Abstract
The interferon (IFN)-inducible double-stranded-RNA (dsRNA)-activated serine-threonine protein kinase (PKR) is a major mediator of the antiviral and antiproliferative activities of IFNs. PKR has been implicated in different stress-induced signaling pathways including dsRNA signaling to nuclear factor kappa B (NF-κB). The mechanism by which PKR mediates activation of NF-κB is unknown. Here we show that in response to poly(rI) · poly(rC) (pIC), PKR activates IκB kinase (IKK), leading to the degradation of the inhibitors IκBα and IκBβ and the concomitant release of NF-κB. The results of kinetic studies revealed that pIC induced a slow and prolonged activation of IKK, which was preceded by PKR activation. In PKR null cell lines, pIC failed to stimulate IKK activity compared to cells from an isogenic background wild type for PKR in accord with the inability of PKR null cells to induce NF-κB in response to pIC. Moreover, PKR was required to establish a sustained response to tumor necrosis factor alpha (TNF-α) and to potentiate activation of NF-κB by cotreatment with TNF-α and IFN-γ. By coimmunoprecipitation, PKR was shown to be physically associated with the IKK complex. Transient expression of a dominant negative mutant of IKKβ or the NF-κB-inducing kinase (NIK) inhibited pIC-induced gene expression from an NF-κB-dependent reporter construct. Taken together, these results demonstrate that PKR-dependent dsRNA induction of NF-κB is mediated by NIK and IKK activation.
The interferon (IFN)-inducible double-stranded-RNA (dsRNA)-activated serine-threonine protein kinase (PKR) is a major mediator of the antiviral and antiproliferative activities of IFNs (11, 12, 19, 29, 34, 38, 53, 55, 70, 75, 77). This ubiquitously expressed kinase is normally inactive but undergoes a conformational change upon binding of its activator, dsRNA, that leads to autophosphorylation and subsequent dsRNA-independent phosphorylation of substrates (7, 71). To date, the alpha subunit of the initiation factor eIF-2 (eIF-2α) is the best-characterized substrate for PKR (16). Indeed, the antiviral effect of PKR is in part mediated through phosphorylation of eIF-2α which results in the sequestration of the recycling factor eIF-2B in an inactive complex together with eIF-2–GDP (33, 44, 64). The net effect is inhibition of protein synthesis. In addition to its role as a regulator of translation, PKR is involved in control of cell proliferation (11, 14, 36, 65–68, 77), differentiation (74), tumor suppression (3, 28, 37, 54), apoptosis (20, 35, 43, 85, 99), and cell cycle progression (101). PKR is also a signaling molecule and a regulator of transcription (12, 80). A PKR inhibitor, 2-aminopurine (2-AP), blocked the induction of c-fos, c-myc, and JE by platelet-derived growth factor. The induction of these genes was also repressed in cells expressing an oncogenic form of the ras gene which induces a cytoplasmic inhibitor of PKR (57, 58). In cells expressing dominant negative forms of PKR or derived from PKR knockout mice, induction of interferon regulatory factor 1 (IRF-1) or guanylate-binding protein (GBP) promoter-reporter gene constructs by IFN-γ or dsRNA were defective, implicating PKR in these signaling pathways (40). This defect was attributed to a diminished activation of IRF-1 and NF-κB DNA binding activity in response to IFN-γ or dsRNA in cells devoid of PKR. Activation of macrophages by lipopolysaccharide (LPS) has also been reported to require PKR (27). Furthermore, PKR null cells failed to activate IRF-1 in response to LPS or tumor necrosis factor alpha (TNF-α) (20). In addition to inhibiting protein synthesis, PKR has recently been shown to restrict cellular proliferation through interaction with p53 (15), enhancing the transcriptional activity of this stress-responsive tumor suppressor protein (14). Although dsRNA functions as an immediate upstream activator of PKR, little is known of upstream regulators of PKR in signaling pathways, and direct downstream targets remain to be identified.
NF-κB is a dimeric transcription factor composed of members of the Rel family. In mammals, these proteins include p50 (NF-κB1), p52 (NF-κB2), p65 (RelA), RelB, c-Rel, p105, and p100 (2, 26, 56). These proteins share homology within a 300-amino-acid Rel homology domain, which mediates homo- and heterodimerization, DNA binding activity, and nuclear localization. A large number of stimuli including proinflammatory cytokines, antigen stimulation of T and B cells, bacterial LPS, UV irradiation, ionizing radiation, viral infection, phorbol esters, and reactive oxygen intermediates can activate NF-κB and its target genes. These target genes include those involved in the immune response (immunoglobulin light chains κ, interleukin-2 [IL-2], and IL-2 receptor α), inflammatory response (TNF-α and -β, IL-1, and IL-6), cell adhesion (I-CAM, V-CAM, and E-selectin), cell growth (p53, Ras, and c-Myc), and apoptosis (TNF receptor-associated factor 1 [TRAF1], TRAF2, cellular inhibitor of apoptosis protein 1 [cIAP1], and cIAP2). The activity of NF-κB is regulated at two levels: DNA binding and transactivation. The DNA binding activity is tightly regulated by a family of inhibitory proteins, IκBs, that sequester NF-κB in the cytoplasm of unstimulated cells (95). IκBs retain NF-κB in the cytosol through the interaction of their ankyrin repeat domain with the Rel homology domain of NF-κB, thus masking the nuclear localization signal and the DNA binding domain. To date, three separate pathways have been identified leading to either degradation or dissociation of IκB. Most NF-κB-inducing signals trigger a cascade of events resulting in the phosphorylation of IκB on two critical serine residues (serines 32 and 36 in IκBα, serines 19 and 23 in IκBβ, and serines 18 and 22 in IκBɛ [6, 81, 87, 94]), initiating polyubiquitination and subsequent degradation by the 26S proteasome (6, 8, 24). The phosphorylation of IκBs is mediated by a large IκB kinase (IKK) complex. UV-C radiation (254-nm-wavelength) signaling to NF-κB also results in an ubiquitin-mediated proteasomal degradation of IκBα; however, this process is not dependent on phosphorylation on serines 32 and 36 and thus is independent of IKK activity (46). In contrast, tyrosine phosphorylation at residue 42 in IκBα which occurs in reoxygenated hypoxic cells or in cells treated with pervanadate induces the dissociation of the inhibitor from NF-κB rather than its degradation (5, 31). The regulatory subunit (p85α) of phosphoinositide 3-kinase (PI3-kinase) stably interacts with tyrosine-phosphorylated IκBα, providing a potential mechanism for sequestering tyrosine-phosphorylated IκBα from NF-κB; however, other IκB proteins lack a site homologous to tyrosine 42 in IκBα (5). Phosphorylation of p65 in its carboxy (C) terminus increases the transcriptional activity of the protein (59, 76, 93, 104). Treatment of cells with LPS leads to phosphorylation on serine residue 276 of p65 by protein kinase A, and this phosphorylation strengthens the interaction between p65 and the transcriptional coactivators CBP (CREB-binding protein) in the nucleus (104). It has recently been reported that in response to IL-1, PI3-kinase induces phosphorylation of the p65/RelA subunit, thus enhancing NF-κB transcriptional activity (78).
IKK is the immediate upstream effector kinase phosphorylating critical serines in IκBs in response to a number of NF-κB-inducing stimuli (21, 42, 52, 69, 96, 102). IKK is a large (>700-kDa) multicomponent enzyme complex containing two closely related kinase subunits with identical structural domains, IKKα (IKK1) and IKKβ (IKK2), which exist as a heterodimer. In addition, the enzyme complex comprises at least two accessory proteins, IKKγ and IKAP. IKKγ (also referred to as NEMO [NF-κB essential modulator] or IKKAP1 [IKK-associated protein 1]), although devoid of a catalytic kinase domain, is essential for linking upstream signals to IKK with a preference for binding to IKKβ (51, 72, 97). IKAP (IKK complex-associated protein) is believed to have a scaffolding property required for the proper assembly of IKK and binds equally well to both IKKα and IKKβ (13). Recent gene knockout and mutagenesis experiments have established clear and distinct roles for IKKα and IKKβ (17, 30, 47, 84). Whereas IKKβ is the kinase subunit responsible for the phosphorylation of IκBs in response to proinflammatory cytokines, such as TNF-α and to a lesser extent IL-1 (17, 47), IKKα responds to as yet unknown morphogenic signals and is crucial for NF-κB activation during embryonic development of the skin and skeletal systems (30, 84). IKKα and IKKβ are both activated by phosphorylation on specific serine residues (serines 176 and 180 in IKKα [49]; serines 177 and 181 in IKKβ [17, 52]). Two members of the MAP kinase family, NIK (NF-κB-inducing kinase) and MEKK1 (mitogen-activated protein/extracellular signal-regulated kinase kinase 1), have been shown to directly interact with IKK (13, 52, 69) and activate the kinase subunits (48, 50, 60, 61, 83, 100). Signals initiated by IL-1, T-cell receptor engagement (CD3/CD28 induction), TNF-α, CD95, Epstein-Barr virus (EBV) latent-infection membrane protein 1 (LMP1), converge on NIK (48, 50, 61, 83). NF-κB induction by the transforming protein (Tax) of human T-cell leukemia virus type 1 is mediated through MEKK1 (100). Additionally, TNF-α and IL-1 have been shown to activate and utilize both NIK and MEKK1 coordinately and synergistically (60). It is important to note that the conclusions that NIK and MEKK1 may be upstream activators of IKK were based on overexpression studies with recombinant proteins and may not necessarily be relevant under physiological conditions. Although bacterial LPS has been shown to activate IKKβ (62), the upstream kinases in this signaling pathway remain to be identified.
Given the importance of NF-κB as a stress-inducible molecule and the central role of PKR in the host defense system, we were interested in elucidating the mechanism by which PKR activates NF-κB. In this paper, we have investigated the role of PKR in the activation of NF-κB by dsRNA and identified NIK and IKK as transducers of PKR-mediated signaling to IκB.
MATERIALS AND METHODS
DNA plasmid constructs.
