Abstract
Objective
To determine whether subconjunctival or intrastromal administration of anti-VEGF agents is more effective on suture-induced corneal neovascularization (CoNV) in rabbits.
Methods
CoNV was induced in 48 eyes of 24 New Zealand white rabbits by using an 8/0 silk suture. On the 7th day after suturing, the rabbits were divided into four treatment groups as follows: six rabbits received subconjunctival bevacizumab (group 1), six rabbits received subconjunctival aflibercept (group 2), six rabbits received intrastromal bevacizumab (group 3) and six rabbits received intrastromal aflibercept (group 4). On the 7th and 14th days after suturing, the CoNV area was calculated by standardised analysis of photographs using the Image-J program. On the 14th day after suturing, all rabbits were sacrificed and then corneal tissue was harvested for the analysis of vascular endothelial growth factor (VEGF)-A, VEGF-B and placental growth factor (PIGF) levels.
Results
On the 7th day after suturing, CoNV areas were 17.10 ± 2.98, 18.88 ± 3.78, 17.36 ± 4.52, 18.57 ± 4.16 and 17.31 ± 2.81 mm2 in the groups 1–4 and control group, respectively. On the 7th day after intervention and removal of suture, CoNV areas were 4.85 ± 1.99, 6.66 ± 1.73, 2.83 ± 1.08, 2.63 ± 1.16 and 11.93 ± 2.64 mm2 in the group 1–4 and control group, respectively. CoNV area was reduced by 88.1% and 82.5% in eyes receiving intrastromal aflibercept and bevacizumab, respectively (both p < 0.001), and by 64.5% and 69.9% in eyes receiving subconjunctival aflibercept and bevacizumab, respectively (both p = 0.001).
Conclusion
Intrastromal anti-VEGF therapy regressed CoNV more effectively than subconjunctival therapy regardless of the type of anti-VEGF agent.
Subject terms: Outcomes research, Corneal diseases
Introduction
Corneal neovascularization (CoNV), characterised by vascular sprouting from the limbus to clear cornea, commonly occurs secondarily to ocular chemical burns, post-infection, ocular surface inflammatory disease and keratoplasty [1]. Management of CoNV is vital because it may result in sight-threatening complications if it grows into central zone of the cornea. Furthermore, vascularised host corneas are at higher risk of corneal transplant rejection with the risk increasing with each quadrant of vascularisation [2].
In recent decades, anti-VEGF agents such as pegabtanib, ranibizumab, bevacizumab and aflibercept have been administered topically or subconjunctivally to treat CoNV [3–5]. Bevacizumab, a humanised monoclonal antibody that inhibits all isoforms of VEF-A, is used more often in the treatment of CoNV compared with other traditional anti-VEGF agents because it is cost-effective, and has strong therapeutic efficacy [6]. Aflibercept, a VEGF-Trap molecule, is a relatively new agent that inhibits VEGF-B and placental growth factor (PIGF) as well as VEGF-A [7]. A limited number of studies have evaluated the efficacies of aflibercept and bevacizumab in the treatment of CoNV; however, some authors have reported that aflibercept is more effective than bevacizumab [8, 9], while others have reported that both agents have similar efficacy in reducing CoNV [5].
Currently, there is no consensus on whether the therapeutic agent should be administered topically, subconjunctivally or intrastromally. Topical administration has some disadvantages such as rapid clearance by tears, variability of corneal penetration and corneal epithelial toxicity [10, 11]. Therefore, subconjunctival administration has been widely adopted in treating CoNV [10, 12, 13]. However, it has a disadvantage of rapid elimination from subconjunctival space by conjunctival blood capillaries.
Experimental studies have focused on topical and subconjunctival administration of therapeutic agents [14]. Knowledge on the intrastromal administration route for the treatment of CoNV remains limited to a small number of clinical case series, and one animal study in which bevacizumab was delivered in the corneal stromal using a bevacizumab-coated microneedle [15–17].
Therefore, we aimed to evaluate the effect of subconjunctival and intrastromal injection of anti-VEGFs on an experimental CoNV model. We also aimed to compare the efficacies of bevacizumab and aflibercept in regressing CoNV.
Materials and methods
This experimental study was conducted in accordance with guidelines on the care and use of animals adopted by the Society for Neuroscience and Association for Research in Vision and Ophthalmology. The Animal Experiments Local Ethics Committee at the University of Abant Izzet Baysal (Bolu, Turkey) approved the study (Approval No: 2019/15).
Animals
Twenty-four adult male New Zealand White rabbits weighing 2500–3000 g were involved in this study. We confirmed that all rabbits had normal avascular corneas prior to the experiment. All rabbits were housed in a room with a 12:12-h light–dark cycle. Intramuscular ketamine hydrochloride (50 mg/kg) and xylazine (5 mg/kg) were used for deep anaesthesia. Proparacaine hydrochloride 0.5% (Alcaine; Alcon, Fort Worth, TX) was used for topical corneal anaesthesia. Euthanasia was performed using cardiac puncture under deep anaesthesia at the end of the study. Corneal sections were extracted following euthanasia.
