Abstract
Seipin is a key protein in assembly of cytoplasmic lipid droplets (LDs) and their maintenance at ER junctions; the absence of seipin results in generalized lipodystrophy. How seipin mediates LD dynamics and prevents lipodystrophy are not well understood. New evidence suggests that seipin attracts triglyceride monomers from the ER to sites of droplet formation. In contrast, seipin may not be directly involved in assembly of nuclear LDs and may actually suppress their formation at a distance. Seipin promotes adipogenesis, but lipodystrophy may involve post-adipogenic effects. We hypothesize that among these are a cycle of runaway lipolysis and lipotoxicity caused by aberrant lipid droplets, resulting in a depletion of fat stores and a failure of adipose and other cells to thrive.
Keywords: seipin, lipodystrophy, lipid droplet, fatty acid, adipogenesis
Introducing the players: seipin, lipid droplets, and lipodystrophy
Lipid droplets (LDs)
Lipid droplets (LDs) which are found in virtually all eukaryotic and many prokaryotic cells, serve several functions [1–3]. First and foremost, they store neutral lipids (“fat”), a rich source of energy, releasing fatty acids according to cellular or organismal needs. They also store lipophilic bioactive molecules such as steroid hormone precursors, acylceramides, and ecosinoids within their core, and proteins such as histones, factors in innate immunity, and viral precursors on their phospholipid monolayer surface. In acylating and sequestering fatty acids, they protect cells from lipotoxicity [4]. Although their home base is the endoplasmic reticulum, they communicate with several other organelles through membrane junctions [5, 6]. LDs exist as subpopulations mainly in the cytoplasm (cLDs), but in liver and in some cultured cell lines they also are found in the nucleus (nLDs) [7]. The popularity and intensity of research in lipid droplets in recent years is suggested by the presence of two other reviews in this journal thus far in early 2021 [6, 8].
Seipin
Seipin is an ER transmembrane protein [9, 10] that is an important constituent of the ER/LD junction [11, 12]. Without seipin, droplets are heterogeneous in size [12, 13], their normal proteome is disturbed [14], and their connection with the ER is disrupted [15, 16]. There are also changes in the cell lipidome [17]. Until recently the molecular mechanism of seipin has been obscure, although many theories have been proposed based on binding and pulldown studies of proteins with clear function [18–21]. However, recent cryo-EM structures [22, 23] have stimulated new hypotheses with exciting supporting data [24, 25]. In the mouse the absence of seipin leads to the lack of development of adipose tissue, fatty liver, and insulin resistance [26]. In humans this defect is severe and is the cause of generalized lipodystrophy, fatty liver, neurological symptoms, and life-threatening metabolic imbalances, among several other organ dysfunctions [27]. How, or whether, the role of seipin in fostering well-behaved lipid droplets is connected to its prevention of severe lipodystrophy is a question that spans the interest of lipid biochemists, cell biologists, and endocrinologists. It is clear that seipin permits PPARγ signaling, which is fundamental to adipogenesis, and there is evidence that altered lipid metabolism, especially related to an increase in phosphatidic acid (PA), may short-circuit the transcriptional cascade of adipogenesis [19]. However, seipin also is necessary for the ability of existing adipocytes to thrive in the animal [28], and this may involve calcium signaling [29], control of lipogenesis and lipolysis [29, 30] and, we imagine, prevention of lipotoxicity. In this review we will discuss new information regarding the role of seipin in harvesting ER neutral lipids, the controversy of whether seipin is always required for lipid droplet formation in cells, and we offer a new idea that may link aberrant lipid droplets in seipin-deficient cells to human lipodystrophy.
Lipid droplets do not need seipin to form
Triacylglycerol (TG) is the major neutral lipid of LDs in most systems and is used most often as a representative of the class. Early work indicated that phospholipid bilayers can accommodate up to 4 or 5 % of TG before they become destabilized, at which point TG oils out of the bilayer and forms drops, emulsified by the membrane phospholipid [31]. Molecular dynamics simulations confirm that as the concentration of TG in a membrane increases, TG molecules aggregate, forming “blisters” among the phospholipid acyl chains [32]. More recently, such blisters, or lenses, have been demonstrated in cells exposed briefly to exogenous fatty acid (oleate), both by transmission electron microscopy [33] and with a sensitive fluorescently labelled probe [34].
Once the lens approaches the combined width of the acyl chains of both leaflets, the latter must distend to accommodate the growing neutral lipid core until a droplet evaginates, coated with the outer phospholipid leaflet from the bilayer. None of this requires proteins. Several recent studies have modeled this system in silico, in giant unilamellar vesicles and in living cells [35–39]. Results have indicated that the nature of phospholipids (head groups and length and degree of unsaturation of acyl chains), asymmetry of the bilayer, ER sheets vs. tubules, and inclusion of sterols or other membrane lipids such as diacylglycerol all can affect the concentration or kinetics of TG aggregation and budding. Theoretical studies have established the basic physical chemistry of lipid droplet budding and have shown that affinity of neutral lipids for themselves vs the acyl chains of phospholipids, surface and lateral tension of the bilayer, and membrane curvature are all key elements in the process [8].
Even though lipid droplets can form on their own in isolation, it is well established that proteins at the site of droplet formation play roles in LD formation in cells. LDs form aberrantly in the absence of seipin [12, 13]. Several proteins, including FIT2 [33], Nem1 [40, 41], Mdm1 [42], LDAF1/promethin [43–46], Pex30 [47, 48], and perilipin [49, 50] also have roles in this process. A recent report has ordered several of these proteins in the pathway of assembly [41]. While none of them is required to form lipid droplets, as droplets still form in their absence, some of these are sufficient (in the context of intact cells), as ectopically expressed Mdm1 [51], seipin [52], or LDAF1 [43] can generate droplets at a foreign site. As the studies with model systems indicate that lipid droplets will be formed as long as neutral lipids are synthesized and accumulate in the ER, outstanding issues in the field are how these proteins collaborate, and what is the value added? And do they function outside the cytoplasm?
Nuclear lipid droplets – subclasses and possible role of seipin
There has been much recent interest in nuclear lipid droplets (nLDs), which are most often observed in liver and liver-derived cultured cells [53, 54]. These particles, which have a higher ratio of cholesteryl esters to TG than cytoplasmic LDs, can result from three processes: invagination of the nuclear envelope, in which cLDs simply get trapped within the nucleus but topologically remain in the cytoplasm; migration of lipoprotein particles (devoid of apoB) from the ER lumen into the nucleoplasmic reticulum (NR); and assembly and budding to droplets directly from the inner nuclear membrane (INM) or NR toward the nucleoplasm [55]. The lipoprotein-related droplets are a result of ER stress and external oleate, depend on microsomal triglyceride transfer protein (MTP, which assembles lipoproteins in the ER) and, once in the nucleus, penetrate the membrane to gain access to the nucleoplasm [7]. As they originally bud into the ER lumen, this process should not involve seipin; their function is likely related to their activation upon binding of CTP:phosphocholine cytidylyltransferase alpha (CCTα), the rate-limiting enzyme in the Kennedy phosphatidylcholine biosynthetic pathway, which thereby promotes phospholipid production in response to ER stress or ER neutral lipid toxicity [7].
The other interesting class of nLDs are those that bud from the INM or NR. Remarkably, nLDs frequently associate with promyelocytic leukemia (PML) bodies, phase-separated organelles that have multiple roles in chromatin maintenance and RNA metabolism [56]. Knockdown of PMLII (a scaffold protein of the organelle) resulted in fewer nLDs, suggesting that nLD function is coupled with PML bodies. New evidence shows that a subpopulation of PML bodies coat the nLD (a lipid droplet within a liquid droplet) and allow lipid biosynthetic proteins such as CCTα and lipin1 to associate with the nLDs [57]. An overarching question in the field regards the role of nLDs in the nucleus. The lipids of nLDs could by critical for growth or remodeling of the nucleoplasmic reticulum, protein fatty acylation, or provision of lipid ligands for transcription factors (such as PPAR members or steroid hormone receptors). Furthermore, phosphatidyl inositol of the nLD bilayer could provide a rich source of signaling molecules including soluble phosphatidyl inositols and inositol polyphosphates [58, 59].
