Abstract
Viral diseases are emerging as global threats. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), that causes coronavirus disease (COVID-19), has severe global impacts. Safety, dosage, and potency of vaccines recently approved for emergency use against SARS-CoV-2 need further evaluation. There is still no effective treatment against COVID-19; therefore, safe, and effective vaccines or therapeutics against SARS-CoV-2 are urgently needed. Oil-in-water nanoemulsions (O/W NEs) are emerging as sophisticated, protective, and therapeutic platforms. Encapsulation capacity, which offers better drug pharmacokinetics, coupled with the tunable surfaces present NEs as promising tools for pharmaceutical applications. The challenges facing drug discovery, and the advancements of NEs in drug delivery demonstrate the potential of NEs against evolving diseases, like COVID-19. Here we summarize current COVID-19 knowledge and discuss the composition, stability, preparation, characterization, and biological fate of O/W NEs. We also provide insights into NE structural-functional properties that may contribute to therapeutic or preventative solutions against COVID-19.
Keywords: Nanoemulsions, Biosurfactants, Drug delivery, COVID-19, SARS-CoV-2
Graphical abstract
1. Introduction
Infectious viral diseases and outbreaks are continuously evolving global threats. Despite successive advances in the medical field, the prevention and treatment of viral outbreaks remain challenging due to the innate characteristics of viral particles, such as the high dynamic rate of genetic mutations. Coronavirus disease of 2019 (COVID-19), caused by severe acute respiratory syndrome 2 (SARS-CoV-2), emerged in December 2019 in Wuhan, China, and grew rapidly into a global pandemic. As of July 2021, severe global health, social, industrial, and economic global impacts are still being caused by COVID-19 including high mortality rates, mandatory lockdowns, and reduced manufacturing leading to global shortages. Collaborative scientific responses to develop effective preventative and therapeutic solutions against COVID-19 using traditional and advanced technologies are being coordinated globally; however, there is still no treatment for COVID-19 and only a few vaccines have been approved recently for emergency use.
Nanoemulsions (NEs) are heterogeneous formulations of two different immiscible liquids that are typically oil and water mixtures stabilized by surface-active agents, surfactants. During emulsification, surfactants minimize or prevent coalescence by adsorbing at the oil-water interface and lowering surface tension. Surfactants also facilitate dispersed phase splitting or disruption by forming an interfacial film at the oil-water interface that delays destabilization mechanisms. The choice of surfactants is critical for preparing NEs with specific physiochemical properties. NEs have droplet sizes ranging from 10 to 1000 nm that make them kinetically stable but thermodynamically unstable systems, due to higher free energy in the system compared to the separated phases [1]. Therefore, phase separation, which depends on the kinetic energy barriers, of homogenized NEs occurs over time leading to destabilization mechanisms including Ostwald ripening and flocculation. However, NEs can possess long-term stability or shelf-life when the system components selected rationally based on their physiochemical properties. NE stability is a crucial parameter for medical applications, where alterations in the physiochemical properties drastically impact pharmacological properties of the formulation.
NEs represent a rapidly growing class of colloidal carriers with unique properties that facilitate successful and affordable applications in agriculture [2], food [3,4], cosmetics [5,6], and pharmaceuticals [1]. NEs are classified by the chemical nature of the dispersed phase (core). For example, when an oil core is dispersed in a continuous phase, like water, oil-in-water emulsions (O/W) are produced. Conversely, water-in-oil (W/O) emulsions are formed when water is dispersed in an oil phase. Both O/W and W/O emulsions are classified as single emulsions. Double emulsions are more complex, like oil-in-water-in-oil (O/W/O) and water-in-oil-in-water (W/O/W) emulsions, and can be manufactured by dispersing a single emulsion within another liquid phase [7]. The advantage of selecting different dispersed phases allows for the encapsulation of various bioactive compounds. The progressive development of NEs with more complex chemical compositions and multiple internal compartments [8], makes NE a multifunctional platform for various drug delivery applications. In medicine, NE platforms are promising and advanced tools with multiple applications that can be harnessed in the fight against current and future viral outbreaks.
The potential of NEs for improving the quality and efficacy of pharmaceutical compounds has been demonstrated in various medical applications [9]. In particular, the large surface area-to-volume ratio of NEs enhances absorption and efficacy of encapsulated drugs, and the tunable interfacial properties of NEs enable targeted delivery of the loaded cargo to the intended site of action [10,11]. For example, NEs can be formulated with surface-displayed targeting biomolecules, such as antibodies, to facilitate targeted delivery. These properties allow optimal doses of encapsulated active ingredients to be delivered with minimal off-site targeting [12]. Encapsulation of drugs within the NE core protects drugs from environmental changes, including pH changes and enzymatic degradation [13,14]. These advantages have led to rapid advances in NEs pharmaceutical applications that highlight the promising future of NEs for solving healthcare problems.
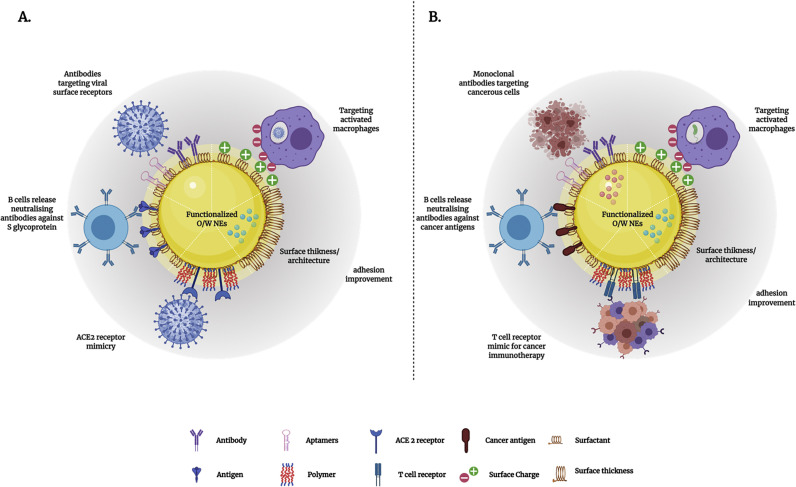
The surface properties of NEs control their physical stability and biointeractions in pharmaceutical applications. These properties include type, concentration, and conformation of surfactant; surface charge; type, thickness, size, and architecture of coating materials; and nature of displayed targeting ligands. Bioconjugation methods are utilized to tune these surface properties, and therefore achieve desired physical stability and pharmacological performance of NEs (Fig. 1 ). For instance, steric hinderance can be achieved through the adsorption or display of polymers such as polyethylene glycol (PEG) at oil-in-water interface, which can alter the thickness and architecture of NEs surfaces. The display of PEG molecules at oil-in-water interface also shields surface charge, therefore prevent unwanted electrostatic interaction of NE with plasma proteins, that lead opsonisation and rapid clearance [15]. These positive surface functionalization impacts on NEs stability and biological interactions can facilitate NE success in drug delivery applications. However, to consider surface functionalization of NE, the process should be feasible in terms of downstream processing, affordable and favourable for encapsulated compounds.
Fig. 1.
Implications of O/W NEs surface properties in viral and other medical applications. A. Illustrates how the surface properties of O/W NEs can be functionalized with different biomolecules to target viral infections using different mechanisms (targeting infected macrophages using electrostatic interactions, surface displayed antibodies targeting virus receptors, evoking immune response using surface displayed antigens/epitopes, virus neutralization via ACE2 receptor mimicry and cell adhesion to enhance drug localization or residence). B. Depicts the tunable surface properties of O/W NEs that can be harnessed toward various drug delivery applications using several strategies including targeting activated macrophages for liver diseases using electrostatic interactions, targeting cancer cells using antibodies, evoking immune response against cancer, immunotherapy via the presentation of T-cell receptors.
Traditional antivirals and other therapeutics are limited by poor solubility and absorption, rapid clearance profiles, low bioavailability, non-specific targeting of viral receptors, off-site toxicity, and limited accessibility to viral depots [16]. NEs have the potential to overcome these limitations due to these systems being tuneable with versatile oil cores (dispersed phase), enhanced encapsulation capacities, easy-to-modify surfaces, and flexible dosage forms. For example, low viscosity dosage formulations are appropriate for parenteral delivery whereas more viscous formulations suit topical administration [17]. Control over NE surface and core properties makes these tuneable systems strong candidates in the development of next generation antivirals.
Here, we summarize the current knowledge of SARS-CoV-2 and provide a comprehensive review of the composition, types, structure, stability, preparation, characterization, and biological fate of O/W NEs. This article also provides mechanistic insights into NE physiochemical properties in drug delivery applications, and how these properties qualify NEs as potential therapeutic and preventative solutions for overcoming the COVID-19 pandemic. Next, we explore NE properties that qualify NEs as potential therapeutic and preventative solutions for overcoming the COVID-19 pandemic. Finally, we discuss the challenges associated with NE drug delivery applications.
2. Severe acute respiratory syndrome coronavirus 2
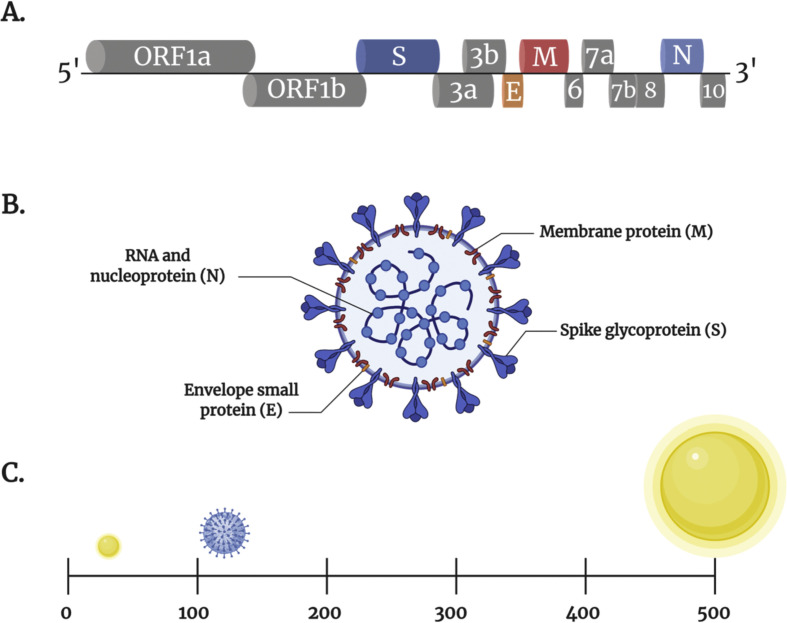
The coronavirus disease, COVID-19, is caused by the novel SARS-CoV-2, which is a strain from the Coronaviridae family of zoonotic viruses that infect mammals and birds. Coronaviruses, named after their crown-like surface structure, are categorized into four genera: α-coronavirus, β-coronavirus, γ-coronavirus, and σ-coronavirus. The most pathogenic β-coronaviruses for humans are SARS-CoV, the Middle Eastern respiratory syndrome coronavirus (MERS-CoV), and the novel SARS-CoV-2. SARS-CoV-2 possesses a 34-kb single-stranded positive-sense RNA genome encoding several open reading frames. According to the reference sequence in NCBI (NC_045512.2), the genome of SARS-CoV-2 consists of 13 open reading frames (ORFs) (Fig. 2 . A). Two-thirds of the genome contains ORF1a and ORF1b, which encode for the non-structural proteins (nsps) for replication [18]. The remaining one-third of the SARS-CoV-2 viral genome encodes the four major structural proteins: spike glycoprotein (S), envelope small membrane protein (E), membrane protein (M), and nucleocapsid proteins (N) [19]. There are also seven accessory proteins encoded by ORF3a, ORF3b, ORF6, ORF7a, ORF7b, ORF8, and ORF10 genes (Fig. 2. A) [18,20]. The viral envelope of SARS-CoV-2 is composed of the S, M, and E proteins, while the N protein is located within the core of the virus (Fig. 2. B) [21,22]. The SARS-CoV-2 virions are relatively large, measuring about 120 nm in diameter, and fall within the same range of commonly studied functionalized nanoparticles (Fig. 2. C) [23]. Understanding the structure, surface properties, and mode of action of SARS-CoV-2 as functional nanomaterials provides insights into the strategies that can be developed to tackle this virus [24].
Fig. 2.
Schematic representation of SARS-CoV-2 genome and particle structure. (A) SARS-CoV-2 genome: 5′ to 3′ terminal sequences. The genome contains the open reading frame ORF1a and ORF1b that encode non-structural proteins (nsps) (grey boxes), structural proteins including spike (dark blue box), envelope (orange box), membrane (maroon box), nucleocapsid (light blue box) proteins, and accessory proteins such as ORF 3a, 3b, 6, 7a, 7b, 8 and 10 (grey boxes). (B) SARS-CoV-2 particle structure representing the envelope composed of S, M and E proteins. The viral core contains the N proteins and RNA. (C) Illustrates the size of SARS-CoV-2 (blue) relative to NEs (yellow). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
SARS-CoVs M protein, which maintains membrane integrity, is the most abundant protein expressed in the viral particle, whereas the E protein, which plays a role in the structure and assembly, is expressed in very low levels within the viral particle [22]. The viral RNA genome is combined with the N protein that is required for the effective packaging of the virion [22,25]. S glycoprotein is the most studied structural protein among SARS-CoV viruses – this structural protein is well known to elicit potent and long-lasting immune responses, making it a potential candidate for targeted therapy or vaccine development [[25], [26], [27]]. S glycoprotein is composed of two domains, S1 and S2 subunits, that facilitate receptor binding and membrane fusion, respectively. SARS-CoV-2 enters the cell either via endosomes or through plasma membrane fusion.
Upon binding host cells through the receptor-binding domain (RBD) of S glycoprotein, SARS-CoV-2 S1 subunit utilizes the peptidase domain (PD) of angiotensin-converting enzyme 2 (ACE-2) [27]. Following virion packaging and complete virus assembly within the endoplasmic reticulum of Golgi apparatus, virions are released through exocytosis. Understanding these virus-host interactions informs how nanomaterials, particularly NEs, can be developed to prevent SARS-CoV-2 binding and cellular entry.
The mechanism of SARS-CoV-2 entry creates targets for many drugs. For instance, an in vitro study demonstrated that clinical-grade soluble human ACE2 can be used to suppress SARS-CoV-2 infection in early stages due to competitive binding with cellular ACE-2 [28]. Interfering with the SARS-CoV-2 lifecycle in early stages of infection also presents potential drug targets. For example, endocytosis and cell membrane fusion could be targeted by endocytosis inhibitors, such as Ikarugamucin, or by blocking membrane fusion with small molecule SSAA09E3 and 25-hydrocholesterol [[29], [30], [31]]. Targeting virus-loaded endosomes provides other early intervention opportunities. For example, repurposed chloroquine and hydroxychloroquine have been used as therapeutics against COVID-19 to inhibit the formation of endosomal compartments and reduce the initial phases of viral infection [32]. Other targets for drug development exist following entry of SARS-CoV-2 into host cells when the viral envelope is cleaved by the main protease (Mpro) to facilitate delivery of genetic material to the cytoplasm and translation of nsps. Targeting Mpro is currently the most promising COVID-19 treatment – Remdesivir, a repurposed antiviral drug that inhibits proteases, is the first COVID-19 drug approved by FDA [33]. A computational study conducted by Grifoni et al. predicted possible epitopes as potential preventive and therapeutic candidates for targeting the S glycoprotein [34,35]. The study identified five regions in the S glycoprotein with high immune response rates and provided a blueprint for the design and development of SARS-CoV-2 anti-viral drugs or vaccines. An in vivo study confirmed that immunization with SARS-CoV-2 RBD stimulated the production of neutralizing antibodies in rodents suggesting that RBD is a potential candidate for the development of an effective vaccine [36]. While one-third of the SARS-CoV-2 genome encodes four structural proteins responsible for the virion assembly and infectivity, the other two-thirds of the genome encodes nsps involved in virion replication [37]. Blocking SARS-CoV-2 replication by protease inhibitors might be a promising therapeutic approach to treat COVID-19 [38,39].
Understanding the origin of SARS-CoV-2 and the zoonotic-human mode of viral transfer are crucial aspects for research into the prevention of future zoonotic infections. Early in the pandemic, COVID-19 was confirmed to be a zoonotic infection; however, transmission from animals to human remains under investigation and requires further research [40,41]. The SARS-CoV-2 virus is mainly transmitted between humans through respiratory aerosols and droplets. Therefore, close contact increases the spread of SARS-CoV-2 making social distancing practices a necessary measure to help contain the virus. Signs and symptoms of COVID-19 differ from patient to patient. Some COVID-19 patients show no symptoms (asymptomatic), while others develop life-threatening symptoms like acute respiratory distress syndrome (ARDS) and organ failure. However, most COVID-19 patients exhibit mild symptoms, and admitted cases recovered within seven to ten days of hospitalization [42,43]. Nonetheless, new variants of SARS-CoV-2 have rapidly spread in the UK, USA, South Africa and India [[44], [45], [46]]. These SARS-CoV-2 variants, characterized by multiple mutations in the spike glycoprotein, have raised concerns regarding vaccine efficacy and the need to monitor for further mutations that might require changes to current vaccines [47].
To date, and despite considerable efforts to develop a range of antiviral drugs and vaccine candidates currently in preclinical or clinical development, only a few vaccine products have been granted emergency use approval for preventing COVID-19. In addition, some concerns have been raised about the safety profiles, number of doses, and potency against new variants of these approved vaccines. Acceptance of vaccines varies throughout the global population and is influenced by public perception and knowledge that creates both positive and negative attitudes toward vaccines. However, it must be highlighted that vaccines are the most effective tool available for stopping the spread of infectious diseases. Containment measures such as lockdown and quarantine, are also needed to reduce SARS-CoV-2 transmission; however, these measures also result in negative social, professional and economic consequences [48]. Moreover, the continuous emergence of new, transmissible SARS-CoV-2 variants exemplifies the obstacles faced by infectious disease surveillance programs. Therefore, there is an urgent need to develop efficient next-generation vaccines or therapeutic platforms against SARS-CoV-2 to overcome the COVID-19 pandemic and any future coronavirus-related outbreaks.
