Abstract
Glyoxalase (GLY) system, comprising of GLYI and GLYII enzymes, has emerged as one of the primary methylglyoxal (MG) detoxification pathways with an indispensable role during abiotic and biotic stresses. MG homeostasis is indeed very closely guarded by the cell as its higher levels are cytotoxic for the organism. The dynamic responsiveness of MG-metabolizing GLY pathway to both endogenous cues such as, phytohormones, nutrient status, etc., as well as external environmental fluctuations (abiotic and biotic stresses) indicates that a tight regulation occurs in the cell to maintain physiological levels of MG in the system. Interestingly, GLY pathway is also manipulated by its substrates and reaction products. Hence, an investigation of signalling and regulatory aspects of GLY pathway would be worthwhile. Herein, we have attempted to converge all known factors acting as signals or directly regulating GLYI/II enzymes in plants. Further, we also discuss how crosstalk between these different signal molecules might facilitate the regulation of glyoxalase pathway. We believe that MG detoxification is controlled by intricate mechanisms involving a plethora of signal molecules.
Keywords: Crosstalk, Glyoxalase, Methylglyoxal, Reaction substrates, Signalling
Introduction
The adaptive responses elicited by plants in response to various abiotic stresses involve complex networks that are maintained by the concerted action of a myriad of signalling molecules which not only involve phytohormones but also reactive oxygen species (ROS), reactive nitrogen species (RNS) and reactive carbonyl species (RCS) (Hossain et al. 2015a; Mittler 2017; Fancy et al. 2017). Methylglyoxal (MG) is one such RCS with a dual role in cellular processes. At lower concentration, it can induce signal transduction pathways while it exhibits cytotoxicity at higher concentrations by forming advanced glycation end products (AGEs) (Hoque et al. 2016; Li 2016; Kaur et al. 2016). Further, there exists an overlap between MG-responsive and stress-responsive signalling events in plants (Kaur et al. 2014a). MG detoxification in the cell occurs primarily by the action of glyoxalase (GLY) enzymes. The glyoxalase system comprises glutathione (GSH)-dependent glyoxalase I (GLYI) and glyoxalase II (GLYII) enzymes and the GSH-independent GLYIII enzyme. In the first step of the GSH- dependent pathway, MG spontaneously reacts with GSH to form hemithioacetal which is used as a substrate by GLYI to convert it into S-lactoylglutathione (SLG). In the subsequent reaction, GLYII hydrolyses SLG to form D-lactate which is further acted upon by D-lactate dehydrogenases into an innocuous molecule, pyruvate, thereby completing the MG detoxification system (Maurino and Engqvist 2015; Bhowal et al. 2020). The coordinated action of GLYI and GLYII is essential for imparting better stress tolerance (Singla-Pareek et al. 2003). In addition, GLYIII enzyme offers a shorter route for MG detoxification directly converting MG to D-lactate in a single step without involving GSH (Ghosh et al. 2016).
In the recent past, detailed studies have been undertaken to decipher the signalling and regulation of this indispensable pathway. In animals, some progress has been achieved in the discovery and development of GLYI regulators. GLYI is of crucial interest in these systems because it is a key rate limiting enzyme linked to various diseases. Thus, an understanding of this enzyme regulation can aid in improved treatment protocols (He et al. 2020). Likewise, in plants, numerous molecules are involved in signalling and regulation of glyoxalase pathway under different environmental and cellular conditions. The present review is thus focussed on a very fundamental question as to what signals the glyoxalase pathway in plants. We have attempted to provide a comprehensive overview of the various typical and atypical molecules involved in signalling of this pathway and a detailed understanding of the cross talk among these molecules.
Reaction substrates as signals
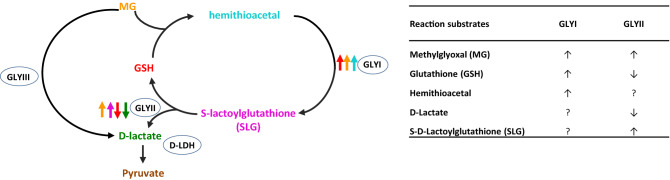
Glyoxalase pathway utilizes hemithioacetal generated from MG and GSH, as substrate, which can regulate GLYI and GLYII expression and/or activity. Moreover, there are indications of SLG, the substrate of GLYII, also being involved in regulation of glyoxalases. Figure 1 summarizes the regulation of glyoxalase pathway by its reaction substrates and same has been discussed in details below.
Fig. 1.
Schematic representation of the glyoxalase pathway involved in MG detoxification and the effect of various reaction substrates on the regulation of the pathway. ↑ indicates positive signalling while ↓ indicates negative signalling of the GLYI and GLYII proteins. Different colours of arrows represents regulation by respective metabolites. The table on the right summarizes these regulations by respective substrates. ‘?’ represents data not known
Glutathione
The role of glutathione (GSH) as a ROS scavenger and its physiological role in regulating various stress responses is well documented (see Hasanuzzaman et al. 2017a). Importantly, GSH can regulate both enzymes of GLY pathway. Addition of GSH in the medium was shown to increase GLYI activity (Basu et al. 1988) whereas the GLYII enzyme activity is inhibited by GSH. End-product inhibition of OsGLYII-2, a GLYII protein from rice, by either of it products, GSH or D-lactate has been demonstrated (Ghosh et al. 2014). While GSH showed a competitive inhibition, D-lactate demonstrated a non-competitive type of inhibition of the OsGLYII-2. Since, GSH is a signalling molecule, and also a part of MG detoxification machinery, a delicate balance is required for proper functioning of both systems particularly during abiotic/biotic stress when redox balance in cell is perturbed. Earlier Thornalley (1990) reported that GLYI may also be induced by high concentrations of lactaldehyde and GLYII by hemithioacetal.
Nahar and his co-workers have shown the protective effects of exogenous GSH application in mung bean seedlings against salinity, drought and high temperature-induced damages by a positive regulation of glyoxalase enzymes and antioxidant systems (Nahar et al. 2015a, c, d). Another study advocates the role of either GSH or GSH + NaHS (H2S donor) in positively co-regulating glyoxalase enzymes and alleviating salinity stress in Capsicum plants (Kaya et al. 2020a). Increase in GSH levels is known to be a general response to biotic stress. In this context, a study by Singh et al. (2020) revealed that the susceptible cultivar of Vigna mungo L. Hepper, cv. KUG253 harboured highest levels of GSH and corresponding high activity of glyoxalase pathway enzyme at a time when the infection in this variety was severe whereas resistant variety MASH114 and a cross variety KUG253 × MASH114, had relatively very low levels of reduced GSH and an absence of infection. Thus, GLYI, GLYII and GLYIII levels were shown to be the highest in the susceptible variety cv. KUG253 and correlated with accumulation of GSH indicating the regulation of enzymes with GSH.
Methylglyoxal
GLYI being the rate-limiting step of the glyoxalase pathway, its activity is important in maintaining threshold levels of MG. Also, glyoxalase genes are induced by MG, the substrate of GLYI. In rice, the expression of a unique GLYI (OsGLYI-11.2) was found to be induced by MG (Mustafiz et al. 2014). Its expression positively correlated with MG concentration, and peaked at 10 mM. Another rice GLYI (OsGLYI-8) was found to localise in the nucleus along with its substrate MG, thus, exhibiting substrate specificity (Kaur et al. 2017). Results from our lab also show up-regulation of other OsGLY genes in response to exogenously applied MG (unpublished work). Substrate inducibility of GLYI is of significance because it implies that an enhanced cellular MG signals the transcription of glyoxalase genes leading to detoxification of MG and thereby maintaining its basal non-toxic levels.
S-lactoylglutathione
S-lactoylglutathione (SLG) acts as signals in various physiological processes in leukocytes and rapidly proliferating cells (Gillespie 1975, 1979). In yeast too, SLG whose levels are controlled by GLYI and GLYII activity was found to modulate physiological signals (Murata et al. 1989). Since, SLG is a substrate for GLYII, addition of 1.0–1.5 mM of SLG caused a significant increase in GLYII activity in human pro myelocytic leukaemia cells indicating a positive regulation of GLY system (Thornalley and Tisdale 1988). In E. coli, SLG pools are linked to changes in activity of GLYI and GLYII enzymes. SLG pool regulates the activity of KefGB potassium efflux system during MG exposure, which controls the acidification of cytoplasm (Ozyamak et al. 2010) and indirectly, affects MG reactivity. Further, as GSH and SLG share common features they offer competition for binding to active site of OsGLYII-2 of rice (Ghosh et al. 2014).
Typical signal molecules in regulating glyoxalases
Phytohormones
Salicylic acid
Exogenous application of the salicylic acid (SA) has been shown to alleviate various stress conditions through concerted action of antioxidant machinery and glyoxalases. Application of 50 µM SA in 10 d old seedlings mitigated drought stress by 83% in B. juncea by regulating anti-oxidant defence along with enhancement of glyoxalase enzyme activities (Alam et al. 2013). Likewise, pre-treatment with 100 μM SA, minimised copper stress in rice by upregulating GLYI and GLYII activities particularly in the roots (Mostofa and Fujita 2013). Further, SA supplementation in B. napus seedlings upregulated GLYI and GLYII enzyme activities under salinity conditions (Hasanuzzaman et al. 2014a). Likewise, in B. parachinensis, SA could counteract salinity damages at up to 200 mM NaCl (Kamran et al. 2020). The mechanisms of SA induced tolerance include co-ordinated activation of antioxidant system and MG detoxification pathways (Kamran et al. 2020). Exposure of mustard plants to Ni stress too elicited a similar response where SA supplementation could co-regulate enzymatic/non-enzymatic antioxidant defence system and glyoxalase system. (Zaid et al. 2019). Another study reports the use of SA to ameliorate the phytotoxic effects of selenium in rice seedlings by co-activation of glyoxalase and antioxidant defence systems (Mostofa et al. 2020). A pre-sowing seed treatment of SA or NaHS (H2S donor) or a combination of both also proved to be beneficial in tolerating Pb stress in maize seedlings with a remarkable upregulation of GLYI activity (Zanganeh et al. 2020). In a similar study, Arsenic (As) stress was alleviated by SA induced NO-mediated coactivation of glyoxalase and antioxidant machinery in maize seedlings (Kaya et al. 2020b).
