Abstract
Objective:
We present findings from the nationally representative Zimbabwe Population-based HIV Impact Assessment (ZIMPHIA) that characterize Zimbabwe’s progress toward the Joint United Nations Programme on HIV/AIDS 90-90-90 targets.
Design:
We conducted a cross-sectional household survey.
Methods:
Consenting adults and children in the household were eligible to participate in ZIMPHIA (October 2015–August 2016). Participants completed face-to-face interviews and provided blood for HIV, CD4, viral load, and syphilis testing. VLS was defined as HIV RNA <1,000 copies/mL. HIV-positive specimens were tested for the presence of selected antiretroviral drugs. Data were weighted. Analysis was restricted to HIV-positive adults aged 15–64 years.
Results:
We enrolled 11,098 men and 14,033 women aged 15–64 years. HIV prevalence was 14.1%. Of those living with HIV, 76.8% (95% confidence interval [CI]: 74.9–78.7) were aware of their HIV status or had detectable antiretroviral levels. Of these, 88.4% (95% CI: 87.1–89.7) were receiving ART, and of these people, 85.3% (95% CI: 83.4–87.1) had VLS. Male sex age 15–34 years and having one or more sexual partners were associated with being unaware of one’s HIV-positive status. Age <50 years and not taking cotrimoxazole were associated with being less likely to be being both aware and taking ART. Male sex, age <50 years, and taking cotrimoxazole were associated with being on ART but not having VLS.
Conclusions:
Zimbabwe has made great strides toward epidemic control. Focusing resources on case finding, particularly among men, people aged<35 years, and sexually active individuals can help Zimbabwe attain 90-90-90 targets.
Keywords: Zimbabwe, 90-90-90, awareness, treatment coverage, viral suppression
INTRODUCTION
In 2014, the Joint United National Programme on HIV and AIDS (UNAIDS) introduced the 90-90-90 targets to help end the global HIV epidemic 1. These targets, which refer to the HIV care continuum, call for 90% of people living with HIV (PLHIV) to know their status; of these, 90% are receiving antiretroviral therapy (ART); and of these, 90% have viral load suppression (VLS) by 2020. Once achieved, these percentages must be sustained for 10 years to decrease the annual number of new HIV infections and AIDS-related deaths by 90% and 80%, respectively 1. The targets serve as both a call to action and a roadmap to end the AIDS public health crisis.
With 1.3 million PLHIV and an adult (age 15–64) HIV prevalence of 12.7% in 2018, Zimbabwe is one of the countries most deeply affected by the HIV epidemic 2. Meeting the 90-90-90 targets for epidemic control is a national priority, exemplified by the government of Zimbabwe’s commitment to the HIV response and the adoption in 2016 of the test and start policy for HIV treatment 3,4. Accordingly, in partnership with global partners including the U.S. President’s Emergency Plan for AIDS Relief, HIV treatment and prevention activities have been scaled up rapidly in the last decade. Programmatic data from public health facilities suggest that both the number of HIV tests performed and the coverage of HIV treatment have increased steadily over the last 10 years with 2.8 million tests performed and 1.1 million people receiving ART as of 2017 5.
However, health facility data are insufficient to quantify progress toward the 90-90-90 targets because they do not include PLHIV who are not engaged with the health system 6. Without knowing the total denominator of people at each step of the cascade, it is impossible to estimate progress toward the 90-90-90 targets. Furthermore, developing programs to reach those currently not accessing HIV services requires identifying factors associated with being unaware of HIV status, not receiving ART, or not having VLS.
Several analyses have explored factors associated with HIV testing in Zimbabwe using data from the Demographic and Health Surveys (DHS); however, because these analyses lack general population data on the second and third targets, they cannot identify factors associated with receiving ART and/or having VLS 7,8. Other analyses use a combination of DHS and program data to model data relevant to the 90-90-90 targets and unreached populations 9,10.
Tafuma et al. used qualitative methods to identify several barriers to HIV service utilization in two provinces of Zimbabwe: health system-related barriers, such as user fees and wait times, and community factors, such as stigma, counterproductive religious messaging, and food insecurity 11. They note that men are inadequately engaged in HIV services. The authors also captured participants’ concern that some patients may discontinue treatment once symptoms improve, suggesting a potential gap in services.
To our knowledge, no previous studies have directly estimated progress toward the 90-90-90 targets and the factors associated with each of the targets at a nationally representative level in Zimbabwe. This is most likely because representative population-level data on both the numerator and denominator for each pillar of the 90-90-90 targets have not previously been available. The Zimbabwe Population-based HIV Impact Assessment (ZIMPHIA; October 2015–August 2016) was the first nationally representative household survey to measure the indicators required to benchmark Zimbabwe’s progress toward the 90-90-90 goals and characterize the population that still needs to be reached. We analyzed ZIMPHIA data to identify the sociodemographic and biological correlates of meeting each of the 90-90-90 targets.
METHODS
Sampling
ZIMPHIA used a two-stage stratified cluster sampling design to select participants. In the first stage, 499 enumeration areas (EA) were selected from 10 strata using the 2012 Zimbabwe Population Census with probability proportional to size. In the second stage, an average of 30 households (range, 15–60) were randomly selected with equal probability from each EA. The sample of households was selected to provide representative provincial estimates of VLS among PLHIV aged 15–64 years with 95% confidence intervals (CI) ± 0.068. The target sample size was 21,257 men and women aged ≥15 years selected from 15,000 households.
Eligibility
The heads of selected households were asked to provide written informed consent to participate in a household interview and list household members. Individuals living in the household and visitors who slept there the night before the survey were eligible to participate in the survey if they were aged ≥16 years or were a 15-year-old emancipated minor and provided written informed consent in English, Shona, or Ndebele. Non-emancipated minors who were similarly staying in the household were required to provide written assent in one of the three languages and obtain written permission from a parent or guardian. Consent was obtained electronically on tablet computers for participation in a face-to-face interview. Following the interview, consent was obtained for blood collection and biomarker testing.
Survey procedures
Participants were interviewed by a trained interviewer using an electronic questionnaire programmed in Open Data Kit. Interview domains included demographics, alcohol use, sexual behaviors, HIV knowledge and attitudes, reproductive health, and uptake of HIV services. After the interview, participants provided written informed consent for biomarker testing. Consenting participants received HIV pre-test counseling according to national guidelines before staff collected 14 mL of venous blood for testing. HIV testing was conducted in the household according to the national serial algorithm of Determine (Alere, Waltham, MA), First Response (Premier Medical Corporation, Maharashtra, India), and Stat-Pak (Chembio, Medford, NY). The DPP Syphilis Screen & Confirm Assay (Chembio, Medford, NY) was used to test for syphilis. HIV and syphilis results were provided the same day to participants. Individuals testing HIV-positive also received same-day CD4 testing and results using the Pima Analyzer and CD4 test (Alere, Waltham, MA). Additionally, a random sample of 5% of HIV-negative participants also received CD4 measurement to mask HIV status from household members.