The luciferase reporter plasmid, pTK81κB2, comprises five copies of the NK-κB2 element (63) from the IP-10 gene upstream of the TK gene promoter and was a kind gift from Yoshihiro Ohmori (Department of Immunology, Cleveland Clinic Foundation). Mutant IKKβ (AA) in which Ser-177 and -181 were replaced by alanine has been previously described (52). Mutant NIK (KK429/430AA) and the vector control, pRK5, were kind gifts from Zhaodan Cao (Tularik, Inc.) (49). Catalytically inactive mutant PKR (pRcCMVK296R) has been previously described (40). Wild-type Stat6 expression plasmid (pcDNA3Stat6) was a kind gift from Yoshihiro Ohmori. Glutathione S-transferase (GST)-IκBα (amino acids 1 to 54) expression plasmid has been previously described (21).
Cell culture and treatments.
T98G human glioblastoma cells (ATCC CRL 1690) and Pkr+/+ and Pkr0/0 cell lines were grown in high-glucose Dulbecco's modified Eagle's medium (DMEM) (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U of penicillin per ml, and 100 mg of streptomycin per ml. Pkr wild-type (Pkr+/+) and null (Pkr0/0) cells were immortalized by continuous subculturing of mouse embryo fibroblasts derived from littermates of C57BL/6 mice carrying homozygous wild-type or knockout alleles (98). To detect NF-κB DNA binding activity, Pkr+/+ and Pkr0/0 cell lines were seeded at a concentration of 106 2 days prior to induction and serum starved (0.3% FBS) overnight. Cells were washed with phosphate-buffered saline (PBS) prior to treatment with induction medium (serum-free DMEM with penicillin and streptomycin) containing 100 μg of poly(I) · poly(C) (pIC) (Sigma) per ml or 5 ng of murine TNF-α (Boehringer Mannheim) per ml. For synergistic activation of NF-κB, 0.025 ng of murine TNF-α per ml and 1,000 U of murine IFN-γ (Boehringer Mannheim) per ml were included in the induction medium. Induction of NF-κB in T98G cells was monitored as described previously (101).
Transfections.
A day before transfection, T98G cells were seeded at 1.2 × 106 or 0.5 × 106 per 100-mm- and 60-mm-diameter tissue culture plates, respectively. Transient transfections were performed using 12 to 50 μl of Lipofectamine reagent (Gibco) and a total of 1.5 to 6.5 μg of DNA for each sample. Each transfection mixture contained 1 to 5 μg of NF-κB luciferase reporter, pTK81κB2, 0.5 μg of Renilla luciferase reporter which served as an internal control for transfection efficiency, and different amounts (5 to 500 ng) of various expression plasmids. pBKS was used to equalize the amount of DNA transfected in each sample. Lipofectamine-DNA complexes were allowed to form for a total of 30 min in serum-free medium (Opti-MEM) before they were added to washed cells. Cells were incubated with the complexes for 3 h before DMEM and FBS were added to a final concentration of 10%. The entire mixture was replaced with complete growth medium the following day. Approximately 36 h after transfection, cells were washed and induced for 8 h with pIC (100 μg/ml) or TNF-α (5 to 10 ng/ml) as described above. Cells were washed twice with PBS, scraped into 400 μl of passive lysis buffer (dual-luciferase reporter assay kit; Promega) and subjected to three cycles of freezing and thawing before clearing at 18,000 × g for 5 min at 4°C. Lysates (30 μl) were processed for luciferase activity according to the manufacturer's instructions (Promega).
Immune complex kinase assays.
Cells derived from a ∼90% confluent 100-mm-diameter tissue culture dish were washed twice in PBS and the pellet was resuspended in 60 to 90 μl of immunoprecipitation (IP) lysis buffer (50 mM Tris-HCl [pH 7.6], 150 mM NaCl, 10% glycerol, 1% NP-40, 5 mM EDTA, 1 mM dithiothreitol, 100 mM NaF, 2 mM sodium pyrophosphate, 2 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 10 μg [each] of aprotinin and leupeptin per ml) and stored on ice for 20 min before clarification by centrifugation (18,000 × g, 20 min, 4°C). PKR was immunoprecipitated and subjected to in vitro kinase assay as described previously (101) except that monoclonal antibody-protein G-Sepharose mixtures were routinely incubated overnight at 4°C before washing. IKK complex was immunoprecipitated using an anti-IKKα antibody (Santa Cruz; SC-7218) and a procedure identical to PKR IPs except that the concentration of magnesium acetate and manganese chloride in DBGA buffer (101) and kinase assay buffer (101) were doubled to 4 and 1.7 mM, respectively. IKK kinase assay mixtures contained 0.5 μg of GST-IκBα (amino acids 1 to 54) as the substrate. Reaction mixtures were incubated for 30 min at 30°C and stopped by the addition of 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. Phosphoproteins were resolved on SDS–10% polyacrylamide gels and transferred onto Immobilon membranes. Following immunodetection of PKR with a polyclonal antibody (101), the blot was subjected to autoradiography once the enhanced chemiluminescence signal had decayed. IKKα protein was detected in human T98G cells and mouse Pkr+/+ and Pkr0/0 IPs using a monoclonal antibody (PharMingen catalogue no. 66781A; 1 in 2,000 dilution) and a polyclonal antibody (1 in 1,000) raised against an N-terminal peptide (amino acids 1 to 15) of human IKKα which cross-reacts with mouse IKKα (KSCN), respectively.
EMSA.
Cells were induced as described above (see “Cell culture and treatments”). Whole-cell extracts were prepared and processed for electrophoretic mobility shift assay (EMSA) as described previously (101) except that the probe was a consensus NF-κB binding site (Santa Cruz; SC-2505).
Western blotting.
IκBα and IκBβ Western blots were performed on 30- to 35-μg samples of whole-cell extracts as described previously (101) using anti-IκBα (Santa Cruz; SC-371) and anti-IκBβ (Santa Cruz; SC-945) rabbit polyclonal antibodies at 1 in 200 dilution. Blots were normalized by stripping and reprobing with an anti-α-actin antibody (Santa Cruz; SC-1615; 1 in 5,000).
Analysis of PKR and IKK association.
A day before transfection, 293T cells were seeded at a concentration of 106 per 60-mm-diameter tissue culture plates. Transient transfections were performed in a final volume of 500 μl using 12 μl of Lipofectamine reagent and 2 μg of an expression plasmid for a catalytically inactive mutant of PKR or wild-type Stat6 (pRcCMVPKRK296R and pcDNA3Stat6, respectively). Lipofectamine-DNA complexes were allowed to form for a total of 30 min in serum-free medium (DMEM) before being added to cells in 2 ml of DMEM. Cells were incubated with the complexes for 4 h before the entire mixture was replaced with normal growth medium (DMEM with 10% FBS and antibiotics). Approximately 24 h after transfection, cells were washed in PBS and extracts were prepared in 300 μl of IP lysis buffer as described above. IPs were performed on 1 mg of total protein using 1 μg of polyclonal antibodies against human IKKα (Santa Cruz; SC-7218) and human Stat6 (Santa Cruz; SC-1698) and a monoclonal antibody against human PKR (25). Normal rabbit immunoglobulin G (IgG) (Santa Cruz; SC-2027) was used as a negative control. IPs were allowed to proceed for 2 h at 4°C with constant rotation before adding protein G-Sepharose and leaving overnight under the same conditions. Immunoprecipitates were washed five times with IP lysis buffer before being subjected to Western blotting. Antibodies used for immunoblotting PKR (1 in 1,000 dilution) and Stat6 (1 in 400) were the same as those used for IP. To detect IKKα, the KSCN antibody described above was used.
RESULTS
Kinetics of PKR-dependent activation of NF-κB.
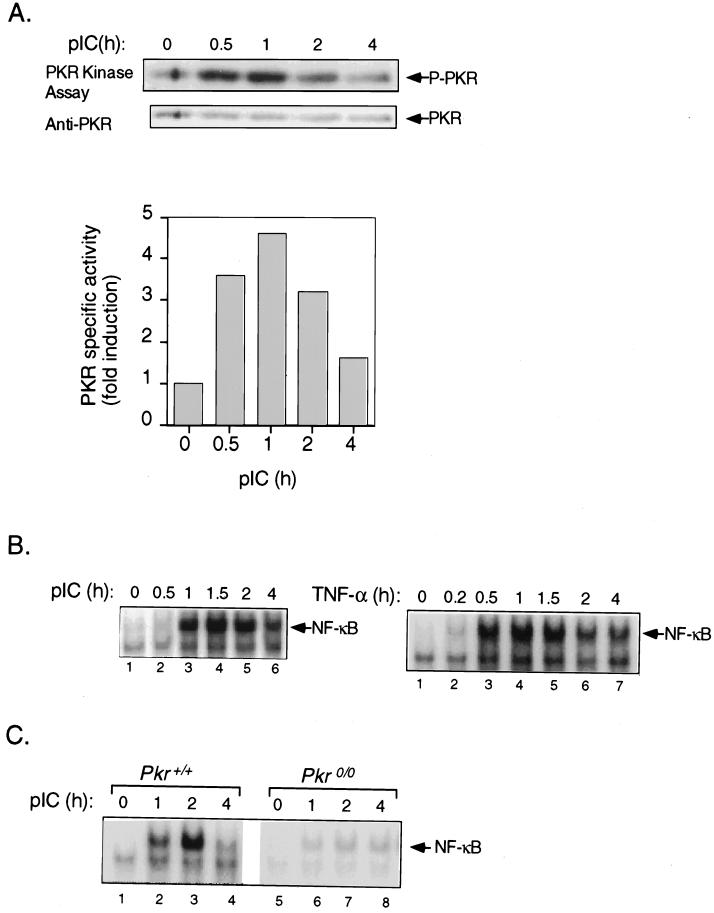
PKR autophosphorylation activity was determined in T98G cells stimulated with pIC following IP with a monoclonal antibody specific for human PKR. A 3.6-fold increase in PKR activity was detectable within 30 min of treatment, with pIC peaking at 1 h before declining to basal levels 4 h subsequent to treatment (Fig. 1A). Extracts were prepared in parallel, and the induction of NF-κB DNA binding activity was determined by EMSA. In response to pIC, NF-κB DNA binding activity was barely detectable at 30 min but was substantially increased by 1 h, peaking at 1.5 h and then declining (Fig. 1B, left gel). Induction of NF-κB by pIC was slower than the response elicited by TNF-α, where DNA binding was detected after 10 min of treatment, reaching a maximum by 30 min (Fig. 1B, right gel). There was no inducible activation of PKR in response to TNF-α at 30 min or later time points (data not shown). The response to pIC was tested in Pkr+/+ and Pkr0/0 cell lines derived from mouse embryo fibroblasts prepared from littermates with an isogenic background. Pkr+/+ cells showed a strong induction of NF-κB by pIC within 1 h, which increased further by 2 h of treatment and declined to very low levels at 4 h (Fig. 1C, lanes 1 to 4) similar to the kinetics observed in T98G cells (Fig. 1B, left gel). In contrast, in Pkr0/0 cells, there was only a minimal induction of NF-κB (Fig. 1C, lanes 5 to 8). The deficiency in induction of NF-κB in PKR null cells is specific to dsRNA signaling, since NF-κB was induced in Pkr0/0 cells to the same level as that in Pkr+/+ cells after 15 min of treatment with TNF-α (data not shown). These results indicated that there is no intrinsic defect in NF-κB in Pkr0/0 cells but that PKR plays a role in dsRNA signaling to NF-κB.