Auxin
The effects of auxin on glyoxalase activity have been reported in soybean cell suspension by Paulus and his co-workers. Normally grown cells were auxin starved for 3 days which resulted in the arrest of cell division. When the cells were transferred to auxin supplemented medium, a dramatic increase in GLYI activity was observed and subsequently, cell division resumed (Paulus et al. 1993).
Gibberellic acid
Transcriptional regulation of glyoxalase family protein in response to both gibberellic acid (GA) and abscisic acid (ABA) was observed in H vulgare aleurone tissues. The protein was 9.48-fold downregulated by GA and 4.2-fold upregulated by ABA (Chen and An 2006). Detailed proteomic study in rice revealed downregulation of GLYI protein in leaf tissue in response to GA treatment (Tanaka et al. 2004).
Cytokinin
A genome-wide transcriptional study of A. thaliana ipt mutants (deficient in cytokinin) reported the expression of several regulatory and functional genes including GLYI to be altered in the mutants under salinity stress. GLYI (AT1G15380) was fivefold upregulated in the mutants as compared to WT under 200 mM salt stress, which could be due to a decrease in active cytokinin (CK) in plants (Nishiyama et al. 2012). Further, exogenously applied kinetin (10 μM) protected S.lycopersicum plants from damages of salinity stress via coordinated up-regulation of antioxidant, Ascorbic acid-Glutathione (AsA-GSH) cycle and glyoxalase system (Ahanger et al. 2018). Indeed, in another transcriptomic study involving transgenic cotton plants harbouring ipt gene GLYI was 8.5-fold upregulated as compared to the non-transgenic G.hirsutum plants (Zhao et al. 2013). In Broccoli application of benzylaminopurine, a cytokinin, maintained GLYI protein levels during storage and ameliorated postharvest yellowing (Liu et al. 2013a). Roy et al. (2004) have reported that MG could replace kinetin in eliciting differentiation in callus cultures. In fact, some results suggest that the effect of both kinetin and MG are similar in nature (reviewed by Li 2016). Ainalidou et al. (2016) found GLYI as one of the upregulated proteins in the outer pericarp of kiwifruit in response to cytokinin N-(2-chloro-4-pyridyl)-N′-phenylurea (CPPU), suggesting protection against MG and ROS stress is required even during the fruit ripening process. The expression of glyoxalase family genes was significantly enhanced in the ipt-overexpressors as compared to WT creeping bent grass plants under drought. This might be due to similar upregulation of sugar metabolising and glycolytic transcripts to cope up with increased energy requirements for mounting stress-defence requirements and coping water deficit by osmotic adjustment via accumulation of sugars and compatible osmolytes (Merewitz et al. 2016).
Brassinosteroids
Brassinosteroids (BR) play important regulatory roles in stress tolerance. Exogenous application of BR was found to further positively regulate GLY pathway enzymes as well as enhance antioxidant capacity (enzymatic and non-enzymatic) of heat stressed Ficus concinna plants, which counteracts the damages caused by severe heat injury (Jin et al. 2015). Likewise, foliar spray of 24-Epibrassinolide (EBR) at 0.1 μM concentration could ameliorate salt-stress in soybean to an appreciable level and also increased glyoxalase activity (Alam et al. 2019). Along with salinity stress, EBR application could successfully mitigate Cr(VI)-associated toxicity in tomato plants via escalating the titre of AsA-GSH, antioxidant system and glyoxalase system (Jan et al. 2020). Another active BR, 28-HBR (homobrassinolide) could impart drought tolerance via reduction of ROS and MG, through upregulating GLYI and GLYII enzymes, and by enhancing antioxidant system for redox homeostasis and decreased lipid peroxidation in soybean seedlings (Hasan et al. 2020).
Ethylene
Ethephon application was reported to counter Zn stress in B.juncea by upregulation of GLYI and GLYII enzymes and a co-ordinated increase in antioxidant defence to detoxify MG and ROS species and also maintain nutrient homeostasis (Khan et al. 2019). Similarly, ethephon application could counter Ni stress in B.juncea system via enhancement of GLYI (by 88%) and GLYII activity (by 178%) as against control plants (Khan et al. 2020).
Jasmonic acid
Jasmonic acid (JA) is known to induce signals for the regulation of ascorbate and GSH metabolism and develop tolerance to water stress (Shan and Liang 2010). Hence, in line with previous reports, exogenous application of JA was found to positively modulate glyoxalase system and antioxidant machinery to impart drought stress tolerance in various species of Brassica. (Alam et al. 2014). However, another study suggests a negative role of JA in drought tolerance. In cpm-2, a JA biosynthesis mutant from rice, DJ-1 (or GLYIII) family protein was more abundant as revealed in root proteome analysis in comparison with WT (Dhakarey et al. 2017). Another protective role of JA application during seed priming has emerged in ameliorating alkaline stress. In this study, JA modulated Na/K ion homeostasis to attenuate Na stress, maintained photosynthetic parameters, enhanced the glyoxalase enzymes to detoxify MG and also maintain Ascorbic acid/dehydrascorbate (AsA/DHA) ratios thereby, conferring tolerance to maize seedlings (Mir et al. 2018). Additionally, exogenous application of JA can alleviate chromium toxicity in choysum plants by enhancing the antioxidant machinery, ascorbate and glutathione pool, and by modulating the glyoxalase system (Kamran et al. 2020). Further, an insilico analysis of the promoter regions of GmGLYI and GmGLYII genes have been predicted to harbour jasmonate elicitor responsive elements (JERE) and methyl jasmonate responsive elements (Ghosh and Islam 2016).
Abscisic acid
Espartero et al. (1995) purified and cloned GLXI from tomato and showed the gene to be responsive to ABA at both transcriptional and translational level. During the desiccation process of Sporobolus stapfianus and also following the application of ABA, a clone resembling GLYI was found to be upregulated (Blomstedt et al. 1998). Similarly, mitochondrially located BjglyII mRNA was strongly induced by 100 μM ABA (Saxena et al. 2005). Interestingly, GLYI family protein has been found to be a direct target of RD26, a dehydration-induced NAC transcription factor known to participate in ABA-dependant stress signalling pathway (Fujita et al. 2004) The promoter of GLYI gene is transcriptionally activated by RD26. The ABA-dependant genes, RD29B and RAB18 were found to be transcriptionally regulated by MG in a concentration-dependant manner in WT plants while in ABA mutant, aba2-2, MG-induced expression was found to be suppressed (Hoque et al. 2012a). Notably, a GLYII-like protein (OsETHE1) from rice but without GLY activity, is also ABA-inducible. The ETHE1 promoter (pOsETHE1: GUS) showed increased enzyme activity in roots in response to 100 μM ABA (Kaur et al. 2014b). Importantly, abscisic acid responsive elements (ABREs) are found to be present in almost all promoters of all GLY genes in G. max indicating a possible regulation of glyoxalase family by endogenous ABA (Ghosh and Islam 2016).
Melatonin
Melatonin (Mel) is a signalling molecule with multiple upcoming functions in plant cells (Back 2020). Banerjee and Roychoudhury (2019) have reported that exogenous melatonin application leads to upregulation of GLYII transcripts to counter the toxic levels of MG produced under fluoride stress. In another study, Mel could, in a similar fashion, counter Pb stress in C.tinctorus plants via activating glyoxalase enzymes and making available more of reduced GSH (Namdjoyan et al. 2020). A positive regulation of glyoxalase system was also achieved by Mel application in high temperature-stressed maize seedlings, indicating its crucial role in Mel-induced stress tolerance mechanisms (Li et al. 2019). In contrast, the use of Mel + GSH to alleviate Zn toxicity showed a decrease in GLY enzymes which might be due to inhibition of signalling in the GLY system (Goodarzi et al. 2020).
Known messenger molecules in regulating glyoxalases
Nitric oxide
Nitric oxide (NO) in optimal concentrations, is an important signalling molecule with a pleiotropic role in multiple stress signalling cascades (Fancy et al. 2017). S-nitrosylation of GLYI, a post-translational modification (PTM) was observed for the first time upon treatment with GSNO (S-Nitrosoglutathione) as well as under low temperature (Sehrawat et al. 2013; Sehrawat and Deswal 2014a). This PTM of GLYI is suggested to be involved in crosstalk between tyrosine residue nitration during cold stress for modulating cellular detoxification (Sehrawat and Deswal 2014b). Cysteine-S-nitrosylation of GLYI enzyme of tea leaves has also been observed (Qiu et al. 2019). However, human GLOI upon interaction with GSNO was shown to have reduced activity (Mitsumoto et al. 2000). The potential of NO as a signalling molecule to augment antioxidant defence and glyoxalase-mediated MG defence has been investigated. In wheat seedlings, SNP (sodium nitoprusside, NO donor) pre-treatment could confer protection against salinity-induced damage (Hasanuzzaman et al. 2011) and even alleviate high temperature stress (Hasanuzzaman et al. 2012). Efficacy of NO pre-treatment against PEG induced-water stress via application of 0.5 mM SNP in B. napus, was shown to be mediated by significant upregulation of GLY activities, antioxidant activities and non-enzymatic antioxidant pool (Hasanuzzaman et al. 2017b). Similar mechanism was reported in S.melongena against Ni stress (Soliman et al. 2019). In rice seedlings, salinity stress was mitigated by priming with H2O2/SA, which is mediated via NO signalling and lead to a concerted increase in glyoxalase and antioxidant host system to counter MG and ROS burst (Mostofa et al. 2015a). In a similar study, supplementation of SNP in tomato plants in response to (As) toxicity markedly decreased MG levels by enhancing GLYI and GLYII enzymatic activity, thus, improving plant performance (Ghorbani et al. 2020).