A rigorous search for seipin on nLDs, or on the nuclear surface of the nuclear envelope of U2OS (human osteosarcoma) cells, failed to detect this protein, even when it was overexpressed [60]. However, seipin knockout strains increased the number and size of nLDs. The authors found that PA was increased in the nuclear envelope in seipin-null cells (consistent with older work [16, 17, 61]) and that this attracted lipin, which would increase the diacylglycerol/PA ratio and thereby foster LD formation. Thus, the authors concluded that seipin is working at a distance to suppress PA and control nLD assembly.
A conflicting result had been reported using yeast cells, in which seipin and many neutral lipid biosynthetic enzymes, as well as nLDs, were shown to exist on the nuclear face of the INM in a mutant in which phospholipid synthesis is attenuated [62]. The presence of seipin on the INM was shown by bimolecular fluorescence complementation with Nup60, a basket protein of the nuclear pore complex. However, nuclear localization of seipin required a high level of overexpression. Under these conditions it is possible that seipin monomers, which may not be filtered by the NPC, appear on the INM in sufficient levels to be detected in the yeast system. Membrane bridges that depended on seipin were also seen between the inner nuclear envelope and nLDs. While this may indicate a direct involvement of seipin in formation of these bridges, the absence of bridges could reflect ectopic budding of LDs as a result of increased neutral lipid in the bilayer [15].
Exogenous oleate was used in these studies probing seipin near nLDs, and the use of this fatty acid is common practice in the field as it enhances the size of droplets. As lipid droplets should spontaneously form locally as neutral lipids derived from oleate challenge bilayer stability, and many cell types are not naturally exposed to high fatty acid concentrations, care must be taken in interpreting results regarding lipid droplet assembly after oleate addition to ensure the mechanisms observed have physiological relevance.
Seipin as a monitor, buffer, and harvester of neutral lipids in the ER bilayer
Seipin contains a large conserved middle region and smaller and more diverse ends [12]. The conserved region (in which secondary structure and several amino acids are preserved) consists of ~225 residues in the ER lumen flanked by a transmembrane domain (TMD) at either end. Ten to twelve monomers assemble to generate a ring-like structure, with the TMDs at the periphery and hydrophobic helices (HHs) facing an aperture in the center ( [22, 23] and unpublished data). The bulk of this luminal domain forms a β-sandwich with 3–4 strands on either side of a narrow space. The central helices are hydrophobic and modeled to lie within the ER bilayer.
Two new reports have exploited the seipin luminal domain structure to model the behavior of neutral lipids in a bilayer in which seipin is present or absent [24, 25]. Both reports used molecular dynamic simulations with human seipin to identify key regions for TG binding. Both confirm previous findings that TG at or above 4% aggregate in the bilayer, while at 2% or less aggregation is rare. However, with seipin present TG again aggregates within the seipin rings even at 2% or lower TG concentration. Accumulation of seipin occurs at both the TMDs and the HHs lining the center aperture. Stronger binding is seen at the HHs, and the kinetics of binding at this site is faster in the absence of the TMDs, suggesting that the TMDs are acting as a gate to restrict entry. Caution is need to interpret the binding of TG to the TMDs as the atomic structure of these seipin elements has not been determined. Both groups identify S165 and S166 are essential for binding to the HH, and experiments in cells confirm that mutations of these two serines strongly inhibit the function of seipin in droplet formation. Binding of TG to the HH is modeled as being important for subsequent binding of LDAF/promethin to seipin, further promoting TG accumulation within the rings and early steps of LD budding. Importantly, diacylglycerol is also shown to bind to seipin. While it is displaced at the HHs by TG, the two lipids in combination promote LD budding [25].
The conclusion from these experiments is that seipin can harvest TG monomers from the ER membrane and helps maintain a low concentration of the neutral lipid elsewhere in the bilayer (Figure 1). It suggests that seipin can be responsible for lens formation, the first step in LD assembly, rather than be attracted to spontaneously formed lenses. Mobile seipin-independent lenses, however, have been shown in living cells upon incubation with oleate, which are then stabilized by seipin [34]. The extent to which seipin accumulates TG monomers vs. stabilizes small TG aggregates, both leading to droplet formation (Figure 1), under physiological conditions may be difficult to determine with current technology.
Figure 1: The first encounter of seipin and triacylglycerol (TG). Developmental pathway of an adipocyte.
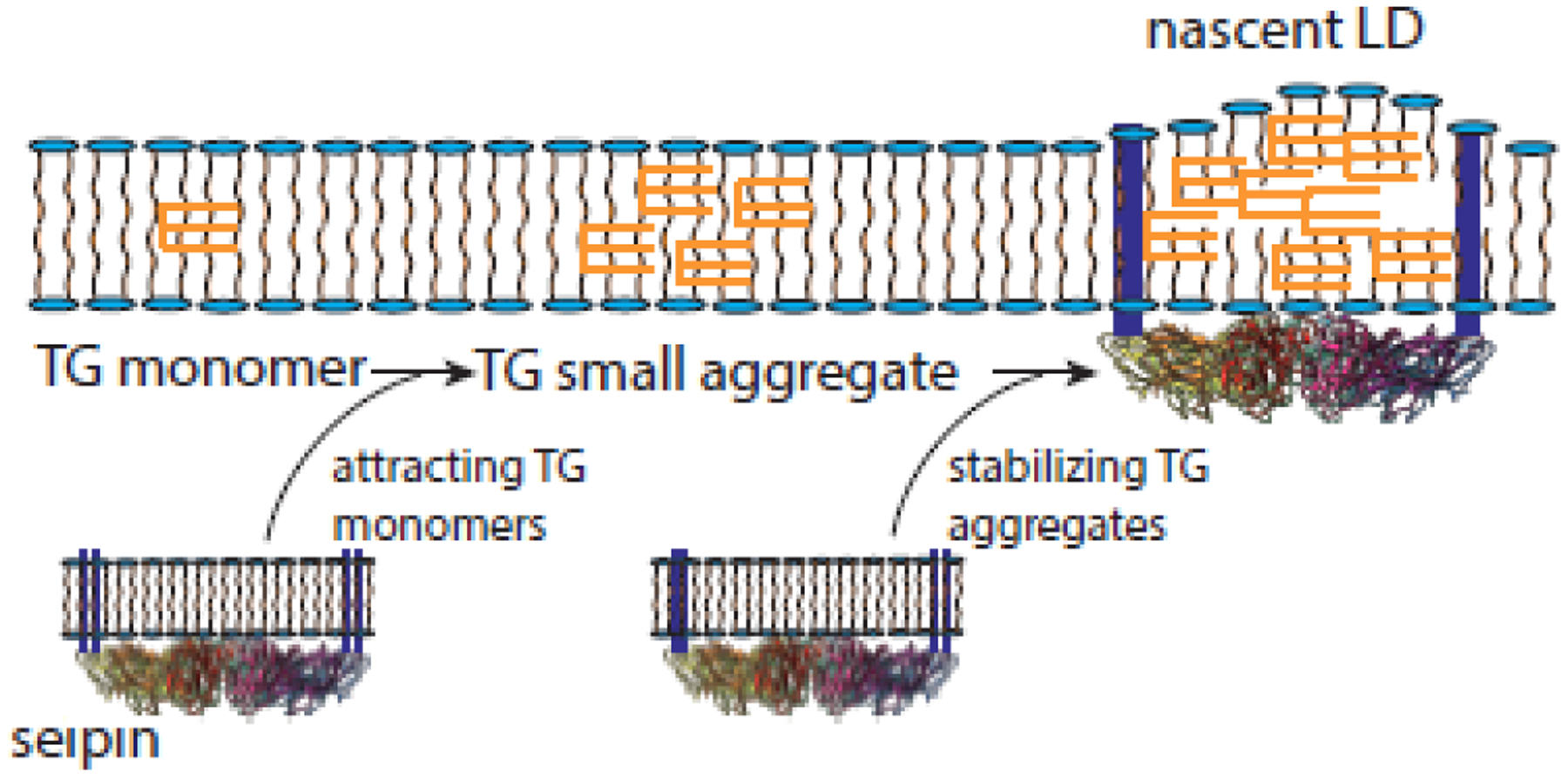
TG will form aggregates in membrane bilayers as its concentration increases. These aggregates will grow in size. Molecular dynamics simulations suggest that seipin promotes aggregation by binding to monomers (first arrow) [24, 25], while short-term incubation of cells with oleate first creates seipin-free aggregates which are then stabilized by seipin (second arrow) and develop further [34]. It is not clear whether one of these pathways is dominant in nature.
