Abstract
Owing to their capacity to rapidly spread across the population, airborne pathogens represent a significant risk to global health. Indeed, several of the past major global pandemics have been instigated by respiratory pathogens. A greater understanding of the immune cells tasked with protecting the airways from infection will allow for the development of strategies that curb the spread and impact of these airborne diseases. A specific subset of memory T-cell resident in both the upper and lower respiratory tract, termed tissue-resident memory (Trm), have been shown to play an instrumental role in local immune responses against a wide breadth of both viral and bacterial infections. In this review, we discuss factors that influence respiratory tract Trm development, longevity, and immune surveillance and explore vaccination regimes that harness these cells, such approaches represent exciting new strategies that may be utilized to tackle the next global pandemic.
Memory T-cell subsets
Immunological memory is defined as the ability of the immune system to respond more rapidly and effectively to pathogens that have been encountered previously. A crucial element of this acquired protective immunity is the generation of memory T cells, which in comparison with their naive counterparts, have increased frequency, effector function, and broader localization. Memory T cells can be divided into three subsets based on localization/trafficking, phenotype, and function; these subsets are termed central memory (Tcm), effector memory (Tem), and tissue-resident memory T cells (Trm)1–5. Tcm are highly proliferative and express the lymph node homing molecules CCR7 and CD62L, which promote homing to secondary lymphoid organs. Conversely, Tem expres slow levels of CCR7 and CD62L, and instead, express integrins and chemokine receptors that facilitate entry into tissues6,7. Studies using tissue transplantation2,8 and parabiosis9–11 demonstrated that an additional non-recirculating, self-sustaining class of memory T-cell persists long term within tissues—these cells were termed Trm.
Trm are the most abundant memory T-cell subset11 residing in barrier tissues such as the skin2,12,13, lung12,14–18, gut5, nasal tissue19, and reproductive tract20–22, non-barrier tissues including the brain23, liver24, and kidney25, as well as lymphoid tissue26–30. The positioning of Trm in diverse tissues with distinct microenvironments drives the development of Trm that are phenotypically and functionally different. At most sites, Trm are characterized by the downregulation of CCR7 and CD62L, which serve to prevent tissue egress, and the upregulation of adhesion molecules including CD103, CD69, CXCR3, and the integrin CD49a that collectively act to maintain tissue localization12,31–33. The co-expression of CD69 and CD103 are Trm signature markers. The constitutive expression of CD69 on Trm limits tissue egress by antagonizing S1P1-mediated extravasation34–36, whereas the expression of CD103 which binds to E-cadherin present on epithelial cells in barrier tissues supports tissue retention37–39. It is important to note that co-expression of CD69 and CD103 does not always mark all bona fide Trm, and hence there is the requirement for additional techniques including parabiosis, intravascular labeling, and tissue transplantation to validate true tissue residency (reviewed in ref. 40–42). Trm are transcriptionally programmed for rapid effector function43,44 and displays prototypic T-cell effector functions such as cytotoxicity and cytokine production2,9,17,22,24,45. Trm also have innate-like “sensing and alarming” properties that can recruit other immune cells to control antimicrobial infections3,46–48. Although Trm are effector-like and thus terminally differentiated, recent evidence shows that Trm can proliferate in situ following local antigen re-encounter49,50. Together, these reports highlight Trm as localized memory T cells that are poised to provide immediate frontline immunity.
Trm can be found in the upper (nasal mucosa) and in the lower (lung) respiratory tract and these cells play a critical role in the local defense against respiratory infections. The co-expression of CD69 and CD103 can be used to identify CD8+ Trm in the lung and nose in both mice and humans15,19, whereas lung CD4+ Trm express CD69, with or without CD10344,45,51. Lung Trm additionally express the adhesion molecules CD11a(LFA-1) for entry and CD49a(VLA-1) for retention51–54, with recent evidence supporting a role for CD49a (and not CD103) in facilitating Trm motility32. Pulmonary Trm can reside in either the airways (epithelium) or parenchyma (interstitium). The cells in these distinct pulmonary compartments have different phenotypic and functional profiles that likely reflect adaption to their local niche. For example, human airway CD8+ T cells have greater expression of CD103 compared with their parenchymal counterparts55. Moreover, murine airway Trm express a distinct transcriptional and epigenetic profile, displaying a pro-apoptotic genetic signature and increased cellular stress levels56. Functionally, in comparison with CD8+ Trm localized to the airways, CD8+ Trm lodged in the parenchyma possess greater cytolytic function, expressing elevated levels of granzyme B as well as an increased capacity to synthesize IFNγ and TNFα57,58. Although Trm lodged in the lung parenchyma are poised for activation, in part owing to their constitutive expression of deployment-ready mRNA encoding effector molecules43,44, the additional expression of inhibitory receptors such as PD-1 on these cells suggests they are also restrained, an approach to potentially minimize unwarranted activation and local immunopathology43,59.
In this review, we will discuss factors that influence respiratory tract Trm development, longevity, and immune surveillance and explore vaccination strategies aimed at evoking this highly protective tissue-bound memory T-cell subset.
The development of respiratory tract Trm
Although there are universal requirements for Trm development across different tissues60, the local microenvironment in which these cells develop heavily influences their differentiation. An intricate assortment of both intrinsic and extrinsic factors, which can act on the T cell either early during priming, or later, during the effector phase, can determine whether selection into the pulmonary Trm pool occurs (Fig. 1).
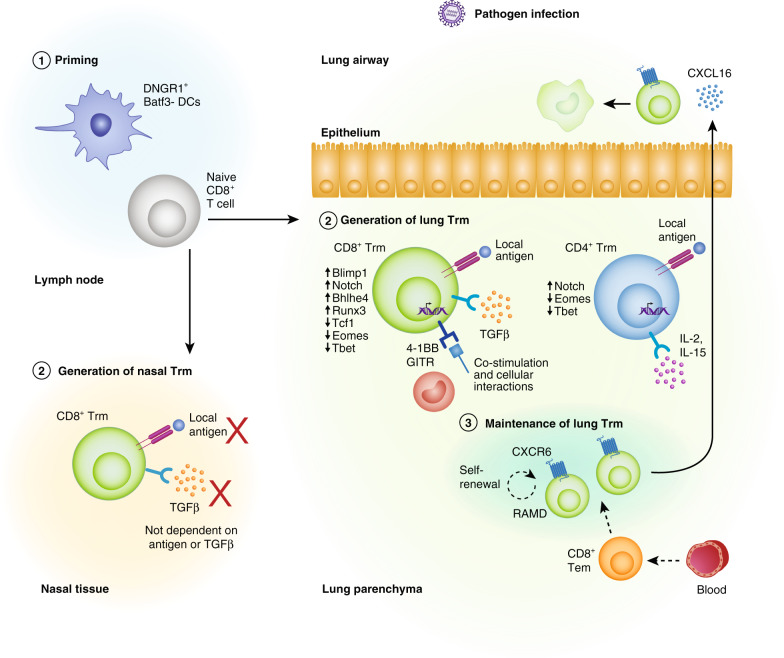
Fig. 1. Respiratory tract Trm generation and maintenance.
1) Upon respiratory pathogen encounter, dendritic cells (DCs) migrate to the mediastinal lymph node to activate naive CD8+ T cells for which effector CD8+ T cells then migrate into the nasal or lung tissue for their conversion into Trm. 2) In the lung, local tissue factors including antigen, cytokines, co-stimulation, and cellular interactions together with a tissue-resident transcriptional profile drive the formation of Trm. The development of nasal CD8+ Trm is independent of local antigen or TGFβ cytokine production. 3) The maintenance of parenchyma lung CD8+ Trm has been proposed to be dependent on replenishment from circulating CD8+ Tem cells or via in situ homeostatic proliferation of lung CD8+ Trm in sites of tissue regeneration called repair-associated memory depots (RAMDs). CD8+ Trm in such sites were shown to replenish the pro-apoptotic airway CD8+ Trm compartment via a CXCR6-CXCL16 axis. The maintenance of lung CD4+ Trm is less studied, though IL-7 has been shown to be required for their maintenance.
