Abstract
Two fungal isolates recovered from the blood of two immunosuppressed patients are described as Phialemonium curvatum. One patient died, while the other, who was infected with Exophiala jeanselmei at the same time, survived after successful treatment with itraconazole. Analysis of internal transcribed spacer sequences demonstrated that the isolates belonged to the same strain and that the source of infection was probably a catheter. The taxonomic position of P. curvatum is discussed, and Phialemonium dimorphosporum is considered a synonym. The in vitro inhibitory activities of six antifungal agents (amphotericin B, itraconazole, ketaconazole, miconazole, flucytosine, and fluconazole) were determined against seven isolates of Phialemonium. Except for flucytosine, all of them were remarkably effective. Phialemonium should be added to the list of potential causes of nosocomial fungemia in cancer patients.
The number of infections caused by hyaline filamentous fungi in immunocompromised patients is continuously increasing. Many fungal genera are responsible for severe infections in these patients and cause a high degree of mortality. Among them there is a group of morphologically related fungi such as Fusarium, Acremonium, Cylindrocarpon, Lecythophora, and Phialemonium. The identification of the form-species level or form-genus level is usually difficult, especially when cultures of clinical isolates are not fully sporulated or are degenerated. Fusarium and Acremonium species are the most common and are the most resistant to antifungal therapy (4, 5).
Phialemonium spp. have been isolated from localized infections, such as peritonitis (7) and endocarditis (15), from an infection in a burned child (9), and from other clinical sources (2). Sometimes the causal relationship of these species with the diseases was not proven. Reports concerning infections in animals also exist (8). We present two cases of Phialemonium curvatum infection from the same hospital.
CASE REPORTS
Patient 1.
A 41-year-old Brazilian man suffering a relapse of acute lymphocytic leukemia was admitted to the Hematology Unit of the Hospital Universitario Clementino Fraga Filho on 21 January 1997, where he was successfully treated with broad-spectrum antibiotics for febrile neutropenia. The final diagnosis was unexplained fever. On 14 February, 5 days after a course of chemotherapy, he was readmitted and suffered from malaise, epigastric pain, and fever. He was severely neutropenic. Blood samples for culture were drawn from a peripheral vein and from a totally implanted catheter that had been in place for 7 months. He was started on treatment with ceftazidine, amikacin, and teicoplanin. On day 4 the catheter was removed, a new blood sample for culture was taken from a peripheral vein, and treatment with amphotericin B (1 mg/kg of body weight daily) was started because of the growth of a mold in the culture of blood taken from the catheter. The blood sample for culture taken on day 4 also tested positive for a morphologically identical fungus. The patient remained febrile and neutropenic and complained of myalgia. Twelve days after the catheter was removed, there were erythema and a secretion in the area of insertion of the previous port. A biopsy of this tissue was performed. Ten days later sinusitis developed. The patient remained febrile, and the dose of amphotericin B was increased to 1.5 mg/kg. The patient subsequently developed abdominal distention, generalized edema, psychosis, and orbital cellulitis. On 12 March a new blood sample for culture taken from a peripheral vein grew a filamentous fungus similar to the previous ones. The patient developed respiratory distress, and orotracheal intubation was performed. He later developed acute renal failure and hypotension and died on 4 April. A Phialemonium sp. grew from all the blood cultures as well as from the biopsied tissue. Histopathologic examination did not show hyphae.
Patient 2.
A 37-year-old man was admitted to the Hematology Unit of the Hospital Universitario Clementino Fraga Filho in January 1997 for an autologous stem cell transplant for the treatment of relapsed Hodgkin’s disease. The patient had a history of high fever 1 month earlier when a Hickman catheter was being manipulated for stem cell collection. A culture of a blood sample taken from the catheter and a culture of a specimen from the stem cell bag grew Exophiala jeanselmei. At the time of admission the patient was afebrile, and physical examination was unremarkable. The patient was given itraconazole (200 mg daily), the catheter was replaced, and high-dose chemotherapy was started. Two days after stem cell infusion the patient became neutropenic and developed a fever. New blood samples for culture were drawn, and treatment with ceftazidime and teicoplanin was started. E. jeanselmei grew in the blood culture, and the dosage of itraconazole was increased to 400 mg daily. Since the patient remained febrile, another blood sample for culture was taken from a peripheral vein and the catheter was removed. Two days later, neutrophil counts rose to 3,000 per mm3, and because the patient was afebrile, he was discharged and itraconazole was discontinued. Later, a Phialemonium sp. grew from the blood culture. No new fever or signs of infection appeared in the 6 months following the patient’s discharge.