Regardless, the simulations suggest that DG may help to expand the lenses, allowing deformation of the outer leaflet, consistent with previous data [25, 41]. The seipin structure may be sufficient to prevent deformation of the inner leaflet, so that budding normally occurs in just one direction (outward), although FIT2 may provide a similar function [33]. By being the way-station for TG, this function may be sufficient to allow flow of TG into droplets as needed even after droplets reach mature size. However, many questions of seipin function at the molecular level remain (see Outstanding Questions box).
Box: Outstanding Questions.
Is the direct molecular function of seipin limited to harvesting TG? Many proteins, including lipid biosynthetic enzymes, LD assembly factors, an ER calcium pump, and a signaling hub, are reported to bind to seipin. How many of these proteins interact directly or physiologically with seipin, and how much of seipin function, or the function of the binding proteins, is dependent on these interactions?
Does the role of seipin in harvesting neutral lipid extend to other neutral lipid classes including steryl esters or acylceramides?
How does seipin affect the LD proteome? Is it a direct effect on filtering proteins that encounter it in the ER membrane or an abnormal monolayer surface on the droplet that changes the affinity of proteins arriving from the cytosol, or both?
The published cryo-EM seipin structures only include the ER luminal domain and not juxtamembrane or transmembrane domains, or the two termini that face the cytosol. Presumably these domains are flexible and difficult to detect by electron microscopy. Does this conformational flexibility reflect different states that are important for seipin function?
The absence of seipin increases the density of nuclear LDs. Does this reflect a role of seipin in nLD production or simply accumulation of neutral lipid in the ER leading to ectopic droplet formation? If seipin does have a role, is it direct or indirect?
How does seipin promote healthy adipocytes? How important is the role of seipin in adipogenesis vs. maintenance of adipocytes in the prevention of lipodystrophy? What is the mechanism of toxicity in tissues and organs beyond adipose?
Seipin and the development of adipose tissue
Before any connection between seipin and lipid droplet dynamics was uncovered, seipin was known for its role in prevention of human generalized lipodystrophy and accompanying dysfunctions of many non-adipose compartments [63, 64]. It is now well established that seipin facilitates adipogenesis, the transcriptional cascade by which mesenchymal stem cells become committed to becoming adipocytes [65]. A key regulator of this process is PPARγ, and early studies using induced mouse fibroblast lines in which seipin expression was knocked down resulted in the inability of PPARγ to signal to downstream factors and promote lipogenesis and appearance of significant numbers of lipid droplets. [66, 67].
There is no obvious mechanism by which seipin, an ER protein found largely at ER/LD junctions [12], could affect PPARγ signaling. However, there are many studies in which seipin influences cell lipidomes, and PPARγ function is dependent on endogenous lipid agonists, mainly fatty acids [68]. Seipin knockdown or elimination results in many changes to fatty acids and glycero- and sphingolipids including degree of unsaturation and chain length of fatty acids and ratio of phospholipids [17, 28]. In a recent report the concentration of sphingolipid intermediates in yeast were increased in seipin KO yeast cells, a result of loss of physical interactions between seipin the sphingolipid biosynthetic enzymes serine palmitoyl transferase and fatty acid elongase, interactions which lowered activities of these enzymes [69]. The importance of seipin in lipid balance has also been recently shown for eggshell development in C. elegans, where seipin promotes the generation of lipids in an inner layer of the shell [70]. In contrast to the action of seipin in animals to promote adipogenesis, seipin actually negatively regulates fat accumulation in other tissues, sometimes by cell autonomous mechanisms [71, 72]. Overall, while the influence of seipin on the lipidome may be the result of direct interactions with enzymes, it is difficult to rule out indirect and compensatory effects caused by the inability of seipin to generate normal lipid droplets.
A mechanism that connects seipin to adipogenesis has been proposed [19] in which seipin promotes PPARγ activation by binding to and inhibiting microsomal glycerol phosphate acyltransferase (GPAT3), a key step in phospholipid and neutral lipid synthesis. Loss of this inhibition is responsible for an increase in phosphatidic acid, as previously observed [17, 73] and its localization into puncta on the ER [16, 61]. PA or a metabolite is hypothesized to serve as an antagonist of PPARγ [19]. A key experiment in support of this hypothesis is that a GPAT inhibitor partially restores adipogenesis in seipin-null pre-adipocytes [19]. However, a role of seipin in maintaining low levels of PA and low activity of GPAT may not universally hold. No change in phospholipid synthesis or phosphatidic acid concentration were observed in seipin knockout clones of Drosophila S2 cells [34], a GPAT knockdown in induced mouse fibroblasts did not reverse the block in adipogenesis [74], and seipin-null mouse embryonic fibroblasts and stromal vascular cells exhibited no defect on initial lipogenesis [75].
Similar to white adipose, development of brown adipose requires PPARγ activation, albeit from a different population of stem cells [76]. Brown fat, more abundant and important in rodents than humans for thermogenesis, especially if challenged by cold temperatures, also is attenuated without seipin. However, mice with a global or a brown fat tissue-specific seipin knockout are born with brown fat stores indistinguishable from wild type, and PPARγ signaling in brown fat precursor cells stimulated to differentiate is normal in the absence of seipin [77, 78].
While the evidence for involvement of seipin in promoting PPARγ signaling is strong and is reinforced by the ability of PPARγ agonists to partially rescue adipogenesis in seipin-null mice [79], a role of seipin in providing an endogenous agonist - for example, from the surface of normal lipid droplets in stem cells - cannot yet be ruled out. Moreover, seipin, in the production of lipid droplets, could be playing other roles in developing and maintaining adipose depots.
Seipin, lipolysis, and a proposed feed-forward pathway that damages adipose tissue
The dearth of adipose depots that results from the loss of seipin is not caused only by poor PPARγ signaling but also by the enhanced lipolysis of stored fat. While differentiating fibroblasts from seipin-null mice initially developed small lipid droplets similar to normal (thus adipogenesis was apparently normal), the droplets became heterogeneous in size, with many tiny ones and a few supersized ones. PPARγ levels in this study subsequently fell, and by day 8 of differentiation, adipocytes and their lipid droplets had disappeared [75]. The loss of lipid droplets was accompanied by massive lipolysis of triacylglycerol; both basal and protein kinase A (PKA)-stimulated lipolysis was elevated. (PKA normally responds to hormones to mobilize fatty acids, which it does by phosphorylating perilipin 1 and activating lipases [80].) Increased lipolysis was also demonstrated in biopsy adipose from the knockout animals [75]. Supporting a mechanistic role of lipolysis in seipin lipodystrophy, genetic or pharmacological block of adipose triglyceride lipase (ATGL), the rate-limiting step in TG breakdown, has recently been demonstrated to reverse the loss of adipose in seipin-knockout mice [81].