During T-cell priming, both the subset of dendritic cell (DC) and the strength of the T-cell receptor (TCR) signal can impact pulmonary Trm development. Specific DC subsets preferentially drive Trm development. Iborra et al.61 show that murine CD8+ T-cell priming in the mediastinal lymph node by cross-presenting DNGR1+ Batf3− DCs is essential for the establishment of lung CD8+ Trm, however, the development of circulating memory T cells was shown to be less reliant on this DC population. TCR signal strength can also influence selection into the lung Trm pool. Utilizing a panel of influenza A virus strains engineered to express defined epitopes of varying degrees of affinity for a fixed TCR transgenic CD8+ T cell, Fiege et al.62 demonstrate a negative correlation between TCR signal strength and lung CD8+ Trm formation, a correlation that was also observed for Trm in other tissues63. The authors propose that this bias in Trm formation for lower affinity cells helps to ensure a broad TCR diversity in the Trm pool, a characteristic that may prevent escape from CD8+ T cell-mediated pathogen control.
Following T-cell priming, effector CD8+ T cells migrate to the respiratory tract where local conditioning events driven by (i) cognate antigen recognition (ii) the cytokine milieu, and (iii) cellular interactions and co-stimulation are required for optimal antiviral effector CD8+ T-cell responses64,65 and for the successful development of pulmonary Trm. The following sections will use predominantly mouse studies to expand on how the above-mentioned local conditioning events drive Trm development.
i) Local cognate antigen recognition
In general, lung Trm requires local cognate antigen recognition for their development16,17,37,66 although, this dependency can be bypassed with the use of certain stimulants that trigger a very specific inflammatory milieu within the lung microenvironment67. The requirement of local antigen recognition for lung CD8+ Trm formation influences the immunodominance hierarchy within the lung Trm pool19. Using an influenza virus mouse model, it was shown that different specificities of influenza-reactive CD8+ T cells were recruited into the lung Trm pool with varying efficiencies. The relative epitope abundance within the lung over the course of the influenza virus infection was identified as a major factor that modulated the immunodominance hierarchy within the Trm compartment. The dependence on local antigen recognition for lung Trm development may serve as a selection process to induct the most “fit” T cells into the Trm pool in a tissue where space is limited. In contrast, nasal CD8+ Trm form without the need for local antigen recognition19, and as such, there appears to be no local bias in T-cell selection, with all T-cell specificities in this region having a comparable Trm conversion rate19.
ii) Local cytokine milieu
Exposure to TGFβ is essential for lung (as well as skin and intestine) Trm development12,68,69 as it promotes the expression of CD10325,37,70. The source of biologically active TGFβ in the lung has been shown to be derived from Type 1 regulatory T cells71 and DCs70,72. It is worth noting that nasal CD8+ Trm, in contrast to their lung counterparts, develops independently of TGFβ19, and to date, the cytokines driving the emergence of this Trm pool remain undefined. In addition, TGFβ exposure serves to cause the downregulation of the T-box transcription factors T-bet and Eomes, the repression of which is required for Trm development73. Though not reported specifically for lung CD8+ Trm, other cytokines such as IL-33 and TNFα have been shown to support the establishment of CD8+ Trm in other tissues36. Exposure to these inflammatory cytokines promotes the downregulation of the transcription factor, Krüppel-like factor 2, and the downregulation of the tissue exit receptor S1PR1, which serves to limit tissue egress36. In regards to lung CD4+ Trm, the cytokines IL-2 and IL-15 were shown to be important for their generation in mouse models of influenza, LCMV, and asthma18,74,75, whereas exposure to IL-7 was crucial for their maintenance in animal models of Klebsiella pneumonia and allergy76,77.
iii) Local cellular interactions and co-stimulation
A diverse network of local cellular interactions within the tissue further supports the differentiation of lung Trm. Early work demonstrated that the presence of IFNγ-producing CD4+ T cells in the lung was necessary for efficient lung airway CD8+ Trm development78. More recently, a lung CD4+ T-cell subset that co-exhibited phenotypic and transcriptional profiles of follicular helper T cells and Trm cells, termed tissue-resident T helper (Trh) cells, were also shown to support the generation of local CD8+ T cells via IL-21-dependent mechanisms79,80. Pulmonary monocytes also promote the differentiation and persistence of lung CD8+ Trm through their interaction with effector T cells and their capacity to locally present antigen81,82. In contrast to the beneficial role of pulmonary monocytes in lung Trm development, tissue-resident alveolar macrophages were reported to be negative regulators of murine CD8+ Trm differentiation83, although this may not be the case for human lung CD8+ and CD4+ Trm84. Work compiled by the Watts group further supports a role for local co-stimulation by inflammatory antigen-presenting cells (APCs) in the generation of lung Trm—a phenomenon they refer to as signal 4. They show that signaling via the TNF receptor family members, 4-1BB (CD137) and GITR are required for the optimal accumulation of lung CD4+ and CD8+ Trm85–87.
The local conditioning events that act on Trm within the local microenvironment of the lung drives the expression of a transcriptional signature within these T cells that encourages tissue residency. Although the transcription factors Hobit and Blimp1, which cooperatively act to suppress the expression of proteins involved in tissue egress (CCR7 and S1PR1), control the generation of CD8+ Trm across different tissues including the skin, liver, kidney, and small intestine88, in the lung, it has been shown that Blimp1 alone regulates CD8+ Trm formation89. Downregulation of T-bet and Eomes is also required for lung Trm development73. Elegant work by the Farber group studying the kinetics of T-bet expression in lung Trm development across different age groups further highlights T-bet as a rheostat for the regulation of effector and Trm cell generation. Investigating in an infant mouse model of influenza and in pediatric patients screened for viral respiratory tract infections (mostly respiratory syncytial virus (RSV)), they show infant T cells expressed greater levels of T-bet compared with adult T cells, and this promoted effector memory T-cell generation but inhibited Trm formation90,91. Additional transcription factors, which are important in lung Trm development have been identified (Table 1), and these collectively regulate the expression of proteins that promote tissue retention and survival.
Table 1.
Transcription factors (TF) that regulate respiratory tract Trm.
| TF | ↑/↓ | Function | Lung Trm population | Ref. |
|---|---|---|---|---|
| Notch | ↑ | Regulates CD103 expression, controls metabolic functions in Trm; required for maintenance of Trm |
Human CD8+CD103+ Human CD4+CD103+ |
43,44 |
| Bhlhe4 | ↑ | Survival and function of Trm; CD103 regulation via acetylation of Itgae; required for Runx3 TF expression |
Mouse CD8+CD69+CD103+ Human CD8+CD103+ |
43,142 |
| Runx3 | ↑ | Required for Trm formation; overexpression enhances lung Trm differentiation; suppresses tissue egress genes (S1pr1, Ccr7) | Mouse CD8+CD69+CD103+ | 143 |
| Blimp1 | ↑ | Suppress expression of tissue egress proteins (CCR7/S1PR1); suppresses Tcf1 TF | Mouse CD8+CD69+CD103+ | 89 |
| Tcf1 | ↓ | Binds to the Itgae locus inhibiting CD103 expression | Mouse CD8+CD103+ | 144 |
| T-bet | ↓ | Downregulation required for TGFb signaling and CD103 expression; residual expression necessary for survival (IL-15) |
Mouse CD8+CD69−/+CD103+ Mouse CD4+CD69+ Human CD8+CD103+ Human CD4+CD103+ |
43,44,73,90,145 |
| Eomes | ↓ | Downregulation required for TGFb signaling |
Mouse CD8+CD103+ Human CD8+CD103+ Human CD4+CD103+ |
43,44,73 |
The protective capacity of respiratory tract trm
The respiratory tract represents an entry point into the body for an array of pathogens. Many studies highlight the importance of respiratory tract Trm in the protection against respiratory pathogens (Table 2). The following sections will highlight studies that show a protective role for respiratory tract Trm against a range of clinically relevant viral and bacterial pulmonary infections.
Table 2.