HIV viral load quantification was conducted at a central laboratory using the COBAS AmpliPrep/COBAS TaqMan System (CAP/CTM) platform and the COBAS AmpliPrep/COBAS TaqMan HIV-1 Test, version 2.0 assay (Indianapolis, IN) using primarily plasma samples. In cases where plasma was not available, dried blood spots (DBS) were tested using a validated protocol. Viral load results were returned to the participant’s health facility where they could be accessed using the participant’s study ID. Participants were notified by SMS when their results were available.
HIV-positive DBS specimens also were tested for the presence of antiretroviral medicines, and qualitative screening for detectable concentrations of antiretrovirals (limit of detection of 20 ng/mL) was conducted on all HIV-positive adult and child samples by means of high-resolution liquid chromatography coupled with tandem mass spectrometry. The method used for antiretroviral drug detection was a modified version of the methodology described by Koal et al. and was carried out by the Division of Clinical Pharmacology of the Department of Medicine at the University of Cape Town in South Africa 12. Three antiretroviral medicines were selected as markers for the most commonly prescribed first- and second-line regimens: efavirenz, nevirapine, and lopinavir. These medicines also were selected based on their relatively long half-lives, allowing for a longer period of detection following intake.
Survey staff were trained to identify and refer all sexually exploited persons younger than 18 years to partner organizations experienced in providing counseling, health, social, and other protective services to these populations.
Data analysis
We restricted this analysis to PLHIV aged 15–64 years. The primary endpoints of interest were the proportion of those with HIV who were unaware of their status, the proportion of those diagnosed who were not receiving ART, and the proportion of those receiving ART who did not have VLS (defined as ≥1,000 copies/mL). Self-reported awareness of HIV status and ART status were modified based on results of testing for the presence of antiretroviral medicines. Individuals were considered aware of their HIV status if they self-reported having an HIV diagnosis, having tested positive for the presence of antiretroviral medicines, or had VLS. Individuals were considered aware and receiving ART if they self-reported receiving ART, tested positive for the presence of antiretroviral medicines, or had VLS.
Data were weighted for survey methods and non-response. We used jackknife replication to estimate variance, and analysis was conducted in SAS (version 9.4; Cary, NC). Variables associated with being outside the 90-90-90 cascade on bivariate analysis at p<0.1 were included in the multivariate model.
Ethical approval
This survey was approved by the Institutional Review Boards (IRB) of the Research Council of Zimbabwe, the Medical Research Council of Zimbabwe (A/1914), WESTAT (IRB# 6317), Columbia University Medical Center (IRB# 6882), and the US Centers for Disease Control and Prevention (CDC; IRB# Y06M00).
Role of the funding source
ZIMPHIA was a PEPFAR initiative. The US Office of the Global AIDS Coordinator and CDC shaped the objectives of our survey. CDC investigators contributed to designing the survey, monitoring data collection, conducting data analysis, and writing this manuscript for publication.
RESULTS
We included data from 11,098 men and 14,033 women aged 15–64 years from 15,009 sampled households. Of these, 83.5% and 94.2% consented to and completed an interview, respectively. Of those who completed an interview, 90.6% of men and 92.2% of women consented to HIV testing. HIV prevalence was estimated at 14.1% and 1,153 men and 2,230 women tested positive for HIV (Table 1). Of those living with HIV, 76.8% (95% CI: 74.9–78.7, n=2,699) were aware of their HIV status (Figure 1). Among those aware of their status, 88.4% (95% CI: 87.1–89.7, n=2,407) were receiving ART, and of these people, 85.3% (95% CI: 83.4–87.1, n=2,085) had VLS. Overall, 42.1% of PLHIV did not have VLS. Geometric mean viral load was 362 (95% CI: 311–422). Among all adults in Zimbabwe, regardless of HIV status, 5.9% (95% CI: 5.5–6.4) have VL≥1,000 copies/mL.
Table 1.
Background characteristics of HIV-positive adults aged 15–64 years in Zimbabwe, 2015–2016
| Characteristic | HIV Positive (N=3,383) | Aware of HIV-positive status (N=2,699) | Aware and on ART (N=2,407) | On ART and have suppressed viral load (N=2,085) |
|---|---|---|---|---|
| n (%) | n (%; 95% CI) | n (%; 95% CI) | n (%; 95% CI) | |
| Total | 3383 | 2699 (76.8; 74.9–78.7) | 2407 (88.4; 87.1–89.7) | 2085 (85.3; 83.4–87.1) |
| Sex | ||||
| Male | 1153 (40.4) | 866 (72.1; 68.9–75.3) | 770 (88.0; 85.5–90.5) | 647 (82.5; 79.1–85.8) |
| Female | 2230 (59.6) | 1833 (80.1; 78.1–82.0) | 1637 (88.6; 87.0–90.3) | 1438 (87.0; 85.1–88.8) |
| Age, years | ||||
| 15–24 | 345 (11.5) | 210 (60.3; 54.2–66.4) | 185 (86.9; 81.5–92.4) | 150 (82.4; 76.4–88.5) |
| 25–34 | 849 (27.3) | 627 (70.0; 66.6–73.4) | 521 (82.1; 79.0–85.3) | 432 (81.5; 77.3–85.6) |
