Abstract
Background
Subjects with mental disorders are at a higher risk of various pandemic, but no specific studies concerning on screening and comparing the risk factors of COVID-19 for subjects with and without mental disorders, and the role of different classes of mental disorders with respect to the COVID-19.
Methods
This study comprised 42,264 subjects with mental disorders and 431,694 subjects without. Logistic regression was used to evaluate the associations of exposure factors with COVID-19 risk. Interaction terms were employed to explore the potential interaction effect between mental disorders and each exposure factor on COVID-19 risk.
Results
Mental disorders increased 1.45-fold risk of COVID-19 compared with non-mental disorders. There were significant interaction effects between mental disorders and age, sex, ethnicity, health ratings, socioeconomic adversity, lifestyle habits or comorbidities on COVID-19 risk. Subjects with and without mental disorders shared some overlapping risk factors of COVID-19, including the non-white ethnicity, socioeconomic adversity and comorbidities. Subjects without mental disorders carry some specific risk and protective factors. Among subjects with mental disorders, the COVID-19 risk was higher in subjects with a diagnosis of organic/symptomatic mental disorders, mood disorders, and neurotic, stress-related and somatoform disorders than that of their counterparts. Age, amount of alcohol consumption, BMI and Townsend deprivation showed non-linear increase with COVID-19 risk.
Limitations
Absence of replication.
Conclusions
Subjects with mental disorders are vulnerable populations to whom more attention should be paid. Public health guidance should focus on reducing the COVID-19 risk by advocating healthy lifestyle habits and preferential policies in populations with comorbidities.
Keywords: COVID-19, Mental disorder, Risk factor, Interaction, Lifestyle, Comorbidity
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has conveyed high rate of morbidity and mortality (Mizumoto et al., 2020; Wu and McGoogan, 2020). As of 22th August 2021, more than two hundred million infected cases and 4 million deaths have been reported worldwide (https://www.worldometers.info/coronavirus/). During the pandemic, global attention has focused on infected patients and frontline responders (Hamer et al., 2020a; Lai et al., 2020; Lu et al., 2020). Several risk factors for the COVID-19 have been reported, including older age, poor lifestyle habits (e.g., smoking, and low physical activity), pre-existing lifestyle diseases (e.g., diabetes, hypertension, and cardiovascular diseases)(Banerjee et al., 2020; Petrilli et al., 2020; Richardson et al., 2020; Simonnet et al., 2020; Tu et al., 2020) and pre-existing a diagnosis of a mental disorder(Wang et al., 2021). However, the potential risk factors among specific vulnerable populations have been largely overlooked during this pandemic.
Identification of high-risk populations or risk factors could allow timely and appropriate medical intervention and early enrollment for prevention strategy such as priority vaccinations. Subjects with mental disorders may be at a higher risk of various pandemic and have worse physical health and treatment outcomes (Rodgers et al., 2018; Sartorious, 2013; Wang et al., 2021; Yao et al., 2020). The lower life expectancy, poor general resilience, poor immune (Glaser and Kiecolt-Glaser, 2005; Minihan et al., 2020) and more susceptibility to infection (Nikolich-Zugich, 2018; Packard et al., 2011; Kelly, 2020) in subjects with mental disorders relative to general population have been widely observed. In addition, socioeconomic adversity and poor lifestyle habits (e.g., smoking, alcoholism and lack of physical activity) are common among subjects with mental disorders (Hailemichael et al., 2019; Jonsdottir et al., 2010; Xu et al., 2010), which were also identified as risk factors associated with development of respiratory diseases and COVID-19 (Hamer et al., 2020a, 2019; Packard et al., 2011). Although these factors might potentially suggest susceptibility towards COVID-19 infection among subjects with mental disorders (Yao et al., 2020), to our knowledge, no specific studies concerning on the risk factors of COVID-19 in this specific vulnerable population. The present cohort study aimed (1) to investigate and compare the risk factors of COVID-19 in subjects with and without mental disorders using a large sample size from the UK Biobank, and (2) to explore the role of different classes of mental disorders with respect to the COVID-19 risk.
2. Materials and methods
2.1. Subjects
A total of 502,505 participants from 22 centers across England, Scotland, and Wales in the UK Biobank were recruited between March 2006 and December 2010. During the available follow-up period, the participants who died before 31-November 2019 were removed (n = 28,547), and the remaining participants (n = 473,958) were grouped into subjects with and without mental disorders. The diagnosis of mental disorder was assessed by International Classification of Diseases, v10 (ICD10) term from UK Biobank data category 41,270 (Fig. 1 ). Ethical approval was obtained from the North West Multi-center Research Ethics Committee (REC reference: 16/NW/0274).
Fig. 1.
Participant flow diagram.
2.2. Categories of mental disorders
Mental disorders comprise ten categories (Supplementary Methods), including the organic disorders (symptomatic disorders and mental disorders) (ICD-10 codes F00-F09); psychoactive substance use-related mental and behavioral disorders (codes F10-F19); schizophrenia, schizotypal and delusional disorders (codes F20-F29); mood [affective] disorders (codes F30-F39); neurotic, stress-related and somatoform disorders (codes F40-F48); behavioral syndromes associated with physiological disturbances and physical factors (codes F50-F59); disorders of adult personality and behavior (codes F60-F69); mental retardation (codes F70-F79); disorders of psychological development (codes F80-F89); and behavioral and emotional disorders with onset usually occurring in childhood and adolescence (codes F90-F99).
2.3. Primary outcomes
Confirmed COVID-19 test results included the specimen date, specimen type (locations/methods used to generate samples), laboratory, origin (inpatient or not) and outcome of confirmed COVID-19 (positive or negative). Confirmed COVID-19 infection was defined as at least one positive test result. We updated the outcome of COVID-19 test results from the UK Biobank on 26th July 2021. Duplicate data were removed by selecting the latest positive test results. Participants who did not report COVID-19 test results were deemed to have negative COVID-19 test results because the COVID-19 test results will be reported if the hospitalized patients tested positive for COVID-19.
2.4. Exposures
Lifestyle habits included smoking status, alcohol consumption, usual walking pace and physical activity. Usual walking pace was grouped into slow, steady average and brisk pace. Physical activity was assessed by the International Physical Activity Questionnaire (IPAQ), which was grouped into low, moderate and high physical activity. Alcohol consumption was quantified as the number of units of alcohol per week by calculating average weekly intake of alcohol. The unit of weekly alcohol consumption was converted into units for beer and cider (1 pint =2 units), wines (1 standard glass = 2 units) and spirits (1 shot = 1 unit) (Hamer et al., 2020b).
Medication use (Field ID 20003) included antidepressants, lithium, cholesterol lowering medication, anticonvulsants, and Benzodiazepine. Socioeconomic status was assessed by education level, employment status, and Townsend deprivation. A higher value of the Townsend deprivation indicated a lower socioeconomic position.
Lifestyle diseases included the cancer, diabetes, obesity (BMI values ≥30 kg/m2), hypertension and coronary artery disease. Other prevalent diseases included respiratory diseases and cerebrovascular disease. The definition of diabetes included self-reported type 1 or type 2 diabetes and those with a primary or secondary hospital diagnoses relating to diabetes (ICD-10 codes E10-E14). The definition of respiratory disease included pulmonary embolism (ICD-10 code I26), other pulmonary heart diseases (ICD-10 code I27), influenza (ICD-10 codes J09-11), pneumonia (ICD-10 codes J12-18), acute bronchitis (ICD-10 code J20), acute bronchiolitis(ICD-10 code J21), unspecified acute lower respiratory infection (ICD-10 code J22), other diseases of upper respiratory tract (ICD-10 codes J30-39), chronic lower respiratory diseases (ICD-10 codes J40-47), lung diseases due to external agents (ICD-10 codes J60-70), other respiratory diseases principally affecting the interstitium (ICD-10 codes J85-86) and other diseases of the pleura and respiratory system (ICD-10 codes J90-99).
