Abstract
Minimally invasive focal therapies for non-viral oncolysis are a cornerstone of cancer therapeutics. Our ability to optimally deploy oncolytic therapies and identify synergistic combination approaches, requires a deeper understanding of elicited biological responses. Extracellular vesicles (EV), which orchestrate a variety of pathophysiological processes and play a critical role in the evolution of primary and disseminated tumors, are now known to be potently modulated by oncolytic focal therapies such as radiotherapy, photodynamic therapy and therapeutic ultrasound. In this review, we summarize the diverse impacts of the aforementioned therapeutic modalities on EV biology, as well as highlight the most recent advances in EV-based drug delivery systems leveraging these modalities.
Keywords: Oncolytic therapy, cancer, extracellular vesicles, exosomes, focal therapy, therapeutic ultrasound
The Growing Importance of Extracellular Vesicles in Oncolytic Therapy
Extracellular vesicles (EVs) (see Glossary) profoundly impact many physiological and pathological processes[1], most notably cancer (details on EV subtypes and their role in cancer are provided in Box 1). EVs facilitate cell-to-cell signaling among a variety of cell types in the tumor microenvironment (TME), mediating resistance, metastasis and immune responses[2]. Given their central role in oncology, there is a pressing need to understand whether and how EV phenotype and function may be altered when tumors undergo various modes of therapy.
Box 1: Overview of EVs and their role in cancer.
The three main classes of EVs are apoptotic bodies, microvesicles and exosomes[3] (Figure I). The largest class of vesicles, apoptotic bodies, are approximately 50–5000 nm in diameter and released during the later stages of apoptosis[2]. Microvesicles are 50–1000 nm in size and originate via direct budding and fission from the plasma membrane[51]. They can be secreted by resting or stimulated cells, but the rate of microvesicle shedding greatly increases upon stimulation by increasing intracellular levels of Ca2+ [51]. Exosomes are typically 30–150 nm in diameter and are distinctly formed from late endosomes and released through exocytosis; they contain many of the transferrin receptors found in large endosomes as well as key proteins – such as tetraspanins (e.g. CD63, CD9, and CD81) – that aid in their identification[46].
These classes of EVs have been shown to facilitate critical pathways of intercellular communication, as enabled by their unique and versatile bioactive molecular payloads that often mirror the composition of their parent cells[51]. In healthy physiology, EVs have been implicated in the processes of blood coagulation[67], immunomodulation[68,69], stem cell differentiation[70], tissue regeneration[71], reproductive biology[72], pregnancy[73], as well as the development of the nervous system[3]. In cancer, the chief focus of this review, the highly concentrated genetic and proteomic payloads of exosomes and microvesicles are capable of mediating immune responses, angiogenesis, hemostasis, and cancer progression[51]. Indeed, circulating tumor-derived exosomes (TEXs) from the primary tumor can reprogram stromal cells in distal tissues, altering cytokine expression and remodeling the extracellular matrix to promote metastasis. For example, TEXs can influence non-tumor cells to alter their extracellular composition within the TME to favor tumor growth and metastasis[3]. TEXs have also been shown to trigger shifts in fibroblasts that favor more pro-angiogenic and pro-tumorigenic phenotypes[3]. Additionally, TEXs in the TME can transfer oncogenic proteins (e.g. pigment epithelium-derived factor, tyrosine protein kinase Met) to non-cancerous cells, activating downstream signaling pathways that are overactive in many cancers, e.g. MAPK and PI3K-AKT-mTOR [3]. TEXs can also enter the bloodstream and interface with other tissues, facilitating formation of pre-metastatic niches at sites distal to the primary tumor[3].
Because EVs are involved in metastasis and contain genetic material, circulating exosomes have the potential to act as cancer biomarkers[3]. Potential biomarker candidates include oncogenic mRNAs, miRNAs and double stranded DNA fragments found in exosomes. Understanding the roles EVs play in cancer can allow them to be used as biomarkers and other identifiers of cancer.
(Box 1) Figure I. Extracellular vesicle (EV) subtypes.
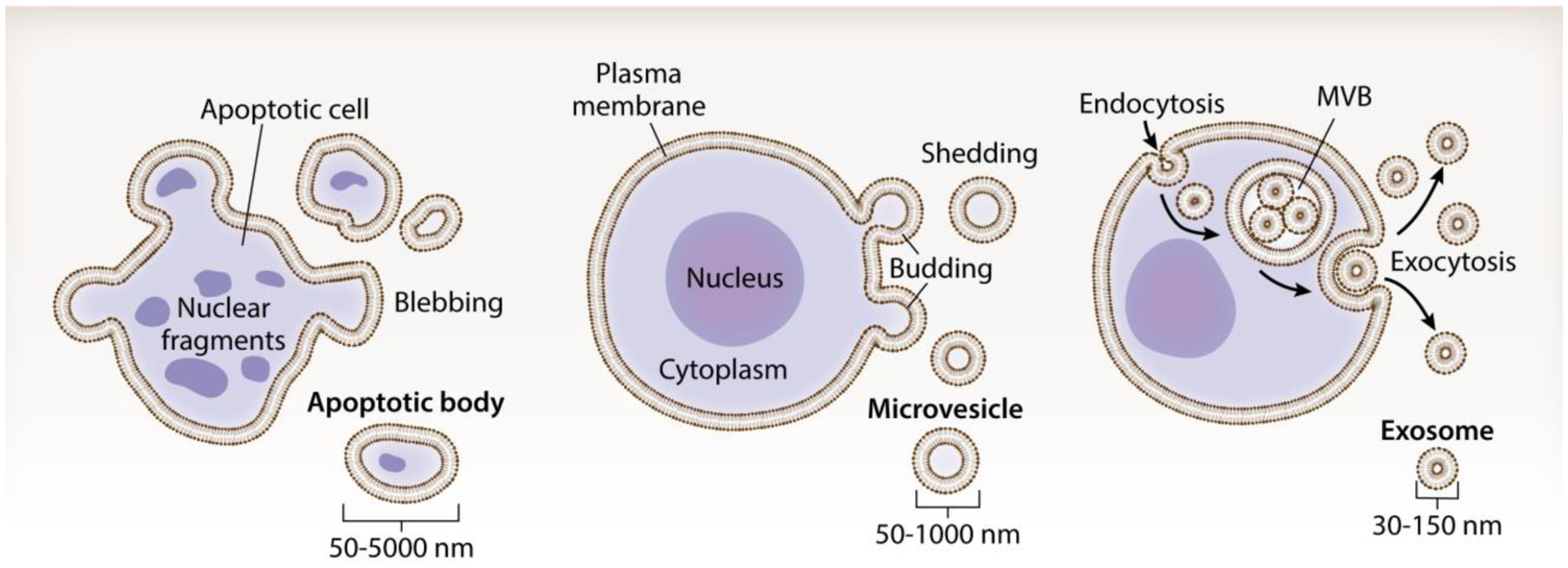
EVs are classified on the basis of their biogenesis and origin. Apoptotic bodies (50–5000 nm) arise when cells undergo apoptosis, typically containing nuclear fragments and cell organelles. Microvesicles (otherwise known as ectosomes or microparticles; 50–1000 nm) are shed via outward budding and fission of the plasma membrane. Exosomes (30–150 nm) are generated within the endosomal network and are released via exocytosis when a multivesicular body (MVB) fuses with the plasma membrane.
Oncolytic cancer therapies such as radiotherapy, photodynamic therapy (PDT), and therapeutic ultrasound (TUS) are non-invasive and focal. For non-ionizing modalities, there also exists the promise of mitigated off-target toxicity (details on focal oncolytic therapy modalities are provided in Box 2). Further, they all center on the localized deposition of energy into tumors, which can produce unique stresses (e.g. genotoxic, oxidative, thermal, mechanical) on cancer cells and surrounding stroma[3]. In turn, these stresses trigger the differential release of EVs and modify their cargo[4–6]. Such alterations to the EV profile of treated cells can yield wide-reaching and long-lasting consequences. An improved understanding of these biological consequences will not only yield important insights regarding the mechanistic underpinnings of oncolytic therapies, but also pathways for improving the deployment and monitoring of these therapies for cancer treatment.
Box 2: Overview of key modalities for focal oncolytic therapy.
Oncolytic therapies are physical or chemical modalities capable of non-viral oncolysis and offer the key advantages of being minimally invasive and locoregional. Ionizing radiation - a staple of modern cancer therapy - extracorporeally transmits x-rays or radioactive particles through the nuclei of cells to disrupt the genetic sequence and cause mutations, leading to genotoxic stresses and cell death in cancer [15,74]. To maximize tumor tissue destruction and mitigate damage to off-target tissues, radiotherapy beams are frequently guided by CT, MRI, or PET imaging[75]. Nonetheless, radiotherapy can damage and destroy cells outside of the targeted zone through a phenomenon known as the bystander effect, which is partially mediated by EVs [14,41,60].