Induction of CoNV
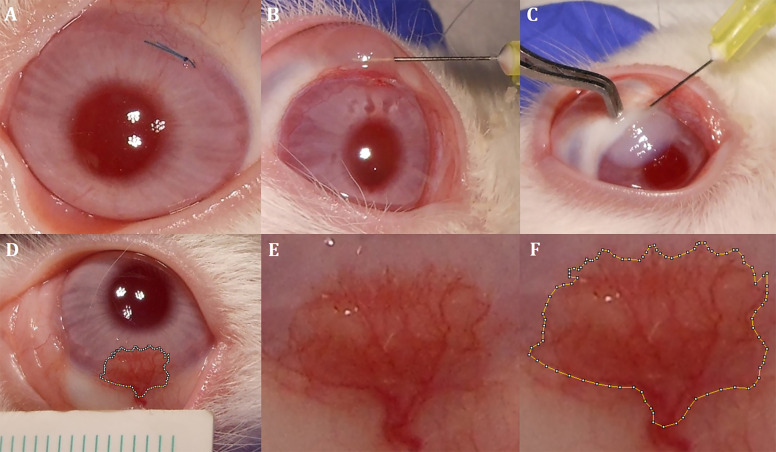
Under general and ocular topical anaesthesia, we placed 8/0 silk sutures (FSSB, Germany) horizontally on the superior part of the peripheral cornea in both eyes of each rabbit to induce CoNV. Sutures were adjusted to 3 mm in length and 1 mm away from the limbus, and the knots and suture ends were left exposed (Fig. 1A). We instilled moxifloxacin 0.5% (Vigamox, Alcon Laboratories) four times a day for 1 week for bacterial prophylaxis. At the end of the first week, we examined all rabbits and verified the development of CoNV.
Fig. 1. Interventions to the cornea and measurement of CoNV area.
A 8/0 silk suture placement on the superior part of the cornea. B Subconjunctival administration technique. C Intrastromal administration technique. D Standard imaging of CoNV area and adjacent ruler. E Magnified CoNV area without marking. F Magnified CoNV area surrounded by manual marking.
Treatment protocols
After confirming of CoNV development, all sutures were removed. We divided the rabbits into four groups according to the type of treatment. Each group consisted of six rabbits. After randomising the eyes to intervention and control groups using flipping a coin method, only one eye of the rabbits in each group received a single dose of 0.05-mL anti-VEGF treatment, which were as follows: subconjunctival bevacizumab (1.25 mg/0.05 mL, Avastin; Genentech and Roche), subconjunctival aflibercept (2 mg/0.05 mL, Eylea; Regeneron Pharmaceuticals, Bayer, Switzerland), intrastromal bevacizumab (1.25 mg/0.05 mL) and intrastromal aflibercept (2 mg/0.05 mL), respectively. The untreated fellow eyes of the rabbits served as the control group and received subconjunctival or intrastromal balanced saline solution. Subconjunctival injections were administered using a Becton–Dickinson (BD, Franklin Lakes, NJ) 30-gauge needle 1 mm posterior of the limbus adjacent to the CoNV area (Fig. 1B). Intrastromal injections were administered using a BD 30-gauge needle 1 mm anterior of the limbus adjacent to the CoNV area (Fig. 1C).
Analysis of CoNV area
On the 7th and 14th days, standard images were recorded at a distance of 10 cm at 1 × 1 magnification using a digital camera (Nikon Coolpix A10, Japan) attached to a light microscope. In order to standardise the calculation of area, a millimetre ruler was placed in the neighbourhood of the CoNV area. Two masked researchers (RKU and AYU) surrounded the CoNV area manually and calculated its area by using the Image-J program (Fig. 1D–F) [18]. The average of two measurements was considered for statistical analysis.
VEGF-A, VEGF-B and PIGF assay
On 14th day after suturing, all 48 eyes of 24 rabbits were enucleated. Neovascularized corneal sections were extracted to a 3 × 3-mm size, as described in previous studies [5, 19, 20], and maintained at −80 °C. Corneal tissues were homogenised mechanically after adding phosphate-buffered saline (0.01 M, pH 7.4). Homogenised samples were centrifuged for 10 min at 8000 rpm and the supernatants were collected. VEGF-A, VEGF-B and PIGF concentrations in supernatants were measured using a sandwich enzyme-linked immunosorbent assay (ELISA), according to manufacturer’s instructions. We used three different ELISA kits for the measurement of VEGF-A (Rabbit VEGF-A ELISA kit, MyBioSource, Cat. No: MBS015064), VEGF-B (Rabbit VEGF-B ELISA kit, MyBioSource, Cat. No: MBS2511947) and PIGF (Rabbit PIGF ELISA kit, MyBioSource, Cat. No: MBS2602252) levels. Absorbance of ELISA test results was read spectrophotometrically at a wavelength of 450 nm. VEGF-A, VEGF-B and PIGF levels were expressed as pg/mL per mg tissue.