3. Nanoemulsions
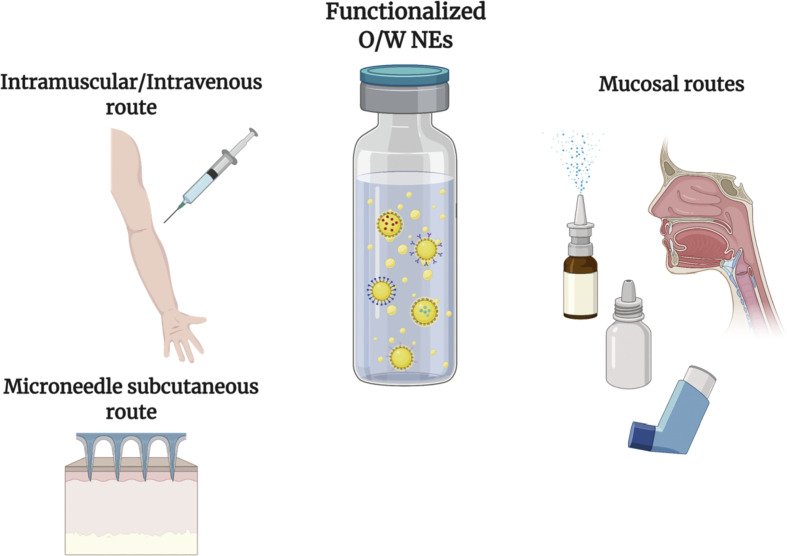
The structural and functional tunability and versatility of NEs have contributed to the application of NEs for vaccine development and for new therapeutic platforms involving subcutaneous, intramuscular, intravenous, and mucosal routes (Fig. 3 ) [[49], [50], [51], [52]]. Subsequently, understanding NE composition and functional properties, along with the surrounding biological systems, is crucial for developing new, sophisticated NE drug delivery platforms.
Fig. 3.
Schematic representation of possible NE administration routes for treating or preventing viral diseases. O/W NEs refers to oil-in-water nanoemulsions.
3.1. Nanoemulsion composition
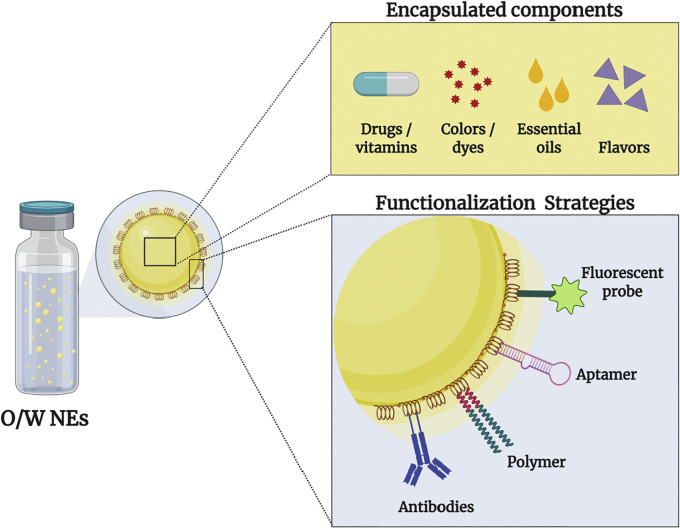
O/W NEs are heterogeneous dispersions mainly composed of three main constituents: a dispersed phase (hydrophobic core), a continuous phase (hydrophilic), and surfactants or stabilizers. The core acts as a reservoir for many hydrophobic materials whereas salts, buffers, or solvents are contained within the hydrophilic phase. Components that help facilitate immune evasion [53] or targeting molecules [54,55], are displayed onto the surface of O/W NEs and directed toward the aqueous continuous phase (Fig. 4 ). Surfactants are amphiphilic surface-active molecules that lower interfacial tension (IFT) between two immiscible fluids during the emulsification process [56,57]. Surfactants rapidly adsorb at O/W interfaces leading to the formation of stable oil droplets by preventing destabilization mechanisms, such as flocculation (i.e. the fusion of droplets forming larger clumps). Surfactants are classified by origin (chemically or biologically derived), electrical charge (cationic, anionic, zwitterionic or non-ionic), and the hydrophilic-lipophilic balance (HLB) value.
Fig. 4.
Schematic representation of encapsulated hydrophobic compounds and surface-functionalized biomolecules of O/W NEs. Drugs, vitamins, colors, dyes, essential oils and flavors can be encapsulated within the oil core. Surface-displayed biomolecules include antibodies, polymers, aptamers and fluorescent probes.
Biosurfactants are derived from or manufactured in living organisms including microorganisms, plants or animals. By contrast, surfactants (or chemical surfactants) are derived from petrochemicals and oleochemicals via chemical synthesis [58,59]. Biosurfactants include glycolipids [60], lipopeptides [61], polysaccharides, peptides [62,63], and proteins [64,65]. Chemical surfactants include Tween 20, Tween 80, and sodium dodecyl sulfate.
Compared to chemical surfactants, biosurfactants are more favored in terms of biocompatibility, biodegradability and sustainability [58,59,[66], [67], [68]]. Biosurfactants can reduce IFT and adsorb at liquid/liquid, air/liquid and solid/liquid interfaces [56]. Biosurfactants stabilize O/W NEs, not only by reducing IFT, but also through steric and electrostatic repulsion. Thus, biosurfactants prevent NE destabilization mechanisms leading to more stable long-lasting formulations which is a crucial property for medical applications.
Biosurfactants have been widely applied in medicine against cancer, microbes, inflammation, immune disorders, and viruses [[69], [70], [71]]. Çelik et al. has summarized the potential strategies for using biosurfactants as therapeutic, environmental, and industrial tools to fight future viral outbreaks [72]. For example, peptide- and lipopeptide-based biosurfactants can interfere with viral life cycles; hence, these compounds are suitable for developing vaccines capable of inducing cytotoxic T-cell responses against viruses [73,74]. Other biosurfactants, such as surfactin, have anti-inflammatory activities. Biosurfactants are also important in the development of preventative tools, including pesticides and disinfectants, which is an essential strategy for minimizing the spread of infectious disease, such as COVID-19.
The amphiphilic nature of biosurfactants qualifies these compounds as essential components of various drug delivery systems, including micelles, liposomes, and emulsions. Biosurfactants also facilitate the fabrication of NEs in different dosage forms, like aerosols, liquids, semi-solids, and solids, and act as solubilizers of active pharmaceutical ingredients (APIs) [71]. Biosurfactants exhibit innate antiviral properties making them defence line candidates for preventing viral invasion through different body entry routes [75]. These properties substantiate biosurfactants as essential structural and functional elements of antiviral drug delivery systems, such as NEs.
Self-assembly is a unique feature of biosurfactants that allows spontaneous adsorption, integration, and arrangement at O/W NEs interfaces. The interfacial activities of self-assembling biosurfactants are governed by hydrophobicity, electrostatic interactions, hydrogen bonds, and van der Waals forces [76]. Peptides and proteins are a major class of biosurfactants that are found naturally or produced artificially with bioengineering. Naturally available peptide or protein biosurfactants are produced by microorganisms and facilitate cell adhesion, growth of organelles, and biofilms. The ability of biosurfactants to interact with cell membranes have led to their use in medical applications as antimicrobials. Stimuli-responsive and self-assembling synthetic biosurfactants can also be rationally designed by chemical and biological engineering allowing for the fabrication of multi-compartment NE surfaces through chemical crosslinking [62,[77], [78], [79]]. This process facilitates the functionalization of NE surfaces with various materials including antibodies, vitamins, and solid nanoparticles-based shells (such as silica nanoparticles) [80]. These properties demonstrate how biosurfactants and NE interfaces are crucial for biological functionalities (Fig. 4) [81].
Surfactant charge impacts NEs stability and interactions with biological materials. Charged surfactants create NEs with electrostatic repulsion providing better colloidal stability compared to non-ionic surfactants that stabilize NEs droplets via steric hindrance [82]. In addition, charged surfactants can be also useful for targeted drug delivery by interacting with cellular membranes of pathogens and other diseased cells. To tune NE surfaces for particular applications, charged surfactants can be modified with charge-shielding and immune-evading biomolecules using bioconjugation chemistry. However, uncharged NEs surfaces are preferred in biological environments because they are less prone to interacting with host plasma proteins and cell membranes leading to better in vivo stability. Charged surfactants can be modified with charge-shielding and immune-evading biomolecules using bioconjugation chemistry to tune NE surfaces for a particular application.
HLB value is another important aspect when selecting surfactants for downstream applications. Ranging from 0 to 20, HLB values describe the level of lipophilicity or hydrophilicity of a surfactant molecule that is determined by hydrophilic and hydrophobic components. Selection of surfactants based on HLB value is determined by oil core requirements and contributes to formation of NEs with a narrow size distribution index [67,83,84]. Understanding biosurfactant structural and functional properties and inter-and intra-molecular interactions at NE interfaces, is crucial in the development of NEs as effective preventative and therapeutic tools.
3.2. Preparation of nanoemulsions
NEs can be prepared using various methods that impact the nature of the resultant NE and are an important parameter to consider in the development of NEs. Energy input is a requirement for preparing O/W NEs and differs between high-energy and low-energy methods [7,85,86]. High-energy approaches, like ultrasonication and high-pressure homogenization, use mechanical devices, that generate disruptive forces to split the dispersed phase into tiny oil droplets [82,87,88]. Currently, ultrasonication is the most common method used in laboratory-based research; however, high-pressure homogenizers are preferred for industrial applications. Low-energy approaches exploit the potential of NE compositions (oil-water-emulsifier mixtures) and use temperature changes to lower IFT and induce spontaneous emulsification. The most commonly used low-energy methods are phase inversion temperature (PIT) and spontaneous or self-emulsification methods [[89], [90], [91]].
Fabrication of NEs using high-energy approaches is determined by the NE composition and required energy input. To overcome energy barriers between selected oil and aqueous phases, shearing forces with high kinetic energy are applied to produce nanosized droplets. Surfactants and co-surfactants are used to stabilize NEs and reduce IFT – the lower the IFT, the lower the amount of external energy needed to split NEs droplets [92]. Varying ultrasonic wave frequency, energy input, and time, O/W NEs can be prepared with specific properties. Constant energy input and residence time of the oil phase in the disruption zone (sonication waves) greatly impact droplet size and dispersity [82,[93], [94], [95]]. For instance, increasing sonication time to a certain level decreases the size of NE droplets [96]. Thus, the parameters that influence physical stability and morphology of NEs should be considered when designing NEs for in vitro and in vivo applications.
High-pressure homogenization is a scalable method used widely to prepare NEs for industrial applications, particularly food production. However, the high-energy consumption and escalation of temperature during NEs preparation are major problems. During homogenization, NE mixtures pass through a valve chamber under high pressure producing shearing energy that fragments the oil core into nanoscale droplets [97]. To prepare NEs with small droplet sizes, hydraulic shear, cavitation, and intense turbulence need to be optimized. Other factors such as homogenizer type, sample composition, and operating conditions (i.e. time, energy intensity and temperature), also affect droplet size during homogenization [[98], [99], [100]]. Increasing homogenization intensity to decrease droplets size is an example of using these factors to control droplet formation [57,101,102]. However, homogenization pressure levels (MPa) should be selected based on the composition of NEs, particularly chemical nature of oil core and surfactant type. A recent study has demonstrated the importance of studying the impact of homogenization pressure on NE composition, and therefore stability and encapsulation efficiency. The study has shown that the increase of homogenization pressure to a certain level, 120 MPa, improves encapsulation efficiency, while exceeding 120 MPa negatively affects encapsulation efficiency owing to the determinantal effect on the structure of soy protein isolate, the vehicle [103]. Surfactant type and concentration, oil percentage and pressure homogenization conditions also influence NEs physical stability [104,105]. Considering the impact of high shearing methods conditions on emulsions properties and physical stability is crucial for the development of stable, functional pharmaceutical NE formulations.
The PIT method, which was reported by Shinoda and Saito [106], prepares NEs by changing the temperature profile of NEs mixture components. This method involves sequential steps including mixing, heating, and fast cooling, and also depends on the solubility of surfactants changing with temperatures that modify surfactant affinities for oil and water phases and affect the overall HLB value of NEs. At high temperatures, surfactants become more soluble in the oil phase; however, surfactants become more soluble in water at lower temperatures [57]. Changes in surfactants composition and concentration also affect their HLB values with higher surfactant-to-oil contents producing smaller droplets [91,93,107]. The spontaneous emulsification method is another way to prepare O/W NEs by exploiting different chemical potentials between the oil core and water phase that enhance solvent diffusion. The industrial production of O/W NEs using spontaneous emulsification is still considered to be in development, even though this method can generate NE droplets with specific sizes [108].
The physiochemical properties of NEs including, rheology, stability, and payload release, are influenced by NE functional interfaces, composition and size, and are important factors when choosing a NE manufacturing method. Viscosity also affects droplet shearing during emulsification; thus, knowledge of different NE formulation methods, compositions, and intended pharmaceutical applications is essential for preparing NEs with desirable properties.
The separation of free and excess surfactant or polymer molecules from prepared NEs is essential for downstream applications, such as vaccines. Centrifugation is commonly used for separating most nanoparticles from excess free molecules; however, it is not suitable for removing excess materials present in NE formulations. In contrast, centrifugation is commonly applied for testing NE stability against phase separation [109]. NEs are kinetically stable formulations of soft nanodroplets that should retain homogeneity during high speed centrifugation. Resistance to centrifugation and phase separation is an indication of O/W NE stability. Once phase separation occurs, NEs undergo one of three destabilization mechanisms: Ostwald ripening, creaming, or sedimentation. Dialysis bag or chromatography-based separation methods are preferred for removing excess materials from NEs with no or minimal effects on physical NE properties. For example, dialysis using snake membranes with different pore sizes allows for removal of excess dyes, surfactants, or polymers such as polyethylene glycol [110].
3.3. Stability of nanoemulsions
Stability during long-term storage is important for developing new drug delivery systems. Stable NEs are in a high-energy state compared to separated phases (oil and water). O/W NE is considered stable if the formulation maintains its physiochemical characteristics over time and under different conditions, including temperature and pH changes. The rate of change in physiochemical properties is determined by energy barrier levels [57,111]. For example, changes in NE physiochemical properties can lead to instability through different mechanisms including Ostwald ripening, coalescence, flocculation, creaming, and cracking. Ostwald ripening is due to differences in Laplace pressure and occurs when small oil droplets migrate toward larger droplets through the continuous phase. This mechanism can be prevented or minimized by selecting a high immiscibility profile between the dispersed and continuous phases and maintaining low IFT. NEs can also undergo coalescence when droplets collide and merge forming larger droplets. To avoid coalescence, repulsive forces between dispersed oil droplets should be maintained. Based on the Derjaguin, Landau, Verwey and Overbeek (DLVO) theory, weak repulsion, and strong attractive forces, such as electrostatic interactions between oil droplets, can lead to flocculation. Creaming occurs when floccules float, while sedimentation is the result of floccules settling. Creaming and sedimentation are determined by whether the dispersed phase is lighter (floating) or denser (creaming) than the continuous phase. The small droplet size of NEs plays a major role in controlling and narrowing density difference between phases that contributes to better stability. Creamed or sedimented NEs can be reconstituted by shaking or mixing that can also recover their physiochemical properties. However, permanent and irreversible phase separation of NEs can occur and is known as cracking [112].
3.4. Nanoemulsions characterization methods
Characterization of NE physiochemical and interfacial properties (i.e. rheology, appearance, viscosity, droplet size, charge, morphology, size distribution, surface tension, elasticity, and stiffness) provides a better understanding of NE behaviour and possible interactions in medical applications. Dynamic light scattering (DLS), based on Brownian motion, is the most common characterization method of NEs and indicates droplets size. In DLS, moving-oil droplets are exposed to light generating scattered light with different intensities that is analyzed with the Stokes-Einstein equation. Droplet size (mean droplet diameter) and polydispersity index (PDI) are important parameters that reflect NE functional properties – stability and dispersibility. Thus, regular monitoring of these parameters is required. When both parameters remain unchanged over time, NEs can be considered physically stable [[113], [114], [115]]. DLS is a convenient and efficient method commonly used for continuous monitoring of nanomaterial stability profiles; however, DLS may provide incomplete information when test samples, contain droplet populations with low percentages. Other techniques, such as microscopic examination, can be used to complement DLS results.
Electron microscopy offers a higher resolution examination of droplet structure and size [[116], [117], [118]]. Conventional transmission electron microscopy (TEM) characterizes the internal structure of the dispersed phase, whereas scanning electron microscopy (SEM) collects topographical data [118]. In addition to conventional TEM and SEM, cryo electron microscopy is more commonly used for a few reasons. For instance, Cryo TEM facilitates direct examination of NEs as frozen-hydrated droplets allowing the original structural properties to be preserved [119]. Thus, cyro TEM analyzes the internal structure and dispersed phase of NEs in their native state [120]. Although cryo TEM provides artefact-free analysis due to its ability to differentiate oil droplets from other objects/structures [118,121], this technique has limitations when detecting droplets with high oil content or viscosity. Cryo SEM overcomes this limitation by analyzing the surface structures of NEs droplets surfaces as aggregates. Cryo SEM can also examine larger sample areas making it the preferred technique for collecting information about overall NE structure and dispersity [122]. The surface morphology of NEs can also be studied by atomic force microscopy (AFM). Although microscopy analysis provides valuable information about NE stability, homogeneity, and morphology, microscopy is a labor intensive and time-consuming technique. Subsequently, monitoring NE shelf-life is usually performed by DLS. NE chemical composition can also be investigated by coupling cryo preparations with electron loss energy spectroscopy. Other NE characterization techniques include differential scanning calorimetry, isothermal titration calorimetry, small-angle X-ray scattering, and x-ray spectroscopy [102,123].
The capacity of NEs to encapsulate a wide range of hydrophobic compounds, like vitamins [124], essential oils (EOs) [83], flavors [125], fluorophores [126] and drugs [127] is a major advantage of NEs in drug delivery applications (Fig. 3). Efficient encapsulation protects cargo compounds from chemical degradation, enhances compound bioavailability, and helps control the release of cargo compounds at the target site [[128], [129], [130]]. The encapsulation efficiency (EE) test is applied to evaluate retention of NEs cargo within the oil core [131]. The EE test can be affected by several factors such as formulation method, NE components, chemical properties of encapsulated drugs including solubility in the oil core, and the overall NE stability. Several strategies are used to estimate the EE of NEs including ultracentrifugation, dialysis bag diffusion, gel filtration, ultrafiltration, and microdialysis. In the ultracentrifugation technique, free molecules are separated from NEs using a membrane with a certain pore size. The dialysis bag technique separates NEs from free or released drug molecules based on diffusion through a semipermeable membrane. To estimate EE using the gel filtration technique, NEs are separated by molecular weight via porous gel particles in an aqueous suspension [132].