Similarly, a recent study in which seipin was knocked out of mature brown cells showed that lipid droplets were rapidly lost, mitochondrial structure and function were disrupted, the brown adipose tissue was subjected to inflammation, and the cells underwent necroptosis [82]. This is consistent with the loss of brown adipose tissue after birth in global seipin knockout mice [26]. The loss of mitochondrial activity may also be exacerbated by low mitochondrial calcium levels in seipin null cells [29].
While a massive increase in lipolysis can account for the disappearance of both white and brown adipose in seipin-null animals, the mechanism by which seipin controls lipolysis and its stimulation through PKA signaling is unknown. We offer a hypothesis shown in Figure 2 to explain this connection and the failure of adipose to thrive in the absence of seipin.
Figure 2: An explanation of runaway lipolysis.
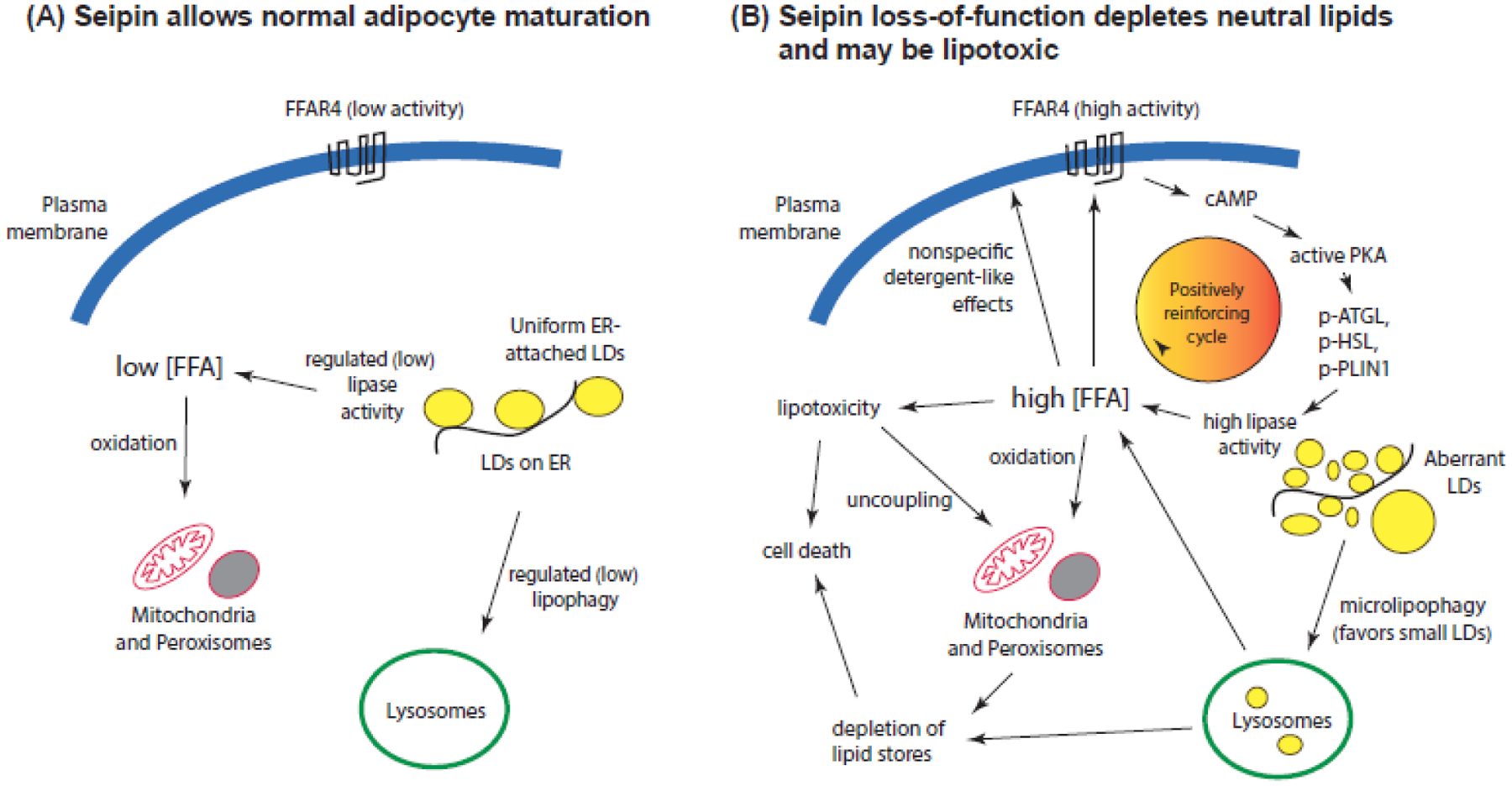
Left: As lipogenesis proceeds in wild type cells, lipase activity is low favoring fat storage, promoting adipocyte maturation. Right: In seipin-deficient cells, lipase activity is stimulated which could lead to an increase in free fatty acids (FFA). The small lipid droplets seen in seipin-null cells may promote microlipophagy, as larger droplets are poor cargo, contributing to the pool of free fatty acids. These can promote cAMP production through several pathways, including FFAR4, which would further stimulate lipolysis. The high concentration of fatty acids could cause lipotoxicity and death of the cells. Note that FFA levels and lipotoxicity have yet to be assessed in seipin-deficient adipocytes. p-ATGL, p-HSL, and p-PLIN1: phosphorylated adipose triglyceride lipase, hormone-sensitive lipase, and perilipin 1, respectively.
In normal adipocytes and other cells (Figure 2, left panel), lipid droplets exist to support energy homeostasis, serving as a depot for neutral lipids and LD-surface proteins. Lipolysis is tightly regulated, such that the steady-state concentration of free fatty acids (FFA) is low. Released FFA can be used for oxidation in mitochondria and peroxisomes to provide energy, or they can be reacylated for phospholipid or neutral lipid synthesis (not shown). In contrast, lipid droplets generated in the absence of seipin are heterogenous in size, typically with many tiny droplets and some supersized ones [12, 14] (right panel). Small lipid droplets are the favored substrates for macrolipophagy, which may partially account for the loss of droplets [83, 84]. On the other hand, the large increase in surface area of small droplets may attract lipases, which would account for the higher basal lipase activity in seipin-null cells.
An increase in lipolysis in seipin-null cells should raise, at least transiently, the FFA concentration. Mitochondrial oxidation may not fully compensate if FFA levels are high, especially if mitochondria are compromised by low calcium levels [29]. Excess free fatty acids will diffuse into membranes including the plasma membrane, where they can both directly and indirectly (through detergent-like effects) stimulate adenylyl cyclase [85, 86] , producing cAMP to activate PKA. Moreover, adipocytes and other cells contain fatty acid-activated receptors (FFARs) such as FFAR4, which is high in adipose, that can directly stimulate adenylyl cyclase [87, 88]. FFA-stimulated cAMP production would form a positively reinforcing loop that would cause the runaway lipolysis observed in seipin-null adipocytes. Furthermore, the FFA released by lipolysis may also cause lipotoxicity, including mitochondrial uncoupling and damage [89]. Lipotoxicity caused by saturated fatty acids are particularly toxic [4, 90], and these are elevated in cell lines from seipin lipodystrophy patients [91]. Moreover, as neutral lipid stores provide the ability of cells to detoxify fatty acids [4], depletion of these stores would add to the toxicity. We hypothesize that lipotoxicity, which has yet to be measured in relation to seipin, contributes to the cell death seen in brown fat tissue in seipin-null animals and may play a role in the function of other cells as well, as virtually all eukaryotic cells, including stem cells, contain lipid droplets [92, 93].