Hydrogen sulphide
Hydrogen sulphide (H2S) exhibits dual effects in plants. It is cytotoxic at high concentrations but acts as a signalling molecule at low concentrations, and has been shown to be intricately involved not only in plant development but also during stress signalling responses (reviewed by Li et al. 2016). H2S application alleviates cadmium (Cd) toxicity via ion homeostasis and successful ROS scavenging and quenching (Mostofa et al. 2015b). MG generated as a consequence of Cd stress in rice seedlings is scavenged by upregulating glyoxalase enzymes. Exogenous application of H2S to the As-treated pea plants also increased the GLYI and GLYII activity (Alsahli et al. 2020). Similarly, application of H2S could also mitigate Cr toxicity in maize seedlings by suppressing NADPH oxidase activity via S-nitrosylation of NAPDH oxidase and reducing MG accumulation by upregulating GLY enzyme activities (Kharbech et al. 2020a). A synergistic effect of H2S and NO also provided Cr tolerance to maize via protection of plasma membrane and also maintaining the GSH pool which could help in efficient MG detoxification by the glyoxalases (Kharbech et al. 2020b).
Hydrogen peroxide
Amongst other pathways, hydrogen peroxide (H2O2) can help the cell to tolerate stress by regulating glyoxalases as well. A global proteome analysis in response to 0.6 mM H2O2 revealed twofold induction of a GLYI enzyme (Wan and Liu 2008). However, in Citrus x paradisi, GLYI protein was downregulated in response to 10 mM H2O2 treatment (Tanou et al. 2010). Interestingly, H2O2 has even been shown to decrease the GLYIII activity of the AtDJ-1B protein in Arabidopsis; the protein thus, being redox-sensitive and recording a lowered glyoxalase activity due to negative regulation by H2O2 (Lewandowska et al. 2019).
Exogenous application of low concentrations of H2O2 could overcome 0.5 mM Cd-induced oxidative stress in B.napus by augmenting the glyoxalase and antioxidant system together (Hasanuzzaman et al. 2017c) and protected against drought exposure by enhancing GLYII activity in B.juncea (Hossain and Fujita 2013). Exogenous priming with H2O2 has been suggested to produce a slight oxidative burst which is beneficial for the plant survival under abiotic stresses (reviewed in Hossain et al. 2015). In fact, over-expression of a sugarbeet GLYI in N.tabacum conferred tolerance to transgenic seedlings against 20 mM H2O2 treatment (Wu et al. 2013).
Calcium
There are reports to indicate the regulation of glyoxalase activity by Ca fluxes in the cell. It was shown earlier that GLYI activity is inhibited following the application of calmodulin inhibitors to leaf discs of B.oleracea in the callus induction media (Bagga et al. 1987). Biochemical evidences also indicated that GLYI from B.juncea is a calmodulin-stimulated protein and is regulated by calcium levels (Deswal and Sopory 1999). Further, GLYI has also been reported to be one of the targets of phosphorylation by Ca-dependant protein kinases in response to GA treatment in rice (Khan et al. 2005). Studies on OsETHE1, one of GLYII family genes in rice, revealed that its promoter activity increases (3–fourfold) remarkably in roots upon application of CaCl2 (Kaur et al. 2014b).
Moreover, exogenous application of calcium can alleviate As stress (Rahman et al. 2015) and mitigate the salinity-induced damages in rice seedlings by positively modulating glyoxalase enzymes and ROS-detoxifying antioxidant systems (Rahman et al. 2016).
Nutrient molecule as signals
Several studies have elucidated newer roles of nutrients in plants especially as signalling molecules (reviewed by Coruzzi and Bush 2001). Nitrogen and Carbon are indeed tightly linked in almost every biochemical pathway in plants. In this context, there are upcoming reports discussing modulation of glyoxalase activity by nutrient availability.
Ammonium and Nitrate signals
Ammonium and nitrate are the predominant forms of nitrogen, extensively used by the plant. While nitrate has been reported as a nutrient and signalling molecule (Krouk et al. 2010; Fredes et al. 2019) ammonia imposes a stress condition referred to as ammonium syndrome (Britto and Kronzucker 2002). In fact, Borysiuk et al. (2018) showed that in Arabidopsis plants grown on ammonia as the sole nitrogen source, MG accumulated in high amounts which consequently led to enhanced formation of MG-derived advanced glycation end products (MAGEs). The higher activities of glyoxalase enzymes in these conditions were attributed to the up-regulation of only GLXI-3 and GLXII-5 expression. Despite higher GLY activity, MG concentration could not be maintained at low levels as observed in nitrate grown plants. The activity of D-LDH which catalyses the conversion of D-lactate to pyruvate using Cytc as an electron acceptor and constitutes the last step of MG detoxification pathway was also found to be lower in NH4+ grown plants as compared to NO3− grown plants Further, various independent transcriptomic studies have also shown the role of nitrate in modulating the levels of glyoxalases in rice and Medicago (Cabeza et al. 2014; Pathak et al. 2020) In rice, nitrate has been found to repress the expression of GLYI and GLYII genes in dark (Pathak et al. 2020). In Medicago, only one of the three GLY genes showed 2.46-fold change upon prolonged exposure to nitrate treatment (Cabeza et al. 2014). Interestingly, results from our lab also show OsGLY family of genes to be differentially expressed upon nitrate treatment under different light regimes (unpublished work).
Sugar
During glycolysis, reactive carbonyls like methylglyoxal, glyoxal and 3-deoxyglucosone, are produced as by-products. Takagi et al. (2014) found that under conditions that enhance photosynthesis such as high light and CO2 concentration, MG levels are elevated due to increased flux of the Calvin cycle. Oxidative stress that results due to the reduction of MG into a superoxide by PSI (Saito et al. 2011) has been referred to as ‘Plant diabetes’ (Shimakawa et al. 2014).
MG being a metabolic by-product of sugar metabolism, it is believed that the expression of glyoxalases involved in MG detoxification could be regulated by the cellular sugar status (Singla-Pareek et al. 2020). In fact, Schmitz et al. (2017) hypothesised that MG might be directly coupled to steady state sugar levels. A significant accumulation of AtGLXI;2 transcript was observed upon supplementation with 60 mM sucrose for 4 days and also in response to increased endogenous sugars in the starch free adg1-1 mutants. In addition, the expression levels of all GLX-I isoforms were found to be downregulated in prolonged dark phases under depleting sugar levels. Furthermore, expression of GLXI;1 and GLXI;2 have been found to be upregulated when exposed to moderately high light intensities (Schmitz et al. 2014) possibly due to increased CO2 fixation and thus higher flux through the Calvin-Benson cycle which consequently leads to higher MG levels.
Secondary metabolites as signals
Of late, there have been many reports suggesting that oxidative damage in plants inflicted by various biotic and abiotic stresses cannot be alleviated by genetic self-defence alone, so exogenous application of diverse group of compounds such as organic acid, phytohormones, nutrient molecules and secondary metabolites is becoming prevalent (Parvin et al. 2019). Herein, we have discussed some of the important secondary metabolites that have been applied to signal glyoxalase systems in plants.
Gamma-amino butyric acid
γ-amino butyric acid (GABA) is a non-protein amino acid which is considered as an intracellular signalling molecule in plants (Ramesh et al. 2017). A recent study reports that MG levels increased by 57–99% under mild to severe stress inflicted by chromium in B.juncea. However, upon supplementation with GABA, MG levels were found to decrease in a dose-dependent manner as a consequence of increased GLYI and GLYII activity thereby, improving plant tolerance to Cr (Mahmud et al. 2017). At present, detailed studies are needed to decipher the role of GABA in signalling and regulating the glyoxalase system in plants.
Flavonoids
Flavonoids are primarily involved in imparting colours to flowers, seed and spore germination and in growth and development (Griesbach 2005). They also function as signal molecules conferring tolerance against drought, heat, and cold. In addition, they also act as UV filters and antimicrobial agents (Samanta et al. 2011). Flavones are one of the subgroups of flavonoids and quercetin is one of the most important flavones found in plants. Parvin et al. (2019) carried out a study in which two different concentrations of quercetin were exogenously applied to 10d old salt-stressed tomato seedlings. It was found that quercetin application stimulated ROS scavenging by upregulating both enzymatic and non-enzymatic antioxidants. Higher concentration of quercetin resulted in improved MG detoxification due to enhanced GLYI and GLYII activity. A reduction in MG content was observed with steady state increase in GSH levels. Thus, this study highlighted the potential role of quercetin in regulating antioxidant and glyoxalase systems.
Phenolic acids
Vanillic acid is a naturally occurring phenolic acid capable of functioning as strong antioxidant owing to its ability to donate hydrogen to stabilize phenoxyl radical (Moran et al. 2014) and it has a potential in improving stress tolerance in plants (Thanh and Xuan 2018). In a recent study, Parvin et al. (2020) found that exogenous application of vanillic acid resulted in increase in the GLYI and GLYII activity by 79 and 18% respectively, with a concomitant decrease in the levels of salinity-induced MG content. Thus, vanillic acid alleviated salt stress-induced damage in tomato plants by the synergistic action of both antioxidant and glyoxalase enzymes. Similarly, vanillic acid enhanced cadmium tolerance in 13d old rice seedlings by up-regulating antioxidant and glyoxalase enzyme activity (Bhuyan et al. 2020).
Osmolytes and polyamines
Osmoprotectants are low molecular weight, highly soluble organic compounds synthesised in plants in response to adverse environmental conditions. Proline, glycine betaine and mannitol are among the most commonly found compatible solutes in plants (Saxena et al. 2013).