Trm responses elicited by respiratory tract pathogens.
| Type | Pathogen | Tissue | Trm markers | Ref. |
|---|---|---|---|---|
| Virus | Influenza | LI |
Mouse CD8+CD69+/−CD103+/−(PD-1hi, IFITM3+, CD11a+, CD49a+, Ly6C−) Mouse CD4+CD69+(CD11a+, PD-1+, FR4lo/hi, PSGL1lo/hi) Human CD8+CD69+/−CD103+/−(HLA-DR+, NKG2A+, CD11a+) |
16,17,19,37,45,47,51,52,56,58,59,66,68,74,78–81,85–87,89,90,92–97,125,128,135,146 |
| LA |
Mouse CD8+CD69+CD103+(CD49a+) Human CD8+CD69+CD103+(CD49a+, CD101+, PD-1hi) Human CD4+CD69+CD103+/−(PD-1hi, CD49a+, CD101+) |
52,55–58,66,84,125 | ||
| Nasal | Mouse CD8+CD69+CD103+ | 19 | ||
| mLN | Mouse CD8+CD69+CD103+(Ly6C−) | 29,94 | ||
| Sendai | LA | Mouse CD8+ | 57 | |
| RSV | LA |
Mouse CD8+CD69+CD103+ Human CD8+CD69+CD103+ Human CD4+CD69+CD103+/− |
99,100,147 | |
| LI |
Mouse CD8+CD69+CD103+ Mouse CD4+CD69+CD103−(CD49d+CD11ahi) |
100,101 | ||
| SARS-CoV-2 | LA |
Human CD8+CD69+CD103+/−(HLA-DR+, PD-1+) Human CD4+CD69+CD103+/−(HLA-DR+, PD-1+) |
104–106 | |
| Vaccina virus | LI | Mouse CD8+CD69+CD103+/−(CXCR3lo/hi) | 82,126 | |
| LA | Mouse CD8+CXCR3hi | 126 | ||
| Bacteria | Streptococcus pneumoniae | LI | Mouse CD4+CD69+(CD11ahi) | 108,113 |
| Nasal | Mouse CD4+CD69+(CD11ahi) | 109 | ||
| Bordetella pertussis | LI | Mouse CD4+CD69+ | 110,148 | |
| Nasal | Mouse CD4+CD69+ | 109,110,148 | ||
| Klebsiella pneumoniae | LA | Mouse CD4+CD69+CD103− | 76 | |
| Mycobacterium tuberculosis | LI | Mouse CD4+CD69+(CXCR3hi, PD-1hi) | 111 | |
| LA | Human CD4+(CD45RO+) | 112 | ||
| Fungi | Aspergillus fumigatus | LI | Mouse CD4+CD69hiCD103lo | 149 |
| Parasite | Nippostrongylus brasiliensis | LI | Mouse CD4+(CD44+CD62L−) | 150 |
LI lung Interstitium, LA lung airway, mLn mediastinal lymph node. Text within brackets indicates additional markers expressed by respiratory tract Trm.
Respiratory viral infections
It is well characterized that respiratory tract Trm have a critical role in the protection against influenza virus infection. An elegant study by Wu et al.17 was the first to highlight the indispensable role of local tissue-bound memory CD8+ T cells in mediating cross-protective immunity against the influenza virus. Using a mouse model, they show that heterosubtypic immunity against influenza virus is lost 6–7 months after primary infection, even though a large population of influenza-specific CD8+ Tem and Tcm remained within the circulation. The waning of protective immunity against secondary influenza challenge tightly correlated with a loss of lung influenza-specific CD8+ Trm17,19,92. Influenza-reactive CD8+ Trm are also present in the upper airways of mice, where they are ideally situated to block the transmission of inhaled influenza virus into the lower respiratory tract, and in doing so, can prevent severe pulmonary disease19. Pulmonary Trm deposit around bronchus-associated lymphoid tissues in the lung parenchyma68 and confer protection via rapid and robust IFNγ and TNFα cytokine production upon reactivation57,93. Interestingly, recent evidence suggests the quality of the Trm re-call response can be influenced by the identity of the APC which triggers their reactivation, with presentation by hematopoietic APCs-regulating chemokine/cytokine production, and presentation by nonhematopoietic APCs-regulating proliferation94. Studies characterizing the immune cell landscape in lung tissue from human organ donors revealed that the human lung harbors a large pool of influenza-specific CD8+ Trm51,93,95–97. These cells were shown to be highly proliferative and polyfunctional, composed of a diverse TCRαβ repertoire, and a proportion were cross-reactive against multiple influenza strains93,98. Influenza virus infection can also be attenuated by lung CD4+ Trm which are positioned within inducible bronchus-associated lymphoid tissues51,68. Experiments that utilized a transgenic CD4+ T cell-specific for influenza hemagglutinin demonstrated the generation of influenza-specific CD4+ Trm and their protective capacity in preventing weight loss and promoting rapid viral clearance in mice45.
Lung Trm have also been shown to protect against RSV infection. Using an elegant experimental human challenge model, Jozwik et al.99 identified RSV-specific CD8+ Trm in the airways and showed that high numbers of virus specific airway CD8+ Trm, but not virus specific circulating blood T cells, correlated with less-severe lower respiratory tract symptoms and reduced viral loads post RSV challenge. Subsequent murine studies corroborate this data and show that airway and parenchyma CD8+ T cells protect against secondary RSV infection100,101.
Of great relevance, pulmonary Trm limit the severity of SARS-coronavirus infection. Early work in mouse models established that the deposition of IFNγ-producing airway CD4+ Trm and parenchymal CD8+ Trm could provide protection against SARS-CoV-1 infection102,103. More recently it has been demonstrated that lung CD4+ and CD8+ Trm were present in SARS-CoV-2-infected patients and reduced disease severity was shown to be associated with increased numbers of airway CD4+ and CD8+ Trm104,105. These Trm cells produced IFNγ upon in vitro stimulation and importantly persisted for at least 10 months in convalescent patients104,106. Although less is known about SARS-CoV-2-specific Trm, it has been reported that the depletion of CD8+ T cells in non-human primates prior to re-challenge with SARS-CoV-2 resulted in elevated viral titers in the nasal tissue, which may indicate a role for nasal CD8+ Trm in protection107. Collectively, this emerging body of work suggests CD4+ and CD8+ respiratory tract Trm may play a key role in the protection against SARS-CoV-2 infection.
Respiratory bacterial infections
In bacterial infection models of Streptococcus pneumoniae108,109, Bordetella pertussis110, Klebsiella pneumoniae76, and Mycobacterium tuberculosis111,112 respiratory tract CD4+ Trm have been shown to contribute to bacterial clearance, typically via the production of the pro-inflammatory cytokines IL-17 and IFNγ. An interesting report by Shenoy et al.113 provides insight into how mouse CD4+ Trm improves bacterial clearance from the lung. They show that IL-17-producing CD4+ Trm can stabilize CXCL5 transcripts produced by lung epithelial cells, which in turn enhanced neutrophil recruitment and pneumococcal clearance. Bystander activation of nonspecific lung Trm cells triggered by a local bacterial infection in mice was also shown to boost neutrophil recruitment into the airways, which attenuated the severity of the S. aureus bacterial pneumonia47. This work highlights the protective role of anti-bacterial Trm through their involvement in accelerating innate immune responses.
The persistence of respiratory tract trm
The capacity to persevere long-term within tissues is an important characteristic of Trm. Although the stable long-term persistence of Trm has been documented in a variety of tissues including the nose, skin, liver, and intestinal mucosal19,21,24,92,114,115, pulmonary CD8+ Trm possess an unusually short half-life (12 days in mice)19,67. This attrition is consequential as animal studies clearly show that a loss of influenza-specific Trm in the parenchyma and airways correlates with waning cross-protective immunity17,116,117. Similarly, influenza-specific CD8+ Trm in human lung tissue wane with advanced age, and this resulted in a lag in the development of an antiviral response following influenza exposure95. The attrition of lung CD8+ Trm is not restricted to influenza-specific Trm cells, as similar findings are observed following Sendai virus116,118 and RSV101 infections in mice. Although murine lung CD4+CD69+ Trm also decline, their decay is less rapid relative to CD8+ Trm18,51,119.
Studies in mouse models have given rise to several theories to explain the attrition of lung Trm, put simply, it has been suggested that lung Trm wane because they either die, leave, or fail to be replenished. Initial work revealed that memory CD8+ T cells located in the airways lose sensitivity to the cytokines IL-7 and IL-15120, a defect that was likely driven by the airway microenvironment as transfer of cells from the spleen into airways resulted in the downregulation of these pro-survival cytokine receptors121. Airway and lung parenchymal CD8+ Trm also display a pro-apoptotic phenotype and express elevated levels of active caspases 3/7 and reduced levels of the anti-apoptotic molecule Bcl-256,92. This then raises the question as to why pulmonary CD8+ Trm are prone to cell death. Evidence suggests that the local microenvironment of the lung, which is oxygen-rich and characterized by cellular stress and amino acid starvation is not suitable for long term T-cell survival or optimal functional capacity56,122, and this is perhaps designed to stop the excessive accumulation of T cells in a delicate site, vital for respiration123.
An alternative explanation for the decay of lung CD8+ Trm was recently explained by the repositioning of lung CD8+ Trm via lymphatic vessels to the draining mediastinal LN29. These cells committed to the residency profile as they did not equilibrate among immunized parabiotic mice and continued to express Trm signature markers CD69 and CD10329. Although this mobility blurs the definition of Trm as sentinels permanently residing in tissues, the authors suggest this feature of Trm serves to provide protection in the draining LN and/or acts to repopulate the downstream non-lymphoid tissue after reinfection, thus serving as a mechanism by which local T cells can contribute to systemic protection26.