PDT induces oxidative stresses to kill tumor cells through the interaction of a chemical photosensitizing agent with extracorporeally administered light[76,77]. In PDT, a photosensitizer (PS) such as porphyrin compounds or aminolevulinic acid is injected systemically and preferentially taken up by cancer cells[76]. Upon PS absorption, cancer cells are illuminated with light of a specific wavelength that reacts with the PS to produce reactive oxygen species (ROS), in turn leading to cell death [77]. PDT is most effective for treating tumors close to the surface of the body as the intensity of light is rapidly attenuated by skin and other connective tissues. Studies of PDT and EVs have focused on the connection between EVs and PS sequestration or the use of engineered EVs for the delivery of PSs through biological barriers.
TUS utilizes non-ionizing acoustic energy to exert a diverse array of localized bioeffects ranging from mechanical to thermal in nature[78]. Typically applied under the guidance of MRI or ultrasound imaging, TUS waveforms most commonly leverage focused transducers that concentrate the acoustic energy into a focal ellipsoid volume, thereby avoiding damage to intervening tissues. Low-intensity TUS regimens generate mild or sublethal cellular stresses, mediating such effects as hyperthermia, drug and gene delivery, neuromodulation, and sonodynamic therapy (SDT) [79]. Meanwhile, high-intensity TUS regimens can drive thermally or mechanically ablative effects within a targeted focal region[80]. The mechanical bioeffects of TUS can be amplified by systemically circulating ultrasound contrast agents (UCAs) such as microbubbles or nanodroplets, which cavitate (i.e. oscillate) in the presence of an acoustic field, thereby augmenting the physical effects of TUS[81]. To date, studies evaluating the connection between EVs and TUS have focused on lower intensity ultrasound regimens such as UCA-assisted ultrasound, hyperthermia, and SDT.
Here, we comprehensively review how localized therapies impact EV biology in the context of cancer, as well as of emerging therapeutic paradigms leveraging EVs in combination with localized therapeutic modalities. Finally, we offer perspectives on future research directions and the promise of continued scientific exploration at the junction of EVs and oncolytic therapy. Note that in the forthcoming sections of this review, EV classifications reflect the terminology used in source literature.
Impacts of Oncolytic Therapies on Extracellular Vesicle Biology
Here, we review oncolytic focal therapy studies that have reported EV-related bioeffects. Despite the broad definitions of ‘oncolytic’ or ‘focal’, this review is limited to treatment modalities that have been investigated to date: radiation, photodynamic therapy (PDT), and therapeutic ultrasound (TUS). As there are notable variations in exposure conditions, experimental methodologies and model systems, we synthesize key information for all modalities in Table 1.
Table 1.
Overview of EV-related bioeffects following oncolytic therapy
| Key Observations | ||||||
|---|---|---|---|---|---|---|
| Lehmann, 2008 | RTx | LNCaP; 22Rv1 human prostate cancer cells in vitro | 0 or 4 Gy irradiation with Cs137 source | Differential ultracentrifugation | Exosomes | Irradiation induces a p53 dependent increase in exosome biogenesis. |
| Al-Mayah, 2012 | RTx | MCF7 breast epithelial cancer in vitro | 2 Gy of X rays | Differential ultracentrifugation with filtration | Exosomes | Exosomes from irradiated breast epithelial cancer cells can induce DNA damage in untreated cells that is similar to DNA damage following direct treatment with radiation. Treating exosomes with RNAse abrogates the DNA damaging effects of these exosomes. |
| Al-Mayah, 2015 | RTx | MCF7 breast epithelial cancer in vitro | 2 Gy of X rays | Differential ultracentrifugation with filtration | Exosomes | Exosomes from progeny of irradiated cells can induce DNA damage in untreated cells. RNAse treatment abolishes the ability of exosomes from irradiated cells to cause DNA damage in untreated cells shortly after exposure to the exosomes, but the RNAse treated exosomes still trigger DNA damage in cells several generations after exposure. |
| Ramakrishnan, 2019 | RTx | Multiple human glioblastoma lines | 2 or 3 Gy/day for 2 days oR 2 Gy/day for 5 days | Total Exosome Isolation Reagent or ExoQuick | EV | EVs appeared to be used to export miR-603 from irradiated cells, allowing cells to express IGF1/R, return to a cancer stem-cell-like state, and acquire resistance to radiation. Export of miR-603 also de-represses MGMT which confers resistance to DNA alkylating agents. |
| Mutschelknaus, 2016 | RTx | BHY and FaDu head and neck cancer cell lines in vitro | 0–9 Gy irradiation with Cs137 source | Differential ultracentrifugation | Exosomes | Exosomes from cancer cells increased proliferation regardless of whether the exosomes came from irradiated or non-irradiated cells. Incubation with exosomes followed by irradiation showed that exosomes conferred some resistance to radiation with peak resistance coming from exosomes isolated from cells treated at 6 Gy. |
| Wen, 2016 | RTx | Bone marrow | 100–500 cGy whole body irradiation prior to harvesting cells for exosome isolation | Differential ultracentrifugation | EV | EVs derived from MSCs can reduce irradiation induced DNA damage to bone marrow. At least part of this effect appears to be mediated by an increase in miRNA content in EVs. |
| Lin, 2020 | RTx | Murine H22/4T1 | 8 Gy | Ultracentrifugation | Small EV (~180 nm) | Radiation treatment can increase HSP70/90 content of small EVs and increase infiltration of CD4 and CD8 T cells that may initiate abscopal effects. |
| Arscott, 2013 | RTx | Human glioblastoma | 0–4 Gy X rays | Differential ultracentrifugation | Exosomes | Radiation led to increased overall uptake of radiation-derived exosomes (1.3-fold). Radiation appears to enhance cell migration. |
| Jella, 2020 | RTx | Murine B16F10 melanoma transduced with lymphocytic choriomeningitis glycoprotein and green fluorescent protein | 0–20 Gy with Cs137 source | Supercentrifugation | Exosomes | Irradiation produced a dose dependent increase in exosome release and content of immunological proteins. Exosomes from irradiated cells increased DC activation. Exosomes from irradiated cells delivered to mice intratumorally conferred greater tumor growth control than exosomes from unirradiated cells. Tumor control was mediated by NK cells and was cD8+ T cell-independent. |
| Diamond, 2018 | RTx | Murine TSA mammary carcinoma | 8 Gy × 3 treatments | Differential ultracentrifugation | Exosomes | Irradiation of tumor cells alters the exosome proteome and increases the tumor dsDNA content. dsDNA in tumor exosomes activates DCs through the cGAS/STING pathway. Vaccination with exosomes from irradiated tumor cells generates adaptive anti-tumor immunity. |
| Li, 2019 | TUS | DC-derived exosomes to treat HUVECs | 1.5 MHz pulses with pulse width 200 μs, repeated at 1 kHz, 30 mW/cm2 | Exo-Quick | Exosomes | Exosomes from LIPUS-treated DCs can reduce inflammation pathways in HUVECs by inhibiting TNFα activation and subsequently lowering NF-kB activation. |
| Yuana, 2017 | TUS | FaDu (human pharyngeal squamous carcinoma) | 1.5 MHz sinusoidal signal with 100 μs pulse length, 1 kHz pulse repetition frequency, microbubble containing medium | None, used ExoCap magnetic capture beads CD9 and CD63 | EV | Proteins CD9, CD63, alix and calnexin enriched in EVs suggest that USMB-treated FaDu cells sort proteins through EVs. |
| Paproski, 2016 | TUS | HT1080 and HT1080-GFP, fibrosarcoma | 1.15 MHz frequency, 1 burst with 10–10,000 US cycles/burst, 30 MPa peak-peak pressure, perfluorobutane nanodroplets | Micro flow cytometry with initial centrifugation steps | EV | FUS with nanodroplets enhanced release and detection of EVs and biomarkers in blood. In vivo data showed apoptosis within small tumor regions within ultrasound focal zones and no further tumor metastasis due to FUS. In vitro data showed enhanced detection of mRNA, miRNA and tumor genomic DNA in released tumor EVs. |
| Li, 2018 | TUS | DCs from C57BL/6 mice | 1.5 MHz frequency pulses, 200 μs pulse, repeated at 1kHz | Exo-Quick | Exosomes | EVs after LIPUS treatment might have inflammatory suppressive effects by blunting the NF-kB signaling pathway in endothelial cells, and inhibiting the TNFα - induced endothelial inflammation. |
| Yuana, 2020 | TUS | FaDu, MDA-MB-123 | 1 kHz pulse repetition, 0.6, 0.7, 0.8 MPa US conditions, SonoVue lipid shelled MB encapsulating sulfur hexafluoride gas | measured by flow cytometry and immuno-magnetic beads | EV | USMB can be used to load model drugs into endothelial cell EVs. |