MATERIALS AND METHODS
Isolate identification.
The fungal isolates from patients 1 and 2 were referred to the Microbiology Unit of the Rovira i Virgili University in Reus, Spain, for identification and susceptibility studies.
Morphological study.
The two isolates (FMR 6321 from patient 1 and FMR 6322 from patient 2) were subcultured on potato dextrose agar and oatmeal agar and were incubated in darkness at 25°C for identification purposes. They were compared with the following strains: Phialemonium obovatum CBS 279.76 and CBS 730.97, P. curvatum CBS 490.82, and Phialemonium dimorphosporum CBS 491.82 and FMR 3940.
Molecular studies.
All strains mentioned above were processed for PCR amplification and restriction fragment length polymorphism (RFLP) analysis. The fungal DNA was isolated as described by Estruch et al. (1), with some modifications. The strains were grown in Sabouraud broth on an orbital shaker at 250 rpm and 25°C. Mycelium was recovered by filtration through nytal mesh (pore size, 42 μm), washed with distilled water, blotted with paper towels, frozen in liquid nitrogen, and ground to a fine powder with a mortar and pestle. The powder was incubated for 1 h at 65°C in 1 to 2 ml of extraction buffer (Tris HCl, 200 mM; NaCl, 250 mM; EDTA, 25 mM; sodium dodecyl sulfate, 0.5% [pH 8]). The lysate was extracted with phenol-chloroform-isoamyl alcohol (25:24:1), and DNA was recovered by isopropanol precipitation. The pellet was washed with 70% (vol/vol) ethanol, dried under vacuum, and resuspended in TE (Tris HCl, 10 mM; EDTA, 1 mM [pH 8]). The internal transcribed spacer (ITS) ribosomal DNA (rDNA) was amplified as described by Henrion et al. (6), with some modifications (3). Primers ITS5 and ITS4 were used to amplify the region corresponding to the ITS, including the 5.8S rDNA, plus a small portion (the primer region) of the 18S and the 28S rDNA genes of the different strains (16). The PCR was performed in a Perkin-Elmer 2400 thermal cycler with the faster ramp times, as follows: 40 cycles of 30 s at 95°C, 60 s at 50°C, and 60 s at 72°C, followed by a final extension of 7 min at 72°C. The molecular sizes of amplified DNA were estimated by comparison with those in a lane with a standard 100-bp DNA ladder (Gibco BRL, Life Technologies, Barcelona, Spain). Restriction endonucleases CfoI (GCG/C) and HinfI (G/ANTC) were used in separate digestion reactions with PCR-amplified products by following the manufacturer’s recommendations (Boehringer Mannheim, Mannheim, Germany). RFLP analysis was performed by loading the digestion reaction mixture onto 2% Agarose MP (Boehringer Mannheim), and the digestion products were stained for 30 min in ethidium bromide as described by Sambrook et al. (14).
The ITS regions of the two isolates from the patients and the type strain of P. dimorphosporum (CBS 491.82) were sequenced. The protocol with the Taq DyeDeoxy Terminator Cycle Sequencing Kit (Applied Biosystems, Gouda, The Netherlands) was used for sequencing. The reactions were performed with primers ITS5 and ITS4 (16) and were run on a 310 DNA sequencer (Applied Biosystems). The sequences were aligned with the Autoassembler computer program (Applied Biosystems).
Antifungal susceptibility testing.
The two isolates from the patients and two additional isolates of P. dimorphosporum, two isolates of P. obovatum, and one isolate of P. curvatum, all from various sources, were tested to determine their susceptibilities to six antifungal drugs (amphotericin B, flucytosine, fluconazole, itraconazole, ketoconazole, and miconazole). Tests were performed by a previously described microdilution method (12) roughly according to the National Committee for Clinical Laboratory Standards’ guidelines (10) by using RPMI 1640 medium buffered to pH 7 with 0.165 M morpholinepropanesulfonic acid, an inoculum of 1.6 × 104 to 2.2 × 104 CFU/ml, an incubation temperature of 30°C, a second-day reading (48 h), and an additive drug dilution procedure.
