses (Thomas and Tran, 2012). Moreover, the association of adherence to the choline supplementation, as a proxy for choline dose, with the volumes of several measured regions in the current study suggests dose-response effects.
The neuroprotective effects of choline in PAE are likely attributable to several mechanisms. PAE has been shown to modulate the methylation of DNA, resulting in epigenetic alterations during cell proliferation and differentiation that have been suggested to underlie the physical and functional alterations observed in FASD (Zeisel, 2011a). As a rich source of methyl groups, choline may be protective against these alterations and may enhance prenatal developmental processes, including cell proliferation and differentiation (Wang et al., 2016). Prenatal choline supplementation has been observed to be associated with increased hippocampal cell proliferation (Glenn et al., 2007). Choline is also a precursor to cell membrane constituents and to cell signalling factors (Zeisel and Niculescu, 2006). Choline supplementation is not effective only during the prenatal period. Considerable brain development occurs after birth (Rice and Barone, 2000) and choline’s roles as a methyl group donor and cell component precursor continue postnatally (Zeisel, 2011a, 2011b). The neuroprotective effect of choline postnatally is evidenced by observations of improvements following postnatal supplementation in rats (Thomas and Tran, 2012) and humans (Kable et al., 2015; Wozniak et al., 2020). However, modification of DNA methylation by choline may occur before neural progenitor cells differentiate, and later choline supplementation may not correct epigenetic changes in those cells (Zeisel, 2011a). Increased availability of choline during critical developmental windows may be protective against PAE-associated reductions in cell proliferation, a process that may contribute to the larger regional volumes observed in choline-supplemented infants in the current study.
Maternal marijuana use during pregnancy was higher in the choline-treated group, and a weak association was observed between marijuana use and volume of the right caudate. Prenatal exposure to marijuana has been associated with reduced cortical gray matter (Rivkin et al., 2008) and reduced volumes of the frontal lobes (Peterson et al., 2020), although El Marroun and colleagues (2016) observed thicker frontal cortex in school-aged children with prenatal marijuana exposure. However, the association of right caudate volume with choline dose and group difference in this region remained significant in the current study following control for marijuana exposure. Interestingly, higher maternal gestational choline levels have been shown to be associated with improved attentional and social outcomes in cannabis-exposed children (Hunter et al., 2021), suggesting that the choline regimen in the current study may have reduced the potential structural damage associated with marijuana exposure as well as with PAE.
A limitation of the current study was the small sample size, due to the original sample’s having been recruited for a pilot study (Jacobson et al., 2018a). 84% of the mothers of infants enrolled in the trial consented to participate in this additional neuroimaging component of the study, and the findings proved to be sufficiently robust to be detected even in this small sample. Whereas in the larger infant clinical trial sample, the choline group performed significantly better on the FTII than the placebo group (Jacobson et al., 2018b), this effect was not significant in the smaller treatment-adherent subset (N = 37) for whom both MRI and FTII data were available. However, when including all of the children for whom both MRI and FTII data were available (N = 41) and after control for treatment adherence, the group difference on the FTII was significant. Although the significance level in the present study was not adjusted for multiple comparisons, the number of significant effects in the group comparison and regression analysis of ROI size with choline adherence (6 of 12 ROIs: 50%) clearly exceeded the 5.0% expected by chance.
An additional limitation is that the study design did not assess whether choline supplementation is universally neuroprotective, as there was no group of infants without PAE whose mothers received the choline treatment. Future human studies are needed to investigate the overall potentially positive effects of choline and to determine whether choline supplementation during pregnancy provides additional specific protection against alcohol exposure.