FIG. 1.
PKR is essential for activation of NF-κB by pIC. (A) T98G cells were treated with pIC (100 μg/ml) for the times indicated in the figure, and PKR was immunoprecipitated using a monoclonal antibody. The immune complex was subjected to in vitro kinase assay as described in Materials and Methods and analyzed by SDS-PAGE followed by Western blotting for PKR protein. The blot was subsequently subjected to autoradiography to detect PKR autophosphorylation. P-PKR, phosphorylated PKR. (B) T98G cells were treated with pIC (100 μg/ml) or TNF-α (5 ng/ml) for the indicated times, and whole-cell extracts were analyzed for NF-κB DNA binding activity by EMSA using 18 μg of protein and a radiolabeled oligonucleotide probe containing a consensus NF-κB binding site. (C) Cell lines derived from Pkr+/+ and Pkr0/0 mouse embryo fibroblasts with isogenic background were treated with pIC (100 μg/ml), and whole-cell extracts were analyzed for NF-κB DNA binding activity by EMSA as described above for panel B.
pIC induces PKR-dependent degradation of IκBα and IκBβ.
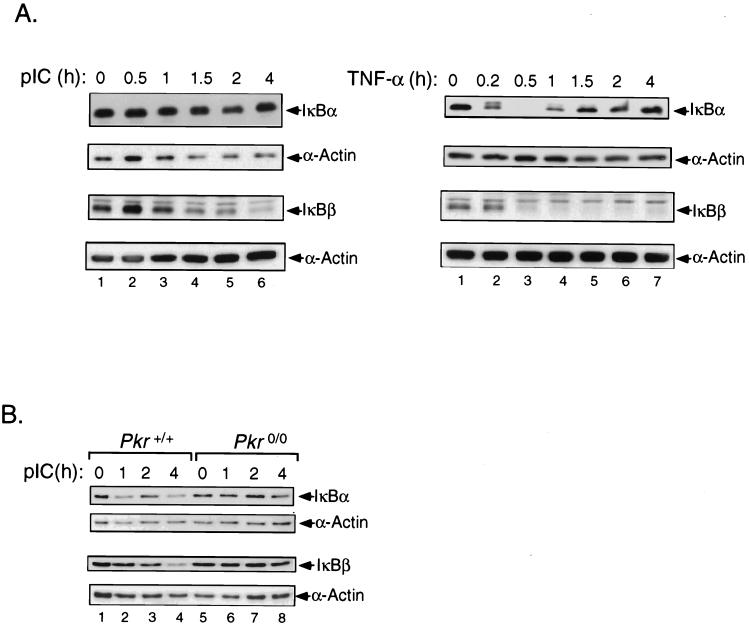
NF-κB is generally retained in the cytoplasm of unstimulated cells by interaction with IκBs. In response to most NF-κB-inducing signals, these inhibitors are targeted for specific phosphorylation and subsequent degradation resulting in the release of NF-κB. However, in some cases, IκB is dissociated from NF-κB without degradation (31). To measure IκB levels in pIC- or TNF-α-stimulated cells, Western blot analyses were performed on T98G extracts using antibodies against IκBα and IκBβ. TNF-α induction of T98G cells leads to the previously described rapid but transient disappearance of IκBα (Fig. 2A, right panel) (4). The slower-migrating form of IκBα at 10 min likely represents a phosphorylated species (Fig. 2A, lane 2). This profile of IκBα protein turnover is attributed to the positive autoregulatory feedback loop of IκBα gene by NF-κB itself (10, 18, 32, 82). IκBβ exhibited different kinetics of degradation in response to TNF-α (Fig. 2A, right panel). IκBβ protein levels declined after 30 min of treatment and remained low through the course of treatment. The delayed kinetics and sustained disappearance are characteristic for IκBβ, which does not contain any NF-κB response elements in its promoter. As expected, the pattern of TNF-α-induced IκB degradation correlated with the activation of NF-κB shown in Fig. 1B. In contrast, there was only a slight decline in the levels of IκBα in response to pIC (Fig. 2A, left panel) compared with TNF-α treatment. However, there was a marked decrease in IκBβ protein levels as early as 1 h of pIC treatment followed by a continued decline at later times (Fig. 2A, left panel). These results suggest that IκBβ is the major target for pIC-mediated degradation in T98G cells. This was confirmed by analyzing IκB protein levels in Pkr+/+ and Pkr0/0 cell lines following pIC treatment. In contrast to T98G cells, there was a noticeable decline in IκBα protein level after 1 h of treatment with levels increasing at 2 h (Fig. 2B) representing a transient nature. As for T98G cells, in Pkr+/+ cells treated with pIC, IκBβ protein levels declined steadily throughout the course of the experiment, confirming this protein as the major target for degradation in pIC signaling (Fig. 2B). To determine whether the targeted degradation of IκB in response to pIC was mediated by PKR, PKR null cells were treated with pIC and extracts were subjected to immunoblotting for IκB. In the absence of PKR, there was little change in the steady-state level of IκBβ or IκBα upon treatment of cells with pIC (Fig. 2B). These results are in accord with the inefficient activation of NF-κB by pIC in Pkr0/0 cells (Fig. 1C). Therefore, PKR is essential for the efficient induction of NF-κB DNA binding activity by pIC.
FIG. 2.
pIC-induced activation of NF-κB targets IκBα and IκBβ for degradation, an effect mediated by PKR. (A) T98G cells were treated with pIC (100 μg/ml) or TNF-α (5 ng/ml) for the times indicated over the lanes, and equal protein amounts from whole-cell extracts were analyzed by Western blotting using antibodies against IκBα and IκBβ. Blots were stripped and reprobed with an antibody against α-actin to normalize for loading. (B) Pkr+/+ and Pkr0/0 cell lines were treated with pIC (100 μg/ml) for the indicated times. Western blot analyses were performed as described above for panel A.
PKR is required for sustained and cooperative TNF-α signaling to NF-κB.
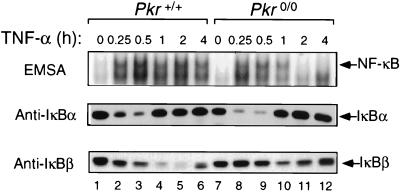
The above results suggest that PKR mediates sustained NF-κB activation. To determine whether PKR was involved in prolonging TNF-α signaling to NF-κB, PKR wild-type and null mouse cells were treated with TNF-α alone or together with IFN-γ and NF-κB activation measured by EMSA. TNF-α (5 ng/ml) in PKR wild-type cells induced NF-κB DNA binding activity within 15 min, peaking at 30 min and remaining high over the 4-h duration of cytokine treatment (Fig. 3, EMSA). In cells devoid of PKR, TNF-α induced NF-κB with identical initial kinetics; however, the signal was not sustained and declined to undetectable levels after 2 h (Fig. 3, compare lanes 5 and 6 with lanes 11 and 12). The inability of PKR null cells to sustain the TNF-α-induced activation of NF-κB was shown to correlate with the accumulation of IκBβ (Fig. 3, compare lanes 4 to 6 with lanes 10 to 12). In contrast, signal-induced IκBα degradation in Pkr0/0 cells was equivalent to that in wild-type cells (Fig. 3, compare lanes 2 and 3 with 8 and 9).
FIG. 3.
PKR is required for sustained TNF-α signaling to NF-κB. Cell lines derived from Pkr+/+ and Pkr0/0 mouse embryo fibroblasts with isogenic background were treated with TNF-α (5 ng/ml) for the times indicated at the top of the figure. Whole-cell extracts were subjected to EMSA for NF-κB DNA binding activity and Western blotting for IκBα and IκBβ protein levels.
A role for PKR in the synergistic activation of NF-κB by cotreatment with TNF-α and IFN-γ in cells of neuronal origin was recently reported and shown to be mediated through degradation of IκBβ (9). These data were generated using either the PKR inhibitor 2-AP or expression of a dominant negative mutant of PKR. To determine whether the absence of PKR resulted in a loss of cooperative NF-κB activation by TNF-α and IFN-γ, we used PKR null cells and compared their response to cotreatment with these two cytokines to that of the wild-type cells. At low TNF-α concentrations (0.025 ng/ml), nuclear translocation of NF-κB was elicited in PKR wild-type cells (Fig. 4, compare lane 1 to lane 2). There was a very slight but reproducible potentiation of this induction when IFN-γ was included in the induction medium (Fig. 4, compare lane 2 to lane 4), even though IFN-γ alone was unable to activate NF-κB (Fig. 4, compare lane 1 to lane 3) in these cells. In PKR null cells, there was no detectable induction of NF-κB by TNF-α alone (Fig. 4, compare lane 5 to lane 6) at the concentration of TNF-α used and cotreatment with IFN-γ resulted in low levels of NF-κB induction (Fig. 4, compare lane 6 to lane 8). Thus, in the absence of PKR, NF-κB induction in response to cotreatment with TNF-α and IFN-γ was reduced (Fig. 4, compare lane 4 to lane 8). Therefore, we conclude that PKR plays a role in the sustained activation of NF-κB by TNF-α and is required for the enhanced activation of NF-κB by IFN-γ in conjunction with TNF-α.
FIG. 4.
PKR is required for the enhanced activation of NF-κB by TNF-α and IFN-γ. Pkr+/+ and Pkr0/0 cell lines were treated with TNF-α (T) (0.025 ng/ml) or IFN-γ (I) (1,000 U/ml) or cotreated with both (T/I) for 1 h or not treated (−) (control). Whole-cell extracts were subjected to EMSA.
IKK is activated in response to pIC.