2.5. Statistical analysis
Continuous variables are presented as the mean ± SD, and categorical variables as a number (percentage). We used the unpaired t-tests, Mann-Whitney U test and χ² test to compare the differences between groups where appropriate.
Logistic regression was used to evaluate the associations of exposure factors with outcomes. Univariate and multivariate models were applied to evaluate the odds ratio (OR) and 95% confidence intervals (95% CIs). Four schemes were performed in the Logistic regression analysis: (1) model 1-adjusted for age, sex, education, and ethnicity; (2) model 2- further adjusted for BMI, overall health rating, employment status, able to confide, and Townsend deprivation if they were significant between groups; (3) model 3- further adjusted for lifestyle habits and medications; (4) model 4-further adjusted for prevalent diseases. Potential confounders for Logistic regression analysis should fulfill the following criteria: (1) imbalance between COVID-19 and non-COVID-19 in each of the exposures (p < 0.05), (2) correlation with primary outcomes, (3) not mediators between exposures and outcomes, and (4) no significant collinearity (correlation coefficient > 0.5) with any other exposures.
The interaction terms were employed for the overall sample to explore the potential interaction effect between mental disorders and exposure factors on the risk of COVID-19. For continuous variables, a non-linearity association between each of exposure factors and the COVID-19 risk were tested using penalized cubic splines in generalized additive model (GAM). Nonlinearity was tested using likelihood ratio test comparing a model with the exposure fitted on a spline with a model assuming a non-linear exposure-outcome relationship. P-values for nonlinearity p < 0.05 suggested evidence against the linearity assumption.
All the analyses were conducted with SPSS version 24.0 and R Statistical Software version 4.0, and a two-tailed p-value < 0.05 was considered significant.
3. Results
3.1. Sample characteristics
A total of 77,217 participants reported COVID-19 test results, including 16,559 participants with positive test results and 60,658 participants with negative test results. Among the participants with positive for COVID-19, 12.2% participants (n = 2027) reported positive COVID-19. Mental disorders increased 1.45-fold risk of COVID-19 infection (OR [95%CI], 1.45 [1.38, 1.52], p < 0.001), this was significant (1.11 [1.04, 1.18], p = 0.001) even after adjusting for age, sex, education, ethnicity, BMI, overall health rating, usual walking pace, IPAQ, antidepressants, and cholesterol lowering medication, cerebrovascular disease, cardiovascular disease, respiratory disease, diabetes, and hypertension.
Compared with other three subgroups (mental disorders without COVID-19, non-mental disorders with and without COVID-19), mental disorders with positive for COVID-19 exhibited the highest proportion of the older age, male sex, poor/fair health ratings, comorbidities (e.g., lifestyle diseases) and socioeconomic adversity (Table 1 ). However, no differences were found in lifestyle habits between those with mental disorders with and without COVID-19.
Table 1.
Characteristics of participants with and without mental disorders who suffer and not suffer from COVID-19 in UK Biobank.
| Characteristics | Mental disorders, n = 42,264 | Non-mental disorders, n = 431,694 | p value a | p value b | p value c | ||
|---|---|---|---|---|---|---|---|
| COVID-19 (n = 2027) | Non-COVID-19 (n = 40,237) | COVID-19 (n = 14,532) | Non-COVID-19 (n = 417,162) | ||||
| Demographics at baseline | |||||||
| Age to July 2021 (years), mean ± SD | 68.2 ± 8.7 | 69.2 ± 8.1⁎⁎⁎ | 66.1 ± 8.6 | 69.3 ± 8.1⁎⁎⁎ | <0.001 | <0.001 | <0.001 |
| Age categories, N (%) | |||||||
| ≤60 years | 498(24.6) | 7545(18.8) ⁎⁎⁎ | 4837(33.3) | 76,762(18.4) ⁎⁎⁎ | Reference | ||
| 61–70 years | 670(33.1) | 13,036(32.4) | 4745(32.7) | 132,825(31.8) | <0.001 | 0.34 | <0.001 |
| ≥71 years | 859(42.4) | 19,656(48.9) | 4950(34.1) | 207,574(49.8) | <0.001 | <0.001 | |
| Sex (male), N (%) | 932(46.0) | 18,098 (45.0) | 6870(47.3) | 186,167(44.6) ⁎⁎⁎ | <0.001 | <0.001 | 0.17 |