| 35–49 | 1535 (45.3) | 1304 (82.8; 80.5–85.2) | 1164 (89.0; 87.2–90.7) | 1005 (84.8; 81.9–87.6) |
| 50–64 | 654 (16.0) | 558 (83.4; 79.8–87.0) | 537 (96.7; 94.9–98.4) | 498 (92.6; 90.1–95.1) |
| Residence | ||||
| Urban | 1049 (36.8) | 819 (74.9; 71.2–78.6) | 719 (87.4; 85.3–89.4) | 629 (85.6; 82.2–89.1) |
| Rural | 2334 (63.2) | 1880 (78.0; 75.9–80.0) | 1688 (89.0; 87.3–90.7) | 1456 (85.1; 83.0–87.2) |
| Highest level of education completed | ||||
| No education | 116 (2.8) | 94 (80.4; 72.6–88.2) | 89 (94.7; 89.8–99.6) | 79 (89.2; 82.3–96.0) |
| Primary | 1285 (34.1) | 1065 (79.9; 77.3–82.6) | 958 (89.8; 87.7–91.9) | 828 (84.3; 81.2–87.3) |
| Secondary | 1857 (58.5) | 1446 (75.4; 72.9–78.0) | 1275 (87.3; 85.6–89.0) | 1106 (85.8; 83.6–88.1) |
| <Secondary | 124 (4.5) | 93 (69.2; 57.5–80.9) | 84 (87.8; 77.4–98.2) | 72 (83.7; 73.6–93.8) |
| Marital status | ||||
| Never married | 342 (10.7) | 244 (69.0; 63.4–74.6) | 224 (90.7; 86.3–95.2) | 186 (80.9; 74 .9–86 .9) |
| Married or living together | 2008 (61.3) | 1597 (7.6.3; 73.7–78.8) | 1413 (87.8; 86.2–89.5) | 1218 (84.7; 82.2–87.2) |
| Divorced or separated | 435 (12.7) | 337 (74.7; 69.8–79.6) | 289 (85.4; 81.1–89.7) | 247 (85.3; 80.5–90.0) |
| Widowed | 591 (15.4) | 515 (86.2; 83.0–89.4) | 476 (91.5; 88.8–94.2) | 430 (89.7; 86.7–92.7) |
| Lives together with partner | ||||
| Yes | 1636 (69.8) | 1312 (77.6; 74.9–80.2) | 1163 (88.4; 86.5–90.2) | 1005 (85.1; 82.5–87.8) |
| No | 690 (30.2) | 507 (69.7; 65.0–74.4) | 429 (83.3; 79.4–87.1) | 366 (84.0; 79.8–88.1) |
| Religious affiliation | ||||
| Protestant | 535 (15 .5) | 451 (83.5; 79.7–87.4) | 409 (89.6; 86.4–92.9) | 357 (87.0; 83.1–90.9) |
| Roman Catholic | 243 (7.6) | 206 (81.6; 75.7–87.4) | 185 (89.1; 84.0–94.3) | 163 (86.4; 80.1–92.6) |
| Pentecostal | 634 (19.2) | 516 (77.8; 73.3–82.2) | 463 (89.2; 85.7–92.8) | 397 (82.8; 78.2–87.5) |
| Apostolic sect | 1104 (32.1) | 877 (76.9; 73.8–80.0) | 776 (88.1; 85.7–90.6) | 672 (85.6; 82.6–88.6) |
| Other | 410 (10.8) | 313 (73.6; 68.0–79.2) | 284 (90.0; 86.1–93.9) | 246 (84.8; 79.7–90.0) |
| None | 455 (14.8) | 336 (68.8; 63.6–74.0) | 290 (84.7; 80.1–89.2) | 250 (85.6; 80.9–90.3) |
| Worked in the last 12 months | ||||
| Yes | 1257 (39.2) | 954 (73.3; 70.2–76.4) | 833 (86.9; 84.5–89.3) | 720 (85.1; 82.1–88.1) |
| No | 2125 (60.8) | 1744 (79.1; 77.0–81.2) | 1573 (89.3; 87.7–91.0) | 1364 (85.3; 83.1–87.6) |
| Wealth quintile | ||||
| Lowest | 843 (20.4) | 690 (79.4; 75.9–82.9) | 623 (89.7; 86.9–92.6) | 535 (83.9; 80.3–87.5) |
| Second | 679 (18.5) | 540 (76.9; 73.2–80.6) | 497 (90.8; 87.8–93.8) | 431 (86.3; 82.9–89.7) |
| Middle | 624 (19.0) | 496 (77.4; 73.2–81.6) | 434 (87.3; 84.1–90.4) | 370 (83.7; 79.4–88.0) |
| Fourth | 627 (22.3) | 491 (74.5; 69.7–79.4) | 434 (88.1; 85.0–91.1) | 377 (85.6; 81.4–89.8) |
| Highest | 610 (19.7) | 482 (76.2; 71.6–80.8) | 419 (86.2; 83.1–89.4) | 372 (87.0; 83.3–90.6) |
| Been away from home in the last 12 months | ||||
| Yes | 508 (15.8) | 402 (75.9; 70.9–80.8) | 350 (86.8; 82.6–91.0) | 302 (84.4; 79.8–89.0) |
| No | 2796 (84.7) | 2235 (77.1; 75.2–79.0) | 2008 (88.9; 87.5–90.4) | 1743 (85.5; 83.5–87.5) |
| Comprehensive knowledge of HIV | ||||
| Yes | 840 (51.2) | 697 (79.8; 76.6–83.0) | 632 (90.5; 87.8–93.3) | 554 (86.8; 83.6–89.9) |
| No | 844 (48.8) | 654 (75.0; 71.3–78.7) | 572 (86.4; 83.2–89.6) | 484 (83.5; 79.5–87.4) |
| Number of sexual partners in the past 12 months | ||||
| 0 | 940 (27.9) | 787 (82.1; 79.3–84.8) | 726 (91.1; 88.8–93.4) | 636 (86.2; 83.1–89.3) |
| 1 | 2046 (62.1) | 1621 (76.6; 74.2–78.9) | 1430 (87.8; 86.0–89.5) | 1237 (85.3; 83.0–87.7) |
| ≥2 | 277 (10.0) | 195 (66.3; 59.5–73.1) | 159 (80.8; 74.7–86.9) | 133 (81.5; 74.1–89.0) |
| Gave birth in last 12 months* | ||||
| Yes | 156 (23.4) | 142 (89.7; 83.9–95.4) | 133 (92.7; 87.3–98.0) | 112 (85.3; 78.5–92.1) |
| No | 502 (76.6) | 404 (77.9; 73.6–82.3) | 358 (88.2; 84.9–91.5) | 305 (84.0; 79.6–88.3) |
| Gave birth in last 3 years* | ||||
| Yes | 484 (74.2) | 409 (82.4; 78.1–86.7) | 376 (91.4; 88.1–94.6) | 321 (84.8; 80.6–89.0) |
| No | 174 (25.8) | 137 (75.8; 68.4–83.2) | 115 (83.1; 77.2–89.0) | 96 (82.7; 74.6–90.8) |
| Been forced to have sex* | ||||
| Yes | 84 (5.1) | 70 (83.1; 74.2–92.0) | 57 (79.0; 67.8–90.3) | 53 (92.5; 85.2–99.9) |
| No | 1517 (94.9) | 1243 (79.8; 77.4–82.1) | 1120 (89.6; 87.7–91.5) | 982 (86.6; 84.5–88.7) |
| STD diagnosis in last 12 months | ||||
| Yes | 156 (5.1) | 142 (89.5; 83.3–95.7) | 108 (76.9; 69.0–84.9) | 85 (76.4; 67.5–85.4) |
| No | 3010 (94.9) | 2386 (76.4; 74.3–78.4) | 2136 (88.7; 87.3–90.1) | 1862 (85.9; 84.1–87.7) |
| CD4 count | ||||
| <200 | 521 (17.0) | 369 (70.0; 65.5–74.6) | 303 (82.4; 78.1–86.7) | 158 (51.5; 44.8–58.3) |
| 200–499 | 1732 (51.0) | 1354 (74.3; 71.9–76.8) | 1208 (88.2; 86.2–90.2) | 1069 (87.6; 85.5–89.8) |
| ≥500 | 1127 (32.0) | 973 (84.4; 81.5–87.2) | 894 (91.5; 89.4–93.5) | 858 (95.7; 94.1–97.3) |
| Taking cotrimoxazole | ||||
| Yes | 1883 (76.2) | 1753 (93.1; 91.7–94.4) | 1510 (84.9; 82.7–87.0) | |
| No | 615 (23.8) | 514 (80.8; 77.4–84.3) | 466 (90.3; 87.2–93.4) |
Includes women only
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; STD, sexually transmitted disease.