Exogenous application of proline and betaine has been shown to provide protective effects in Camellia sinensis (L.) O. Kuntze against cold stress by restricting the increase in MG and malondialdehyde levels but increasing glutathione-S-transferase (GST) and glutathione reductase (GR) activity, maintaining thiol/disulfide ratio close to the homeostatic state, restricting the decline in GLYI activity and enhancing GLYII activity in response to cold stress induced damage (Kumar and Yadav 2009). Similarly, up-regulation in GST and GLYI activity by exogenous application of 15 mM proline and betaine reduced H2O2 levels, thereby protecting against drought-induced oxidative stress in Lens culinaris (Molla et al. 2014). Further, a coordinated action of antioxidant and glyoxalase system induced upon proline and betaine supplementation conferred cadmium tolerance to mung bean (Hossain et al. 2010). Similar results were also observed in rice varieties (Hasanuzzaman et al. 2014b).
In addition to proline and betaine, polyamines such as spermidine could induce cold tolerance (Nahar et al. 2015b) and also confer combined heat and drought tolerance in mung bean through the synergistic effects of antioxidant and glyoxalase system much like the other osmolytes (Nahar et al. 2017). Spermine has also been successfully employed in alleviating prolonged fluoride stress in soil grown rice plants through the up-regulation of glyoxalase activity (Banerjee et al. 2020). Likewise, spermine was found to confer heat tolerance to lettuce seedlings employing similar mechanisms (Li et al. 2020).
Crosstalk of signals in regulating glyoxalases
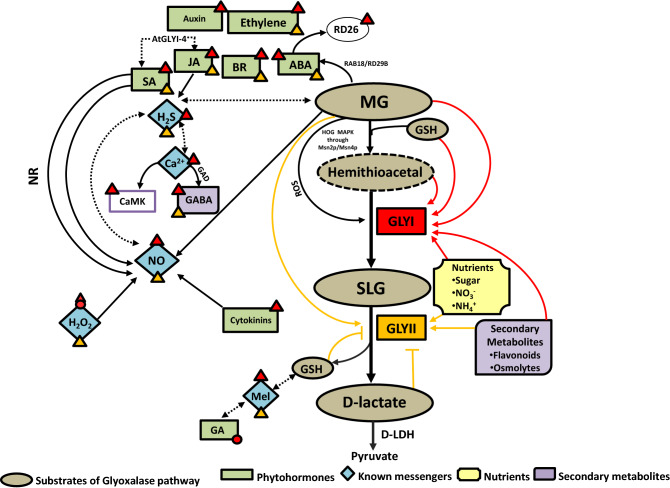
Though many signals, both endogenous as well as exogenous, regulate glyoxalase system in plants, yet these signals also interact with each other, to bring about modulation of glyoxalase levels (summarized in Fig. 2). For instance, GSH in combination with H2S acts synergistically in providing salinity tolerance to C.annum seedlings by the concerted action of glyoxalase pathway and antioxidant system. GSH acts via increasing endogenous H2S levels which subsequently, modulates GLY and antioxidant enzyme activities to bring about stress tolerance (Kaya et al. 2020a). Melatonin and GSH showed synergistic effect on relieving Zn toxicity of the C.tinctorus seedlings. However, glyoxalase activities decreased considerably in melatonin + GSH treated Zn-stressed seedlings as compared to Zn-stressed seedlings alone (Goodarzi et al. 2020). Another study suggests that a signalling crosstalk between MG and H2S is essential for imparting MG-mediated thermotolerance to maize seedlings and that the impairment of either MG or H2S signalling resulted in loss of thermotolerance (Li et al. 2018). Notably, MG stimulates L-cysteine desulfhydrase, a key enzyme in H2S biosynthesis to elevate endogenous H2S levels. The interaction between MG and H2S also resulted in modulation of glyoxalase system (Li 2020a). Since both MG and GSH, being reaction substrates, are also known to induce GLY activity, it is possible such interaction via H2S involves glyoxalase pathway. Kharbech et al. (2020b) report a synergistic effect of H2S and NO in MG detoxification of stressed maize seedlings.
Fig. 2.
Schematic representation of crosstalk among various molecules involved in signalling of glyoxalases in plants. Glyoxalase pathway mediated MG detoxification in plants is controlled by intricate mechanisms involving a plethora of signalling molecules such as phytohormones, messenger molecules, nutrients, reaction substrates and secondary metabolites. A crosstalk between these molecules facilitates optimal regulation of glyoxalases that are best suited for plant survival. Red and yellow arrows and/or arrowheads indicate upregulation of GLYI and GLYII, respectively whereas corresponding coloured circles and/or flatheads represent downregulation/inhibition, combination of arrowhead and circle represent both up and downregulation (as reports pertaining to both conditions available). NR, Nitrate reductase; CaMK, Calmodulin Kinase; GA, Gibberellin; JA, Jasmonic acid; ABA, Abscisic acid; SA, Salicylic acid; BR, Brassinosteroid; H2O2, Hydrogen peroxide; NO, Nitric oxide; H2S, Hydrogen sulphide; Ca2+, Calcium; Mel, Melatonin; NO3−, Nitrate; NH4+, Ammonia; MG, Methylglyoxal; GSH, reduced glutathione; SLG, S-D-lactoylglutathione; HOG-MAPK, High osmolarity glycerol-Mitogen activated protein kinase pathway; D-LDH, D-lactate Dehydrogenase; GABA, γ-Aminobutyric acid; GAD, Glutamate decarboxylase; ROS, Reactive Oxygen Species; GLYI, Glyoxalase I; GLYII, Glyoxalase II; RD26, Responsive to Desiccation 26; RAB18, Responsive to ABA 18; RD29B, Responsive to Desiccation 29B; Msn2p/Msn4p, Multicopy suppressor of SNF1 (Sucrose Non-fermentable) mutation 2/4p. Dashed line indicates synergistic interactions
A known messenger Ca2+ too, has been implicated in MG-mediated signalling crosstalk. The interaction of Ca2+ and MG in mediating thermotolerance has been recently shown where exogenous addition of MG resulted in improved thermotolerance in maize seedlings (Li 2020b). Likewise, oscillations in cytosolic calcium levels in guard cells have also been reported to play a role in mediating high levels of MG-induced stomatal closure in stressed Arabidopsis plants in ABA and MeJA independent manner (Hoque et al. 2012b). These studies are similar to those reported earlier in animal systems where MG caused cell death through ROS production, mitochondrial membrane potential loss and a concomitant increase in intracellular Ca levels in retinal pigment epithelial cells (Chan et al. 2016). In yeast, MG initiates a high osmolarity glycerol (HOG)-MAPK cascade and signals the influx of extracellular calcium via Ca2+ channels (Maeta et al. 2005).
Among the phytohormones, cytokinins have been shown to directly interact with NO to cause a reduction in its endogenous levels which affects NO-based signalling (Liu et al. 2013b). Likewise, in the H2O2 and/or SA priming-mediated stress tolerance signalling pathway, NO was found to be a key player in mounting defence mechanisms against salt stress-induced oxidative damage in rice seedlings (Mostofa et al. 2015a). In the presence of Hb, a NO scavenger, SA could not induce salinity tolerance. Evidences indicating NO acting downstream of SA-induced signalling cascade have been demonstrated (Naser Alavi et al. 2014). In fact, a combination treatment of SA and NO donor, SNP, results in marked tolerance to zinc stress in C.tinctorus plants and increase in GLY activities as compared to single SA or NO treatments (Namdjoyan et al. 2017). Recently, SA-mediated tolerance to drought stress has been shown to be dependent on NO and nitrate reductase (NR) (Kaya 2020c). In this study, SA/SNP (NO donor) were used singly or in combination to water stressed capsicum plants and it was found SA could initiate biosynthesis of NO by activating NR. The NO acts as a secondary messenger to upregulate MG detoxification pathways and antioxidant system (both enzymatic and non-enzymatic) which increases GSH pool and in turn co-regulates the GSH-dependant glyoxalase enzymes to detoxify the accumulated MG (Kaya 2020c). Also, efficient MG detoxification during drought stress in plants is suggested to involve a crosstalk of ABA, JA, SA and BR to provide MG homeostasis (Askari-Khorasgani and Pessarakli 2019). In fact, in Arabidopsis, through genome wide association studies it was shown for the first time that a glyoxalase family protein AtGLYI-4 was involved in crosstalk between SA and JA mediated pathways (Proietti et al. 2018). The glyI4 mutants had reduced MG scavenging, compromised fitness, ROS accumulation and stomatal closure (Proietti et al. 2019). Further, Siddiqui et al. (2020) reported a synergistic effect of GA and Mel on tomato seedling growth under salinity by modulating both enzymatic and non-enzymatic components of antioxidant defence, upregulation of glyoxalase system and accumulation of compatible osmolytes (Siddiqui et al. 2020).
Conclusion
Several years of research into plant glyoxalases have established their crucial and significant role in MG detoxification and abiotic/biotic stress tolerance making them indispensable for plant growth and development. The signalling components and the subsequent transduction pathways that regulate the expression of different members of glyoxalase genes and also the enzyme activity have remained poorly understood. In this regard, we believe that this review will provide an emerging picture of the various typical and atypical molecules involved in the signalling of this pathway. It also provides an overview of the crosstalk that exists between these molecules resulting in a convergent pattern best suited for plant survival. Future research in this direction can be aimed at understanding the detailed mechanism by which the molecules regulate the glyoxalase pathway giving deeper insights into methods by which the glyoxalase proteins can be optimally modulated for raising durable stress tolerant crops.
Acknowledgements
SKS acknowledges grant from DBT (Grant No. BT/PR20626/BPA/118/194/2016) and from SERB Distinguished Fellow award. SG and BB were supported by grant from DBT. CK acknowledges Department of Science and Technology (DST) for the grant (IFA-14/LSPA-24) received from the DST-INSPIRE Faculty award. SLS-P acknowledges grants from ICGEB and DBT.
Authors’ contribution
SKS conceived the concept and designed the outline; Literature review: SKS, SG, BB; Writing: SG and BB; Critical revision and editing of the manuscript: SKS, CK, SLS-P.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sampurna Garai and Bidisha Bhowal have contributed equally.