Statistical analysis
Before the experiment, the sample size of each group was defined considering the power of each analysis ≥0.8 in Gpower 1.3.9.4. version software. We used the SPSS software for Windows version 22.0 (Chicago, IL) to analyse the collected data from the present study. Wilcoxon signed-rank test was used to compare the CoNV areas at first week with those at second week. Kruskal–Wallis test was used to compare the levels of VEGF-A, VEGF-B and PIGF among the groups, and to determine effect of the interventions on CoNV compared with control eyes. The Mann–Whitney U test was also used for pairwise comparison. We presented the outcomes by using boxplots because of the nonparametric distribution of the collected data. A p value < 0.05 was considered as statistically significant.
Results
Morphologic evaluation of CoNV area
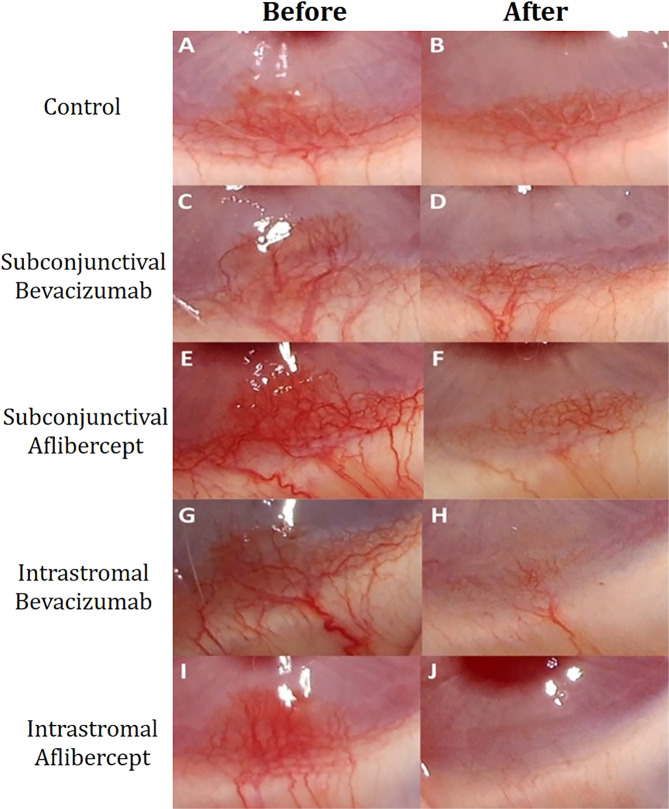
In all eyes, the development of the CoNV in the quadrant where the suture is placed validated the model employed. CoNV areas decreased significantly in all groups. In control group, eyes received subconjunctival injection and eyes received intrastromal injection were similar with regard to CoNV areas at first and second weeks (18.02 ± 4.40 mm2 vs. 16.59 ± 2.01 mm2 at first week, p = 0.776; 12.72 ± 2.81 mm2 vs. 11.15 ± 3.91 mm2 at second week, p = 0.847). Combining the data from these eyes, we considered a single control group, when comparing them with intervention groups. One week after the induction of neovascularization, CoNV areas were calculated as 17.10 ± 2.98, 18.88 ± 3.78, 17.36 ± 4.52, 18.57 ± 4.16 and 17.31 ± 2.81 mm2 in the groups 1–4 and control group, respectively. One week after the intervention and removal of suture, CoNV areas were calculated as 4.85 ± 1.99, 6.66 ± 1.73, 2.83 ± 1.08, 2.63 ± 1.16 and 11.93 ± 2.64 mm2 in the groups 1–4 and control group, respectively. Extent of CoNV was significantly decreased in eyes receiving intrastromal anti-VEGF administration. CoNV area was reduced by 88.1% and 82.5% in eyes receiving intrastromal aflibercept and bevacizumab, respectively (both p < 0.001), and by 64.5% and 69.9% in eyes receiving subconjunctival aflibercept and bevacizumab, respectively (both p = 0.001). Table 1 shows the change in CoNV areas for each group. Furthermore, change of CoNV areas in each group is represented by images in Fig. 2. In pairwise comparison with the control group, all intervention groups showed higher change in CoNV area (p = 0.010 for subconjunctival bevacizumab vs. control, p = 0.005 for subconjuctival aflibercept vs. control, p = 0.003 for intrastromal bevacizumab vs. control, and p < 0.001 for intrastromal aflibercept vs. control) (Fig. 3A).
Table 1.