Further studies are needed to determine whether these findings are generalizable to other populations. Gestational choline supplementation is likely to be most effective in ameliorating alcohol-related damage in women who are choline deficient (Zeisel, 2011a), given findings in animal laboratory studies (Idrus et al., 2017; Thomas et al., 2000). More than 70% of women in the supplementation trial from which the current infant cohort was drawn showed inadequate choline intake (Jacobson et al., 2018b), a finding which reflects the high prevalence of dietary choline inadequacy in this population (>88%; Carter et al., 2017). However, mean dietary choline intake for this study was comparable to that reported in a study of pregnant women in the U.S. (Shaw et al., 2004). High levels of inadequate choline intake in pregnant women have been reported in the U.S. (Jensen et al., 2007; Shaw et al., 2004), Canada (Lewis et al., 2014) and New Zealand (Mygind et al., 2013), suggesting that choline supplementation may be effective in other populations. It remains to be determined to what extent a maternal choline supplementation intervention may be effective in heavy-drinking women who are not choline deficient.
This study is the first to provide evidence that high-dose choline supplementation initiated early in pregnancy may mitigate the effects of PAE on brain structure in human neonates and that these volumetric changes may partially mediate the effects of choline on an important long-term cognitive outcome. These results, in conjunction with our previously reported findings of improved cognition and postnatal growth (Jacobson et al., 2018b), not only suggest that choline may be neuroprotective against a number of PAE-related cognitive and growth deficits, but also identify specific brain regions, the right putamen and corpus callosum, that may mediate these effects and may, therefore, be particularly amenable to early choline treatment of PAE exposure. The findings of the current study need to be confirmed and extended to other metrics of brain structure and function, including metabolism and connectivity, in a fully powered trial, and longitudinal investigations are warranted to determine whether these beneficial effects of choline supplementation persist into childhood.
ACKNOWLEDGMENTS
We thank A. Hess and A. Mareyam for their work in constructing the birdcage RF coil used in this study under the supervision of L. Wald, Director MRI Core, Martinos Center for Biomedical Imaging, Radiology, Massachusetts General Hospital; the Cape Universities Brain Imaging Centre radiographers N. Maroof and A. Siljeur; and our University of Cape Town research staff M. September, B. Arendse, M. Raatz, and P. Solomon. We greatly appreciate the participation of the mothers and infants in our choline supplementation study.
Funding/ Support:
This work was funded by National Institutes of Health (NIH) grants R01-AA016781 (SJ), R21-AA020332 (SJ), R21-AA020037 (SJ, EM, AvdK), R01-HD085813 (EM, AvdK, Laughton), supplemental funding from the Lycaki-Young Fund from the State of Michigan (SJ and JJ), and the National Research Foundation of South Africa (Grant Number: 48337; EM). FW was supported by a South African National Research Foundation (NRF) Innovative Scholarship and the Duncan Baxter Scholarship from the University of Cape Town. LZ was supported by R00-HD061485, R01-HD065762, and Shared Instrumentation Grants 1S10RR023401, 1S10RR019307, and 1S10RR023043.
Role of Funders/Sponsors:
The funders and sponsors did not participate in the work.
Abbreviations:
- PAE
Prenatal alcohol exposure
- MRI
Magnetic resonance imaging
- FTII
Fagan Test of Infant Intelligence
- FASD
Fetal alcohol spectrum disorders
- AA
Absolute alcohol
- GA
Gestational age
- MEF
Multi-echo FLASH
- TIV
Total intracranial volume
Footnotes
Declarations of Interest: None.