References
- Ahanger MA, Alyemeni MN, Wijaya L, et al. Potential of exogenously sourced kinetin in protecting Solanum lycopersicum from NaCl-induced oxidative stress through up-regulation of the antioxidant system, ascorbate-glutathione cycle and glyoxalase system. PLoS ONE. 2018;13:e0202175. doi: 10.1371/journal.pone.0202175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainalidou A, Tanou G, Belghazi M, et al. Integrated analysis of metabolites and proteins reveal aspects of the tissue-specific function of synthetic cytokinin in kiwifruit development and ripening. J Proteomics. 2016;143:318–333. doi: 10.1016/j.jprot.2016.02.013. [DOI] [PubMed] [Google Scholar]
- Alam MM, Hasanuzzaman M, Nahar K, Fujita M. Exogenous salicylic acid ameliorates short-term drought stress in mustard (Brassica juncea L.) seedlings by up-regulating the antioxidant defense and glyoxalase system. Aust J Crop Sci. 2013 doi: 10.4172/2376-0354.1000139. [DOI] [Google Scholar]
- Alam MM, Nahar K, Hasanuzzaman M, Fujita M. Exogenous jasmonic acid modulates the physiology, antioxidant defense and glyoxalase systems in imparting drought stress tolerance in different Brassica species. Plant Biotechnol Rep. 2014;8:279–293. doi: 10.1007/s11816-014-0321-8. [DOI] [Google Scholar]
- Alam P, Albalawi TH, Altalayan FH, et al. 24-Epibrassinolide (EBR) confers tolerance against NaCl stress in soybean plants by up-regulating antioxidant system, ascorbate-glutathione cycle, and glyoxalase system. Biomolecules. 2019;9:640. doi: 10.3390/biom9110640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsahli A, Bhat J, Alyemeni M, et al. Hydrogen sulfide (H2S) mitigates Arsenic (As)-induced toxicity in Pea (Pisum sativum L.) plants by regulating osmoregulation, antioxidant defense system, ascorbate glutathione cycle and glyoxalase system. J Plant Growth Regul. 2020;21:89–145. doi: 10.1007/s00344-020-10254-6. [DOI] [Google Scholar]
- Askari-Khorasgani O, Pessarakli M. Manipulation of plant methylglyoxal metabolic and signaling pathways for improving tolerance to drought stress. J Plant Nutr. 2019;42:1268–1275. doi: 10.1080/01904167.2019.1589502. [DOI] [Google Scholar]
- Back K. Melatonin metabolism, signaling and possible roles in plants. Plant J. 2020 doi: 10.1111/tpj.14915. [DOI] [PubMed] [Google Scholar]
- Bagga S, Das R, Sopory SK. Inhibition of cell proliferation and glyoxalase-I activity by calmodulin inhibitors and lithium in Brassica oleracea. J Plant Physiol. 1987;129:149–153. doi: 10.1016/S0176-1617(87)80111-6. [DOI] [Google Scholar]
- Banerjee A, Roychoudhury A. Melatonin application reduces fluoride uptake and toxicity in rice seedlings by altering abscisic acid, gibberellin, auxin and antioxidant homeostasis. Plant Physiol Biochem. 2019;145:164–173. doi: 10.1016/j.plaphy.2019.10.033. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Samanta S, Roychoudhury A. Spermine ameliorates prolonged fluoride toxicity in soil-grown rice seedlings by activating the antioxidant machinery and glyoxalase system. Ecotox Environ Safe. 2020;189:109737. doi: 10.1016/j.ecoenv.2019.109737. [DOI] [PubMed] [Google Scholar]
- Basu A, Sethi U, Guha-Mukherjee S. Induction of cell division in leaf cells of coconut palm by alteration of pH and its correlation with glyoxalase-I activity. J Exp Bot. 1988;39:1735–1742. doi: 10.1093/jxb/39.12.1735. [DOI] [Google Scholar]
- Bhowal B, Singla-Pareek SL, Sopory SK, Kaur C. From methylglyoxal to pyruvate: a genome-wide study for the identification of glyoxalases and D-lactate dehydrogenases in Sorghum bicolor. BMC Genomics. 2020;21:145. doi: 10.1186/s12864-020-6547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuyan MHMB, Parvin K, Mohsin SM, et al. Modulation of cadmium tolerance in rice: Insight into vanillic acid-induced upregulation of antioxidant defense and glyoxalase systems. Plants. 2020;9:188. doi: 10.3390/plants9020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomstedt CK, Gianello R, Hamill JD, et al. Drought-stimulated genes correlated with desiccation tolerance of the resurrection grass Sporobolus stapfianus. Plant Growth Regul. 1998;24:153–161. doi: 10.1023/A:1005923528109. [DOI] [Google Scholar]
- Borysiuk K, Ostaszewska-Bugajska M, Vaultier M-N, et al. Enhanced formation of methylglyoxal-derived advanced glycation end products in Arabidopsis under ammonium nutrition. Front Plant Sci. 2018;9:667. doi: 10.3389/fpls.2018.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britto D, Kronzucker H. NH4+ toxicity in higher plants: a critical review. J Plant Physiol. 2002;159:567–584. doi: 10.1078/0176-1617-0774. [DOI] [Google Scholar]
- Cabeza R, Koester B, Liese R, et al. An RNA sequencing transcriptome analysis reveals novel insights into molecular aspects of the nitrate impact on the nodule activity of Medicago truncatula. Plant Physiol. 2014;164:400–411. doi: 10.1104/pp.113.228312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C, Huang D, Huang Y, et al. Methylglyoxal induces cell death through endoplasmic reticulum stress-associated ROS production and mitochondrial dysfunction. J Cell Mol Med. 2016;20:1749–1760. doi: 10.1111/jcmm.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, An YC. Transcriptional responses to gibberellin and abscisic acid in barley aleurone. J Integr Plant Biol. 2006;48:591–612. doi: 10.1111/j.1744-7909.2006.00270.x. [DOI] [Google Scholar]
- Coruzzi G, Bush DR. Nitrogen and Carbon Nutrient and Metabolite Signaling in Plants. Plant Physiol. 2001;125:61. doi: 10.1104/pp.125.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deswal R, Sopory SK. Glyoxalase I from Brassica juncea is a calmodulin stimulated protein. BBA-Mol Cell Res. 1999;1450:460–467. doi: 10.1016/s0167-4889(99)00047-6. [DOI] [PubMed] [Google Scholar]
- Dhakarey R, Raorane ML, Treumann A, et al. Physiological and proteomic analysis of the rice mutant cpm2 suggests a negative regulatory role of jasmonic acid in drought tolerance. Front Plant Sci. 2017;8:1903. doi: 10.3389/fpls.2017.01903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espartero J, Sánchez-Aguayo I, Pardo JM. Molecular characterization of glyoxalase-I from a higher plant; upregulation by stress. Plant Mol Biol. 1995;29:1223–1233. doi: 10.1007/BF00020464. [DOI] [PubMed] [Google Scholar]
- Fancy NN, Bahlmann A-K, Loake GJ. Nitric oxide function in plant abiotic stress. Plant Cell Environ. 2017;40:462–472. doi: 10.1111/pce.12707. [DOI] [PubMed] [Google Scholar]
- Fredes I, Moreno S, Díaz FP, Gutiérrez RA. Nitrate signaling and the control of Arabidopsis growth and development. Curr Opin Plant Biol. 2019;47:112–118. doi: 10.1016/j.pbi.2018.10.004. [DOI] [PubMed] [Google Scholar]
- Fujita M, Fujita Y, Maruyama K, et al. A dehydration-induced NAC protein, RD26, is involved in a novel ABA-dependent stress-signaling pathway. Plant J. 2004;39:863–876. doi: 10.1111/j.1365-313X.2004.02171.x. [DOI] [PubMed] [Google Scholar]
- Ghorbani A, Pishkar L, Roudbari N, et al. Nitric oxide could allay arsenic phytotoxicity in tomato (Solanum lycopersicum L.) by modulating photosynthetic pigments, phytochelatin metabolism, molecular redox status and arsenic sequestration. Res Square. 2020 doi: 10.21203/rs.3.rs-129421/v1. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Islam T. Genome-wide analysis and expression profiling of glyoxalase gene families in soybean (Glycine max) indicate their development and abiotic stress specific response. BMC Plant Biol. 2016;16:87–87. doi: 10.1186/s12870-016-0773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Pareek A, Sopory SK, Singla-Pareek SL. A glutathione responsive rice glyoxalase II, Os GLYII-2, functions in salinity adaptation by maintaining better photosynthesis efficiency and anti-oxidant pool. Plant J. 2014;80:93–105. doi: 10.1111/tpj.12621. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Kushwaha HR, Hasan MR, et al. Presence of unique glyoxalase III proteins in plants indicates the existence of shorter route for methylglyoxal detoxification. Sci Rep. 2016;6:18358. doi: 10.1038/srep18358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie, E (1975) Cell-free microtubule assembly-evidence for control by glyoxalase. In Federation Proceedings, Federation Amer Soc Exp Biol Rockville Pike, Bethesda,34(3):541
- Gillespie E. Effects of S-lactoylglutathione and inhibitors of glyoxalase I on histamine release from human leukocytes. Nature. 1979;277(5692):135–137. doi: 10.1038/277135a0. [DOI] [PubMed] [Google Scholar]
- Goodarzi A, Namdjoyan S, Soorki AA. Effects of exogenous melatonin and glutathione on zinc toxicity in safflower (Carthamus tinctorius L.) seedlings. Ecotox Environ Safe. 2020;201:110853. doi: 10.1016/j.ecoenv.2020.110853. [DOI] [PubMed] [Google Scholar]
- Griesbach RJ. Biochemistry and genetics of flower color. Plant Breed Rev. 2005;25:89–114. doi: 10.1002/9780470650301.ch4. [DOI] [Google Scholar]