4. Effect of drug administration route on nanoemulsion fate
The route of drug administration and physiochemical properties determine the fate of O/W NEs. Depending on the administration route, NEs must overcome different biological challenges to facilitate efficient drug delivery [9,93]. These challenges include renal clearance, phagocytosis, pH changes, and enzymatic degradation. Upon parenteral administration, NEs interact with complex biological environments containing plasma proteins, immune cells (phagocytes), and erythrocytes [133]. These interactions are influenced by the interfacial properties of NEs, including size, charge, and displayed biomolecules, which determine the NE biodistribution profile. NEs with approximately 10 nm oil droplets undergo glomerular filtration, while oil droplets larger than 200 nm are prone to phagocytosis. Droplet size also impacts cytotoxicity levels – large oil droplets tend to cause hemolysis. Surface charge also impacts NE interactions with plasma proteins. Charged NEs are more likely to be engulfed by phagocytes due to the adsorption of opsonins on their surfaces through electrostatic interactions, leading to rapid clearance [134]. The variation in flow rates in the blood stream can affect NE formulations as it induces shear stress and removal of NEs surface coatings and damage the encapsulated materials. It can also prevent NEs from reaching their target tissue by disrupting their localization to vessel walls. Controlling the surface properties, composition and size is important to ensure that NEs can exhibit prolonged blood circulation.
Epidermal layers in the skin act as barriers against the surrounding environment hindering the diffusion of topically applied drug formulations. Topically applied NEs can be delivered locally (dermal) or systemically (transdermal) depending on the depth of penetration [135]. Accumulation of NEs within dermal layers results in local drug delivery whereas stratum corneum disruption via high thermodynamic activity using surfactants or penetration enhancers leads to systemic delivery [136,137]. Surface charge, surfactants, co-surfactants, and penetration enhancers play major roles in determining the fate of NEs following topical drug delivery.
Administration of NE via the nasal route can result in local or systemic delivery. Locally administered NEs are delivered to nasal cavities and sinuses, whereas systemic administration of NEs occurs through the mucosa [9]. The nasal mucosa is also utilized for nose-to-brain drug delivery by passing the blood-brain barrier using NE surface properties [138]. NEs can also be delivered to the lung through pulmonary drug delivery using different aerosolization methods. Orally administered NEs face many challenges, such as pH changes and gastrointestinal enzymes. When NEs overcome these initial gastrointestinal barriers, oil droplets reside longer within the intestinal tract leading to activation of the intestinal lymphatic pathway that facilitates evasion from first path metabolism by the liver [9,139].
Controlling core and surface properties is crucial for overcoming the biological obstacles NEs face when delivered through different administration routes. Desired physiochemical properties can be achieved through the fabrication of NEs with biocompatible oil cores, surfactants, and functional biomolecules. Bioavailability across the gastrointestinal system is one property impacted by the chemical composition of the oil core [140]. For example, O/W NEs prepared with long chain fatty acids have better bioavailability profiles compared to NEs manufactured with medium chain fatty acids-based core [141]. In addition, O/W NEs created with monounsaturated fatty acids also exhibit greater bioaccessibility and bioavailability than O/W NEs made with polyunsaturated fatty acids [142].
Surface mechanical properties, particularly surface stiffness, also impact immune evasion and cellular uptake of silica-templated NEs stabilized with peptide biosurfactants. A recent study reported that soft silica-templated NEs are less prone to phagocytosis when compared to harder or stiffer NEs; however, PEGylation reduced the effect of stiffness on cellular uptake [143]. This study demonstrates how tuneable functional interfaces, dependent on peptide surfactants, can help researchers learn more about the interactions of NEs with biological surfaces. Another example is functionalizing NE surfaces with immune shielding biomolecules, such as PEG, that can prevent adsorption of opsonin and subsequent phagocytosis [15]. PEGylation can protect NE surfaces from physical degradation caused by enzyme and antibody secretion while stabilization of NEs with biocompatible surfactants can prevent or minimize cytotoxic effects [144]. Surfactants can also improve NE stability in biological settings and help present various biomolecules for the creation of multifunctional NEs. Finally, surfactant adsorption at the oil-water interface helps tune NE size and surface charge parameters that can be harnessed to overcome the barriers encountered through different drug delivery routes [77,135].
5. Insights into nanoemulsion drug delivery mechanisms
The feasibility of NEs for developing effective preventative and therapeutic tools against COVID-19 lies in other drug delivery platforms that use NEs to effectively deliver safe and effective treatments. NEs have shown great potential for delivering a variety of hydrophobic therapeutics by enhancing bioavailability, protecting therapeutics from the surrounding environment, facilitating release, and improving efficacies with no or minimal side effects. NEs can be prepared with various functional interfaces enabling administration through topical, injectable, ingestible, and inhalable formulations [9,145]. O/W NEs confer anticancer, antiproliferative [146], antioxidative [147], and antimicrobial [148] effects. Pharmaceutical NEs can be found in research, pre-clinical, and clinical settings demonstrating NE to be safe, stable, and efficient nanocarrier platforms that are promising candidates for tackling viral outbreaks, such as COVID-19.
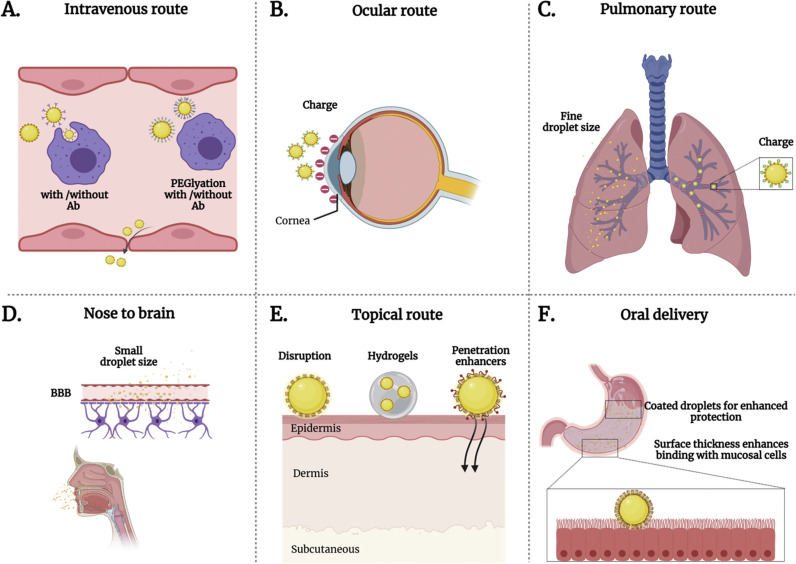
The advantages of NEs in various physical forms have been demonstrated through different routes of administration (Fig. 5 ).
Fig. 5.
Administration routes with highlighted strategies using NEs surface designs to overcome biological barriers. A. Intravenously administered NEs functionalized with PEG to escape from macrophages and achieve therapeutic delivery. B. Cationic NEs targeting cornea for potential intracellular drug delivery. C. Pulmonary and nasal drug delivery through cationic NEs with fine droplet size. D. Nose to brain delivery of therapeutic NEs crossing the blood-brain barrier with small droplet size and hydrophobic interactions. E. Dermal drug delivery of NEs through surface disruption (surface tension, thermodynamic activity), hydrogels, and penetration enhancers. F. Surfactant-stabilized NEs orally administered to overcome harsh gastric environment, e.g., gastric juices, and increased surface thickness for enhanced adhesion with targeted tissues.
The intrinsic properties of topically applied NEs improve the absorption of encapsulated hydrophobic compounds through skin layers. Surface displayed surfactants and the low surface tension of NEs promote penetration and permeation of loaded cargos through skin layers (Fig. 5). The nano-scale droplet size, thermodynamic activity, and low surface tension of NEs can disrupt skin barrier layers to facilitate drug delivery [149]. Tuning NEs droplet size, surface, viscosity facilitated successful topical ocular drug delivery by enhancing corneal permeation and drug absorption [145,150]. Ingestible NEs have been used to protect orally administered drugs from pH changes, enzymatic degradation, and the interaction with food byproducts. Droplet size impacts solubility, bioavailability, and bioaccessibility of ingestible NE formulations [151]. The lipophilic nature of NEs improves absorption and initiates the intestinal lymphatic pathway. Because of this NEs evade first-pathway metabolism and subsequently have prolonged residence in the gastrointestinal tract [[152], [153], [154]].
Aerosolised NEs are promising alternatives for non-invasive drug delivery via the pulmonary route. The fine droplet size of aerosolised NEs provides a large surface area that can enhance interactions with mucosal surfaces of the respiratory system (Fig. 5). The enhanced bio-interactions of NEs with targeted cells improves drug absorption and bioavailability [155]. Inhalable NEs have been formulated toward active targeting to treat respiratory disorders such as lung cancer and asthma [156,157]. Aerosolized NEs have been formulated as vaccines against respiratory pathogens and proven to evoke robust immune responses [158].
Pharmaceutical intravenously administered NEs emerged as containers for chemotherapeutics to minimize their side effects, enhance therapeutic efficacy, and overcome multidrug resistance (MDR) through passive and active targeting (Fig. 5). Tumors can be targeted passively by exploiting NE droplet size and taking advantage of the enhanced permeability and retention (EPR) effect. Extensive blood circulation is essential for passive targeting and can be achieved by coating the surface of NEs with immune evading molecules, like PEG. Since passive targeting is based on the size of drug delivery systems, the specificity of passive targeting is limited, particularly when cancerous and normal cells cannot be effectively distinguished [159]. However, active targeting is another approach based on functionalized nanomaterials surfaces that display targeting moieties [11,160], attached through bioconjugation techniques. O/W NEs are also applied as stimuli-responsive platforms to overcome MDR and improve cancer therapeutics [161]. NEs have also shown great potential in theranostic platforms enabling imaging-guided therapy against cancer [162].
NEs have been administered through different routes for treating cancers. For example, topical application of NEs functionalized with selected surfactants and penetration enhancers improved skin penetration, delivery, and accumulation of drugs against melanoma through passive targeting [163]. Cationic NEs delivered C6 ceramide through the intraductal route exhibited sustained localization and retention due to improved interactions with negatively charged proteins found in the mammary duct. This action was attributed to coating NEs with chitosan [164]. Considerations including the pharmacodynamics and pharmacokinetics of a drug administered topically, along with the surface properties of NEs, are crucial to producing effective topical, non-invasive formulations. Oral NEs encapsulating curcumin are in clinical trials for cancer prevention and supportive care [165]. Many studies investigating different cancers have shown that NEs encapsulated drugs can be efficiently localized in tumors, suppressing tumor growth, minimizing undesired cytotoxicity and side effects, and preventing metastasis.
Anti-inflammatory and antioxidative NEs have been developed as protective and therapeutic formulations for wound healing, and treating neurological, and dermatological diseases [[166], [167], [168], [169]]. Anti-inflammatory effects of P-selectin targeted NEs loaded with dexamethasone were studied in animal models. These NEs reduced vascular inflammation by minimizing endothelium activation and monocyte adhesion [170]. NEs functionalized with co-surfactants or penetration enhancers, Transcutol P, have also been found to enhance skin permeation, and improve delivery of lipophilic, anti-inflammatory compounds. In this study, surface coating biopolymers, hyaluronic acid, with variable molecular weights were utilized to tune the physical form of NE, viscosity. NE coated with low molecular weight hyaluronic acid was found to achieve better skin permeation on pig skin [171].
NEs have shown promising antimicrobial activities against bacteria, fungi, and viruses [[172], [173], [174]]. The potential of NEs for improving the efficacies of many lipophilic active ingredients, including EOs, natural extracts and antibiotics is attributed to the tunable physiochemical properties of NEs [175]. These properties, that include droplet size, internal pressure, surface charge, and loading capacity, facilitate NE interactions with microbial cell membranes and subsequent payload release. Antimicrobial NEs offer broad-spectrum activities that limit the possibility of bacterial resistance induced by antibiotics [176]. EOs have been used as antimicrobials; however, EO instability and susceptibility to degradation reduces efficacy. Moreover, EOs are highly volatile and water-insoluble, and difficult to handle. Emulsions have been utilized to enhance EOs water dispersibility, stability, and prolong the release of EOs for improving the delivery of their active ingredients [83,177]. Optimal surfactant concentration also enhances the antimicrobial activity of cinnamon oil-loaded NEs. Varying surfactant concentration can facilitate the preparation of small-sized droplets leading to better EO dispersibility in aqueous media [178]. Overall, NEs have significantly improved the effectiveness of natural compounds; however, surfactant types and concentration should be chosen carefully to avoid unwanted cytotoxicity.
Exploiting NE physiochemical properties and loading capacities has led to successful antiviral applications administered through different routes in various in vitro and in vivo studies. A recent in vitro study showed that curcumin-loaded cationic NEs enhanced curcumin antiviral activity against dengue virus serotypes and improved biocompatibility with targeted cells. Cationic NE surfaces facilitated binding to the negatively charged infected and non-infected cells improving cargo delivery and therapeutic efficiency [179]. Intravenously administered O/W NEs stabilized with non-ionic surfactant Tween 80 exhibited better absorption and retention of the loaded antiviral agent Indinavir (i.e., a protease inhibitor) in brain tissues. Localization in brain capillaries was attributed to the mechanical properties of the formulation, particularly the elasticity or flexibility of oil droplets that enables them to squeeze through the endothelial lining [180]. Topical administration of NEs has also led to many successful antiviral drug delivery applications. Topically applied curcumin-loaded NEs have exhibited efficacy against human papillomavirus infections [181]. Topical administration of NEs has also led to many successful antiviral drug delivery applications. Penciclovir–lavender oil NEs-based gels have shown promising results as topical anti-herpes virus treatments, particularly for herpes labialis disease due to improved skin permeation of protonated surfaces [182].
NEs have also been formulated to treat viral diseases through intranasal administration. Konek et al. prepared a whole inactivated respiratory syncytial virus (RSV) vaccine based on NEs for immunization of animals [183]. Surface displayed poloxamer surfactants with polar protrusions enhanced mucoadhesion of the NE vaccine in the nasal cavities. The formulation also demonstrated an effective immune response with no harmful effects observed in the lung tissues. HIV infections, that inhabit phagocytic cells, have been also targeted nasally using cationic NEs to help overcome the permeability challenges of antiviral drugs associated with blood-brain barrier. The cationic surfaces of NEs facilitated the biointeractions with negatively-charged macrophages, therefore site of infection [184]. These successful studies demonstrate the versatility of NEs in many pharmaceutical applications, particularly in the development of antivirals through different administration routes.
6. Potential roles of tunable nanoemulsions in preventing and treating COVID-19
6.1. Nanoemulsion as potential therapeutic platforms against COVID-19
Rapid repurposing of existing drugs for treating COVID-19 is currently underway in clinical trials. These repurposed therapeutics can be divided into various groups including drugs that directly interferes with the SARS-CoV-2 replication cycle, by inhibiting viral enzymes [[185], [186], [187], [188], [189]] or blocking viral entry into human cells [190,191], and drugs intended to enhance innate immune responses or reduce damage caused by acute inflammatory processes. However, even though some of these repurposed drugs are promising candidates, no effective treatments are currently available for COVID-19.
Repurposed drugs may alleviate the symptoms of COVID-19; however, these drugs tend to produce high cytotoxicity in normal body tissues and impact vital organs. For example, adverse cardiac and cerebral effects have been reported following the administration of protease inhibitors as antiviral therapies [192,193]. Also, administration of immunomodulators, like chloroquine, is associated with mild to severe side effects causing gastrointestinal problems, retinopathies and cardiomyopathies [194]. The inadequate clinical evaluation of repurposed drug candidates to determine drug efficacy and safety is another major limitation for using these drugs to treat COVID-19 patients.
Designing new drug delivery systems based on NE platforms could improve pharmacokinetics and pharmacodynamics of repurposed drugs and enable versatile therapeutic strategies against the ongoing COVID-19 public health crisis (Fig. 6 ). NEs can enhance drug efficacy by extending drug release profiles and help minimize drug side effects by lowering the required administered dosage. NE surfaces can also be engineered for passive or active targeting to improve drug localization and bioavailability. Biological barriers, like the skin and blood-brain barriers, have also been overcome by NEs to enable administration of encapsulated drugs through different entry routes. For effective drug delivery through different entry routes, NEs can be prepared in different dosage forms, like liquid, semi-liquid, solid and aerosols. These characteristics demonstrate the huge potential of NEs as therapeutics in the fight against COVID-19 and any future viral outbreaks.
Fig. 6.
O/W NE formulations as possible strategies in the fight against COVID-19.
The fabrication of NEs for inhibiting the SARS-CoV-2 replication cycle is possible. For example, Hoeller et al. studied the delivery of coumestrol, an inhibitor of Herpes Simplex Virus-1 (HSV-1) replication, using NEs to enhance antiviral activity via skin permeation [195]. Another strategy against COVID-19 is disrupting cell recognition and preventing virus access. Surfaces of NEs can be designed to prevent or minimize SARS-CoV-2 cell entry by binding S glycoprotein or blocking its receptor, ACE-2 [196]. For instance, NEs can be fabricated and stabilized with human pulmonary surfactant-A that can interact with the viral surface glycoproteins and possibly prevent further virus attachment and transmission of infection. The delivery of antiviral compounds to viruses using NEs stabilized by surfactants with antiviral properties, like human pulmonary surfactant-D, might create synergistic effects against SARS-CoV-2 infection [181,197,198].
NEs stabilized with peptide or protein surfactants containing histidines can interact with and incorporate metals such as zinc and copper [199]. These metals are considered innate and adaptive antiviral immunity boosters [200] and could maximise the fight against COVID-19 in conjunction with the action of administered drugs.