Figure 1: Zimbabwe’s achievements toward 90-90-90 targets.
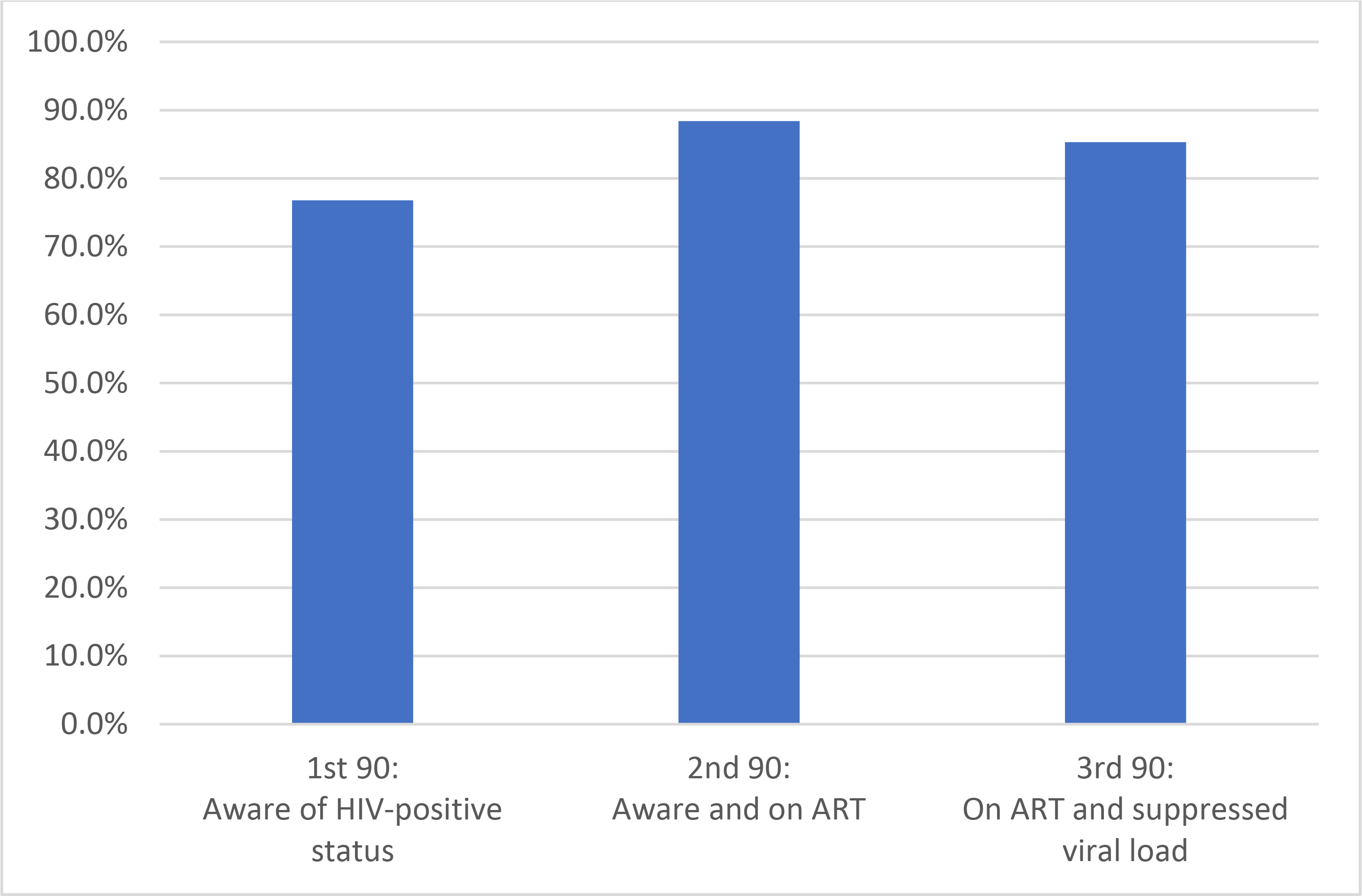
*1st and 2nd 90s are based on self-report or detection of the presence of antiretroviral drugs.
The first 90: Awareness of HIV-positive status
A higher proportion of HIV-positive women were aware of their status (80.1%) than men (72.1%; Table 1). Awareness of HIV-positive status was lowest among those aged 15–24 years (60.3%). In contrast, 70.0% of PLHIV aged 25–34 years and 82.8% of PLHIV aged 35–49 years were aware of their HIV-positive status. Awareness of HIV-positive status did not differ by urban or rural residence or wealth quintile. In terms of sexual behavior, 82.1% of those who had not had sex in the last 12 months were aware of their HIV-positive status compared to 66.3% of those who had ≥2 partners.
More women who gave birth in the last 12 months (89.7%) were aware of their HIV-positive status than those who had not (77.9%). Similarly, a higher proportion of people who had been diagnosed with a sexually transmitted disease (STD) in the last 12 months were aware of their HIV-positive status (89.5%) than those who had not (76.4%). Awareness was higher among those with CD4≥500 (84.4%) than those with CD4 of 200–499 (74.3%) or <200 (70.0%).
Among people who were unaware of their HIV-positive status, 37.0% had seen a healthcare provider in the last 12 months, and of them, 47.6% were offered an HIV test (Table 2). People who have never tested for HIV (41.7%) or last tested >2 years ago (35.9%) accounted for three-quarters of those unaware of their HIV status.
Table 2.