REFERENCES
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL (2001) Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol 43:148–54. [PubMed] [Google Scholar]
- Biffen SC, Warton CMR, Lindinger NM, Randall SR, Lewis CE, Molteno CD, Jacobson JL, Jacobson SW, Meintjes EM (2017) Reductions in corpus callosum volume partially mediate effects of prenatal alcohol exposure on IQ. Front Neuroanat 11:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd L, Klug MG, Martsolf JT, Kerbeshian J (2003) Fetal alcohol syndrome: neuropsychiatric phenomics. Neurotoxicol Teratol 25:697–705. [DOI] [PubMed] [Google Scholar]
- Carter RC, Senekal M, Dodge NC, Bechard LJ, Meintjes EM, Molteno CD, Duggan CP, Jacobson JL, Jacobson SW (2017) Maternal alcohol use and nutrition during pregnancy: diet and anthropometry. Alcohol Clin Exp Res 41:2114–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clogg CC (1995) Latent Class Models In: Handbook of Statistical Modeling for the Social and Behavioral Sciences , pp 311–359. Boston, MA, Springer US. [Google Scholar]
- Coles CD, Goldstein FC, Lynch ME, Chen X, Kable JA, Johnson KC, Hu X (2011) Memory and brain volume in adults prenatally exposed to alcohol. Brain Cogn 75:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CD, Kable JA, Keen CL, Jones KL, Wertelecki W, Granovska I V., Pashtepa AO, Chambers CD, CIFASD (2015) Dose and timing of prenatal alcohol exposure and maternal nutritional supplements: developmental effects on 6-month-old infants. Matern Child Health J 19:2605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Macedo Rodrigues K, Ben-Avi E, Sliva DD, Choe M-S, Drottar M, Wang R, Fischl B, Grant PE, Zöllei L (2015) A FreeSurfer-compliant consistent manual segmentation of infant brains spanning the 0–2 year age range. Front Hum Neurosci 9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Marroun H, Tiemeier H, Franken IHA, Jaddoe VW V, van der Lugt A, Verhulst FC, Lahey BB, White T (2016) Prenatal Cannabis and Tobacco Exposure in Relation to Brain Morphology: A Prospective Neuroimaging Study in Young Children. Biol Psychiatry 79:971–9. [DOI] [PubMed] [Google Scholar]
- Fagan JF, McGrath SK (1981) Infant recognition memory and later intelligence. Intelligence 5:121–130. [Google Scholar]
- Fagan JF, Singer LT (1983) Infant recognition memory as a measure of intelligence. Adv Infancy Res 2:31–78. [Google Scholar]
- Fan J, Jacobson SW, Taylor PA, Molteno CD, Dodge NC, Stanton ME, Jacobson JL, Meintjes EM (2016) White matter deficits mediate effects of prenatal alcohol exposure on cognitive development in childhood. Hum Brain Mapp 37:2943–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Meintjes EM, Molteno CD, Spottiswoode BS, Dodge NC, Alhamud AA, Stanton ME, Peterson BS, Jacobson JL, Jacobson SW (2015) White matter integrity of the cerebellar peduncles as a mediator of effects of prenatal alcohol exposure on eyeblink conditioning. Hum Brain Mapp 36:2470–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJW, Makris N, Ségonne F, Quinn BT, Dale AM (2004) Sequence-independent segmentation of magnetic resonance images. Neuroimage 23 Suppl 1:S69–84. [DOI] [PubMed] [Google Scholar]
- Glenn MJ, Gibson EM, Kirby ED, Mellott TJ, Blusztajn JK, Williams CL (2007) Prenatal choline availability modulates hippocampal neurogenesis and neurogenic responses to enriching experiences in adult female rats. Eur J Neurosci 25:2473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CR, Mihic AM, Nikkel SM, Stade BC, Rasmussen C, Munoz DP, Reynolds JN (2009) Executive function deficits in children with fetal alcohol spectrum disorders (FASD) measured using the Cambridge Neuropsychological Tests Automated Battery (CANTAB). J Child Psychol Psychiatry 50:688–97. [DOI] [PubMed] [Google Scholar]
- Gross LA, Moore EM, Wozniak JR, Coles CD, Kable JA, Sowell ER, Jones KL, Riley EP, Mattson SN, CIFASD (2018) Neural correlates of verbal memory in youth with heavy prenatal alcohol exposure. Brain Imaging Behav 12:806–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson TJ, Mueller BA, Sowell ER, Mattson SN, Coles CD, Kable JA, Jones KL, Boys CJ, Lim KO, Riley EP, Wozniak JR (2017) Cortical gyrification is abnormal in children with prenatal alcohol exposure. NeuroImage Clin 15:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland D, Chang L, Ernst TM, Curran M, Buchthal SD, Alicata D, Skranes J, Johansen H, Hernandez A, Yamakawa R, Kuperman JM, Dale AM (2014) Structural growth trajectories and rates of change in the first 3 months of infant brain development. JAMA Neurol 71:1266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB (2011) Four factor index of social status. Yale J Sociol 8:21–52. [Google Scholar]