NEs can mediate targeted anti-inflammatory responses, and potentially minimize tissue damage when administered to treat acute inflammatory responses caused by SARS-CoV-2 infection [201]. A non-invasive nucleic acid-based NE treatment was developed to silence CD73 by administering short interfering RNA (siRNA) through the intranasal route demonstrating a novel therapeutic strategy for suppressing enzyme activities via gene knockdown [202]. Intravenously administered fish oil loaded-emulsions are also under investigation as immunomodulators to minimize the severity of COVID-19 symptoms [203]. NE can also be utilized as a safe tool for food fortification to lower inflammation and support immunity. For example, Akkam et al. have prepared pea protein-stabilized NE loaded with vitamin D, which can lower viral replication cycles and inflammatory responses, as a supportive therapy during the COVID-19 pandemic [204]. The aerosolization capacity of NEs contributes to the potential of these emulsions as non-invasive drug delivery systems through the pulmonary route for treating respiratory diseases, such as ARDS, and facilitating drug delivery to lung tissues and other body tissues affected by COVID-19 [[205], [206], [207]]. A study by Nasr et al. (2012) characterized commercially available lipid NEs, Intralipid® and Clinoleic®, loaded with an antifungal drug, Amphotericin B (AmB) [207]. In vitro analysis showed enhanced delivery of the antifungal drug through respiratory airways suggesting that aerosolized NEs can improve the therapeutic outcome of the loaded drug. Based on this evidence, NEs as inhalable formulations are promising therapeutic candidates for tackling the COVID-19 pandemic.
There is an urgent need for ventilators and oxygen supplies in the treatment of COVID-19. NEs have also been explored in oxygen delivery and as tissue preservatives. Perfluorocarbon (PFC)-based emulsions are promising oxygen delivery vehicles with a high capacity to solubilize gases owing to the PFC hydrophobic nature and chemical structure (which accommodates 20-fold higher levels of dissolved oxygen compared to water molecules). A recent study reported successful preparation of promising biomimetic PFC-NEs stabilized with RBC-membrane components for oxygen delivery and as blood components substitutes to address the global demand for hospital blood transfusions [208]. NEs have been also loaded with an FDA approved, high capacity oxygen carrier, Chlorin (e6), to enhance tumor treatment in hypoxic conditions. The FDA has recently authorized Propofol, an emulsion formulation, for an emergency use to maintain sedation during oxygen ventilation of COVID-19 patients [209]. Other products containing PFCs were commercialized as oxygen-based therapeutics. However, some of these products were discontinued due to handling difficulties, storage requirements, and unacceptable clearance profiles [210]. Given these issues, there is a need to explore new PFC-loaded NEs with better physiochemical properties to overcome the disadvantages of the previously marketed formulations and accelerate their development process.
A novel approach for identifying promising therapeutic candidates for treating COVID-19 involves the development of surface-epitope-targeting neutralizing antibodies specific for SARS-CoV-2. These antibodies are expected to exert both protective and therapeutic effects [211]. However to date, there is insufficient evidence to suggest these antibodies are an effective COVID-19 treatment. The development of antibody delivery systems using functionalized NEs might allow for effective, safe, and targeted delivery of therapeutic candidates against SARS-CoV-2 without damaging healthy cells. These potential therapeutic strategies highlight NEs as useful components of multifunctional platforms; however, more research is needed.
6.2. Nanoemulsions as potential preventative tools against COVID-19
Finding a vaccine for preventing COVID-19 pandemic is an active research area as vaccines are well-known to provide a long-term strategy to prevent future viral outbreaks. Over 30 pharmaceutical companies and academic institutions are striving to create the most effective vaccine for COVID-19. Only a few of these companies, including Pfizer and AstraZeneca, have received emergency use approval of their vaccines. The emergency approval of these vaccines prompted social concerns about vaccine safety profiles, administered dosage and frequency, and potency against evolving SARS-CoV-2 mutants. Acceptance of vaccines varies throughout the global population and is influenced by public perception and knowledge that creates both positive and negative attitudes toward vaccines. However, it must be highlighted that vaccines are the most effective tool available for stopping the spread of infectious diseases. An adequate supply of manufactured vaccines for the global market is another challenge. Other vaccine candidates, such as Novavax, are still under investigation in clinical trials. Thus, the development of safe, affordable, and effective vaccines that can be easily manufactured is urgently needed.
Different strategies have been adopted for developing COVID-19 vaccines, including whole virus, protein subunit, and nucleic acid vaccines. Traditional vaccine platforms using inactivated viruses are renowned for eliciting a strong immune reaction, particularly that encode SARS-CoV-2 S glycoprotein, such as CanSino and the University of Oxford [212]. After the SARS-CoV-2 genome sequence became available in January 2020, nucleic acid-based vaccine candidates, mostly based on the S glycoprotein epitope, are also being tested [19].
Vaccine platforms based on nanotechnology hold great promise. For instance, two messenger RNA (mRNA)-based vaccines have been approved by the US FDA – Moderna Therapuetics and Pfizer. These vaccines contain lipid nanoparticles carrying mRNA that encode the SARS-CoV-2 S glycoprotein and can stimulate a high level of protein expression and long-term stability that will lead to strong immune responses and provoke antibody production specifically against the S glycoprotein of SARS-CoV-2 [213]. A similar approach has been taken by another two pharmaceutical companies, NovaVax and Curevac, that have developed their own COVID-19 lipid nanoparticle-based vaccines which encode the S glycoprotein [213,214]. Nucleic acid-based lipid nanoparticle vaccines offer great advantages over conventional vaccines. For instance, these vaccines do contain the whole virus and are therefore relatively safe. Furthermore, the high production capacity of these vaccines is more appropriate for a pandemic than conventional vaccine production [215].
NEs have been proven safe as vaccines and provide many advantages including antigen protection, increasing the surface area for greater antigen presentation, slow release of the antigen, enhanced stability, better bioavailability, and prolonged blood circulation. [216]. Moreover, O/W NEs adjuvant vaccines elicit immunity through multiple pathways that offer effective protection against viruses [217,218]. Adjuvants are essential components in vaccine formulations as they reduce the number of antigen epitopes needed to elicit an immune response [219,220]. The adaptable compositions of NEs systems enable new strategies and novel platforms for developing vaccines with high efficacies (Table 1 ).
Table 1.
Nanoemulsion-based vaccine platforms developed against several infectious diseases.
| Technology | Active ingredient (antigen) | Oil core | Surfactants and stabilizers | Route | Reference |
|---|---|---|---|---|---|
| NEs adjuvant | H5N1 virus | Squalene | Tween 80 | Intramuscular | [217] |
| Epitope-loaded NEs | Helicobacter pylori | Isopropyl myristate | EL-35®, PEG | Intranasal | [221] |
| Cationic mRNA NEs | Respiratory syncytial virus (RSV), Human cytomegalovirus (hCMV), Human immunodeficiency virus (HIV). | Squalene | DOTAP, sorbitan trioleate. | Intramuscular | [222] |
| W805EC NEs adjuvant | Hepatitis B | Soybean oil | Cetylpyridinium chloride, Tween 80 | Intranasal | [223] |
| W805EC NE adjuvant | Fluzone/Fluvirin influenza vaccine | Soybean oil | Tween 80, cetylpyridinium chloride | Intranasal | [224] |
| W805EC NEs adjuvant | Respiratory syncytial virus (RSV) | Soybean oil | Cetylpyridinium chloride, CPC | Intranasal | [225] |
| mRNA formulated with cationic NEs | Venezuelan equine encephalitis virus (VEEV) | Squalene | DOTAP, sorbitan trioleate. | Intramuscular | [226] |
| NEs adjuvant, Golden03 | Rabies | Squalene | Tween 80, Span 80 | Intramuscular | [130] |
NE platforms provide novel strategies for adjuvant encapsulation and antigen surface presentation. Through encapsulation or covalent functionalization, NE surfaces can mimic viruses via epitope functionalization, and elicit cellular and humoral immune reactions. Surfactants are required to stabilize NEs and prolong shelf life. In addition to stabilization property, surfactants play a major role in creating a functional interface for the bioconjugation or presentation of different biomolecules such as antigenic epitopes, offering an adaptable template for medical applications (Fig. 6) [227]. Chemical modifications of NE surfactants for changing the charge, altering the structure of polar headgroup, and attaching polymers, targeting moieties or antigenic epitopes, can improve the cellular uptake of nanodroplets and the overall prophylactic effects [52,[228], [229], [230]].
In vaccine applications, early emulsion formulations were approved for use as adjuvant systems. Freund's complete adjuvant, with inactivated mycobacterial cells, and the nonbacterial Freund's incomplete adjuvant, have been used as animal and human vaccines [231]. However, the major issue in Freund's adjuvants is the presence of mineral oil that is considered to be highly reactogenic [232].
Today, popular modern emulsion adjuvants contain squalene oil, instead of mineral oils, and non-ionic surfactants as emulsifiers. Squalene, a naturally occurring and readily metabolized oil, has been reported as an efficient adjuvant, provoking both cellular and humoral immune responses [233,234]. The modern squalene-based NEs MF59® and AS03®, are being used in the influenza vaccines Focetria® and Prepandrix®, respectively [235,236]. In January 2020, the FDA approved the first-ever adjuvanted cell-based influenza vaccine against H5N1, called AUDENZ™, that enhances and prolongs immune responses [50,202]. The formulation of MF59C – an emulsion-COVID-19 vaccine by Seqirus – contains as an adjuvant along with the SARS-CoV-2 sclamp antigen. This vaccine has recently entered clinical trials in Australia [237]. This study highlights the potential of NE as adjuvant and vaccine platforms having safety and immunogenicity profiles that show compatibility with other vaccine platforms.
Vaccination through oral or intranasal delivery routes is a non-invasive method that can stimulate systemic immune reactions via mucosal surfaces [202]. This application showcases the potential of NEs as therapeutic or protective tools against COVID-19. Oral administration of NE vaccines, one of the mucosal routes of administration for NE vaccines, is an attractive needle-free approach to elicit immune responses and enhance protection against infectious agents and pathologies. A recent study has developed a tumor NE vaccine encapsulating a melanoma-specific antigen that provoked mucosal immune responses and inhibited tumor growth in vivo demonstrating immunization protection [238]. This study promoted oral delivery of NE vaccines as feasible for a broad range of applications in the medical field. Intranasal NEs vaccines have also produced promising results in recent years. For example, findings from an in vivo study exploring the intranasal NanoVax® vaccine, based on W805EC (NE01), showed this vaccine to be a successful prophylactic and therapeutic tool against Herpes Simplex Virus (HSV) in in-vivo studies [239]. Another in -vivo study developed an intranasal NE vaccine formulated against the flu virus and showed promising results by eliciting both systemic and mucosal immunity against the H5N1 virus strain [240].
Antiviral and antimicrobial agents developed with nanotechnology can also be exploited as surface disinfectants. SARS-CoV-2 was found to be viable for up to 72 h on different surfaces ranging from plastic to stainless steel; thus, disinfectants are required to battle COVID-19 and prevent both contamination and contagion [241]. The current pandemic requires extreme preventive measures. Effective and safe protective and therapeutic tools must be created to overcome the COVID-19 pandemic and avoid other viral outbreaks and future pandemics. The application of nanotechnology in the current public health crisis holds promise for the prevention or treatment of COVID-19. NEs have been formulated as disinfectants and sanitizers due to their aerosolization capacity [242,243]. NEs have also been formulated with various EOs that have broad antibacterial and antiviral properties [244] and could be used to produce surface disinfectants; however, this area requires further research.
7. Challenges associated with nanoemulsion therapies
Despite the huge potential of NEs as drug carriers, many challenges must be overcome concerning the production, physical and biological stability, clinical translation, and industrial scalability of NEs. In the production of NEs, selecting the most appropriate drug encapsulation method is an extremely important decision. Pre-loading bioactive compounds into the oil core of NEs generally provides high entrapment efficiency; however, loaded cargos are prone to heat and shearing energy conditions that may negatively affect cargo release and bioactivity. Conversely, bioconjugated drugs displayed on the surface of NEs are not protected within the dispersed phase and are therefore exposed to biointeractions that can chemically modify or degrade the drug.
Stability and shelf-life are important considerations in the development of pharmaceutical NE formulations. Due to the thermodynamic instability of NEs, phase separation occurs over time altering physical NE properties like size and surface charge. These physical changes severely impact pharmacological performance of NEs and can cause adverse healthcare outcomes. Surfactants are used to stabilize NEs, preserve physiochemical properties, and prevent deterioration of encapsulated bioactive compounds, like EOs, during NE processing, application, and storage. Thorough stability testing studies to help understand NE behaviour during in vivo applications should be performed in various pH, temperature, and salt environments that mimic biological conditions.
Nanomedicines, including NEs, encounter many complex barriers through different administration routes. Degradation, non-specificity, off-site toxicity, and rapid clearance are common biological barriers nanomedicines must overcome. Chemical composition and surface properties determine NE behaviour in biological applications. Non-specific interactions with biological components are challenging and may be prevented through NE design that considers the intended route of administration. The selection of surface functional additives, such as polymers, influences NE stability and surface functionalization. NEs formulated with non-biodegradable polymers may cause toxicity, increase ROS, free radical reactions, and generate inflammatory mediators that can damage healthy cells. Biodegradable, biocompatible, and FDA-approved polymers are preferred in the manufacturing process of pharmaceutical NEs. Surfactant type and concentration impact stability of NE droplets and functionality of excipients. Surfactants also play a major role in the functionalization and modification of NE surfaces.
Industrial scalability and reproducibility are major hurdles for the clinical translation of nanomedicines. The conjugation of functional molecules to surfactants by chemical or genetic modifications creates promising drug delivery strategies; however, scalability, reproducibility, and quantification of surface-adsorbed biomolecules are challenging. Complex surface modifications of NEs increase the complexity of manufacturing and production costs. Excessive chemical surface modification might also lead to detrimental effects on encapsulated compounds. Although, accelerating large-scale and continuous manufacturing of functionalized surfactants may help facilitate clinical translation of pre-clinically promising multifunctional NE formulations. The current COVID-19 pandemic, and the aforementioned hurdles should be taken into consideration by researchers and pharmaceutical companies undertaking development of therapeutic or preventive NE systems.
8. Conclusion and future directions
The development of effective treatments for viral outbreaks is challenging due to limited safety, efficacy, specificity, precision, and scalability of the currently available therapeutics. Also, viruses are rapidly and continuously mutating impacting the efficacies of the currently available vaccines and drugs. Stable, scalable, tunable, and adaptable generic platforms that can deliver drugs, adjuvants, and antigens are urgently needed. Safe and targeted delivery of therapeutics against SARS-CoV2 can help to accelerate the development of effective therapeutics against COVID-19.
Despite recent advances in nanotechnology and the development of formulations for various medical applications, there is an ongoing need for optimized delivery platforms. NEs are promising platforms for various research areas including diagnostics, surveillance and monitoring, and therapeutics, and prophylactics [245]. NEs hold a great promise as drug carriers and vaccine platforms through different administration routes (Fig. 3) [[49], [50], [51], [52]]. NEs are tunable and versatile nanomaterials with high loading capacities that can improve drugs solubility and bioavailability. NEs are easy to manufacture, generally recognized as safe ingredients (GRAS), and offer long-term stability. Emulsions have a safe history as vaccines effectively delivering adjuvants and surface-displayed antigens or epitopes. NEs can encapsulate various antivirals, and mimic or target viruses via epitopes or targeting moieties displayed on NE surfaces. NEs can be developed as protective (e.g. disinfectants and vaccines), diagnostic, therapeutic, and theranostic formulations in different dosage forms, demonstrating NEs to be promising platforms for viral diseases, such as COVID-19.
Advances in the development of NEs is a research area aimed at improving NE precision and reproducibility. 3D printing has recently emerged as a promising technology for fabricating nanomaterials. Although 3D printing of NEs is still a relatively unexplored area, it has the potential to precisely control the physiochemical and functional properties of NEs. For instance, oil droplet size may be better controlled and tailored by selecting appropriate 3D printhead nozzles [[246], [247], [248], [249]]. Only a few publications have reported the preparation of NEs using 3D printing technologies. Hsiao et al. prepared mesoporous hydrogels with flexible functionalities and complex shapes by using O/W NEs as 3D thermoresponsive ink [250]. This study highlights the bright future of NEs that could be precisely manufactured using 3D printing.
Advances in surface functionalization of NE are required at research, clinical and industrial levels to progress their applications as therapeutic and preventative alternatives to current antivirals. In particular, large-scale production of biocompatible surfactants that can be easily adapted for various NE pharmaceutical applications is needed. Facile production methodologies are essential for avoiding complex and harsh post-emulsification processing. Next-generation NEs as multimodal imaging, vaccines, and therapeutic platforms are currently under investigation and development. Despite the huge success of NEs in pre-clinical and clinical settings, a fundamental understanding of NE physiochemical properties and how these properties determine NE biointeractions through various routes of administration is needed to rapidly advance the clinical translation of NEs toward COVID-19 and other global health concerns.
Author contributions
H.T, S.A, R.F and N·H wrote and revised the manuscript. The manuscript was proposed and supervised by H.T who also significantly contributed to the manuscript structure, context, revision, editing and reference formatting. All Authors contributed to figure design. Figures were produced by S.A and reviewed by H.T. S.A contributed to writing the following manuscript sections; introduction and potential roles of tunable nanoemulsions in preventing and treating COVID-19, including Table. 1. Nanoemulsion composition, types and structure, and stability of nanoemulsions sections were written by H.T and N·H. R.F and N.H. wrote the nanoemulsions preparation and characterization sections, as well as discussing advances in nanoemulsion preparation (i.e. 3D printing). N.H and R.F and H.T wrote the following section: effect of administration route on nanoemulsion fate. All authors wrote and revise sections; Insights into nanoemulsion drug delivery mechanisms and Challenges associated with nanoemulsion therapies. All authors have read and agreed to the present manuscript. No writing assistance was utilized in the production of this manuscript.
Funding
This research did not receive any specific grants from any funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare no conflict of interest.
