Abstract
Fifty-six Pasteurella multocida strains (40 P. multocida subsp. septica and 16 P. multocida subsp. multocida strains) isolated from the mouths of 56 dogs among the 134 living in a French canine military training center (132e Groupe Cynophile de l’Armée de Terre, Suippes, France) were studied by use of enterobacterial repetitive intergenic consensus-PCR (ERIC-PCR) and restriction fragment length polymorphism (RFLP) techniques. Both techniques showed genomic heterogeneity of the strains studied. However, RFLP was more discriminatory than ERIC-PCR for differentiating P. multocida strains. All but three pairs of strains were discriminated by RFLP, suggesting a limited circulation of strains between these dogs living in proximity. Although ERIC-PCR is easier and faster to perform, it cannot be recommended for epidemiological studies of P. multocida strains.
Pasteurella multocida is a commensal of digestive and respiratory tracts of warm-blooded animals and is responsible for diseases chiefly in reared animals (bovine animals, porcine animals, rabbits, and poultry) weakened by stresses such as viral infections, cold, or humidity (5). The bacterium spreads from animal to animal by aerosols. Diseases consist mainly of hemorrhagic septicemia and pneumonia (11). Thus, enzootic pasteurellosis and epizootic pasteurellosis affect the livestock industry and are responsible for important economic losses in cattle farming (10, 29).
Pasteurellosis is a zoonosis, and humans are accidental hosts, acquiring P. multocida infections from animals, primarily after cat bites and, to a lesser extent, dog bites (26). The latter infections are, however, more prevalent, as they represent 80% of animal-bite wounds (8). In most cases, infections remain limited to the wound site and have a favorable outcome. However, septic arthritis may occur.
The dogs living in a canine military training center (132e Groupe Cynophile de l’Armée de Terre, Suippes, France [132e GCAT]) are responsible for about 200 bite wounds on dog attendants per year. Most of these wounds have no consequences, as amoxicillin-clavulanic acid is systematically administered. Less than 5% become infected and necessitate hospital consultation. To evaluate the risk of pasteurellosis in the staff of this center, we studied the aerobic bacterial flora of the 134 dogs present in the center in 1996 (17). P. multocida was the main species isolated, and 56 strains were recovered from 56 dogs.
Serotyping is commonly used for the epidemiological study of P. multocida. Five capsule groups (A, B, D, E, and F) and 16 somatic types (1 through 16) have been described (1, 9, 19). Serotypes of P. multocida can be associated with specific diseases in animals (e.g., B:2 and E:2 with hemorrhagic septicemia in cattle and buffaloes) (30). Like other phenotypic markers, the serotype is of limited value for epidemiological studies of P. multocida infections (24, 30, 31). To our knowledge, serotyping is no longer used in France and therefore was not used in this study. To characterize the genetic relationship among P. multocida strains isolated from the mouths of these dogs living in proximity, we performed enterobacterial repetitive intergenic consensus-PCR (ERIC-PCR). This technique consists of the amplification of genomic DNA enclosed between conserved repetitive regions scattered all over the bacterial genome. These conserved repetitive elements (ERIC sequences) were described first for the genomes of enterobacteria and later for those of many other bacterial species. A consensus sequence has been defined (13, 28). The number and the location of ERIC sequences vary not only between species but also between strains of the same species (13). Electrophoresis of amplified fragments provides band patterns which permit the differentiation of strains. We evaluated this technique for P. multocida strains and compared the results to those obtained by use of the restriction fragment length polymorphism (RFLP) technique, a previously validated technique for the epidemiological study of P. multocida (24, 27, 30, 31). P. multocida is divided into three subspecies (P. multocida subsp. multocida, P. multocida subsp. septica, and P. multocida subsp. gallicida) which have different ecologies (e.g., P. multocida subsp. gallicida is isolated from avian origins) (5, 12). Thus, the strains of P. multocida subsp. multocida and P. multocida subsp. septica isolated were studied separately.
(Part of this work was presented at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, 1997.)
MATERIALS AND METHODS
Dogs.
