Abstract
Introduction
The ability to predict which patients with a history of coronavirus disease (COVID-19) will exhibit a high antibody titer is necessary for more efficient screening of potential convalescent plasma donors. We aimed to identify factors associated with a high immunoglobulin G (IgG) titer in Japanese convalescent plasma donors after COVID-19.
Methods
This cross-sectional study included volunteers undergoing screening for convalescent plasma donation after COVID-19. Serum anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) S-protein IgG antibodies were measured using a high-sensitivity chemiluminescence enzyme immunoassay.
Results
IgG antibodies were measured in 581 patients, 534 of whom had full information of selected independent variables. Multiple linear regression analysis revealed that increasing age (1.037 [1,025, 1.048]), days from symptom onset to sampling (0.997 [0.995, 0.998]), fever (1.664 [1.226, 2.259]), systemic corticosteroid use during SARS-CoV-2 infection (2.382 [1.576, 3.601]), and blood type AB (1.478 [1.032, 2.117]) predict antibody titer.
Conclusion
Older participants, those who experienced fever during infection, those treated with systemic corticosteroids during infection, those from whom samples were obtained earlier after symptom onset, and those with blood type AB are the best candidates for convalescent plasma donation. Therefore, these factors should be incorporated into the screening criteria for convalescent plasma donation after SARS-CoV-2 infection.
Keywords: Coronavirus disease, COVID-19, Convalescent plasma, Antibody, Screening criteria
Abbreviations
- COVID-19
coronavirus disease 2019
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- s-IgG
spike protein immunoglobulin G
- IQR
interquartile range
1. Introduction
Since December 2019, coronavirus disease (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread worldwide. As of June 15, 2021, more than 175 million people have been infected with SARS-CoV-2, and more than 3 million have died [1]. Previous studies have reported the efficacy of medications including remdesivir [2], dexamethasone [3], tocilizumab [4], and baricitinib [5] in treating COVID-19. Since the beginning of the pandemic, studies conducted in various countries have also investigated the efficacy of convalescent plasma therapy in patients with COVID-19 [6], reporting mixed results [7,8], but mostly without clear benefit. Given that new variants of COVID-19 continue to emerge, the efficacy of current treatment strategies may fluctuate over time, making it essential to continue investigating various treatment options.
Although SARS-CoV-2 antibody titers are higher in patients with severe disease than in those with mild disease [9], they decrease over time, with a reported half-life of approximately 40 days after reaching their peak [10,11]. Continuing to screen participants with low antibody titers is inefficient and burdens both patients and medical institutions. The ability to predict which patients will exhibit higher antibody titers will enable more efficient screening for convalescent plasma donation, thus reducing this burden.
As previously mentioned, both the severity of illness and the time after the onset of illness are important factors influencing antibody titers after SARS-CoV-2 infection [[9], [10], [11]]. However, knowledge regarding the factors associated with a higher antibody titer remains limited. In this study, we aimed to identify factors associated with a high antibody titer in the Japanese population by analyzing data collected from individuals who voluntarily participated in screening for convalescent plasma donation.
2. Methods
2.1. Study design and ethics
The Ethics Committee of Japan's National Center for Global Health and Medicine (approval no.: NCGM-G-003536-01) approved this single-center, cross-sectional, retrospective study, and we obtained written informed consent from all participants.
2.2. Setting and participants
The present study included individuals who participated in the Convalescent Plasma Donation Study at Japan's National Center for Global Health and Medicine between April 30, 2020, and April 8, 2021. An overview of this study is included in a previously published report [12]. In the early phase, we recruited patients admitted to our hospital, following which we recruited external participants via social network services and websites. During their first visit conducted 3 weeks after the onset of COVID-19, volunteers between 20 and 69 years of age completed a self-reported questionnaire that included items related to the date of COVID-19 onset, medical history, symptoms, date of diagnosis, hospitalization status, and treatment. Physicians and research assistants confirmed details regarding unclear information via interviews. After confirming their eligibility, participants underwent blood sampling for blood typing and antibody titer measurements. Participants who met the criteria for eligibility [12] subsequently proceeded with plasma donation. In this study, only the results of the first measurement were used for the data analysis when multiple samples were obtained.
2.3. Evaluation of serum anti-severe acute respiratory syndrome coronavirus 2 immunoglobulin G antibody titers
Anti-SARS-CoV-2 spike protein IgG (S-IgG) antibodies were evaluated using a fully automated high-sensitivity chemiluminescence enzyme immunoassay (Sysmex Corporation, Kobe, Japan). More details on this measurement method and its performance can be found in a previous paper [13].