References
- 1.Wilson R.J., Li Y., Yang G., Zhao C.-X. Nanoemulsions for drug delivery. Particuology. 2021 doi: 10.1016/j.partic.2021.05.009. [DOI] [Google Scholar]
- 2.Kaur P., Gupta S., Kaur K., Kaur N., Kumar R., Bhullar M.S. Nanoemulsion of Foeniculum vulgare essential oil: a propitious striver against weeds of Triticum aestivum. Ind. Crop. Prod. 2021;168:113601. doi: 10.1016/j.indcrop.2021.113601. [DOI] [Google Scholar]
- 3.Jamali S.N., Assadpour E., Feng J., Jafari S.M. Natural antimicrobial-loaded nanoemulsions for the control of food spoilage/pathogenic microorganisms. Adv. Colloid Interf. Sci. 2021;295:102504. doi: 10.1016/j.cis.2021.102504. [DOI] [PubMed] [Google Scholar]
- 4.Li G., Zhang Z., Liu H., Hu L. Nanoemulsion-based delivery approaches for nutraceuticals: fabrication, application, characterization, biological fate, potential toxicity and future trends. Food Funct. 2021;12(5):1933–1953. doi: 10.1039/D0FO02686G. [DOI] [PubMed] [Google Scholar]
- 5.Tiwari U., Ganesan N.G., Junnarkar J., Rangarajan V. Toward the formulation of bio-cosmetic nanoemulsions: from plant-derived to microbial-derived ingredients. J. Dispers. Sci. Technol. 2020:1–18. doi: 10.1080/01932691.2020.1847664. [DOI] [Google Scholar]
- 6.Pandey V., Shukla R., Garg A., Kori M.L., Rai G. In: Nanocosmetics. Nanda A., Nanda S., Nguyen T.A., Rajendran S., Slimani Y., editors. Elsevier; Amsterdam: 2020. Chapter 17 - Nanoemulsion in cosmetic: From laboratory to market; pp. 327–347. [Google Scholar]
- 7.McClements D.J., Jafari S.M. In: Nanoemulsions. Jafari S.M., McClements D.J., editors. Academic Press; Cambridge: 2018. Chapter 1 - general aspects of nanoemulsions and their formulation; pp. 3–20. [Google Scholar]
- 8.Sheth T., Seshadri S., Prileszky T., Helgeson M.E. Multiple nanoemulsions. Nat. Rev. Mater. 2020;5(3):214–228. doi: 10.1038/s41578-019-0161-9. [DOI] [Google Scholar]
- 9.Tayeb H.H., Sainsbury F. Nanoemulsions in drug delivery: formulation to medical application. Nanomedicine (Lond) 2018;13(19):2507–2525. doi: 10.2217/nnm-2018-0088. [DOI] [PubMed] [Google Scholar]
- 10.Ismail A., Nasr M., Sammour O. Nanoemulsion as a feasible and biocompatible carrier for ocular delivery of travoprost: improved pharmacokinetic/pharmacodynamic properties. Int. J. Pharm. 2020;583:119402. doi: 10.1016/j.ijpharm.2020.119402. [DOI] [PubMed] [Google Scholar]
- 11.Zaichik S., Steinbring C., Friedl J.D., Bernkop-Schnürch A. Development and in vitro characterization of transferrin-decorated nanoemulsion utilizing hydrophobic ion pairing for targeted cellular uptake. J. Pharm. Innov. 2021 doi: 10.1007/s12247-021-09549-2. [DOI] [Google Scholar]
- 12.Bonnet S., Prévot G., Mornet S., Jacobin-Valat M.-J., Mousli Y., Hemadou A., Duttine M., Trotier A., Sanchez S., Duonor-Cérutti M., Crauste-Manciet S., Clofent-Sanchez G. A Nano-emulsion platform functionalized with a fully human scFv-fc antibody for atheroma targeting: towards a theranostic approach to atherosclerosis. Int. J. Mol. Sci. 2021;22(10):5188. doi: 10.3390/ijms22105188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jirwankar P., Shah D., Shao J. Protection of protein drugs by self-emulsified nanoemulsion against proteolysis. J Pharm Sci. 2020;109(8):2615–2621. doi: 10.1016/j.xphs.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y., Liu C., Zhang S., Li J., Zheng H., Jin H., Xu J. Comparison of different protein emulsifiers on physicochemical properties of β-carotene-loaded Nanoemulsion: effect on formation, stability, and in vitro digestion. Nanomaterials. 2021;11(1):167. doi: 10.3390/nano11010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elsewedy H.S., Aldhubiab B.E., Mahdy M.A., Elnahas H.M. Brucine PEGylated nanoemulsion: in vitro and in vivo evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2021;608:125618. doi: 10.1016/j.colsurfa.2020.125618. [DOI] [Google Scholar]
- 16.Shah S., Chougule M.B., Kotha A.K., Kashikar R., Godugu C., Raghuvanshi R.S., Singh S.B., Srivastava S. Nanomedicine based approaches for combating viral infections. J. Control. Release. 2021;338:80–104. doi: 10.1016/j.jconrel.2021.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delshadi R., Bahrami A., McClements D.J., Moore M.D., Williams L. Development of nanoparticle-delivery systems for antiviral agents: a review. J. Control. Release. 2021;331:30–44. doi: 10.1016/j.jconrel.2021.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khailany R.A., Safdar M., Ozaslan M. Genomic characterization of a novel SARS-CoV-2. Gene Rep. 2020;19:100682. doi: 10.1016/j.genrep.2020.100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., Yuan M.L., Zhang Y.L., Dai F.H., Liu Y., Wang Q.M., Zheng J.J., Xu L., Holmes E.C., Zhang Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim D., Lee J.-Y., Yang J.-S., Kim J.W., Kim V.N., Chang H. The architecture of SARS-CoV-2 transcriptome. Cell J. 2020;181(4):914–921. doi: 10.1016/j.cell.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siu Y.L., Teoh K.T., Lo J., Chan C.M., Kien F., Escriou N., Tsao S.W., Nicholls J.M., Altmeyer R., Peiris J.S., Bruzzone R., Nal B. The M, E, and N structural proteins of the severe acute respiratory syndrome coronavirus are required for efficient assembly, trafficking, and release of virus-like particles. J. Virol. 2008;82(22):11318–11330. doi: 10.1128/JVI.01052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nal B., Chan C., Kien F., Siu L., Tse J., Chu K., Kam J., Staropoli I., Crescenzo-Chaigne B., Escriou N., van der Werf S., Yuen K.Y., Altmeyer R. Differential maturation and subcellular localization of severe acute respiratory syndrome coronavirus surface proteins S, M and E. J Gen Virol. 2005;86(Pt 5):1423–1434. doi: 10.1099/vir.0.80671-0. [DOI] [PubMed] [Google Scholar]
- 23.Fathi M. The crystallography of SARS-CoV2 suggests its deactivation, ran. Red Crescent Med. J. 2020;(3):22. doi: 10.5812/IRCMJ.102812. [DOI] [Google Scholar]
- 24.Rahman M.M., Williams S.J. Membrane tension may define the deadliest virus infection. Colloids Interface Sci. Commun. 2021;40:100338. doi: 10.1016/j.colcom.2020.100338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol. Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed S.F., Quadeer A.A., McKay M.R. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12(3):254. doi: 10.3390/v12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell J. 2020;181(2):281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M., Leopoldi A., Garreta E., Hurtado del Pozo C., Prosper F., Romero J.P., Wirnsberger G., Zhang H., Slutsky A.S., Conder R., Montserrat N., Mirazimi A., Penninger J.M. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell J. 2020;181(4) doi: 10.1016/j.cell.2020.04.004. 905-913.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elkin S.R., Oswald N.W., Reed D.K., Mettlen M., MacMillan J.B., Schmid S.L. Ikarugamycin: a natural product inhibitor of clathrin-mediated endocytosis. Traffic. 2016;17(10):1139–1149. doi: 10.1111/tra.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adedeji A.O., Severson W., Jonsson C., Singh K., Weiss S.R., Sarafianos S.G. Novel inhibitors of severe acute respiratory syndrome coronavirus entry that act by three distinct mechanisms. Virol. J. 2013;87(14):8017–8028. doi: 10.1128/JVI.00998-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S., Li W., Hui H., Tiwari S.K., Zhang Q., Croker B.A., Rawlings S., Smith D., Carlin A.F., Rana T.M. Cholesterol 25-Hydroxylase inhibits SARS-CoV-2 and other coronaviruses by depleting membrane cholesterol. EMBO J. 2020;39:e106057. doi: 10.15252/embj.2020106057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopes M.I., Bonjorno L.P., Giannini M.C., Amaral N.B., Menezes P.I., Dib S.M., Gigante S.L., Benatti M.N., Rezek U.C., Emrich-Filho L.L., Sousa B.A.A., Almeida S.C.L., Assad R. Luppino, Veras F.P., Schneider A., Rodrigues T.S., Leiria L.O.S., Cunha L.D., Alves-Filho J.C., Cunha T.M., Arruda E., Miranda C.H., Pazin-Filho A., Auxiliadora-Martins M., Borges M.C., Fonseca B.A.L., Bollela V.R., Del-Ben C.M., Cunha F.Q., Zamboni D.S., Santana R.C., Vilar F.C., Louzada-Junior P., Oliveira R.D.R. Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial. RMD Open. 2021;7:e001455. doi: 10.1136/rmdopen-2020-001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashemian S.M., Farhadi T., Velayati A.A. A review on remdesivir: a possible promising agent for the treatment of COVID-19. Drug Des Devel Ther. 2020;14:3215–3222. doi: 10.2147/DDDT.S261154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grifoni A., Sidney J., Zhang Y., Scheuermann R.H., Peters B., Sette A. A sequence homology and bioinformatic approach can predict candidate targets for immune responses to SARS-CoV-2. Cell Host Microbe. 2020;27(4):671–680. doi: 10.1016/j.chom.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graham R.L., Baric R.S. Recombination, reservoirs, and the modular spike: mechanisms of coronavirus cross-species transmission. Virol. J. 2010;84(7):3134. doi: 10.1128/JVI.01394-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quinlan B.D., Mou H., Zhang L., Guo Y., He W., Ojha A., Parcells M.S., Luo G., Li W., Zhong G. The SARS-CoV-2 receptor-binding domain elicits a potent neutralizing response without antibody-dependent enhancement. BioRxiv. 2020 doi: 10.1101/2020.04.10.036418. [DOI] [Google Scholar]
- 37.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92(4):418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hilgenfeld R. From SARS to MERS: crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J. 2014;281(18):4085–4096. doi: 10.1111/febs.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L., Becker S., Rox K., Hilgenfeld R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368(6489):409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X., Zai J., Zhao Q., Nie Q., Li Y., Foley B.T., Chaillon A. Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2. J. Med. Virol. 2020;92(6):602–611. doi: 10.1002/jmv.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koopmans M. SARS-CoV-2 and the human-animal interface: outbreaks on mink farms. Lancet Infect. Dis. 2021;21(1):18–19. doi: 10.1016/S1473-3099(20)30912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menni C., Valdes A.M., Freidin M.B., Sudre C.H., Nguyen L.H., Drew D.A., Ganesh S., Varsavsky T., Cardoso M.J., Moustafa J.S.E.-S. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat. Med. 2020;26(7):1037–1040. doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X., Ma X. Acute respiratory failure in COVID-19: is it “typical” ARDS? Crit. Care. 2020;24:1–5. doi: 10.1186/s13054-020-02911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davies N.G., Abbott S., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J., Pearson C.A.B., Russell T.W., Tully D.C., Washburne A.D., Wenseleers T., Gimma A., Waites W., Wong K.L.M., van Zandvoort K., Silverman J.D., Diaz-Ordaz K., Keogh R., Eggo R.M., Funk S., Jit M., Atkins K.E., Edmunds W.J. Estimated transmissibility and severity of novel SARS-CoV-2 Variant of Concern 202012/01 in England. medRxiv. 2021:20248822. doi: 10.1101/2020.12.24.20248822. [DOI] [Google Scholar]
- 45.Zhang W., Davis B.D., Chen S.S., Martinez J.M.S., Plummer J.T., Vail E. Emergence of a novel SARS-CoV-2 strain in Southern California, USA. medRxiv. 2021 doi: 10.1001/jama.2021.1612. 2021.01.18.21249786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tegally H., Wilkinson E., Giovanetti M., Iranzadeh A., Fonseca V., Giandhari J., Doolabh D., Pillay S., San E.J., Msomi N., Mlisana K., von Gottberg A., Walaza S., Allam M., Ismail A., Mohale T., Glass A.J., Engelbrecht S., Van Zyl G., Preiser W., Petruccione F., Sigal A., Hardie D., Marais G., Hsiao M., Korsman S., Davies M.-A., Tyers L., Mudau I., York D., Maslo C., Goedhals D., Abrahams S., Laguda-Akingba O., Alisoltani-Dehkordi A., Godzik A., Wibmer C.K., Sewell B.T., Lourenço J., Alcantara L.C.J., Pond S.L.K., Weaver S., Martin D., Lessells R.J., Bhiman J.N., Williamson C., de Oliveira T. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. medRxiv. 2020:20248640. doi: 10.1101/2020.12.21.20248640. [DOI] [Google Scholar]
- 47.Williams T.C., Burgers W.A. SARS-CoV-2 evolution and vaccines: cause for concern? LANCET RESP MED. 2020;9(4):333–335. doi: 10.1016/S2213-2600(21)00075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He X., Lau E.H., Wu P., Deng X., Wang J., Hao X., Lau Y.C., Wong J.Y., Guan Y., Tan X. Temporal dynamics in viral shedding and transmissibility of COVID-19. medRxiv. 2020:20036707. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J., Miao J., Han X., Lu Y., Deng B., Lv F., Zhao Y., Ding C., Hou J. Development of a novel oil-in-water emulsion and evaluation of its potential adjuvant function in a swine influenza vaccine in mice. BMC Vet. Res. 2018;14(1):415. doi: 10.1186/s12917-018-1719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao L., Zhu Z., Ma L., Li Y. O/W Nanoemulsion as an adjuvant for an inactivated H3N2 influenza vaccine: based on particle properties and mode of carrying. International Int. J. Nanomedicine. 2020;15:2071. doi: 10.2147/IJN.S232677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang S.H., Chen J., Smith D., Cao Z., Acosta H., Fan Y., Ciotti S., Fattom A., Baker J. A novel combination of intramuscular vaccine adjuvants, nanoemulsion and CpG produces an effective immune response against influenza a virus. Vaccine. 2020;38(19):3537–3544. doi: 10.1016/j.vaccine.2020.03.026. [DOI] [PubMed] [Google Scholar]
- 52.Wong P.T., Wang S.H., Ciotti S., Makidon P.E., Smith D.M., Fan Y., Schuler C.F., IV, Baker J.R., Jr. Formulation and characterization of nanoemulsion intranasal adjuvants: effects of surfactant composition on mucoadhesion and immunogenicity. Mol. Pharm. 2014;11(2):531–544. doi: 10.1021/mp4005029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hak S., Garaiova Z., Olsen L.T., Nilsen A.M., de Lange Davies C. The effects of oil-in-water Nanoemulsion polyethylene glycol surface density on intracellular stability, pharmacokinetics, and biodistribution in tumor bearing mice. Pharm. Res. 2015;32(4):1475–1485. doi: 10.1007/s11095-014-1553-6. [DOI] [PubMed] [Google Scholar]
- 54.Tayeb H.H., Piantavigna S., Howard C.B., Nouwens A., Mahler S.M., Middelberg A.P., He L., Holt S.A., Sainsbury F. Insights into the interfacial structure–function of poly (ethylene glycol)-decorated peptide-stabilised nanoscale emulsions. Soft Matter. 2017;13(43):7953–7961. doi: 10.1039/c7sm01614j. [DOI] [PubMed] [Google Scholar]
- 55.Ganta S., Singh A., Patel N.R., Cacaccio J., Rawal Y.H., Davis B.J., Amiji M.M., Coleman T.P. Development of EGFR-targeted Nanoemulsion for imaging and novel platinum therapy of ovarian cancer. Pharm. Res. 2014;31:2490–2502. doi: 10.1007/s11095-014-1345-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eastoe J., Tabor R.F. In: Colloidal Foundations of Nanoscience. Berti D., Palazzo G., editors. Elsevier; Amsterdam: 2014. Chapter 6 - surfactants and nanoscience; pp. 135–157. [Google Scholar]
- 57.Tadros T., Izquierdo P., Esquena J., Solans C. Formation and stability of nano-emulsions. Adv. Colloid Interf. Sci. 2004;108–109:303–318. doi: 10.1016/j.cis.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 58.Desai J.D., Banat I.M. Microbial production of surfactants and their commercial potential. Microbiol. Mol. Biol. Rev: MMBR. 1997;61(1):47–64. doi: 10.1128/mmbr.61.1.47-64.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marchant R., Banat I.M. Biosurfactants: a sustainable replacement for chemical surfactants? Biotechnol. Lett. 2012;34(9):1597–1605. doi: 10.1007/s10529-012-0956-x. [DOI] [PubMed] [Google Scholar]
- 60.Abdel-Mawgoud A.M., Lepine F., Deziel E. Rhamnolipids: diversity of structures, microbial origins and roles. Appl. Microbiol. Biotechnol. 2010;86(5):1323–1336. doi: 10.1007/s00253-010-2498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kowall M., Vater J., Kluge B., Stein T., Franke P., Ziessow D. Separation and characterization of Surfactin isoforms produced byBacillus subtilis OKB 105. J. Colloid Interface Sci. 1998;204(1):1–8. doi: 10.1006/jcis.1998.5558. [DOI] [PubMed] [Google Scholar]
- 62.Malcolm A.S., Dexter A.F., Middelberg A.P. Foaming properties of a peptide designed to form stimuli-responsive interfacial films. Soft Matter. 2006;2(12):1057–1066. doi: 10.1039/b609960b. [DOI] [PubMed] [Google Scholar]