| Education level (degree), N (%) | 320 (16.3) | 9050 (23.2) ⁎⁎⁎ | 3739(26.3) | 141,538(34.6) ⁎⁎⁎ | <0.001 | <0.001 | 0.48 |
| Ethnicity (white), N (%) | 1860(92.4) | 37,803(94.7) ⁎⁎⁎ | 12,904(89.3) | 392,761(94.6) ⁎⁎⁎ | <0.001 | <0.001 | <0.001 |
| Obesity (BMI ≥30 kg/m2), N (%) | 722(36.0) | 12,593(31.6) ⁎⁎⁎ | 4349(30.2) | 95,919(23.1) ⁎⁎⁎ | <0.001 | <0.001 | 0.001 |
| Smoking (Previous or current), N (%) | 1278(63.7) | 26,050(65.3) | 6411(44.4) | 174,721 (42.1) ⁎⁎⁎ | <0.001 | <0.001 | 0.001 |
| Overall health rating, N (%) | |||||||
| Excellent or good | 923(46.2) | 20,549 (51.8) ⁎⁎⁎ | 10,346(71.7) | 323,571(78.0) ⁎⁎⁎ | Reference | ||
| Fair or poor | 1073(53.8) | 19,142(48.2) | 4078(28.3) | 91,260(22.0) | <0.001 | <0.001 | 0.023 |
| Alcohol consumption (UK unit), mean ± SD | 15.8 ± 26.5 | 16.1 ± 23.8 | 14.0 ± 17.8 | 14.6 ± 17.1 | <0.001 | <0.001 | 0.18 |
| Employment status (not in paid), N (%) | 1070(53.2) | 21,387(53.7) | 4426(30.7) | 166,176 (40.1) ⁎⁎⁎ | <0.001 | <0.001 | <0.001 |
| Townsend deprivation, mean ± SD | 0.2 ± 3.4 | −0.2 ± 3.5 ⁎⁎⁎ | −0.8 ± 3.2 | −1.5 ± 3.0 ⁎⁎⁎ | <0.001 | <0.001 | <0.001 |
| Able to confide (<once a week), N (%) | 687(34.4) | 13,503(33.9) | 4054(28.2) | 112,090(27.1) ⁎⁎ | 0.003 | <0.001 | 0.51 |
| Leisure/social activity (any activities), N (%) | 1312(65.1) | 25,609(64.2) | 10,125(70.2) | 291,889(70.3) | 0.68 | <0.001 | 0.35 |
| Physical activity, N (%) | |||||||
| Low | 414(28.0) | 7231(23.7) ⁎⁎⁎ | 2229(19.5) | 60,975(18.1) ⁎⁎⁎ | <0.001 | <0.001 | 0.12 |
| Moderate | 532(35.9) | 11,698(38.4) | 4340(38.0) | 138,808(41.2) | Reference | ||
| High | 535(36.1) | 11,556(37.9) | 4850(42.5) | 137,436(40.8) | <0.001 | 0.31 | |
| Usual walking pace, N (%) | |||||||
| Slow pace | 427(21.6) | 6993(17.7) ⁎⁎⁎ | 1211(8.4) | 24,241(6.3) ⁎⁎⁎ | <0.001 | <0.001 | 0.16 |
| Steady average pace | 1031(52.1) | 21,150(53.6) | 7838(54.6) | 218,033(52.6) | Reference | ||
| Brisk pace | 520(26.3) | 11,310(28.7) | 5314(37.0) | 170,013(41.0) | <0.001 | 0.71 | |
| Prevalent diseases at baseline | |||||||
| Respiratory disease, N (%) | 836(41.2) | 14,414(35.8) ⁎⁎⁎ | 2561(17.6) | 57,717(13.8) ⁎⁎⁎ | <0.001 | <0.001 | 0.26 |
| Cancer, N (%) | 329(16.2) | 6641(16.5) | 1536(10.6) | 52,251(12.5) ⁎⁎⁎ | <0.001 | <0.001 | 0.011 |
| Cerebrovascular disease, N (%) | 221(10.9) | 2407(6.0) ⁎⁎⁎ | 268(1.8) | 6366(1.5) ⁎⁎ | 0.002 | <0.001 | <0.001 |
| Diabetes, N (%) | 345(17.0) | 5151(12.8) ⁎⁎⁎ | 1079(7.4) | 19,679(4.7) ⁎⁎⁎ | <0.001 | <0.001 | 0.032 |
| Hypertension, N (%) | 943(46.5) | 16,801(41.8) ⁎⁎⁎ | 3100(21.3) | 79,339(19.0) ⁎⁎⁎ | <0.001 | <0.001 | 0.32 |
| Cardiovascular disease,N (%) | 381(18.8) | 6352(15.8) ⁎⁎⁎ | 977(6.7) | 24,633(5.9) ⁎⁎⁎ | <0.001 | <0.001 | 0.28 |
| Medication use | |||||||
| Antidepressants, N (%) | 616(30.4) | 11,176(27.8) ⁎⁎ | 890(6.1) | 23,086(5.5) ⁎⁎ | 0.39 | <0.001 | 0.77 |
| Lithium, N (%) | 16(0.8) | 482(1.2) # | 7(0.0) | 234(0.1) | 0.69 | <0.001 | 0.67 |
| Anticonvulsants, N (%) | 132(6.5) | 2302(5.7) | 198(1.4) | 5087(1.2) | 0.12 | <0.001 | 0.83 |
| Benzodiazepine, N (%) | 72(3.6) | 1681(4.2) | 98(0.7) | 3110(0.7) | 0.33 | <0.001 | 0.4 |
| Cholesterol lowering medication, N (%) | 559(27.6) | 9511(23.6) ⁎⁎⁎ | 2344(16.1) | 68,711(16.5) | 0.28 | <0.001 | <0.001 |
0.05≤p < 0.1
*p < 0.05.
p ≤ 0.01.
p ≤ 0.001 between COVID-19 and non-COVID-19 in mental disorders subgroup or non-mental disorders subgroup.
The pa, pb, pc indicated p values of main effect of each factor and mental disorders, and interaction effect between each factor and mental disorders on risk of COVID-19 infection.
3.2. Interaction effect
There were significant main effects of mental disorders and some potentially exposure factors, and significant interaction effects between the two items (mental disorders × exposure factors) on the COVID-19 risk (Table 1). There were significant interaction effects between mental disorders and age (interaction quadratic p < 0.001), sex (p < 0.001), ethnicity (p < 0.001), health ratings (p = 0.023), socioeconomic adversity (employment status, p < 0.001; Townsend deprivation, p < 0.001), lifestyle habits (obesity, p = 0.001; smoking, p = 0.001), lifestyle diseases (cancer, p = 0.011; diabetes, p = 0.032; cerebrovascular diseases, p < 0.001) and medication use (cholesterol lowering medication, p < 0.001) on the COVID-19 risk (Table 1).