| Lucchetti, 2020 | TUS | CaCo2 and HT29 | 2400 kHz frequency | Ultracentrifugation and flow cytometry | EV | LIPUS influences colorectal cancer phenotype, increase of E-cadherin and decrease of Vimentin reverses EMT pathway. |
| Zhao, 2020 | TUS | A2780 and SKOV3 | 0.5–1 W/cm2 for 10, 30, or 60 minutes | Exo-Quick | Exosomes | LIUS irradiation increases exosome secretion via increased CHMP2B, CHMP5, and YKT6, both in vitro and in vivo. |
| Sheybani, 2020 | TUS | GL261, DCs | 1.1 MHz transducer, evenly spaced 252 sonications for 5 seconds at 5W power | Differential ultracentrifugation | EV | Glioma derived EVs biogenesis after FUS was 46% higher and also induced an upregulation of IL-12p70 production in sdendritic cells compared to untreated glioma-derived EVs. |
| Meng 2021 | TUS | WHO grade IV glioblastoma | 220 kHz transducer, magnetic resonance guided ultrasound (MRgFUS) | Nanoscale flow cytometry | EV | Transient blood brain barrier opening increases neuron-derived EVs and other brain-derived biomarkers by 3.2-fold compared to unopened barrier. |
| Goler-Baron, 2012 | PDT | MCF7 and MCF7-MR | IA photosensitizer, 512 or 630 nm emission wavelength | N/A | EV | Upon photosensitization, IA-loaded EVs release their IAs due to the lysis of the cell membrane and form MSIS. |
| Baydoun, 2020 | PDT | OVCAR3, SKOV3 | 1mW/cm2, 668 nm laser | Differential ultracentrifugation | EV | PDT induced apoptosis of ovarian tumor cells very rapidly, 90% of tumor cells died after 1 hour illumination, supernatants of OVCAR3 activated PBMCs, and can activate CD4+ and CD8+ T cells. |
| Cheng, 2019 | PDT | 4T1, murine breast cancer | 630 nm LED light (in vitro), 630 nm He-Ne laser (in vivo), PDT + ChiP | Ultracentrifugation | Exosomes | Exosomes conjugated ChiP enhanced the generation of ROS when used with dual-stage photodynamic therapy. Nucleus targeting ability of ChiP-Exo enhanced intranuclear PDT effect and significantly inhibited tumor growth. |
Abbreviations: cGAS: Cyclic GMP-AMP synthase; ChiP: Chimeric peptide; DC: Dendritic cell; dsDNA: Double stranded DNA; EMT: Epithelial-to-mesenchymal transition; EV: Extracellular vesicle; HUVEC: Human umbilical vein endothelial cell; IA: Imidazoacridinone; LIUS: Low-intensity ultrasound; LIPUS: Low-intensity pulsed ultrasound; MRgFUS: MR-guided focused ultrasound; MSIS: Multiple small intravesicular structures; NK: Natural killer; PBMC: Peripheral blood mononuclear cell; PDT: Photodynamic therapy; ROS: Reactive oxygen species; RTx: Radiotherapy; STING: Stimulator of interferon genes; TUS: Therapeutic ultrasound;
Radiotherapy.
Radiotherapy damages target cell DNA, produces reactive oxygen species (ROS), and elicits cell death[7]. Radiotherapy also induces a “bystander effect”, wherein distal cells experience similar radiation-induced bioeffects, including death of unirradiated cells[8–10]. The bystander effect is mediated by both juxtacrine and paracrine signaling[9]. Juxtacrine signaling is largely driven by gap junctional connections between cells[11], while paracrine signaling produces a bystander effect through the transfer of EVs from irradiated cells to bystander cells[12,13].
The cargo of EVs from irradiated cells is involved in the bystander effect. It has been demonstrated that incubation of epithelial breast cancer cells with conditioned media from irradiated cells results in DNA damage and increased mutation rate consistent with genotoxic damage which persisted for many generations, and later confirmed these effects were mediated by exosomes[12,13]. Notably, even exosomes derived from the unirradiated progeny of irradiated breast cancer cells increased the rate of chromosomal damage in naïve breast cancer cells[13]. RNase treatment of exosomes from irradiated cells reduced the DNA damage sustained by recipient cells, but their progeny displayed DNA damage[12,13]. However, treatment of exosomes from irradiated cells with both RNase and boiling to denature protein prevented the development of DNA damage in unirradiated cells suggesting that both the RNA and protein cargo of tumor-derived exosomes (TEXs) are involved in the anti-tumor bystander effects of radiotherapy on unirradiated tumor cells. More in-depth discussion of the roles of EVs in the radiotherapy bystander effect is available elsewhere[9,14,15].
Notably, exosomes from irradiated tumor cells also have the potential to enhance tumor survival and metastasis through enhancement of DNA repair and cell migration pathways in recipient cells [16,17]. Incubation with TEXs from either irradiated or untreated squamous head and neck cancer (HNC) cells conferred radiation resistance to multiple lineages of HNC via DNA repair[16]. The development of radiation resistance was interrupted by treatment of exosomes with RNase, suggesting that exosomes delivered DNA stabilizing RNA molecules. These results are similar to studies in glioblastoma, wherein irradiation stimulated exosomal enrichment of miR-603, which has a role in repairing DNA damage[18]. In glioblastoma and squamous HNCs, irradiation stimulated an increase in cellular uptake of exosomes, enhancing the effectiveness of DNA-repair RNA molecules[16,17]. Furthermore, a hepatocellular carcinoma cell line showed a radiotherapy-triggered, dose dependent increase in exosome release that returned to baseline at high doses [16]. Similarly, multiple human glioblastoma exhibit cell lines produce irradiation dose (up to 8 Gy) dependent increases in exosome abundance within 24–48 hours of treatment[17]. In another study, irradiation decreased the total number of EVs released by glioma cells, but the released EVs contained more miR-603 copies[18]. The reason for this decrease in EV release following radiotherapy is unclear and further study is needed as the dose-dependent effects of irradiation on EV release has not been previously studied in the cell lines used. Irradiated glioblastoma cells also release exosomes that promote migration and invasion of recipient cancer cells through transfer of mRNAs and proteins that activate the focal adhesion kinase (FAK) pathway.[17]. Activation of the FAK pathway is frequently observed in cancer and triggers increased motility which supports subsequent metastasis.
Modifications to the protein and nucleic acid cargo of TEXs can also regulate the host immune response to tumors[19]. Irradiating melanoma cells increases TEX content of immunological proteins such as calreticulin and HMGB1 in a dose dependent manner[20]. These proteins support adaptive anti-cancer immune responses through the activation of dendritic cells (DCs)[21]. TEXs of irradiated breast cancer cells also activate DCs through cyclic GMP-AMP synthase (cGAS) stimulation, presumably by delivering double stranded DNA to DCs[22]. Further, these cGAS-activating TEXs conferred resistance to tumor implantation in vivo in vaccination experiments[22]. TEXs from irradiated melanoma cells also show in vivo delay of tumor outgrowth mediated primarily by increased natural killer cell effector function[20]. Further, EVs derived from metastatic breast cancer cells were also enriched for the proteins HSP70 and HSP90 which can act as damage associated molecular patterns following irradiation[23]. In contrast, irradiation enriches B7-H3 inhuman prostate cancer-derived TEXs, an immune checkpoint molecule related to metastasis that inhibits tumoricidal T cells[24,25]. Looking ahead, modification of the immunological protein content of exosomes in response to irradiation bears significant implications for cancer immunotherapy. We submit that harnessing of these EV cargo modifications could substantially aid cancer diagnosis and therapy.
Photodynamic Therapy.
PDT uses targeted delivery of light in combination with a PS to treat cancers and non-malignant disorders[26]. PDT has been used with and without chemotherapies to achieve pronounced cytotoxic effects within tumor tissues, serving as a non-ionizing, non-invasive method for tumor destruction. In an in vitro human prostate cancer study comparing doxorubicin (DOX) monotherapy and PDT, both treatments induced EV release[5]. However, cells photosensitized with meta-tetra(hydroxyphenyl) chlorine (mTHPC) and treated with PDT released significantly more EVs than those treated with DOX. Moreover, the released EVs contained the drug (either PS or chemotherapy) to which the parent cells had been exposed and transferred their contents to naive cells. When the study was repeated in tumor-bearing mice, both PDT and DOX independently induced the release of EVs carrying oncogenes and oncoproteins[5], underscoring the potential contribution of EVs to the dissemination of oncogenes and oncoproteins to distal naïve cells and conferral of drug resistance through reduction of intracellular drug concentrations.