2.4. Statistical analyses
Continuous variables are presented as medians and interquartile ranges (IQRs), whereas categorical variables are presented as numbers of cases and percentages. We performed a multiple linear regression analysis to identify the factors associated with a high antibody titer. The study investigators selected clinically important factors (i.e., age, sex, days from symptom onset to sampling, fever, mechanical ventilation, systemic corticosteroid use during SARS-CoV-2 infection, hypertension, diabetes mellitus, and blood type AB) as variables. We approximated the residuals to a normal distribution by transforming the antibody titer to the log10 scale. R (version 4.0.2) was used for all analyses. A p value less than 0.05 was considered statistically significant.
3. Results
During the study period, 586 patients underwent antibody titer measurements. Antibody titer measurements were unavailable for 5 of the 586 participants; thus, we evaluated serum antibody titers in 581 participants. The demographic characteristics of the 581 participants included in the analysis are shown in Table 1 .
Table 1.
Participant characteristics (n = 581).
| Overall (n = 581) | |
|---|---|
| Agea | 46 (38–54) |
| Male sex | 287 (49.4) |
| ABO blood type | |
| A | 249 (43.1) |
| B | 131 (22.7) |
| O | 134 (23.2) |
| AB | 64 (9.9) |
| Rh blood type-positive | 574 (99.3) |
| Symptoms during disease onset | |
| Fever | 458 (84.2) |
| Headache | 205 (37.6) |
| Arthralgia | 133 (24.4) |
| Myalgia | 97 (17.8) |
| Loss of appetite | 117 (21.5) |
| Cough | 235 (42.9) |
| Sputum | 79 (14.5) |
| Hyperemic conjunctiva | 16 (2.9) |
| Sore throat | 121 (22.2) |
| Nasal discharge | 81 (14.9) |
| Chest pain | 62 (11.4) |
| Dyspnea | 105 (19.3) |
| Dysgeusia | 171 (31.4) |
| Dysosmia | 202 (36.9) |
| Diarrhea | 96 (17.6) |
| Nausea and vomiting | 23 (4.2) |
| Fatigue | 255 (46.8) |
| Baseline conditions | |
| Diabetes | 41(7.1) |
| Hypertension | 89 (15.5) |
| Dyslipidemia | 70 (12.1) |
| Highest facilities during disease onset | |
| Hospital admission | 318 (54.8) |
| Isolation facility (e.g., hotels) | 121 (20.9) |
| Home | 141 (24.3) |
| Pneumonia on radiography | 222 (45.9) |
| Severity | |
| Oxygen supplementation | 90 (15.5) |
| Mechanical ventilation | 20 (3.5) |
| ECMO | 7 (1.3) |
| Systemic corticosteroid therapy | 53 (9.3) |
| Days from onset to samplingb | 63 (38–102) |
| Antibody titer (AU/mL) | 33.7 (13.2–73.3) |
Numbers of missing data and denominators differ for each item.
In the 12 asymptomatic participants, the day of sampling on which the first positive specimen was identified was regarded as the day of onset.ECMO, extracorporeal membrane oxygenation.
Among the 581 participants, the median age was 46 (IQR: 38–54) years, 49.4% were men, and 9.9% had blood type AB. At the time of illness, 84.2% and 42.9% of participants had fever and cough, respectively. Hospitalization was required for 54.8% of participants, whereas oxygen support was not required for 84.5% of participants. The median time from symptom onset to collection of antibody titer samples was 63 (IQR: 38–102) days, and the median antibody titer was 33.7 (IQR: 13.2–73.3) AU/mL. None of the participants had been previously vaccinated against COVID-19 at the time of sampling.