- Hoyme HE, Kalberg WO, Elliott AJ, Blankenship J, Buckley D, Marais A-S, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Jewett T, Coles CD, Chambers C, Jones KL, Adnams CM, Shah PE, Riley EP, Charness ME, Warren KR, May PA (2016) Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics 138:e20154256–e20154256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SK, Hoffman MC, D’Alessandro A, Wyrwa A, Noonan K, Zeisel SH, Law AJ, Freedman R (2021) Prenatal choline, cannabis, and infection, and their association with offspring development of attention and social problems through 4 years of age. Psychol Med 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idrus NM, Breit KR, Thomas JD (2017) Dietary choline levels modify the effects of prenatal alcohol exposure in rats. Neurotoxicol Teratol 59:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante MA, Moore EM, Bischoff-Grethe A, Migliorini R, Mattson SN, Riley EP (2015) Atypical cortical gyrification in adolescents with histories of heavy prenatal alcohol exposure. Brain Res 1624:446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Carter RC, Molteno CD, Meintjes EM, Senekal MS, Lindinger NM, Dodge NC, Zeisel SH, Duggan CP, Jacobson JL (2018a) Feasibility and acceptability of maternal choline supplementation in heavy drinking pregnant women: a randomized, double-blind, placebo-controlled clinical trial. Alcohol Clin Exp Res 42:1315–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Carter RC, Molteno CD, Stanton ME, Herbert JS, Lindinger NM, Lewis CE, Dodge NC, Hoyme HE, Zeisel SH, Meintjes EM, Duggan CP, Jacobson JL (2018b) Efficacy of maternal choline supplementation during pregnancy in mitigating adverse effects of prenatal alcohol exposure on growth and cognitive function: a randomized, double-blind, placebo-controlled clinical trial. Alcohol Clin Exp Res 42:1327–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL (2002) Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics 109:815–25. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Fein GG, Jacobson JL, Schwartz PM, Dowler JK (1985) The effect of intrauterine PCB exposure on visual recognition memory. Child Dev 56:853–60. [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Molteno CD, Warton CMR, Wintermark P, Hoyme HE, De Jong G, Taylor P, Warton F, Lindinger NM, Carter RC, Dodge NC, Grant E, Warfield SK, Zöllei L, van der Kouwe AJW, Meintjes EM (2017) Heavy prenatal alcohol exposure is related to smaller corpus callosum in newborn MRI scans. Alcohol Clin Exp Res 41:965–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, O’Neill JM, Padgett RJ, Frankowski JJ, Bihun JT (1992) Visual expectation and dimensions of infant information processing. Child Dev 63:711–24. [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Ager JW (1993) Prenatal alcohol exposure and infant information processing ability. Child Dev 64:1706–21. [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Chiodo LM (1996) New evidence for neurobehavioral effects of in utero cocaine exposure. J Pediatr 129:581–90. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Dodge NC, Pienaar M, Fuller DS, Molteno CD, Meintjes EM, Hoyme HE, Robinson LK, Khaole N, Jacobson JL (2011) Impaired delay and trace eyeblink conditioning in school-age children with fetal alcohol syndrome. Alcohol Clin Exp Res 35:250–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Robinson LK, Khaole N, Jacobson JL (2008) Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res 32:365–72. [DOI] [PubMed] [Google Scholar]
- Jensen HH, Batres-Marquez SP, Carriquiry A, Schalinske KL (2007) Choline in the diets of the US population: NHANES, 2003–2004. FASEB J 21:LB46–LB46. [Google Scholar]
- Kable JA, Coles CD, Keen CL, Uriu-Adams JY, Jones KL, Yevtushok L, Kulikovsky Y, Wertelecki W, Pedersen TL, Chambers CD (2015) The impact of micronutrient supplementation in alcohol-exposed pregnancies on information processing skills in Ukrainian infants. Alcohol 49:647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavšek M (2004) Predicting later IQ from infant visual habituation and dishabituation: A meta-analysis. J Appl Dev Psychol 25:369–393. [Google Scholar]