Another study in an ex vivo HNC model found that exosomes isolated from post-therapy plasma harbored decreased concentrations of EV proteins relative to those in pre-therapy plasma[27]. These exosomes contained both N-Cadherin and E-Cadherin, important markers of epithelial-to-mesenchymal transition (EMT). Naive exosomes had the highest concentrations of N-Cadherin whereas exosome cargo following therapy reflected high E-Cadherin concentrations. In the same model, untreated tumor cells exposed to exosomes isolated from post-PDT plasma displayed reduced motility, proliferation, and invasiveness compared to tumor cells exposed to pre-PDT exosomes[27]. Taken together, PDT may reduce the growth and metastasis of HNC tumors through the reversal of EMT, specifically through upregulation of E-cadherin in PDT-exposed exosomes.
PDT has also been demonstrated to potentiate sterile inflammatory mechanisms[28]. In an in vivo model of squamous cell carcinoma (SCC), PDT with aminolevulinic acid (ALA-PDT) resulted in exosomes that stimulated dendritic cell (DC) maturation; moreover, exosomes derived from SCCs exposed to ALA-PDT promoted TGF-β1 secretion by fibroblasts and ultimately increased proliferation of the cancer cells[5]. In another study evaluating the impact of the secretome of PDT-treated ovarian cancer cells on human peripheral blood mononuclear cells, PDT favored the release of EVs prone to activating immune cells - most notably CD4+ and CD8+ T cells[28]. In a human cervical cancer model, in vivo ALA-PDT treatment significantly elevated exosomes containing HMGB1, and this effect was reversible by transfection with a miR-34a mimic. As it was observed that this also reversed PDT-mediated elevation in IFNα, TNFα, IL-6, IL-12, and IL-18 secretion by mature DCs, ALA-PDT promotes DC maturation by modulating miR-34a-mediated secretion of HMGB1-bearing exosomes[29].
The use of PDT in conjunction with exosomes is also being explored as a method for overcoming multidrug resistance[26]. In a mitoxantrone-resistant human breast cancer model, photoexcitation of imidazoacridinones (IAs) that accumulated specifically within EVs increased cytotoxicity[26]. Meanwhile, in a human colon cancer model, mTHPC loaded in EVs (mTHPC-EV) was more cytotoxic in the setting of PDT - with an LD50 nearly 2.5 times lower than that for free mTHPC[30]. This increased photocytotoxicity corresponded with a greater degree of apoptosis in mTHPC-EV treated tumor-spheroids relative to those treated with free mTHPC.
Therapeutic Ultrasound.
TUS is the most common non-invasive, non-ionizing technique for disease screening, assessment and treatment. A diverse array of thermal and mechanical TUS regimens is currently being evaluated pre-clinically and clinically for solid tumor therapy[31]. To date, a number of studies have explored the impact of low-intensity TUS regimens on EV release and profile, as well as the ability of TUS to potentiate delivery of EV-encapsulated therapeutic agents in tumor settings. The latter is discussed in the “Extracellular Vesicles as Drug Delivery Vehicles” section of the paper.
Sonoporation is a TUS mechanism of action commonly leveraged for drug delivery owing to its formation of transient cell membrane pores and enhancement of endocytosis after US-induced cavitation of microbubbles (USMB)[32]. Treatment of human umbilical vein endothelial cells (HUVEC) with this approach triggers the increased release of EVs in an acoustic pressure-dependent manner[33]. Across multiple cancer models – i.e. colorectal cancer and fibrosarcoma– TUS exposure enhances the release of TEXs[34,35]. In a human fibrosarcoma models, TUS in the presence of perfluorobutane nanodroplets increased the release of EVs and augmented diverse payloads including mRNA, miR21-5p, tumor specific N921 RAC1 mutation, and tumor genomic DNAs; no enhancement of tumor metastasis was observed[34]. Similar results were found in two human colorectal adenocarcinoma models exposed to pulsed TUS, wherein EV release was augmented (by 1.4- and 2-fold in the HT29 and CaCo2 lines, respectively) 24 hours after treatment - despite noteworthy reductions in cell viability at higher pressures[35]. The same study also demonstrated that pulsed TUS modulates cancer cell architecture via the actin cytoskeleton and EV trafficking. Similar findings relating to the increase of exosome yield following low-intensity TUS have been noted in models of human ovarian cancer[36]; in this study, exosome augmentation was underpinned by increased levels of the endosomal sorting and exosome secretion markers CHMP2B, CHMP5, and YKT6[36]. Yet another study evaluating murine glioma cells unveiled that a TUS hyperthermia regimen not only increased EV release by nearly 50%, but also promoted downregulation of EV protein markers associated with cancer progression and resistance[37].
Finally, there is emerging evidence for the role of TUS-exposed EVs in immunity. Indeed, a recent study tested this by incubating bone marrow DC-derived exosomes with HUVECs to evaluate TNFα-induced endothelial inflammation. Pre-treatment of these exosomes with low-intensity pulsed TUS elicited anti-inflammatory responses in the endothelial cells specifically by blunting TNFα-induced endothelial inflammation, thereby inhibiting activation of the NF-κB signaling pathway. DC-derived exosomes also saw enhanced after TUS [38]. Meanwhile, in an in vitro glioma model, immortalized DCs exposed to EVs from glioma cells treated with TUS hyperthermia significantly upregulated production of IL-12p70, a pro-inflammatory cytokine[37]. This finding not only underscores the possibility that TUS might modulate the tumor-immune landscape via EVs, but also illuminates a potential role for tumor-derived EVs as an asset in the monitoring of response to FUS therapies. In order to harness this potential fully, however, we must improve our understanding of the complex role that EVs can play in tuning the immune landscape[2,39].
Chemotherapy.
Chemotherapies – though not explicitly a mode of focal therapy - are often sequentially or concomitantly administered with focal oncolytic therapies. As there is a growing appreciation of the interface between chemotherapies and EV biology, we briefly touch on their impacts in monotherapy form. As with other cellular stressors, chemotherapy generates a dose-dependent release of EVs both in vitro and in vivo[5,40]. EVs released in response to chemotherapy contribute to several tumor-promoting functions including multi-drug resistance and tumor metastasis[41]. EVs enable cancer cells to resist chemotherapeutic treatment by sequestering and removing drugs from the intracellular space[5]. In addition to sequestration of chemotherapy, EVs from chemoresistant cancer cells can deliver resistance-conferring protein and RNA cargoes to chemo-sensitive cells[42,43]. EV-related mechanisms underscoring the evolution of chemotherapy resistance are reviewed elsewhere [41].
Extracellular Vesicles as Drug Delivery Vehicles
Nanotechnology-based drug delivery systems leveraging synthetic or biological nanoparticles to target anti-cancer drugs to tumors are under exploration for wide range of cancers. Under this umbrella, EVs represent promising nanomedicines owing to physicochemical properties that enable them to bypass several traditional barriers to the transport and release of cancer drugs [44]. Exosomes are the most commonly used EV for drug delivery, in large part owing to their small (40–100 nm) size[45]. EVs are biocompatible and non-toxic, as evidenced by their role in biological signaling and release from most cell types[46]. Importantly, EVs are also protected from elements of the immune response such as complement cascade, which can pose challenges for traditional nanoparticle delivery[47]. Furthermore, exosomes are readily uptaken by cancer cells and the fusion of exosomes with the cell membrane to deposit exosome contents into cells avoids specific barriers to nanoparticle delivery including the P-glycoprotein (PGP) transporter overexpressed in cancer and involved in drug efflux [16,48,49]. Exosomes naturally carry proteins and RNAs and can be engineered to incorporate hydrophobic or hydrophilic small molecules[45,50],including chemotherapeutics such as DOX and cytosine deaminase[45]. Endogenously derived exosomes with recombinant proteins for targeting and therapy have also been used to induce anti-tumor effects and modulate immune responses in different models[51]. Exosomes can be loaded with diverse cargo through a variety of methods including electroporation, sonoporation, transfection of the exosome donor cells, and incubation with cargo[49,52]. Pre-clinical studies investigating EV-facilitated drug delivery approaches for oncolytic therapy are summarized in Table 2. As will be discussed herein, engineered exosomes offer exciting opportunities for use in conjunction with PDT or ultrasound to deliver sensitizing agents or adjuvants for combination therapy.
Table 2.