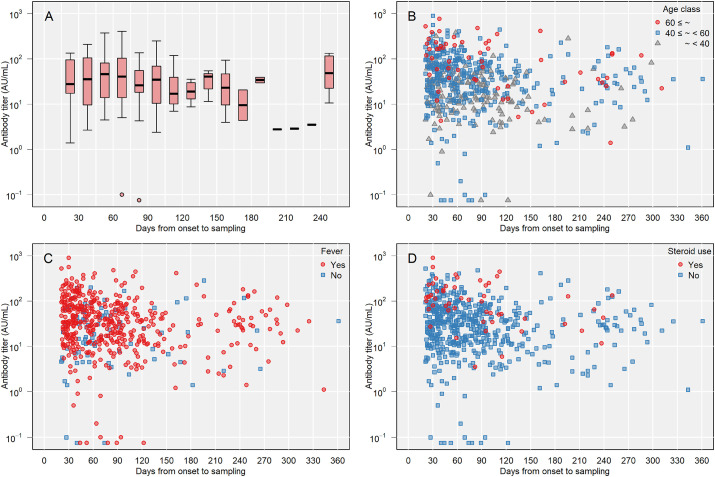
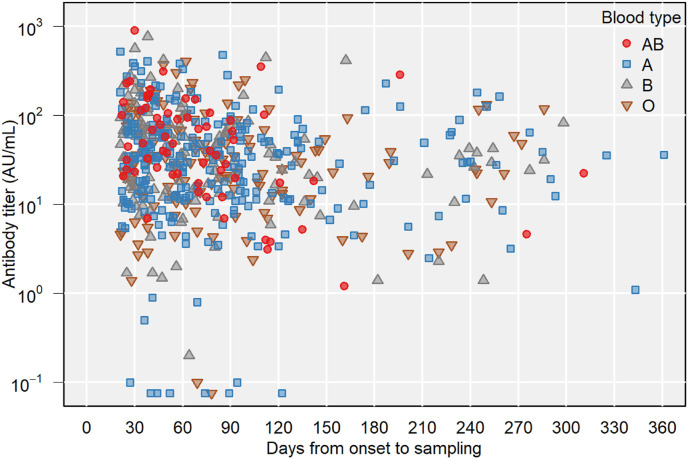
Multiple linear regression was performed to predict antibody titer based on the independent variables shown above. In total, 534 participants had full information of the independent variables. The estimated amount of antibody titer increase according to each independent variable is shown in Table 2 . Higher age, increasing days from symptom onset to sampling, presence of fever, systemic corticosteroid use during COVID-19, and blood type AB were significant predictors of antibody titer. Fig. 1 A–D and Fig. 2 present the association between days from the onset and antibody titer for these significant variables.
Table 2.
Multiple linear regression analysis (n = 534).
| Estimate | 95% CI | p value | |
|---|---|---|---|
| (Intercept) | 3.741 | 2.163–6.469 | <0.001 |
| Age | 1.037 | 1.025–1.048 | <0.001 |
| Male sex | 1.055 | 0.835–1.332 | 0.655 |
| Days from symptom onset to sampling | 0.997 | 0.995–0.998 | <0.001 |
| Fever | 1.664 | 1.226–2.259 | 0.001 |
| Mechanical ventilation | 1.708 | 0.771–3.782 | 0.188 |
| Systemic corticosteroid use | 2.382 | 1.576–3.601 | <0.001 |
| Hypertension | 1.317 | 0.939–1.847 | 0.111 |
| Diabetes mellitus | 1.176 | 0.735–1.883 | 0.499 |
| Blood type AB | 1.478 | 1.032–2.117 | 0.033 |
CI: confidence interval.
Fig. 1.
Antibody titer and days from onset to sampling. A) By sampling days. B) By age class. C) By fever during disease onset. D) By systemic corticosteroid use during coronavirus disease 2019 infection.
Fig. 2.
Antibody titer and days from onset to sampling by ABO blood type.
4. Discussion
To identify factors associated with a high IgG antibody titer in Japanese convalescent plasma donors after SARS-CoV-2 infection, we examined IgG in 581 volunteers with a history of SARS-CoV-2 infection. The analysis revealed that older age, earlier sampling, fever, systemic corticosteroid use during SARS-CoV-2 infection, and blood type AB were associated with a high antibody titer. To the best of our knowledge, this is the largest study to date regarding factors associated with high antibody titers among convalescent plasma donors in Japan. A previous Chinese study conducted from February to March 2020 examined antibody titers in 49 participants in their 30s and 40s, who were tested at an average of 37.8 days from onset [14]. This Chinese study indicated that high IgG titer is correlated with fever but not with age, sex, or ABO blood type. A study conducted in Germany from April 2020 included 206 participants with a median age of 37.4 years who were tested at a median of 58 days from onset [15]. This study reported that the need for hospitalization due to COVID-19 and fever affects SARS-CoV-2 neutralizing antibody titers. The present study included more participants than the previous study, and our participants were slightly older and had a longer duration from onset to sampling than those in the previous studies.