Engagement with health services
| Variable | n (%) | % (95% CI) |
|---|---|---|
| Unaware of HIV status | ||
| Saw a doctor or nurse in a health facility in the last 12 months | ||
| Yes | 252 (36.8) | 37.0 (32.6–41.4) |
| No | 432 (63.2) | 63.0 (58.6–67.4) |
| Among those receiving health services, offered an HIV test during any of these visits | ||
| Yes | 122 (48.4) | 47.6 (40.9–54.3) |
| No | 130 (51.6) | 52.4 (45.7–59.1) |
| Time since last HIV test | ||
| Never tested | 231 (41.4) | 41.7 (36.8–46.7) |
| <6 months | 23 (4.1) | 4.6 (2.6–6.6) |
| 6–12 months | 34 (6.1) | 5.9 (3.9–8.0) |
| 1–2 years | 67 (12.0) | 11.8 (8.9–14.7) |
| >2 years | 203 (36.4) | 35.9 (31.5–40.3) |
| Reason never tested for HIV | ||
| Don’t know where to test | 2 (0.3) | 0.3 (0.0–0.8) |
| Cost | 13 (1.9) | |
| Too far away | 9 (1.3) | 1.2 (0.3–2.0) |
| Afraid others will know about the result | 14 (2.0) | 2.3 (0.9–3.6) |
| Don’t need to test/low risk | 56 (8.2) | 8.3 (5.8–10.8) |
| Family reasons | 7 (1.0) | |
| Don’t want to know I have HIV | 17 (2.5) | 3.0 (1.6–4.4) |
| Cannot get treatment for HIV | -- | -- |
| Test kits not available | 2 (0.3) | 0.2 (0.0–0.6) |
| Religious reasons | 2 (0.3) | 0.3 (0.0–0.8) |
| Other | 121 (17.7) | 17.1 (14.0–20.2) |
| Aware and not on treatment: | ||
| Time since HIV+ diagnosis | ||
| <6 months | 31 (13.4) | 13.8 (8.6–19.0) |
| 6–12 months | 31 (13.4) | 13.1 (8.3–17.9) |
| >1 year | 170 (73.3) | 73.1 (66.1–80.2) |
| Received HIV care | ||
| Yes | 198 (67.8) | 66.4 (60.3–72.4) |
| No | 94 (32.2) | 33.6 (27.6–39.7) |
| Ever taken ART | ||
| Yes | 32 (13.8) | 14.9 (9.7–20.2) |
| No | 200 (86.2) | 85.1 (79.8–90.3) |
| Main reason never took ART | ||
| Not eligible | 92 (46.9) | 44.4 (36.3–52.4) |
| Not prescribed | 51 (26.0) | 26.9 (21.0–32.9) |
| Other | 53 (27.0) | 28.7 (21.3–36.2) |
| Taking cotrimoxazole | ||
| Yes | 130 (56.3) | 53.7 (46.1–61.4) |
| No | 101 (43.7) | 46.3 (38.6–53.9) |
| Reason not taking cotrimoxazole | ||
| Was not prescribed | 34 (33.7) | 34.7 (24.6–44.8) |
| I have trouble taking a tablet everyday | -- | -- |
| I had side effects/rash | 9 (8.9) | 8.0 (2.5–13.5) |
| Facility too far away for me to get cotrimoxazole regularly | 1 (1.0) | 1.0 (0.0–2.9) |
| Do not need it/not sick | 11 (10.9) | 11.6 (3.9–19.4) |
| Cost of medications | 4 (4.0) | 5.7 (0.0–12.2) |
| Cost of transport | 1 (1.0) | 0.9 (0.0–2.9) |
| Doctor said no longer needed | 9 (8.9) | 7.8 (2.2–13.4) |
| Other | 32 (31.7) | 30.3 (21.1–39.4) |
Abbreviations: CI, confidence interval; ART, antiretroviral therapy.
On multivariable analysis, those least likely to be aware of their HIV-positive status included men (adjusted odds ratio [aOR], 0.6 [95% confidence interval (CI): 0.5–0.8]) and those aged 15–24 years (aOR, 0.2 [95% CI: 0.2–0.4]) or 25–34 years (aOR, 0.5 [95% CI: 0.4–0.8]) compared to individuals aged 50–64 years (Table 3). People with one (aOR, 0.7 [95% CI: 0.5–0.9]) or ≥2 sexual partners (aOR, 0.5 [95% CI: 0.3–0.7]) in the last 12 months were less likely to know their HIV status than those who had no sexual partners.
Table 3:
Correlates of awareness of HIV-positive status, aware and on ART, and on ART with suppressed VL
| Correlates of awareness of HIV-positive status | Correlates of being aware of HIV status and on ART | Correlates of being on ART and having suppressed viral load | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | OR (95% CI) | p-value | aOR (95% CI) | p-value | OR (95% CI) | p-value | aOR (95% CI) | p-value | OR (95% CI) | p-value | aOR (95% CI) | p-value |
| Sex | <0.0001 | <0.0001 | 0.6950 | 0.0113 | 0.0115 | |||||||
| Male | 0.6 (0.5–0.8) | 0.6 (0.5–0.8) | 0.9 (0.7–1.3) | 0.7 (0.5–0.9) | 0.7 (0.5–0.9) | |||||||
| Female | Ref | Ref | ref | ref | ||||||||
| Age (years) | <0.0001 | <0.0001 | <0.0001 | 0.0001 | 0.0005 | 0.0004 | ||||||
| 15–24 | 0.3 (0.2–0.4) | 0.2 (0.2–0.4) | 0.2 (0.1–0.5) | 0.2 (0.1–0.4) | 0.4 (0.2–0.6) | 0.4 (0.2–0.7) | ||||||
| 25–34 | 0.5 (0.3–0.6) | 0.5 (0.3–0.7) | 0.2 (0.1–0.3) | 0.2 (0.1–0.4) | 0.4 (0.2–0.6) | 0.3 (0.2–0.5) | ||||||
| 35–49 | 1.0 (0.7–1.3) | 1.1 (0.8–1.5) | 0.3 (0.2–0.5) | 0.3 (0.2–0.6) | 0.4 (0.3–0.7) | 0.4 (0.3–0.6) | ||||||
| 50–64 | Ref | Ref | Ref | Ref | Ref | Ref | ||||||
| Residence | 0.1380 | 0.2212 | 0.7822 | |||||||||
| Urban | Ref | Ref | 1.0 (0.7–1.3) | |||||||||
| Rural | 1.2 (0.9–1.5) | 1.2 (0.9–1.5) | ||||||||||
| Highest level of education completed | 0.0408 | 0.1029 | 0.1101 | 0.6144 | ||||||||
| No education | Ref | Ref | Ref | Ref | ||||||||
| Primary | 1.0 (0.6–1.6) | 1.7 (1.0–2.9) | 0.5 (0.2–1.3) | 0.7 (0.3–1.4) | ||||||||
| Secondary | 0.8 (0.5–1.2) | 1.5 (0.8–2.6) | 0.4 (0.1–1.0) | 0.7 (0.4–1.5) | ||||||||
| >Secondary | 0.5 (0.3–1.1) | 1.1 (0.5–2.2) | 0.4 (0.1–1.7) | 0.6 (0.2–1.8) | ||||||||