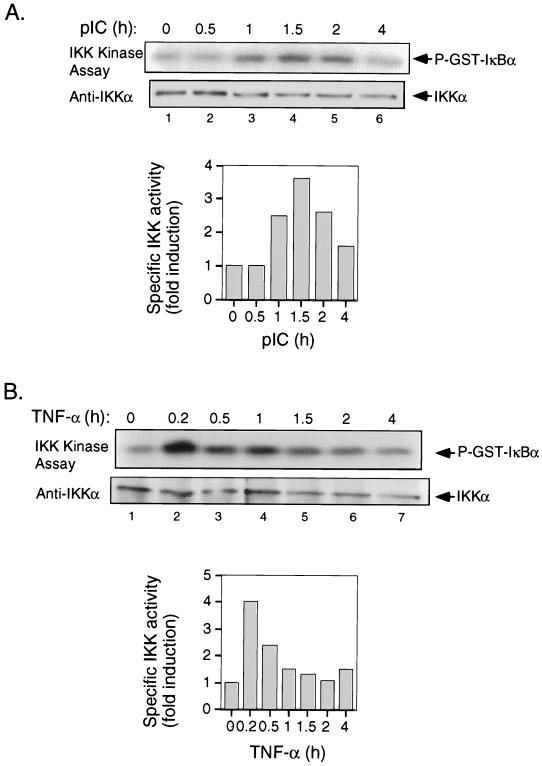
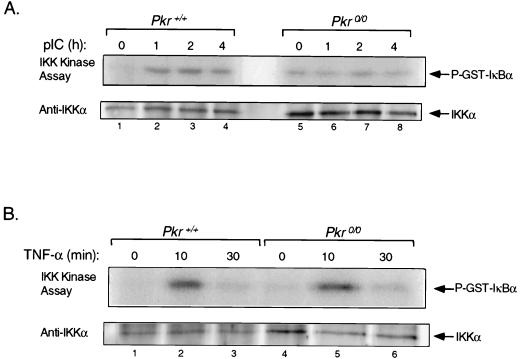
Signal-induced serine phosphorylation and degradation of IκB proteins are primarily mediated by IKK. To determine whether PKR-dependent dsRNA signaling to NF-κB was channelled through IKK, we first examined whether pIC treatment results in activation of endogenous IKK. T98G cell extracts prepared at various time intervals after treatment with pIC were immunoprecipitated using a monoclonal antibody against IKKα and subjected to an immune-complex kinase assay using GST-IκBα (amino acids 1 to 54) as the substrate. Antibodies directed to IKKα have previously been shown to precipitate the entire IKK complex (72). While TNF-α stimulated IKK activity within 10 min by approximately 4-fold (Fig. 5B), IKK activity was stimulated by pIC more slowly, first detectable after 1 h and reaching a maximum (3.6-fold increase) after 1.5 h (Fig. 5A). The kinetics of IKK activation in response to TNF and pIC also differed with respect to the decay in IKK activity. Whereas IKK activation elicited by TNF was transient (Fig. 5B), pIC resulted in a more prolonged activation of IKK (Fig. 5A).
FIG. 5.
IKK is activated in response to pIC. (A) T98G cells were treated with pIC (100 μg/ml) for the indicated times. IKK complex was immunoprecipitated with an anti-IKKα monoclonal antibody, and the immune complex was subjected to in vitro kinase assay using GST-IκBα (amino acids 1 to 54) as a substrate. Following SDS-PAGE, the portion of the gel containing the substrate was dried and processed for autoradiography. The portion containing IKK was analyzed by Western blotting for IKKα protein. (B) T98G cells were treated with TNF-α (5 ng/ml) for the indicated times. IKK kinase assay and immunoblotting were performed as described above for panel A. P-GST-IκBα, phosphorylated GST-IκBα.
pIC-induced activation of IKK is PKR dependent.
Having established that IKK is activated in response to pIC, we next addressed whether this activation was mediated by PKR. Pkr+/+ and Pkr0/0 mouse cell lines were treated with pIC and examined for IKK activity as described above. Whereas a significant induction in IKK kinase activity was observed in cells containing PKR, no stimulation of IKK activity was detectable in PKR null cells (Fig. 6A). While the basal level of IKK activity in Pkr0/0 cells was higher than in their wild-type counterparts, this correlated with higher levels of IKK protein (Fig. 6A). To rule out any inherent defect in inducibility of IKK in response to NF-κB-activating stimuli in Pkr0/0 cells, the cells were treated with TNF-α and assayed for IKK activation. The results (Fig. 6B) show these cells were competent in IKK activation in response to TNF-α, yielding a fourfold induction in IKK activity within 10 min of treatment with TNF compared with a threefold increase in the PKR wild-type cells (Fig. 6B). Taken together, these results show that in the absence of PKR, pIC is unable to signal to NF-κB because IKK is not activated.
FIG. 6.
IKK activation in response to pIC is PKR dependent. (A) Cell lines derived from Pkr+/+ and knockout mice with isogenic background were treated with pIC (100 μg/ml) for the indicated times. IKK kinase assay and immunoblotting were performed as described in the legend to Fig. 5A. (B) Pkr+/+ and Pkr0/0 cell lines were treated with TNF-α (5 ng/ml) and processed for IKK kinase assay and immunoblotting as described for panel A.
PKR physically associates with the IKK complex.
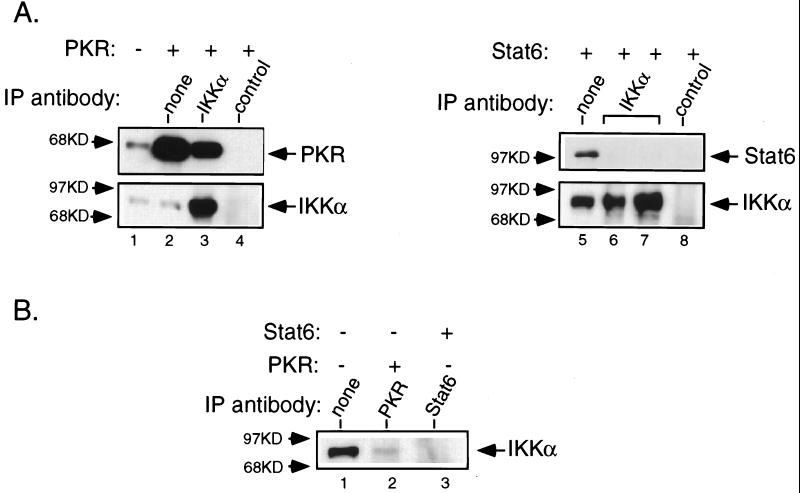
We reasoned that if the activation of IKK by pIC was PKR dependent, there may be a physical association between these two kinases. To address this possibility, a catalytically inactive mutant of PKR was transiently overexpressed in 293T cells and the endogenous IKK complex immunoprecipitated with anti-IKKα antibody followed by immunoblotting for PKR. Mutant PKR was used rather than wild-type PKR, since the latter is growth suppressive and therefore cannot be overexpressed. The ectopically expressed mutant PKR immunoprecipitated with anti-IKKα antibody (Fig. 7A, lane 3). The specificity of this interaction was demonstrated by the failure of PKR to coimmunoprecipitate with a control rabbit polyclonal antibody (Fig. 7A, lane 4) and from the lack of interaction of overexpressed Stat6 with IKK (Fig. 7A, lanes 6 and 7). The association of PKR with the IKK complex was confirmed by immunoprecipitating PKR from cell extracts and subjecting the immunoprecipitate to immunoblotting for IKKα. The endogenous IKKα associated with PKR (Fig. 7B, lane 2) and IKKα was not present in Stat6 immunocomplexes from Stat6-overexpressing cell extracts (Fig. 7B, lane 3). These results demonstrate that the association of PKR with the IKK complex is stimulus independent.
FIG. 7.
PKR interacts with IKK complex in vivo. Cells (293T) were transiently transfected with 2 μg of plasmids expressing a catalytically inactive mutant of human PKR or human Stat6 as described in Materials and Methods. (A) IKK complex was immunoprecipitated from extracts (0.5 mg [lane 6] or 1 mg [lanes 2 to 4 and 7 to 8]) using an anti-IKKα antibody, and the immune complex was subjected to Western blotting for PKR or Stat6 as indicated. A rabbit IgG polyclonal antibody was used as the control. The blots were subsequently stripped and reprobed for IKKα. (B) PKR and Stat6 were immunoprecipitated from PKR- or Stat6-overexpressing extracts (1 mg), respectively, and the immunocomplexes were subjected to analysis by Western blotting for endogenous IKKα.
A kinase-deficient mutant of IKKβ inhibits pIC-stimulated NF-κB-dependent transcription.
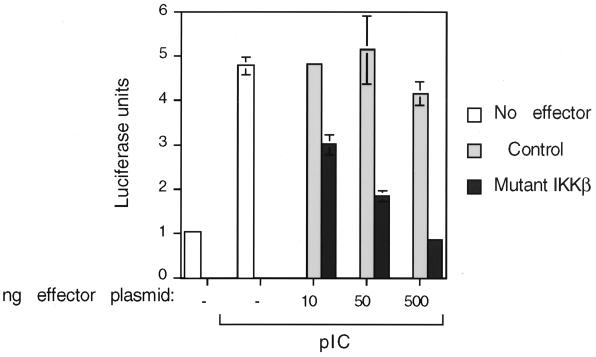
To directly demonstrate the requirement for IKK in dsRNA signaling to NF-κB, we transiently expressed a dominant negative mutant of IKKβ and examined its effect on pIC-induced gene expression. T98G cells were cotransfected with an NF-κB-dependent luciferase reporter plasmid containing 5 NF-κB sites upstream of a minimal reporter. The results (Fig. 8) show that reporter gene expression in response to pIC was inhibited by the catalytically inactive mutant of IKKβ in a dose-dependent manner. These results indicate that pIC-induced activation of NF-κB is mediated by the IKK complex.
FIG. 8.
Dominant negative IKKβ inhibits pIC-stimulated NF-κB-dependent transcription in a dose-dependent manner. T98G cells were transiently cotransfected with an NF-κB-dependent luciferase reporter (5 NF-κB sites) and increasing amounts of a mutant IKKβ expression plasmid or the corresponding empty vector as the control. Approximately 36 h posttransfection, cells were treated with pIC (100 μg/ml for 8 h) and extracts were prepared and analyzed for luciferase activity. The graph reflects firefly luciferase units corrected for transfection efficiency by expression from a Renilla luciferase plasmid included as an internal control in the dual-luciferase assay system. The experiment was performed in duplicate and is representative of several separate experiments.