While CD8+ Trm in most tissues exist independently of the circulating memory T-cell pool2,5,42,51, it has been proposed that the maintenance of both airway and interstitial CD8+ Trm is reliant on continual replenishment by circulating CD8+ Tem cells92,116,124. With time, the frequency of circulating Trm precursors waned, this, coupled with the resolution of the inflammatory signature within the lung, was proposed to contribute to the gradual loss of lung Trm92. In contrast to this model, other studies report that lung interstitial and airway CD8+ Trm are essentially maintained without recruitment from the circulating T-cell pool56,58,125. Instead, these studies show that lung interstitial CD8+ Trm are maintained in sites of tissue regeneration by homeostatic proliferation, and it is these local T cells that replenish the airway CD8+ Trm compartment58,66,125,126. Migration into the airways was dependent on the expression of the chemokine receptor CXCR6 on interstitial CD8+ Trm (and not CD8+ Tem), which mobilized these cells in a process driven by an airway epithelial cell generated CXCL16 gradient58. Although it remains unclear whether local or circulating memory T cells reseed the pulmonary Trm pool (Fig. 1), this necessity for continued supplementation together with a time-dependent loss of Trm precursors and the resolution of pulmonary inflammation may underlie the impermanence of the lung Trm compartment17,92,125,127.
To compensate for the transient nature of lung CD8+ Trm, researchers have investigated different approaches that can extend the longevity of these cells. Utilizing a consecutive adoptive transfer model to generate CD8+ T cells with varying levels of antigen exposure, Van Braechkel-Budimir et al.128 demonstrated that quaternary-boosted influenza-specific CD8+ Trm persisted significantly longer in the lung tissue compared with primary-boosted lung Trm. This improved stability in the lung Trm compartment due to multiple antigenic exposures extended the duration of cross-protective immunity against influenza challenge128. This work highlights that repeated antigen exposure improves the durability and survival of CD8+ Trm within the microenvironment of the lung. In line with this, studies delivering into the lung a replication-defective adenovirus expressing the influenza virus nucleoprotein resulted in persistent local antigen exposure and this evoked a population of influenza-specific CD8+ Trm in the respiratory tract that remained stable for at least 1 year following vaccination129. Importantly, these cells did not become exhausted and could provide long-term protection against secondary influenza virus infection. In humans, this repeated stimulation or cross-reactivity from a lifetime of infections and/or annual vaccinations may also account for the observation of donor-derived CD8+ Trm persisting for over a year post-transplantation in the airways of lung transplant recipients55. Since the size of the lung Trm compartment often correlates with protection against respiratory pathogens17,99, it is important to understand mechanisms that account for lung Trm decay and identify approaches that can circumvent this attrition.
Vaccine strategies to generate respiratory tract trm
Trm deposited along the respiratory tract convey protective immunity against respiratory infections. As such, vaccines that evoke respiratory tract Trm are likely to provide potent protection against airborne pathogens. Insights gained from the study of respiratory tract Trm development has revealed key factors that should be considered when developing a vaccine aimed at promoting pulmonary Trm development. For example, as local cognate antigen recognition in the lung significantly improves the induction of pulmonary Trm16,37,70, vaccines aiming to generate pulmonary Trm should deliver vaccine antigen into the airways. Vaccination strategies tested in mice support this theory, as intranasal but not systemic/parenteral immunizations generated pulmonary Trm against influenza130, RSV131, M. tuberculosis132, vaccina virus126, and SARS-CoV-2133. Thus, the route of vaccine administration is a critical factor that determines whether pulmonary Trm develops.
Several types of vaccine formulations have been tested for their capacity to trigger respiratory tract Trm (Table 3) and most have shown excellent efficacy in animal studies. Many studies have investigated the use of vaccine vectors that utilize viruses such as adenovirus, Modified Vaccina Ankara (MVA), and murine cytomegalovirus (MCMV) to deliver antigens intracellularly and to evoke Trm development. However, careful consideration is warranted when selecting these vectors as the route of administration and/or the inflammatory milieu evoked by different vaccine vectors can influence the localization of memory CD8+ T cells and in turn impact lung Trm differentiation134,135. Furthermore, the use of vaccine vectors must additionally factor in pre-existing vector immunity that may impair vaccine efficacy136 and safety concerns of using live replicating vectors in the elderly and immunocompromised will also need to be addressed.
Table 3.
Vaccine strategies that generate respiratory tract Trm.
| Strategy | Type | Respiratory pathogen |
|---|---|---|
| Vaccine vectors | Adenovirus | Influenza129,151, SARS-CoV-2133, Mycobacterium tuberculosis152 |
| MCMV | Influenza153, RSV131,154 | |
| MVA | Influenza155 | |
| Influenza A virus | Mycobacterium tuberculosis156 | |
| CMV | Mycobacterium tuberculosis157 | |
| Sendai virus | Mycobacterium tuberculosis158 | |
| Vaccina virus | SARS-CoV102 | |
| Particulate vaccines | Nanoparticles | Influenza137,139,159,160, Mycobacterium tuberculosis161 |
| Virus-like particles | Influenza162, RSV163 | |
| Other | Attenuation | Influenza130, Brucella abortus164, Mycobacterium tuberculosis132, Streptococcus pneumoniae109 |
| Antibody-targeted vaccination | Influenza70 | |
| Chitosan-hydrogel | Influenza140 | |
| Outer membrane vesicles | Bordetella pertussis165 | |
| Virus replicon particle | SARS-CoV/MERS-CoV103 |
To bypass issues associated with viral vectors, an alternative vaccination approach to generate pulmonary Trm cells is to directly deposit antigen in the lung through antibody-targeted vaccination (ATV) to lung DCs70 or through the use of particulate vaccines (reviewed in ref. 137). The latter of which includes nanoparticles that offer the advantage of shielding antigen from degradation compared with naked antigen delivery by ATV. Nanoparticles confer further advantages by engineering properties that promote Trm development such as antigen persistence138. Recent work demonstrated a single intranasal dose of a nanoparticle vaccine incorporating influenza virus nucleoprotein induced numbers of lung CD8+ Trm cells exceeding natural infection and this vaccination regime conferred protection against lethal influenza challenge139.
Studies on respiratory tract Trm vaccination strategies have predominately focused on lodging Trm in the lung. However, the uncontrolled deposition of Trm in this delicate, vital organ, may unintentionally cause lung tissue damage and compromise respiratory function59. To mitigate the potential challenges associated with depositing Trm in the lower airways, we recommend the consideration of the nasal mucosa as an alternative site for induction of airborne pathogen-specific Trm. We have previously shown that influenza-specific Trm can be deposited in stable quantities in the nasal tissue of mice, these cells do not decline as aggressive as lung Trm, and in turn, offer long-term protective immunity19. In a proof of principle study, we further demonstrate that nasal CD8+ Trm can be induced by a chitosan-hydrogel vaccine that sustains antigen retention in the nasal cavity and that these cells can provide potent protection in a murine influenza virus challenge model140.
Collectively, mouse studies showcase a range of immunization approaches to evoke respiratory tract Trm with a common requirement of delivering the immunization agent into the respiratory tract to facilitate optimal Trm formation. The appropriate selection of adjuvant, delivered by the correct route, can also greatly improve vaccines that aim to elicit lung Trm development67,141. For example, in a proof of concept study, we show that zymosan, an adjuvant derived from yeast cell walls, when co-administered to mice intranasally with influenza vaccines can significantly boost lung Trm development67. A greater understanding of the appropriate local inflammatory milieu triggered by these Trm promoting adjuvants will allow for the generation of refined adjuvants that evoke conditions that favor lung Trm development with minimal side effects.
Conclusion
The events of the past 12 months exemplify the catastrophic impact newly emerging respiratory pathogens can have on global health and world economies. Then again, they also demonstrate that vaccination is an extraordinarily effective strategy to curb the spread and impact of airborne diseases. To develop vaccines that protect against respiratory pathogens, it is essential to identify the immune cells that best protect the airways from infection. Trm lodged along the respiratory tract provide exquisite protection against respiratory pathogens. Although vaccines that evoke respiratory tract Trm hold significant therapeutic potential, care should be taken to ensure that elevating these cells does not increase the risk of immunopathology, allergy, or chronic airway inflammation. Vaccines that can safely deposit stable populations of Trm along the respiratory tract represent exciting new approaches that may be utilized to tackle the next global pandemic.