Our results indicate that actively involving older participants, those with a shorter duration from onset to sampling, and those with febrile illness may improve the efficiency of the convalescent plasma donation process. A previous study conducted in the United States reported that older age, male sex, and hospitalization were associated with higher levels of anti-spike avidity in convalescent plasma candidates [16]. Another Chinese study indicated that antibody titers were higher in patients with fever than in those without [14]. The results of the present study are generally consistent with these previous findings, but notable for showing the similar findings in Japanese populations. Given that antibody titers are also correlated with neutralizing antibodies [13], these candidates are expected to have better neutralizing titers.
Our results also suggest that collecting plasma from patients treated with systemic corticosteroids during SARS-CoV-2 infection can improve the efficiency of the screening process. Although it is a notable finding in this study, systemic corticosteroid use was not discussed in previous reports [[14], [15], [16]]. Systemic corticosteroids generally function by suppressing the immune response; however, based on the results of the RECOVERY study [3], they have become standard in the treatment of patients requiring oxygen therapy to receive dexamethasone or other corticosteroids. Therefore, the results of the present study suggest that antibody titers are high in patients whose illness is sufficiently severe to warrant corticosteroid treatment. The present results regarding corticosteroid use may essentially represent the fact that severely ill patients tend to have higher antibody titers. Although it is not mandatory to limit plasma donation to patients treated with corticosteroids, including patients with a moderate or severe disease rather than those with mild disease may be more efficient.
Given that levels of neutralizing antibodies decrease over time [10] and that the results of this study show that days from symptom onset to sampling negatively correlate with antibody titer, it is also reasonable to collect plasma from participants as early as possible after symptom onset. Thus, it may be necessary to add the number of days from symptom onset to the screening criteria for plasma donation. Further, new participants should be recruited continually.
Another notable finding of the current study is that antibody titers were higher in patients with blood type AB than in those with other blood types. Approximately 10% of the Japanese population is estimated to have blood type AB [17], and a similar percentage of this blood type was observed in the present study. In accordance with our findings, previous studies have also reported that donors with blood type AB have higher antibody titers than those with blood type O [18,19] and that patients with COVID-19 have lower levels of ABO antibodies than uninfected individuals [20]. These findings indicate that blood type ABO may have some influence on the development of SARS-CoV-2 infection and antibody production. Given that plasma from donors with blood type AB can theoretically be provided to patients with any blood type, individuals with blood type AB should be encouraged to participate in plasma donation.
In the current analysis of factors related to high antibody titers, mechanical ventilation was not statistically significant. This may be due to the small number of patients and the possibility that systemic corticosteroids were utilized for longer and at higher doses in ventilated patients than in non-ventilated patients. It is also likely that various immunosuppressive medications such as tocilizumab and baricitinib were used, which may have further influenced the results.
The present study has several limitations, including its single-center design, which limited our study population to individuals visiting our hospital. Therefore, caution is required when attempting to generalize our findings to other populations. In addition, information related to each patient's background and medical history was collected using a self-reported questionnaire, and we did not contact each institution to inquire about their medical records. Thus, it is difficult to exclude recall bias. However, we believe that such bias was minimal because the place of treatment and history of oxygen administration are unlikely to be mistaken. Our study is also limited by the variation in antibody titer among participants, with some participants having less than detectable levels of antibody titer. Although we approximated the residuals to a normal distribution, this deviated sample data may have influenced the results.
5. Conclusion
Our analysis indicated that older age, days from symptom onset to sampling, fever, systemic corticosteroid use during SARS-CoV-2 infection, and blood type AB were associated with a high antibody titer in convalescent plasma following SARS-CoV-2 infection. Therefore, to avoid excessive blood collection, healthcare professionals should refer to these factors when selecting convalescent plasma donors. Given that the level of antibody titer decreases with days from onset to sampling and increases when the illness is sufficiently severe to necessitate treatment with systemic corticosteroids, these factors should be incorporated into the screening criteria for convalescent plasma donation. Moreover, as the number of participants who experienced vaccination increases, further studies are warranted to elucidate the trend of antibody production in the convalescent phase among post-vaccination participants.
Conflict-of-interest disclosure
None.
Funding
This research was supported by AMED under Grant Number JP20fk0108502 (Kutsuna).
Data availability
Dr. Kutsuna has full access to all data and takes responsibility for this study.
Authorship statement
Concept and design: Suzuki, Asai, and Kutsuna, Data collection: Suzuki, Ide, and Fukuda, Statistical analysis: Suzuki, Asai, and Kutsuna, Material sampling: All authors, Sample measurement: Tanaka, Drafting of the manuscript: Suzuki, Revision of the manuscript: All authors, Procurement of funding: Kutsuna, Supervision: Kutsuna and Ohmagari, All authors meet the ICMJE authorship criteria.