A kinase-deficient mutant of NIK inhibits pIC-stimulated NF-κB-dependent transcription.
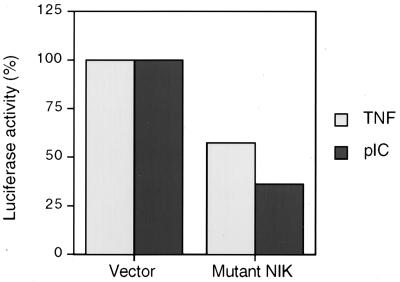
Most NF-κB-inducing signals (IL-1, T-cell receptor engagement, TNF-α, CD95, EBV LMP1) appear to converge on the NF-κB-inducing kinase (NIK) (50, 83), while NF-κB induction by Tax protein has been shown to be mediated through another member of the MAP kinase kinase kinase family (100). IL-1 and TNF-α have been reported to activate both NIK and MEKK1 (60). These two members of the MAP kinase kinase kinase family have been reported to physically associate with the IKK complex (13, 52, 69). Therefore, activation of IKKβ by PKR in pIC signaling to NF-κB may be direct or may be mediated through NIK, MEKK1, or another unidentified kinase. Therefore, we examined whether NIK may be involved in dsRNA signaling to NF-κB by transiently expressing a dominant negative mutant of this kinase and examining the effect on NF-κB-dependent reporter gene expression. A kinase-deficient mutant of NIK (KK429/430AA) inhibited both TNF-α and pIC signaling pathways leading to NF-κB activation (Fig. 9). This mutant (NIK KK429/430AA) did not affect activation of JNK1 in response to TNF-α (data not shown) as previously reported (79). These results suggest that NIK is a required component of pIC signaling pathway to NF-κB.
FIG. 9.
Dominant negative NIK inhibits pIC-stimulated NF-κB-dependent transcription T98G cells were transiently cotransfected with an NF-κB-dependent luciferase reporter (5 NF-κB sites) and 500 ng of a plasmid expressing a kinase-dead mutant of NIK or the corresponding empty vector as the control. Approximately 36 h posttransfection, cells were treated with pIC or TNF-α (100 μg/ml and 10 ng/ml, respectively, for 8 h) and extracts were prepared and analyzed for luciferase activity. The graph reflects firefly luciferase units corrected for transfection efficiency by expression from a Renilla luciferase plasmid included as an internal control in the dual-luciferase assay system. The experiment was performed in duplicate and is representative of several separate experiments.
DISCUSSION
The unravelling of the signaling cascades that activate the transcription factor NF-κB in response to a wide array of extracellular factors has been intensively studied. NF-κB drives the expression of genes involved in inflammation and the immune response as well as those with antiapoptotic functions and promoters of cell proliferation, transformation, and tumor development. Thus, a clear understanding of the molecular mechanisms involved in these signaling pathways would allow the targeting of critical components to prevent activation of NF-κB in undesirable situations, such as protection of tumor cells against apoptosis by anticancer agents, the onset of the inflammatory response, or cell cycle progression in tumor cells. In this study, we addressed the mechanism by which synthetic pIC, a viral mimic, activates NF-κB. We demonstrate that PKR is an essential mediator of pIC signaling to NF-κB, transducing the signal through IKK. Furthermore, we identify NIK as a component of the pIC signaling cascade to NF-κB. These conclusions are based on several pieces of evidence. (i) The kinetics of activation of PKR in response to pIC correlates with the induction of NF-κB DNA binding activity, with an initial increase in kinase activity observed prior to nuclear translocation of NF-κB. (ii) Isogenic cell lines derived from PKR null mice fail to induce NF-κB in response to pIC compared to PKR wild-type cells. (iii) pIC leads to degradation of IκBα and IκBβ in PKR wild-type cells, while these inhibitors are unaffected in PKR null cells. (iv) IKK is activated by pIC, and the kinetics of activation lag behind that of PKR. (v) pIC is unable to activate IKK in PKR-deficient cell lines. (vi) PKR can physically associate with the IKK complex. (vii) A catalytically inactive IKKβ mutant, previously shown to inhibit NF-κB activation by TNF, blocks pIC-induced, NF-κB-dependent gene expression from a reporter plasmid. (viii) A kinase-deficient mutant of NIK inhibits pIC-induced, NF-κB-dependent gene expression. Although the absence of PKR severely compromised the induction by pIC of NF-κB DNA binding activity, nevertheless, we did consistently observe a minor activation of NF-κB in PKR null cells (Fig. 1C). This is in accord with our previous observations that priming of PKR null cells with IFN overcomes the defect in PKR-dependent activation of NF-κB by dsRNA, arguing for the presence of a separate pathway which is PKR independent and IFN inducible (98). Thus, small amounts of IFN produced by the PKR null cells allow for low-level induction of NF-κB.
The work presented here establishes PKR as a major mediator of dsRNA signaling to NF-κB. More importantly, our study identifies NIK and IKK as two kinases transducing the PKR-mediated signal to IκBα, IκBβ, and NF-κB. Thus, for the first time, downstream targets in a PKR-dependent signaling pathway have been identified. We previously reported an in vitro assay system in which NF-κB DNA binding activity was induced in cell extracts by recombinant wild-type PKR but not a catalytically inactive mutant of PKR (39). Our present study provides the mechanism by which this phenomenon may occur, namely, the activation of IKK by PKR leading to IκB phosphorylation and the release of NF-κB. Although recombinant PKR can directly phosphorylate IκBα in vitro (39), the phosphorylation sites have not been mapped and shown to be identical to those phosphorylated in an intact cell in response to dsRNA. It seems more likely that phosphorylation of IκBs by PKR is indirect: our present study indicates that PKR-mediated activation of NF-κB is mediated through IKK. Furthermore, a cell line has been described in which PKR was fully functional, but pIC-mediated NF-κB activation was deficient, providing further evidence for indirect phosphorylation of IκBs by PKR (41).
Furthermore, we demonstrate that in addition to dsRNA, PKR is involved in sustained TNF-α signaling to NF-κB and the enhanced activation of this transcription factor by IFN-γ in conjunction with TNF-α. The NF-κB inhibitor IκBβ and not IκBα is targeted by PKR in these PKR-dependent TNF-α signaling pathways.
Although in most cases, NF-κB is induced via the classical activation pathway which depends on phosphorylation of IκBα and IκBβ by IKK or equivalent kinase complexes, alternate activation pathways have been identified. For instance, UV-C radiation was shown to lead to ubiquitin-mediated proteasomal degradation of IκBα; however, this process was noted to be independent of phosphorylation of IκBα on the critical serine residues at positions 32 and 36 and thus not mediated by IKK (46). In contrast, tyrosine phosphorylation at residue 42 in IκBα has been demonstrated to lead to the dissociation rather than degradation of this inhibitor (31). Our finding that IKK mediates pIC-induced activation of NF-κB suggests that degradation of IκBα and IκBβ is probably triggered by phosphorylation on critical serine residues. There is some degree of cell type specificity with respect to IκBα degradation in response to pIC. In contrast to the Pkr+/+ mouse cell line where there is modest targeting of IκBα, T98G cells do not exhibit IκBα degradation in response to pIC. Selective degradation of a subset of IκBs in response to a stimulus has been previously reported. Thus, infection of intestinal epithelial cells HT-29 and T84 with enteroinvasive bacteria lead to the partial degradation of IκBα with no effect on the levels of IκBβ (23). Thompson et al. reported that treatment of 70Z/3 cells with phorbol myristate acetate and Jurkat cells with TNF-α resulted in the transient disappearance of IκBα but had no effect on the steady-state levels of IκBβ (86). Previously, cell type specificity with respect to the degradation of IκBβ in the synergistic activation of NF-κB by TNF-α and IFN-γ mediated by PKR had been observed (9). Thus, inactivation of PKR in cells of neuronal but not endothelial origin blocked IκBβ degradation by TNF-α and IFN-γ cotreatment (9). Furthermore, we show here that PKR null cell lines are compromised in their ability to sustain a prolonged activation of NF-κB in response to TNF-α as a consequence of accumulation of IκBβ in these cells compared to PKR wild-type cells. Clearly there is a role for PKR in TNF-α signaling to NF-κB which is uncovered only when NF-κB activation is examined over an extended time course. In previous studies with cells with compromised PKR activity or devoid of PKR protein, we had noted a potential involvement of this kinase in TNF-α signaling. For example, cells expressing a dominant negative mutant of PKR showed a small but consistent decrease in transcriptional activity of promoter-reporter constructs induced by TNF-α (40). PKR null mouse embryo fibroblasts which were resistant to apoptotic cell death in response to TNF-α were deficient in activation of IRF-1 in response to TNF-α but also exhibited slightly diminished levels of NF-κB induction in response to this cytokine compared to those of the wild-type cells (20). The kinetic studies with isogenic cells derived from Pkr+/+ and Pkr0/0 mice clearly show that PKR is required for sustained NF-κB DNA binding activity in response to TNF-α. We are currently investigating the nature of the signal that activates PKR in response to TNF-α.
IFN-γ is usually not an efficient activator of NF-κB on its own. However, under defined conditions and in a cell type-specific manner, IFN-γ can induce NF-κB (A. Deb and B. R. G. Williams, unpublished results). We have previously shown that in response to IFN-γ, a posttranslational modification of PKR consistent with phosphorylation is detectable (40). In the preneuronal derived cell line, PC12, transiently transfected with a transdominant negative mutant of PKR or treated with the PKR inhibitor 2-AP, synergistic activation of NF-κB by TNF-α and IFN-γ is blocked. This PKR-dependent activation of NF-κB is channelled through IκBβ and not IκBα (9). In this study, the PKR null cells exhibited a deficiency in the potentiation of activation of NF-κB by TNF-α and IFN-γ consistent with a requirement for PKR.