Seipin lipodystrophy extends beyond effects on adipose
Loss of adipose in patients with seipin loss-of-function alleles results in low concentration of leptin, adiponectin, and presumably other adipokines which interact with the hypothalamus and other tissues to directly or indirectly control metabolic activities [94]. Patients not only have scarce adipose tissue but also significant fat storage in liver and muscle, splenomegaly, advanced bone age, disrupted brain functions, metabolic disease including diabetes, and low fertility. Involvement of so many organs might explain Berardinelli’s first report of children with a syndrome (later labeled as lipodystrophy and found to be caused by lack of seipin), in which he did not even mention changes in adipose tissue [95]! Adipose-specific knockout in mice recapitulates the loss of adipose and fatty liver and some metabolic defects, but they are not as severe as a global knockout, suggesting tissue-specific effects outside of the adipose compartment [28, 30, 96], and a recent knockout in both liver and adipose rule out the liver for many effects [97]. Patients with seipin loss-of-function mutations typically have severe neurological issues including neurodegeneration even in the absence of lipodystrophy [98], and several recent studies are concentrated on these. A neuron-specific seipin knockout shows effects on behavior and motor coordination [99, 100], and an increase in inflammation [101]. Animals lack proliferation of neuronal stem cells [102] and display an increase in tau protein phosphorylation [103], both of which could account for the neurological symptoms displayed in patients. These papers, demonstrating severe effects from a neuron-specific seipin knockout, implicate the presence and importance of LDs in neurons, even though they are seldomly observed there, perhaps due to a fast turnover rate, and therefore relatively unstudied in this cell type. Seipin is abundant in neural tissue [63], and mutations that prevent glycosylation and result in seipin aggregation are usually manifest as motor neuropathies [104]. Along this line, the concentration of wild type seipin increases in a mouse Parkinson’s Disease model, and suppression of seipin results in amelioration of disease [105]. Thus, seipin can be a curse as well as a blessing.
Concluding Remarks
As our knowledge of the roles of lipid droplets has expanded [1], so has interest into the basic mechanisms of droplet assembly and maintenance. Both ER lipids and proteins are involved in these processes but the requirement for proteinaceous machinery in assembly and maintenance for all cellular lipid droplets under physiological conditions remains uncertain. Answering this question will be technically difficult, but one possible avenue to resolve this issue would be to interrogate cells such as enterocytes taken from an organism in fasted and fed states for proximity of proteins such as seipin to all newly formed lipid droplets.
Establishing the molecular function of seipin (Figure 3) is of prime concern in the field and we have outlined progress made in this review. How exactly does the protein work to facilitate droplet formation? An atomic structure of the entire protein, not just the luminal domain, would be informative as the transmembrane regions may be key to gating entry of lipids and proteins. Is the major function for assembly the attraction and accumulation of triacylglycerol monomers, as the recent modeling studies strongly suggest [24, 25]? Does this extend to other neutral lipids? There have been no reports of the reconstitution of seipin into bilayers, although attempts at this are certainly underway. Is seipin sufficient in this context, or do other proteins, such as LDAF [43–46] or perilipin [49, 50] greatly enhance activity in purified systems. A reconstituted system involving seipin and other relevant proteins would greatly accelerate progress and should be technically feasible.
Figure 3 (Key Figure): Demonstrated and other possible roles of seipin.
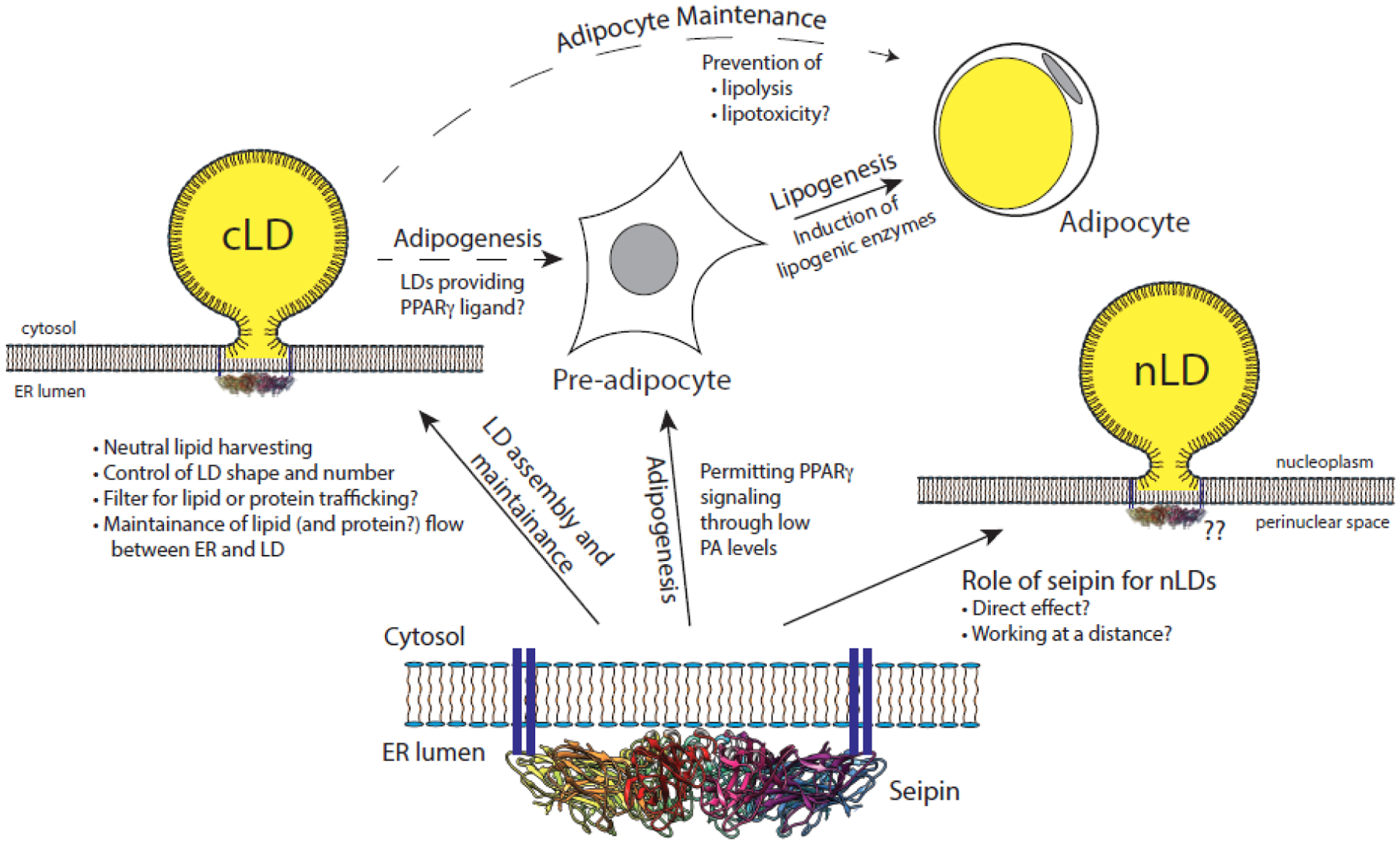
Seipin (at bottom) is necessary for controlling cytoplasmic lipid droplet assembly and maintenance (at left). Its role in nuclear lipid droplets may be direct or indirect (at right). Seipin is also essential for adipogenesis, where PPARγ signaling is disrupted. This likely involves suppression of phosphatidic acid (PA) by inhibiting glycerol phosphate acyltransferase. We imagine it could also involve release of a PPARγ ligand from lipid droplets to induce pre-adipocytes from mesenchymal stem cells. Some lipogenesis occurs even in the absence of seipin but it is not sustained, due in part to lipolysis. Seipin maintains low lipolysis in nascent adipocytes, and we imagine that the low concentration of released free fatty acids prevents lipotoxicity and promotes the health of these cells (at top, cf. Fig. 2 for details).