3.3. Multivariate risk factors for incident COVID-19
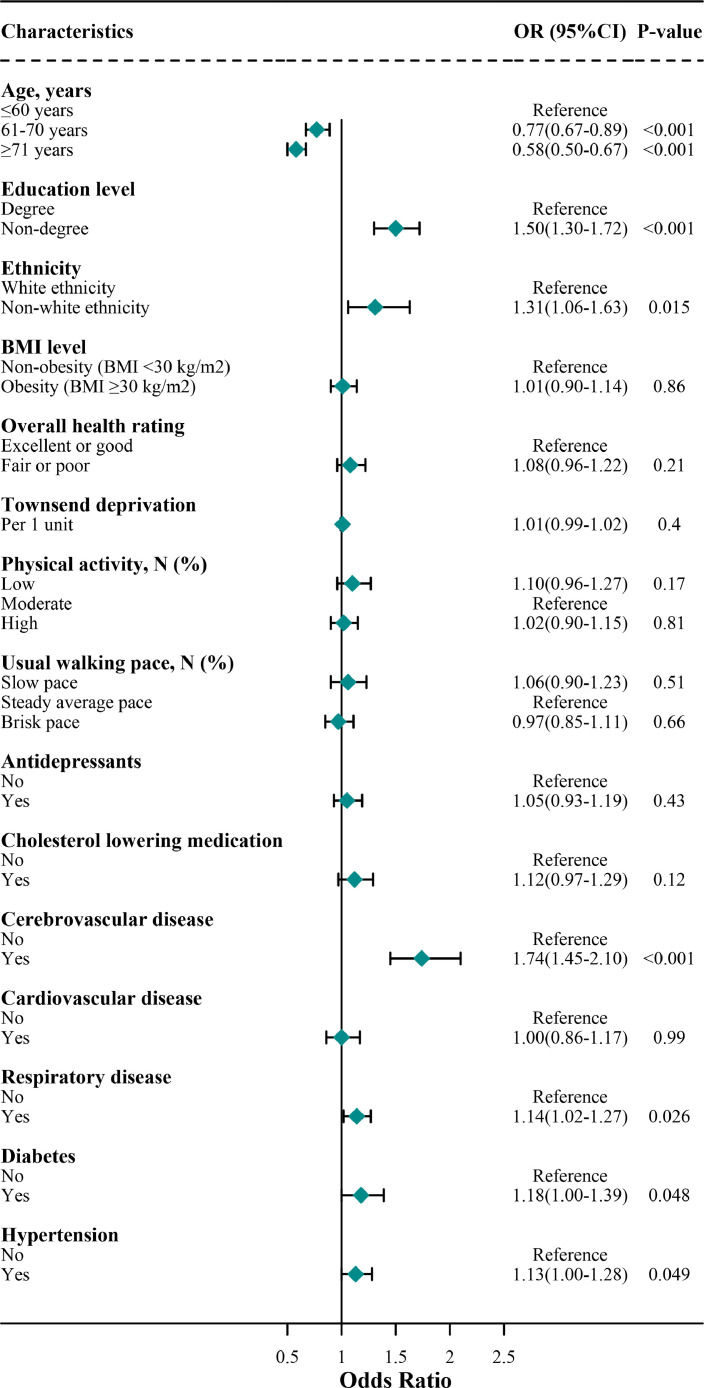
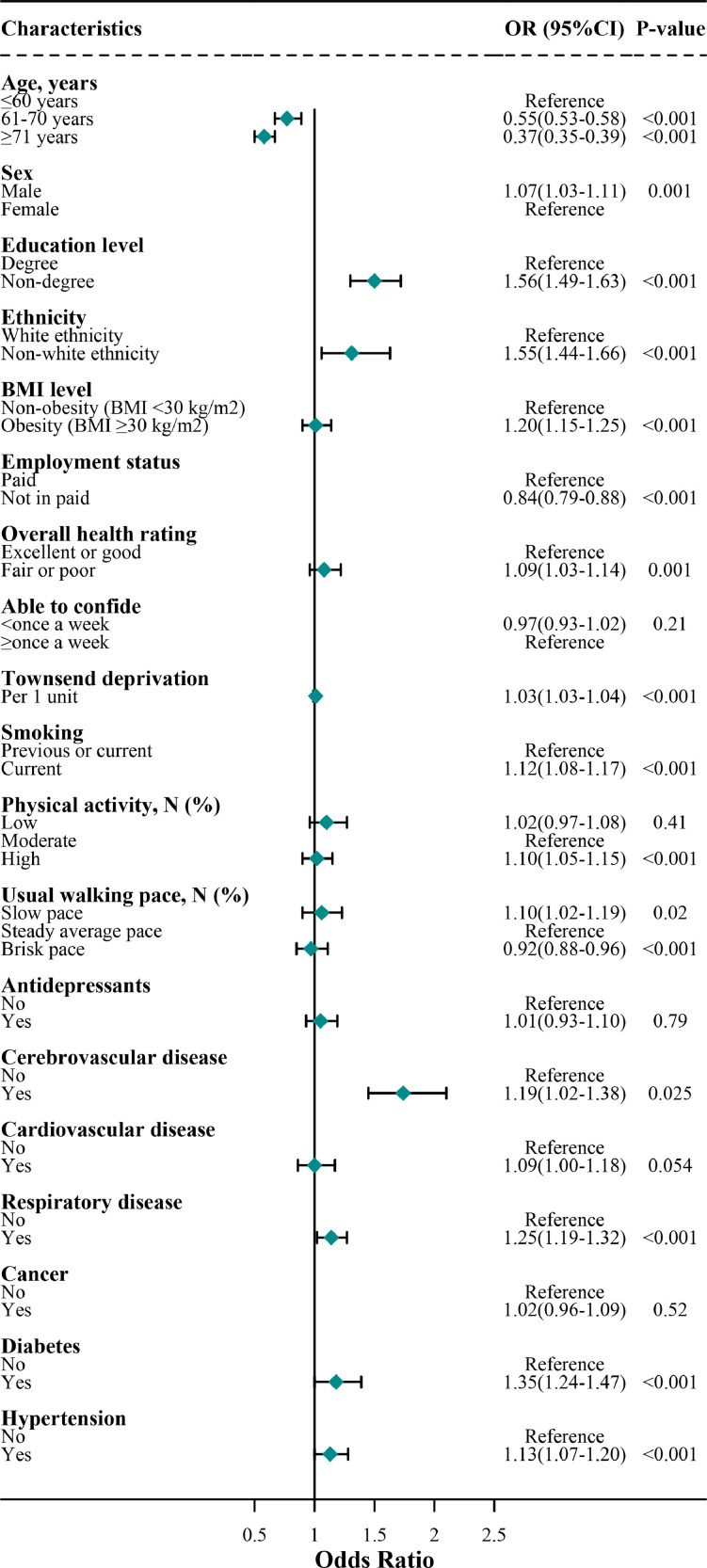
In the multivariate final model, the two populations shared several overlapping key independent risk factors for COVID-19 (Tables 2 , 3 , Figs. 2 , 3 ). These factors included socioeconomic factors (low education level, 1.50 [1.30, 1.72] and 1.56 [1.49, 1.63] in mental disorders and non-mental disorders, respectively), non-white ethnicity (1.31 [1.06, 1.63] and 1.56 [1.49, 1.63]), and some comorbidities (respiratory disease, 1.14 [1.02, 1.27] and 1.25 [1.19, 1.32]; cerebrovascular disease, 1.74 [1.45–2.10] and 1.19 [1.02–1.38]; diabetes, 1.18 [1.00, 1.39] and 1.35 [1.24, 1.47]; hypertension, 1.13 [1.00–1.28] and 1.13 [1.07, 1.20]).
Table 2.
Association of risk factors for COVID-19 cases with mental disorders using multivariate Logistic regression analysis.
| Variables | model 1 | model 2 | model 3 | model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95%Cl) | p value | OR (95%Cl) | p value | OR (95%Cl) | p value | OR (95%Cl) | p value | |
| Age | ||||||||
| ≤60 years | Reference | Reference | Reference | Reference | ||||
| 61–70 years | 0.79(0.70–0.89) | <0.001 | 0.80(0.71–0.90) | <0.001 | 0.79(0.69–0.92) | 0.001 | 0.77(0.67–0.89) | <0.001 |
| ≥71 years | 0.67(0.59–0.75) | <0.001 | 0.68(0.60–0.76) | <0.001 | 0.62(0.54–0.72) | <0.001 | 0.58(0.50–0.67) | <0.001 |
| Education level | ||||||||
| Degree | Reference | Reference | Reference | |||||
| Non-degree | 1.60(1.42–1.81) | <0.001 | 1.56(1.38–1.77) | <0.001 | 1.52(1.32–1.75) | <0.001 | 1.50(1.30–1.72) | <0.001 |
| Ethnicity | ||||||||
| White ethnicity | Reference | Reference | Reference | Reference | ||||
| Non-white ethnicity | 1.44(1.21–1.71) | <0.001 | 1.33(1.11–1.61) | 0.002 | 1.36(1.09–1.69) | 0.006 | 1.31(1.06–1.63) | 0.015 |
| BMI level | ||||||||
| Non-obesity (BMI <30 kg/m2) | Reference | Reference | Reference | Reference | ||||
| Obesity (BMI ≥30 kg/m2) | 1.19(1.09–1.31) | <0.001 | 1.15(1.05–1.27) | 0.004 | 1.05(0.93–1.18) | 0.43 | 1.01(0.90–1.14) | 0.86 |
| Overall health rating | ||||||||
| Excellent or good | Reference | Reference | Reference | Reference | ||||
| Fair or poor | 1.20(1.09–1.31) | <0.001 | 1.15(1.04–1.26) | 0.005 | 1.13(1.00–1.27) | 0.049 | 1.08(0.96–1.22) | 0.21 |
| Townsend deprivation | 1.02(1.00–1.03) | 0.01 | 1.15(1.04–1.26) | 0.05 | 1.01(0.99–1.03) | 0.26 | 1.01(0.99–1.02) | 0.40 |
| Physical activity, N (%) | ||||||||
| Low | 1.20(1.05–1.37) | 0.008 | 1.13(0.99–1.28) | 0.064 | 1.11(0.95–1.29) | 0.18 | 1.10(0.96–1.27) | 0.17 |
| Moderate | Reference | Reference | Reference | Reference | ||||
| High | 0.97(0.86–1.10) | 0.68 | 0.99(0.89–1.11) | 0.89 | 0.96(0.85–1.09) | 0.55 | 1.02(0.90–1.15) | 0.81 |
| Usual walking pace, N (%) | ||||||||
| Slow pace | 1.25(1.11–1.41) | <0.001 | 1.12(0.98–1.28) | 0.10 | 1.10(0.96–1.27) | 0.17 | 1.06(0.90–1.23) | 0.51 |
| Steady average pace | Reference | Reference | Reference | Reference | ||||
| Brisk pace | 0.96(0.86–1.07) | 0.43 | 1.00(0.89–1.14) | 0.97 | 1.01(0.89–1.15) | 0.84 | 0.97(0.85–1.11) | 0.66 |
| Antidepressants | 1.16(1.05–1.28) | 0.004 | 1.10(1.00–1.22) | 0.061 | 1.04(0.92–1.18) | 0.50 | 1.05(0.93–1.19) | 0.43 |
| Cholesterol lowering medication | 1.34(1.20–1.49) | <0.001 | 1.27 (1.14–1.42) | <0.001 | 1.25(1.10–1.43) | 0.001 | 1.12(0.97–1.29) | 0.12 |
| Cerebrovascular disease | 2.05(1.76–2.38) | <0.001 | 2.01(1.73–2.35) | <0.001 | 1.81(1.51–2.18) | <0.001 | 1.74(1.45–2.10) | <0.001 |
| Cardiovascular disease | 1.30(1.15–1.47) | <0.001 | 1.24(1.10–1.41) | 0.001 | 1.08(0.93–1.26) | 0.33 | 1.00(0.86–1.17) | 0.99 |
| Respiratory disease | 1.26(1.15–1.38) | <0.001 | 1.21(1.10–1.33) | <0.001 | 1.18(1.05–1.31) | 0.005 | 1.14(1.02–1.27) | 0.026 |
| Diabetes | 1.40(1.23–1.58) | <0.001 | 1.30(1.14–1.49) | <0.001 | 1.23(1.05–1.45) | 0.011 | 1.18(1.00–1.39) | 0.048 |
| Hypertension | 1.30(1.18–1.43) | <0.001 | 1.23(1.12–1.36) | <0.001 | 1.21(1.07–1.36) | 0.002 | 1.13(1.00–1.28) | 0.049 |
Model 1Analyses were adjusted for age, sex, education, and ethnicity.