- Lange S, Probst C, Gmel G, Rehm J, Burd L, Popova S (2017) Global prevalence of Fetal Alcohol Spectrum Disorder among children and youth: a systematic review and meta-analysis. JAMA Pediatr 171:948–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laswad T, Wintermark P, Alamo L, Moessinger A, Meuli R, Gudinchet F (2009) Method for performing cerebral perfusion-weighted MRI in neonates. Pediatr Radiol 39:260–4. [DOI] [PubMed] [Google Scholar]
- Lebel C, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, Bookheimer SY, O’Connor MJ, Narr KL, Kan E, Abaryan Z, Sowell ER (2012) A longitudinal study of the long-term consequences of drinking during pregnancy: heavy in utero alcohol exposure disrupts the normal processes of brain development. J Neurosci 32:15243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CE, Thomas KGF, Dodge NC, Molteno CD, Meintjes EM, Jacobson JL, Jacobson SW (2015) Verbal learning and memory impairment in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 39:724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis ED, Subhan FB, Bell RC, McCargar LJ, Curtis JM, Jacobs RL, Field CJ, APrON team (2014) Estimation of choline intake from 24 h dietary intake recalls and contribution of egg and milk consumption to intake among pregnant and lactating women in Alberta. Br J Nutr 112:112–21. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Bernes GA, Doyle LR (2019) Fetal alcohol spectrum disorders: A review of the neurobehavioral deficits associated with prenatal alcohol exposure. Alcohol Clin Exp Res 43:1046–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Blankenship J, Marais A-S, Gossage JP, Kalberg WO, Barnard R, De Vries M, Robinson LK, Adnams CM, Buckley D, Manning M, Jones KL, Parry C, Hoyme HE, Seedat S (2013) Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a South African population-based study. Alcohol Clin Exp Res 37:818–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH, Smith RA, Williams CL (1989) Organizational changes in cholinergic activity and enhanced visuospatial memory as a function of choline administered prenatally or postnatally or both. Behav Neurosci 103:1234–41. [DOI] [PubMed] [Google Scholar]
- Meintjes EM, Narr KL, van der Kouwe AJW, Molteno CD, Pirnia T, Gutman B, Woods RP, Thompson PM, Jacobson JL, Jacobson SW (2014) A tensor-based morphometry analysis of regional differences in brain volume in relation to prenatal alcohol exposure. NeuroImage Clin 5:152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mygind VL, Evans SE, Peddie MC, Miller JC, Houghton LA (2013) Estimation of usual intake and food sources of choline and betaine in New Zealand reproductive age women. Asia Pac J Clin Nutr 22:319–24. [DOI] [PubMed] [Google Scholar]
- Nardelli A, Lebel C, Rasmussen C, Andrew G, Beaulieu C (2011) Extensive deep gray matter volume reductions in children and adolescents with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 35:1404–17. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Risbud RD, Mattson SN, Chambers CD, Thomas JD (2016) Randomized, double-blind, placebo-controlled clinical trial of choline supplementation in school-aged children with fetal alcohol spectrum disorders1. Am J Clin Nutr 104:1683–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panczakiewicz AL, Glass L, Coles CD, Kable JA, Sowell ER, Wozniak JR, Jones KL, Riley EP, Mattson SN, CIFASD (2016) Neurobehavioral Deficits Consistent Across Age and Sex in Youth with Prenatal Alcohol Exposure. Alcohol Clin Exp Res 40:1971–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangelinan MM, Zhang G, VanMeter JW, Clark JE, Hatfield BD, Haufler AJ (2011) Beyond age and gender: relationships between cortical and subcortical brain volume and cognitive-motor abilities in school-age children. Neuroimage 54:3093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Rosen T, Dingman S, Toth ZR, Sawardekar S, Hao X, Liu F, Xu D, Dong Z, Peterson JB, Ryoo JH, Serino D, Branch CA, Bansal R (2020) Associations of Maternal Prenatal Drug Abuse With Measures of Newborn Brain Structure, Tissue Organization, and Metabolite Concentrations. JAMA Pediatr 174:831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resseguie M, Song J, Niculescu MD, da Costa K-A, Randall TA, Zeisel SH (2007) Phosphatidylethanolamine N-methyltransferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. FASEB J 21:2622–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, Barone S (2000) Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 108 Suppl:511–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivkin MJ, Davis PE, Lemaster JL, Cabral HJ, Warfield SK, Mulkern