Reduction in CoNV area in the groups.
| Groups | CoNV area (mm2) (first week) | CoNV area (mm2) (second week) | Change in CoNV area (mm2) | Percentage of reduction in CoNV area (%) | Pa |
|---|---|---|---|---|---|
| Control | 17.31 ± 2.81 | 11.93 ± 2.64 | 5.29 ± 3.20 | 30.3% | 0.028* |
| Subconjunctival bevacizumab | 17.10 ± 2.98 | 4.85 ± 1.99 | 12.25 ± 4.28 | 69.9% | 0.001*** |
| Subconjunctival aflibercept | 18.88 ± 3.78 | 6.66 ± 1.73 | 12.21 ± 2.91 | 64.5% | 0.001*** |
| Intrastromal bevacizumab | 17.36 ± 4.52 | 2.83 ± 1.08 | 14.53 ± 4.93 | 82.5% | <0.001*** |
| Intrastromal aflibercept | 18.57 ± 4.16 | 2.63 ± 1.16 | 16.43 ± 4.19 | 88.1% | <0.001*** |
| Pb | 0.851 | <0.001*** | 0.007** |
Bold values indicate statistical significance.
aWilcoxon signed-rank test was used to compare the CoNV areas at first week (before the treatment) with those at second week (1 week after the treatment).
bKruskal–Wallis test was used to compare the CoNV areas at first and second week, and the change in CoNV area among the groups.
Fig. 2. Representative images of each group before and 1 week after the treatment.
A, B CoNV areas in the control group before and 1 week after the saline injection. C, D CoNV areas in the subconjunctival bevacizumab group before and 1 week after the injection. E, F CoNV areas in the subconjunctival aflibercept group before and 1 week after the injection. G, H CoNV areas in the intrastromal bevacizumab group before and 1 week after the injection. I, J CoNV areas in the intrastromal aflibercept group before and 1 week after the injection.
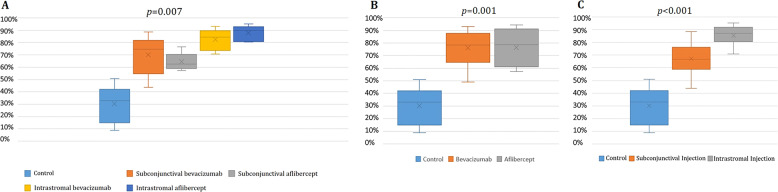
Fig. 3. Percent reductions in CoNV areas in different treatment groups.
Percent reductions in CoNV area were 30.3% in the control group, 69.9% in the subconjuctival bevacizumab group, 64.5% in the subconjunctival aflibercept group, 82.5% in the intrastromal bevacizumab group and 88.1% in the intrastromal aflibercept group (p* = 0.007). In pairwise comparison of each group with the control group, p# = 0.010 for subconjunctival bevacizumab vs. control, p# = 0.005 for subconjuctival aflibercept vs. control, p# = 0.003 for intrastromal bevacizumab vs. control, and p# < 0.001 for intrastromal aflibercept vs. control. A Percent reductions in CoNV area were 76.2% in the bevacizumab group, and 76.3% in the aflibercept group, regardless of the administration route (p* = 0.001), p# = 0.002 for bevacizumab vs. control, p# = 0.001 for aflibercept vs. control, p# = 0.609 for bevacizumab vs. aflibercept). B Percent reductions in CoNV area were 67.2% in the subconjunctival group, and 85.3% in the intrastromal group, regardless of the type of anti-VEGF. (p* < 0.001). In pairwise comparison, p# = 0.005 for subconjunctival injection vs. control, p# < 0.001 for intrastromal injection vs. control, p# = 0.039 for subconjunctival injection vs. aflibercept injection). *Kruskal–Wallis test, #Mann–Whitney U test.
Regardless of the administration route, when eyes treated with bevacizumab were compared with those treated with aflibercept, changes in CoNV areas were similar (13.39 ± 4.56 mm2 and 14.32 ± 4.08 mm2, respectively, p = 0.602, Fig. 3B). Regardless the type of anti-VEGF received, eyes treated with intrastromal injection showed higher change in CoNV area compared with those with subconjunctival injection (15.48 ± 4.47 mm2 and 12.23 ± 3.49 mm2 respectively, p = 0.039, Fig. 3C).
Evaluation of VEGF-A, VEGF-B and PIGF levels
The highest VEGF-A levels (439.6 ± 52.7 pg/mL/mg tissue) were observed in the control group, and the lowest VEGF-A levels (221.6 ± 57.5 pg/mL per mg tissue) were in the intrastromal bevacizumab group. The highest VEGF-B levels (601.7 ± 115.2 pg/mL per mg tissue) were observed in the subconjunctival bevacizumab group, and the lowest VEGF-B levels (246.2 ± 54.5 pg/mL per mg tissue) were in the intrastromal aflibercept group. The highest PIGF levels (34.0 ± 5.2 pg/mL per mg tissue) were observed in the intrastromal bevacizumab group, and the lowest were in the intrastromal aflibercept group (19.3 ± 4.1 pg/mL per mg tissue). Table 2 shows the VEGF-A, VEGF-B and PIGF levels for each group. Regardless of the administration route, eyes treated with aflibercept showed the lowest VEGF-B and PIGF levels (295.9 ± 91.6 and 21.9 ± 5.4 pg/mL per mg tissue). Both the aflibercept- and bevacizumab-treated groups had similar VEGF-A levels (p = 0.619). Regardless the type of anti-VEGF received, eyes treated with intrastromal injections showed lower VEGF-A levels compared with those with subconjunctival injections (230.3 ± 50.3 and 301.5 ± 31.7 pg/mL per mg tissue, respectively, p = 0.001).