Acknowledgements
L.M.W. is supported by funding from the National Health and Medical Research Council (NHMRC) and Australian Research Council (ARC).
Author contributions
M.Z. and L.M.W. contributed to the conception, writing, critical review, and final approval of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 2.Gebhardt T, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 3.Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity. 2014;41:886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat. Rev. Immunol. 2016;16:79–89. doi: 10.1038/nri.2015.3. [DOI] [PubMed] [Google Scholar]
- 5.Masopust D, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 2010;207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 7.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 8.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 9.Jiang X, et al. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iijima N, Iwasaki A. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science. 2014;346:93–98. doi: 10.1126/science.1257530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinert EM, et al. Quantifying Memory CD8 T Cells Reveals Regionalization of Immunosurveillance. Cell. 2015;161:737–749. doi: 10.1016/j.cell.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackay LK, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 2013;14:1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- 13.Glennie ND, et al. Skin-resident memory CD4+ T cells enhance protection against Leishmania major infection. J. Exp. Med. 2015;212:1405–1414. doi: 10.1084/jem.20142101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson KG, et al. Cutting edge: intravascular staining redefines lung CD8 T cell responses. J. Immunol. 2012;189:2702–2706. doi: 10.4049/jimmunol.1201682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Purwar R, et al. Resident memory T cells (T(RM)) are abundant in human lung: diversity, function, and antigen specificity. PLoS One. 2011;6:e16245. doi: 10.1371/journal.pone.0016245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wakim LM, Gupta N, Mintern JD, Villadangos JA. Enhanced survival of lung tissue-resident memory CD8(+) T cells during infection with influenza virus due to selective expression of IFITM3. Nat. Immunol. 2013;14:238–245. doi: 10.1038/ni.2525. [DOI] [PubMed] [Google Scholar]
- 17.Wu T, et al. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J. Leukoc. Biol. 2014;95:215–224. doi: 10.1189/jlb.0313180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hondowicz BD, et al. Interleukin-2-dependent allergen-specific tissue-resident memory cells drive asthma. Immunity. 2016;44:155–166. doi: 10.1016/j.immuni.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pizzolla, A. et al. Resident memory CD8(+) T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci. Immunol.2, eaam6970 (2017). [DOI] [PubMed]
- 20.Cuburu N, et al. Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses. J. Clin. Invest. 2012;122:4606–4620. doi: 10.1172/JCI63287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mackay LK, et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc. Natl. Acad. Sci. USA. 2012;109:7037–7042. doi: 10.1073/pnas.1202288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012;491:463–467. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc. Natl. Acad. Sci. USA. 2010;107:17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez-Ruiz D, et al. Liver-resident memory CD8(+) T cells form a front-line defense against malaria liver-stage infection. Immunity. 2016;45:889–902. doi: 10.1016/j.immuni.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Casey KA, et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J. Immunol. 2012;188:4866–4875. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beura LK, et al. T cells in nonlymphoid tissues give rise to lymph-node-resident memory T cells. Immunity. 2018;48:327–338 e325. doi: 10.1016/j.immuni.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beura LK, et al. CD4(+) resident memory T cells dominate immunosurveillance and orchestrate local recall responses. J. Exp. Med. 2019;216:1214–1229. doi: 10.1084/jem.20181365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonseca R, et al. Developmental plasticity allows outside-in immune responses by resident memory T cells. Nat. Immunol. 2020;21:412–421. doi: 10.1038/s41590-020-0607-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stolley, J. M. et al. Retrograde migration supplies resident memory T cells to lung-draining LN after influenza infection. J. Exp. Med. 217, e20192197 (2020). [DOI] [PMC free article] [PubMed]
- 30.Klicznik, M. M. et al. Human CD4(+)CD103(+) cutaneous resident memory T cells are found in the circulation of healthy individuals. Sci. Immunol.4, eaav8995 (2019). [DOI] [PMC free article] [PubMed]
- 31.Topham DJ, Reilly EC. Tissue-resident memory CD8(+) T cells: from phenotype to function. Front. Immunol. 2018;9:515. doi: 10.3389/fimmu.2018.00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reilly EC, et al. TRM integrins CD103 and CD49a differentially support adherence and motility after resolution of influenza virus infection. Proc. Natl. Acad. Sci. USA. 2020;117:12306–12314. doi: 10.1073/pnas.1915681117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar BV, et al. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep. 2017;20:2921–2934. doi: 10.1016/j.celrep.2017.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiow LR, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 35.Mackay LK, et al. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J. Immunol. 2015;194:2059–2063. doi: 10.4049/jimmunol.1402256. [DOI] [PubMed] [Google Scholar]
- 36.Skon CN, et al. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat. Immunol. 2013;14:1285–1293. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee YT, et al. Environmental and antigen receptor-derived signals support sustained surveillance of the lungs by pathogen-specific cytotoxic T lymphocytes. J. Virol. 2011;85:4085–4094. doi: 10.1128/JVI.02493-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hadley GA, Bartlett ST, Via CS, Rostapshova EA, Moainie S. The epithelial cell-specific integrin, CD103 (alpha E integrin), defines a novel subset of alloreactive CD8+ CTL. J. Immunol. 1997;159:3748–3756. [PubMed] [Google Scholar]
- 39.Cepek KL, et al. Adhesion between epithelial-cells and T-lymphocytes mediated by E-cadherin and the alpha(E)beta(7) integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 40.Szabo, P. A., Miron, M. & Farber, D. L. Location, location, location: tissue resident memory T cells in mice and humans. Sci. Immunol.4, eaas9673 (2019). [DOI] [PMC free article] [PubMed]
- 41.Reagin KL, Klonowski KD. Incomplete memories: the natural suppression of tissue-resident memory CD8 T cells in the lung. Front. Immunol. 2018;9:17. doi: 10.3389/fimmu.2018.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masopust D, Soerens AG. Tissue-resident T cells and other resident leukocytes. Annu. Rev. Immunol. 2019;37:521–546. doi: 10.1146/annurev-immunol-042617-053214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hombrink P, et al. Programs for the persistence, vigilance and control of human CD8(+) lung-resident memory T cells. Nat. Immunol. 2016;17:1467–1478. doi: 10.1038/ni.3589. [DOI] [PubMed] [Google Scholar]
- 44.Oja AE, Piet B, Helbig C, Stark R, van der Zwan D, Blaauwgeers H, et al. Trigger-happy resident memory CD4(+) T cells inhabit the human lungs. Mucosal Immunol. 2018;11:654–667. doi: 10.1038/mi.2017.94. [DOI] [PubMed] [Google Scholar]
- 45.Teijaro JR, et al. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol. 2011;187:5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8(+) T cells. Nat. Immunol. 2013;14:509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ge C, et al. Bystander activation of pulmonary Trm cells attenuates the severity of bacterial pneumonia by enhancing neutrophil recruitment. Cell Rep. 2019;29:4236–4244 e4233. doi: 10.1016/j.celrep.2019.11.103. [DOI] [PubMed] [Google Scholar]
- 48.Ariotti S, et al. T cell memory. Skin-resident memory CD8(+) T cells trigger a state of tissue-wide pathogen alert. Science. 2014;346:101–105. doi: 10.1126/science.1254803. [DOI] [PubMed] [Google Scholar]
- 49.Beura LK, et al. Intravital mucosal imaging of CD8(+) resident memory T cells shows tissue-autonomous recall responses that amplify secondary memory. Nat. Immunol. 2018;19:173–182. doi: 10.1038/s41590-017-0029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park SL, et al. Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat. Immunol. 2018;19:183–191. doi: 10.1038/s41590-017-0027-5. [DOI] [PubMed] [Google Scholar]