At the time of the study, 134 1- to 11-year-old healthy male dogs had been present at 132e GCAT from 1 month to 8 years. The population consisted of German and Belgian shepherds. The dogs were separated into three groups dwelling in three different enclosures: 109 watch dogs in training, 19 resident dogs belonging to staff, and 6 dogs in specific training (explosives or drugs). Each dog was fed in its own cage.
P. multocida strains.
P. multocida strains were isolated from dogs by gingival swabbing as previously described (17). Briefly, two cotton-tipped swabs were rubbed vigorously on lateral gums through the muzzle of each dog. The samples were immediately inoculated on sheep blood agar plates and chocolate agar plates and incubated in a 5% CO2 atmosphere at 37°C. Pasteurella strains were identified as described by Holmes et al. (11). Fifty-six P. multocida strains were isolated and designated P1 to P56. P. multocida subspecies were determined by study of the fermentation of sorbitol and dulcitol. Eight epidemiologically unrelated P. multocida strains isolated from eight different patients between 1994 and 1997 at the University Hospital Center of Nancy were also studied (dog-bite wounds, four strains; respiratory tract infections, four strains); they were designated NER1 to NER8 (for “not epidemiologically related” [NER]). P. multocida subsp. multocida CIP959 (Institut Pasteur, Paris, France) was included as a reference. Ten different colonies were analyzed separately to test the reproducibility of ERIC-PCR and RFLP. Strains were stored at −80°C in brain heart infusion broth with 15% (vol/vol) glycerol.
ERIC-PCR.
Bacteria were grown overnight at 37°C in brain heart infusion broth, and suspensions were adjusted to an absorbance at 600 nm of 0.5. Each 25-μl reaction mixture contained 10 μl of bacterial suspension and final amounts of 67 mM Tris-HCl (pH 8.8), 16.6 mM (NH4)2SO4, 6.7 mM MgCl2, 6.7 μM EDTA, 30 mM β-mercaptoethanol, 0.17 mg of bovine serum albumin per ml, 10% (vol/vol) dimethyl sulfoxide, 1.25 mM each deoxynucleoside triphosphate (Boehringer Mannheim Biochemicals, Mannheim, Germany), 2 μM each primer, and 1.5 U of Taq DNA polymerase (Gibco-BRL Life Technologies, Paisley, United Kingdom). PCR was performed by use of thermal cycler (GeneAmp PCR System 2400; Perkin-Elmer, Norwalk, Conn.) with an initial denaturation step (95°C, 10 min), with 30 cycles of denaturation (94°C, 1 min), annealing (52°C, 1 min), and extension (65°C, 8 min), and with a final extension (65°C, 16 min). The primers were ERIC 1R (5′-ATG TAA GCT CCT GGG GAT TCA C-3′) and ERIC 2 (5′-AAG TAA GTG ACT GGG GTG AGC G-3′) (28). A negative control without template DNA was included in each run. Amplified products were resolved by electrophoresis in 1.5% (wt/vol) agarose gels containing ethidium bromide (1.6 mg/ml) at 11 V/cm for 90 min in TBE buffer (0.089 M Tris, 0.089 M boric acid, 0.002 M EDTA [pH 8]). A 1-kb DNA ladder was used as a size marker (Gibco-BRL Life Technologies).
RFLP.
Preliminary studies of restriction endonuclease digestion of P. multocida DNA with BamHI, BglII, EcoRI, HhaI, HindIII, HpaII, PstI, and SmaI confirmed that HhaI gave the most discriminant fingerprint profiles. Therefore, DNA was extracted and digested with HhaI (New England BioLabs, Beverly, Mass.) as described by Wilson et al. (30). DNA fragments were separated by electrophoresis in 0.8% (wt/vol) agarose gels containing ethidium bromide (1.6 mg/ml) at 6 V/cm for 7 h in TBE buffer. Bacteriophage λ DNA digested with HindIII was used as a size marker (Gibco-BRL Life Technologies).
Analysis of band patterns.