Table 2.
VEGF-A, VEGF-B and PIGF levels in each group.
| Groups | VEGF-A (pg/mL per mg tissue) | VEGF-B (pg/mL per mg tissue) | PIGF (pg/mL per mg tissue) |
|---|---|---|---|
| Control | 439.6 ± 52.7 | 573.7 ± 102.3 | 30.4 ± 5.5 |
| Subconjunctival bevacizumab | 298.1 ± 31.9 | 601.7 ± 115.2 | 32.7 ± 7.3 |
| Subconjunctival aflibercept | 304.8 ± 49.2 | 345.7 ± 97.8 | 24.6 ± 5.4 |
| Intrastromal bevacizumab | 221.6 ± 57.5 | 560.5 ± 93.0 | 34.0 ± 5.2 |
| Intrastromal aflibercept | 239.0 ± 45.7 | 246.2 ± 54.5 | 19.3 ± 4.1 |
| pa | <0.001*** | <0.001*** | 0.003** |
Bold values indicate statistical significance. Regarding VEGF-A level, in pairwise comparison with the control group, all intervention groups showed lower VEGF-A levels than the control group (p = 0.004 for subconjunctival bevacizumab vs. control, p = 0.010 for subconjuctival aflibercept vs control, p < 0.001 for intrastromal bevacizumab vs. control, and p < 0.001 for intrastromal aflibercept vs. control). Regarding VEGF-B level, in pairwise comparison with the control group, aflibercept groups showed lower VEGF-B levels than the control group (p = 1.000 for subconjunctival bevacizumab vs. control, p = 0.027 for subconjuctival aflibercept vs. control, p = 1.000 for intrastromal bevacizumab vs. control, and p = 0.002 for intrastromal aflibercept vs. control). Regarding PIGF level, in pairwise comparison with the control group, only the intrastromal aflibercept group showed lower PIGF levels than the control group (p = 1.000 for subconjunctival bevacizumab vs. control, p = 0.636 for subconjuctival aflibercept vs. control, p = 0.964 for intrastromal bevacizumab vs. control, and p = 0.031 for intrastromal aflibercept vs. control).
aKruskal–Wallis test.
Discussion
In the present study, the highest percentage reduction in CoNV (88.1%) was observed in the intrastromal aflibercept group, and the lowest percentage reduction in CoNV (30.3%) was observed in the control group. Intrastromal intervention groups showed more decrease in CoNV area compared with the subconjunctival intervention groups. Furthermore, both bevacizumab- and aflibercept-treated groups showed similar decrease in CoNV area.The vessels continue to mature into the second week post suturing, and therefore earlier suture removal results in some spontaneous regression, as observed in our control group. However, retained sutures require antibiotic drops for bacterial prophylaxis. Suture removal was performed on the 7th day in order to avoid the possible interaction of antibiotic drops with bevacizumab, aflibercept or angiogenesis process. The present study is the first to investigate the effectiveness of the intrastromal injection of anti-VEGFs by comparing it with the subconjunctival injection. We used both anti-VEGF agents at their intravitreal concentrations, which were commercially available.
In recent decades, various anti-neovascular or anti-inflammatory agents including pegaptanib [4], ranibizumab [21], infliximab [20], methotrexate [22], suramin [23], doxycycline [24] and steroids [25] have been tried in the treatment of CoNV; however, bevacizumab has been more accepted because it is easily accessible, inexpensive and effectively inhibits CoNV [14]. Previous studies reported favourable outcomes in the treatment of CoNV when bevacizumab was used alone [26] or in combination with angio-occlusive treatments such as argon laser photocoagulation [27] or photodynamic therapy [19]. Moreover, Petsoglou et al. [28], in a randomised controlled clinical trial, found that three subconjunctival injections of 2.5 mg/0.1 mL bevacizumab were more effective than placebo in regressing of recent-onset CoNV. Recently, aflibercept, a relatively new agent, has been shown to have successful outcomes in regressing choroidal neovascularization [29]. This prompted corneal specialists to investigate the efficacy of aflibercept in the treatment of CoNV. Some authors studying on rabbits model of suture-induced CoNV reported that, similar to the present study, aflibercept was similarly effective to bevacizumab in treating CoNV [5], whereas others studying on rats alkali burn model reported that aflibercept was more effective than bevacizumab [8, 9]. However, Yu et al. [30] revealed that bevacizumab poorly interacted with murine VEGF-A using western bolt analysis. This study might explain why aflibercept was more effective in previous experiments using rats. In the present study, although aflibercept, unlike bevacizumab, effectively inhibited VEGF-B and PIGF, this appeared to make no significant contribution to CoNV reduction.