Nucleotide sequence accession numbers.
The ITS sequences were deposited in the European Molecular Biology Laboratory (EMBL) under the following accession numbers: AJ 012298 for strain CBS 491.82, AJ 012299 for strain FMR 6321, and AJ 012300 for strain FMR 6322.
RESULTS
Morphological study.
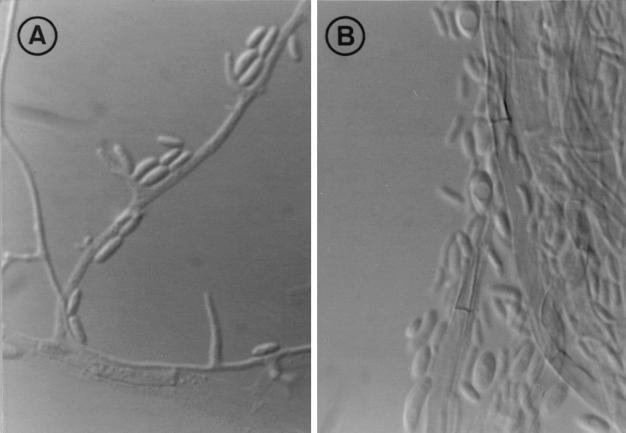
The colonies of both isolates on potato dextrose agar were white and cottony, reaching 36 to 38 mm in diameter after 7 days. They then became cream colored, and small areas of the colonies became light brown, and these light brown areas were also visible on the reverse. No diffusible pigment was produced. Colonies on oatmeal agar were flat and white with sparse aerial mycelia and reached a diameter of 39 to 40 mm. Microscopically, vegetative hyphae were hyaline to pale brown and ranged from 2 to 4 μm in width, conidiogenous cells (phialides and adelophialides) ranged from 2 to 30 μm in length (Fig. 1A), and conidia were hyaline, cylindrical to allantoid (3 to 6 by 0.8 to 2 μm), or ellipsoidal to obovate (2.8 to 4.5 by 1.5 to 2.5 μm) (Fig. 1B). These characteristics were compared with those of the three described species of Phialemonium, and the clinical isolates were morphologically identified as P. dimorphosporum, although the RFLP patterns of this species are identical to those of P. curvatum.
FIG. 1.
P. curvatum FMR 6321. (A) Conidiogenous cells and conidia. (B) Conidial morphology. Magnification, with Nomarski optics, ×1,600.
Molecular study.
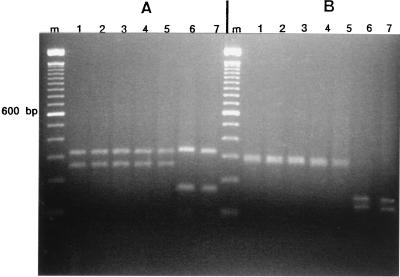
All the PCR products had an approximate molecular size of 600 bp. The restriction digests are shown in Fig. 2A and B. Digestion with CfoI (Fig. 2A) showed two very different band patterns. One of them was shared by the isolates of P. dimorphosporum (isolates CBS 491.82 and FMR 3940), the two strains from the patients (strains FMR 6322 and FMR 6321), and the strain of P. curvatum (strain CBS 490.82) and consisted of two bands of approximately 325 and 260 bp. The other pattern, corresponding to the strains of P. obovatum (strains CBS 730.97 and CBS 279.76), consisted of two bands of approximately 325 bp (identical to that of the other species) and another one of 180 bp. The pattern groups were similar by HinfI digestion (Fig. 2B), which yielded a wide band of 300 bp (probably a doublet) shared by the patient isolates and the strains of P. dimorphosporum and P. curvatum, while the P. obovatum isolates showed two bands of 150 and 125 bp. From the alignments of the sequences (Fig. 3), we can see only 1 nucleotide difference (position 549) between the two patient isolates (isolates FMR 6321 and FMR 6322) and 10 nucleotide differences between those isolates and the type strain of P. dimorphosporum (strain CBS 491.82).