Overview of oncolytic therapy-mediated drug delivery studies leveraging EVs
| Key Observations | ||||||||
|---|---|---|---|---|---|---|---|---|
| Piffoux, 2018 | PDT | CT26, human colon carcinoma | mTHPC, 10 J/cm2 by a 650 nm laser | HUVEC and MSC cells fused with PEG and liposomes | mTHPC | PDT + mTHPC | 2.5, 0.5, and 0.1 μM | PEGylation of EVs with liposomes resulted in 3–4-fold increased ability to deliver mTHPC to tumor cells compared to liposomes alone. |
| Fuhrman n, 2015 | PDT | MDA-MB231, breast cancer | 633 nm for 200 or 400 seconds (0.3–0.6 J/well) | hMSCs, HUVECs, hESCs, and MDAs | por, TMP, porBA | PDT + por | 0.1 μM | por encapsulated EVs greatly reduced cell viability upon laser irradiation and increased uptake by MDA-MB231 cells by 60%. Total drug concentration was comparable or lower to photosensitizing drugs, suggesting a more effective treatment. |
| Cheng, 2019 | PDT | 4T1, murine breast cancer | 630 nm LED light (in vitro), 630 nm He-Ne laser (in vivo) | Murine blood-derived | ChiP | PDT + ChiP | 30 μM | Exosomes conjugated with chimeric peptide (ChiP) enhanced the generation of ROS when used with dual-stage photodynamic therapy. Nucleus targeting ability of ChiP-Exo enhanced intranuclear PDT effect and significantly inhibited tumor growth. |
| Bai, 2019 | TUS | GL261, glioma | 1.0 MHz, 0.6 W/cm2, duration of 1 minute | RAW264.7 cells, serum from C57BL mice | Dox | TUS BBB disruption for Exos-Dox delivery; TUS-triggered release of Dox | 10 μg/mL | Exosomes isolated from different tissues and loaded with Dox have better delivery with TUS than free Dox. When treated with TUS, Exos-Dox can effectively cross the BBB both in vitro and in vivo, and improved anti-glioma activity. |
| Liu, 2019 | TUS | 4T1, MDA-MB-231, MCF-7 | 1.0 MHz in vitro: 60 second duration with load power 1–6 W in vivo: SonoVUE, 2 W for 3 minutes | 4T1 | DVDMS | SDT | 5 μg/mL | Exosomes loaded with DVDMS enhanced intracellular ROS production compared to free-DVDMS plus US. Combination ultrasound (guided and therapeutic) in conjunction with DVDMS loaded exosomes inhibited tumor growth in both volume and weight, and inhibited metastasis. |
| Yuana, 2020 | TUS | FaDu, MDA-MB-123 | 1 kHz pulse repetition, 0.6, 0.7, 0.8 MPa US, SonoVue lipid shelled MB encapsulating sulfur hexafluoride gas | HUVEC cells | CTG, BSA FITC | USMB | 0.7–3.5×108 MB | HUVEC derived EVs were loaded with model drugs by way of the cell’s mechanism to generate and package EVs. Co-culturing these EVs with FaDu and MDA-MB-231 cells resulted in the uptake and release of drug cargo in these cells. However, differing drugs may affect how cargo is endocytosed and sorted by cells, potentially leading to the lysosomal degradative pathway and reducing drug encapsulation in EVs. |
| Zhao, 2020 | TUS | C57BL/6 in vivo | 0.5–1 W/cm2, 0–60 minutes | A2780 and SKOV3 ovarian cancer lines | N/A | LIUS | N/A | LIUS led to increased exosome release compared to controls or 1W/cm2 treatment. Increased release related to ESCRT pathway and could be suppressed by knockdown of that pathway with silencing RNAs. |
| Sun, 2019 | TUS | C56BL/6 in vivo | 0.66 MHz at 0.22–1.8 W/cm2 | Mouse tissue | N/A | USMB (SonoVue) | 100 μL | UTMD improved exosome infiltration into targeted organs. Infiltration occurs with UTMD during or after exosome injection but UTMD before exosome treatment does little to localize exosomes. |
Abbreviations: BBB: Blood-brain barrier; BSA FITC: Bovine serum albumin conjugated with fluorescein isothiocyanate; ChiP: Chimeric peptide; CTG: CellTracker™ green fluorescent dye; DVDMS: Sinoporphyrin sodium; Dox: Doxorubicin; EV: Extracellular vesicle; Exos-Dox: Exosomes loaded with doxorubicin; hESC: Human embryonic stem cell; hMSC: Human mesenchymal stem cell; HUVEC: Human umbilical vein endothelial cell; LED: Light emitting diode; mTHPC: Meta-tetra(hydroxyphenyl)chlorin; MSC: Mesenchymal stem cell; PDT: Photodynamic therapy; PEG: Polyethylene glycol; Por: 2,7,12,17-Tetra-tert-butyl-5,10,15,20-tetraaza-21H,23H-porphine; PorBA: 4,4′,4″,4‴-(porphine-5,10,15,20-tetrayl) tetrakis(benzoic acid); ROS: Reactive oxygen species; SDT: Sonodynamic therapy; TMP: 5,10,15,20-tetrakis(1-methyl-4-pyridinio) porphyrin tetra(p-toluenesulfonate); TUS: Therapeutic ultrasound; USMB: Ultrasound therapy with microbubbles; UTMD: Ultrasound-mediated microbubble destruction
Applications in Photodynamic Therapy.
EVs have been explored as a delivery vehicle in combination with PDT to enhance the tumor-directed delivery of PSs. For example, fusing mesenchymal stem cell (MSC)-derived EVs with liposomes formed hybrid EVs containing the liposomal contents while retaining the favorable biological features of EVs [30]. Use of hybrid EVs resulted in a 3–4 fold increase in the uptake of mTHPC by tumor cells compared to mTHPC encapsulated in liposomes alone[53].The addition of polyethylene glycol (PEGylation) to EVs increased their circulation time, which led to greater accumulation of PS-loaded PEGylated EVs by way of the enhanced permeability and retention (EPR) effect[54]. When administered in the setting of PDT, hybrid EVs fused with PEGylated liposomes enhanced PS delivery, increasing ROS production and cytotoxicity[53]. These hybrid EVs serve as just one example of innovative approaches in drug delivery that are combining the properties of conventional drug delivery vehicles with those of EVs.
The transport benefits of using EVs for PS delivery to cancer cells translate to improved cytotoxicity following activation of the PSs. Delivery of porphyrin-containing EVs derived from endothelial cells, breast cancer cells, MSCs, and embryonic stem cells reduced the viability of breast cancer cells following PDT compared to free porphyrin or porphyrin-loaded liposomes[55]. Moreover, uptake of porphyrin encapsulated in EVs from breast cancer cells was over 60% higher than free drug[55]. This study also found that model drugs could be loaded in EVs using various active encapsulation techniques including electroporation, saponin treatment, or hypotonic dialysis without significantly impairing vesicle composition or functionality. In the example of porphyrins, EV loading with these techniques could lend value for theranostic purposes, such as enabling non-invasive in vivo imaging in addition to PDT.
Another benefit of PDT in conjunction with engineered exosomes is increased phototoxicity. For example, dual-stage light PDT with engineered chimeric peptide conjugated exosomes (ChiP-Exo) exhibited a significantly higher phototoxicity than single-stage light at various doses in mammary carcinoma cells; additionally, nuclear translocation of ChiP-Exo drastically amplified nuclear PDT efficiency upon second-stage light[56]. These results were validated in vivo as dual-stage light PDT treatment with ChiP-Exo enabled targeting of tumor tissues while keeping normal tissue intact, ultimately achieving significant inhibition of tumor growth.
Applications in Therapeutic Ultrasound.
The aforementioned uses of EVs for drug delivery extend to TUS as well. For example, low-intensity TUS in combination with systemically circulating microbubbles can produce reversible and localized blood brain barrier (BBB) disruption, allowing more efficient therapeutic delivery to the brain [57]. TUS has been demonstrated to mediate exosomal drug delivery across the BBB, leading to improved control of orthotopic gliomas, and gliomas treated with fluorescent-dye-labeled exosomes derived from blood serum showed a significantly higher accumulation of dye compared to tumors treated with dye alone[58]. These findings suggest that TUS enhances the accumulation of exosomes in gliomas and has the potential to improve exosome-mediated drug delivery. When this study was repeated with two sessions of DOX-bearing exosome delivery across the BBB with TUS, tumor control and increased survival were observed. Indeed, the second TUS session significantly improved tumor control and survival compared to a single treatment, potentially due to the second round of TUS disrupting the exosome carriers and liberating DOX in the TME. This study highlights not only the potential for leveraging TUS to alleviate physical barriers (e.g. the BBB) to nanotherapeutic delivery with EVs, but also the potential role for TUS in uncaging the payloads within these EVs at the target site.
Exosomal drug delivery is under investigation for other TUS mechanisms of action as well. Analogous in principle to PDT, sonodynamic therapy (SDT) makes use of a similar class of drugs to PSs - known as sonosensitizers. These agents conjugate to stimulate ROS production and, ultimately, cell death when activated with low intensity TUS[59,60]. In a murine model of metastatic breast cancer, TEXs loaded with the sonosensitizer sinoporphyrin sodium (DVDMS) enhanced intracellular ROS production in the setting of SDT compared to SDT with free-DVDMS[61]. These findings were corroborated in vivo, as application of ultrasound-guided SDT in combination with DVDMS-loaded exosomes in an analogous mouse model inhibited tumor growth and pulmonary metastases.