R V, Robson CD, Rose-Jacobs R, Frank DA (2008) Volumetric MRI study of brain in children with intrauterine exposure to cocaine, alcohol, tobacco, and marijuana. Pediatrics 121:741–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussotte FF, Sulik KK, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, O’Connor MJ, Narr KL, Sowell ER (2012) Regional brain volume reductions relate to facial dysmorphology and neurocognitive function in fetal alcohol spectrum disorders. Hum Brain Mapp 33:920–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SH, Williams JK, Thomas JD (2008) Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: Effects of varying the timing of choline administration. Brain Res 1237:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw GM, Carmichael SL, Yang W, Selvin S, Schaffer DM (2004) Periconceptional Dietary Intake of Choline and Betaine and Neural Tube Defects in Offspring. Am J Epidemiol 160:102–109. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Kan E, Thompson PM, Riley EP, Toga AW (2008) Abnormal cortical thickness and brain-behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cereb Cortex 18:136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttie M, Wozniak JR, Parnell SE, Wetherill L, Mattson SN, Sowell ER, Kan E, Riley EP, Jones KL, Coles C, Foroud T, Hammond P, CIFASD (2018) Combined Face-Brain Morphology and Associated Neurocognitive Correlates in Fetal Alcohol Spectrum Disorders. Alcohol Clin Exp Res 42:1769–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PA, Jacobson SW, van der Kouwe A, Molteno CD, Chen G, Wintermark P, Alhamud A, Jacobson JL, Meintjes EM (2015) A DTI-based tractography study of effects on brain structure associated with prenatal alcohol exposure in newborns. Hum Brain Mapp 36:170–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Abou EJ, Dominguez HD (2009) Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicol Teratol 31:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Garrison M, O’Neill TM (2004) Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol 26:35–45. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Idrus NM, Monk BR, Dominguez HD (2010) Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth Defects Res A Clin Mol Teratol 88:827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, La Fiette MH, Quinn VRE, Riley EP (2000) Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicol Teratol 22:703–711. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Tran TD (2012) Choline supplementation mitigates trace, but not delay, eyeblink conditioning deficits in rats exposed to alcohol during development. Hippocampus 22:619–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kouwe AJW, Benner T, Salat DH, Fischl B (2008) Brain morphometry with multiecho MPRAGE. Neuroimage 40:559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J, Mooney SM (2017) Choline and working memory training improve cognitive deficits caused by prenatal exposure to ethanol. Nutrients 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Surzenko N, Friday WB, Zeisel SH (2016) Maternal dietary intake of choline in mice regulates development of the cerebral cortex in the offspring. FASEB J 30:1566–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warton FL, Meintjes EM, Warton CMR, Molteno CD, Lindinger NM, Carter RC, Zöllei L, Wintermark P, Jacobson JL, van der Kouwe A, Jacobson SW (2018) Prenatal methamphetamine exposure is associated with reduced subcortical volumes in neonates. Neurotoxicol Teratol 65:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer B (1971) Statistical principles in experimental design, 2nd ed. McGraw-Hill. [Google Scholar]
- Wozniak JR, Fink BA, Fuglestad AJ, Eckerle JK, Boys CJ, Sandness KE, Radke JP, Miller NC, Lindgren C, Brearley AM, Zeisel SH, Georgieff MK (2020) Four-year follow-up of a randomized controlled trial of choline for neurodevelopment in fetal alcohol spectrum disorder. J Neurodev Disord 12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH (2011a) What choline metabolism can tell us about the underlying mechanisms of fetal alcohol spectrum disorders. Mol Neurobiol 44:185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH (2011b) The supply of choline is important for fetal progenitor cells. Semin Cell Dev Biol 22:624–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH, Niculescu MD (2006) Perinatal choline influences brain structure and function. Nutr Rev 64:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]