The mechanisms and physiological importance of seipin binding partners also need clarification. Binding to LDAF is conserved from yeast to humans and its role has been reported by several groups [43–46]. Binding to SERCA was reported as the primary mechanism of seipin for lipid droplet production, and effects on calcium flux are persuasive [18], but more work needs to be done, in our opinion, to reconcile this model with a more direct role at the ER/LD junction. Similarly, the notion of seipin as a scaffold for lipid biosynthetic enzymes or lipid intermediates is an attractive one (building droplets at the sign of lipid synthesis, or negatively regulating activities to adjust to the LD rate of synthesis) [19, 20, 23, 73]. With the availability of seipin cryo-EM structures, it should be feasible to identify binding surfaces with other proteins and make the appropriate mutants to directly test or confirm such models in cell-free systems and in vivo.
Finally, relating the role of seipin in LD dynamics to its suppression of lipodystrophy, with the accompanying pathologies, is likely not explained completely by suppression of GPAT activity and low PA levels [19]. Our idea that increased FFA levels accounts for both the runaway lipolysis and cell death of at least brown adipocytes links aberrant LDs to lipodystrophy and may be worthy of investigation. This pathway could extend to non-adipose tissue as well but would not be universal as some non-adipose tissues show lower lipolysis without seipin [71, 94]. Seipin-null adipocytes may be a suitable first model to look for high FFA levels and lipotoxicity.
The biological importance of lipid droplets, the complexity of generalized lipodystrophy, and the beautiful symmetric structure of seipin continue to inspire work in the field. Considering present efforts, we expect to understand their relationship in the next several years.
Box: Highlights.
Cytoplasmic lipid droplets (cLDs), assembled in the ER, store energy and perform other functions. Recent work in model systems shows the importance of membrane lipid determinants and shape in droplet formation.
Seipin, the molecular role of which has been unclear, facilitates cLD formation. Seipin forms oligomeric rings in the ER, and new data have revealed that triacylglycerol accumulates within them, probably an initial step in cLD formation.
Lipid droplets also appear in the nucleus, a process often mediated by PML bodies. Seipin affects the density of nuclear LDs, but there is no consensus regarding mechanism.
Humans lacking seipin develop severe lipodystrophy. While phosphatidic acid may be important for disrupting adipogenesis, other factors must be involved as adipocytes fail to thrive. Other organs are affected by seipin lipodystrophy as well, and mechanisms are being worked out with tissue-specific seipin knockout animals. A new hypothesis is presented that involves lipolysis and lipotoxicity to link poor lipid droplets generated without seipin to lipodystrophy.
Acknowledgements
Many important contributions could not be included in this review due to limitations on references, and the authors apologize for omissions. This work was supported by NIH grant GM084210.
Box: Glossary
- Adipogenesis
The process by which stem cells are committed to differentiate into adipocytes. It involves a transcriptional cascade in which PPARγ is the master regulator
- FFARs, FFAR4
Free fatty acid receptors, a family of G protein-coupled receptors belonging to the rhodopsin family that respond to fatty acids. FFAT4 is abundant in adipose and participates in suppressing anti-inflammatory responses and insulin sensitizing
- GPAT3
Mammalian glycerol-3-phosphate acyltransferase in the ER, the initial step of de novo glycerolipid synthesis that converts glycerol-3-phosphate to lysophosphatidic acid. Activation of GPAT leads to an increase in phosphatidic acid, implicated in seipin lipodystrophy
- Lipid droplet (LD, cLDs, nLD)
An organelle containing stored neutral lipids, mainly fatty acids stored as esters of triacylglycerol and sterol, which are enwrapped in a phospholipid monolayer derived from the ER. Lipid droplets are usually cytoplasmic (cLDs) but also are in the nucleus (nLDs) in some tissues and cells
- Lipodystrophy
A condition in mammals characterized by progressive loss or redistribution of adipose. The most severe form of human lipodystrophy, in which nearly all adipose is absent, is caused by a loss-of-function of seipin in the germ line
- Lipolysis
The general term used for enzymatic hydrolysis of lipids, often liberating free fatty acids
- Nucleoplasmic reticulum (NR)
An extension of the inner nuclear membrane (INM) that extends in sheets or tubules from the nuclear envelop into the deep interior of the nucleoplasm. The space within the NR is continuous with the perinuclear space, which in turn is continuous with the ER lumen
- PA
phosphatidic acid, a key highly regulated branch point in glycerolipid metabolism, which can give rise to both phospholipid and triacylglycerol synthesis
- PML bodies
Promyelocytic leukemia bodies, nuclear non-membrane-bound organelles that control many aspects of chromatin dynamics and interact with nuclear lipid droplets
- PPARγ
Peroxisome proliferator-activated receptor gamma, the transcriptional master regulator of adipogenesis
- Seipin
A transmembrane protein in the ER that forms oligomeric rings and is localized to ER/LD junctions. The seipin gene is mutated in severe cases of human lipodystrophy (loss-of-function) and in seipinopathies (gain-of-function)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
References
- 1.Olzmann JA and Carvalho P (2019) Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol 20 (3), 137–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welte MA and Gould AP (2017) Lipid droplet functions beyond energy storage. Biochimica Et Biophysica Acta-Molecular and Cell Biology of Lipids 1862 (10), 1260–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thiam AR and Beller M (2017) The why, when and how of lipid droplet diversity. J Cell Sci 130 (2), 315–324. [DOI] [PubMed] [Google Scholar]
- 4.Petschnigg J et al. (2009) Good fat, essential cellular requirements for triacylglycerol synthesis to maintain membrane homeostasis in yeast. J Biol Chem 284 (45), 30981–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao Q and Goodman JM (2015) The lipid droplet-a well-connected organelle. Front Cell Dev Biol 3, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herker E et al. (2021) Lipid Droplet Contact Sites in Health and Disease. Trends Cell Biol. [DOI] [PubMed] [Google Scholar]
- 7.Soltysik K et al. (2019) Nuclear lipid droplets derive from a lipoprotein precursor and regulate phosphatidylcholine synthesis. Nat Commun 10 (1), 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thiam AR and Ikonen E (2021) Lipid Droplet Nucleation. Trends Cell Biol 31 (2), 108–118. [DOI] [PubMed] [Google Scholar]
- 9.Lundin C et al. (2006) Membrane topology of the human seipin protein. FEBS Lett 580 (9), 2281–4. [DOI] [PubMed] [Google Scholar]
- 10.Windpassinger C et al. (2004) Heterozygous missense mutations in BSCL2 are associated with distal hereditary motor neuropathy and Silver syndrome. Nat Genet 36 (3), 271–6. [DOI] [PubMed] [Google Scholar]
- 11.Salo VT et al. (2016) Seipin regulates ER-lipid droplet contacts and cargo delivery. EMBO J 35 (24), 2699–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szymanski KM et al. (2007) The lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology. Proc Natl Acad Sci U S A 104 (52), 20890–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fei W et al. (2008) Fld1p, a functional homologue of human seipin, regulates the size of lipid droplets in yeast. J Cell Biol 180 (3), 473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolinski H et al. (2011) A role for seipin in lipid droplet dynamics and inheritance in yeast. J Cell Sci 124 (Pt 22), 3894–904. [DOI] [PubMed] [Google Scholar]
- 15.Cartwright BR et al. (2015) Seipin performs dissectible functions in promoting lipid droplet biogenesis and regulating droplet morphology. Mol Biol Cell 26 (4), 726–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolinski H et al. (2015) Seipin is involved in the regulation of phosphatidic acid metabolism at a subdomain of the nuclear envelope in yeast. Biochim Biophys Acta 1851 (11), 1450–64. [DOI] [PubMed] [Google Scholar]