Upstream of its important role in exosomal therapeutic delivery, TUS is also seeing use as a tool for loading EVs with cargo. For instance, in a study evaluating TUS for generation of EVs containing model fluorescent reporter drugs, USMB at varying acoustic pressures was applied to HUVECs loaded with these model drugs. Not only did USMB facilitate the loading of endothelial cells with model drugs, but it also triggered release of EVs carrying the drugs. These drug-loaded EVs were effectively taken up by recipient tumor cells within 4 hours. Finally, the degree of USMB-triggered EV release corresponded with acoustic pressure in a dose-dependent manner[33]. This study also highlighted the importance of cargo properties for the efficiency of EV packaging, as the concentration of model drug compounds at the target site differed upon EV-mediated delivery; one mechanistic explanation lies in how these drugs were taken up and sorted once internalized, which could differ based on their proclivity for endosomal entrapment and cargo degradation after internalization. Such findings highlight the importance of drug selection for focal drug delivery efforts leveraging EV carriers.
Concluding Remarks & Future Perspectives
EVs represent a burgeoning facet of the study of molecular biology; however, they remain underexplored in the context of oncolytic therapy, despite their vast potential to serve as tools for drug delivery, disease prognostication, and even monitoring of treatment response [62]. Here, we reviewed (i) the available evidence for how EVs interface with TME-modulating oncolytic therapies and (ii) the emerging role of EVs as tool to facilitate drug delivery in combination with these modalities. We summarize these mechanisms in Key Figure 1.
Key Figure 1. Summary of oncolytic focal therapies and their potential mechanisms of interaction with extracellular vesicles (EVs).
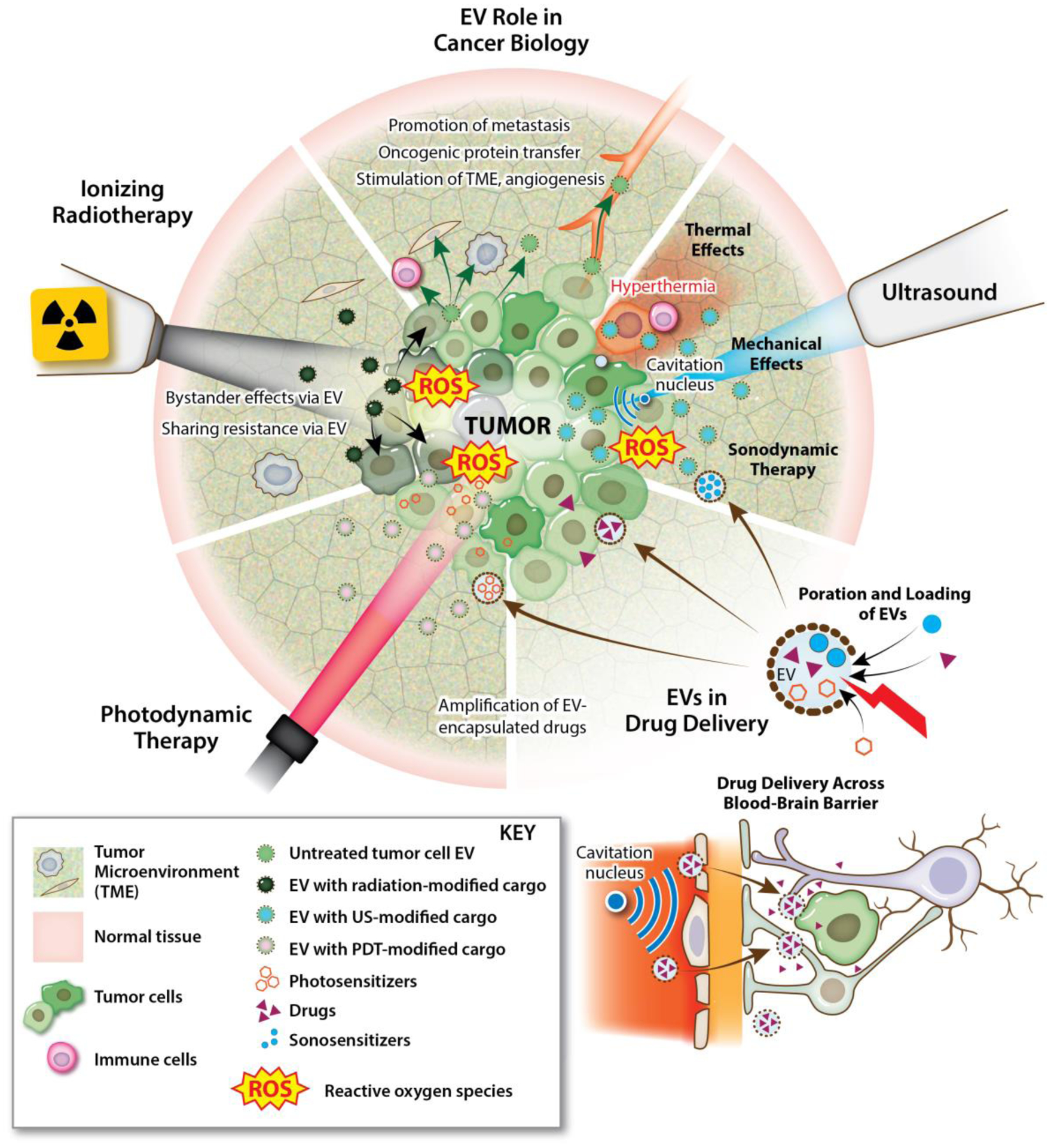
EVs play a diverse role in the evolution of cancer, including facilitation of tumor progression and metastasis, transfer of oncogenic payloads, and stimulation of other pathophysiological processes such as angiogenesis. Oncolytic focal therapies can intervene on the tumor microenvironment (TME), and thereby EVs, in a variety of ways. Ionizing radiation, photodynamic therapy (PDT) and therapeutic ultrasound (TUS) can differentially influence EV amplitude and cargo, immunological responses, and therapeutic delivery within the TME.
Despite their similarities, radiotherapy, PDT and TUS also diverge significantly in how they influence the amplitude of EV release, EV cargo and downstream biological mechanisms. These differences underscore the importance of continued study at the interface of oncolytic therapy and EVs, as it is evident that observed bioeffects are not generalizable. Rather, they are susceptible to cancer type, exposure conditions, choice of adjuvant, and even method of EV isolation. As such, it is critical that consensus guidelines for standardization of EV isolation and classification be adopted where available [63].
EVs have an established foothold in the liquid biopsy domain, which is drawing considerable interest as a non-invasive approach for diagnosis and monitoring of cancer [64]. While there is exciting potential to leverage EVs as a repository of established or yet undiscovered cancer biomarkers, certain tumor settings – such as the central nervous system – remain notoriously difficult to survey by this method owing to low abundance of EVs and other circulating biomarkers within their circulome[65]. Interestingly, the oncolytic focal therapies reviewed herein harbor similarities in their ability to augment EV release, irrespective of the cellular stress induced. Going forward, this may open up a fascinating possibility for enriching the tumor circulome with EVs and thus rendering it more amenable to liquid biopsy assays. Encouragingly, this concept has already been explored clinically. A recent first-in-human study demonstrated that following BBB opening with TUS and microbubbles, neuron-derived extracellular vesicles were acutely enriched (by over 3-fold) in the circulation of glioblastoma patients[66]. Going forward, systematic pre-clinical studies comparing oncolytic modalities and/or exposure conditions (with greater emphasis on in vivo EV dynamics) can offer us the necessary insights into how energy deposition must be tuned to yield clinically valuable EV deposits in the circulome in an inert, controlled, and reproducible manner (see Outstanding Questions).
Outstanding Questions.
Can EVs serve as a reliable source of cancer biomarkers for deploying and monitoring response to oncolytic focal therapies?
Can oncolytic focal therapies such as TUS reproducibly augment EVs within the circulome as a strategy for potentiating liquid biopsy? Is this strategy safe and clinically valuable?
What roles do EVs play in the established immuno-modulatory mechanisms underpinning oncolytic focal therapies?
Where are oncolytic focal therapies and/or exposure conditions consistent in their influence on EV biology?
What drugs or sensitizing agents are most rational to deliver using EV-based vehicles and focal therapy approaches?
Highlights/Trends.
The past decade has seen marked advancement in the sophisticated technologies available for focal therapy in cancer. Among the emerging bioeffects of these therapies is modulation of extracellular vesicles (EVs) within the tumor microenvironment.
Radiation, therapeutic ultrasound and photodynamic therapy can each differentially alter the concentration and/or profile of tumor-associated EVs. In some cases, these bioeffects exert secondary impacts on surrounding cancer or immune cells. The ability of oncolytic therapies to modulate EVs holds important implications for liquid biopsy and biomarker discovery in cancer.