Since its initial characterization, IKK has progressively been identified as the point of signal convergence in a variety of NF-κB-inducing signal transduction pathways. All these external signals have been shown to activate IKK by one or two members of the MAP3-kinase family of proteins, NIK and MEKK1. More recently, two other members of this family, MEKK2 and MEKK3, have been reported to induce IKK activation and site-specific IκBα phosphorylation (103). Thus, the consensus with regards to signal-induced activation of NF-κB appears to be that the core element of the signaling cascade is a MAP3K and an IKK. A mutant cell line derived from the JAK-minus U4C cells which is deficient in pIC-induced NF-κB activation despite being wild type for PKR and IKK activity has been described (41), suggesting the possibility of an indirect means of activation of IKK by PKR. In the current study, we have shown a potential role for NIK in the pIC signaling cascade to NF-κB. NIK is present in the IKK complex (13, 69) and has been shown to be the immediate upstream kinase activating IKK in TNF-α, IL-1, CD95, CD28/CD3, and EBV LMP1 signaling cascades to NF-κB (50, 61, 73, 83). In at least three signaling pathways to NF-κB, including IL-1 (61), TNF (73), and CD3/CD28 (48), a member of the MAP3-kinase family has been shown to activate NIK. For example, the proto-oncogene, Cot (also called Tpl-2), which is a serine-threonine kinase, was shown to be upstream of NIK in CD3/CD28 but not in the TNF-α signaling cascade leading to NF-κB induction (48). The kinase TAK1 was demonstrated to be upstream of NIK in the IL-1 (61) and TNF-α (73) signaling pathways. It remains to be determined whether TAK1 or Cot/Tpl-2 may participate upstream of NIK in the pIC-induced PKR-mediated signaling pathway to NF-κB. NF-κB gene expression results from two distinct but sequential events: (i) release of NF-κB from its inhibitory proteins (IκBs), which in the case of most NF-κB-inducing signals depends on activation of IKK; (ii) transactivation of NF-κB (on p65/RelA) by phosphorylation. We demonstrate in Fig. 9 that NIK is an important component of pIC signaling to NF-κB, since disruption of its activity through overexpression of a dominant negative variant of NIK leads to an inhibition in pIC-induced NF-κB-dependent gene expression. We have observed that in cells expressing a catalytically inactive mutant of NIK, IKK activation and NF-κB release from inhibitory complexes were not affected despite a clear inhibition in TNF-α-induced NF-κB-dependent gene expression (unpublished data). Therefore, despite numerous publications implicating NIK as the direct upstream activator of IKK, we are obliged to exclude NIK as having any role in the release of NF-κB but rather place it in the pathway leading to the transactivation of NF-κB.
PKR plays a pivotal role in the antiviral activity of IFNs. Activation of PKR by virus has several consequences including inhibition of host-dependent viral replication through eIF-2α phosphorylation, the expression of genes involved in the inflammatory response, antigen presentation, chemotaxis, cell adhesion, antimicrobial activities, and induction of apoptosis. The pro-apoptotic role for PKR (1, 20, 22, 43, 45, 99) and the anti-apoptotic activity of NF-κB (89, 91) lead to a delicate balance between survival and death. Similar to dsRNA, TNF-α can both initiate apoptosis and activate NF-κB, which suppresses apoptosis by blocking the activation of caspase 8. This effect is achieved through NF-κB-dependent transcription including a group of genes: TRAF1, TRAF2, cIAP1, and cIAP2 (92). Thus, activation of NF-κB may provide a rapid defense mechanism to block death signaling in response to TNF-α challenge. At present, the components of PKR-mediated dsRNA- or stress-induced apoptosis are unknown. However, the observation that PKR null cells which are deficient in dsRNA-induced apoptosis have reduced levels of Fas/CD95 death receptor mRNA (20) suggests that PKR-mediated apoptosis may also involve caspase 8, the initiator caspase downstream of Fas. Furthermore, overexpression of wild-type PKR causes apoptosis which can be blocked by overexpression of Bcl2 (45) and leads to increased Fas mRNA levels, suggesting that this member of the TNF and nerve growth factor receptor family may be a downstream effector of PKR during viral infection (22). A role for Fas in virus-induced apoptosis has been suggested for influenza virus (85, 90) and EBV (88). The central role of PKR as a mediator of dsRNA-induced activation of NF-κB makes this kinase a clear target for inhibition by viruses which strive to prevent an inflammatory response and suppress a pro-apoptotic response in the host. Accordingly, viruses have developed several mechanisms including inhibitory viral RNA, inhibitory viral or cellular proteins, and proteolytic cleavage to subvert the activity of PKR.
ACKNOWLEDGMENTS
This work was supported by a grant to B.R.G.W. from the National Institutes of Health (AI34039). T.H.M. was supported by a grant from the Danish Cancer Society.
We thank Sandy Der and Aylin Ozdemir for generating the Pkr+/+ and Pkr0/0 cell lines.
REFERENCES
- 1.Balachandran S, Kim C N, Yeh W C, Mak T W, Bhalla K, Barber G N. Activation of the dsRNA-dependent protein kinase, PKR, induces apoptosis through FADD-mediated death signaling. EMBO J. 1998;17:6888–6902. doi: 10.1093/emboj/17.23.6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldwin A S., Jr The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 3.Barber G N, Wambach M, Thompson S, Jagus R, Katze M G. Mutants of the RNA-dependent protein kinase (PKR) lacking double-stranded RNA binding domain I can act as transdominant inhibitors and induce malignant transformation. Mol Cell Biol. 1995;15:3138–3146. doi: 10.1128/mcb.15.6.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beg A A, Finco T S, Nantermet P V, Baldwin A S., Jr Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of IκBα: a mechanism for NF-κB activation. Mol Cell Biol. 1993;13:3301–3310. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beraud C, Henzel W J, Baeuerle P A. Involvement of regulatory and catalytic subunits of phosphoinositide 3-kinase in NF-κB activation. Proc Natl Acad Sci USA. 1999;96:429–434. doi: 10.1073/pnas.96.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brockman J A, Scherer D C, McKinsey T A, Hall S M, Qi X, Lee W Y, Ballard D W. Coupling of a signal response domain in IκBκ to multiple pathways for NF-κB activation. Mol Cell Biol. 1995;15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carpick B W, Graziano V, Schneider D, Maitra R K, Lee X, Williams B R G. Characterization of the solution complex between the interferon-induced, double-stranded RNA-activated protein kinase and HIV-I transactivating region RNA. J Biol Chem. 1997;272:9510–9516. doi: 10.1074/jbc.272.14.9510. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Hagler J, Palombella V J, Melandri F, Scherer D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets IκBα to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 9.Cheshire J L, Williams B R, Baldwin A S., Jr Involvement of double-stranded RNA-activated protein kinase in the synergistic activation of nuclear factor-κB by tumor necrosis factor-alpha and gamma-interferon in preneuronal cells. J Biol Chem. 1999;274:4801–4806. doi: 10.1074/jbc.274.8.4801. [DOI] [PubMed] [Google Scholar]
- 10.Chiao P J, Miyamoto S, Verma I M. Autoregulation of IκBα activity. Proc Natl Acad Sci USA. 1994;91:28–32. doi: 10.1073/pnas.91.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chong K L, Feng L, Schappert K, Meurs E, Donahue T F, Friesen J D, Hovanessian A G, Williams B R. Human p68 kinase exhibits growth suppression in yeast and homology to the translational regulator GCN2. EMBO J. 1992;11:1553–1562. doi: 10.1002/j.1460-2075.1992.tb05200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clemens M J, Elia A. The double-stranded RNA-dependent protein kinase PKR: structure and function. J Interferon Cytokine Res. 1997;17:503–524. doi: 10.1089/jir.1997.17.503. [DOI] [PubMed] [Google Scholar]
- 13.Cohen L, Henzel W J, Baeuerle P A. IKAP is a scaffold protein of the IκB kinase complex. Nature. 1998;395:292–296. doi: 10.1038/26254. [DOI] [PubMed] [Google Scholar]
- 14.Cuddihy A R, Li S, Tam N W, Wong A H, Taya Y, Abraham N, Bell J C, Koromilas A E. Double-stranded-RNA-activated protein kinase PKR enhances transcriptional activation by tumor suppressor p53. Mol Cell Biol. 1999;19:2475–2484. doi: 10.1128/mcb.19.4.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuddihy A R, Wong A H, Tam N W, Li S, Koromilas A E. The double-stranded RNA activated protein kinase PKR physically associates with the tumor suppressor p53 protein and phosphorylates human p53 on serine 392 in vitro. Oncogene. 1999;18:2690–2702. doi: 10.1038/sj.onc.1202620. [DOI] [PubMed] [Google Scholar]
- 16.de Haro C, Mendez R, Santoyo J. The eIF-2α kinases and the control of protein synthesis. FASEB J. 1996;10:1378–1387. doi: 10.1096/fasebj.10.12.8903508. [DOI] [PubMed] [Google Scholar]
- 17.Delhase M, Hayakawa M, Chen Y, Karin M. Positive and negative regulation of IκB kinase activity through IKKβ subunit phosphorylation. Science. 1999;284:309–313. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- 18.de Martin R, Vanhove B, Cheng Q, Hofer E, Csizmadia V, Winkler H, Bach F H. Cytokine-inducible expression in endothelial cells of an IκBα-like gene is regulated by NF-κB. EMBO J. 1993;12:2773–2779. doi: 10.1002/j.1460-2075.1993.tb05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Der S D, Lau A S. Involvement of the double-stranded-RNA-dependent kinase PKR in interferon expression and interferon-mediated antiviral activity. Proc Natl Acad Sci USA. 1995;92:8841–8845. doi: 10.1073/pnas.92.19.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Der S D, Yang Y L, Weissmann C, Williams B R. A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:3279–3283. doi: 10.1073/pnas.94.7.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 22.Donze O, Dostie J, Sonenberg N. Regulatable expression of the interferon-induced double-stranded RNA dependent protein kinase PKR induces apoptosis and fas receptor expression. Virology. 1999;256:322–329. doi: 10.1006/viro.1999.9618. [DOI] [PubMed] [Google Scholar]