In the treatment of CoNV, the route of drug administration is as important as determining the most effective drug. Numerous studies have been conducted in order to investigate more effective and safer administration route of the therapeutic agents for treatment of CoNV. A limited number of experimental studies reported that topical bevacizumab and subconjunctival bevacizumab were similarly effective in regressing CoNV [31, 32]. By contrast, Dastjerdi et al. [10] revealed that subconjunctival bevacizumab penetrated into the cornea more than topical bevacizumab in an experimental study. Rocher et al. [12] reported that subconjunctival bevacizumab was more effective than topical bevacizumab in binding to VEGF-A. Furthermore, Bhatti et al. [13] showed that subconjunctival bevacizumab was more effective compared with topical bevacizumab in patients with CoNV. Therefore, the subconjunctival route for bevacizumab has been accepted more.
Recently, some researchers have been investigating new administration routes for the treatment of CoNV because of the association of subconjunctival administration with systemic adverse effects and rapid elimination of drug from the subconjunctival space. Accordingly, Sarah et al. [16] reported that intrastromal bevacizumab successfully regressed CoNV in their case series involving 25 patients. Gupta et al. [15] reported that intrastromal administration of bevacizumab offered effective and durable inhibition of VEGF-A in patients with CoNV. Kim et al. [17] delivered bevacizumab intrastromally using a bevacizumab-coated microneedle, not injecting it directly into the corneal stroma, and reported intrastromal delivery of bevacizumab inhibited the CoNV strongly. Furthermore, Yeung et al. [33] reported favourable outcomes after combined intrastromal and subconjunctival administration of bevacizumab in patients with CoNV. Taking the aforementioned studies and the present study together, the intrastromal administration route seems to have greater efficacy compared with other application routes in regressing CoNV. All these favourable outcomes after intrastromal bevacizumab administration might be explained by the direct injection of bevacizumab into the neovascularization area and long-term elimination of the bevacizumab because of the avascular structure of cornea.
The present study possesses two major limitations. First, we evaluated corneas according to their clinical appearance, not by using a confocal microscope; therefore, we could not report whether the intrastromal agents had an effect on endothelial cells. However, Lichtinger et al. [34] demonstrated that both subconjunctival and intrastromal bevacizumab caused no change in endothelial cell count and morphology with repetitive administration in patients with CoNV. Second, we reported the effect of anti-VEGFs on early-formed CoNV. However, previous studies demonstrated that the effect of anti-VEGFs on late-formed CoNV was limited compared with early-formed CoNV [35–37].
Although this study suggests that intrastromal route for administration of anti-VEGFs may be more effective than subconjunctival route in regressing CoNV, in clinical practice, it should be born in mind that Descemet’ membrane perforation and detachment may occur during the intrastromal injection. However, these complications can be easily eliminated by performing intrastromal injection under the operating microscope and injecting the drug slowly.
In conclusion, the present study showed that intrastromal administration of anti-VEGF agents was more effective in regressing CoNV compared with subconjunctival administration. Although aflibercept inhibited VEGF-B and PIGF as well VEGF-A, this did not make any contribution to the reduction of CoNV. Further clinical studies comparing different administration routes of anti-VEGFs in the treatment of early- and late-formed CoNV should be designed.
Summary
What was known before
Subconjunctival injection of bevacizumab was accepted as the mainstay of the medical treatment of CoNV.
What this study adds
Intrastromal injection of anti-VEGF agents give rise to greater regression of CoNV compared with subconjunctival injection of them.
Both aflibercept and bevacizumab seem to have similar efficacy on early-formed CoNV.
Author contributions
RKU, SC and AYU designed and conducted the study. NSY and NB made substantial contributions to acquisition of biochemical data. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that the research was conducted in the absence of any commercialor financial relationships that could be construed as a potential conflict of interest.