FIG. 2.
(A) PCR products cleaved by CfoI and separated on a 2% agarose gel. Lane m, 100-bp DNA ladder (Gibco BRL) used as a size marker; lanes 1 and 4, P. dimorphosporum CBS 491.82 and FMR 3940, respectively; lanes 2 and 3, patient isolates FMR 6322 and FMR 6321, respectively; lane 5, P. curvatum CBS 490.82; lanes 6 and 7, P. obovatum CBS 730.97 and CBS 279.76, respectively. (B) PCR products cleaved by HinfI and separated on a 2% agarose gel. Lane m, 100-bp DNA ladder (Gibco BRL) used as a size marker; lanes 1 to 7 are as described above for panel A.
FIG. 3.
Alignment of ITS1, 5.8S gene, and ITS2 of P. dimorphosporum CBS 491.82 and patient isolates FMR 6321 and FMR 6322. CBS 491.82 is used as the leading strand. Dots indicate nucleotides identical to the reference sequence (the CBS 491.82 sequence).
In vitro antifungal susceptibility.
The data from the antifungal susceptibility testing of all Phialemonium isolates generally demonstrated low MICs except for those of flucytosine. Examination of the minimal fungicidal concentrations (MFCs) revealed that the majority of isolates displayed a higher degree of resistance. The MICs and MFCs for P. obovatum, with some exceptions, were higher than those for the three species tested (Table 1).
TABLE 1.
Antifungal susceptibilities of Phialemonium sp. strainsa
| Strain | AMB
|
MON
|
ITRA
|
FLU
|
KETO
|
5-FC
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (μg/ml) | MFC (μg/ml) | MIC (μg/ml) | MFC (μg/ml) | MIC (μg/ml) | MFC (μg/ml) | MIC (μg/ml) | MFC (μg/ml) | MIC (μg/ml) | MFC (μg/ml) | MIC (μg/ml) | MFC (μg/ml) | |
| P. obovatum CBS 279.76 | 16 | 32 | 1 | 31 | 1 | 32 | 16 | 64 | 1 | 4 | 256 | 256 |
| P. obovatum CBS 730.97 | 0.5 | 32 | 0.5 | 32 | 0.5 | 32 | 32 | 128 | 1 | 4 | 256 | 256 |
| P. curvatum CBS 490.82 | 0.125 | 2 | 0.5 | 32 | 0.06 | 0.5 | 16 | 64 | 0.25 | 2 | 256 | 256 |
| P. curvatum FMR 3940 | 0.5 | 0.5 | 0.5 | 2 | 0.06 | 0.5 | 16 | 64 | 0.25 | 1 | 256 | 256 |
| P. curvatum CBS 491.82 | 0.5 | 1 | 2 | 32 | 0.25 | 16 | 16 | 32 | 0.5 | 32 | 256 | 256 |
| P. curvatum FMR 6321 | 0.25 | 8 | 0.5 | 4 | 0.06 | 32 | 16 | 16 | 0.25 | 1 | 256 | 256 |
| P. curvatum FMR 6322 | 0.5 | 1 | 0.5 | 4 | 0.06 | 0.5 | 16 | 32 | 0.25 | 2 | 256 | 256 |
AMB, amphotericin B; 5-FC, flucytosine; FLU, fluconazole; ITRA, itraconazole; KETO, ketoconazole; MON, miconazole.