- 63.Malcolm A.S., Dexter A.F., Middelberg A.P. Peptide surfactants (Pepfactants) for switchable foams and emulsions. Asia Pac. J. Chem. Eng. 2007;2(5):362–367. doi: 10.1002/apj.66. [DOI] [Google Scholar]
- 64.Middelberg A.P.J., Dimitrijev-Dwyer M. A designed biosurfactant protein for switchable foam control. ChemPhysChem. 2011;12(8):1426–1429. doi: 10.1002/cphc.201100082. [DOI] [PubMed] [Google Scholar]
- 65.Cheung L.D., Samantray S. Molecular dynamics simulation of protein biosurfactants. Colloids and Interfaces. 2018;2(3):39. doi: 10.3390/colloids2030039. [DOI] [Google Scholar]
- 66.Jahan R., Bodratti A.M., Tsianou M., Alexandridis P. Biosurfactants, natural alternatives to synthetic surfactants: physicochemical properties and applications. Adv. Colloid Interf. Sci. 2020;275:102061. doi: 10.1016/j.cis.2019.102061. [DOI] [PubMed] [Google Scholar]
- 67.Hasenhuettl G.L., Hartel R.W. second ed. Springer; Newyork: 2008. Food Emulsifiers and their Applications. [Google Scholar]
- 68.Marchant R., Banat I.M. Microbial biosurfactants: challenges and opportunities for future exploitation. Trends Biotechnol. 2012;30(11):558–565. doi: 10.1016/j.tibtech.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 69.Rodrigues L., Banat I.M., Teixeira J., Oliveira R. Biosurfactants: potential applications in medicine. J. Antimicrob. Chemother. 2006;57(4):609–618. doi: 10.1093/jac/dkl024. [DOI] [PubMed] [Google Scholar]
- 70.Rodrigues L.R., Teixeira J.A. Biosurfactants. Vol. 672. Springer; 2010. Biomedical and therapeutic applications of biosurfactants; pp. 75–87. [DOI] [PubMed] [Google Scholar]
- 71.Gudiña E.J., Rangarajan V., Sen R., Rodrigues L.R. Potential therapeutic applications of biosurfactants. Trends Pharmacological Sci. 2013;34(12):667–675. doi: 10.1016/j.tips.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 72.Çelik P.A., Manga E.B., Çabuk A., Banat I.M. Biosurfactants’ potential role in combating COVID-19 and similar future microbial threats. Appl. Sci. 2021;11(1):334. doi: 10.3390/app11010334. [DOI] [Google Scholar]
- 73.Vollenbroich D., Özel M., Vater J., Kamp R.M., Pauli G. Mechanism of inactivation of enveloped viruses by the biosurfactant Surfactin fromBacillus subtilis. Biologicals. 1997;25(3):289–297. doi: 10.1006/biol.1997.0099. [DOI] [PubMed] [Google Scholar]
- 74.Yuan L., Zhang S., Peng J., Li Y., Yang Q. Synthetic surfactin analogues have improved anti-PEDV properties. PLoS One. 2019;14(4):e0215227. doi: 10.1371/journal.pone.0215227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith M.L., Gandolfi S., Coshall P.M., Rahman P.K.S.M. Biosurfactants: a Covid-19 perspective. Front. Microbiol. 2020;11(1341) doi: 10.3389/fmicb.2020.01341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berti D., Montis C., Baglioni P. Self-assembly of designer biosurfactants. Soft Matter. 2011;7(16):7150–7158. doi: 10.1039/C1SM05197K. [DOI] [Google Scholar]
- 77.Tayeb H.H., Stienecker M., Middelberg A.P.J., Sainsbury F. Impact of site-specific bioconjugation on the interfacial activity of a protein biosurfactant. Langmuir. 2019;35(42):13588–13594. doi: 10.1021/acs.langmuir.9b01684. [DOI] [PubMed] [Google Scholar]
- 78.Otzen D.E. Biosurfactants and surfactants interacting with membranes and proteins: same but different? Biochim. Biophys. Acta Biomembr. 2017;1859(4):639–649. doi: 10.1016/j.bbamem.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 79.Yeh C.-M., Jarrett T., Gao Y., Zhao C.-X., Whittaker A., Sainsbury F., White A.L. Templating core-shell particles using metal ion-chelating biosurfactants. Particuology. 2021 doi: 10.1016/j.partic.2021.06.003. [DOI] [Google Scholar]
- 80.Wibowo D., Zhao C.-X., Middelberg A.P.J. Emulsion-templated silica nanocapsules formed using bio-inspired silicification. ChemComm. 2014;50(77):11325–11328. doi: 10.1039/C4CC04904G. [DOI] [PubMed] [Google Scholar]
- 81.Sainsbury F., Zeng B., Middelberg A.P. Towards designer nanoemulsions for precision delivery of therapeutics. Curr. Opin. Chem. 2014;4:11–17. doi: 10.1016/j.coche.2013.12.007. [DOI] [Google Scholar]
- 82.Salvia-Trujillo L., Soliva-Fortuny R., Rojas-Grau M.A., McClements D.J., Martin-Belloso O. Edible Nanoemulsions as carriers of active ingredients: a review. Annu. Rev. Food Sci. Technol. 2017;8:439–466. doi: 10.1146/annurev-food-030216-025908. [DOI] [PubMed] [Google Scholar]
- 83.Pavoni L., Perinelli D.R., Bonacucina G., Cespi M., Palmieri G.F. An overview of micro- and Nanoemulsions as vehicles for essential oils: formulation, preparation and stability. Nanomaterials. 2020;10(1):135. doi: 10.3390/nano10010135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pasquali R.C., Taurozzi M.P., Bregni C. Some considerations about the hydrophilic–lipophilic balance system. Int. J. Pharm. 2008;356(1):44–51. doi: 10.1016/j.ijpharm.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 85.Gupta A., Eral H.B., Hatton T.A., Doyle P.S. Nanoemulsions: formation, properties and applications. Soft Matter. 2016;12(11):2826–2841. doi: 10.1039/C5SM02958A. [DOI] [PubMed] [Google Scholar]
- 86.Shams N., Sahari M.A. Nanoemulsions: Preparation, structure, functional properties and their antimicrobial effects. Appl. Food Biotechnol. 2016;3:138–149. doi: 10.22037/afb.v3i3.11773. [DOI] [Google Scholar]
- 87.Anton N., Benoit J.-P., Saulnier P. Design and production of nanoparticles formulated from nano-emulsion templates—a review. J. Control. Release. 2008;128(3):185–199. doi: 10.1016/j.jconrel.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 88.McClements D.J., Rao J. Food-grade nanoemulsions: formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011;51(4):285–330. doi: 10.1080/10408398.2011.559558. [DOI] [PubMed] [Google Scholar]
- 89.Kumar M., Bishnoi R.S., Shukla A.K., Jain C.P. Techniques for formulation of Nanoemulsion drug delivery system: a review. Prev Nutr Food Sci. 2019;24(3):225–234. doi: 10.3746/pnf.2019.24.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kotta S., Khan A.W., Ansari S.H., Sharma R.K., Ali J. Formulation of nanoemulsion: a comparison between phase inversion composition method and high-pressure homogenization method. Drug Deliv. 2015;22(4):455–466. doi: 10.3109/10717544.2013.866992. [DOI] [PubMed] [Google Scholar]
- 91.Solans C., Solé I. Nano-emulsions: formation by low-energy methods. Curr. Opin. Colloid Interface Sci. 2012;17(5):246–254. doi: 10.1016/j.cocis.2012.07.003. [DOI] [Google Scholar]
- 92.Yukuyama M.N., Ghisleni D.D., Pinto T.J., Bou-Chacra N.A. Nanoemulsion: process selection and application in cosmetics--a review. Int. J. Cosmet. Sci. 2016;38(1):13–24. doi: 10.1111/ics.12260. [DOI] [PubMed] [Google Scholar]
- 93.Singh Y., Meher J.G., Raval K., Khan F.A., Chaurasia M., Jain N.K., Chourasia M.K. Nanoemulsion: concepts, development and applications in drug delivery. J. Control. Release. 2017;252:28–49. doi: 10.1016/j.jconrel.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 94.Mohsin M., Meribout M. Oil–water de-emulsification using ultrasonic technology. Ultrason. Sonochem. 2015;22:573–579. doi: 10.1016/j.ultsonch.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 95.McClements D.J. Nanoscale nutrient delivery systems for food applications: improving bioactive dispersibility, stability, and bioavailability. J. Food Sci. 2015;80(7):1602–1611. doi: 10.1111/1750-3841.12919. [DOI] [PubMed] [Google Scholar]
- 96.Kentish S., Wooster T., Ashokkumar M., Balachandran S., Mawson R., Simons L. The use of ultrasonics for nanoemulsion preparation. Innov. Food Sci. Emerg. Technol. 2008;9(2):170–175. doi: 10.1016/j.ifset.2007.07.005. [DOI] [Google Scholar]
- 97.Saffarionpour S. Preparation of food flavor Nanoemulsions by high- and low-energy emulsification approaches. Food Eng. Rev. 2019;11(4):259–289. doi: 10.1007/s12393-019-09201-3. [DOI] [Google Scholar]
- 98.Håkansson A., Rayner M. In: Nanoemulsions. Jafari S.M., McClements D.J., editors. Academic Press; Cambridge: 2018. Chapter 5 - general principles of Nanoemulsion formation by high-energy mechanical methods; pp. 103–139. [Google Scholar]
- 99.Akbas E., Soyler B., Oztop M.H. Formation of capsaicin loaded nanoemulsions with high pressure homogenization and ultrasonication. LWT. 2018;96:266–273. doi: 10.1016/j.lwt.2018.05.043. [DOI] [Google Scholar]
- 100.Donsì F., Sessa M., Ferrari G. Effect of emulsifier type and disruption chamber geometry on the fabrication of food Nanoemulsions by high pressure homogenization. Ind. Eng. Chem. Res. 2012;51(22):7606–7618. doi: 10.1021/ie2017898. [DOI] [Google Scholar]
- 101.Galvão K.C.S., Vicente A.A., Sobral P.J.A. Development, characterization, and stability of O/W pepper Nanoemulsions produced by high-pressure homogenization. Food Bioprocess Technol. 2018;11(2):355–367. doi: 10.1007/s11947-017-2016-y. [DOI] [Google Scholar]
- 102.Espitia P.J.P., Fuenmayor C.A., Otoni C.G. Nanoemulsions: synthesis, characterization, and application in bio-based active food packaging. Compr. Rev. Food Sci. 2019;18(1):264–285. doi: 10.1111/1541-4337.12405. [DOI] [PubMed] [Google Scholar]
- 103.Zhang A., Wang X., Zhao X.-h. Effect of homogenizing pressure on the properties of soy protein isolate-vitamin D3 nanoemulsion. J. Food Process Eng. 2021;44(8) doi: 10.1111/jfpe.13757. [DOI] [Google Scholar]
- 104.Silva H.D., Cerqueira M.A., Vicente A.A. Influence of surfactant and processing conditions in the stability of oil-in-water nanoemulsions. J. Food Eng. 2015;167:89–98. doi: 10.1016/j.jfoodeng.2015.07.037. [DOI] [Google Scholar]
- 105.Elwell M.W., Roberts R.F., Coupland J.N. Effect of homogenization and surfactant type on the exchange of oil between emulsion droplets. Food Hydrocoll. 2004;18(3):413–418. doi: 10.1016/j.foodhyd.2003.05.001. [DOI] [Google Scholar]
- 106.Shinoda K., Saito H. The stability of O/W type emulsions as functions of temperature and the HLB of emulsifiers: the emulsification by PIT-method. J. Colloid Interface Sci. 1969;30(2):258–263. doi: 10.1016/S0021-9797(69)80012-3. [DOI] [Google Scholar]
- 107.Solans C., Izquierdo P., Nolla J., Azemar N., Garcia-Celma M. Nano-emulsions. Curr. Opin. Colloid Interface Sci. 2005;10(3):102–110. doi: 10.1016/j.cocis.2005.06.004. [DOI] [Google Scholar]
- 108.Solans C., Morales D., Homs M. Spontaneous emulsification. Curr. Opin. Colloid Interface Sci. 2016;22:88–93. doi: 10.1016/j.cocis.2016.03.002. [DOI] [Google Scholar]
- 109.Ghosh V., Mukherjee A., Chandrasekaran N. Ultrasonic emulsification of food-grade nanoemulsion formulation and evaluation of its bactericidal activity. Ultrason. Sonochem. 2013;20(1):338–344. doi: 10.1016/j.ultsonch.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 110.Chuacharoen T., Prasongsuk S., Sabliov C.M. Effect of surfactant concentrations on physicochemical properties and functionality of curcumin Nanoemulsions under conditions relevant to commercial utilization. Molecules. 2019;24(15):2744. doi: 10.3390/molecules24152744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gupta A. In: Nanoparticles for Biomedical Applications. Chung E.J., Leon L., Rinaldi C., editors. Elsevier; Amsterdam: 2020. Nanoemulsions; pp. 371–384. [Google Scholar]
- 112.Zhang Z., McClements D.J. In: Nanoemulsions. Jafari S.M., McClements D.J., editors. Academic Press; Cambrige: 2018. Overview of Nanoemulsion properties: Stability, rheology, and appearance; pp. 21–49. [Google Scholar]
- 113.Jaiswal M., Dudhe R., Sharma P.K. Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech. 2015;5(2):123–127. doi: 10.1007/s13205-014-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McClements J., McClements D.J. Standardization of nanoparticle characterization: methods for testing properties, stability, and functionality of edible nanoparticles. Crit Rev Food Sci and Nutr. 2016;56(8):1334–1362. doi: 10.1080/10408398.2014.970267. [DOI] [PubMed] [Google Scholar]
- 115.Faria M., Björnmalm M., Thurecht K.J., Kent S.J., Parton R.G., Kavallaris M., Johnston A.P.R., Gooding J.J., Corrie S.R., Boyd B.J., Thordarson P., Whittaker A.K., Stevens M.M., Prestidge C.A., Porter C.J.H., Parak W.J., Davis T.P., Crampin E.J., Caruso F. Minimum information reporting in bio–nano experimental literature. Nat. Nanotechnol. 2018;13(9):777–785. doi: 10.1038/s41565-018-0246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Seki J., Sonoke S., Saheki A., Fukui H., Sasaki H., Mayumi T. A nanometer lipid emulsion, lipid nano-sphere (LNS), as a parenteral drug carrier for passive drug targeting. Int. J. Pharm. 2004;273(1–2):75–83. doi: 10.1016/j.ijpharm.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 117.Ganta S., Amiji M. Coadministration of paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol. Pharm. 2009;6(3):928–939. doi: 10.1021/mp800240j. [DOI] [PubMed] [Google Scholar]
- 118.Klang V., Matsko N.B., Valenta C., Hofer F. Electron microscopy of nanoemulsions: an essential tool for characterisation and stability assessment. Micron. 2012;43(2):85–103. doi: 10.1016/j.micron.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 119.Klang V., Matsko N., Raupach K., El-Hagin N., Valenta C. Development of sucrose stearate-based nanoemulsions and optimisation through gamma-cyclodextrin. Eur. J. Pharm. Biopharm. 2011;79(1):58–67. doi: 10.1016/j.ejpb.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 120.Kuntsche J., Horst J.C., Bunjes H. Cryogenic transmission electron microscopy (cryo-TEM) for studying the morphology of colloidal drug delivery systems. Int. J. Pharm. 2011;417(1–2):120–137. doi: 10.1016/j.ijpharm.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 121.Fox C.B. Squalene emulsions for parenteral vaccine and drug delivery. Molecules. 2009;14(9):3286–3312. doi: 10.3390/molecules14093286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Severs N.J., Robenek H. Freeze-fracture Cytochemistry in cell biology. Methods Cell Biol. 2008;88:181–204. doi: 10.1016/S0091-679X(08)00411-1. [DOI] [PubMed] [Google Scholar]
- 123.Jin W., Xu W., Liang H., Li Y., Liu S., Li B. In: Nanotechnology in the Agri-Food Industry. Grumezescu A.M., editor. Academic Press; Cambrige: 2016. pp. 1–36. [Google Scholar]
- 124.Morais Diane J.M., Burgess J. Vitamin E nanoemulsions characterization and analysis. Int. J. Pharm. 2014;465(1–2):455–463. doi: 10.1016/j.ijpharm.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 125.Liu Q., Huang H., Chen H., Lin J., Wang Q. Food-grade Nanoemulsions: preparation, stability and application in encapsulation of bioactive compounds. Molecules. 2019;24(23):4242. doi: 10.3390/molecules24234242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Patel S.K., Beaino W., Anderson C.J., Janjic J.M. Theranostic nanoemulsions for macrophage COX-2 inhibition in a murine inflammation model. Clin. Immunol. 2015;160(1):59–70. doi: 10.1016/j.clim.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Meng L., Xia X., Yang Y., Ye J., Dong W., Ma P., Jin Y., Liu Y. Co-encapsulation of paclitaxel and baicalein in nanoemulsions to overcome multidrug resistance via oxidative stress augmentation and P-glycoprotein inhibition. Int. J. Pharm. 2016;513(1–2):8–16. doi: 10.1016/j.ijpharm.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 128.Salvia-Trujillo L., Martín-Belloso O., McClements D.J. Excipient nanoemulsions for improving oral bioavailability of bioactives. Nanomaterials. 2016;6(1):17. doi: 10.3390/nano6010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tran L.T.C., Gueutin C., Frebourg G., Burucoa C., Faivre V. Erythromycin encapsulation in nanoemulsion-based delivery systems for treatment of helicobacter pylori infection: protection and synergy. Biochem. Biophys. 2017;493(1):146–151. doi: 10.1016/j.bbrc.2017.09.060. [DOI] [PubMed] [Google Scholar]
- 130.Streck L., Sarmento V.H.V., de Menezes R.P.R.P.B., Fernandes-Pedrosa M.F., Martins A.M.C., da Silva-Júnior A.A. Tailoring microstructural, drug release properties, and antichagasic efficacy of biocompatible oil-in-water benznidazol-loaded nanoemulsions. Int. J. Pharm. 2019;555:36–48. doi: 10.1016/j.ijpharm.2018.11.041. [DOI] [PubMed] [Google Scholar]
- 131.da Rocha Neto A.C., da Rocha A.B.D.O., Maraschin M., Di Piero R.M., Almenar E. Factors affecting the entrapment efficiency of β-cyclodextrins and their effects on the formation of inclusion complexes containing essential oils. Food Hydrocoll. 2018;77:509–523. doi: 10.1016/j.foodhyd.2017.10.029. [DOI] [Google Scholar]