Ethics approval
The Animal Experiments Local Ethics Committee at the University of Abant Izzet Baysal approved the study.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Curr Opin Ophthalmol. 2001;12:242–9. doi: 10.1097/00055735-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann B, Taylor RS, Cursiefen C. Corneal neovascularization as a risk factor for graft failure and rejection after keratoplasty: an evidence-based meta-analysis. Ophthalmology. 2010;117:1300–5.e7. doi: 10.1016/j.ophtha.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 3.Sener E, Yuksel N, Yildiz DK, Yilmaz B, Ozdemir O, Caglar Y, et al. The impact of subconjuctivally injected EGF and VEGF inhibitors on experimental corneal neovascularization in rat model. Curr Eye Res. 2011;36:1005–13. doi: 10.3109/02713683.2011.601840. [DOI] [PubMed] [Google Scholar]
- 4.Akar EE, Oner V, Kucukerdonmez C, Aydin Akova Y. Comparison of subconjunctivally injected bevacizumab, ranibizumab, and pegaptanib for inhibition of corneal neovascularization in a rat model. Int J Ophthalmol. 2013;6:136–40. doi: 10.3980/j.issn.2222-3959.2013.02.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park YR, Chung SK. Inhibitory effect of topical aflibercept on corneal neovascularization in rabbits. Cornea. 2015;34:1303–7. doi: 10.1097/ICO.0000000000000507. [DOI] [PubMed] [Google Scholar]
- 6.Fogli S, Del ReM, Rofi E, Posarelli C, Figus M, Danesi R. Clinical pharmacology of intravitreal anti-VEGF drugs. Eye. 2018;32:1010–20. doi: 10.1038/s41433-018-0021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai S, Yang Q, Li X, Zhang Y. The efficacy and safety of aflibercept and conbercept in diabetic macular edema. Drug Des Devel Ther. 2018;12:3471–83. doi: 10.2147/DDDT.S177192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gal-Or O, Livny E, Sella R, Nisgav Y, Weinberger D, Livnat T, et al. Efficacy of Subconjunctival aflibercept versus bevacizumab for prevention of corneal neovascularization in a rat model. Cornea. 2016;35:991–6. doi: 10.1097/ICO.0000000000000849. [DOI] [PubMed] [Google Scholar]
- 9.Sella R, Gal-Or O, Livny E, Dachbash M, Nisgav Y, Weinberger D, et al. Efficacy of topical aflibercept versus topical bevacizumab for the prevention of corneal neovascularization in a rat model. Exp Eye Res. 2016;146:224–32. doi: 10.1016/j.exer.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 10.Dastjerdi MH, Sadrai Z, Saban DR, Zhang Q, Dana R. Corneal penetration of topical and subconjunctival bevacizumab. Invest Ophthalmol Vis Sci. 2011;52:8718–23. doi: 10.1167/iovs.11-7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SW, Ha BJ, Kim EK, Tchah H, Kim TI. The effect of topical bevacizumab on corneal neovascularization. Ophthalmology. 2008;115:e33–38. doi: 10.1016/j.ophtha.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Rocher N, Behar-Cohen F, Pournaras JA, Naud MC, Jeanny JC, Jonet L, et al. Effects of rat anti-VEGF antibody in a rat model of corneal graft rejection by topical and subconjunctival routes. Mol Vis. 2011;17:104–12. [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatti N, Qidwai U, Hussain M, Kazi A. Efficacy of sub-conjunctival and topical bevacizumab in high-risk corneal transplant survival. J Pak Med Assoc. 2013;63:1256–9. [PubMed] [Google Scholar]
- 14.Sharif Z, Sharif W. Corneal neovascularization: updates on pathophysiology, investigations & management. Rom J Ophthalmol. 2019;63:15–22. doi: 10.22336/rjo.2019.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta AA, Mammo DA, Page MA. Intrastromal bevacizumab in the management of corneal neovascularization: a retrospective review. Graefes Arch Clin Exp Ophthalmol. 2020;258:167–73. doi: 10.1007/s00417-019-04519-4. [DOI] [PubMed] [Google Scholar]
- 16.Sarah B, Ibtissam H, Mohammed B, Hasna S, Abdeljalil M. Intrastromal injection of bevacizumab in the management of corneal neovascularization: about 25 eyes. J Ophthalmol. 2016;2016:6084270. doi: 10.1155/2016/6084270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim YC, Grossniklaus HE, Edelhauser HF, Prausnitz MR. Intrastromal delivery of bevacizumab using microneedles to treat corneal neovascularization. Invest Ophthalmol Vis Sci. 2014;55:7376–86. doi: 10.1167/iovs.14-15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim RY, Chung SK, Kim MS, Ra H. Effects of combined photodynamic therapy and topical bevacizumab treatment on corneal neovascularization in rabbits. Cornea. 2016;35:1615–20. doi: 10.1097/ICO.0000000000001016. [DOI] [PubMed] [Google Scholar]