- Hasan MM, Ali MA, Soliman MH, et al. Insights into 28-homobrassinolide (HBR)-mediated redox homeostasis, AsA–GSH cycle, and methylglyoxal detoxification in soybean under drought-induced oxidative stress. J Plant Interact. 2020;15:371–385. doi: 10.1080/17429145.2020.1832267. [DOI] [Google Scholar]
- Hasanuzzaman M, Hossain MA, Fujita M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol Rep. 2011;5:353. doi: 10.1007/s11816-011-0189-9. [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman M, Nahar K, Alam MM, Fujita M. Exogenous nitric oxide alleviates high temperature induced oxidative stress in wheat (Triticum aestivum L.) seedlings by modulating the antioxidant defense and glyoxalase system. Aust J Crop Sci. 2012;6:1314. doi: 10.1007/s10646-013-1050-4. [DOI] [Google Scholar]
- Hasanuzzaman M, Alam MM, Nahar K, et al. Exogenous salicylic acid alleviates salt stress-induced oxidative damage in Brassica napus by enhancing the antioxidant defense and glyoxalase systems. Aust J Crop Sci. 2014;8:631–639. [Google Scholar]
- Hasanuzzaman M, Alam MdM, Rahman A, et al. Exogenous proline and glycinebetaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two Rice (Oryza sativa L.) Varieties. Biomed Res Int. 2014;2014:757219. doi: 10.1155/2014/757219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanuzzaman M, Nahar K, Hossain MS, et al. Nitric oxide pretreatment enhances antioxidant defense and glyoxalase systems to confer PEG-induced oxidative stress in rapeseed. J Plant Interact. 2017;12:323–331. doi: 10.1080/17429145.2017.1362052. [DOI] [Google Scholar]
- Hasanuzzaman M, Nahar K, Anee TI, Fujita M. Glutathione in plants: biosynthesis and physiological role in environmental stress tolerance. Physiol Mol Biol Pla. 2017;23:249–268. doi: 10.1007/s12298-017-0422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanuzzaman M, Nahar K, Gill SS, et al. Hydrogen peroxide pretreatment mitigates cadmium-induced oxidative stress in Brassica napus L.: an intrinsic study on antioxidant defense and glyoxalase systems. Front Plant Sci. 2017;8:115. doi: 10.3389/fpls.2017.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Zhou C, Huang M, et al. Glyoxalase system: a systematic review of its biological activity, related-diseases, screening methods and small molecule regulators. Biomed Pharmacother. 2020;131:110663. doi: 10.1016/j.biopha.2020.110663. [DOI] [PubMed] [Google Scholar]
- Hoque TS, Uraji M, Ye W, et al. Methylglyoxal-induced stomatal closure accompanied by peroxidase-mediated ROS production in Arabidopsis. J Plant Physiol. 2012;169:979–986. doi: 10.1016/j.jplph.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Hoque T, Uraji M, Tuya A, et al. Methylglyoxal inhibits seed germination and root elongation and up-regulates transcription of stress-responsive genes in ABA-dependent pathway in Arabidopsis. Plant Biol. 2012;14:854–858. doi: 10.1111/j.1438-8677.2012.00607.x. [DOI] [PubMed] [Google Scholar]
- Hoque TS, Hossain MA, Mostofa MG, et al. Methylglyoxal: an emerging signaling molecule in plant abiotic stress responses and tolerance. Front Plant Sci. 2016;7:1341. doi: 10.3389/fpls.2016.01341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MA, Fujita M. Hydrogen peroxide priming stimulates drought tolerance in mustard (Brassica juncea L.) seedlings. Plant Gene Trait. 2013;4:20. doi: 10.5376/pgt.2013.04.0020. [DOI] [Google Scholar]
- Hossain MA, Hasanuzzaman M, Fujita M. Up-regulation of antioxidant and glyoxalase systems by exogenous glycinebetaine and proline in mung bean confer tolerance to cadmium stress. Physiol Mol Biol Pla. 2010;16:259–272. doi: 10.1007/s12298-010-0028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MA, Bhattacharjee S, Armin S-M, et al. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front Plant Sci. 2015;6:420. doi: 10.3389/fpls.2015.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan S, Noman A, Kaya C, et al. 24-Epibrassinolide alleviates the injurious effects of Cr (VI) toxicity in tomato plants: Insights into growth, physio-biochemical attributes, antioxidant activity and regulation of Ascorbate–glutathione and Glyoxalase cycles. J Plant Growth Regul. 2020 doi: 10.1007/s00344-020-10169-2. [DOI] [Google Scholar]
- Jin SH, Li XQ, Wang GG, Zhu XT. Brassinosteroids alleviate high-temperature injury in Ficus concinna seedlings via maintaining higher antioxidant defence and glyoxalase systems. AoB P. 2015 doi: 10.1093/aobpla/plv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamran M, Xie K, Sun J, et al. Modulation of growth performance and coordinated induction of ascorbate-glutathione and methylglyoxal detoxification systems by salicylic acid mitigates salt toxicity in choysum (Brassica parachinensis L.) Ecotox Environ Safe. 2020;188:109877. doi: 10.1016/j.ecoenv.2019.109877. [DOI] [PubMed] [Google Scholar]
- Kaur C, Mustafiz A, Sarkar AK, et al. Expression of abiotic stress inducible ETHE1-like protein from rice is higher in roots and is regulated by calcium. Physiol Plant. 2014;152:1–16. doi: 10.1111/ppl.12147. [DOI] [PubMed] [Google Scholar]
- Kaur C, Singla-Pareek SL, Sopory SK. Glyoxalase and methylglyoxal as biomarkers for plant stress tolerance. Crit Rev Plant Sci. 2014;33(6):429–456. doi: 10.1080/07352689.2014.904147. [DOI] [Google Scholar]
- Kaur C, Sharma S, Singla-Pareek SL, Sopory SK. Methylglyoxal detoxification in plants: role of glyoxalase pathway. Indian J Plant Physiol. 2016;21:377–390. doi: 10.1007/s40502-016-0260-1. [DOI] [Google Scholar]
- Kaur C, Tripathi AK, Nutan KK, et al. A nuclear-localized rice glyoxalase I enzyme, OsGLYI-8, functions in the detoxification of methylglyoxal in the nucleus. Plant J. 2017;89:565–576. doi: 10.1111/tpj.13407. [DOI] [PubMed] [Google Scholar]
- Kaya C. Nitrate reductase is required for salicylic acid-induced water stress tolerance of pepper by upraising the AsA-GSH pathway and glyoxalase system. Phys Plant. 2020 doi: 10.1111/ppl.13153. [DOI] [PubMed] [Google Scholar]
- Kaya C, Ashraf M, Alyemeni MN, et al. Salicylic acid-induced nitric oxide enhances arsenic toxicity tolerance in maize plants by upregulating the ascorbate-glutathione cycle and glyoxalase system. J Hazard Mater. 2020;399(2020):123020. doi: 10.1016/j.jhazmat.2020.123020. [DOI] [PubMed] [Google Scholar]
- Kaya C, Murillo-Amador B, Ashraf M. Involvement of L-cysteine desulfhydrase and hydrogen sulfide in glutathione-induced tolerance to salinity by accelerating ascorbate-glutathione cycle and glyoxalase system in capsicum. Antioxidants. 2020;9:603. doi: 10.3390/antiox9070603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Takasaki H, Komatsu S. Comprehensive phosphoproteome analysis in rice and identification of phosphoproteins responsive to different hormones/stresses. J Proteome Res. 2005;4:1592–1599. doi: 10.1021/pr0501160. [DOI] [PubMed] [Google Scholar]
- Khan MIR, Jahan B, Alajmi MF, et al. Exogenously-sourced ethylene modulates defense mechanisms and promotes tolerance to zinc stress in mustard (Brassica juncea L.) Plants. 2019;8:540. doi: 10.3390/plants8120540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MIR, Jahan B, AlAjmi MF, et al. Ethephon mitigates nickel stress by modulating antioxidant system, glyoxalase system and proline metabolism in Indian mustard. Physiol Mol Biol Plants. 2020;26(6):1201–1213. doi: 10.1007/s12298-020-00806-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharbech O, Sakouhi L, Massoud MB, et al. Nitric oxide and hydrogen sulfide protect plasma membrane integrity and mitigate chromium-induced methylglyoxal toxicity in maize seedlings. Plant Phys Biochem. 2020;157:244–255. doi: 10.1016/j.plaphy.2020.10.017. [DOI] [PubMed] [Google Scholar]
- Kharbech O, Massoud MB, Sakouhi L, et al. Exogenous application of hydrogen sulfide reduces chromium toxicity in maize seedlings by suppressing NADPH oxidase activities and methylglyoxal accumulation. Plant Phys Biochem. 2020;154:646–656. doi: 10.1016/j.plaphy.2020.06.002. [DOI] [PubMed] [Google Scholar]
- Krouk G, Lacombe B, Bielach A, et al. Nitrate-Regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev. 2010;18:927–937. doi: 10.1016/j.devcel.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Kumar V, Yadav SK. Proline and betaine provide protection to antioxidant and methylglyoxal detoxification systems during cold stress in Camellia sinensis (L.) O. Kuntze Acta Physiol Plant. 2009;31:261–269. doi: 10.1007/s11738-008-0227-6. [DOI] [Google Scholar]
- Lewandowska A, Vo TN, Nguyen T-DH, et al. Bifunctional chloroplastic DJ-1B from arabidopsis thaliana is an oxidation-robust holdase and a glyoxalase sensitive to H2O2. Antioxidants. 2019;8(1):8. doi: 10.3390/antiox8010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z-G. Methylglyoxal and glyoxalase system in plants: old players, new concepts. Bot Rev. 2016;82:183–203. doi: 10.1007/s12229-016-9167-9. [DOI] [Google Scholar]
- Li Z-G. Regulative role of calcium signaling on methylglyoxal-improved heat tolerance in maize (Zea mays L) seedlings. Plant Signal Behav. 2020;15:1788303. doi: 10.1080/15592324.2020.1788303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z-G. Mechanisms of plant adaptation and tolerance to heat stress. In: Hasanuzzaman M, editor. Plant ecophysiology and adaptation under climate change: mechanisms and perspectives II. Singapore: Springer Nature; 2020. [Google Scholar]