- 132.Che Marzuki N.H., Wahab R.A., Abdul Hamid M. An overview of nanoemulsion: concepts of development and cosmeceutical applications. Biotechnol. Biotechnol. Equip. 2019;33(1):779–797. doi: 10.1080/13102818.2019.1620124. [DOI] [Google Scholar]
- 133.Sánchez-López E., Guerra M., Dias-Ferreira J., Lopez-Machado A., Ettcheto M., Cano A., Espina M., Camins A., Garcia M.L., Souto E.B. Current applications of Nanoemulsions in cancer therapeutics. Nanomaterials. 2019;9(6):821. doi: 10.3390/nano9060821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gustafson H.H., Holt-Casper D., Grainger D.W., Ghandehari H. Nanoparticle uptake: the phagocyte problem. Nano Today. 2015;10(4):487–510. doi: 10.1016/j.nantod.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yamada M., Tayeb H., Wang H., Dang N., Mohammed Y.H., Osseiran S., Belt P.J., Roberts M.S., Evans C.L., Sainsbury F., Prow T.W. Using elongated microparticles to enhance tailorable nanoemulsion delivery in excised human skin and volunteers. J. Control. Release. 2018;288:264–276. doi: 10.1016/j.jconrel.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shaker S.D., Ishak A.H.R., Ghoneim A., Elhuoni A.M. Nanoemulsion: a review on mechanisms for the transdermal delivery of hydrophobic and hydrophilic drugs. Sci. Pharm. 2019;87(3):17. doi: 10.3390/scipharm87030017. [DOI] [Google Scholar]
- 137.Rai V.K., Mishra N., Yadav K.S., Yadav N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: formulation development, stability issues, basic considerations and applications. J. Control. Release. 2018;270:203–225. doi: 10.1016/j.jconrel.2017.11.049. [DOI] [PubMed] [Google Scholar]
- 138.Bonferoni M.C., Rossi S., Sandri G., Ferrari F., Gavini E., Rassu G., Giunchedi P. Nanoemulsions for “nose-to-brain” drug delivery. Pharmaceutics. 2019;11(2):84. doi: 10.3390/pharmaceutics11020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Basha S.K., Muzammil M.S., Dhandayuthabani R., Kumari V.S., Kaviyarasu K. Nanoemulsion as oral drug delivery - a review. Curr Drug Res Rev. 2019 doi: 10.2174/2589977511666191024173508. [DOI] [Google Scholar]
- 140.Ozturk B., Argin S., Ozilgen M., McClements D.J. Nanoemulsion delivery systems for oil-soluble vitamins: influence of carrier oil type on lipid digestion and vitamin D3 bioaccessibility. Food Chem. 2015;187:499–506. doi: 10.1016/j.foodchem.2015.04.065. [DOI] [PubMed] [Google Scholar]
- 141.Salvia-Trujillo L., Sun Q., Um B.H., Park Y., McClements D.J. In vitro and in vivo study of fucoxanthin bioavailability from nanoemulsion-based delivery systems: impact of lipid carrier type. J. Funct. Foods. 2015;17:293–304. doi: 10.1016/j.jff.2015.05.035. [DOI] [Google Scholar]
- 142.Xia Z., Han Y., Du H., McClements D.J., Tang Z., Xiao H. Exploring the effects of carrier oil type on in vitro bioavailability of β-carotene: a cell culture study of carotenoid-enriched nanoemulsions. LWT. 2020;134:110224. doi: 10.1016/j.lwt.2020.110224. [DOI] [Google Scholar]
- 143.Hui Y., Wibowo D., Liu Y., Ran R., Wang H.-F., Seth A., Middelberg A.P.J., Zhao C.-X. Understanding the effects of Nanocapsular mechanical property on passive and active tumor targeting. ACS Nano. 2018;12(3):2846–2857. doi: 10.1021/acsnano.8b00242. [DOI] [PubMed] [Google Scholar]
- 144.Mitchell M.J., Billingsley M.M., Haley R.M., Wechsler M.E., Peppas N.A., Langer R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021;20(2):101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Choradiya B.R., Patil S.B. A comprehensive review on nanoemulsion as an ophthalmic drug delivery system. J. Mol. Liq. 2021;339:116751. doi: 10.1016/j.molliq.2021.116751. [DOI] [Google Scholar]
- 146.Chrastina A., Baron V.T., Abedinpour P., Rondeau G., Welsh J., Borgström P. Plumbagin-loaded nanoemulsion drug delivery formulation and evaluation of antiproliferative effect on prostate cancer cells. Biomed. Res. Int. 2018;2018:7. doi: 10.1155/2018/9035452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sharma N., Kaur G., Khatkar S.K. Optimization of emulsification conditions for designing ultrasound assisted curcumin loaded nanoemulsion: characterization, antioxidant assay and release kinetics. LWT. 2021;141:110962. doi: 10.1016/j.lwt.2021.110962. [DOI] [Google Scholar]
- 148.Lima T.S., Silva M.F.S., Nunes X.P., Colombo A.V., Oliveira H.P., Goto P.L., Blanzat M., Piva H.L., Tedesco A.C., Siqueira-Moura M.P. Cineole-containing nanoemulsion: development, stability, and antibacterial activity. Chem. Phys. Lipids. 2021;239:105113. doi: 10.1016/j.chemphyslip.2021.105113. [DOI] [PubMed] [Google Scholar]
- 149.Klang V., Schwarz J.C., Valenta C. In: Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Drug Manipulation Strategies and Vehicle Effects. Dragicevic N., Maibach H.I., editors. Springer; Berlin, Heidelberg: 2015. Nanoemulsions in dermal drug delivery; pp. 255–266. [Google Scholar]
- 150.Youssef A.A., Cai C., Dudhipala N., Majumdar S. Design of topical ocular ciprofloxacin nanoemulsion for the management of bacterial keratitis. Pharmaceuticals. 2021;14(3) doi: 10.3390/ph14030210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Salvia-Trujillo L., Fumiaki B., Park Y., McClements D.J. The influence of lipid droplet size on the oral bioavailability of vitamin D2 encapsulated in emulsions: an in vitro and in vivo study. Food Funct. 2017;8(2):767–777. doi: 10.1039/c6fo01565d. [DOI] [PubMed] [Google Scholar]
- 152.Yao M., Li Z., Julian McClements D., Tang Z., Xiao H. Design of nanoemulsion-based delivery systems to enhance intestinal lymphatic transport of lipophilic food bioactives: influence of oil type. Food Chem. 2020;317:126229. doi: 10.1016/j.foodchem.2020.126229. [DOI] [PubMed] [Google Scholar]
- 153.McClements D.J. Advances in edible nanoemulsions: digestion, bioavailability, and potential toxicity. Prog. Lipid Res. 2021;81:101081. doi: 10.1016/j.plipres.2020.101081. [DOI] [PubMed] [Google Scholar]
- 154.Zeng F., Wang D., Tian Y., Wang M., Liu R., Xia Z., Huang Y. Nanoemulsion for improving the Oral bioavailability of Hesperetin: formulation optimization and absorption mechanism. J. Pharm. Sci. 2021;110(6):2555–2561. doi: 10.1016/j.xphs.2021.02.030. [DOI] [PubMed] [Google Scholar]
- 155.Abdulbaqi I.M., Assi R.A., Yaghmur A., Darwis Y., Mohtar N., Parumasivam T., Saqallah F.G., Wahab H.A. Pulmonary delivery of anticancer drugs via lipid-based Nanocarriers for the treatment of lung cancer: an update. Pharmaceuticals. 2021;14(8) doi: 10.3390/ph14080725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Koh J., Kim S., Lee S.N., Kim S.-Y., Kim J.-E., Lee K.Y., Kim M.S., Heo J.Y., Park Y.M., Ku B.M., Sun J.-M., Lee S.-H., Ahn J.S., Park K., Yang S., Ha S.-J., Lim Y.T., Ahn M.-J. Therapeutic efficacy of cancer vaccine adjuvanted with nanoemulsion loaded with TLR7/8 agonist in lung cancer model. Nanomed.: Nanotechnol. Biol. Med. 2021;37:102415. doi: 10.1016/j.nano.2021.102415. [DOI] [PubMed] [Google Scholar]
- 157.Freitas M.M., Cavalcante P.M., Duarte-Filho L.A.M.S., Macedo C.A.F., Brito M.C., Menezes P.M.N., Ribeiro T.F., Costa S.M., Carvalho B.A.G., Ribeiro F.P.R.A., Moura M.P.S., Silva F.S., Ribeiro L.A.A. Investigation of the relaxing effect of a camphor nanoemulsion on rat isolated trachea. Chem. Biol. Interact. 2021;348:109656. doi: 10.1016/j.cbi.2021.109656. [DOI] [PubMed] [Google Scholar]
- 158.Ahmed M., Smith D.M., Hamouda T., Rangel-Moreno J., Fattom A., Khader S.A. A novel nanoemulsion vaccine induces mucosal Interleukin-17 responses and confers protection upon mycobacterium tuberculosis challenge in mice. Vaccine. 2017;35(37):4983–4989. doi: 10.1016/j.vaccine.2017.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Attia M.F., Anton N., Wallyn J., Omran Z., Vandamme T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019;71(8):1185–1198. doi: 10.1111/jphp.13098. [DOI] [PubMed] [Google Scholar]
- 160.Yang Y., Ge S., Song Z., Zhao A., Zhao L., Hu Z., Cai D., Zhang Z., Peng L., Lu D., Luo P., Zhang W., Sun H., Zou Q., Zeng H. A novel self-assembled epitope peptide nanoemulsion vaccine targeting nasal mucosal epithelial cell for reinvigorating CD8+ T cell immune activity and inhibiting tumor progression. Int. J. Biol. Macromol. 2021;183:1891–1902. doi: 10.1016/j.ijbiomac.2021.05.158. [DOI] [PubMed] [Google Scholar]
- 161.Marques M.B., Machado A.P., Santos P.A., Carrett-Dias M., Araújo G.S., da Silva Alves B., de Oliveira B.S., da Silva Júnior F.M.R., Dora C.L., Cañedo A.D., Filgueira D., Fernandes E.S.E., de Souza Votto A.P. Anti-MDR effects of quercetin and its Nanoemulsion in multidrug-resistant human Leukemia cells. Anti Cancer Agents Med. Chem. 2021;21(14):1911–1920. doi: 10.2174/1871520621999210104200722. [DOI] [PubMed] [Google Scholar]
- 162.Zhang Y., Bo S., Feng T., Qin X., Wan Y., Jiang S., Li C., Lin J., Wang T., Zhou X., Jiang Z.-X., Huang P. A versatile Theranostic Nanoemulsion for architecture-dependent multimodal imaging and dually augmented photodynamic therapy. Adv. Mater. 2019;31(21):1806444. doi: 10.1002/adma.201806444. [DOI] [PubMed] [Google Scholar]
- 163.Carvalho V.F.M., Migotto A., Giacone D.V., de Lemos D.P., Zanoni T.B., Maria-Engler S.S., Costa-Lotufo L.V., Lopes L.B. Co-encapsulation of paclitaxel and C6 ceramide in tributyrin-containing nanocarriers improve co-localization in the skin and potentiate cytotoxic effects in 2D and 3D models. Eur J Pharm Sci. 2017;109:131–143. doi: 10.1016/j.ejps.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 164.Migotto A., Carvalho V.F.M., Salata G.C., da Silva F.W.M., Yan C.Y.I., Ishida K., Costa-Lotufo L.V., Steiner A.A., Lopes L.B. Multifunctional nanoemulsions for intraductal delivery as a new platform for local treatment of breast cancer. Drug Deliv. 2018;25(1):654–667. doi: 10.1080/10717544.2018.1440665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.clinicaltrials.gov Curcumin in Reducing Joint Pain in Breast Cancer Survivors With Aromatase Inhibitor-Induced Joint Disease. 2019. https://clinicaltrials.gov/ct2/show/NCT03865992 (Accessed 03 October 2021)
- 166.Shafaei N., Barkhordar S.M.A., Rahmani F., Nabi S., Idliki R.B., Alimirzaei M., Karimi E., Oskoueian E. Protective effects of Anethum graveolens Seed’s oil Nanoemulsion against cadmium-induced oxidative stress in mice. Biol. Trace Elem. Res. 2020;198(2):583–591. doi: 10.1007/s12011-020-02093-z. [DOI] [PubMed] [Google Scholar]
- 167.Akrawi S.H., Gorain B., Nair A.B., Choudhury H., Pandey M., Shah J.N., Venugopala K.N. Development and optimization of Naringenin-loaded chitosan-coated Nanoemulsion for topical therapy in wound healing. Pharmaceutics. 2020;12(9):893. doi: 10.3390/pharmaceutics12090893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Alkhatib M.H., Alyamani S.A., Abdu F. Incorporation of methotrexate into coconut oil nanoemulsion potentiates its antiproliferation activity and attenuates its oxidative stress. Drug Deliv. 2020;27(1):422–430. doi: 10.1080/10717544.2020.1736209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ghiasi Z., Esmaeli F., Aghajani M., Ghazi-Khansari M., Faramarzi M.A., Amani A. Enhancing analgesic and anti-inflammatory effects of capsaicin when loaded into olive oil nanoemulsion: an in vivo study. Int. J. Pharm. 2019;559:341–347. doi: 10.1016/j.ijpharm.2019.01.043. [DOI] [PubMed] [Google Scholar]
- 170.Simion V., Constantinescu C.A., Stan D., Deleanu M., Tucureanu M.M., Butoi E., Manduteanu I., Simionescu M., Calin M. P-selectin targeted dexamethasone-loaded lipid Nanoemulsions: a novel therapy to reduce vascular inflammation. Mediat. Inflamm. 2016;2016:15. doi: 10.1155/2016/1625149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pleguezuelos-Villa M., Nacher A., Hernandez M.J., Buso M.A. Ofelia Vila, Sauri A. Ruiz, Diez-Sales O. Mangiferin nanoemulsions in treatment of inflammatory disorders and skin regeneration. Int J Pharm. 2019;564:299–307. doi: 10.1016/j.ijpharm.2019.04.056. [DOI] [PubMed] [Google Scholar]
- 172.Jeong Y.-J., Kim H.-E., Han S.-J., Choi J.-S. Antibacterial and antibiofilm activities of cinnamon essential oil nanoemulsion against multi-species oral biofilms. Sci. Rep. 2021;11(1):5911. doi: 10.1038/s41598-021-85375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yang Q., Liu S., Gu Y., Tang X., Wang T., Wu J., Liu J. Development of sulconazole-loaded nanoemulsions for enhancement of transdermal permeation and antifungal activity. Int. J. Nanomedicine. 2019;14:3955–3966. doi: 10.2147/IJN.S206657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mahmoud D.B., Ismail W.M., Moatasim Y., Kutkat O., ElMeshad A.N., Ezzat S.M., El Deeb K.S., El-Fishawy A.M., Gomaa M.R., Kandeil A., Al-karmalawy A.A., Ali M.A., Mostafa A. Delineating a potent antiviral activity of Cuphea ignea extract loaded nano-formulation against SARS-CoV-2: in silico and in vitro studies. J Drug Deliv Sci Technol. 2021;66:102845. doi: 10.1016/j.jddst.2021.102845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Duarte J.L., Bezerra D.C., da Conceição E.C., Mourão R.H.V., Fernandes C.P. Self-nano-emulsification of chamomile essential oil: a novel approach for a high value phytochemical. Colloids Interface Sci. Commun. 2020;34:100225. doi: 10.1016/j.colcom.2019.100225. [DOI] [Google Scholar]
- 176.Hwang Y.Y., Ramalingam K., Bienek D.R., Lee V., You T., Alvarez R. Antimicrobial activity of nanoemulsion in combination with cetylpyridinium chloride in multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2013;57(8):3568–3575. doi: 10.1128/AAC.02109-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tiwari N., Ebenazer A., Franklyne J.S., Sivakumar A., Mukherjee A., Chandrasekaran N. Drug loaded essential oil microemulsions enhance photostability and evaluation of in vitro efficacy. Photodiagn. Photodyn. Ther. 2020;29:101638. doi: 10.1016/j.pdpdt.2019.101638. [DOI] [PubMed] [Google Scholar]
- 178.Chuesiang P., Siripatrawan U., Sanguandeekul R., McClements D.J., McLandsborough L. Antimicrobial activity of PIT-fabricated cinnamon oil nanoemulsions: effect of surfactant concentration on morphology of foodborne pathogens. Food Control. 2019;98:405–411. doi: 10.1016/j.foodcont.2018.11.024. [DOI] [Google Scholar]
- 179.Nabila N., Hassan S.R., Larasati G.P., Yohan B., Sasmono R.T., Adi A.C., Iskandar F., Rachmawati H. The influence of surface charge on the antiviral effect of curcumin loaded in Nanocarrier system. Pharm Nanotechnol. 2021;9(3):210–216. doi: 10.2174/2211738509666210204121258. [DOI] [PubMed] [Google Scholar]
- 180.Prabhakar K., Afzal S.M., Surender G., Kishan V. Tween 80 containing lipid nanoemulsions for delivery of indinavir to brain. Acta Pharm. Sin. B. 2013;3(5):345–353. doi: 10.1016/j.apsb.2013.08.001. [DOI] [Google Scholar]
- 181.Bonfim C.M.D., Monteleoni L.F., Calmon M.D.F., Cândido N.M., Provazzi P.J.S., Lino V.D.S., Rabachini T., Sichero L., Villa L.L., Quintana S.M., Melli P.P.D.S., Primo F.L., Amantino C.F., Tedesco A.C., Boccardo E., Rahal P. Antiviral activity of curcumin-nanoemulsion associated with photodynamic therapy in vulvar cell lines transducing different variants of HPV-16. Artif Cells Nanomed Biotechnol. 2020;48(1):515–524. doi: 10.1080/21691401.2020.1725023. [DOI] [PubMed] [Google Scholar]
- 182.Hosny K.M., Sindi A.M., Alkhalidi H.M., Kurakula M., Alruwaili N.K., Alhakamy N.A., Abualsunun W.A., Bakhaidar R.B., Bahmdan R.H., Rizg W.Y., Ali S.A., Abdulaal W.H., Nassar M.S., Alsuabeyl M.S., Alghaith A.F., Alshehri S. Oral gel loaded with penciclovir–lavender oil nanoemulsion to enhance bioavailability and alleviate pain associated with herpes labialis. Drug Deliv. 2021;28(1):1043–1054. doi: 10.1080/10717544.2021.1931561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.O'Konek J.J., Makidon P.E., Landers J.J., Cao Z., Malinczak C.-A., Pannu J., Sun J., Bitko V., Ciotti S., Hamouda T., Wojcinski Z.W., Lukacs N.W., Fattom A., Baker J.R. Intranasal nanoemulsion-based inactivated respiratory syncytial virus vaccines protect against viral challenge in cotton rats. Hu Vaccin Immunother. 2015;11(12):2904–2912. doi: 10.1080/21645515.2015.1075680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mahajan H.S., Mahajan M.S., Nerkar P.P., Agrawal A. Nanoemulsion-based intranasal drug delivery system of saquinavir mesylate for brain targeting. Drug Deliv. 2014;21(2):148–154. doi: 10.3109/10717544.2013.838014. [DOI] [PubMed] [Google Scholar]