- 51.Turner DL, et al. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 2014;7:501–510. doi: 10.1038/mi.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McMaster SR, et al. Pulmonary antigen encounter regulates the establishment of tissue-resident CD8 memory T cells in the lung airways and parenchyma. Mucosal Immunol. 2018;11:1071–1078. doi: 10.1038/s41385-018-0003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ray SJ, et al. The collagen binding α1β1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity. 2004;20:167–179. doi: 10.1016/S1074-7613(04)00021-4. [DOI] [PubMed] [Google Scholar]
- 54.Galkina E, et al. Preferential migration of effector CD8+ T cells into the interstitium of the normal lung. J. Clin. Invest. 2005;115:3473–3483. doi: 10.1172/JCI24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Snyder, M. E. et al. Generation and persistence of human tissue-resident memory T cells in lung transplantation. Sci. Immunol.4, eaav5581 (2019). [DOI] [PMC free article] [PubMed]
- 56.Hayward SL, et al. Environmental cues regulate epigenetic reprogramming of airway-resident memory CD8(+) T cells. Nat. Immunol. 2020;21:309–320. doi: 10.1038/s41590-019-0584-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McMaster SR, Wilson JJ, Wang H, Kohlmeier JE. Airway-resident memory CD8 T. Cells provide antigen-specific protection against respiratory virus challenge through rapid IFN-gamma production. J. Immunol. 2015;195:203–209. doi: 10.4049/jimmunol.1402975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wein AN, et al. CXCR6 regulates localization of tissue-resident memory CD8 T cells to the airways. J. Exp. Med. 2019;216:2748–2762. doi: 10.1084/jem.20181308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang, Z. et al. PD-1(hi) CD8(+) resident memory T cells balance immunity and fibrotic sequelae. Sci. Immunol. 4, eaaw1217 (2019). [DOI] [PMC free article] [PubMed]
- 60.Mackay LK, Kallies A. Transcriptional regulation of tissue-resident lymphocytes. Trends Immunol. 2017;38:94–103. doi: 10.1016/j.it.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 61.Iborra S, et al. Optimal generation of tissue-resident but not circulating memory T cells during viral infection requires crosspriming by DNGR-1(+) dendritic cells. Immunity. 2016;45:847–860. doi: 10.1016/j.immuni.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fiege JK, et al. The impact of TCR signal strength on resident memory T cell formation during influenza virus infection. J. Immunol. 2019;203:936–945. doi: 10.4049/jimmunol.1900093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maru S, Jin G, Schell TD, Lukacher AE. TCR stimulation strength is inversely associated with establishment of functional brain-resident memory CD8 T cells during persistent viral infection. PLoS Pathog. 2017;13:e1006318. doi: 10.1371/journal.ppat.1006318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McGill J, Van Rooijen N, Legge KL. IL-15 trans-presentation by pulmonary dendritic cells promotes effector CD8 T cell survival during influenza virus infection. J. Exp. Med. 2010;207:521–534. doi: 10.1084/jem.20091711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McGill J, Van Rooijen N, Legge KL. Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. J. Exp. Med. 2008;205:1635–1646. doi: 10.1084/jem.20080314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takamura S, et al. Specific niches for lung-resident memory CD8+ T cells at the site of tissue regeneration enable CD69-independent maintenance. J. Exp. Med. 2016;213:3057–3073. doi: 10.1084/jem.20160938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caminschi I, Lahoud MH, Pizzolla A, Wakim LM. Zymosan by-passes the requirement for pulmonary antigen encounter in lung tissue-resident memory CD8(+) T cell development. Mucosal Immunol. 2019;12:403–412. doi: 10.1038/s41385-018-0124-2. [DOI] [PubMed] [Google Scholar]
- 68.Yoshizawa A, et al. TCR-pMHC encounter differentially regulates transcriptomes of tissue-resident CD8 T cells. Eur. J. Immunol. 2018;48:128–150. doi: 10.1002/eji.201747174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang N, Bevan MJ. Transforming growth factor-beta signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity. 2013;39:687–696. doi: 10.1016/j.immuni.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wakim LM, Smith J, Caminschi I, Lahoud MH, Villadangos JA. Antibody-targeted vaccination to lung dendritic cells generates tissue-resident memory CD8 T cells that are highly protective against influenza virus infection. Mucosal Immunol. 2015;8:1060–1071. doi: 10.1038/mi.2014.133. [DOI] [PubMed] [Google Scholar]
- 71.Ferreira C, et al. Type 1 Treg cells promote the generation of CD8(+) tissue-resident memory T cells. Nat. Immunol. 2020;21:766–776. doi: 10.1038/s41590-020-0674-9. [DOI] [PubMed] [Google Scholar]
- 72.Yu CI, et al. Human CD1c+ dendritic cells drive the differentiation of CD103+ CD8+ mucosal effector T cells via the cytokine TGF-beta. Immunity. 2013;38:818–830. doi: 10.1016/j.immuni.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mackay LK, et al. T-box transcription factors combine with the cytokines TGF-beta and IL-15 to control tissue-resident memory T cell fate. Immunity. 2015;43:1101–1111. doi: 10.1016/j.immuni.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 74.Strutt TM, et al. IL-15 supports the generation of protective lung-resident memory CD4 T cells. Mucosal Immunol. 2018;11:668–680. doi: 10.1038/mi.2017.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hondowicz BD, Kim KS, Ruterbusch MJ, Keitany GJ, Pepper M. IL-2 is required for the generation of viral-specific CD4(+) Th1 tissue-resident memory cells and B cells are essential for maintenance in the lung. Eur. J. Immunol. 2018;48:80–86. doi: 10.1002/eji.201746928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Amezcua Vesely MC, et al. Effector TH17 cells give rise to long-lived TRM cells that are essential for an immediate response against bacterial infection. Cell. 2019;178:1176–1188 e1115. doi: 10.1016/j.cell.2019.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yeon SM, et al. IL-7 plays a critical role for the homeostasis of allergen-specific memory CD4 T cells in the lung and airways. Sci. Rep. 2017;7:11155. doi: 10.1038/s41598-017-11492-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Laidlaw BJ, et al. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity. 2014;41:633–645. doi: 10.1016/j.immuni.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Son, Y. M. et al. Tissue-resident CD4(+) T helper cells assist the development of protective respiratory B and CD8(+) T cell memory responses. Sci. Immunol. 6, eabb6852 (2021). [DOI] [PMC free article] [PubMed]
- 80.Swarnalekha, N. et al. T resident helper cells promote humoral responses in the lung. Sci. Immunol. 6, eabb6808 (2021). [DOI] [PMC free article] [PubMed]
- 81.Dunbar PR, et al. Pulmonary monocytes interact with effector T cells in the lung tissue to drive TRM differentiation following viral infection. Mucosal Immunol. 2019;13:161–171. doi: 10.1038/s41385-019-0224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Desai P, Tahiliani V, Stanfield J, Abboud G, Salek-Ardakani S. Inflammatory monocytes contribute to the persistence of CXCR3(hi) CX3CR1(lo) circulating and lung-resident memory CD8(+) T cells following respiratory virus infection. Immunol. Cell Biol. 2018;96:370–378. doi: 10.1111/imcb.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goplen NP, et al. Tissue-resident macrophages limit pulmonary CD8 resident memory T cell establishment. Front. Immunol. 2019;10:2332. doi: 10.3389/fimmu.2019.02332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Snyder ME, et al. Human lung-resident macrophages colocalize with and provide costimulation to PD1(hi) tissue-resident memory T cells. Am. J. Respir. Crit. Care Med. 2021;203:1230–1244. doi: 10.1164/rccm.202006-2403OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou AC, Wagar LE, Wortzman ME, Watts TH. Intrinsic 4-1BB signals are indispensable for the establishment of an influenza-specific tissue-resident memory CD8 T-cell population in the lung. Mucosal Immunol. 2017;10:1294–1309. doi: 10.1038/mi.2016.124. [DOI] [PubMed] [Google Scholar]
- 86.Zhou AC, Batista NV, Watts TH. 4-1BB regulates effector CD8 T cell accumulation in the lung tissue through a TRAF1-, mTOR-, and antigen-dependent mechanism to enhance tissue-resident memory T cell formation during respiratory influenza infection. J. Immunol. 2019;202:2482–2492. doi: 10.4049/jimmunol.1800795. [DOI] [PubMed] [Google Scholar]
- 87.Chu KL, Batista NV, Wang KC, Zhou AC, Watts TH. GITRL on inflammatory antigen presenting cells in the lung parenchyma provides signal 4 for T-cell accumulation and tissue-resident memory T-cell formation. Mucosal Immunol. 2019;12:363–377. doi: 10.1038/s41385-018-0105-5. [DOI] [PubMed] [Google Scholar]
- 88.Mackay LK, et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science. 2016;352:459–463. doi: 10.1126/science.aad2035. [DOI] [PubMed] [Google Scholar]