Model 2 Analyses were further adjusted for BMI, overall health rating, and Townsend deprivation.
Model 3 Analyses were further adjusted for usual walking pace, IPAQ, antidepressants, and cholesterol lowering medication.
Model 4 Analyses were further adjusted for cerebrovascular disease, cardiovascular disease, respiratory disease, diabetes, and hypertension.
Table 3.
Association of risk factors for COVID-19 cases without mental disorders using multivariate Logistic regression analysis.
| Variables | model 1 | model 2 | model 3 | model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95%Cl) | p value | OR (95%Cl) | p value | OR (95%Cl) | p value | OR (95%Cl) | p value | |
| Age | ||||||||
| ≤60 years | Reference | Reference | Reference | Reference | ||||
| 61–70 years | 0.57(0.55–0.59) | <0.001 | 0.57(0.55–0.60) | <0.001 | 0.57(0.54–0.59) | <0.001 | 0.55(0.53–0.58) | <0.001 |
| ≥71 years | 0.37(0.36–0.39) | <0.001 | 0.40(0.38–0.42) | <0.001 | 0.40(0.38–0.42) | <0.001 | 0.37(0.35–0.39) | <0.001 |
| Sex | ||||||||
| Male | 1.13(1.09–1.16) | <0.001 | 1.10(1.06–1.14) | <0.001 | 1.09(1.05–1.13) | <0.001 | 1.07(1.03–1.11) | 0.001 |
| Female | Reference | Reference | Reference | Reference | ||||
| Education level | ||||||||
| Degree | Reference | Reference | Reference | Reference | ||||
| Non-degree | 1.66(1.59–1.72) | <0.001 | 1.59(1.53–1.65) | <0.001 | 1.58(1.51–1.64) | <0.001 | 1.56(1.49–1.63) | <0.001 |
| Ethnicity | ||||||||
| White ethnicity | Reference | Reference | Reference | Reference | ||||
| Non-white ethnicity | 1.80(1.70–1.90) | <0.001 | 1.59(1.49–1.69) | <0.001 | 1.58(1.47–1.69) | <0.001 | 1.55(1.44–1.66) | <0.001 |
| BMI level | ||||||||
| Non-obesity (BMI <30 kg/m2) | Reference | Reference | Reference | Reference | ||||
| Obesity (BMI ≥30 kg/m2) | 1.39(1.34–1.44) | <0.001 | 1.31(1.26–1.36) | <0.001 | 1.25(1.19–1.31) | <0.001 | 1.20(1.15–1.25) | <0.001 |
| Employment status | ||||||||
| Paid | Reference | Reference | Reference | Reference | ||||
| Not in paid | 0.91(0.87–0.95) | <0.001 | 0.87(0.83–0.91) | <0.001 | 0.85(0.81–0.90) | <0.001 | 0.84(0.79–0.88) | <0.001 |
| Overall health rating | ||||||||
| Excellent or good | Reference | Reference | Reference | Reference | ||||
| Fair or poor | 1.28(1.24–1.33) | <0.001 | 1.19(1.14–1.24) | <0.001 | 1.15(1.10–1.21) | <0.001 | 1.09(1.03–1.14) | 0.001 |
| Able to confide | ||||||||
| <once a week | 1.02(0.98–1.06) | 0.41 | 0.98(0.95–1.02) | 0.35 | 0.97(0.93–1.01) | 0.17 | 0.97(0.93–1.02) | 0.21 |
| ≥once a week | Reference | Reference | Reference | Reference | ||||
| Townsend deprivation | 1.04(1.04–1.05) | <0.001 | 1.04(1.03–1.04) | <0.001 | 1.03(1.03–1.04) | <0.001 | 1.03(1.03–1.04) | <0.001 |
| Smoking | ||||||||
| Previous or current | Reference | Reference | Reference | Reference | ||||
| Current | 1.17(1.13–1.21) | <0.001 | 1.13(1.09–1.17) | <0.001 | 1.13(1.09–1.17) | <0.001 | 1.12(1.08–1.17) | <0.001 |
| Physical activity, N (%) | ||||||||
| Low | 1.11(1.05–1.17) | <0.001 | 1.05(0.99–1.11) | 0.093 | 1.02(0.97–1.08) | 0.39 | 1.02(0.97–1.08) | 0.41 |
| Moderate | Reference | Reference | Reference | Reference | ||||
| High | 1.07(1.03–1.12) | 0.001 | 1.09(1.05–1.14) | <0.001 | 1.10(1.05–1.15) | <0.001 | 1.10(1.05–1.15) | <0.001 |
| Usual walking pace, N (%) | ||||||||
| Slow pace | 1.29(1.21–1.38) | <0.001 | 1.14(1.07–1.22) | <0.001 | 1.16(1.07–1.26) | <0.001 | 1.10(1.02–1.19) | 0.02 |
| Steady average pace | Reference | Reference | Reference | Reference | ||||
| Brisk pace | 0.86(0.83–0.90) | <0.001 | 0.92(0.89–0.95) | <0.001 | 0.91(0.87–0.95) | <0.001 | 0.92(0.88–0.96) | <0.001 |
| Antidepressants | 1.14(1.06–1.22) | <0.001 | 1.06(0.99–1.14) | 0.11 | 1.02(0.94–1.11) | 0.65 | 1.01(0.93–1.10) | 0.79 |
| Cerebrovascular disease | 1.43(1.26–1.62) | <0.001 | 1.35(1.18–1.53) | <0.001 | 1.33(1.14–1.54) | <0.001 | 1.19(1.02–1.38) | 0.025 |
| Cardiovascular disease | 1.38(1.29–1.48) | <0.001 | 1.28(1.19–1.37) | <0.001 | 1.24(1.14–1.34) | <0.001 | 1.09(1.00–1.18) | 0.054 |
| Respiratory disease | 1.38(1.32–1.44) | <0.001 | 1.31(1.25–1.37) | <0.001 | 1.30(1.24–1.37) | <0.001 | 1.25(1.19–1.32) | <0.001 |
| Cancer | 1.03(0.97–1.09) | 0.37 | 1.03(0.98–1.09) | 0.25 | 1.05(0.99–1.12) | 0.11 | 1.02(0.96–1.09) | 0.52 |
| Diabetes | 1.73(1.62–1.85) | <0.001 | 1.50(1.40–1.61) | <0.001 | 1.49(1.37–1.61) | <0.001 | 1.35(1.24–1.47) | <0.001 |
| Hypertension | 1.41(1.35–1.47) | <0.001 | 1.29(1.23–1.35) | <0.001 | 1.25(1.19–1.32) | <0.001 | 1.13(1.07–1.20) | <0.001 |
Model 1Analyses were adjusted for age, sex, education, and ethnicity.
Model 2 Analyses were further adjusted for BMI, overall health rating, employment status, able to confide, and Townsend deprivation.