Given their recognition as “self” by the immune system, EVs offer superior biocompatibility and low immunogenicity. EVs are gaining traction as nanocarriers for drug delivery in the settings of therapeutic ultrasound and photodynamic therapy.
Acknowledgements
N.D.S. was supported by a National Cancer Institute F99/K00 Predoctoral to Postdoctoral Fellow Transition Award (K00CA234954). R.J.P. was supported by NIH R01 CA204968, R01EB030409, R01EB030744, R01EB030007, R21CA230088, and R21NS118278. The authors thank Anita Impagliazzo Medical Illustration (impag1@lumos.net) for the generation of all figures in this manuscript.
Glossary
- Anti-inflammatory
Subduing the immunological response against harmful stimuli such as cancer cells.
- Cavitation
The formation, expansion, and collapse of microbubbles in an ultrasound field.
- Extracellular Vesicle
Particles with a lipid bilayer that are secreted by cells into the extracellular space.
- Extracorporeal
Originating from a device located outside the patient’s body.
- Exosome
A specific class of extracellular vesicle that is generated within endosomes called multivesicular bodies. Multivesicular bodies bud inward to form intraluminal vesicles that can contain cytosolic contents or proteins incorporated into the membrane of the intraluminal vesicle. Exosomes are released into the extracellular space after fusion with the plasma membrane.
- Genotoxic Stress
Cellular damage resulting from exposure to DNA-damaging agents and subsequent attempts to repair the damaged DNA.
- Hyperthermia
Temperatures above normal physiological temperature, but not high enough to be immediately lethal to the majority of cells. Hyperthermia is generally defined as 40–47°C, but temperatures above 43°C are more lethal than those below 43°C.
- Liquid Biopsy
Analysis of a blood sample to identify circulating cancer biomarkers that can aid in clinical diagnosis or prognosis of disease.
- Microbubble
Microscale particles that encapsulate a gas with a shell commonly made of lipids, albumin, or polymers. Microbubbles can expand and contract in response to ultrasound waves.
- MicroRNA (miR)
Short, single-stranded, noncoding RNA molecules that regulate gene expression by binding to complementary mRNA and triggering translational repression or mRNA degradation.
- Nanodroplet
Nanoscale particles consisting of encapsulated liquid perfluorocarbon emulsions. The emulsions in nanodroplets commonly undergo phase-transition to a gas core during therapeutic ultrasound treatment.
- Oncogenic
Relating to the development and survival of tumors.
- Oxidative Stress
Cellular damage resulting from exposure to and accumulation of free radicals beyond a cell’s ROS-clearing capacity.
- Pro-inflammatory
Promoting the immunological response against harmful stimuli such as cancer cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simons M and Raposo G (2009) Exosomes - vesicular carriers for intercellular communication. Curr. Opin. Cell Biol 21, 575–581 [DOI] [PubMed] [Google Scholar]
- 2.Xie F et al. (2019) Extracellular Vesicles in Cancer Immune Microenvironment and Cancer Immunotherapy. Adv. Sci 6, 1901779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu R et al. (2018) Extracellular vesicles in cancer — implications for future improvements in cancer care. Nat. Rev. Clin. Oncol 15, 617–638 [DOI] [PubMed] [Google Scholar]
- 4.Szatmári T et al. (2018) Extracellular vesicles mediate low dose ionizing radiation-induced immune and inflammatory responses in the blood. Int. J. Radiat. Biol 95, 12–22 [DOI] [PubMed] [Google Scholar]
- 5.Aubertin K et al. (2016) Massive release of extracellular vesicles from cancer cells after photodynamic treatment or chemotherapy. Sci. Rep 6, 35376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Q et al. (2017) Low-intensity ultrasound-induced anti-inflammatory effects are mediated by several new mechanisms including gene induction, immunosuppressor cell promotion, and enhancement of exosome biogenesis and docking. Front. Physiol 8, 818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jabbari N et al. (2020) Tumor-derived extracellular vesicles: insights into bystander effects of exosomes after irradiation. Lasers Med. Sci 35, 531–545 [DOI] [PubMed] [Google Scholar]
- 8.Azzam EI and Little JB (2004) The radiation-induced bystander effect: evidence and significance. Hum. Exp. Toxicol 23, 61–65 [DOI] [PubMed] [Google Scholar]
- 9.Marín A et al. (2015) Bystander effects and radiotherapy. Reports Pract. Oncol. Radiother 20, 12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heeran AB et al. (2019) The Radiation-Induced Bystander Effect (RIBE) and its Connections with the Hallmarks of Cancer. Radiat. Res 192, 668–679 [DOI] [PubMed] [Google Scholar]
- 11.Gaillard S et al. (2009) Propagation Distance of the α-Particle-Induced Bystander Effect: The Role of Nuclear Traversal and Gap Junction Communication. Radiat. Res 171, 513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Mayah AHJ et al. (2012) Possible role of exosomes containing RNA in mediating nontargeted effect of ionizing radiation. Radiat. Res 177, 539–545 [DOI] [PubMed] [Google Scholar]
- 13.Al-Mayah A et al. (2015) The non-targeted effects of radiation are perpetuated by exosomes. Mutat. Res. - Fundam. Mol. Mech. Mutagen 772, 38–45 [DOI] [PubMed] [Google Scholar]
- 14.Szatmári T et al. (2019) Extracellular Vesicles in Modifying the Effects of Ionizing Radiation. Int. J. Mol. Sci 20, 5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Formenti SC and Demaria S (2009) Systemic effects of local radiotherapy. Lancet Oncol 10, 718–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mutschelknaus L et al. (2016) Exosomes derived from squamous head and neck cancer promote cell survival after ionizing radiation. PLoS One 11, e0152213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arscott WT et al. (2013) Ionizing radiation and glioblastoma exosomes: Implications in tumor biology and cell migration. Transl. Oncol 6, 638–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramakrishnan V et al. (2020) Radiation-induced extracellular vesicle (EV) release of miR-603 promotes IGF1-mediated stem cell state in glioblastomas. EBioMedicine 55, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abramowicz A et al. (2019) Ionizing radiation affects the composition of the proteome of extracellular vesicles released by head-and-neck cancer cells in vitro. J. Radiat. Res 60, 289–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jella KK et al. (2020) Exosome-Containing Preparations From Postirradiated Mouse Melanoma Cells Delay Melanoma Growth In Vivo by a Natural Killer Cell–Dependent Mechanism. Int. J. Radiat. Oncol 108, 104–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galluzzi L et al. (2016) Immunogenic cell death in cancer and infectious disease DOI: 10.1038/nri.2016.107 [DOI] [PubMed] [Google Scholar]
- 22.Diamond JM et al. (2018) Exosomes Shuttle TREX1-Sensitive IFN-Stimulatory dsDNA from Irradiated Cancer Cells to DCs. Cancer Immunol Res 6, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin W et al. (2020) Radiation-induced small extracellular vesicles as “carriages” promote tumor antigen release and trigger antitumor immunity. Theranostics 10, 4871–4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehmann BD et al. (2008) Senescence-Associated Exosome Release from Human Prostate Cancer Cells. Cancer Res 68, 7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castellanos JR et al. (2017) B7-H3 role in the immune landscape of cancer. Am. J. Clin. Exp. Immunol 6, 66. [PMC free article] [PubMed] [Google Scholar]
- 26.Goler-Baron V and Assaraf YG (2012) Overcoming Multidrug Resistance via Photodestruction of ABCG2-Rich Extracellular Vesicles Sequestering Photosensitive Chemotherapeutics. PLoS One 7, e35487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Theodoraki M-N et al. (2018) Plasma-derived Exosomes Reverse Epithelial-to-Mesenchymal Transition after Photodynamic Therapy of Patients with Head and Neck Cancer. Oncoscience 5, 75–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baydoun M et al. (2020) Photodynamic Therapy Using a New Folate Receptor-Targeted Photosensitizer on Peritoneal Ovarian Cancer Cells Induces the Release of Extracellular Vesicles with Immunoactivating Properties. J. Clin. Med 9, 1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin Y et al. (2018) ALA-PDT promotes HPV-positive cervical cancer cells apoptosis and DCs maturation via miR-34a regulated HMGB1 exosomes secretion. Photodiagnosis Photodyn. Ther 24, 27–35 [DOI] [PubMed] [Google Scholar]