Amplified products or restriction fragment patterns obtained by ERIC-PCR or RFLP, respectively, were visualized by UV transillumination, and pictures were taken with a charge-coupled device camera (Bio-Rad Laboratories, Richmond, Calif.). Fingerprints were stored in tagged-image-file format with Molecular Analyst 2.1 software (Bio-Rad Laboratories). Images were then processed with Molecular Analyst/PC Fingerprinting software (Bio-Rad Laboratories). Two fingerprints were considered identical if the same numbers of bands at the same positions were observed; variations in intensity were not considered. Percentages of similarity between two profiles were calculated by use of the Dice coefficient × 100 {i.e., [2nAB/(nA + nB)] × 100, where nAB is the number of bands common to both strains and (nA + nB) is the total number of bands found for both strains} (4). The percentage of similarity ranged from 0% (complete dissimilarity) to 100% (identity). Cluster analysis was performed by use of the unweighted-pair-group method with arithmetic linkages (UPGMA) (14). Strains with a percentage of similarity above 90% were considered only potentially related.
RESULTS
Molecular typing by ERIC-PCR.
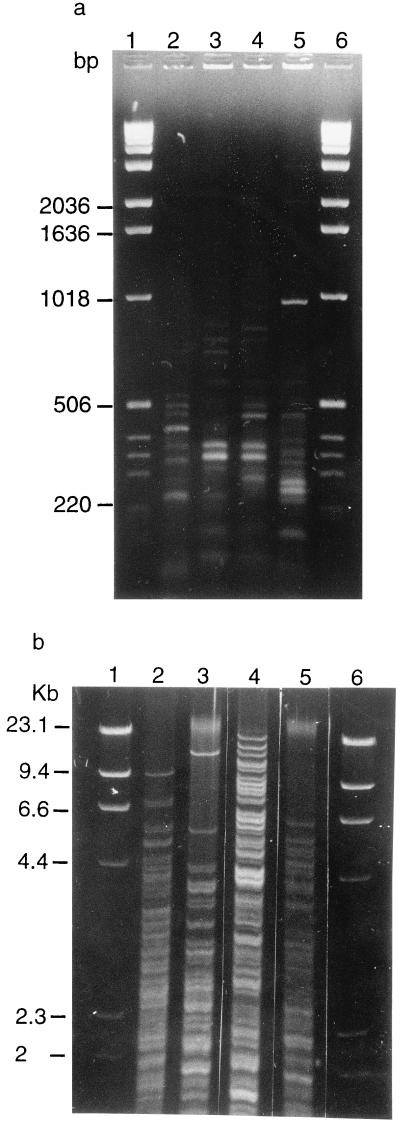
The DNA of all the isolates of P. multocida was amplified by the consensus primers. The fingerprints obtained consisted of 5 to 15 amplification bands ranging in size from 100 bp to 5 kb (Fig. 1a). Sixty-two different patterns were obtained with the 56 dog strains and the 8 NER strains. The 56 dog strains were distributed into 40 P. multocida subsp. septica and 16 P. multocida subsp. multocida strains. The NER strains included five P. multocida subsp. multocida and three P. multocida subsp. septica strains. The subspecies of P. multocida were differentiated by a similarity between their patterns of <78% (Table 1). Among strains of P. multocida subsp. septica, a similarity of >90% was obtained for two pairs of dog strains: 92% for P6 and P55 and 100% for P21 and P22 (data not shown). Among strains of P. multocida subsp. multocida, a similarity of >90% was obtained for three pairs of dog strains: 94% for P28 and P41, 96% for P42 and P49, and 100% for P2 and P5 (data not shown). The NER strains were differentiated from each other and from dog strains by a similarity of <83% (Table 1). The 10 patterns obtained with reference strain CIP959 were all identical. They differed clearly from those of dog or NER strains by <64% similarity.
FIG. 1.
Genomic fingerprints of P. multocida strains obtained by ERIC-PCR (a) and RFLP (b). Lanes 1 and 6, size markers (1-kb DNA ladder and bacteriophage λ DNA digested with HindIII for ERIC-PCR and RFLP, respectively); lanes 2 to 5, strains of P. multocida subsp. septica isolated from dogs (strains P30, P31, P32, and P33 respectively).
TABLE 1.