Model 3 Analyses were further adjusted for smoking, usual walking pace, IPAQ, and antidepressants.
Model 4 Analyses were further adjusted for cerebrovascular disease, cardiovascular disease, respiratory disease, cancer, diabetes, and hypertension.
Fig. 2.
Association of risk factors for COVID-19 cases with mental disorders in the final model
Note: this figure shows the results of the final model, which is the same as the model 4 in Table 2.
Fig. 3.
Association of risk factors for COVID-19 cases without mental disorders in the final model
Note: this figure shows the results of the final model, which is the same as the model 4 in Table 3.
Subjects without mental disorders carry some specific risk factors contributing to a higher risk of COVID-19 (Table 3). These risk factors included male sex (1.07 [1.03, 1.11]), fair or poor health rating (1.09 [1.03, 1.14]), Townsend deprivation (1.03 [1.03, 1.04]), lifestyle habits (smoking, 1.12 [1.08, 1.17]; high physical activity, 1.10 [1.05, 1.15]; slow walking pace, 1.10 [1.02, 1.19]; obesity, 1.20 [1.15, 1.25]). Furthermore, subjects without mental disorders carry some specific protective factors contributing to a lower risk of COVID-19. These protective factors included not in paid employment status (0.84 [0.79, 0.88]) and brisk walking pace (0.92 [0.88, 0.96]).
3.4. Multivariate risk factors for mental disorder subtypes
In the multivariate final model, the organic/symptomatic mental disorders (3.00 [2.47, 3.63]), mood [affective] disorders (1.17 [1.07, 1.27]), and neurotic, stress-related and somatoform disorders (1.18 [1.06, 1.31]) respectively predisposed to a higher risk of COVID-19 when compared with their counterparts (Table 4 ). However, other subgroups of mental disorders were not associated with COVID-19 risk.
Table 4.
Association of subgroup of mental disorders with COVID-19 risk.
| Variables | COVID-19, N (%) | None-COVID-19, N (%) | p value | model 1 | model 2 | model 3 | model 4 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95%Cl) | p value | OR (95%Cl) | p value | OR (95%Cl) | p value | OR (95%Cl) | p value | ||||
| Organic and symptomatic mental disorders | 194 (1.2) | 1626 (0.4) | <0.001 | 4.01 (3.43–4.68) | <0.001 | 3.70 (3.16–4.33) | <0.001 | 3.54 (2.93–4.28) | <0.001 | 3.00 (2.47–3.63) | <0.001 |
| Mental and behavioral disorders due to psychoactive substance use | 893 (5.4) | 18,643 (4.1) | <0.001 | 1.21 (1.13–1.30) | <0.001 | 1.09 (1.01–1.17) | 0.021 | 1.08 (1.00–1.18) | 0.064 | 0.99(0.91–1.07) | 0.74 |
| Schizophrenia, schizotypal and delusional disorders | 56 (0.3) | 1165 (0.3) | 0.037 | 1.01 (0.76–1.34) | 0.95 | 0.75 (0.55–1.02) | 0.065 | 0.77 (0.55–1.09) | 0.15 | 0.73 (0.51–1.03) | 0.07 |
| Mood [affective] disorders | 912 (5.5) | 16,396 (3.6) | <0.001 | 1.48 (1.38–1.59) | <0.001 | 1.31 (1.22–1.41) | <0.001 | 1.28 (1.17–1.40) | <0.001 | 1.17 (1.07–1.27) | 0.001 |
| Neurotic, stress-related and somatoform disorders | 573 (3.5) | 10,704 (2.3) | <0.001 | 1.48 (1.36–1.62) | <0.001 | 1.34 (1.23–1.47) | <0.001 | 1.28 (1.15–1.43) | <0.001 | 1.18 (1.06–1.31) | 0.003 |
| Behavioral syndromes associated with physiological disturbances and physical factors | 33 (0.2) | 562 (0.1) | 0.006 | 1.54 (1.07–2.20) | 0.019 | 1.33 (0.91–1.94) | 0.14 | 1.22 (0.79–1.90) | 0.38 | 1.12 (0.72–1.74) | 0.62 |
| Disorders of adult personality and behavior | 30 (0.2) | 482 (0.1) | 0.004 | 1.36 (0.93–1.99) | 0.11 | 1.11 (0.76–1.64) | 0.59 | 1.03 (0.65–1.63) | 0.89 | 0.94 (0.59–1.48) | 0.77 |
| Mental retardation | 6 (0.0) | 70 (0.0) | 0.037 | 1.71(0.68–4.26) | 0.25 | 0.87 (0.27–2.78) | 0.81 | 0.48 (0.07–3.54) | 0.47 | 0.41 (0.06–2.98) | 0.38 |
| Disorders of psychological development | 25 (0.2) | 362 (0.1) | 0.001 | 1.55 (1.00–2.39) | 0.049 | 1.20 (0.75–1.91) | 0.45 | 1.49 (0.88–2.54) | 0.14 | 1.26 (0.74–2.14) | 0.4 |
| Behavioral and emotional disorders with onset usually occurring in childhood and adolescence, and unspecified mental disorder | 22 (0.1) | 273 (0.1) | <0.001 | 2.07 (1.33–3.20) | 0.001 | 1.68 (1.06–2.66) | 0.027 | 1.60 (0.92–2.76) | 0.095 | 1.50(0.86–2.59) | 0.15 |
Model 1Analyses were adjusted for age, sex, education, and ethnicity.
Model 2 Analyses were further adjusted for BMI, overall health rating, and Townsend deprivation.
Model 3 Analyses were further adjusted for usual walking pace, IPAQ, antidepressants, and cholesterol lowering medication.
Model 4 Analyses were further adjusted for cerebrovascular disease, cardiovascular disease, respiratory disease, diabetes, and hypertension.
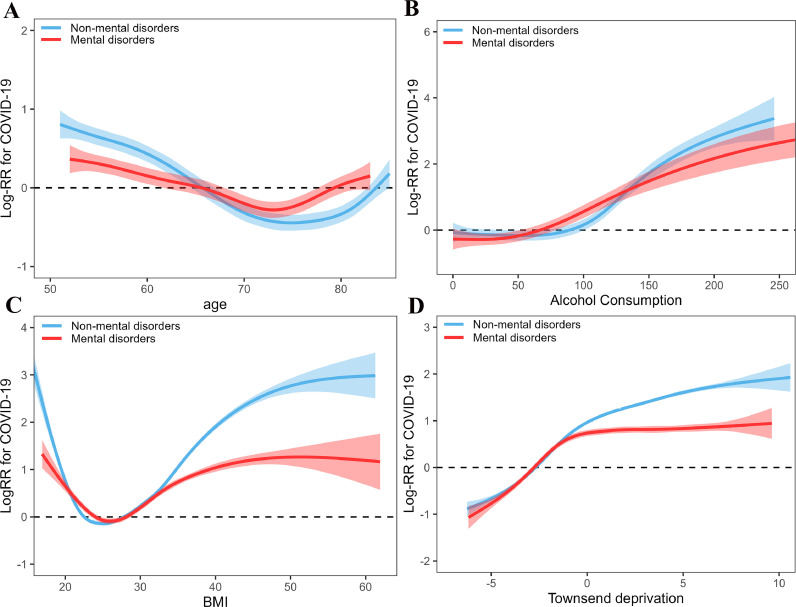
3.5. Nonlinear associations
Nonlinear associations of continuous factors with COVID-19 risk are shown in Fig. 2. Subjects with and without mental disorders shared similar curve with COVID-19 risk across these factors. Age was associated with COVID-19 curvilinearly (Fig. 4 A), where participants aged about 50–75 years had a gradually decreased risk of COVID-19 with increase of age and the risk was gradually increased among participants aged over 75 years (mental disorders: R2 = 0.69, deviance explained = 73.4%, p < 0.001; non-mental disorders: R2 = 0.91, deviance explained = 92.5%, p < 0.001). The amount of alcohol consumption had a curvilinearly J-shaped correlation with risk of COVID-19 (Fig. 4B), where participants had a higher risk with the increase of amount of alcohol consumption (mental disorders: R2 = 0.55, deviance explained = 56.1%, p < 0.001; non-mental disorders: R2 = 0.73, deviance explained = 73.5%, p < 0.001). BMI showed a U-shaped correlation with risk of COVID-19 (Fig. 4C), where the risk was first decreased and then increased with BMI (mental disorders: R2 = 0.30, deviance explained = 29.8%, p < 0.001; non-mental disorders: R2 = 0.35, deviance explained = 34.8%, p < 0.001). Townsend deprivation showed curvilinear increase with risk of COVID-19 (Fig. 4D), where the risk of COVID-19 reached a threshold and was not significant increased with Townsend deprivation (mental disorders: R2 = 0.42, deviance explained = 41.7%, p < 0.001; non-mental disorders: R2 = 0.50, deviance explained = 49.4%, p < 0.001).