DISCUSSION
The anamorphic genus Phialemonium was proposed in 1983 to accommodate some filamentous fungi with morphological features that fell between those of Acremonium and Phialophora (2). Three species, P. obovatum, P. curvatum, and P. dimorphosporum, were distinguished in the genus, mainly on the basis of the shape of their conidia, which are obovate in P. obovatum, allantoid in P. curvatum, and both allantoid and obovate or ellipsoidal in P. dimorphosporum. An additional characteristic used to separate the species was the color of the colony, which is greenish in P. obovatum and white but becoming grayish in P. curvatum. The colonies of P. dimorphosporum are similar to those of P. curvatum, although they may become pale vinaceous buff. However, further reports describing clinical isolates of this genus demonstrated that the distinction of the species was not so easy in practice. Important phenotypic variations, which mainly depend on the culture conditions, are shown by the isolates of this genus (7). This confusion was reported in recent articles, in which King et al. (7) pointed out that one clinical isolate morphologically resembled both P. dimorphosporum and P. curvatum, and Schonheyder et al. (15) also found difficulties in characterizing another clinical isolate tentatively identified as P. curvatum. Our study corroborates these findings and demonstrates that only two species in the genus Phialemonium can be considered distinct. The two patient isolates were initially identified morphologically as P. dimorphosporum, but RFLP analysis of PCR products obtained with the enzymes HinfI and CfoI confirmed that P. dimorphosporum and P. curvatum are the same species and that this species can easily be differentiated with molecular markers from P. obovatum. Because P. curvatum has priority over P. dimorphosporum, the second species should be considered a synonym of the first one.
Only a few previously documented cases of Phialemonium infections exist. In 1975, P. obovatum was involved in an infection in a burned child. The fungus was recovered from biopsy specimens of cutaneous and subcutaneous tissue obtained antemortem from thermal burn wounds and postmortem from spleen tissue and three burn sites. No antifungal treatment for this patient was mentioned (9). More recently, a few new cases have been described. Two of them were in renal transplant recipients, one of whom suffered peritonitis and the other of whom had a phaeohyphomycotic cyst on his foot. The species involved were P. obovatum and probably P. curvatum, respectively (7). Another strain probably belonging to the species P. curvatum was found to be associated with Streptococcus sanguis in a patient with endocarditis involving a porcine aortic valve prosthesis. It was suggested that the Phialemonium infection evolved insidiously during open heart surgery by contaminating the prosthesis. This then led to a hematogenous streptococcal infection which caused the death of the patient (15).
To our knowledge, the cases of fungemia described here are the first cases of fungemia caused by Phialemonium ever reported. It is worth mentioning that both patients were in the same ward of the bone marrow transplant unit at the same time (patient 1 was admitted in January 1997 for a short period, while patient 2 was already in the hospital). This suggests that they acquired the infection from the same source, which was probably the catheters, since the isolates from the two patients grew from blood taken from central venous catheters. Moreover, molecular data showed that both isolates were actually identical. So, their genetic identities and the fact that both patients were in the same hospital unit confirm our hypothesis of a nosocomial acquisition of the infections.
For only two of the previously reported isolates from patients with Phialemonium infection, which were described in the same article (7), were in vitro susceptibility data provided. The tests were performed in two different laboratories, and the MICs of amphotericin B, itraconazole, and ketoconazole were completely different for both isolates. One of them was susceptible to amphotericin B and resistant to itraconazole and ketoconazole and the susceptibility of the other one was the exact opposite of that of the first one. Phialemonium spp. are morphologically similar to other well-known opportunistic pathogens such as Fusarium spp. and Acremonium spp. However, the susceptibilities of the isolates of these two genera, at least in vitro, are very different, and members of both genera display universal antifungal resistance (4, 13). Due to the lack of practical data concerning the antifungal treatment of infections caused by Phialemonium spp., it is impossible to predict the most adequate treatment. Only one of the previous reports of Phialemonium infections mentioned the treatment that was applied. This was for a patient with peritonitis whose dialysate catheter was removed and who was then treated with amphotericin B, ketoconazole, flucytosine, and, finally, fluconazole until repeated cultures of peritoneal fluid yielded no growth (7). On the basis of the good in vitro results of our study, it seems that several alternative treatments could be used, although further study is required.
The outcomes for our patients were quite different. The first patient, who developed multiple organ failure and who had had positive blood cultures on three previous occasions, died, despite removal of the catheter and the administration of high doses of amphotericin B, which had been demonstrated to have efficacy in vitro. The second patient improved with catheter removal and oral treatment with itraconazole, which had also been demonstrated to have efficacy in vitro. However, the most important difference was that in the second patient the abone marrow recovered, but the first patient died with profound and prolonged neutropenia. We realize that the infection in the second patient was not fully substantiated on the basis of only a single positive culture for P. curvatum. Fungi present on the skin or other surfaces can be picked up through transdermal hypodermic or catheter ports and can contaminate blood cultures, and we demonstrated that this fungus was present in the hospital environment. Unfortunately, it was not possible to perform a second blood culture because 2 days later the patient was afebrile and was discharged. Bone marrow recovery for determination of the prognosis for cancer patients with fungemia has been shown to be very important (11).