- 30.Millard M et al. (2018) mTHPC-loaded extracellular vesicles outperform liposomal and free mTHPC formulations by an increased stability drug delivery efficiency and cytotoxic effect in tridimensional model of tumors. Drug Deliv 25, 1790–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sengupta S and Balla VK A review on the use of magnetic fields and ultrasound for non-invasive cancer treatment. , Journal of Advanced Research, 14. 01-November-(2018) , Elsevier B.V., 97–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuana Y et al. (2017) Microbubbles-Assisted Ultrasound Triggers the Release of Extracellular Vesicles. Int. J. Mol. Sci 18, 1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuana Y et al. (2020) Potential Use of Extracellular Vesicles Generated by Microbubble-Assisted Ultrasound as Drug Nanocarriers for Cancer Treatment. Int. J. Mol. Sci 21, 3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paproski RJ et al. (2017) Enhanced detection of cancer biomarkers in blood-borne extracellular vesicles using nanodroplets and focused ultrasound. Cancer Res 77, 3–13 [DOI] [PubMed] [Google Scholar]
- 35.Lucchetti D et al. (2020) Low‐intensity pulsed ultrasound affects growth, differentiation, migration, and epithelial‐to‐mesenchymal transition of colorectal cancer cells. J. Cell. Physiol 235, 5363–5377 [DOI] [PubMed] [Google Scholar]
- 36.Zhao Z et al. (2020) Low-intensity ultrasound radiation increases exosome yield for efficient drug delivery. J. Drug Deliv. Sci. Technol 57, 101713 [Google Scholar]
- 37.Sheybani ND et al. (2020) Focused Ultrasound Hyperthermia augments release of Glioma-derived Extracellular Vesicles with differential Immunomodulatory Capacity. Theranostics 10, 7436–7447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X et al. (2019) Exosomes Derived From Low‐Intensity Pulsed Ultrasound‐Treated Dendritic Cells Suppress Tumor Necrosis Factor–Induced Endothelial Inflammation. J. Ultrasound Med 38, 2081–2091 [DOI] [PubMed] [Google Scholar]
- 39.Gehrmann U et al. (2014) Harnessing the exosome-induced immune response for cancer immunotherapy. Semin. Cancer Biol 28, 58–67 [DOI] [PubMed] [Google Scholar]
- 40.Keklikoglou I et al. (2019) Chemotherapy elicits pro-metastatic extracellular vesicles in breast cancer models. Nat. Cell Biol 21, 190–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suchorska WM and Lach MS (2016) The role of exosomes in tumor progression and metastasis. Oncol. Rep 35, 1237–1244 [DOI] [PubMed] [Google Scholar]
- 42.Ma X et al. (2014) Essential role for TrpC5-containing extracellular vesicles in breast cancer with chemotherapeutic resistance. Proc. Natl. Acad. Sci. U. S. A 111, 6389–6394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takahashi K et al. (2014) Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer. FEBS Open Bio 4, 458–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fais S et al. Evidence-Based Clinical Use of Nanoscale Extracellular Vesicles in Nanomedicine. , ACS Nano, 10. 26-April-(2016) , American Chemical Society, 3886–3899 [DOI] [PubMed] [Google Scholar]
- 45.Wang J et al. (2017) Exosome-based cancer therapy: Implication for targeting cancer stem cells. Front. Pharmacol 7, 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Théry C et al. (2002) Exosomes: composition, biogenesis and function. Nat. Rev. Immunol 2, 569–579 [DOI] [PubMed] [Google Scholar]
- 47.Clayton A et al. (2003) Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59. Eur. J. Immunol 33, 522–531 [DOI] [PubMed] [Google Scholar]
- 48.Yong T et al. (2019) Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat. Commun 10, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim MS et al. (2016) Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine Nanotechnology, Biol. Med 12, 655–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munagala R et al. (2016) Bovine milk-derived exosomes for drug delivery. Cancer Lett 371, 48–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kooijmans SAA et al. (2012) Exosome mimetics: A novel class of drug delivery systems. Int. J. Nanomedicine 7, 1525–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hood JL et al. (2014) Maximizing exosome colloidal stability following electroporation. Anal. Biochem 448, 41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piffoux M et al. (2018) Modification of Extracellular Vesicles by Fusion with Liposomes for the Design of Personalized Biogenic Drug Delivery Systems. ACS Nano 12, 6830–6842 [DOI] [PubMed] [Google Scholar]
- 54.Kooijmans SAA et al. (2016) PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J. Control. Release 224, 77–85 [DOI] [PubMed] [Google Scholar]
- 55.Fuhrmann G et al. (2015) Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release 205, 35–44 [DOI] [PubMed] [Google Scholar]
- 56.Cheng H et al. (2019) Chimeric peptide engineered exosomes for dual-stage light guided plasma membrane and nucleus targeted photodynamic therapy. Biomaterials 211, 14–24 [DOI] [PubMed] [Google Scholar]
- 57.Fisher DG and Price RJ (2019) Recent Advances in the Use of Focused Ultrasound for Magnetic Resonance Image-Guided Therapeutic Nanoparticle Delivery to the Central Nervous System. Front. Pharmacol 10, 1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bai L et al. (2019) Ultrasound Facilitates Naturally Equipped Exosomes Derived from Macrophages and Blood Serum for Orthotopic Glioma Treatment. ACS Appl. Mater. Interfaces 11, 14576–14587 [DOI] [PubMed] [Google Scholar]
- 59.Costley D et al. (2015) Treating cancer with sonodynamic therapy: A review. Int. J. Hyperth 31, 107–117 [DOI] [PubMed] [Google Scholar]
- 60.Jelonek K et al. (2016) The Influence of Ionizing Radiation on Exosome Composition, Secretion and Intercellular Communication. Protein Pept. Lett 23, 656–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y et al. (2019) Focused ultrasound-augmented targeting delivery of nanosonosensitizers from homogenous exosomes for enhanced sonodynamic cancer therapy. Theranostics 9, 5261–5281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rahbarghazi R et al. (2019) Tumor-derived extracellular vesicles: reliable tools for Cancer diagnosis and clinical applications. Cell Commun. Signal 17, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Théry C et al. (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. vesicles 7, 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao Z et al. (2019) Extracellular vesicles as cancer liquid biopsies: from discovery, validation, to clinical application. Lab Chip 19, 1114–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aili Y et al. (2020) Liquid biopsy in central nervous system tumors: the potential roles of circulating miRNA and exosomes. Am. J. Cancer Res 10, 4134–4150 [PMC free article] [PubMed] [Google Scholar]
- 66.Meng Y et al. (2021) MR-guided focused ultrasound liquid biopsy enriches circulating biomarkers in patients with brain tumors. Neuro. Oncol DOI: 10.1093/neuonc/noab057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heijnen HFG et al. (1999) Activated platelets release two types of membrane vesicles: Microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and α-granules. Blood 94, 3791–3799 [PubMed] [Google Scholar]
- 68.Greening DW et al. (2015) Exosomes and their roles in immune regulation and cancer. Semin. Cell Dev. Biol 40, 72–81 [DOI] [PubMed] [Google Scholar]
- 69.Robbins PD and Morelli AE Regulation of immune responses by extracellular vesicles. , Nature Reviews Immunology, 14. March-(2014) , 195–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nair R et al. (2014) Extracellular vesicles derived from preosteoblasts influence embryonic stem cell differentiation. Stem Cells Dev 23, 1625–1635 [DOI] [PubMed] [Google Scholar]
- 71.Wen S et al. (2016) Mesenchymal stromal cell-derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia 30, 2221–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simon C et al. (2018) Extracellular vesicles in human reproduction in health and disease. Endocr. Rev 39, 292–332 [DOI] [PubMed] [Google Scholar]
- 73.Mitchell MD et al. (2015) Placental exosomes in normal and complicated pregnancy. Am. J. Obstet. Gynecol 213, S173–S181 [DOI] [PubMed] [Google Scholar]
- 74.Martins PN (2018) A brief history about radiotherapy. Int. J. Latest Res. Eng. Technol 04, 8–11 [Google Scholar]
- 75.Schmidt MA and Payne GS (2015) Radiotherapy planning using MRI. Phys. Med. Biol 60, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Allison RR and Moghissi K (2013) Photodynamic therapy (PDT): PDT mechanisms. Clin. Endosc 46, 24–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Celli JP et al. (2010) Imaging and photodynamic therapy: Mechanisms, monitoring, and optimization. Chem. Rev 110, 2795–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Curley CT et al. (2017) Focused ultrasound immunotherapy for central nervous system pathologies: challenges and opportunities. Theranostics 7, 3608–3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meng Y et al. (2021) Applications of focused ultrasound in the brain: from thermoablation to drug delivery. Nat. Rev. Neurol 17, 7–22 [DOI] [PubMed] [Google Scholar]
- 80.Bystritsky A et al. (2011) A review of low-intensity focused ultrasound pulsation. Brain Stimul 4, 125–136 [DOI] [PubMed] [Google Scholar]
- 81.Shibaguchi H et al. (2011) Sonodynamic Cancer Therapy: A Non-invasive and Repeatable Approach Using Low-intensity Ultrasound with a Sonosensitizer. Anticancer Res 31, 2425–2430 [PubMed] [Google Scholar]