| Marital status | 0.0001 | 0.3633 | 0.0555 | 0.3133 | 0.0387 | 0.8815 | ||||||
| Never married | Ref | Ref | Ref | Ref | Ref | Ref | ||||||
| Married or living together | 1.4 (1.1–1.9) | 0.9 (0.6–1.4) | 0.7 (0.4–1.3) | 0.6 (0.3–1.2) | 1.3 (0.9–2.0) | 0.9 (0.5–1.7) | ||||||
| Divorced or separated | 1.3 (0.9–1.9) | 0.7 (0.5–1.1) | 0.6 (0.3–1.1) | 0.5 (0.2–1.1) | 1.4 (0.8–2.3) | 0.8 (0.4–1.6) | ||||||
| Widowed | 2.8 (1.9–4.2) | 0.9 (0.5–1.6) | 1.1 (0.6–2.0) | 0.6 (0.2–1.3) | 2.1 (1.3–3.4) | 1.0 (0.5–2.0) | ||||||
| Worked in the last 12 months | 0.0020 | 0.1239 | 0.1056 | 0.3521 | 0.9030 | |||||||
| Yes | Ref | Ref | Ref | Ref | Ref | |||||||
| No | 1.4 (1.1–1.7) | 1.2 (1.0–1.4) | 1.3 (0.9–1.7) | 1.2 (0.8–1.6) | 1.0 (0.8–1.4) | |||||||
| Wealth quintile | 0.5193 | 0.2187 | ||||||||||
| Lowest | Ref | Ref | Ref | 0.6156 | ||||||||
| Second | 0.9 (0.6–1.2) | 1.1 (0.7–1.8) | 1.2 (0.8–1.7) | |||||||||
| Middle | 0.9 (0.6–1.2) | 0.8 (0.5–1.2) | 1.0 (0.7–1.5) | |||||||||
| Fourth | 0.8 (0.5–1.1) | 0.8 (0.6–1.3) | 1.1 (0.7–1.7) | |||||||||
| Highest | 0.8 (0.6–1.2) | 0.7 (0.5–1.1) | 1.3 (0.8–1.9) | |||||||||
| Been away from home in the last 12 months | 0.5982 | 0.3089 | 0.6363 | |||||||||
| Yes | Ref | Ref | Ref | |||||||||
| No | 1.1 (0.8–1.4) | 1.2 (0.8–1.8) | 1.1 (0.7–1.6) | |||||||||
| Number of sexual partners in the past 12 months | 0.0002 | 0.0031 | 0.0032. | 0.6955 | 0.4483 | |||||||
| 0 | Ref | Ref | Ref | Ref | Ref | |||||||
| 1 | 0.7 (0.6–0.9) | 0.7 (0.5–0.9) | 0.7 (0.5–1.0) | 1.0 (0.6–1.5) | 0.9 (0.7–1.3) | |||||||
| ≥2 | 0.4 (0.3–0.6) | 0.5 (0.3–0.7) | 0.4 (0.3–0.7) | 0.8 (0.4–1.5) | 0.7 (0.4–1.2) | |||||||
| Gave birth in last 12 months* | 0.0072 | 0.1902 | 0.7420 | |||||||||
| Yes | Ref | Ref | Ref | |||||||||
| No | 0.4 (0.2–0.8) | 0.6 (0.3–1.3) | 0.9 (0.5–1.7) | |||||||||
| Gave birth in last 3 years* | 0.1081 | 0.0122 | 0.6330 | |||||||||
| Yes | Ref | Ref | Ref | |||||||||
| No | 0.7 (0.4–1.1) | 0.5 (0.3–0.8) | 0.9 (0.4–1.7) | |||||||||
| Been forced to have sex* | 0.4936 | 0.0218 | 0.2809 | |||||||||
| Yes | Ref | Ref | Ref | |||||||||
| No | 0.8 (0.4–1.5) | 2.3 (1.1–4.6) | 0.5 (0.2–1.8) | |||||||||
| STD diagnosis in last 12 months | 0.0085 | 0.0012 | 0.0165 | |||||||||
| Yes | Ref | Ref | Ref | |||||||||
| No | 0.4 (0.2–0.8) | 2.4 (1.5–3.8) | 1.9 (1.1–3.1) | |||||||||
| Taking cotrimoxazole | <.0001 | <.0001 | 0.0180 | 0.0124 | ||||||||
| Yes | Ref | Ref | Ref | Ref | ||||||||
| No | 0.3 (0.2–0.4) | 0.3 (0.2–0.5) | 1.6 (1.1–2.5) | 1.7 (1.1–2.5) | ||||||||
Abbreviations: aOR, adjusted odds ratio; CI, confidence intervals; ART, antiretroviral therapy; OR, odds ratio; STD, sexually transmitted disease.
The second 90: Aware of HIV-positive status and receiving ART
Among people who were aware of their HIV-positive status, ART status was similar across sex, age, urban or rural residence, and wealth quintiles (Table 1). More individuals who did not have an STD diagnosis in the last 12 months (88.7%) were receiving ART than those who had an STD (76.9%).
Nearly three-quarters (73.1%) of people who were aware of their HIV-positive status but were not on ART were diagnosed >1 year ago (Table 2). Approximately one-third (33.6%) of those aware but not receiving ART had not received HIV care, and 14.9% had stopped ART. Main reasons for never taking ART included not being eligible (44.4%) and not being prescribed ART (26.9%). Half (53.7%) of those not on ART were receiving cotrimoxazole, while 34.7% of those who were not receiving cotrimoxazole had not been prescribed it.
On bivariate analysis, the odds of receiving ART were higher among women who had not been forced to have sex than among those who had (OR, 2.3 [95% CI: 1.1–4.6]; Table 3). On multivariable analysis, those who were aware of their HIV-positive status and younger than 50–64 years were least likely to be receiving ART. Compared to people aged 50–64 years, the adjusted odds of receiving ART among those who were aware of their HIV-positive status was 0.2 (95% CI: 0.1–0.4) for those aged 15–24 years, 0.2 (95% CI: 0.1–0.4) for those aged 35–34 years, and 0.3 (95% CI: 0.2–0.6) for those aged 35–49 years. People who were not taking cotrimoxazole were 70% less likely to be receiving ART than those taking cotrimoxazole (aOR, 0.3 [95% CI: 0.2–0.5]).
The third 90: Receiving ART and having VLS
Among those receiving ART, VLS was similar across sex, age, urban or rural residence, and wealth quintiles. Among women who gave birth in the last 12 months and were receiving ART, 84.8% had VLS. Though not statistically significant, a smaller proportion of those with an STD diagnosis in the last 12 months (76.4%) had VLS than those who did not have an STD (85.9%).