- 17.Fei W et al. (2011) A role for phosphatidic acid in the formation of “supersized” lipid droplets. PLoS Genet 7 (7), e1002201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bi JF et al. (2014) Seipin Promotes Adipose Tissue Fat Storage through the ER Ca2+-ATPase SERCA. Cell Metabolism 19 (5), 861–871. [DOI] [PubMed] [Google Scholar]
- 19.Pagac M et al. (2016) SEIPIN Regulates Lipid Droplet Expansion and Adipocyte Development by Modulating the Activity of Glycerol-3-phosphate Acyltransferase. Cell Reports 17 (6), 1546–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talukder MMU et al. (2015) Seipin oligomers can interact directly with AGPAT2 and lipin 1, physically scaffolding critical regulators of adipogenesis. Molecular Metabolism 4 (3), 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang WL et al. (2014) BSCL2/seipin regulates adipogenesis through actin cytoskeleton remodelling. Human Molecular Genetics 23 (2), 502–513. [DOI] [PubMed] [Google Scholar]
- 22.Sui XW et al. (2018) Cryo-electron microscopy structure of the lipid droplet-formation protein seipin. Journal of Cell Biology 217 (12), 4080–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan RH et al. (2018) Human SEIPIN Binds Anionic Phospholipids. Developmental Cell 47 (2), 248-+. [DOI] [PubMed] [Google Scholar]
- 24.Prasanna X et al. (2021) Seipin traps triacylglycerols to facilitate their nanoscale clustering in the endoplasmic reticulum membrane. PLoS Biol 19 (1), e3000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zoni V et al. (2021) Seipin accumulates and traps diacylglycerols and triglycerides in its ring-like structure. Proc Natl Acad Sci U S A 118 (10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui X et al. (2011) Seipin ablation in mice results in severe generalized lipodystrophy. Hum Mol Genet 20 (15), 3022–30. [DOI] [PubMed] [Google Scholar]
- 27.Patni N and Garg A (2015) Congenital generalized lipodystrophies-new insights into metabolic dysfunction. Nature Reviews Endocrinology 11 (9), 522–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu L et al. (2014) Adipose-specific knockout of SEIPIN/BSCL2 results in progressive lipodystrophy. Diabetes 63 (7), 2320–31. [DOI] [PubMed] [Google Scholar]
- 29.Ding L et al. (2018) Seipin regulates lipid homeostasis by ensuring calcium-dependent mitochondrial metabolism. EMBO J 37 (17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou H et al. (2015) Berardinelli-Seip congenital lipodystrophy 2 regulates adipocyte lipolysis, browning, and energy balance in adult animals. J Lipid Res 56 (10), 1912–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamilton JA (1989) Interactions of Triglycerides with Phospholipids - Incorporation into the Bilayer Structure and Formation of Emulsions. Biochemistry 28 (6), 2514–2520. [DOI] [PubMed] [Google Scholar]
- 32.Khandelia H et al. (2010) Triglyceride blisters in lipid bilayers: implications for lipid droplet biogenesis and the mobile lipid signal in cancer cell membranes. PLoS One 5 (9), e12811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choudhary V et al. (2015) A conserved family of proteins facilitates nascent lipid droplet budding from the ER. J Cell Biol 211 (2), 261–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang HJ et al. (2016) Seipin is required for converting nascent to mature lipid droplets. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chorlay A et al. (2019) Membrane Asymmetry Imposes Directionality on Lipid Droplet Emergence from the ER. Dev Cell 50 (1), 25–42 e7. [DOI] [PubMed] [Google Scholar]
- 36.Santinho A et al. (2021) Fat inclusions strongly alter membrane mechanics. Biophys J 120 (4), 607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santinho A et al. (2020) Membrane Curvature Catalyzes Lipid Droplet Assembly. Curr Biol 30 (13), 2481–2494 e6. [DOI] [PubMed] [Google Scholar]
- 38.Zoni V et al. (2021) Pre-existing bilayer stresses modulate triglyceride accumulation in the ER versus lipid droplets. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chorlay A and Thiam AR (2018) An Asymmetry in Monolayer Tension Regulates Lipid Droplet Budding Direction. Biophys J 114 (3), 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adeyo O et al. (2011) The yeast lipin orthologue Pah1p is important for biogenesis of lipid droplets. J Cell Biol 192 (6), 1043–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choudhary V et al. (2020) Seipin and Nem1 establish discrete ER subdomains to initiate yeast lipid droplet biogenesis. J Cell Biol 219 (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hariri H et al. (2018) Lipid droplet biogenesis is spatially coordinated at ER-vacuole contacts under nutritional stress. EMBO Rep 19 (1), 57–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chung J et al. (2019) LDAF1 and Seipin Form a Lipid Droplet Assembly Complex. Dev Cell 51 (5), 551–563 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eisenberg-Bord M et al. (2018) Identification of seipin-linked factors that act as determinants of a lipid droplet subpopulation. J Cell Biol 217 (1), 269–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teixeira V et al. (2018) Regulation of lipid droplets by metabolically controlled Ldo isoforms. J Cell Biol 217 (1), 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castro IG et al. (2019) Promethin Is a Conserved Seipin Partner Protein. Cells 8 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joshi AS et al. (2018) Lipid droplet and peroxisome biogenesis occur at the same ER subdomains. Nat Commun 9 (1), 2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang S et al. (2018) Seipin and the membrane-shaping protein Pex30 cooperate in organelle budding from the endoplasmic reticulum. Nat Commun 9 (1), 2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao Q et al. (2017) Pet10p is a yeast perilipin that stabilizes lipid droplets and promotes their assembly. J Cell Biol 216 (10), 3199–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takahashi Y et al. (2013) Perilipin-mediated lipid droplet formation in adipocytes promotes sterol regulatory element-binding protein-1 processing and triacylglyceride accumulation. PLoS One 8 (5), e64605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hariri H et al. (2019) Mdm1 maintains endoplasmic reticulum homeostasis by spatially regulating lipid droplet biogenesis. J Cell Biol 218 (4), 1319–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salo VT et al. (2019) Seipin Facilitates Triglyceride Flow to Lipid Droplet and Counteracts Droplet Ripening via Endoplasmic Reticulum Contact. Dev Cell 50 (4), 478–493 e9. [DOI] [PubMed] [Google Scholar]
- 53.Layerenza JP et al. (2013) Nuclear lipid droplets: a novel nuclear domain. Biochim Biophys Acta 1831 (2), 327–40. [DOI] [PubMed] [Google Scholar]
- 54.Wang L et al. (2013) Specific accumulation of lipid droplets in hepatocyte nuclei of PFOA-exposed BALB/c mice. Sci Rep 3, 2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soltysik K et al. (2018) Duo in a mystical realm — nuclear lipid droplets and the inner nuclear membrane. Contact (Thousand Oaks) 2, 1–11. [Google Scholar]
- 56.Ohsaki Y et al. (2016) PML isoform II plays a critical role in nuclear lipid droplet formation. J Cell Biol 212 (1), 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee J et al. (2020) Lipid-associated PML structures assemble nuclear lipid droplets containing CCTalpha and Lipin1. Life Sci Alliance 3 (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bryant JM and Blind RD (2019) Signaling through non-membrane nuclear phosphoinositide binding proteins in human health and disease. J Lipid Res 60 (2), 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.York JD (2006) Regulation of nuclear processes by inositol polyphosphates. Biochim Biophys Acta 1761 (5–6), 552–9. [DOI] [PubMed] [Google Scholar]
- 60.Soltysik K et al. (2021) Nuclear lipid droplets form in the inner nuclear membrane in a seipin-independent manner. J Cell Biol 220 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han S et al. (2015) Dissecting seipin function: the localized accumulation of phosphatidic acid at ER/LD junctions in the absence of seipin is suppressed by Sei1p(DeltaNterm) only in combination with Ldb16p. BMC Cell Biol 16, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Romanauska A and Kohler A (2018) The Inner Nuclear Membrane Is a Metabolically Active Territory that Generates Nuclear Lipid Droplets. Cell 174 (3), 700–715 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Magre J et al. (2001) Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat Genet 28 (4), 365–70. [DOI] [PubMed] [Google Scholar]