Fig. 4.
Nonlinear associations of continuous variables with COVID-19.
4. Discussion
In this cohort study with a large sample size in the UK Biobank, three main novel findings are worthy noting. First, subjects with mental disorders are vulnerable populations with a 1.45-fold higher risk of COVID-19 compared with subjects without mental disorders. Second, subjects with and without mental disorders shared some overlapping risk factors, including non-white ethnicity, socioeconomic adversity, and comorbidities (respiratory disease, cerebrovascular disease, diabetes, hypertension). Furthermore, subjects without mental disorders carry some specific risk factors contributing to higher risks of COVID-19 and some specific protective factors contributing to lower risks of COVID-19. Third, subgroup analysis of mental disorders showed that the organic/symptomatic mental disorders, mood [affective] disorders, and neurotic, stress-related and somatoform disorders respectively predisposed to a risk of COVID-19 infection. Fourth, the continuous variables of age, amount of alcohol consumption, BMI and Townsend deprivation showed non-linear correlations with risk of COVID-19.
Our study suggests that mental disorders posed a higher risk in predisposing to COVID-19 infection. Mental disorders have been reported to be associated with a higher risk of developing respiratory diseases (Seminog and Goldacre, 2013). Subjects with mental disorders had lower awareness of personal protection and a higher risk of COVID-19 (Muruganandam et al., 2020; Wang et al., 2021; Yao et al., 2020), worse health outcomes, and poor overall resilience compared with subjects without mental disorders, which may also increase exposure and susceptibility to COVID-19 for those subjects with mental disorders.
Age has been suggested to be a risk factor of COVID-19 (Pan et al., 2020). However, some studies from UK Biobank did not find an association of age with the risk of COVID-19 (Khawaja et al., 2020; Raisi-Estabragh et al., 2020). We speculated that a curvilinear association of age with COVID-19 may explain this discrepancy. Our study draws the trajectory of COVID-19 risk with age among subjects with and without mental disorders, and found different curvilinear associations between age and the risk of COVID-19 in the two groups. The immune system of older adults possesses numerous age-related changes, which is called immune senescence (Nikolich-Zugich, 2018). We speculated that the higher risk before 65 years old may be associated with a higher exposure due to daily work, and increased risk of COVID-19 with age after 75 years old may be associated with immune senescence, poor physical health and more comorbidities. Subjects with mental disorders exhibited worse physical health and immune system, and lower awareness of infection precautions than those without mental disorders, which may explain the earlier immune senescence in subjects with mental disorders compared with subjects without mental disorders.
Unhealthy lifestyle behaviors and lifestyle diseases were considered primary intervention target for reducing the gap in physical health between subjects with and without mental disorders (Scott and Happell, 2011). Our study demonstrated that subjects without mental disorders were more likely to be affected by unhealthy lifestyle behaviors and lifestyle diseases in predisposing to future COVID-19 infection. Consistent findings of increased COVID-19 risk of unhealthy lifestyle behaviors in the general population have been reported (2020b; Ho et al., 2020). However, the association between the lifestyle practices or lifestyle diseases and the COVID-19 risk were not found among subjects with mental disorders. The exact reasons for these unexpected findings were unclear. The association between lifestyle factors and risk of respiratory infection among subjects with mental disorders has been less studied.
Our findings also suggest that we should pay specific attentions to subjects who have a diagnosis of organic/symptomatic mental disorders (mainly presented as dementia, and brain damage and dysfunction), mood [affective] disorders and neurotic, stress-related or somatoform disorders among subjects with mental disorders, which respectively showed a higher risk of COVID-19. Subjects with mood [affective] disorders or dementia who have a higher risk of COVID-19 have been reported in our previous study (Wang et al., 2021). Those with severe neurological dysfunction and injury are more likely to develop pulmonary diseases and further worsen clinical outcomes (Baumann et al., 2007; Wang et al., 2021), and this has been proposed as the brain-lung-brain axis theory (Stevens and Puybasset, 2011).
The major strengths of this study are the prospective large UK population-based cohort and a focus on the subgroup analyses among the population with mental disorders. However, there are several limitations that should be addressed. First, the UK Biobank has a restricted age range (48–85 years in 2021), and therefore did not represent the entire population. Second, the relatively small sample size of mental disorders comorbid with COVID-19, and the absence of replication in other cohorts should also be considered. Third, most exposures were measured at baseline. Although the lifestyle behaviors and socioeconomic conditions are not easily modified factors, the potential changes are not known.
5. Conclusions
In conclusion, our study demonstrated that apart from sharing some overlapping risk factors for COVID-19, subjects without mental disorders also exhibit some specific risk factors with enhanced risk of COVID-19 infection. public health guidance should take prioritized preventive actions, e.g., future vaccinations when available, to the susceptible populations, including older age population, subjects with a diagnosis of mental disorders, especially those with unhealthy lifestyle habits and comorbidities.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had final responsibility for the decision to submit for publication.
Data availability
The data that support the findings of this study are available from the UKB team.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Statement
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Author statement
XJ. D and Y.W had the idea for and designed this study, had full access to all the data in this study, take responsibility for the integrity of the data and the accuracy of the data analysis, and critically revised the manuscript for important intellectual content and gave final approval for the version to be published. XJ. D drafted the paper and did the analysis. Y. W, L. R, Y. S, and W. T takes responsibility for double check of the data analysis. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately resolved.
Declaration of Competing Interest
We declare no competing interests.
Acknowledgment
This work was supported by China Postdoctoral Science Foundation (grant No, 2020M670052), Guangdong Basic and Applied Basic Research Foundation (grant No, 2020A1515011469) and Sanming Project of Medicine in Shenzhen (grant No, SZSM201812052).
References
- Banerjee, A., Pasea, L., Harris, S., Gonzalez-Izquierdo, A., Torralbo, A., Shallcross, L., Noursadeghi, M., Pillay, D., Pagel, C., Wong, W.K., Langenberg, C., Williams, B., Denaxas, S., Hemingway, H., 2020. Estimating excess 1- year mortality from COVID-19 according to underlying conditions and age in England: a rapid analysis using NHS health records in 3.8 million adults. medRxiv.