Comparison of the discriminating values of ERIC-PCR and RFLP
| Organism | Strains | Method | Similarity (% range) of:
|
|||
|---|---|---|---|---|---|---|
|
P. multocida subsp. septica strains
|
P. multocida subsp. multocida strains
|
|||||
| Dog | NER | Dog | NER | |||
| P. multocida subsp. septica | Dog | ERIC-PCR | 30–100 | 30–77 | 23–77 | 36–72 |
| RFLP | 36–100 | 36–75 | 38–86 | 38–87 | ||
| NER | ERIC-PCR | 45–82 | 36–72 | 36–72 | ||
| RFLP | 51–57 | 38–87 | 51–64 | |||
| P. multocida subsp. multocida | Dog | ERIC-PCR | 38–100 | 38–82 | ||
| RFLP | 54–100 | 54–74 | ||||
| NER | ERIC-PCR | 41–79 | ||||
| RFLP | 55–81 | |||||
Molecular typing by RFLP.
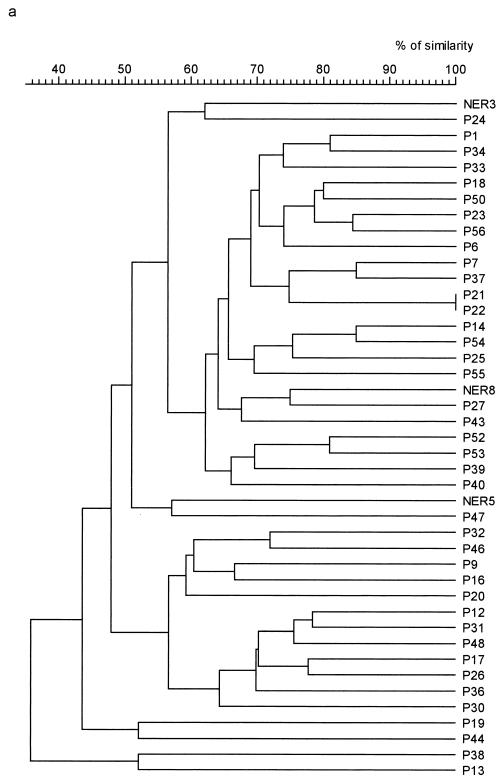
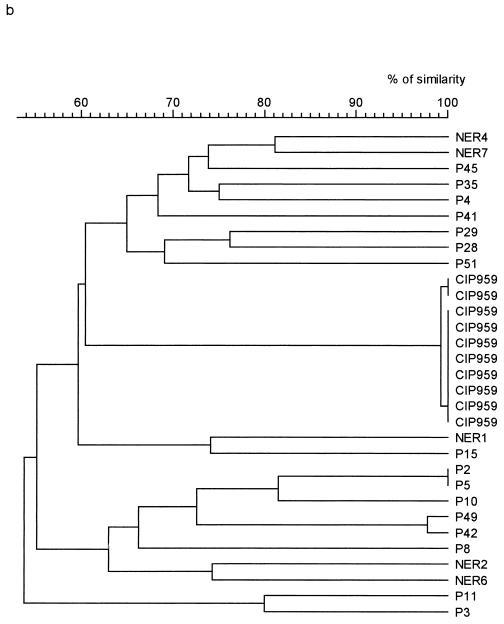
Fingerprints obtained with RFLP consisted of 15 to 35 amplification bands ranging in size from 2 to 23 kb (Fig. 1b). Strains P21 and P22 of P. multocida subsp. septica showed 100% similarity between their patterns (Fig. 2a). A similarity of >90% was obtained for two pairs of P. multocida subsp. multocida strains: 98% for P42 and P49 and 100% for P2 and P5 (Fig. 2b). The NER strains were differentiated from each other and from dog strains by a similarity of <88% (Table 1). Reference strain CIP959 gave eight identical fingerprints and two patterns showing 99% similarity with the other eight patterns. These 10 patterns differed from those of dog or NER strains by a similarity of <61% (Fig. 2b).
FIG. 2.
Dendrograms obtained from RFLP patterns. Percentages of similarity between patterns were calculated by use of the Dice coefficient. Dendrograms were constructed by use of UPGMA. (a) Pasteurella multocida subsp. septica. (b) Pasteurella multocida subsp. multocida.