- 20.Kim JW, Chung SK. The effect of topical infliximab on corneal neovascularization in rabbits. Cornea. 2013;32:185–90. doi: 10.1097/ICO.0b013e318271cc2a. [DOI] [PubMed] [Google Scholar]
- 21.Kim EK, Kong SJ, Chung SK. Comparative study of ranibizumab and bevacizumab on corneal neovascularization in rabbits. Cornea. 2014;33:60–64. doi: 10.1097/ICO.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 22.Byun YS, Chung SK. The effect of methotrexate on corneal neovascularization in rabbits. Cornea. 2011;30:442–6. doi: 10.1097/IAE.0b013e3181e46ad8. [DOI] [PubMed] [Google Scholar]
- 23.Lee HS, Chung SK. The effect of subconjunctival suramin on corneal neovascularization in rabbits. Cornea. 2010;29:86–92. doi: 10.1097/ICO.0b013e3181ae91e3. [DOI] [PubMed] [Google Scholar]
- 24.Jovanovic V, Nikolic L. The effect of topical doxycycline on corneal neovascularization. Curr Eye Res. 2014;39:142–8. doi: 10.3109/02713683.2013.833246. [DOI] [PubMed] [Google Scholar]
- 25.Murata M, Shimizu S, Horiuchi S, Taira M. Inhibitory effect of triamcinolone acetonide on corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2006;244:205–9. doi: 10.1007/s00417-005-0036-1. [DOI] [PubMed] [Google Scholar]
- 26.You IC, Kang IS, Lee SH, Yoon KC. Therapeutic effect of subconjunctival injection of bevacizumab in the treatment of corneal neovascularization. Acta Ophthalmol. 2009;87:653–8. doi: 10.1111/j.1755-3768.2008.01399.x. [DOI] [PubMed] [Google Scholar]
- 27.Lakshmipathy M, Susvar P, Popet K, Rajagopal R. Subconjunctival bevacizumab and argon laser photocoagulation for preexisting neovascularization following deep lamellar anterior keratoplasty. Indian J Ophthalmol. 2019;67:1193–4. doi: 10.4103/ijo.IJO_1583_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petsoglou C, Balaggan KS, Dart JK, Bunce C, Xing W, Ali RR, et al. Subconjunctival bevacizumab induces regression of corneal neovascularisation: a pilot randomised placebo-controlled double-masked trial. Br J Ophthalmol. 2013;97:28–32. doi: 10.1136/bjophthalmol-2012-302137. [DOI] [PubMed] [Google Scholar]
- 29.Papadopoulos Z. Aflibercept: a review of its effect on the treatment of exudative age-related macular degeneration. Eur J Ophthalmol. 2019;29:368–78. doi: 10.1177/1120672119832432. [DOI] [PubMed] [Google Scholar]
- 30.Yu L, Wu X, Cheng Z, Lee CV, LeCouter J, Campa C, et al. Interaction between bevacizumab and murine VEGF-A: a reassessment. Invest Ophthalmol Vis Sci. 2008;49:522–7. doi: 10.1167/iovs.07-1175. [DOI] [PubMed] [Google Scholar]
- 31.Ozdemir O, Altintas O, Altintas L, Ozkan B, Akdag C, Yuksel N. Comparison of the effects of subconjunctival and topical anti-VEGF therapy (bevacizumab) on experimental corneal neovascularization. Arq Bras Oftalmol. 2014;77:209–13.. doi: 10.5935/0004-2749.20140054. [DOI] [PubMed] [Google Scholar]
- 32.Lopes GJA, Casella AMB, Oguido AP, Matsuo T. Effects of topical and subconjunctival use of bevacizumab on corneal neovascularization in rabbits’ eyes. Arq Bras Oftalmol. 2017;80:252–6.. doi: 10.5935/0004-2749.20170061. [DOI] [PubMed] [Google Scholar]
- 33.Yeung SN, Lichtinger A, Kim P, Amiran MD, Slomovic AR. Combined use of subconjunctival and intracorneal bevacizumab injection for corneal neovascularization. Cornea. 2011;30:1110–4. doi: 10.1097/ICO.0b013e31821379aa. [DOI] [PubMed] [Google Scholar]
- 34.Lichtinger A, Yeung SN, Kim P, Amiran MD, Elbaz U, Slomovic AR. Corneal endothelial safety following subconjunctival and intrastromal injection of bevacizumab for corneal neovascularization. Int Ophthalmol. 2014;34:597–601. doi: 10.1007/s10792-013-9807-6. [DOI] [PubMed] [Google Scholar]
- 35.Cursiefen C, Hofmann-Rummelt C, Kuchle M, Schlotzer-Schrehardt U. Pericyte recruitment in human corneal angiogenesis: an ultrastructural study with clinicopathological correlation. Br J Ophthalmol. 2003;87:101–6. doi: 10.1136/bjo.87.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin CT, Hu FR, Kuo KT, Chen YM, Chu HS, Lin YH, et al. The different effects of early and late bevacizumab (Avastin) injection on inhibiting corneal neovascularization and conjunctivalization in rabbit limbal insufficiency. Invest Ophthalmol Vis Sci. 2010;51:6277–85. doi: 10.1167/iovs.09-4571. [DOI] [PubMed] [Google Scholar]
- 37.Chen WL, Chen YM, Chu HS, Lin CT, Chow LP, Chen CT, et al. Mechanisms controlling the effects of bevacizumab (avastin) on the inhibition of early but not late formed corneal neovascularization. PLoS ONE. 2014;9:e94205. doi: 10.1371/journal.pone.0094205. [DOI] [PMC free article] [PubMed] [Google Scholar]