- Baumann A., Audibert G., McDonnell J., Mertes P.M. Neurogenic pulmonary edema. Acta Anaesthesiol. Scand. 2007;51:447–455. doi: 10.1111/j.1399-6576.2007.01276.x. [DOI] [PubMed] [Google Scholar]
- Glaser R., Kiecolt-Glaser J.K. Stress-induced immune dysfunction: implications for health. Nat. Rev. Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Hailemichael Y., Hanlon C., Tirfessa K., Docrat S., Alem A., Medhin G., Fekadu A., Lund C., Chisholm D., Hailemariam D. Mental health problems and socioeconomic disadvantage: a controlled household study in rural Ethiopia. Int. J. Equity Health. 2019;18:121. doi: 10.1186/s12939-019-1020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer, M., Kivimaki, M., Gale, C.R., Batty, G.D., 2020. Lifestyle risk factors for cardiovascular disease in relation to COVID-19 hospitalization: a community-based cohort study of 387,109 adults in UK. [DOI] [PMC free article] [PubMed]
- Hamer M., Kivimaki M., Gale C.R., Batty G.D. Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: a community-based cohort study of 387,109 adults in UK. Brain Behav. Immun. 2020;87:184–187. doi: 10.1016/j.bbi.2020.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M., O'Donovan G., Stamatakis E. Lifestyle risk factors, obesity and infectious disease mortality in the general population: linkage study of 97,844 adults from England and Scotland. Prev. Med. 2019;123:65–70. doi: 10.1016/j.ypmed.2019.03.002. [DOI] [PubMed] [Google Scholar]
- Ho, F.K., Celis-Morales, C.A., Gray, S.R., Katikireddi, S.V., Niedzwiedz, C.L., Hastie, C., Lyall, D.M., Ferguson, L.D., Berry, C., Mackay, D.F., Gill, J.M.R., Pell, J.P., Sattar, N., Welsh, P.I., 2020. Modifiable and non-modifiable risk factors for COVID-19: results from UK Biobank. [DOI] [PMC free article] [PubMed]
- Jonsdottir I.H., Rodjer L., Hadzibajramovic E., Borjesson M., Ahlborg G. A prospective study of leisure-time physical activity and mental health in Swedish health care workers and social insurance officers. Prev. Med. 2010;51:373–377. doi: 10.1016/j.ypmed.2010.07.019. [DOI] [PubMed] [Google Scholar]
- Kelly B.D. Coronavirus disease: challenges for psychiatry. Br. J. Psychiatry. 2020;217:352–353. doi: 10.1192/bjp.2020.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khawaja, A.P., Warwick, A.N., Hysi, P.G., Kastner, A., Dick, A., Khaw, P.T., Tufail, A., Foster, P.J., Khaw, K.T., 2020. Associations with covid-19 hospitalisation amongst 406,793 adults: the UK Biobank prospective cohort study.
- Lai J., Ma S., Wang Y., Cai Z., Hu J., Wei N., Wu J., Du H., Chen T., Li R., Tan H., Kang L., Yao L., Huang M., Wang H., Wang G., Liu Z., Hu S. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W., Wang H., Lin Y., Li L. Psychological status of medical workforce during the COVID-19 pandemic: a cross-sectional study. Psychiatry Res. 2020;288 doi: 10.1016/j.psychres.2020.112936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minihan E., Gavin B., Kelly B.D., McNicholas F. Covid-19, mental health and psychological first aid. Ir J Psychol Med. 2020;37(4):259–263. doi: 10.1017/ipm.2020.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the diamond princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25(10):2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muruganandam P., Neelamegam S., Menon V., Alexander J., Chaturvedi S.K. COVID-19 and severe mental illness: impact on patients and its relation with their awareness about COVID-19. Psychiatry Res. 2020;291:113265. doi: 10.1016/j.psychres.2020.113265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolich-Zugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat. Immunol. 2018;19:10–19. doi: 10.1038/s41590-017-0006-x. [DOI] [PubMed] [Google Scholar]
- Packard C.J., Bezlyak V., McLean J.S., Batty G.D., Ford I., Burns H., Cavanagh J., Deans K.A., Henderson M., McGinty A., Millar K., Sattar N., Shiels P.G., Velupillai Y.N., Tannahill C. Early life socioeconomic adversity is associated in adult life with chronic inflammation, carotid atherosclerosis, poorer lung function and decreased cognitive performance: a cross-sectional, population-based study. BMC Public Health. 2011;11:42. doi: 10.1186/1471-2458-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan A., Liu L., Wang C., Guo H., Hao X., Wang Q., Huang J., He N., Yu H., Lin X., Wei S., Wu T. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. JAMA. 2020;323(19):1915–1923. doi: 10.1001/jama.2020.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrilli, C.M., Jones, S.A., Yang, J., Rajagopalan, H., O'Donnell, L.F., Chernyak, Y., Tobin, K., Cerfolio, R.J., Francois, F., Horwitz, L.I., 2020. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. medRxiv, 2020.2004.2008.20057794.
- Raisi-Estabragh, Z., McCracken, C., Ardissino, M., Bethell, M.S., Cooper, J., Cooper, C., Harvey, N.C., Petersen, S.E., 2020. Non-white ethnicity, male sex, and higher body mass index, but not medications acting on the renin-angiotensin system are associated with coronavirus disease 2019 (COVID-19) Hospitalisation sation: review of the first 669 cases from the UK Biobank.
- Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., the Northwell C.R.C., Barnaby D.P., Becker L.B., Chelico J.D., Cohen S.L., Cookingham J., Coppa K., Diefenbach M.A., Dominello A.J., Duer-Hefele J., Falzon L., Gitlin J., Hajizadeh N., Harvin T.G., Hirschwerk D.A., Kim E.J., Kozel Z.M., Marrast L.M., Mogavero J.N., Osorio G.A., Qiu M., Zanos T.P. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers M., Dalton J., Harden M., Street A., Parker G., Eastwood A. Integrated care to address the physical health needs of people with severe mental illness: a mapping review of the recent evidence on barriers, facilitators and evaluations. Int. J. Integr. Care. 2018;18:9. doi: 10.5334/ijic.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorious N. Comorbidity of mental and physical diseases: a main challenge for medicine of the 21st century. Shanghai Arch. Psychiatry. 2013;25:68–69. doi: 10.3969/j.issn.1002-0829.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D., Happell B. The high prevalence of poor physical health and unhealthy lifestyle behaviours in individuals with severe mental illness. Issues Ment. Health Nurs. 2011;32:589–597. doi: 10.3109/01612840.2011.569846. [DOI] [PubMed] [Google Scholar]
- Seminog O.O., Goldacre M.J. Risk of pneumonia and pneumococcal disease in people with severe mental illness: english record linkage studies. Thorax. 2013;68:171–176. doi: 10.1136/thoraxjnl-2012-202480. [DOI] [PubMed] [Google Scholar]
- Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., Labreuche J., Mathieu D., Pattou F., Jourdain M., LICORN and the Lille COVID-19 and Obesity study group High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity Silver Spring. 2020;28(7):1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens R., Puybasset L. The brain-lung-brain axis. Intensive Care Med. 2011;37:1054–1056. doi: 10.1007/s00134-011-2233-1. [DOI] [PubMed] [Google Scholar]
- Tu H., Tu S., Gao S., Shao A., Sheng J. Current epidemiological and clinical features of COVID-19; a global perspective from China. J. Infect. 2020;81(1):1–9. doi: 10.1016/j.jinf.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Yang Y., Ren L., Shao Y., Tao W., Dai X.J. Preexisting mental disorders increase the risk of COVID-19 infection and associated mortality. Front. Public Health. 2021;9 doi: 10.3389/fpubh.2021.684112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Xu Q., Anderson D., Courtney M. A longitudinal study of the relationship between lifestyle and mental health among midlife and older women in Australia: findings from the healthy aging of women study. Health Care Women Int. 2010;31:1082–1096. doi: 10.1080/07399332.2010.486096. [DOI] [PubMed] [Google Scholar]
- Yao H., Chen J.H., Xu Y.F. Patients with mental health disorders in the COVID-19 epidemic. Lancet Psychiatry. 2020;7:e21. doi: 10.1016/S2215-0366(20)30090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the UKB team.