DISCUSSION
Two molecular epidemiological techniques have been recently applied to P. multocida: random amplified polymorphic DNA (2, 22) and RFLP (24, 27, 30, 31). However, random amplified polymorphic DNA, which gives less than six amplification bands, is less discriminatory than RFLP, which yields patterns consisting of more than 10 restriction fragments. RFLP has been shown to be of value for the differentiation of field and vaccine isolates (30, 31) and for evidencing the probable source of infection in a patient who died of endocarditis due to P. multocida (27).
ERIC-PCR, a recently described epidemiological technique (28), has been previously used for various species belonging to α-proteobacteria (21), γ-proteobacteria (3, 7, 15, 16, 18, 20, 28), and ɛ-proteobacteria (6). ERIC sequences have also been described for the genomes of Staphylococcus aureus (25) and Mycobacterium tuberculosis (23). As ERIC sequences are present in most γ-proteobacteria, it is not surprising that they are also present in the genome of P. multocida.
P. multocida subsp. septica and P. multocida subsp. multocida were differentiated by a similarity of <78% with ERIC-PCR. For these subspecies, differentiation between dog strains and NER strains was satisfactory, as the similarity between strains belonging to these different groups was never >82%. Good reproducibility of this technique was also observed, as the 10 patterns obtained with the reference strain were all identical. However, differentiation between strains belonging to the same subspecies was less satisfactory. Five pairs of strains showed >90% similarity in ERIC-PCR. This apparent relation was not confirmed by RFLP for two of these pairs, as strains P6 and P55 and strains P28 and P41 showed 65% similarity between their patterns, clearly differentiating them. The three other pairs (P42 and P49; P2 and P5; and P21 and P22) had closely related patterns in ERIC-PCR (96, 100, and 100% similarity, respectively) and in RFLP (98, 100, and 100% similarity, respectively). Among these five pairs of strains, only strains P42 and P49, strains P2 and P5, and strains P21 and P22, which had similar or identical patterns with both techniques, were epidemiologically related. They were isolated from resident dogs (P42 and P49; and P21 and P22) which dwell in the same enclosure or from watch dogs in training (P2 and P5). Thus, they may be considered as belonging to the same clones. This information indicates that at 132e GCAT, transmission between animals is a possibility. These dogs do not share food or water; however, transmission could take place during training (e.g., biting of the same stick) or by aerosol transmission between dogs dwelling in the same enclosure.
In a preliminary study, gingival swabbing of 25 dogs at 132e GCAT was performed. Isolation plates for samples from 10 of these dogs yielded three to five colonies of P. multocida per dog. The corresponding 39 strains were studied by RFLP. All the strains from each dog had the same profile, suggesting that a dog may harbor only one strain of P. multocida. All 10 profiles were different (unpublished results). Thus, a dog may retain the P. multocida strain that it harbored when entering the training center. This suggestion would explain the clonal diversity of P. multocida strains in a situation in which transmission between dogs would be limited. If few strains had circulated at 132e GCAT, a higher prevalence of identical or closely related patterns would have been obtained. However, it would have been necessary to collect iterative samples from the same dogs to study the evolution of P. multocida colonization of the mouths of the dogs.
ERIC-PCR is a valuable technique for epidemiological studies. It provides reproducible results and has satisfactory discriminatory power, as all NER strains and most dog strains were differentiated by this technique. However, the thresholds of identity (96%) and difference (94%) between strains are very close, impairing the interpretation of results. Such limited discrimination has also been reported for Enterobacter aerogenes (7). ERIC-PCR is easier and faster to perform than RFLP; however, it cannot be recommended at the present time for epidemiological investigations of P. multocida strains. Further studies are necessary for establishing its usefulness as a screening tool.
ACKNOWLEDGMENT
We thank the Veterinary Unit of 132e Groupe Cynophile de l’Armée de Terre for providing bacteriological samples.