- 89.Behr FM, et al. Blimp-1 rather than hobit drives the formation of tissue-resident memory CD8(+) T cells in the lungs. Front. Immunol. 2019;10:400. doi: 10.3389/fimmu.2019.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zens KD, et al. Reduced generation of lung tissue-resident memory T cells during infancy. J. Exp. Med. 2017;214:2915–2932. doi: 10.1084/jem.20170521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Connors TJ, et al. Developmental regulation of effector and resident memory T cell generation during pediatric viral respiratory tract infection. J. Immunol. 2018;201:432–439. doi: 10.4049/jimmunol.1800396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Slutter, B. et al. Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Sci. Immunol. 2, eaag2031 (2017). [DOI] [PMC free article] [PubMed]
- 93.Pizzolla A, et al. Influenza-specific lung-resident memory T cells are proliferative and polyfunctional and maintain diverse TCR profiles. J. Clin. Invest. 2018;128:721–733. doi: 10.1172/JCI96957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Low, J. S. et al. Tissue-resident memory T cell reactivation by diverse antigen-presenting cells imparts distinct functional responses. J. Exp. Med. 217, e20192291 (2020). [DOI] [PMC free article] [PubMed]
- 95.Nguyen TH, et al. Influenza, but not SARS-CoV-2, infection induces a rapid interferon response that wanes with age and diminished tissue-resident memory CD8(+) T cells. Clin. Transl. Immunol. 2021;10:e1242. doi: 10.1002/cti2.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.de Bree GJ, et al. Selective accumulation of differentiated CD8+ T cells specific for respiratory viruses in the human lung. J. Exp. Med. 2005;202:1433–1442. doi: 10.1084/jem.20051365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Piet B, et al. CD8(+) T cells with an intraepithelial phenotype upregulate cytotoxic function upon influenza infection in human lung. J. Clin. Invest. 2011;121:2254–2263. doi: 10.1172/JCI44675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koutsakos M, et al. Human CD8(+) T cell cross-reactivity across influenza A, B and C viruses. Nat. Immunol. 2019;20:613–625. doi: 10.1038/s41590-019-0320-6. [DOI] [PubMed] [Google Scholar]
- 99.Jozwik A, et al. RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat. Commun. 2015;6:10224. doi: 10.1038/ncomms10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kinnear E, et al. Airway T cells protect against RSV infection in the absence of antibody. Mucosal Immunol. 2018;11:249–256. doi: 10.1038/mi.2017.46. [DOI] [PubMed] [Google Scholar]
- 101.Luangrath MA, Schmidt ME, Hartwig SM, Varga SM, Tissue-Resident Memory T. Cells in the lungs protect against acute respiratory syncytial virus infection. Immunohorizons. 2021;5:59–69. doi: 10.4049/immunohorizons.2000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Channappanavar R, Fett C, Zhao J, Meyerholz DK, Perlman S. Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection. J. Virol. 2014;88:11034–11044. doi: 10.1128/JVI.01505-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhao J, et al. Airway memory CD4(+) T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity. 2016;44:1379–1391. doi: 10.1016/j.immuni.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Szabo, P. A. et al. Longitudinal profiling of respiratory and systemic immune responses reveals myeloid cell-driven lung inflammation in severe COVID-19. Immunity54, 797–814.e6 (2021). [DOI] [PMC free article] [PubMed]
- 105.Liao M, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 106.Grau-Exposito J, et al. Peripheral and lung resident memory T cell responses against SARS-CoV-2. Nat. Commun. 2021;12:3010. doi: 10.1038/s41467-021-23333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McMahan K, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590:630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smith NM, et al. Regionally compartmentalized resident memory T cells mediate naturally acquired protection against pneumococcal pneumonia. Mucosal Immunol. 2018;11:220–235. doi: 10.1038/mi.2017.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.O’Hara JM, et al. Generation of protective pneumococcal-specific nasal resident memory CD4(+) T cells via parenteral immunization. Mucosal Immunol. 2020;13:172–182. doi: 10.1038/s41385-019-0218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wilk MM, et al. Immunization with whole cell but not acellular pertussis vaccines primes CD4 TRM cells that sustain protective immunity against nasal colonization with Bordetella pertussis. Emerg. Microbes Infect. 2019;8:169–185. doi: 10.1080/22221751.2018.1564630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sakai S, et al. Cutting edge: control of Mycobacterium tuberculosis infection by a subset of lung parenchyma-homing CD4 T cells. J. Immunol. 2014;192:2965–2969. doi: 10.4049/jimmunol.1400019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Walrath J, Zukowski L, Krywiak A, Silver RF. Resident Th1-like effector memory cells in pulmonary recall responses to Mycobacterium tuberculosis. Am. J. Respir. Cell Mol. Biol. 2005;33:48–55. doi: 10.1165/rcmb.2005-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shenoy AT, et al. Lung CD4(+) resident memory T cells remodel epithelial responses to accelerate neutrophil recruitment during pneumonia. Mucosal Immunol. 2020;13:334–343. doi: 10.1038/s41385-019-0229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bergsbaken T, Bevan MJ. Proinflammatory microenvironments within the intestine regulate the differentiation of tissue-resident CD8(+) T cells responding to infection. Nat. Immunol. 2015;16:406–414. doi: 10.1038/ni.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sheridan BS, et al. Oral infection drives a distinct population of intestinal resident memory CD8(+) T cells with enhanced protective function. Immunity. 2014;40:747–757. doi: 10.1016/j.immuni.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ely KH, Cookenham T, Roberts AD, Woodland DL. Memory T cell populations in the lung airways are maintained by continual recruitment. J. Immunol. 2005;176:537–543. doi: 10.4049/jimmunol.176.1.537. [DOI] [PubMed] [Google Scholar]
- 117.Flynn KJ, et al. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/S1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 118.Hogan RJ, et al. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J. Immunol. 2001;166:1813–1822. doi: 10.4049/jimmunol.166.3.1813. [DOI] [PubMed] [Google Scholar]
- 119.Turner DL, et al. Biased generation and in situ activation of lung tissue-resident memory CD4 T cells in the pathogenesis of allergic asthma. J. Immunol. 2018;200:1561–1569. doi: 10.4049/jimmunol.1700257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shen CH, et al. Loss of IL-7R and IL-15R expression is associated with disappearance of memory T cells in respiratory tract following influenza infection. J. Immunol. 2008;180:171–178. doi: 10.4049/jimmunol.180.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kohlmeier JE, Miller SC, Woodland DL. Cutting edge: antigen is not required for the activation and maintenance of virus-specific memory CD8+ T cells in the lung airways. J. Immunol. 2007;178:4721–4725. doi: 10.4049/jimmunol.178.8.4721. [DOI] [PubMed] [Google Scholar]
- 122.Clever D, et al. Oxygen sensing by T cells establishes an immunologically tolerant metastatic niche. Cell. 2016;166:1117–1131 e1114. doi: 10.1016/j.cell.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Van Braeckel-Budimir N, Harty JT. Influenza-induced lung Trm: not all memories last forever. Immunol. Cell Biol. 2017;95:651–655. doi: 10.1038/icb.2017.32. [DOI] [PubMed] [Google Scholar]
- 124.Wijeyesinghe, S. et al. Expansible residence decentralizes immune homeostasis. Nature592, 457–462 (2021). [DOI] [PMC free article] [PubMed]
- 125.Takamura S, et al. Interstitial-resident memory CD8(+) T cells sustain frontline epithelial memory in the lung. J. Exp. Med. 2019;216:2736–2747. doi: 10.1084/jem.20190557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gilchuk P, et al. A distinct lung-interstitium-resident memory CD8(+) T cell subset confers enhanced protection to lower respiratory tract infection. Cell Rep. 2016;16:1800–1809. doi: 10.1016/j.celrep.2016.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Takamura S, Kohlmeier JE. Establishment and maintenance of conventional and circulation-driven lung-resident memory CD8(+) T cells following respiratory virus infections. Front. Immunol. 2019;10:733. doi: 10.3389/fimmu.2019.00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Van Braeckel-Budimir N, Varga SM, Badovinac VP, Harty JT. Repeated antigen exposure extends the durability of influenza-specific lung-resident memory CD8(+) T cells and heterosubtypic immunity. Cell Rep. 2018;24:3374–3382 e3373. doi: 10.1016/j.celrep.2018.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Uddbäck, I. et al. Long-term maintenance of lung resident memory T cells is mediated by persistent antigen. Mucosal Immunol.14, 92–99 (2020). [DOI] [PMC free article] [PubMed]