- 185.Furuta Y., Komeno T., Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Pro Jap Acad Ser B Phys Biol Sci. 2017;93(7):449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J., Liao X., Gu Y., Cai Q., Yang Y. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering (Beijing). 2020;6(10):1192–1198. doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kylie M., Wagstaff H. Sivakumaran, Heaton Steven M., Harrich D., Jans David A. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J. 2012;443(3):851–856. doi: 10.1042/BJ20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chan J.F.-W., Yao Y., Yeung M.-L., Deng W., Bao L., Jia L., Li F., Xiao C., Gao H., Yu P. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J. Infect. Dis. 2015;212(12):1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yao T.T., Qian J.D., Zhu W.Y., Wang Y., Wang G.Q. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus—A possible reference for coronavirus disease-19 treatment option. J Med Virol. 2020;92(6):556–563. doi: 10.1002/jmv.25729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases. Lancet Infect. Dis. 2003;3(11):722–727. doi: 10.1016/s1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Seidah N.G., Nichol S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rhew D.C., Bernal M., Aguilar D., Iloeje U., Goetz M.B. Association between protease inhibitor use and increased cardiovascular risk in patients infected with human immunodeficiency virus: a systematic review. Clin. Infect. Dis. 2003;37(7):959–972. doi: 10.1086/378064. [DOI] [PubMed] [Google Scholar]
- 193.Gupta S., Knight A.G., Losso B.Y., Ingram D.K., Keller J.N., Bruce-Keller A.J. Brain injury caused by HIV protease inhibitors: role of lipodystrophy and insulin resistance. Antivir. Res. 2012;95(1):19–29. doi: 10.1016/j.antiviral.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Braga C.B.E., Martins A.C., Cayotopa A.D.E., Klein W.W., Schlosser A.R., da Silva A.F., de Souza M.N., Andrade B.W.B., Filgueira-Júnior J.A., Pinto W.D.J., da Silva-Nunes M. Side effects of chloroquine and primaquine and symptom reduction in malaria endemic area (Mâncio Lima, Acre, Brazil) Interdiscip Perspect Infect Dis. 2015;2015:346853. doi: 10.1155/2015/346853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Argenta D.F., Bidone J., Koester L.S., Bassani V.L., Simões C.M.O., Teixeira H.F. Topical delivery of Coumestrol from lipid Nanoemulsions thickened with Hydroxyethylcellulose for Antiherpes treatment. AAPS PharmSciTech. 2018;19(1):192–200. doi: 10.1208/s12249-017-0828-8. [DOI] [PubMed] [Google Scholar]
- 196.Szunerits S., Barras A., Khanal M., Pagneux Q., Boukherroub R. Nanostructures for the inhibition of viral infections. Molecules. 2015;20(8):14051–14081. doi: 10.3390/molecules200814051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hartshorn K.L. Role of surfactant protein a and D (SP-A and SP-D) in human antiviral host defense. Front. Biosci. 2010;2:527–546. doi: 10.2741/s83. [DOI] [PubMed] [Google Scholar]
- 198.Guagliardo R., Pérez-Gil J., De Smedt S., Raemdonck K. Pulmonary surfactant and drug delivery: focusing on the role of surfactant proteins. J. Control. Release. 2018;291:116–126. doi: 10.1016/j.jconrel.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 199.Zeng B., Chuan Y.P., O'sullivan B., Caminschi I., Lahoud M., Thomas R., Middelberg A. Receptor-specific delivery of protein antigen to dendritic cells by a nanoemulsion formed using top-down non-covalent click self-assembly. Small. 2013;9(22):3736–3742. doi: 10.1002/smll.201300078. [DOI] [PubMed] [Google Scholar]
- 200.Raha S., Mallick R., Basak S., Duttaroy A.K. Is copper beneficial for COVID-19 patients? Med. Hypotheses. 2020;142:109814. doi: 10.1016/j.mehy.2020.109814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Janjic J.M., Vasudeva K., Saleem M., Stevens A., Liu L., Patel S., Pollock J.A. Low-dose NSAIDs reduce pain via macrophage targeted nanoemulsion delivery to neuroinflammation of the sciatic nerve in rat. J. Neuroimmunol. 2018;318:72–79. doi: 10.1016/j.jneuroim.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Azambuja J., Schuh R., Michels L., Gelsleichter N., Beckenkamp L., Iser I., Lenz G., De Oliveira F., Venturin G., Greggio S. Nasal administration of cationic nanoemulsions as CD73-siRNA delivery system for glioblastoma treatment: a new therapeutical approach. Mol. Neurobiol. 2020;57(2):635–649. doi: 10.1007/s12035-019-01730-6. [DOI] [PubMed] [Google Scholar]
- 203.Torrinhas R.S., Calder P.C., Waitzberg D.L. Response to Bistrian BR. Parenteral Fish-Oil Emulsions in Critically Ill COVID-19 Emulsions. JPEN. J Parenter Enteral Nutr. 2020;44(7):1169–1170. doi: 10.1002/jpen.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Akkam Y., Rababah T., Costa R., Almajwal A., Feng H., Laborde J.E.A., Abulmeaty M.M., Razak S. Pea protein Nanoemulsion effectively stabilizes vitamin D in food products: a potential supplementation during the COVID-19 pandemic. J. Nanomater. 2021;11(4):887. doi: 10.3390/nano11040887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Azarmi S., Roa W.H., Löbenberg R. Targeted delivery of nanoparticles for the treatment of lung diseases. Adv. Drug Deliv. Rev. 2008;60(8):863–875. doi: 10.1016/j.addr.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 206.Martin A.R., Finlay W.H. Nebulizers for drug delivery to the lungs. Expert Opin Drug Deliv. 2015;12(6):889–900. doi: 10.1517/17425247.2015.995087. [DOI] [PubMed] [Google Scholar]
- 207.Nasr M., Nawaz S., Elhissi A. Amphotericin B lipid nanoemulsion aerosols for targeting peripheral respiratory airways via nebulization. Int. J. Pharm. 2012;436(1–2):611–616. doi: 10.1016/j.ijpharm.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 208.Zhuang J., Ying M., Spiekermann K., Holay M., Zhang Y., Chen F., Gong H., Lee J.H., Gao W., Fang R.H., Zhang L. Biomimetic Nanoemulsions for oxygen delivery in vivo. Adv. Mater. 2018;30(49):1804693. doi: 10.1002/adma.201804693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.A.H. Association FDA Authorizes Propofol Emulsion for Emergency Use for COVID-19 Ventilator Sedation. 2021. https://www.aha.org/news/headline/2020-05-12-fda-authorizes-propofol-emulsion-emergency-use-covid-19-ventilator (Accessed 28 May 2021)
- 210.Lambert E., Gorantla V.S., Janjic J.M. Pharmaceutical design and development of perfluorocarbon nanocolloids for oxygen delivery in regenerative medicine. Nanomedicine (Lond). 2019;14(20):2697–2712. doi: 10.2217/nnm-2019-0260. [DOI] [PubMed] [Google Scholar]
- 211.Zhou G., Zhao Q. Perspectives on therapeutic neutralizing antibodies against the novel coronavirus SARS-CoV-2. Int. J. Biol. Sci. 2020;16(10):1718–1723. doi: 10.7150/ijbs.45123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Cohen J. Vaccine designers take first shots at COVID-19. Science. 2020;368:14–16. doi: 10.1126/science.368.6486.14. [DOI] [PubMed] [Google Scholar]
- 213.Chen W.-H., Strych U., Hotez P.J., Bottazzi M.E. The SARS-CoV-2 vaccine pipeline: an overview. Curr. Trop. Med. Rep. 2020:1–4. doi: 10.1007/s40475-020-00201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Al-Halifa S., Gauthier L., Arpin D., Bourgault S., Archambault D. Nanoparticle-based vaccines against respiratory viruses. Front. Immunol. 2019;10:22. doi: 10.3389/fimmu.2019.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Maruggi G., Zhang C., Li J., Ulmer J.B., Yu D. mRNA as a transformative technology for vaccine development to control infectious diseases. Mol. Ther. 2019;27(4):757–772. doi: 10.1016/j.ymthe.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.O’Hagan D.T., Podda A. In: Influenza Vaccines for the Future. Rappuoli R., Del Giudice G., editors. Birkhäuser Basel; Basel: 2008. MF59: A safe and potent oil in water emulsion adjuvant for influenza vaccines, which induces enhanced protection against virus challenge; pp. 221–244. [Google Scholar]
- 217.Cao W., Davis W.G., Kim J.H., Juan A., Taylor A., Hendrickson G.R., Kumar A., Ranjan P., Lyon L.A., Katz J.M. An oil-in-water nanoemulsion enhances immunogenicity of H5N1 vaccine in mice, nanomedicine: nanotechnology. Biol. Med. 2016;12(7):1909–1917. doi: 10.1016/j.nano.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Shah P., Bhalodia D., Shelat P. Nanoemulsion: a pharmaceutical review. Syst. Rev. Pharm. 2010;1(1):24–32. doi: 10.4103/0975-8453.59509. [DOI] [Google Scholar]
- 219.Reed S.G., Bertholet S., Coler R.N., Friede M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009;30(1):23–32. doi: 10.1016/j.it.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 220.O’Hagan D.T., Ott G.S., Nest G.V., Rappuoli R., Giudice G.D. The history of MF59® adjuvant: a phoenix that arose from the ashes. Expert Rev. Vaccines. 2013;12(1):13–30. doi: 10.1586/erv.12.140. [DOI] [PubMed] [Google Scholar]
- 221.Yang Y., Chen L., Sun H.-w., Guo H., Song Z., You Y., Yang L.-y., Tong Y.-n., Gao J.-n., Zeng H. Epitope-loaded nanoemulsion delivery system with ability of extending antigen release elicits potent Th1 response for intranasal vaccine against Helicobacter pylori. J. Nanobiotechnology. 2019;17(1):1–15. doi: 10.1186/s12951-019-0441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Brito L.A., Chan M., Shaw C.A., Hekele A., Carsillo T., Schaefer M., Archer J., Seubert A., Otten G.R., Beard C.W. A cationic nanoemulsion for the delivery of next-generation RNA vaccines. Mol. Ther. 2014;22(12):2118–2129. doi: 10.1038/mt.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Makidon P.E., Bielinska A.U., Nigavekar S.S., Janczak K.W., Knowlton J., Scott A.J., Mank N., Cao Z., Rathinavelu S., Beer M.R. Pre-clinical evaluation of a novel nanoemulsion-based hepatitis B mucosal vaccine. PLoS One. 2008;3(8) doi: 10.1371/journal.pone.0002954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hamouda T., Sutcliffe J.A., Ciotti S., Baker J.R. Intranasal immunization of ferrets with commercial trivalent influenza vaccines formulated in a nanoemulsion-based adjuvant. Clin. Vaccine Immunol. 2011;18(7):1167–1175. doi: 10.1128/CVI.00035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lindell D.M., Morris S.B., White M.P., Kallal L.E., Lundy P.K., Hamouda T., Baker J.R., Jr., Lukacs N.W. A novel inactivated intranasal respiratory syncytial virus vaccine promotes viral clearance without Th2 associated vaccine-enhanced disease. PLoS One. 2011;6(7) doi: 10.1371/journal.pone.0021823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Samsa M.M., Dupuy L.C., Beard C.W., Six C.M., Schmaljohn C.S., Mason P.W., Geall A.J., Ulmer J.B., Yu D. Self-amplifying RNA vaccines for venezuelan equine encephalitis virus induce robust protective immunogenicity in mice. Mol. Ther. 2019;27(4):850–865. doi: 10.1016/j.ymthe.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chuan Y.P., Zeng B.Y., O’Sullivan B., Thomas R., Middelberg A.P. Co-delivery of antigen and a lipophilic anti-inflammatory drug to cells via a tailorable nanocarrier emulsion. J. Colloid Interface Sci. 2012;368(1):616–624. doi: 10.1016/j.jcis.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 228.Khachane P.V., Jain A.S., Dhawan V.V., Joshi G.V., Date A.A., Mulherkar R., Nagarsenker M.S. Cationic nanoemulsions as potential carriers for intracellular delivery. Saudi Pharm J. 2015;23(2):188–194. doi: 10.1016/j.jsps.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Aswathanarayan J.B., Vittal R.R. Nanoemulsions and their potential applications in food industry. Front. Sustain. Food Syst. 2019;3(95) doi: 10.3389/fsufs.2019.00095. [DOI] [Google Scholar]
- 230.Tian Y., Chen L., Zhang W. Influence of ionic surfactants on the properties of nanoemulsions emulsified by nonionic surfactants span 80/tween 80. J. Dispers. Sci. Technol. 2016;37(10):1511–1517. doi: 10.1080/01932691.2015.1048806. [DOI] [Google Scholar]
- 231.Allison A., Gregoriadis G. Liposomes as immunological adjuvants. Nature. 1974;252(5480):252. doi: 10.1038/252252a0. [DOI] [PubMed] [Google Scholar]
- 232.Chapel H., August P. Report of nine cases of accidental injury to man with Freund's complete adjuvant. Clin. Exp. Immunol. 1976;24(3):538. [PMC free article] [PubMed] [Google Scholar]
- 233.Kelly G.S. Squalene and its potential clinical uses. Altern. Med. Rev. 1999;4(1):29–36. [PubMed] [Google Scholar]
- 234.Allison A.C. Squalene and squalane emulsions as adjuvants. Methods. 1999;19(1):87–93. doi: 10.1006/meth.1999.0832. [DOI] [PubMed] [Google Scholar]
- 235.Carter N.J., Plosker G.L. Prepandemic influenza vaccine H5N1 (split virion, inactivated, adjuvanted)[Prepandrix™] BioDrugs. 2008;22(5):279–292. doi: 10.2165/00063030-200822050-00001. [DOI] [PubMed] [Google Scholar]
- 236.O'Hagan D.T. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev. Vaccines. 2007;6(5):699–710. doi: 10.1586/14760584.6.5.699. [DOI] [PubMed] [Google Scholar]
- 237.Chappell K.J., Mordant F.L., Li Z., Wijesundara D.K., Ellenberg P., Lackenby J.A., Cheung S.T.M., Modhiran N., Avumegah M.S., Henderson C.L., Hoger K., Griffin P., Bennet J., Hensen L., Zhang W., Nguyen T.H.O., Marrero-Hernandez S., Selva K.J., Chung A.W., Tran M.H., Tapley P., Barnes J., Reading P.C., Nicholson S., Corby S., Holgate T., Wines B.D., Hogarth P.M., Kedzierska K., Purcell D.F.J., Ranasinghe C., Subbarao K., Watterson D., Young P.R., Munro T.P. Safety and immunogenicity of an MF59-adjuvanted spike glycoprotein-clamp vaccine for SARS-CoV-2: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect Dis. 2021;21(10):1383–1394. doi: 10.1016/S1473-3099(21)00200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Long P., Zhang Q., Xue M., Cao G., Li C., Chen W., Jin F., Li Z., Li R., Wang X., Ge W. Tomato lectin-modified nanoemulsion-encapsulated MAGE1-HSP70/SEA complex protein vaccine: targeting intestinal M cells following peroral administration. Biomed. Pharmacother. 2019;115:108886. doi: 10.1016/j.biopha.2019.108886. [DOI] [PubMed] [Google Scholar]
- 239.Bernstein D.I., Cardin R.D., Bravo F.J., Hamouda T., Pullum D.A., Cohen G., Bitko V., Fattom A. Intranasal nanoemulsion-adjuvanted HSV-2 subunit vaccine is effective as a prophylactic and therapeutic vaccine using the Guinea pig model of genital herpes. Vaccine. 2019;37(43):6470–6477. doi: 10.1016/j.vaccine.2019.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Smith D., Streatfield S.J., Acosta H., Ganesan S., Fattom A. A nanoemulsion-adjuvanted intranasal H5N1 influenza vaccine protects ferrets against homologous and heterologous H5N1 lethal challenge. Vaccine. 2019;37(42):6162–6170. doi: 10.1016/j.vaccine.2019.08.071. [DOI] [PubMed] [Google Scholar]
- 241.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Arbain N.H., Basri M., Salim N., Wui W.T., Abdul Rahman M.B. Development and characterization of aerosol nanoemulsion system encapsulating low water soluble quercetin for lung cancer treatment. Mater. Today: Proc. 2018;5:S137–S142. doi: 10.1016/j.matpr.2018.08.055. [DOI] [Google Scholar]
- 243.Szalay S.F.D. 2013. Skin Sanitizer Compositions Comprising Alcohol Based Emulsion. [Google Scholar]
- 244.Franklyne J.S., Gopinath P.M., Mukherjee A., Chandrasekaran N. Nanoemulsions: the rising star of antiviral therapeutics and nanodelivery system. Curr Opin Colloid Interface Sci. 2021;54:101458. doi: 10.1016/j.cocis.2021.101458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Chan W.C. Nano research for COVID-19. ACS Nano. 2020;14(4):3719–3720. doi: 10.1021/acsnano.0c02540. [DOI] [PubMed] [Google Scholar]
- 246.Tao J., Zhang J., Du T., Xu X., Deng X., Chen S., Liu J., Chen Y., Liu X., Xiong M., Luo Y., Cheng H., Mao J., Cardon L., Gou M., Wei Y. Rapid 3D printing of functional nanoparticle-enhanced conduits for effective nerve repair. Acta Biomater. 2019;90:49–59. doi: 10.1016/j.actbio.2019.03.047. [DOI] [PubMed] [Google Scholar]
- 247.Zhu W., Webster T.J., Zhang L.G. How can 3D printing be a powerful tool in nanomedicine? Nanomedicine (Lond). 2018;13(3):251–253. doi: 10.2217/nnm-2017-0369. [DOI] [PubMed] [Google Scholar]
- 248.O’Brien C.M., Holmes B., Faucett S., Zhang L.G. Three-dimensional printing of nanomaterial scaffolds for complex tissue regeneration. Tissue Eng. Part B Rev. 2015;21(1):103–114. doi: 10.1089/ten.TEB.2014.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Liaw C.Y., Guvendiren M. Current and emerging applications of 3D printing in medicine. Biofabrication. 2017;9(2) doi: 10.1088/1758-5090/aa7279. [DOI] [PubMed] [Google Scholar]
- 250.Hsiao L.C., Badruddoza A.Z.M., Cheng L.-C., Doyle P.S. 3D printing of self-assembling thermoresponsive nanoemulsions into hierarchical mesostructured hydrogels. Soft Matter. 2017;13(5):921–929. doi: 10.1039/C6SM02208A. [DOI] [PubMed] [Google Scholar]