REFERENCES
- 1.Carter G R. Studies on Pasteurella multocida. I. A hemagglutination test for the identification of serological types. Am J Vet Res. 1955;16:481–484. [PubMed] [Google Scholar]
- 2.Chaslus-Dancla E, Lesage-Descauses M C, Leroy-Stérin S, Martel J L, Coudert P, Lafont J P. Validation of random amplified polymorphic DNA assays by ribotyping as tools for epidemiological surveys of Pasteurella from animals. Vet Microbiol. 1996;52:91–102. doi: 10.1016/0378-1135(96)00065-x. [DOI] [PubMed] [Google Scholar]
- 3.Chatelut M, Dournes J L, Chabanon G, Marty N. Epidemiological typing of Stenotrophomonas (Xanthomonas) maltophilia by PCR. J Clin Microbiol. 1995;33:912–914. doi: 10.1128/jcm.33.4.912-914.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dice L R. Measures of the amount of ecological association between species. Ecology. 1945;26:297–302. [Google Scholar]
- 5.Donnio P Y. Pasteurella. In: Freney J, Renaud F, Hansen W, Bollet C, editors. Manual of clinical bacteriology. 2nd ed. Paris, France: Elsevier; 1994. pp. 1371–1395. [Google Scholar]
- 6.Endtz H P, Giesendorf B A J, van Belkum A, Lauwers S J M, Jansen W H, Quint W G V. PCR-mediated DNA typing of Campylobacter jejuni isolated from patients with recurrent infections. Res Microbiol. 1993;144:703–708. doi: 10.1016/0923-2508(93)90034-y. [DOI] [PubMed] [Google Scholar]
- 7.Georghiou P R, Hamill R J, Wright C E, Versalovic J, Koeuth T, Watson D A, Lupski J R. Molecular epidemiology of infections due to Enterobacter aerogenes: identification of hospital outbreak-associated strains by molecular techniques. Clin Infect Dis. 1995;20:84–94. doi: 10.1093/clinids/20.1.84. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein E J C. Bite wounds and infection. Clin Infect Dis. 1992;14:633–640. doi: 10.1093/clinids/14.3.633. [DOI] [PubMed] [Google Scholar]
- 9.Heddleston K L, Gallagher J E, Rebers P A. Fowl cholera: gel diffusion precipitin test for serotyping Pasteurella multocida from avian species. Avian Dis. 1972;16:925–936. [PubMed] [Google Scholar]
- 10.Hirsh D C, Hansen L M, Dorfman L C, Snipes K P, Carpenter T E, Hird D W, McCapes R H. Resistance to antimicrobial agents and prevalence of R plasmids in Pasteurella multocida from turkeys. Antimicrob Agents Chemother. 1989;33:670–673. doi: 10.1128/aac.33.5.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmes B, Pickett M J, Hollis D G. Unusual gram-negative bacteria, including Capnocytophaga, Eikenella, Pasteurella, and Streptobacillus. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: American Society for Microbiology; 1995. pp. 499–508. [Google Scholar]
- 12.Holst E, Rollof J, Larsson L, Nielsen J P. Characterization and distribution of Pasteurella species recovered from infected humans. J Clin Microbiol. 1992;30:2984–2987. doi: 10.1128/jcm.30.11.2984-2987.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hulton C S J, Higgins C F, Sharp P M. ERIC sequences: a novel family of repetitive elements in the genomes of Escherichia coli, Salmonella typhimurium and other enterobacteria. Mol Microbiol. 1991;5:825–834. doi: 10.1111/j.1365-2958.1991.tb00755.x. [DOI] [PubMed] [Google Scholar]
- 14.Li W H. Simple method for constructing phylogenetic trees from distance matrices. Proc Natl Acad Sci USA. 1981;78:1085–1089. doi: 10.1073/pnas.78.2.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipman L J A, de Nijs A, Lam T J G M, Gaastra W. Identification of Escherichia coli strains from cows with clinical mastitis by serotyping and DNA polymorphism patterns with REP and ERIC primers. Vet Microbiol. 1995;43:13–19. doi: 10.1016/0378-1135(94)00070-d. [DOI] [PubMed] [Google Scholar]
- 16.Liu P Y-F, Lau Y-J, Hu B-S, Shyr J-M, Shi Z-Y, Tsai W-S, Lin Y-H, Tseng C-Y. Analysis of clonal relationships among isolates of Shigella sonnei by different molecular typing methods. J Clin Microbiol. 1995;33:1779–1783. doi: 10.1128/jcm.33.7.1779-1783.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loubinoux J, Garin D, Ulmer P, Richard S, Vignot J L, Courrier P L. Study of oral main aerobic bacterial flora able to contaminate bites among a military dog population. Int Rev Armed Forces Med Serv. 1997;70:102–108. [Google Scholar]