- 130.Zens, K. D., Chen, J. K. & Farber, D. L. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight. 1, e85832 (2016). [DOI] [PMC free article] [PubMed]
- 131.Morabito KM, et al. Intranasal administration of RSV antigen-expressing MCMV elicits robust tissue-resident effector and effector memory CD8+ T cells in the lung. Mucosal Immunol. 2017;10:545–554. doi: 10.1038/mi.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Perdomo, C. et al. Mucosal BCG vaccination induces protective lung-resident memory T cell populations against tuberculosis. mBio7, e01686-16 (2016). [DOI] [PMC free article] [PubMed]
- 133.Hassan AO, et al. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell. 2020;183:169–184 e113. doi: 10.1016/j.cell.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Slutter B, Pewe LL, Kaech SM, Harty JT. Lung airway-surveilling CXCR3(hi) memory CD8(+) T cells are critical for protection against influenza A virus. Immunity. 2013;39:939–948. doi: 10.1016/j.immuni.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Welten, S. P. M. et al. Influenza- and MCMV-induced memory CD8 T cells control respiratory vaccinia virus infection despite residence in distinct anatomical niches. Mucosal Immunol. 14, 728–742 (2021). [DOI] [PMC free article] [PubMed]
- 136.Matsuda, K. et al. A replication-competent adenovirus-vectored influenza vaccine induces durable systemic and mucosal immunity. J. Clin. Invest.131, e140794 (2021). [DOI] [PMC free article] [PubMed]
- 137.Knight, F. C. & Wilson, J. T. Engineering vaccines for tissue-resident memory T cells. Adv. Ther.4, 2000230 (2021). [DOI] [PMC free article] [PubMed]
- 138.Deng L, et al. Heterosubtypic influenza protection elicited by double-layered polypeptide nanoparticles in mice. Proc. Natl. Acad. Sci. USA. 2018;115:E7758–E7767. doi: 10.1073/pnas.1805713115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Knight FC, et al. Mucosal immunization with a pH-responsive nanoparticle vaccine induces protective CD8(+) lung-resident memory T cells. ACS Nano. 2019;13:10939–10960. doi: 10.1021/acsnano.9b00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bedford, J. G., Caminschi, I. & Wakim, L. M. Intranasal delivery of a chitosan-hydrogel vaccine generates nasal tissue resident memory CD8(+) T cells that are protective against influenza virus infection. Vaccines (Basel)8, 572 (2020). [DOI] [PMC free article] [PubMed]
- 141.Wang, J. et al. Pulmonary surfactant-biomimetic nanoparticles potentiate heterosubtypic influenza immunity. Science367, eaau0810 (2020). [DOI] [PMC free article] [PubMed]
- 142.Li C, et al. The transcription factor Bhlhe40 programs mitochondrial regulation of resident CD8(+) T cell fitness and functionality. Immunity. 2019;51:491–507 e497. doi: 10.1016/j.immuni.2019.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Milner JJ, et al. Runx3 programs CD8(+) T cell residency in non-lymphoid tissues and tumours. Nature. 2017;552:253–257. doi: 10.1038/nature24993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wu J, et al. T cell factor 1 suppresses CD103+ lung tissue-resident memory T cell development. Cell Rep. 2020;31:107484. doi: 10.1016/j.celrep.2020.03.048. [DOI] [PubMed] [Google Scholar]
- 145.Dhume K, Finn CM, Strutt TM, Sell S, McKinstry KK. T-bet optimizes CD4 T-cell responses against influenza through CXCR3-dependent lung trafficking but not functional programming. Mucosal Immunol. 2019;12:1220–1230. doi: 10.1038/s41385-019-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Goplen, N. P. et al. Tissue-resident CD8(+) T cells drive age-associated chronic lung sequelae after viral pneumonia. Sci. Immunol. 5, eabc4557 (2020). [DOI] [PMC free article] [PubMed]
- 147.Guvenel A, et al. Epitope-specific airway-resident CD4+ T cell dynamics during experimental human RSV infection. J. Clin. Invest. 2020;130:523–538. doi: 10.1172/JCI131696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Allen AC, et al. Sustained protective immunity against Bordetella pertussis nasal colonization by intranasal immunization with a vaccine-adjuvant combination that induces IL-17-secreting TRM cells. Mucosal Immunol. 2018;11:1763–1776. doi: 10.1038/s41385-018-0080-x. [DOI] [PubMed] [Google Scholar]
- 149.Ichikawa T, et al. CD103(hi) Treg cells constrain lung fibrosis induced by CD103(lo) tissue-resident pathogenic CD4 T cells. Nat. Immunol. 2019;20:1469–1480. doi: 10.1038/s41590-019-0494-y. [DOI] [PubMed] [Google Scholar]
- 150.Thawer SG, et al. Lung-resident CD4(+) T cells are sufficient for IL-4Ralpha-dependent recall immunity to Nippostrongylus brasiliensis infection. Mucosal Immunol. 2014;7:239–248. doi: 10.1038/mi.2013.40. [DOI] [PubMed] [Google Scholar]
- 151.Uddback IE, et al. Combined local and systemic immunization is essential for durable T-cell mediated heterosubtypic immunity against influenza A virus. Sci. Rep. 2016;6:20137. doi: 10.1038/srep20137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Haddadi S, et al. Expression and role of VLA-1 in resident memory CD8 T cell responses to respiratory mucosal viral-vectored immunization against tuberculosis. Sci. Rep. 2017;7:9525. doi: 10.1038/s41598-017-09909-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zheng X, et al. Mucosal CD8+ T cell responses induced by an MCMV based vaccine vector confer protection against influenza challenge. PLoS Pathog. 2019;15:e1008036. doi: 10.1371/journal.ppat.1008036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Morabito KM, et al. Memory inflation drives tissue-resident memory CD8(+) T cell maintenance in the lung after intranasal vaccination with murine cytomegalovirus. Front. Immunol. 2018;9:1861. doi: 10.3389/fimmu.2018.01861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Puksuriwong S, et al. Modified vaccinia ankara-vectored vaccine expressing nucleoprotein and matrix protein 1 (M1) activates mucosal M1-specific T-cell immunity and tissue-resident memory T cells in human nasopharynx-associated lymphoid tissue. J. Infect. Dis. 2020;222:807–819. doi: 10.1093/infdis/jiz593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Florido M, et al. Pulmonary immunization with a recombinant influenza A virus vaccine induces lung-resident CD4(+) memory T cells that are associated with protection against tuberculosis. Mucosal Immunol. 2018;11:1743–1752. doi: 10.1038/s41385-018-0065-9. [DOI] [PubMed] [Google Scholar]
- 157.Hansen SG, et al. Prevention of tuberculosis in rhesus macaques by a cytomegalovirus-based vaccine. Nat. Med. 2018;24:130–143. doi: 10.1038/nm.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hu Z, et al. Sendai virus mucosal vaccination establishes lung-resident memory CD8 T cell immunity and boosts BCG-primed protection against TB in mice. Mol. Ther. 2017;25:1222–1233. doi: 10.1016/j.ymthe.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lin PH, et al. Robust induction of TRMs by combinatorial nanoshells confers cross-strain sterilizing immunity against lethal influenza viruses. Mol. Ther. Methods Clin. Dev. 2021;21:299–314. doi: 10.1016/j.omtm.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zacharias ZR, et al. Polyanhydride nanovaccine induces robust pulmonary B and T cell immunity and confers protection against homologous and heterologous influenza A virus infections. Front. Immunol. 2018;9:1953. doi: 10.3389/fimmu.2018.01953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hart P, et al. Nanoparticle-fusion protein complexes protect against mycobacterium tuberculosis infection. Mol. Ther. 2018;26:822–833. doi: 10.1016/j.ymthe.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lee YN, Lee YT, Kim MC, Gewirtz AT, Kang SM. A novel vaccination strategy mediating the induction of lung-resident memory CD8 T cells confers heterosubtypic immunity against future pandemic influenza virus. J. Immunol. 2016;196:2637–2645. doi: 10.4049/jimmunol.1501637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Schwarz B, et al. Viruslike particles encapsidating respiratory syncytial virus M and M2 proteins induce robust T cell responses. ACS Biomater. Sci. Eng. 2016;2:2324–2332. doi: 10.1021/acsbiomaterials.6b00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang H, Hoffman C, Yang X, Clapp B, Pascual DW. Targeting resident memory T cell immunity culminates in pulmonary and systemic protection against Brucella infection. PLoS Pathog. 2020;16:e1008176. doi: 10.1371/journal.ppat.1008176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zurita ME, et al. A pertussis outer membrane vesicle-based vaccine induces lung-resident memory CD4 T cells and protection against bordetella pertussis, including pertactin deficient strains. Front. Cell Infect. Microbiol. 2019;9:125. doi: 10.3389/fcimb.2019.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]