Acknowledgments
None.
References
- 1.World Health Organization . 2021. Weekly epidemiological update on COVID-19 - 15 June 2021.https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---15-june-2021 [Google Scholar]
- 2.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., et al. ACTT-1 study group members. Remdesivir for the treatment of covid-19 - final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Recovery Collaborative Group, Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salama C., Han J., Yau L., Reiss W.G., Kramer B., Neidhart J.D., et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;384:20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalil A.C., Patterson T.F., Mehta A.K., Tomashek K.M., Wolfe C.R., Ghazaryan V., et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kutsuna S. Coronavirus disease 2019 (COVID-19): research progress and clinical practice. Glob Health Med. 2020;2:78–88. doi: 10.35772/ghm.2020.01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J., et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. J Am Med Assoc. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kutsuna S., Asai Y., Matsunaga A. Loss of anti-SARS-CoV-2 antibodies in mild Covid-19. N Engl J Med. 2020;383:1695–1696. doi: 10.1056/NEJMc2027051. [DOI] [PubMed] [Google Scholar]
- 10.Gontu A., Srinivasan S., Salazar E., Nair M.S., Nissly R.H., Greenawalt D., et al. Limited window for donation of convalescent plasma with high live-virus neutralizing antibody titers for COVID-19 immunotherapy. Commun Biol. 2021;4:267. doi: 10.1038/s42003-021-01813-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamayoshi S., Yasuhara A., Ito M., Akasaka O., Nakamura M., Nakachi I., et al. Antibody titers against SARS-CoV-2 decline, but do not disappear for several months. EClinicalMedicine. 2021;32:100734. doi: 10.1016/j.eclinm.2021.100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terada M., Kutsuna S., Togano T., Saita S., Kinoshita N., Shimanishi Y., et al. How we secured a COVID-19 convalescent plasma procurement scheme in Japan. Transfusion. 2021 doi: 10.1111/trf.16541. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noda K., Matsuda K., Yagishita S., Maeda K., Akiyama Y., Terada-Hirashima J., et al. A novel highly quantitative and reproducible assay for the detection of anti-SARS-CoV-2 IgG and IgM antibodies. Sci Rep. 2021;11:5198. doi: 10.1038/s41598-021-84387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L., Tong X., Chen H., He R., Lv Q., Yang R., et al. Characteristics and serological patterns of COVID-19 convalescent plasma donors: optimal donors and timing of donation. Transfusion. 2020;60:1765–1772. doi: 10.1111/trf.15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlickeiser S., Schwarz T., Steiner S., Wittke K., Al Besher N., Meyer O., et al. Disease severity, fever, age, and sex correlate with SARS-CoV-2 neutralizing antibody responses. Front Immunol. 2021;11:628971. doi: 10.3389/fimmu.2020.628971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benner S.E., Patel E.U., Laeyendecker O., Pekosz A., Littlefield K., Eby Y., et al. SARS-CoV-2 antibody avidity responses in COVID-19 patients and convalescent plasma donors. J Infect Dis. 2020;222:1974–1984. doi: 10.1093/infdis/jiaa581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujita Y., Tanimura M., Tanaka K. The distribution of the ABO blood groups in Japan. Jinrui Idengaku Zasshi. 1978;23:63–109. doi: 10.1007/BF02001790. [DOI] [PubMed] [Google Scholar]
- 18.Madariaga M.L.L., Guthmiller J.J., Schrantz S., Jansen M.O., Christensen C., Kumar M., et al. Clinical predictors of donor antibody titre and correlation with recipient antibody response in a COVID-19 convalescent plasma clinical trial. J Intern Med. 2021;289:559–573. doi: 10.1111/joim.13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayes C., Rubenstein W., Gibb D., Klapper E., Tanaka J., Pepkowitz S., et al. Blood group O convalescent plasma donations have significantly lower levels of SARS-CoV-2 IgG antibodies compared to blood group A donations. Transfusion. 2021 doi: 10.1111/trf.16524. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deleers M., Breiman A., Daubie V., Maggetto C., Barreau T., Besse T., et al. Covid-19 and blood groups: ABO antibody levels may also matter. Int J Infect Dis. 2021;104:242–249. doi: 10.1016/j.ijid.2020.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Dr. Kutsuna has full access to all data and takes responsibility for this study.