- 18.Louws F J, Fulbright D W, Stephens C T, de Bruijn F J. Specific genomic fingerprints of phytopathogenic Xanthomonas and Pseudomonas pathovars and strains generated with repetitive sequences and PCR. Appl Environ Microbiol. 1994;60:2286–2295. doi: 10.1128/aem.60.7.2286-2295.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rimler R B, Rhoades K R. Serogroup F, a new capsule serogroup of Pasteurella multocida. J Clin Microbiol. 1987;25:615–618. doi: 10.1128/jcm.25.4.615-618.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivera I G, Chowdhury M A R, Huq A, Jacobs D, Martins M T, Colwell R R. Enterobacterial repetitive intergenic consensus sequences and the PCR to generate fingerprints of genomic DNAs from Vibrio cholerae O1, O139, and non-O1 strains. Appl Environ Microbiol. 1995;61:2898–2904. doi: 10.1128/aem.61.8.2898-2904.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Barradas M C, Hamill R J, Houston E D, Georghiou P R, Clarridge J E, Regnery R L, Koehler J E. Genomic fingerprinting of Bartonella species by repetitive-element PCR for distinguishing species and isolates. J Clin Microbiol. 1995;33:1089–1093. doi: 10.1128/jcm.33.5.1089-1093.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuur P M H, Haring A J P, van Belkum A, Draaisma J M T, Buiting A G M. Use of random amplification of polymorphic DNA in a case of Pasteurella multocida meningitis that occurred following a cat scratch on the head. Clin Infect Dis. 1997;24:1004–1006. doi: 10.1093/clinids/24.5.1004. [DOI] [PubMed] [Google Scholar]
- 23.Sechi L A, Zanetti S, Dupré I, Delogu G, Fadda G. Enterobacterial repetitive intergenic consensus sequences as molecular targets for typing of Mycobacterium tuberculosis strains. J Clin Microbiol. 1998;36:128–132. doi: 10.1128/jcm.36.1.128-132.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snipes K P, Hirsh D C, Kasten R W, Hansen L M, Hird D W, Carpenter T E, McCapes R H. Use of an rRNA probe and restriction endonuclease analysis to fingerprint Pasteurella multocida isolated from turkeys and wildlife. J Clin Microbiol. 1989;27:1847–1853. doi: 10.1128/jcm.27.8.1847-1853.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Struelens M J, Bax R, Deplano A, Quint W G V, van Belkum A. Concordant clonal delineation of methicillin-resistant Staphylococcus aureus by macrorestriction analysis and polymerase chain reaction genome fingerprinting. J Clin Microbiol. 1993;31:1964–1970. doi: 10.1128/jcm.31.8.1964-1970.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talan D A, Citron D M, Abrahamian F M, Moran G J, Goldstein E J C. Bacteriologic analysis of infected dog and cat bites. N Engl J Med. 1999;340:85–92. doi: 10.1056/NEJM199901143400202. [DOI] [PubMed] [Google Scholar]
- 27.Vasquez J E, Ferguson D A, Bin-Sagheer S, Myers J W, Ramsak A, Wilson M A, Sarubbi F A. Pasteurella multocida endocarditis: a molecular epidemiological study. Clin Infect Dis. 1998;26:518–520. doi: 10.1086/517105. [DOI] [PubMed] [Google Scholar]
- 28.Versalovic J, Koeuth T, Lupski J R. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watts J L, Yancey R J, Salmon S A, Case C A. A 4-year survey of antimicrobial susceptibility trends for isolates from cattle with bovine respiratory disease in North America. J Clin Microbiol. 1994;32:725–731. doi: 10.1128/jcm.32.3.725-731.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson M A, Rimler R B, Hoffman L J. Comparison of DNA fingerprints and somatic serotypes of serogroup B and E Pasteurella multocida isolates. J Clin Microbiol. 1992;30:1518–1524. doi: 10.1128/jcm.30.6.1518-1524.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson M A, Morgan M J, Barger G E. Comparison of DNA fingerprinting and serotyping for identification of avian Pasteurella multocida isolates. J Clin Microbiol. 1993;31:255–259. doi: 10.1128/jcm.31.2.255-259.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]