- Li Z-G, Min X, Zhou Z-H. Hydrogen sulfide: a signal molecule in plant cross-adaptation. Front Plant Sci. 2016;7:1621. doi: 10.3389/fpls.2016.01621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z-G, Long W-B, Yang S-Z, et al. Signaling molecule methylglyoxal-induced thermotolerance is partly mediated by hydrogen sulfide in maize (Zea mays L.) seedlings. Acta Physiol Plant. 2018;40:76. doi: 10.1007/s11738-018-2653-4. [DOI] [Google Scholar]
- Li Z-G, Xu Y, Bai L-K, et al. Melatonin enhances thermotolerance of maize seedlings (Zea mays L.) by modulating antioxidant defense, methylglyoxal detoxification, and osmoregulation systems. Protoplasma. 2019;256:471–490. doi: 10.1007/s00709-018-1311-4. [DOI] [PubMed] [Google Scholar]
- Li C, Han Y, Hao J, et al. Effects of exogenous spermidine on antioxidants and glyoxalase system of lettuce seedlings under high temperature. Plant Signal Behav. 2020;15(12):1824697. doi: 10.1080/15592324.2020.1824697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W-Z, Kong D-D, Gu X-X, et al. Cytokinins can act as suppressors of nitric oxide in Arabidopsis. Proc Natl Acad Sci U S A. 2013;110:1548–1553. doi: 10.1073/pnas.1213235110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M-S, Li H-C, Lai Y-M, et al. Proteomics and transcriptomics of broccoli subjected to exogenously supplied and transgenic senescence-induced cytokinin for amelioration of postharvest yellowing. J Proteom. 2013;93:133–144. doi: 10.1016/j.jprot.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Maeta K, Izawa S, Inoue Y. Methylglyoxal, a metabolite derived from glycolysis, functions as a signal initiator of the high osmolarity glycerol-mitogen-activated protein kinase cascade and calcineurin/Crz1-mediated pathway in Saccharomyces cerevisiae. J Biol Chem. 2005;280:253–260. doi: 10.1074/jbc.M408061200. [DOI] [PubMed] [Google Scholar]
- Mahmud JA, Hasanuzzaman M, Nahar K, et al. γ-aminobutyric acid (GABA) confers chromium stress tolerance in Brassica juncea L. by modulating the antioxidant defense and glyoxalase systems. Ecotoxicology. 2017;26:1–16. doi: 10.1007/s10646-017-1800-9. [DOI] [PubMed] [Google Scholar]
- Maurino VG, Engqvist MKM. 2-hydroxy acids in plant metabolism. Arabidopsis Book. 2015;13:e0182. doi: 10.1199/tab.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merewitz E, Xu Y, Huang B. Differentially expressed genes associated with improved drought tolerance in creeping bentgrass overexpressing a gene for cytokinin biosynthesis. PLoS ONE. 2016;11:e0166676. doi: 10.1371/journal.pone.0166676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir MA, John R, Alyemeni MN, et al. Jasmonic acid ameliorates alkaline stress by improving growth performance, ascorbate glutathione cycle and glyoxylase system in maize seedlings. Sci Rep. 2018;8:1–13. doi: 10.1038/s41598-018-21097-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsumoto A, Kim K-R, Oshima G, et al. Nitric oxide inactivates glyoxalase I in cooperation with glutathione. J Biochem. 2000;128:647–654. doi: 10.1093/oxfordjournals.jbchem.a022797. [DOI] [PubMed] [Google Scholar]
- Mittler R. ROS Are Good. Trends Plant Sci. 2017;22:11–19. doi: 10.1016/j.tplants.2016.08.002. [DOI] [PubMed] [Google Scholar]
- Molla MdR, Ali M, Hasanuzzaman M, et al. Exogenous proline and betaine-induced upregulation of glutathione transferase and glyoxalase I in lentil (Lens culinaris) under drought stress. Not Bot Horti Agrobot Cluj Napoca. 2014;42(1):73–80. [Google Scholar]
- Moran E, Zamora-Álvarez L, Stephens-Camacho N, et al. Antioxidant capacity, radical scavenging kinetics and phenolic profile of methanol extracts of wild plants of southern Sonora, Mexico. Trop J of Pharm Res. 2014;13:1487–1493. doi: 10.4314/tjpr.v13i9.15. [DOI] [Google Scholar]
- Mostofa MG, Fujita M. Salicylic acid alleviates copper toxicity in rice (Oryza sativa L.) seedlings by up-regulating antioxidative and glyoxalase systems. Ecotoxicology. 2013;22:959–973. doi: 10.1007/s10646-013-1073-x. [DOI] [PubMed] [Google Scholar]
- Mostofa MG, Fujita M, Tran L-SP. Nitric oxide mediates hydrogen peroxide-and salicylic acid-induced salt tolerance in rice (Oryza sativa L.) seedlings. Plant Growth Regul. 2015;77:265–277. doi: 10.1007/s10725-015-0061-y. [DOI] [Google Scholar]
- Mostofa MG, Rahman A, Ansary MMU, et al. Hydrogen sulfide modulates cadmium-induced physiological and biochemical responses to alleviate cadmium toxicity in rice. Sci Rep. 2015;5:1–17. doi: 10.1038/srep14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofa MG, Rahman MM, Siddiqui MN, et al. Salicylic acid antagonizes selenium phytotoxicity in rice: selenium homeostasis, oxidative stress metabolism and methylglyoxal detoxification. J Hazard Mater. 2020;394:122572. doi: 10.1016/j.jhazmat.2020.122572. [DOI] [PubMed] [Google Scholar]
- Murata K, Sato N, Inoue Y, Kimura A. S-d-Lactoylglutathione: control of the cellular level by a yeast glyoxalase system. Agr Biol Chem. 1989;53:1999–2000. doi: 10.1080/00021369.1989.10869573. [DOI] [Google Scholar]
- Mustafiz A, Ghosh A, Tripathi A, et al. A unique Ni2 +-dependent and methylglyoxal-inducible rice glyoxalase I possesses a single active site and functions in abiotic stress response. Plant J. 2014;78(6):951–963. doi: 10.1111/tpj.12521. [DOI] [PubMed] [Google Scholar]
- Nahar K, Hasanuzzaman M, Alam MdM, Fujita M. Exogenous glutathione confers high temperature stress tolerance in mung bean (Vigna radiata L.) by modulating antioxidant defense and methylglyoxal detoxification system. Environ Exper Bot. 2015;112:44–54. doi: 10.1016/j.envexpbot.2014.12.001. [DOI] [Google Scholar]
- Nahar K, Hasanuzzaman M, Alam M, Fujita M. Roles of exogenous glutathione in antioxidant defense system and methylglyoxal detoxification during salt stress in mung bean. Biol Plant. 2015;59:745–756. doi: 10.1007/s10535-015-0542-x. [DOI] [Google Scholar]
- Nahar K, Hasanuzzaman M, Alam MdM, Fujita M. Exogenous spermidine alleviates low temperature injury in Mung Bean (Vigna radiata L.) seedlings by modulating ascorbate-glutathione and glyoxalase pathway. Int J Mol Sci. 2015;16(12):30117–30132. doi: 10.3390/ijms161226220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahar K, Hasanuzzaman M, Alam MM, Fujita M. Glutathione-induced drought stress tolerance in mung bean: coordinated roles of the antioxidant defence and methylglyoxal detoxification systems. AoB Plants. 2015 doi: 10.1093/aobpla/plv069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahar K, Hasanuzzaman M, Alam MM, et al. Insights into spermine-induced combined high temperature and drought tolerance in mung bean: osmoregulation and roles of antioxidant and glyoxalase system. Protoplasma. 2017;254:445–460. doi: 10.1007/s00709-016-0965-z. [DOI] [PubMed] [Google Scholar]
- Namdjoyan S, Kermanian H, Soorki AA, et al. Interactive effects of salicylic acid and nitric oxide in alleviating zinc toxicity of Safflower (Carthamus tinctorius L.) Ecotoxicology. 2017;26:752–761. doi: 10.1007/s10646-017-1806-3. [DOI] [PubMed] [Google Scholar]
- Namdjoyan S, Soorki AA, Elyasi N, et al. Melatonin alleviates lead-induced oxidative damage in safflower (Carthamus tinctorius L.) seedlings. Ecotoxicology. 2020;29:108–118. doi: 10.1007/s10646-019-02136-9. [DOI] [PubMed] [Google Scholar]
- Naser Alavi SM, Arvin MJ, Manoochehri Kalantari K. Salicylic acid and nitric oxide alleviate osmotic stress in wheat (Triticum aestivum L.) seedlings. J Plant Interact. 2014;9(1):683–688. doi: 10.1080/17429145.2014.900120. [DOI] [Google Scholar]
- Nishiyama R, Le DT, Watanabe Y, et al. Transcriptome analyses of a salt-tolerant cytokinin-deficient mutant reveal differential regulation of salt stress response by cytokinin deficiency. PLoS One. 2012 doi: 10.1371/journal.pone.0032124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozyamak E, Black SS, Walker CA, et al. The critical role of S-lactoylglutathione formation during methylglyoxal detoxification in Escherichia coli. Mol Microbiol. 2010;78:1577–1590. doi: 10.1111/j.1365-2958.2010.07426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvin K, Hasanuzzaman M, Bhuyan MHM, et al. Quercetin mediated salt tolerance in tomato through the enhancement of plant antioxidant defense and glyoxalase systems. Plants. 2019;8:247. doi: 10.3390/plants8080247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvin K, Nahar K, Hasanuzzaman M, et al. Exogenous vanillic acid enhances salt tolerance of tomato: Insight into plant antioxidant defense and glyoxalase systems. Plant Physiol Biochem. 2020;50:109–120. doi: 10.1016/j.plaphy.2020.02.03. [DOI] [PubMed] [Google Scholar]