REFERENCES
- 1.Estruch J J, Antuña C, Ferrer S, Ramón D. Aislamiento de DNA genómico de Trichophyton mentagrophytes. Rev Iber Micol. 1989;5:62–66. [Google Scholar]
- 2.Gams W, McGinnis M R. Phialemonium, a new anamorph genus intermediate between Phialophora and Acremonium. Mycologia. 1983;75:977–987. [Google Scholar]
- 3.Gené J, Guillamón J M, Guarro J, Pujol I, Ulfig K. Hormographiella aspergillata anamorph of Coprinus cinereus, a human opportunistic fungus: molecular characterization and antifungal susceptibility. Antonie Leeuwenhoek. 1996;70:49–57. doi: 10.1007/BF00393569. [DOI] [PubMed] [Google Scholar]
- 4.Guarro J, Gams W, Pujol I, Gené J. Acremonium species: new emerging fungal opportunists. In vitro antifungal susceptibilities and review. Clin Infect Dis. 1997;25:1222–1229. doi: 10.1086/516098. [DOI] [PubMed] [Google Scholar]
- 5.Guarro J, Gené J. Opportunistic fusarial infections in humans. Eur J Clin Microbiol Infect Dis. 1995;14:741–754. doi: 10.1007/BF01690988. [DOI] [PubMed] [Google Scholar]
- 6.Henrion B, Chevalier G, Martin F. Typing truffle species by PCR amplification of the ribosomal DNA spacers. Mycol Res. 1994;98:37–43. [Google Scholar]
- 7.King D, Pasarell L, Dixon D M, McGinnis M R, Merz W G. A phaeohyphomycotic cyst and peritonitis caused by Phialemonium species and a reevaluation of its taxonomy. J Clin Microbiol. 1993;31:1804–1810. doi: 10.1128/jcm.31.7.1804-1810.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lomax L G, Cole J R, Padhye A A, Ajello L, Chanler F W, Smith B R. Osteolytic phaeohyphomycosis in a German shepherd dog caused by Phialemonium obovatum. J Clin Microbiol. 1986;23:987–991. doi: 10.1128/jcm.23.5.987-991.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGinnis M R, Gams W, Goodwin M N., Jr Phialemonium obovatum infection in a burned child. J Med Vet Mycol. 1986;24:51–55. [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeast. Standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 11.Nucci M, Silveira M I, Spector N, Silveira F, Velasco E, Akiti T, Barreiros G, Derossi A, Colombo A L, Pulcheri W. Risk factors for death in cancer patients with fungemia. Clin Infect Dis. 1998;27:107–111. doi: 10.1086/514609. [DOI] [PubMed] [Google Scholar]
- 12.Pujol I, Guarro J, Llop C, Soler L, Fernández-Ballart J. Comparison of broth macrodilution and microdilution antifungal susceptibility tests for the filamentous fungi. Antimicrob Agents Chemother. 1996;40:2103–2110. doi: 10.1128/aac.40.9.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pujol I, Guarro J, Gené J, Sala J. In-vitro antifungal susceptibility of clinical and environmental Fusarium spp. strains. J Antimicrob Chemother. 1997;39:163–167. doi: 10.1093/jac/39.2.163. [DOI] [PubMed] [Google Scholar]
- 14.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 15.Schonheyder H C, Jensen H E, Gams W, Nyvad O, Van Nga P, Aalbaek B, Stenderup J. Late bioprosthetic valve endocarditis caused by Phialemonium aff. curvatum. J Med Vet Mycol. 1996;34:209–214. [PubMed] [Google Scholar]
- 16.White T J, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M A, Gelfand D H, Sninsky J J, White T J, editors. PCR protocols. A guide to methods and applications. San Diego, Calif: Academic Press, Inc.; 1990. pp. 315–322. [Google Scholar]