On multivariable analysis, among those receiving ART, men (aOR, 0.7 [95% CI: 0.5–0.9]) were less likely than women to have VLS. Compared to people aged 50–64 years, the adjusted odds of having VLS were lower for those aged 15–24 years (aOR, 0.4 [95% CI: 0.2–0.7]), those aged 25–34 years (aOR, 0.3 [95% CI: 0.2–0.5]), and those aged 35–49 years (aOR, 0.4 [95% CI: 0.3–0.6]). People who were not taking cotrimoxazole were more likely to have VLS than those who were (aOR, 1.7 [95% CI: 1.1–2.5]).
DISCUSSION
ZIMPHIA was the first nationally representative survey conducted to estimate progress toward the UNAIDS 90-90-90 targets. Our findings reveal that Zimbabwe has the potential to achieve the 90-90-90 targets by the end of 2020. With 76.8% of PLHIV aware of their HIV status and 88.4% of them receiving ART in 2016, our findings are consistent with the hypothesis that retention may be better than anticipated in many countries, allowing HIV service providers to prioritize case-finding efforts 13. On the basis of the estimated number of PLHIV in Zimbabwe in 2015, we estimate that 282,000 people in Zimbabwe were unaware of their HIV-positive status, 390,000 people were not receiving ART, and 512,000 people did not have VLS 14.
We found that men and adults younger than 35 years were most likely to be unaware of their HIV-positive status. These populations are also the least likely to access healthcare services where they may be reached by provider-initiated testing and counseling services. Case-finding strategies that extend beyond health facilities could increase awareness of HIV-positive status among these populations 15. These strategies also can support HIV diagnosis among people with a higher CD4 count. Strategies are also needed to identify the larger share of PLHIV who are unaware of their HIV status and have CD4 < 500 cells/μL.
Service integration including mother-to-child transmission prevention and provider-initiated HIV testing and counseling help increase HIV case finding 16–18. Our findings suggest the same may be true in Zimbabwe as women who gave birth >12 months ago were more likely to be unaware of their HIV-positive status than those who gave birth in the last year. Similarly, awareness was lower among people who did not have an STD diagnosis in the last 12 months than those who had an STD. Better risk screening, including the use of screening tools that do not indicate the client’s HIV risk behaviors to the provider but inform the client that they should undergo HIV testing, could help identify individuals who may not otherwise receive testing services in the health facility.
People who had sex in the last 12 months were less likely to be aware of their HIV-positive status than those who had not, possibly indicating that people change their sexual behaviors upon learning they have HIV 19–21. The larger number of sexual partners among people who were unaware of their HIV-positive status reflects a higher transmission potential. As people with no sexual partners theoretically have no transmission potential, diagnosing them would facilitate reaching 90-90-90 targets but may have a limited impact on epidemic control 22,23. Case-finding efforts including index testing may have more impact if they prioritize people with the most sexual partners in a defined period.
Although men were more likely than women to be unaware of their HIV-positive status, ART initiation did not differ by sex, reflecting improvements in Zimbabwe’s ART program. A retrospective analysis of ART retention among people who were initiated on ART (2007–2009) found that the adjusted hazards ratio (aHR) for attrition risk was greater for men than women (aHR, 1.2 [95% CI: 1.1–1.4]) 24. A similar analysis of those initiated on ART from 2012–2015 did not find sex to be a predictor of retention 16 but found age 15–24 years (compared to age ≥25 years) to be a negative predictor (aHR, 0.6 [95% CI: 0.5–0.9) of retention. We found the odds of receiving ART were lower for all age groups compared to those aged 50–64 years, reflecting the importance of disaggregating data to the finest level possible.
Though decentralizing ART to primary health clinics and male involvement in mother-to-child transmission prevention programs may have helped increase ART uptake among men, we found gender differences in VLS among those receiving ART 16,25. Men receiving ART were less likely than women to have VLS. Compared to those aged 50–64 years, younger age groups were also less likely to have VLS. This may be because those in the oldest age group have been receiving ART the longest and consequently had the most time to find the most appropriate regimen. This interpretation is borne out in a recent meta-analysis that found no difference in the relative risk (RR) of VLS between those aged ≥50 years and those aged 13–49 years at 6, 12, and 24 months after ART initiation but found a small increase in the RR of VLS among those aged ≥50 years 36 months after initiation (RR, 1.04 [95% CI: 1.01–1.08]) 26.
Taken together, the treatment as prevention trials highlighted the need for earlier diagnosis and rapid linkage to treatment 27. Our findings reveal the importance of focusing on earlier diagnosis in Zimbabwe. Where TasP/ANRS 12249 revealed the challenges of ART initiation and retention in rural Africa, we found no difference in ART status or viral suppression by urban or rural residence 28. Efforts to initiate treatment appear to be effective as approximately 90% of PLHIV with CD4 > 200 copies/μL were on ART and had achieved viral suppression. Zimbabwe’s transition to test and start could further improve ART coverage and achievement of viral suppression.
One strength of our study was including self-reported HIV-positive status, self-reported ART status, and antiretroviral biomarkers to inform 90-90-90 results. Although we were unable to test for all antiretroviral medicines used in Zimbabwe, testing accounted for antiretroviral medicines used by >90% of PLHIV receiving ART.
Our study has several limitations. Our results are limited by the cross-sectional nature of ZIMPHIA, the self-reported nature of data collected through face-to-face interviews, and our inability to collect reliable data for all key populations. We were also unable to determine if participants with known HIV-positive status but who were not prescribed ART were not prescribed it because they were ineligible at their last clinic visit or for another reason. Together with those who were ineligible, this group comprised 71.3% of people not receiving ART. Because Zimbabwe broadened ART eligibility criteria to include all HIV-positive individuals by adopting the Test and Start policy just after ZIMPHIA, ART coverage has likely increased since our survey was conducted. Finally, we did not include CD4 in our models because we did not know CD4 at diagnosis for participants with a previous HIV diagnosis.
Additionally, viral load was measured for all PLHIV, regardless of ART duration. VLS correlates were assessed on this broader population, and some people may have newly initiated ART and not had adequate time for VLS. We expect this to have had a minimal effect on findings.
Our findings reveal awareness of HIV-positive status is a critical gap in Zimbabwe’s HIV response. Furthermore, with one in seven PLHIV having CD4<200, late diagnosis remains a problem. Reaching people who are not aware of their HIV-positive status requires innovative testing strategies, such as index testing, outside health facilities. However, case finding will become more difficult as awareness of HIV status increases among PLHIV. Characterizing those who are outside the 90-90-90 cascade (i.e., people who are not aware of their HIV-positive status, those not receiving ART, those who do not have VLS) will remain essential for targeting interventions to these groups.