- Pathak RR, Jangam AP, Malik A, et al. Transcriptomic and network analyses reveal distinct nitrate responses in light and dark in rice leaves (Oryza sativa Indica var. Panvel1) Sci Rep. 2020;10:12228. doi: 10.1038/s41598-020-68917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus C, Köllner B, Jacobsen H-J. Physiological and biochemical characterization of glyoxalase I, a general marker for cell proliferation, from a soybean cell suspension. Planta. 1993;189:561–566. doi: 10.1007/BF00198220. [DOI] [PubMed] [Google Scholar]
- Proietti S, Caarls L, Coolen S, et al. Genome-wide association study reveals novel players in defense hormone crosstalk in Arabidopsis. Plant Cell Environ. 2018;41:2342–2356. doi: 10.1111/pce.13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proietti S, Falconieri G, Bertini L, et al. GLYI4 plays a role in methylglyoxal detoxification and jasmonate-mediated stress responses in Arabidopsis thaliana. Biomolecules. 2019;9:635. doi: 10.3390/biom9100635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C, Sun J, Wang Y, et al. First nitrosoproteomic profiling deciphers the cysteine S-nitrosylation involved in multiple metabolic pathways of tea leaves. Sci Rep. 2019;9:17525. doi: 10.1038/s41598-019-54077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Mostofa MG, Alam M, et al. Calcium mitigates arsenic toxicity in rice seedlings by reducing arsenic uptake and modulating the antioxidant defense and glyoxalase systems and stress markers. Biomed Res Int. 2015;2015:340812. doi: 10.1155/2015/340812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Nahar K, Hasanuzzaman M, Fujita M. Calcium supplementation improves Na+/K+ ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Front Plant Sci. 2016;7:609. doi: 10.3389/fpls.2016.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh SA, Tyerman SD, Gilliham M, Xu B. γ-Aminobutyric acid (GABA) signalling in plants. Cell Mol Life Sci. 2017;74:1577–1603. doi: 10.1007/s00018-016-2415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy K, De S, Ray M, Ray S. Methylglyoxal can completely replace the requirement of kinetin to induce differentiation of plantlets from some plant calluses. Plant Growth Regul. 2004;44:33–45. doi: 10.1007/s10725-004-1634-3. [DOI] [Google Scholar]
- Saito R, Yamamoto H, Makino A, et al. Methylglyoxal functions as Hill oxidant and stimulates the photoreduction of O(2) at photosystem I: a symptom of plant diabetes. Plant Cell Environ. 2011;34:1454–1464. doi: 10.1111/j.1365-3040.2011.02344.x. [DOI] [PubMed] [Google Scholar]
- Samanta A, Das G, Das SK. Roles of flavonoids in plants. Novel Sci Int J Pharm Sci. 2011;6:12–35. doi: 10.1017/jns.2016.41. [DOI] [Google Scholar]
- Saxena M, Bisht R, Roy SD, et al. Cloning and characterization of a mitochondrial glyoxalase II from Brassica juncea that is upregulated by NaCl, Zn, and ABA. Biochem Biophys Res Commun. 2005;336:813–819. doi: 10.1016/j.bbrc.2005.08.178. [DOI] [PubMed] [Google Scholar]
- Saxena SC, Kaur H, Verma P, Petla BP, Andugula VR, Majee M. Osmoprotectants: potential for crop improvement under adverse conditions. In: Tuteja N, Singh Gill S, editors. Plant acclimation to environmental stress. New York, NY: Springer; 2013. [Google Scholar]
- Schmitz J, Heinrichs L, Scossa F, Fernie AR, Oelze ML, Dietz KJ, Rothbart M, Grimm B, Flügge UI, Häusler RE. The essential role of sugar metabolism in the acclimation response of Arabidopsis thaliana to high light intensities. J Exp Bot. 2014;65(6):1619–1636. doi: 10.1093/jxb/eru027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J, Dittmar IC, Brockmann JD, et al. Defense against reactive carbonyl species involves at least three subcellular compartments where individual components of the system respond to cellular sugar status. Plant Cell. 2017;29:3234. doi: 10.1105/tpc.17.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehrawat A, Deswal R. Sub-proteome S-nitrosylation analysis in Brassica juncea hints at the regulation of Brassicaceae specific as well as other vital metabolic pathway (s) by nitric oxide and suggests post-translational modifications cross-talk. Nitric Oxide. 2014;43:97–111. doi: 10.1016/j.niox.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Sehrawat A, Deswal R. S-nitrosylation analysis in Brassica juncea apoplast highlights the importance of nitric oxide in cold-stress signaling. J Proteome Res. 2014;13:2599–2619. doi: 10.1021/pr500082u. [DOI] [PubMed] [Google Scholar]
- Sehrawat A, Abat JK, Deswal R. RuBisCO depletion improved proteome coverage of cold responsive S-nitrosylated targets in Brassica juncea. Front Plant Sci. 2013;4:342. doi: 10.3389/fpls.2013.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan C, Liang Z. Jasmonic acid regulates ascorbate and glutathione metabolism in Agropyron cristatum leaves under water stress. Plant Sci J. 2010;178:130–139. doi: 10.1016/j.plantsci.2009.11.002. [DOI] [Google Scholar]
- Shimakawa G, Suzuki M, Yamamoto E, et al. Why don’t plants have diabetes? Systems for scavenging reactive carbonyls in photosynthetic organisms: figure 1. Biochem Soc Trans. 2014;42:543–547. doi: 10.1042/BST20130273. [DOI] [PubMed] [Google Scholar]
- Siddiqui MH, Alamri S, Alsubaie QD, Ali HM. Melatonin and gibberellic acid promote growth and chlorophyll biosynthesis by regulating antioxidant and methylglyoxal detoxification system in tomato seedlings under salinity. J Plant Growth Regul. 2020 doi: 10.1007/s00344-020-10122-3. [DOI] [Google Scholar]
- Singh YJ, Grewal SK, Gill RK. Role of glutathione in methylglyoxal detoxification pathway during yellow mosaic virus (YMV) infection in black gram (Vigna mungo (L.) Hepper) Physiol Mol Plant Pathol. 2020;111:101513. doi: 10.1016/j.pmpp.2020.101513. [DOI] [Google Scholar]
- Singla-Pareek SL, Reddy MK, Sopory SK. Genetic engineering of the glyoxalase pathway in tobacco leads to enhanced salinity tolerance. Proc Natl Acad Sci USA. 2003;100:14672. doi: 10.1073/pnas.2034667100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla-Pareek SL, Kaur C, Kumar B, et al. Reassessing plant glyoxalases: large family and expanding functions. New Phytol. 2020;227:714–721. doi: 10.1111/nph.16576. [DOI] [PubMed] [Google Scholar]
- Soliman M, Alhaithloul HA, Hakeem KR, et al. Exogenous nitric oxide mitigates nickel-induced oxidative damage in eggplant by upregulating antioxidants, osmolyte metabolism, and glyoxalase systems. Plants. 2019;8:562. doi: 10.3390/plants8120562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi D, Inoue H, Odawara M, et al. The calvin cycle inevitably produces sugar-derived reactive carbonyl methylglyoxal during photosynthesis: a potential cause of plant diabetes. Plant Cell Physiol. 2014;55:333–340. doi: 10.1093/pcp/pcu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Konishi H, Khan M, Komatsu S. Proteome analysis of rice tissues by two-dimensional electrophoresis: an approach to the investigation of gibberellin regulated proteins. Mol Genet Genomics. 2004;270:485–496. doi: 10.1007/s00438-003-0929-9. [DOI] [PubMed] [Google Scholar]
- Tanou G, Job C, Belghazi M, et al. Proteomic signatures uncover hydrogen peroxide and nitric oxide cross-talk signaling network in citrus plants. J Proteome Res. 2010;9:5994–6006. doi: 10.1021/pr100782h. [DOI] [PubMed] [Google Scholar]
- Thanh Q, Xuan T. Foliar application of vanillic and p-hydroxybenzoic acids enhanced drought tolerance and formation of phytoalexin momilactones in rice. Arch Agron Soil Sci. 2018 doi: 10.1080/03650340.2018.1463520. [DOI] [Google Scholar]
- Thornalley PJ. The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem J. 1990;269:1–11. doi: 10.1042/bj2690001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornalley P, Tisdale M. Inhibition of proliferation of human promyelocytic leukaemia HL60 cells by SD-lactoylglutathione in vitro. Leuk Res. 1988;12:897–904. doi: 10.1016/0145-2126(88)90016-1. [DOI] [PubMed] [Google Scholar]
- Wan X-Y, Liu J-Y. Comparative proteomics analysis reveals an intimate protein network provoked by hydrogen peroxide stress in rice seedling leaves. Mol Cell Proteomics. 2008;7:1469–1488. doi: 10.1074/mcp.M700488-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Ma C, Pan Y, et al. Sugar beet M14 glyoxalase I gene can enhance plant tolerance to abiotic stresses. Int J Plant Res. 2013;126:415–425. doi: 10.1007/s10265-012-0532-4. [DOI] [PubMed] [Google Scholar]
- Zaid A, Mohammad F, Wani SH, Siddique KM. Salicylic acid enhances nickel stress tolerance by up-regulating antioxidant defense and glyoxalase systems in mustard plants. ECOTOX ENVIRON SAFE. 2019;180:575–587. doi: 10.1016/j.ecoenv.2019.05.042. [DOI] [PubMed] [Google Scholar]
- Zanganeh R, Jamei R, Rahmani F. Pre-sowing seed treatment with salicylic acid and sodium hydrosulfide confers Pb toxicity tolerance in maize (Zea mays L.) ECOTOX ENVIRON SAFE. 2020;206:111392. doi: 10.1016/j.ecoenv.2020.111392. [DOI] [PubMed] [Google Scholar]
- Zhao P, Zhang N, Yin Z, et al. Analysis of differentially expressed genes in response to endogenous cytokinins during cotton leaf senescence. Biol plant. 2013;57:425–432. doi: 10.1007/s10535-013-0324-2. [DOI] [Google Scholar]