- 23.Elewaut D, DiDonato J A, Kim J M, Truong F, Eckmann L, Kagnoff M F. NF-κB is a central regulator of the intestinal epithelial cell innate immune response induced by infection with enteroinvasive bacteria. J Immunol. 1999;163:1457–1466. [PubMed] [Google Scholar]
- 24.Finco T S, Baldwin A S. Mechanistic aspects of NF-κB regulation: the emerging role of phosphorylation and proteolysis. Immunity. 1995;3:263–272. doi: 10.1016/1074-7613(95)90112-4. [DOI] [PubMed] [Google Scholar]
- 25.Galabru J, Hovanessian A G. Two interferon-induced proteins are involved in the protein kinase complex dependent on double-stranded RNA. Cell. 1985;43:685–694. doi: 10.1016/0092-8674(85)90241-7. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh S, May M J, Kopp E B. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 27.Gusella G L, Musso T, Rottschafer S E, Pulkki K, Varesio L. Potential requirement of a functional double-stranded RNA-dependent protein kinase (PKR) for the tumoricidal activation of macrophages by lipopolysaccharide or IFN-α/β but not IFN-γ. J Immunol. 1995;154:345–354. [PubMed] [Google Scholar]
- 28.He J, Olson J J, Ekstrand A J, Serbanescu A, Yang J, Offermann M K, James C D. Transfection of IFN-α in human glioblastoma cells and tumorigenicity in association with induction of PKR and OAS gene expression. J Neurosurg. 1996;85:1085–1090. doi: 10.3171/jns.1996.85.6.1085. [DOI] [PubMed] [Google Scholar]
- 29.Hovanessian A G. The double stranded RNA-activated protein kinase induced by interferon: dsRNA-PK. J Interferon Res. 1989;9:641–647. doi: 10.1089/jir.1989.9.641. [DOI] [PubMed] [Google Scholar]
- 30.Hu Y, Baud V, Delhase M, Zhang P, Deerinck T, Ellisman M, Johnson R, Karin M. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKα subunit of IκB kinase. Science. 1999;284:316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- 31.Imbert V, Rupec R A, Livolsi A, Pahl H L, Traenckner E B, Mueller-Dieckmann C, Farahifar D, Rossi B, Auberger P, Baeuerle P A, Peyron J F. Tyrosine phosphorylation of IκB-α activates NF-κB without proteolytic degradation of IκB-α. Cell. 1996;86:787–798. doi: 10.1016/s0092-8674(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 32.Ito C Y, Kazantsev A G, Baldwin A S., Jr Three NF-κB sites in the IκB-α promoter are required for induction of gene expression by TNF α. Nucleic Acids Res. 1994;22:3787–3792. doi: 10.1093/nar/22.18.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katze M G. Regulation of the interferon-induced PKR: can viruses cope? Trends Microbiol. 1995;3:75–78. doi: 10.1016/s0966-842x(00)88880-0. [DOI] [PubMed] [Google Scholar]
- 34.Kerr I M, Brown R E, Hovanessian A G. Nature of inhibitor of cell-free protein synthesis formed in response to interferon and double-stranded RNA. Nature. 1977;268:540–542. doi: 10.1038/268540a0. [DOI] [PubMed] [Google Scholar]
- 35.Kibler K V, Shors T, Perkins K B, Zeman C C, Banaszak M P, Biesterfeldt J, Langland J O, Jacobs B L. Double-stranded RNA is a trigger for apoptosis in vaccinia virus-infected cells. J Virol. 1997;71:1992–2003. doi: 10.1128/jvi.71.3.1992-2003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirchhoff S, Koromilas A E, Schaper F, Grashoff M, Sonenberg N, Hauser H. IRF-1 induced cell growth inhibition and interferon induction requires the activity of the protein kinase PKR. Oncogene. 1995;11:439–445. [PubMed] [Google Scholar]
- 37.Koromilas A E, Roy S, Barber G N, Katze M G, Sonenberg N. Malignant transformation by a mutant of the IFN-inducible dsRNA-dependent protein kinase. Science. 1992;257:1685–1689. doi: 10.1126/science.1382315. [DOI] [PubMed] [Google Scholar]
- 38.Krust B, Galabru J, Hovanessian A G. Further characterization of the protein kinase activity mediated by interferon in mouse and human cells. J Biol Chem. 1984;259:8494–8498. [PubMed] [Google Scholar]
- 39.Kumar A, Haque J, Lacoste J, Hiscott J, Williams B R G. Double-stranded RNA-dependent protein kinase activates transcription factor NF-κB by phosphorylating IκB. Proc Natl Acad Sci USA. 1994;91:6288–6292. doi: 10.1073/pnas.91.14.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar A, Yang Y L, Flati V, Der S, Kadereit S, Deb A, Haque J, Reis L, Weissmann C, Williams B R. Deficient cytokine signaling in mouse embryo fibroblasts with a targeted deletion in the PKR gene: role of IRF-1 and NF-κB. EMBO J. 1997;16:406–416. doi: 10.1093/emboj/16.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leaman D W, Salvekar A, Patel R, Sen G C, Stark G R. A mutant cell line defective in response to double-stranded RNA and in regulating basal expression of interferon-stimulated genes. Proc Natl Acad Sci USA. 1998;95:9442–9447. doi: 10.1073/pnas.95.16.9442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee F S, Hagler J, Chen Z J, Maniatis T. Activation of the IκB α kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 43.Lee S B, Esteban M. The interferon-induced double-stranded RNA-activated protein kinase induces apoptosis. Virology. 1994;199:491–496. doi: 10.1006/viro.1994.1151. [DOI] [PubMed] [Google Scholar]
- 44.Lee S B, Melkova Z, Yan W, Williams B R, Hovanessian A G, Esteban M. The interferon-induced double-stranded RNA-activated human p68 protein kinase potently inhibits protein synthesis in cultured cells. Virology. 1993;192:380–385. doi: 10.1006/viro.1993.1048. [DOI] [PubMed] [Google Scholar]
- 45.Lee S B, Rodriguez D, Rodriguez J R, Esteban M. The apoptosis pathway triggered by the interferon-induced protein kinase PKR requires the third basic domain, initiates upstream of Bcl-2, and involves ICE-like proteases. Virology. 1997;231:81–88. doi: 10.1006/viro.1997.8494. [DOI] [PubMed] [Google Scholar]
- 46.Li N, Karin M. Ionizing radiation and short wavelength UV activate NF-κB through two distinct mechanisms. Proc Natl Acad Sci USA. 1998;95:13012–13017. doi: 10.1073/pnas.95.22.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Q, Van Antwerp D, Mercurio F, Lee K F, Verma I M. Severe liver degeneration in mice lacking the IκB kinase 2 gene. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- 48.Lin X, Cunningham E T, Jr, Mu Y, Geleziunas R, Greene W C. The proto-oncogene Cot kinase participates in CD3/CD28 induction of NF-κB acting through the NF-κB-inducing kinase and IκB kinases. Immunity. 1999;10:271–280. doi: 10.1016/s1074-7613(00)80027-8. [DOI] [PubMed] [Google Scholar]
- 49.Ling L, Cao Z, Goeddel D V. NF-κB-inducing kinase activates IKK-α by phosphorylation of Ser-176. Proc Natl Acad Sci USA. 1998;95:3792–3797. doi: 10.1073/pnas.95.7.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malinin N L, Boldin M P, Kovalenko A V, Wallach D. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 51.Mercurio F, Murray B W, Shevchenko A, Bennett B L, Young D B, Li J W, Pascual G, Motiwala A, Zhu H, Mann M, Manning A M. IκB kinase (IKK)-associated protein 1, a common component of the heterogeneous IKK complex. Mol Cell Biol. 1999;19:1526–1538. doi: 10.1128/mcb.19.2.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mercurio F, Zhu H, Murray B W, Shevchenko A, Bennett B L, Li J, Young D B, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 53.Meurs E, Chong K, Galabru J, Thomas N S, Kerr I M, Williams B R, Hovanessian A G. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990;62:379–390. doi: 10.1016/0092-8674(90)90374-n. [DOI] [PubMed] [Google Scholar]
- 54.Meurs E F, Galabru J, Barber G N, Katze M G, Hovanessian A G. Tumor suppressor function of the interferon-induced double-stranded RNA-activated protein kinase. Proc Natl Acad Sci USA. 1993;90:232–236. doi: 10.1073/pnas.90.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meurs E F, Watanabe Y, Kadereit S, Barber G N, Katze M G, Chong K, Williams B R, Hovanessian A G. Constitutive expression of human double-stranded RNA-activated p68 kinase in murine cells mediates phosphorylation of eukaryotic initiation factor 2 and partial resistance to encephalomyocarditis virus growth. J Virol. 1992;66:5804–5814. doi: 10.1128/jvi.66.10.5805-5814.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miyamoto S, Verma I M. Rel/NF-κB/IκB story. Adv Cancer Res. 1995;66:255–292. [PubMed] [Google Scholar]
- 57.Mundschau L J, Faller D V. Oncogenic ras induces an inhibitor of double-stranded RNA-dependent eukaryotic initiation factor 2 alpha-kinase activation. J Biol Chem. 1992;267:23092–23098. [PubMed] [Google Scholar]
- 58.Mundschau L J, Faller D V. Platelet-derived growth factor signal transduction through the interferon-inducible kinase PKR. Immediate early gene induction. J Biol Chem. 1995;270:3100–3106. doi: 10.1074/jbc.270.7.3100. [DOI] [PubMed] [Google Scholar]
- 59.Naumann M, Scheidereit C. Activation of NF-κB in vivo is regulated by multiple phosphorylations. EMBO J. 1994;13:4597–4607. doi: 10.1002/j.1460-2075.1994.tb06781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nemoto S, DiDonato J A, Lin A. Coordinate regulation of IκB kinases by mitogen-activated protein kinase kinase kinase 1 and NF-κB-inducing kinase. Mol Cell Biol. 1998;18:7336–7343. doi: 10.1128/mcb.18.12.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-I κB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 62.O'Connell M A, Bennett B L, Mercurio F, Manning A M, Mackman N. Role of IKK1 and IKK2 in lipopolysaccharide signaling in human monocytic cells. J Biol Chem. 1998;273:30410–30414. doi: 10.1074/jbc.273.46.30410. [DOI] [PubMed] [Google Scholar]
- 63.Ohmori Y, Tebo J, Nedospasov S, Hamilton T A. κB binding activity in a murine macrophage-like cell line. Sequence-specific differences in κB binding and transcriptional activation functions. J Biol Chem. 1994;269:17684–17690. [PubMed] [Google Scholar]