Acknowledgments:
We wish to thank Jill Russell for her editing support.
Funding: This project has been supported by the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the terms of Cooperative Agreement Number U2GGH001226 to ICAP at Columbia University.
Disclaimer: The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the funding agencies.
Footnotes
Competing interests: We have no competing interests to report.
REFERENCES
- 1.UNAIDS. 90-90-90: An ambitious treatment target to help end the AIDS epidemic. Geneva: 2014. [Google Scholar]
- 2.UNAIDS. UNAIDS Data 2019. Geneva: 2019. [Google Scholar]
- 3.Ministry of Health and Child Care (MOHCC) Z. Extended Zimbabwe National HIV and AIDS Strategic Plan (ZNASP) 2015–2020. Harare: 2015. [Google Scholar]
- 4.Ministry of Health and Child Care (MOHCC) Z. Joint HIV/AIDS/STI Strategy 2021–2025. Harare: 2020. [Google Scholar]
- 5.Ministry of Health and Child Care (MOHCC) Z. Zimbabwe Global AIDS Response Progress Report 2018. Harare: 2018. [Google Scholar]
- 6.Hladik W, Benech I, Bateganya M, Hakim AJ. The utility of population-based surveys to describe the continuum of HIV services for key and general populations. Int J STD AIDS. 2016;27(1):5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleming PJ, Rosen JG, Wong VJ, Carrasco MA. Shedding light on a HIV blind spot: Factors associated with men’s HIV testing in five African countries. Glob Public Health. 2019;14(9):1241–1251. [DOI] [PubMed] [Google Scholar]
- 8.Staveteig S, Croft TN, Kampa KT, Head SK. Reaching the ‘first 90’: Gaps in coverage of HIV testing among people living with HIV in 16 African countries. PLoS One. 2017;12(10):e0186316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuadros DF, Li J, Mukandavire Z, et al. Towards UNAIDS Fast-Track goals: targeting priority geographic areas for HIV prevention and care in Zimbabwe. Aids. 2019;33(2):305–314. [DOI] [PubMed] [Google Scholar]
- 10.Bansi-Matharu L, Cambiano V, Apollo T, et al. 90-90-90 by 2020? Estimation and projection of the adult HIV epidemic and ART programme in Zimbabwe - 2017 to 2020. J Int AIDS Soc 2018;21(11):e25205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tafuma TA, Mahachi N, Dziwa C, et al. Barriers to HIV service utilisation by people living with HIV in two provinces of Zimbabwe: Results from 2016 baseline assessment. South Afr J HIV Med 2018;19(1):721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koal T, Burhenne H, Romling R, Svoboda M, Resch K, Kaever V. Quantification of antiretroviral drugs in dried blood spot samples by means of liquid chromatography/tandem mass spectrometry. Rapid communications in mass spectrometry: RCM. 2005;19(21):2995–3001. [DOI] [PubMed] [Google Scholar]
- 13.Geng EH, Nash D, Kambugu A, et al. Retention in care among HIV-infected patients in resource-limited settings: emerging insights and new directions. Curr HIV/AIDS Rep 2010;7(4):234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ministry of Health and Child Care (MOHCC) Z. Zimbabwe National and Sub-National HIV Estimates Report, 2017. Harare, Zimbabwe: 2018. [Google Scholar]
- 15.Sharma M, Ying R, Tarr G, Barnabas R. Systematic review and meta-analysis of community and facility-based HIV testing to address linkage to care gaps in sub-Saharan Africa. Nature. 2015;528(7580):S77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makurumidze R, Mutasa-Apollo T, Decroo T, et al. Retention and predictors of attrition among patients who started antiretroviral therapy in Zimbabwe’s national antiretroviral therapy programme between 2012 and 2015. PLoS One. 2020;15(1):e0222309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gamell A, Luwanda LB, Kalinjuma AV, et al. Prevention of mother-to-child transmission of HIV Option B+ cascade in rural Tanzania: The One Stop Clinic model. PLoS One. 2017;12(7):e0181096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dzangare J, Takarinda KC, Harries AD, et al. HIV testing uptake and retention in care of HIV-infected pregnant and breastfeeding women initiated on ‘Option B+’ in rural Zimbabwe. Trop Med Int Health. 2016;21(2):202–209. [DOI] [PubMed] [Google Scholar]
- 19.Rucinski KB, Rutstein SE, Powers KA, et al. Sustained Sexual Behavior Change After Acute HIV Diagnosis in Malawi. Sex Transm Dis 2018;45(11):741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venkatesh KK, de Bruyn G, Mayer KH, et al. Changes in sexual risk behavior before and after HIV seroconversion in Southern African women enrolled in a HIV prevention trial. J Acquir Immune Defic Syndr 2011;57(5):435–441. [DOI] [PubMed] [Google Scholar]
- 21.Turner AN, Miller WC, Padian NS, et al. Unprotected sex following HIV testing among women in Uganda and Zimbabwe: short- and long-term comparisons with pre-test behaviour. Int J Epidemiol 2009;38(4):997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akullian A, Bershteyn A, Jewell B, Camlin CS. The missing 27%. Aids. 2017;31(17):2427–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baral S, Rao A, Sullivan P, et al. The disconnect between individual-level and population-level HIV prevention benefits of antiretroviral treatment. The Lancet HIV. 2019;6(9):e632–e638. [DOI] [PubMed] [Google Scholar]
- 24.Mutasa-Apollo T, Shiraishi RW, Takarinda KC, et al. Patient retention, clinical outcomes and attrition-associated factors of HIV-infected patients enrolled in Zimbabwe’s National Antiretroviral Therapy Programme, 2007–2010. PLoS One. 2014;9(1):e86305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ministry of Health and Child Care (MOHCC) Z. ART Outcomes of People Living with HIV Enrolled in the National ART Programme, Zimbabwe, October 2012 –January 2015. Harare, Zimbabwe: Ministry of Health and Child Care (MOHCC), Zimbabwe;2016. [Google Scholar]
- 26.Zhang Q, Yu X, Wu T, Shang H, Jiang Y. Immunological and Virological Responses in Older HIV-Infected Adults Receiving Antiretroviral Therapy: An Evidence-Based Meta-Analysis. J Acquir Immune Defic Syndr 2020. [DOI] [PubMed] [Google Scholar]
- 27.Brault MA, et al. (2020). “Integrating and Interpreting Findings from the Latest Treatment as Prevention Trials.” Curr HIV/AIDS Rep 17(3): 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwuji CC, et al. (2018). “Universal test and treat and the HIV epidemic in rural South Africa: a phase 4, open-label, community cluster randomised trial.” The Lancet HIV 5(3): e116–e125. [DOI] [PubMed] [Google Scholar]