- 64.Agarwal AK and Garg A (2006) Genetic basis of lipodystrophies and management of metabolic complications. Annu Rev Med 57, 297–311. [DOI] [PubMed] [Google Scholar]
- 65.Rosen ED and MacDougald OA (2006) Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 7 (12), 885–96. [DOI] [PubMed] [Google Scholar]
- 66.Chen W et al. (2009) The human lipodystrophy gene product Berardinelli-Seip congenital lipodystrophy 2/seipin plays a key role in adipocyte differentiation. Endocrinology 150 (10), 4552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Payne VA et al. (2008) The human lipodystrophy gene BSCL2/seipin may be essential for normal adipocyte differentiation. Diabetes 57 (8), 2055–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim YG et al. (2011) A metabolomics strategy for detecting protein-metabolite interactions to identify natural nuclear receptor ligands. Mol Biosyst 7 (4), 1046–9. [DOI] [PubMed] [Google Scholar]
- 69.Su WC et al. (2019) Seipin negatively regulates sphingolipid production at the ER-LD contact site. J Cell Biol 218 (11), 3663–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bai X et al. (2020) Loss of the seipin gene perturbs eggshell formation in Caenorhabditis elegans. Development 147 (20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tian Y et al. (2011) Tissue-autonomous function of Drosophila seipin in preventing ectopic lipid droplet formation. PLoS Genet 7 (4), e1001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu W et al. (2019) Novel metabolic disorders in skeletal muscle of Lipodystrophic Bscl2/Seipin deficient mice. Mol Cell Endocrinol 482, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sim MF et al. (2012) The human lipodystrophy protein seipin is an ER membrane adaptor for the adipogenic PA phosphatase lipin 1. Mol Metab 2 (1), 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sim MFM et al. (2020) Oligomers of the lipodystrophy protein seipin may co-ordinate GPAT3 and AGPAT2 enzymes to facilitate adipocyte differentiation. Sci Rep 10 (1), 3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen W et al. (2012) Berardinelli-seip congenital lipodystrophy 2/seipin is a cell-autonomous regulator of lipolysis essential for adipocyte differentiation. Mol Cell Biol 32 (6), 1099–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seale P et al. (2008) PRDM16 controls a brown fat/skeletal muscle switch. Nature 454 (7207), 961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou H et al. (2016) Berardinelli-Seip Congenital Lipodystrophy 2/Seipin Is Not Required for Brown Adipogenesis but Regulates Brown Adipose Tissue Development and Function. Mol Cell Biol 36 (15), 2027–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dollet L et al. (2016) Seipin deficiency alters brown adipose tissue thermogenesis and insulin sensitivity in a non-cell autonomous mode. Sci Rep 6, 35487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prieur X et al. (2013) Thiazolidinediones partially reverse the metabolic disturbances observed in Bscl2/seipin-deficient mice. Diabetologia 56 (8), 1813–25. [DOI] [PubMed] [Google Scholar]
- 80.Duncan RE et al. (2007) Regulation of lipolysis in adipocytes. Annu Rev Nutr 27, 79–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou H et al. (2019) Targeting ATGL to rescue BSCL2 lipodystrophy and its associated cardiomyopathy. JCI Insight 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou H et al. (2020) Berardinelli-Seip congenital lipodystrophy 2/SEIPIN determines brown adipose tissue maintenance and thermogenic programing. Mol Metab 36, 100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schott MB et al. (2019) Lipid droplet size directs lipolysis and lipophagy catabolism in hepatocytes. J Cell Biol 218 (10), 3320–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schulze RJ et al. (2020) Direct lysosome-based autophagy of lipid droplets in hepatocytes. Proc Natl Acad Sci U S A 117 (51), 32443–32452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Perkins JP and Moore MM (1971) Adenyl cyclase of rat cerebral cortex. Activation of sodium fluoride and detergents. J Biol Chem 246 (1), 62–8. [PubMed] [Google Scholar]
- 86.Resnick RJ and Tomaska L (1994) Stimulation of yeast adenylyl cyclase activity by lysophospholipids and fatty acids. Implications for the regulation of Ras/effector function by lipids. J Biol Chem 269 (51), 32336–41. [PubMed] [Google Scholar]
- 87.Grundmann M et al. (2021) Pharmacology of Free Fatty Acid Receptors and Their Allosteric Modulators. Int J Mol Sci 22 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hilgendorf KI et al. (2019) Omega-3 Fatty Acids Activate Ciliary FFAR4 to Control Adipogenesis. Cell 179 (6), 1289–1305 e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maassen JA et al. (2007) Fatty acid-induced mitochondrial uncoupling in adipocytes as a key protective factor against insulin resistance and beta cell dysfunction: a new concept in the pathogenesis of obesity-associated type 2 diabetes mellitus. Diabetologia 50 (10), 2036–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Piccolis M et al. (2019) Probing the Global Cellular Responses to Lipotoxicity Caused by Saturated Fatty Acids. Mol Cell 74 (1), 32–44 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boutet E et al. (2009) Seipin deficiency alters fatty acid Delta9 desaturation and lipid droplet formation in Berardinelli-Seip congenital lipodystrophy. Biochimie 91 (6), 796–803. [DOI] [PubMed] [Google Scholar]
- 92.Hershey BJ et al. (2019) Lipid Droplets Define a Sub-Population of Breast Cancer Stem Cells. J Clin Med 9 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tirinato L et al. (2015) Lipid droplets: a new player in colorectal cancer stem cells unveiled by spectroscopic imaging. Stem Cells 33 (1), 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Craveiro Sarmento AS et al. (2018) Exploring Seipin: From Biochemistry to Bioinformatics Predictions. Int J Cell Biol 2018, 5207608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Berardinelli W (1954) An undiagnosed endocrinometabolic syndrome: report of 2 cases. J Clin Endocrinol Metab 14 (2), 193–204. [DOI] [PubMed] [Google Scholar]
- 96.McIlroy GD et al. (2018) Adipose specific disruption of seipin causes early-onset generalised lipodystrophy and altered fuel utilisation without severe metabolic disease. Mol Metab 10, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McIlroy GD et al. (2020) Ablation of Bscl2/seipin in hepatocytes does not cause metabolic dysfunction in congenital generalised lipodystrophy. Dis Model Mech 13 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guillen-Navarro E et al. (2013) A new seipin-associated neurodegenerative syndrome. J Med Genet 50 (6), 401–9. [DOI] [PubMed] [Google Scholar]
- 99.Wang L et al. (2018) Seipin deficiency in mice causes loss of dopaminergic neurons via aggregation and phosphorylation of alpha-synuclein and neuroinflammation. Cell Death Dis 9 (5), 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhou L et al. (2014) Lack of seipin in neurons results in anxiety- and depression-like behaviors via down regulation of PPARgamma. Hum Mol Genet 23 (15), 4094–102. [DOI] [PubMed] [Google Scholar]
- 101.Qian Y et al. (2016) Neuronal seipin knockout facilitates Abeta-induced neuroinflammation and neurotoxicity via reduction of PPARgamma in hippocampus of mouse. J Neuroinflammation 13 (1), 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li G et al. (2015) Seipin knockout in mice impairs stem cell proliferation and progenitor cell differentiation in the adult hippocampal dentate gyrus via reduced levels of PPARgamma. Dis Model Mech 8 (12), 1615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chang H et al. (2019) Seipin deletion in mice enhances phosphorylation and aggregation of tau protein through reduced neuronal PPARgamma and insulin resistance. Neurobiol Dis 127, 350–361. [DOI] [PubMed] [Google Scholar]
- 104.Ito D and Suzuki N (2009) Seipinopathy: a novel endoplasmic reticulum stress-associated disease. Brain 132 (Pt 1), 8–15. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Y et al. (2017) Echinacoside’s nigrostriatal dopaminergic protection against 6-OHDA-Induced endoplasmic reticulum stress through reducing the accumulation of Seipin. J Cell Mol Med 21 (12), 3761–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]