- 64.Pain V M. Initiation of protein synthesis in eukaryotic cells. Eur J Biochem. 1996;236:747–771. doi: 10.1111/j.1432-1033.1996.00747.x. [DOI] [PubMed] [Google Scholar]
- 65.Petryshyn R, Chen J J, Danley L, Matts R L. Effect of interferon on protein translation during growth stages of 3T3 cells. Arch Biochem Biophys. 1996;326:290–297. doi: 10.1006/abbi.1996.0078. [DOI] [PubMed] [Google Scholar]
- 66.Petryshyn R, Chen J J, London I M. Detection of activated double-stranded RNA-dependent protein kinase in 3T3-F442A cells. Proc Natl Acad Sci USA. 1988;85:1427–1431. doi: 10.1073/pnas.85.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Petryshyn R, Chen J J, London I M. Growth-related expression of a double-stranded RNA-dependent protein kinase in 3T3 cells. J Biol Chem. 1984;259:14736–14742. [PubMed] [Google Scholar]
- 68.Raveh T, Hovanessian A G, Meurs E F, Sonenberg N, Kimchi A. Double-stranded RNA-dependent protein kinase mediates c-Myc suppression induced by type I interferons. J Biol Chem. 1996;271:25479–25484. doi: 10.1074/jbc.271.41.25479. [DOI] [PubMed] [Google Scholar]
- 69.Regnier C H, Song H Y, Gao X, Goeddel D V, Cao Z, Rothe M. Identification and characterization of an IκB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 70.Rice A P, Duncan R, Hershey J W, Kerr I M. Double-stranded RNA-dependent protein kinase and 2-5A system are both activated in interferon-treated, encephalomyocarditis virus-infected HeLa cells. J Virol. 1985;54:894–898. doi: 10.1128/jvi.54.3.894-898.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Romano P R, Green S R, Barber G N, Mathews M B, Hinnebusch A G. Structural requirements for double-stranded RNA binding, dimerization, and activation of the human eIF-2α kinase DAI in Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:365–378. doi: 10.1128/mcb.15.1.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rothwarf D M, Zandi E, Natoli G, Karin M. IKK-γ is an essential regulatory subunit of the IκB kinase complex. Nature. 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 73.Sakurai H, Miyoshi H, Toriumi W, Sugita T. Functional interactions of transforming growth factor beta-activated kinase 1 with IκB kinases to stimulate NF-κB activation. J Biol Chem. 1999;274:10641–10648. doi: 10.1074/jbc.274.15.10641. [DOI] [PubMed] [Google Scholar]
- 74.Salzberg S, Mandelboim M, Zalcberg M, Shainberg A, Mandelbaum M. Interruption of myogenesis by transforming growth factor beta 1 or EGTA inhibits expression and activity of the myogenic-associated (2′-5′) oligoadenylate synthetase and PKR. Exp Cell Res. 1995;219:223–232. doi: 10.1006/excr.1995.1222. [DOI] [PubMed] [Google Scholar]
- 75.Samuel C E, Duncan R, Knutson G S, Hershey J W. Mechanism of interferon action. Increased phosphorylation of protein synthesis initiation factor eIF-2α in interferon-treated, reovirus-infected mouse L929 fibroblasts in vitro and in vivo. J Biol Chem. 1984;259:13451–13457. [PubMed] [Google Scholar]
- 76.Schmitz M L, dos Santos Silva M A, Baeuerle P A. Transactivation domain 2 (TA2) of p65 NF-κB. Similarity to TA1 and phorbol ester-stimulated activity and phosphorylation in intact cells. J Biol Chem. 1995;270:15576–15584. doi: 10.1074/jbc.270.26.15576. [DOI] [PubMed] [Google Scholar]
- 77.Shang Y, Baumrucker C R, Green M H. c-myc is a major mediator of the synergistic growth inhibitory effects of retinoic acid and interferon in breast cancer cells. J Biol Chem. 1998;273:30608–30613. doi: 10.1074/jbc.273.46.30608. [DOI] [PubMed] [Google Scholar]
- 78.Sizemore N, Leung S, Stark G R. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-κB p65/RelA subunit. Mol Cell Biol. 1999;19:4798–4805. doi: 10.1128/mcb.19.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Song H Y, Regnier C H, Kirschning C J, Goeddel D V, Rothe M. Tumor necrosis factor (TNF)-mediated kinase cascades: bifurcation of nuclear factor-κB and c-jun N-terminal kinase (JNK/SAPK) pathways at TNF receptor-associated factor 2. Proc Natl Acad Sci USA. 1997;94:9792–9796. doi: 10.1073/pnas.94.18.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stark G R, Kerr I M, Williams B R, Silverman R H, Schreiber R D. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 81.Sun S, Elwood J, Greene W C. Both amino- and carboxyl-terminal sequences within IκBα regulate its inducible degradation. Mol Cell Biol. 1996;16:1058–1065. doi: 10.1128/mcb.16.3.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun S C, Ganchi P A, Ballard D W, Greene W C. NF-κB controls expression of inhibitor IκBα: evidence for an inducible autoregulatory pathway. Science. 1993;259:1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- 83.Sylla B S, Hung S C, Davidson D M, Hatzivassiliou E, Malinin N L, Wallach D, Gilmore T D, Kieff E, Mosialos G. Epstein-Barr virus-transforming protein latent infection membrane protein 1 activates transcription factor NF-κB through a pathway that includes the NF-κB-inducing kinase and the IκB kinases IKKα and IKKβ. Proc Natl Acad Sci USA. 1998;95:10106–10111. doi: 10.1073/pnas.95.17.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takeda K, Takeuchi O, Tsujimura T, Itami S, Adachi O, Kawai T, Sanjo H, Yoshikawa K, Terada N, Akira S. Limb and skin abnormalities in mice lacking IKKα. Science. 1999;284:313–316. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- 85.Takizawa T, Ohashi K, Nakanishi Y. Possible involvement of double-stranded RNA-activated protein kinase in cell death by influenza virus infection. J Virol. 1996;70:8128–8132. doi: 10.1128/jvi.70.11.8128-8132.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thompson J E, Phillips R J, Erdjument-Bromage H, Tempst P, Ghosh S. IκB-β regulates the persistent response in a biphasic activation of NF-κB. Cell. 1995;80:573–582. doi: 10.1016/0092-8674(95)90511-1. [DOI] [PubMed] [Google Scholar]
- 87.Traenckner E B, Pahl H L, Henkel T, Schmidt K N, Wilk S, Baeuerle P A. Phosphorylation of human IκB-α on serines 32 and 36 controls IκB-α proteolysis and NF-κB activation in response to diverse stimuli. EMBO J. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Uehara T, Miyawaki T, Ohta K, Tamaru Y, Yokoi T, Nakamura S, Taniguchi N. Apoptotic cell death of primed CD45RO+ T lymphocytes in Epstein-Barr virus-induced infectious mononucleosis. Blood. 1992;80:452–458. [PubMed] [Google Scholar]
- 89.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 90.Wada N, Matsumura M, Ohba Y, Kobayashi N, Takizawa T, Nakanishi Y. Transcription stimulation of the Fas-encoding gene by nuclear factor for interleukin-6 expression upon influenza virus infection. J Biol Chem. 1995;270:18007–18012. doi: 10.1074/jbc.270.30.18007. [DOI] [PubMed] [Google Scholar]
- 91.Wang C Y, Mayo M W, Baldwin A S., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 92.Wang C Y, Mayo M W, Korneluk R G, Goeddel D V, Baldwin A S., Jr NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 93.Wang D, Baldwin A S., Jr Activation of nuclear factor-κB-dependent transcription by tumor necrosis factor-alpha is mediated through phosphorylation of RelA/p65 on serine 529. J Biol Chem. 1998;273:29411–29416. doi: 10.1074/jbc.273.45.29411. [DOI] [PubMed] [Google Scholar]
- 94.Whiteside S T, Ernst M K, LeBail O, Laurent-Winter C, Rice N, Israel A. N- and C-terminal sequences control degradation of MAD3/IκBα in response to inducers of NF-κB activity. Mol Cell Biol. 1995;15:5339–5345. doi: 10.1128/mcb.15.10.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Whiteside S T, Israel A. IκB proteins: structure, function and regulation. Semin Cancer Biol. 1997;8:75–82. doi: 10.1006/scbi.1997.0058. [DOI] [PubMed] [Google Scholar]
- 96.Woronicz J D, Gao X, Cao Z, Rothe M, Goeddel D V. IκB kinase-beta: NF-κB activation and complex formation with IκB kinase-alpha and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 97.Yamaoka S, Courtois G, Bessia C, Whiteside S T, Weil R, Agou F, Kirk H E, Kay R J, Israel A. Complementation cloning of NEMO, a component of the IκB kinase complex essential for NF-κB activation. Cell. 1998;93:1231–1240. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- 98.Yang Y L, Reis L F, Pavlovic J, Aguzzi A, Schafer R, Kumar A, Williams B R, Aguet M, Weissmann C. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 1995;14:6095–6106. doi: 10.1002/j.1460-2075.1995.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yeung M C, Liu J, Lau A S. An essential role for the interferon-inducible, double-stranded RNA-activated protein kinase PKR in the tumor necrosis factor-induced apoptosis in U937 cells. Proc Natl Acad Sci USA. 1996;93:12451–12455. doi: 10.1073/pnas.93.22.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yin M J, Christerson L B, Yamamoto Y, Kwak Y T, Xu S, Mercurio F, Barbosa M, Cobb M H, Gaynor R B. HTLV-I Tax protein binds to MEKK1 to stimulate IκB kinase activity and NF-κB activation. Cell. 1998;93:875–884. doi: 10.1016/s0092-8674(00)81447-6. [DOI] [PubMed] [Google Scholar]
- 101.Zamanian-Daryoush M, Der S D, Williams B R. Cell cycle regulation of the double stranded RNA activated protein kinase, PKR. Oncogene. 1999;18:315–326. doi: 10.1038/sj.onc.1202293. [DOI] [PubMed] [Google Scholar]
- 102.Zandi E, Rothwarf D M, Delhase M, Hayakawa M, Karin M. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ necessary for IκB phosphorylation and NF-κB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 103.Zhao Q, Lee F S. Mitogen-activated protein kinase/ERK kinase kinases 2 and 3 activate nuclear factor-κB through IκB kinase-alpha and IκB kinase-beta. J Biol Chem. 1999;274:8355–8358. doi: 10.1074/jbc.274.13.8355. [DOI] [PubMed] [Google Scholar]
- 104.Zhong H, Voll R E, Ghosh S. Phosphorylation of NF-κB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell. 1998;1:661–671. doi: 10.1016/s1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]